Abstract
Indoxacarb and metaflumizone are two sodium channel blocker insecticides (SCBIs). They preferably bind to and trap sodium channels in the slow-inactivated non-conducting state, a mode of action similar to that of local anesthetics (LAs). Recently, two sodium channel mutations, F1845Y (F4i15Y) and V1848I (V4i18I), in the transmembrane segment 6 of domain IV (IVS6), were identified to be associated with indoxacarb resistance in Plutella xylostella. F4i15 is known to be critical for the action of LAs on mammalian sodium channels. Previously, mutation F4i15A in a cockroach sodium channel, BgNav1-1a, has been shown to reduce the action of lidocaine, a LA, but not the action of SCBIs. In this study, we introduced mutations F4i15Y and V4i18A/I individually into the cockroach sodium channel, BgNav1-1a, and conducted functional analysis of the three mutants in Xenopus oocytes. We found that both the F4i15Y and V4i18I mutations reduced the inhibition of sodium current by indoxacarb, DCJW (an active metabolite of indoxacarb) and metaflumizone. F4i15Y and V4i18I mutations also reduced the use-dependent block of sodium current by lidocaine. In contrast, substitution V4i18A enhanced the action metaflumizone and lidocaine. These results show that both F4i15Y and V4i18I mutations may contribute to target-site resistance to SCBIs, and provide the first molecular evidence for common amino acid determinants on insect sodium channels involved in action of SCBIs and LA.
Introduction
Voltage-gated sodium channels are critical for the initiation and propagation of action potentials in nerves and other excitable cells. Like mammalian sodium channels, insect sodium channels are comprised by four homologous domains (I-IV), each having six membrane spanning helical segments (S1-S6) (Catterall, 2014; Dong et al., 2014). In response to membrane depolarization, the S4 segments move outward, initiating the opening of the activation gate, which is formed by cytoplasmic ends of each S6 (i.e., activation). Within a few milliseconds, sodium channels close or inactivate, which is known as fast inactivation. Prolonged depolarization, however, causes sodium channels enter into a different inactivated state, slow inactivation, that is distinct from fast inactivation. Recovery from fast inactivation takes tens of milliseconds, whereas recovery from slow inactivation requires seconds to minutes of membrane repolarization to return to a resting state (Goldin, 2003; Vilin and Ruben, 2001).
Indoxacarb and metaflumizone are two sodium channel blocker insecticides (SCBIs; Fig. 1). Indoxacarb, the first registered insecticide of this class, causes cessation of feeding, poor coordination, paralysis, and death (Harder et al., 1996; Narahashi, 2001; Silver and Soderlund, 2005; Wing et al., 2005) in a wide range of agricultural pests. Indoxacarb, a proinsecticide, is activated within insects to its more potent, N-decarbomethoxylated metabolite, DCJW (Fig. 1) (Wing et al., 2005; Wing et al., 2000; Wing et al., 1998). Metaflumizone, the second commercialized SCBI, causes poisoning symptoms that are similar to those produced by indoxacarb (Salgado and Hayashi, 2007). Interestingly, SCBIs share a similar mode of action with local anesthetics (LAs), such as lidocaine, anticonvulsants and antiarrhythmics (Salgado, 1992; Salgado and Hayashi, 2007; Wing et al., 2005; Wing et al., 2000; Wing et al., 1998). LAs and related drugs interrupt the initiation and propagation of nerve impulses (i.e., action potentials) by blocking sodium channels, thereby relieving or preventing pain (Catterall, 1987). These compounds preferentially block open and inactivated states of the sodium channel and have a lower affinity to channels in the resting state (Fozzard et al., 2005; Hille, 2001). Similarly, SCBIs inhibit sodium channel function by binding selectively to slow-inactivated states (Silver et al., 2010).
Figure 1.
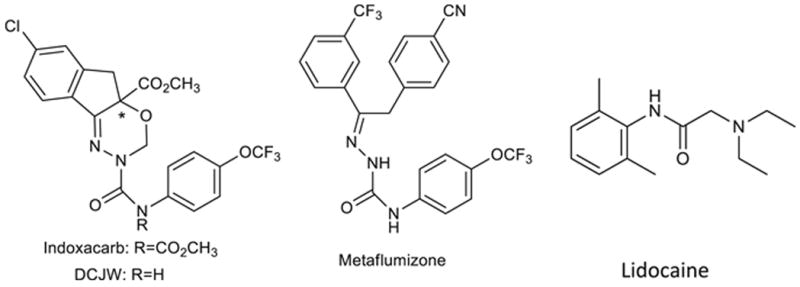
Chemical structures of indoxacarb, DCJW, metaflumizone and lidocaine.
LAs, anticonvulsants and antiarrhythmics bind to a receptor in the inner pore of sodium channels and impede ion permeation (Catterall, 2012). Site-directed mutagenesis studies with mammalian sodium channels revealed that the receptor site for these compounds is formed by amino acid residues in the S6 segments in domains I, III and IV (Catterall, 2012; Mike and Lukacs, 2010). In particular, two LA-sensing residues in IVS6, i.e., F1764 and Y1771 in rat Nav1.2 and F1579 and Y1586 in Nav1.4, are critical for the binding and action of LAs and related drugs on mammalian sodium channels. To facilitate recognition of these mutations among sodium channels from various species, here we use a nomenclature universal for P-loop ion channels (Du et al., 2013; Zhorov and Tikhonov, 2004). It provides a common designation of the two residues in various sodium channels as F4i15 and Y4i21, where 4i denotes the domain 4 inner helix (IVS6), and 15 and 21 are the relative numbers of the residues in IVS6.
Soderlund and associates have investigated the role of F4i15 and Y4i21 in the action of SCBIs on mammalian sodium channels (Silver and Soderlund, 2007; von Stein et al., 2013). Similar to the effect on the action of LAs, alanine substitution, F4i15A, resulted in a significant reduction in the ability of DCJW and RH3421, a different experimental SCBI, to inhibit Nav1.4 sodium channels expressed in Xenopus oocytes (Silver and Soderlund, 2007). In contrast, mutation of the tyrosine residue, Y4i21, to alanine in Nav1.4 channels resulted in a significant increase in the potency of indoxacarb, DCJW, and RH3421 (Silver and Soderlund, 2007). Mutational analysis of F4i15 and Y4i21 in a cockroach sodium channel, BgNav1-1a, revealed that neither F4i15A or Y4i21A reduce the action of SCBIs on BgNav1-1a channels (Silver et al., 2009). Nevertheless, both F4i15A and Y4i21A reduce the use-dependent block by lidocaine of BgNav1-1a channels (Song et al., 2011). These results suggest that these two residues contribute to the LA receptor site in insect sodium channels, but have a limited role in the action of SCBIs.
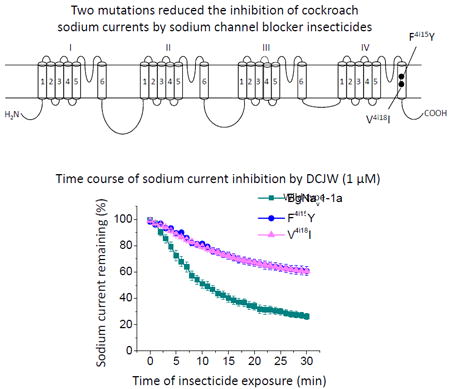
Recently, we identified two sodium channel mutations, F4i15Y and V4i18I (Fig. 2), which were associated with high levels of resistance to SCBIs in field populations of the diamondback moth (P. xylostella) in China (Wang et al., 2015). Particularly, one population of P. xylostella (BY12) collected from Baiyun, Guangdong province of China in 2012, was 750-fold more resistant to indoxacarb and 70-fold more resistant to metaflumizone compared with a susceptible strain (Wang et al., 2015). Both mutations, F1845Y and V1848I in IVS6 (i.e., F4i15Y and V4i18I), were detected in the BY12 population. Furthermore, a significant correlation between allele frequencies of the two mutations and levels of resistance to both indoxacarb and metaflumizone was observed in multiple field-collected populations (Wang et al., 2015). Interestingly, F4i15 corresponds to the major LA-sensing residue in mammalian and cockroach sodium channels (Fig. 2). Valine V4i18 is three positions downstream of F4i15. The F4i15A substitution did not confer BgNav1-1a channels resistance to SCBIs (Silver et al., 2009). However, it remains unknown whether F4i15Y and/or V4i18I mutations alter the action of SCBIs on sodium channels.
Figure 2.
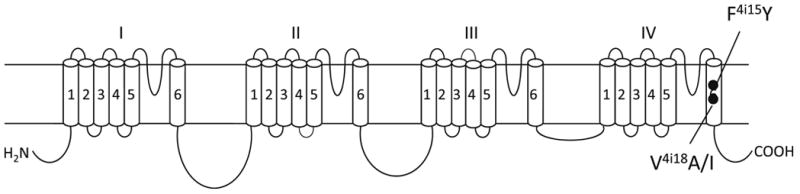
The topology of BgNav1-1a indicating the positions of two naturally occurring mutations, F4i15Y and V4i18I, which are associated with resistance to indoxacarb in P. xylostella (Wang et al., 2015). F4i15Y and V4i18I are labeled based on the nomenclature universal for P-loop ion channels (Du et al., 2013; Zhorov and Tikhonov, 2004). A residue label includes the domain number (1– 4), segment type (k, the linker-helix between S4 and S5; i, the inner helix S6; and o, the outer helix S5), and relative number of the residue in the segment.
Functional expression of sodium channels from the diamondback moth has not been established yet. Therefore, in this study we introduced the F4i15Y and V4i18A/I mutations into a well-characterized cockroach sodium channel, BgNav1-1a, and conducted functional analysis of the mutant channels in Xenopus oocytes using two-electrode voltage clamp. Both naturally occurring mutations, F4i15Y and V4i18I, introduced individually were found to reduce the ability of indoxacarb, DCJW and metaflumizone to inhibit sodium current. In contrast, the V4i18A mutation did not alter the action of indoxacarb and DCJW, but enhanced the inhibitory effect by metaflumizone. In addition, mutations F4i15Y and V4i18I were found to reduce the use-dependent block of sodium current by lidocaine, whereas the V4i18A mutation enhanced the blocking affect by lidocaine. These results demonstrate that F4i15 and V4i18 are involved in the action of both SCBIs and lidocaine, suggesting that SCBIs and lidocaine share overlapping receptor sites on the sodium channel.
Materials and Methods
Site-directed mutagenesis
Site-directed mutagenesis was performed by PCR using specific primers and Phusion High-Fidelity DNA polymerase (NEB, Ipswich, MA). All mutants were verified by DNA sequencing.
Expression of BgNav Sodium Channels in Xenopus laevis Oocytes
The procedures for oocyte preparation, cRNA synthesis and injection are identical to those described previously (Tan et al., 2002). For robust expression of the BgNav sodium channel, cRNA was co-injected into oocytes with Drosophila melanogaster tipE cRNA (1:1 ratio), which enhances the expression of insect sodium channels in oocytes (Feng et al., 1995; Warmke et al., 1997).
Electrophysiological Recording and Analysis
Sodium currents were recorded using the two-electrode voltage clamp technique. Electrodes were pulled from borosilicate glass and filled with 3 M KCl and 0.5% agarose. Resistances ranged between 0.5 and 1.5 MΩ. Currents were measured with an oocyte clamp amplifier OC725C (Warner Instrument Corp., Hamden, CT), Digidata 1440A (Axon Instruments, Foster City, CA), and pClamp 10.2 software (Axon Instruments). Capacitive transient and leak currents were subtracted using the P/N (N = 4) subtraction method.
Examination of BgNav channel sensitivity to SCBIs and lidocaine
The methods for measuring the effects of SCBIs and lidocaine on BgNav1-1a channels are similar to those described in Silver et al. (2009) and Song et al. (2011), respectively. Briefly, we measured the onset of block by SCBIs at or near the potential of 50% steady-state inactivation. After establishing a stable voltage clamp near the half-inactivation potential specific to a channel variant, insecticide-containing solution was perfused into the bath at a rate of 3 ml/min over the first 7-8 min whereas the time course of onset of block was recorded for 30 min.
For use- and frequency dependence of block, we measured peak current by delivering a train of 50 pulses (a 20 ms test pulse to −10 mV from the holding potential of −120 mV) at a frequency of 20 Hz or at a range of frequencies between 1 and 20 Hz, respectively. The amplitude of sodium current elicited by each pulse was then normalized to the amplitude of the peak sodium current generated by the initial pulse.
All experiments were performed at room temperature. Indoxacarb and DCJW were provided by K. D. Wing and D. Cordova (DuPont Agrochemicals) and metaflumizone was provided by V. Salgado (BASF Agricultural Products). Lidocaine was purchased from Sigma (L-7757). Drugs and insecticides were perfused onto oocytes in a manner similar to that previously described (Tatebayashi and Narahashi, 1994).
Data are presented as mean ± SEM. Statistical analysis was determined using a one-way ANOVA test and Scheffe’s post hoc analysis. Significance values were set at p< 0.05 or as indicated in the table and figure legends.
Molecular modeling
A homology model of the cockroach sodium channel variant BgNav1-1 was constructed based on the crystal structure of the NavAb sodium channel (PMID: 22678295) as described elsewhere (Du et al., 2011) .
Results
3.1. F4i15Y and V4i18I mutant channels are more resistant to indoxacarb, DCJW and metaflumizone than wild type channels
We introduced F4i15Y and V4i18A/I into a cockroach sodium channel, BgNav1-1a, and first examined the effects of the mutations on the gating properties. All three mutant channels generated sodium currents in Xenopus oocytes that were sufficient for functional analysis. Compared to the wild-type, none of the mutations altered the voltage dependence of activation or fast or slow inactivation (Figs. 3; Table 1). However, both F4i15Y and V4i18I mutations caused incomplete slow inactivation. As shown in Figure 3B, about 30% of sodium currents remained at -30 mV and beyond for F4i15Y channels and the modification in slow activation was less extensive for V4i18I channels.
Figure 3.
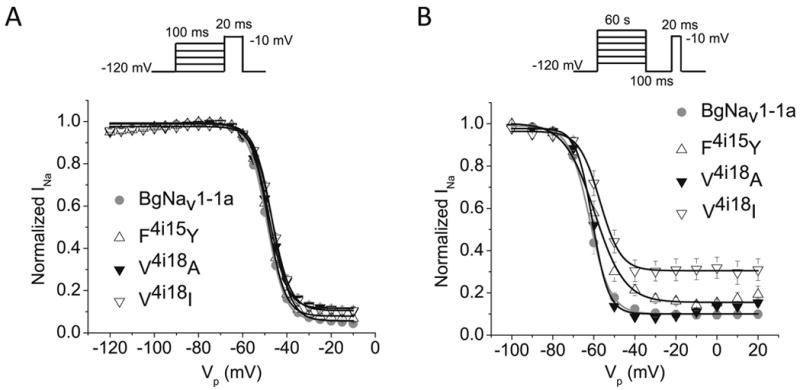
Effect of F4i15Y and V4i18A/I substitutions on the voltage dependence of fast (A) and slow (B) inactivation of BgNav1-1a channels. A. Voltage dependence of fast inactivation. B. Voltage dependence of slow inactivation. The voltage dependences were measured using a series of prepulse potentials (Vp) as indicated in the recording protocols.
Table 1.
Voltage-dependence of activation, fast and slow inactivation of BgNav1-1a and mutant channels at the holding potential of -120 mV.
| Activation | Fast Inactivation | Slow Inactivation | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| V1/2 (mV) | k | V1/2 (mV) | k | V1/2 (mV) | k | |
| BgNav1-1a | -28.2 ± 0.5 | 5.1 ± 0.9 | -49.0 ± 0.5 | 4.2 ± 0.1 | -54.6 ± 0.9 | 4.2 ± 0.2 |
| F4i15Y | -30.7 ± 1.3 | 5.4 ± 0.4 | -48.4 ± 0.5 | 4.0 ± 0.1 | -58.5 ± 1.3 | 5.5 ± 0.3 |
| V4i18I | -27.4 ± 1.4 | 5.4 ± 0.6 | -46.8 ± 0.5 | 4.1 ± 0.1 | -56.7 ± 1.5 | 3.9 ± 0.2 |
| V4i18A | -33.6 ±1.5 | 6.3 ± 0.9 | -50.0 ± 1.1 | 4.9 ± 0.5 | 60.7 ± 0.4 | 3.7 ± 0.3 |
The voltage dependences of conductance and inactivation were fitted with a two-state Boltzmann equation to determine V1/2, the voltage for half- maximal conductance or inactivation, and k, the slope factor for conductance or inactivation. The values in the table represent the mean ± S.E.M. and the number of oocytes was 6-13.
Figure 4A shows a representative trace of BgNav1-1a sodium current elicited by a 20 ms depolarization to -10 mV from a holding potential of -120 mV. Perfusion of oocytes with 1 μM DCJW for 30 min at a holding potential of -120 mV, did not alter the amplitude of BgNav1-1a currents (Fig. 4A), indicating DCJW had no effect on sodium channels in the resting state. The same results were observed from the three mutants (data not shown). However, perfusion with 1 μM of DCJW for 30 min at -55 mV (the V1/2 of slow inactivation for BgNav1-1a channels) or -60 mV (the V1/2 of slow inactivation for mutant BgNav1-1a channels) caused a gradual decrease in BgNav1-1a current (Fig. 4B). This is consistent with findings from previous studies (Silver et al., 2009), confirming that SCBIs inhibit BgNav1-1a channels by binding to inactivated states. We then compared the state-dependent inhibition of sodium channels by indoxacarb, DCJW, or metaflumizone between wild-type, F4i15Y and V4i18I/A channels at depolarized holding potentials during 30 min of insecticide exposure.
Figure 4.
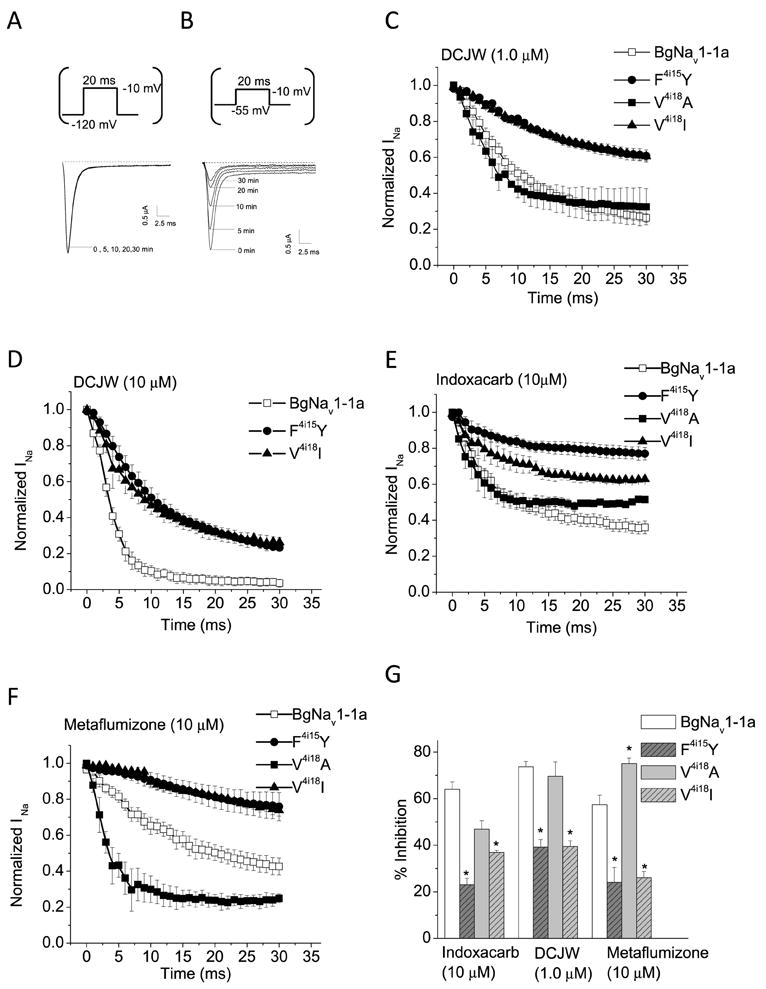
Time course of inhibition of BgNav1-1a, F4i15Y, and V4i18A/I sodium channels by indoxacarb, DCJW, and metaflumizone. A and B. Representative BgNav1-1a currents recorded with test pulses to -10 mV from the hyperpolarizing holding potential of -120 mV (A) or the depolarizing holding potential of -55 mV (B) at different time points in the presence of 1 μM DCJW. The recording trace immediately preceding the sodium currents was a large capacity current which is not shown and the baseline is indicated with a dash line. C and D. Inhibition of peak sodium currents by 1 μM (C) and 10 μM DCJW (D). E and F. Inhibition of peak sodium currents by 10 μM indoxacarb and 10 μM metaflumizone. To measure the inhibition of peak current by SCBIs, test pulses (20 ms) to -10 mV from a depolarizing holding potential (-55 mV for BgNav1-1a and −60 mV for F4i15Y and V4i18A/I channels) were given once every minute to record the remaining sodium current. The remaining sodium current was then normalized to the current measured prior to application of insecticide. Reduction in “Normalized INa” reflects the progress of channel inhibition by SCBIs.
Figure 4C shows the time courses of inhibition of BgNav1-1a wild-type and F4i15Y and V4i18A/I mutant channels by 1.0 μM DCJW. For both BgNav1-1a and V4i18A channels, inhibition of sodium current increased steadily after DCJW exposure. No inhibition was observed in the absence of DCJW (data not shown). After 30 min exposure of WT and V4i18A channels to 1.0 μM DCJW, peak sodium currents decreased by ~74% and 70%, respectively, whereas peak sodium current in the F4i15Y and V4i18I channels decreased by only 39 - 40% (Fig. 4C and Table 2). However, 10 μM DCJW reduced the currents in the F4i15Y and V4i18I mutants by about 77% and 74%, respectively (Fig. 4D and Table 2). This effect was comparable with inhibition of sodium currents by 1 μM DCJW on the WT channels (Table 2), indicating that the mutants were ~10- fold less sensitive to DCJW than the WT channel. Furthermore, unlike the naturally occurring mutations, F4i15Y and V4i18I, the V4i18A mutation did not alter the inhibition by DCJW (Fig. 4C).
Table 2.
Percentage of inhibition of BgNav1-1a and mutant channels by indoxacarb (10 μM), DCJW (1 and 10 μM) and metaflumizone (10 μM) at the end of 30 min insecticide exposure.
| Indoxacarb | DCJW | Metaflumizone | |||
|---|---|---|---|---|---|
|
| |||||
| 10.0 | 0.1 | 1.0 | 10.0 | 10.0 | |
| BgNav1-1a | 64.0 ± 3.2 | 39.0 ± 4.2 | 73.7 ± 2.3 | ND | 57.4 ± 4.1 |
| F4i15Y | 23.0 ± 2.8* | ND | 39.2 ± 3.3* | 76.7 ± 1.3 | 24.1 ± 6.4* |
| V4i18I | 36.9 ± 0.9* | ND | 39.5 ± 2.4* | 73.8 ± 2.2 | 26.1 ± 2.6* |
| V4i18A | 46.9 ± 3.7 | ND | 69.6 ± 6.2 | ND | 75.3 ± 3.1* |
The values of percentage of inhibition were determined by comparing values of “normalized INa” of channels treated with insecticide to untreated channels at the end of the 30 minutes recording period (See Fig.3). The values in the table represent the mean ± S.E.M. and the number of oocytes was 4-10. The asterisks indicate significant differences from the BgNav1-1a channel as determined by one-way ANOVA (p<0.05) with Scheffe’s post hoc analysis. ND: not determined.
Apparently, the extent of inhibition by indoxacarb and metaflumizone on the wild-type and F4i15Y and V4i18I mutant channels are similar. Following 30 min of exposure to 10 μM indoxacarb, BgNav1-1a sodium currents were reduced by about 64% (Fig. 4E), whereas F4i15Y and V4i18I currents were reduced only by about 23% and 37%, respectively (Fig. 4E and 4G). 10 μM metaflumizone inhibited peak sodium currents in the WT, F4i15Y and V4i18I channels by ~ 57, 24 and 26%, respectively (Fig. 4F, G and Table 2). Unlike the V4i18I mutant channels, the degree of inhibition of V4i18A channels by indoxacarb was similar to that of BgNav1-1a channels (Fig, 4E and 4G). In contrast, 10 μM metaflumizone inhibited the V4i18A channels by 75%, which is more than that in WT channels (Fig. 4F and 4G).
3.2. F4i15Y and V4i18I mutant channels were resistant to lidocaine, but V4i18A channels were more sensitive to lidocaine
It is well established that LAs preferentially bind to open and inactivated states of the mammalian sodium channel and cause their use-dependent and frequency-dependent block (Li et al., 1999). Here we employed rapid trains of stimulus pulses to promote binding of lidocaine to open and inactivated channels and compared the responses of F4i15Y and V4i18I/A mutant channels with those of BgNav1-1a. As shown in Fig. 5, inhibition of BgNav1-1a channels by lidocaine gradually enhanced with the increase of pulse numbers or frequency. However, little enhancement was observed for the F4i15Y and V4i18I channels with either changes in pulse number or frequency. In contrast, inhibition of V4i18A mutant channels by lidocaine was stronger than that for the WT channel at all pulse numbers or frequencies (Fig. 5).
Figure 5.
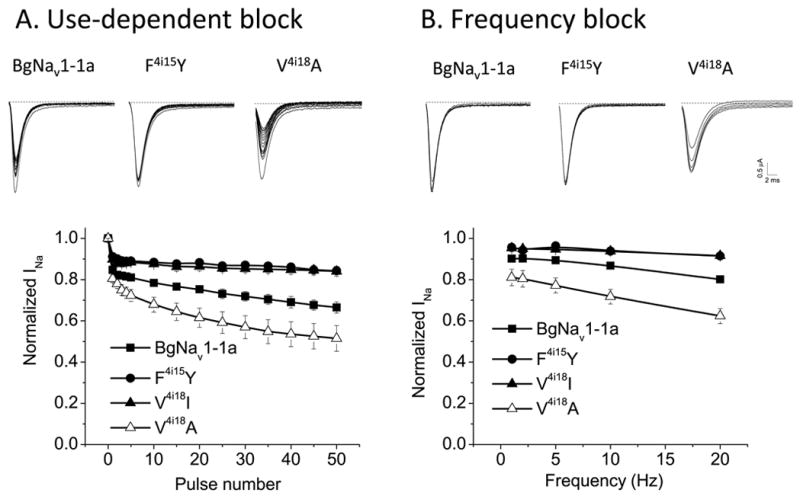
Use-dependent block (A) and frequency-dependent block (B) of wildtype and mutant channels by lidocaine (2 mM).The recording protocol are shown and details are provided under Materials and Methods. The recording trace immediately preceding the sodium currents was a large capacity current which is not shown and the baseline is indicated with a dash line.
3.3. Possible location of binding sites for SCBIs
Figure 6 shows side (Fig. 6A) and top (Fig. 6B) views of the NavAb-based homology model of the BgNav1-1 channel with space-filled sidechains of F4i15 and V4i18. For comparison, the space-filled models of DCJW are shown in two projections, in the same scale as the channel model. The V4i18 sidechain faces the inner pore, whereas the F4i15 sidechain may move between the inner pore and the III/IV domain interface. Location of F4i15 and V4i18 suggests that SCBIs bind in the inner pore and may expand a hydrophobic moiety into the III/IV domain interface.
Figure 6.
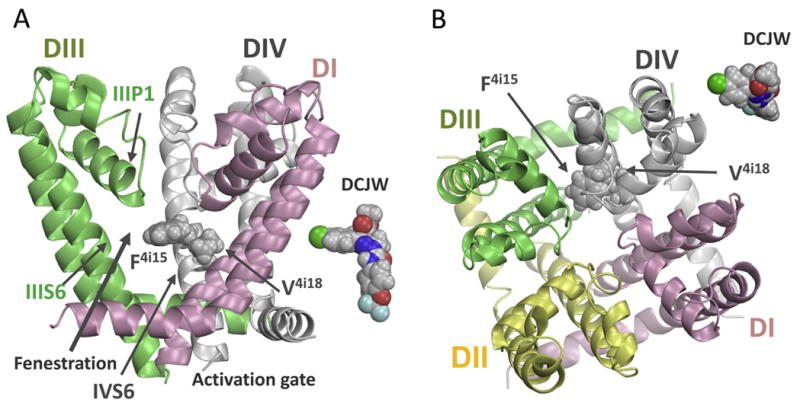
Side (A) and extracellular (B) views of the pore module in the NavAb-based homology model of the insect sodium channel BgNav1-1a. Domains DI, DII, DIII, and DIV are shown by pink, yellow, green, and gray ribbons, respectively. Domain DII is removed at the side view for clarity. Side chains of residues F4i15 and V4i18 are space-filled. For comparison, “side” and “top” views of a DCJW conformer are placed next to respective views of the channel. Mutations F4i15Y and V4i18I enlarge respective residues suggesting that DCJW binds tightly in the inner pore, forms close contacts with F4i15 and V4i18, and may expose its hydrophobic moiety to F4i15.
Discussion
The mode of action of SCBIs is different from those of other classes of insecticides that act on sodium channels, including pyrethroid insecticides. Therefore, SCBIs have been excellent alternatives for controlling insect pest populations which have developed resistance to pyrethroid insecticides due to target-site modifications (Wing et al., 2005). However, in recent years, resistance to SCBIs began to emerge in field populations of various lepidopteran pests, including Plutella xylostella (Khakame et al., 2013; Santos et al., 2011; Sayyed and Wright, 2006; Zhao et al., 2006), Spodoptera litura (Shad et al., 2012; Tong et al., 2013) and Spodoptera exigua (Che et al., 2013; Tong et al., 2013; Zhou et al., 2011). More recently, two sodium channel mutations, F1845Y and V1848 (i.e., F4i15Y and V4i18I), were found to be associated with SCBI resistance in diamondback moth populations in China (Wang et al., 2015). This study represents the first effort to characterize the effect of naturally occurring sodium channel mutations on the action of SCBIs. Our functional analysis of the mutations in cockroach sodium channels expressed in Xenopus oocytes show that both F4i15Y and V4i18I mutations reduced the potency of indoxacarb, DCJW and metaflumizone, indicating that these mutations likely contribute to SCBI resistance in diamondback moth populations. The findings from Wang et al. (2015) and this study provide the molecular evidence for target-site modification as a major mechanism of SCBI resistance, and the two mutations could be used as molecular markers for resistance monitoring in field populations of the diamondback moth and possibly in other pest species.
In insects, indoxacarb is metabolically converted to the more active metabolite DCJW (Wing et al., 2005). While DCJW is a more potent blocker of sodium channels, it is well-documented in the literature that indoxacarb exhibits a modest level of blocking effect on most mammalian sodium channel isoforms expressed in Xenopus oocytes (von Stein et al., 2013) and in mammalian neurons (Zhao et al., 2003). Indoxacarb also inhibits cockroach sodium channels in primary neurons (Zhao et al., 2005) and cockroach sodium channels expressed in Xenopus oocytes (Silver et al., 2009). The effects of indoxacarb on wild-type cockroach sodium channels from our current study are consistent with those reported in our previous study (Silver et al., 2009).
Identification of these naturally occurring mutations in sodium channels are also valuable resources for elucidating the molecular basis of binding and action of SCBIs on sodium channels. Systematic site-directed mutagenesis of residues using alanine substitutions have been successfully employed in identification of major residues for LA binding in mammalian sodium channels. A number of residues in the S6 transmembrane segments of domains I, III, and IV are thought to affect therapeutic drug activity directly in rat Nav1.2 sodium channels (Ragsdale et al., 1994, 1996; Yarov-Yarovoy et al., 2001; Yarov-Yarovoy et al., 2002), and similar results have been reported for other mammalian sodium channel isoforms, including the Nav1.4 sodium channel (Nau et al., 1999; Wang et al., 2004; Wang et al., 2000; Wang and Wang, 1998). Because of similar modes of action between LA and SCBIs, residues necessary for LA activity have been used as a road map to determine their potential roles in the binding and action of SCBIs on insect sodium channels. However, alanine substitution of the key LA-sensing residue, F4i15, did not reduce the BgNav1-1a channel sensitivity to SCBIs (Silver et al., 2009), but reduced the effect of lidocaine (Song et al., 2011). In fact, the F4i15A substitution caused a slight (1.3-fold) increase in BgNav1-1a sodium channel sensitivity to DCJW. These earlier findings demand further evaluation on the role of F4i15 in the action of SCBIs on insect sodium channels (Silver et al., 2009). Here by examining a different substitution, F4i15Y, which emerged naturally due to intensive use of indoxacarb in controlling agricultural insect pests, we demonstrated that F4i15 is involved in the action of SCBIs on insect sodium channels.
Another significant finding of this study is that both F4i15Y and V4i18I mutations also confer BgNav channels resistance to a local anesthetic, providing further molecular evidence for overlapping receptors for SCBI and LA on sodium channels (Salgado and Hayashi, 2007; von Stein et al., 2013; Wing et al., 2005). Different substitutions at V4i18 affect the sensitivity of BgNav1-1a channels to different SCBIs and lidocaine differently. The V4i18I substitution confers resistance to lidocaine and all three SCBIs, whereas V4i18A substitution enhanced the sensitivity of BgNav1-1a channels to metaflumizone and lidocaine, but had little effect on the sensitivity of BgNav1-1a channels to indoxacarb or DCJW, indicating that changes at this amino acid affect both SCBI and LA activity and imply that both SCBIs and LA occupy this space in voltage-sensitive sodium channels.
Our mutational and electrophysiological analyses suggest that the SCBIs may interact with valine V4i18 and phenylalanine F4i15. Same-scale images of the NavAb-based model of the insect sodium channel pore module (Du et al., 2013) and DCJW suggest that the ligand may directly interact with the pore-facing F4i15 and V4i18 (Fig. 6). The finding that mutation F4i15A has little effect on the action of indoxacarb and DCJW, but enhances the action of metaflumizone (Silver et al., 2009) suggests that repulsing and attractive forces may be balanced for interactions of F4i15 with indoxacarb and DCJW, while metaflumizone repulsion from F4i15 may overbear attraction to F4i15. This proposition would be consistent with the large size of SCBI molecules that could tightly fit into the inner pore. Mutation F4i15Y may destabilize SCBIs due to unfavorable interactions of their hydrophobic moieties with the hydrophilic hydroxyl of tyrosine. The fact that mutation V4i18I decreases the potency of SCBIs also suggests that large SCBIs fit tightly into the inner pore. A ligand may form a close contact with V4i18, but repel from the bigger I4i18. Mutation V4i18A increases the potency of metaflumizone indicating that the ligand experiences repulsion from the bulky V4i18, but not from the small A4i18. Further research is needed to test these hypotheses.
The effects of BgNav1-1 mutations on the use- and frequency-dependent block by lidocaine (Fig. 5) are consistent with the open-state model of Nav1.4 in which the ligand binds in the inner pore between IS6, IIIS6 and IVS6, whereas its diethylamine, amide and dimethylphenyl moieties approach the pore-facing residues F4i15, V4i18 and Y4i22, respectively (Tikhonov and Zhorov, 2007). Lidocaine, which is much smaller than SCBIs (Fig. 1), is unlikely to experience van der Waals repulsions from inner pore residues. Mutation F4i15Y would impose unfavorable contact between the tyrosine hydrophilic group and hydrophobic groups of lidocaine. Similarly, the V4i18I mutation may shift the ligand farther from IVS6, thus deteriorating its interactions with F4i15 and Y4i22, whereas mutation V4i18A would allow a closer contacts of the ligand with IVS6 and stronger interactions with F4i15 and Y4i22.
In conclusion, here we functionally established that the potency of SCBIs on BgNav1-1a channels was reduced by two naturally occurring sodium channel mutations that are associated with SCBI resistance in the diamondback moth. Our study also provides important information on ligand-channel interactions, suggesting that the two mutations likely alter the binding of SCBIs to its receptor site on voltage-sensitive sodium channels. Our results also provide molecular evidence for the notion that the receptor sites of SCBIs and LAs overlap on insect sodium channels. Further modeling and mutational analysis are needed to define the receptor sites of these compounds on insect sodium channels.
Mutations F4i15Y and V4i18I reduced the sensitivity of cockroach sodium channels to SCBIs.
The two mutations also confer cockroach sodium channel resistance to lidocaine.
SCBIs and lidocaine share a common receptor site on cockroach sodium channels.
Acknowledgments
The study was supported by grants from the National Institutes of Health (GM057440 to KD and BSZ), the Ministry of Agriculture of China (No.201203038 to YW), the “111” project of the Ministry of Education of China (B07030 to YW). Dingxin Jiang was supported by a scholarship from the China Scholar Council. We thank Drs. Kris Silver, Daniel Cordova and Vincent Salgado for critical review of this manuscript. We would like to thank Drs. Keith D. Wing and Daniel Cordova (DuPont Agrochemicals) and Vincent Salgado (BASF Agricultural Products) for providing indoxacarb, DCJW and metaflumizone for this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Catterall M. “Controlled” clinical trials in neutron therapy. IntJ Radiat Oncol. 1987;13:1961–1965. doi: 10.1016/0360-3016(87)90367-1. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Voltage-gated sodium channels at 60: structure, function and pathophysiology. J Physiol. 2012;590:2577–2589. doi: 10.1113/jphysiol.2011.224204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA. Structure and function of voltage-gated sodium channels at atomic resolution. Exp Physiol. 2014;99:35–51. doi: 10.1113/expphysiol.2013.071969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che W, Shi T, Wu Y, Yang Y. Insecticide resistance status of field populations of Spodoptera exigua (Lepidoptera: Noctuidae) from China. J Econ Entomol. 2013;106:1855–1862. doi: 10.1603/ec13128. [DOI] [PubMed] [Google Scholar]
- Dong K, Du Y, Rinkevich F, Nomura Y, Xu P, Wang L, Silver K, Zhorov BS. Molecular biology of insect sodium channels and pyrethroid resistance. Insect Biochem Mol Biol. 2014;50:1–17. doi: 10.1016/j.ibmb.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Garden DP, Wang L, Zhorov BS, Dong K. Identification of new batrachotoxin-sensing residues in segment IIIS6 of the sodium channel. J Biol Chem. 2011;286:13151–13160. doi: 10.1074/jbc.M110.208496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Nomura Y, Satar G, Hu Z, Nauen R, He SY, Zhorov BS, Dong K. Molecular evidence for dual pyrethroid-receptor sites on a mosquito sodium channel. Proc Nat Acad Sci. 2013;110:11785–11790. doi: 10.1073/pnas.1305118110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng G, Deak Pt, Chopra M, Hall LM. Cloning and functional analysis of tipE, a novel membrane protein that enhances drosophila para sodium channel function. Cell. 1995;82:1001–1011. doi: 10.1016/0092-8674(95)90279-1. [DOI] [PubMed] [Google Scholar]
- Fozzard HA, Lee PJ, Lipkind GM. Mechanism of local anesthetic drug action on voltage-gated sodium channels. Curr Pharm Des. 2005;11:2671–2686. doi: 10.2174/1381612054546833. [DOI] [PubMed] [Google Scholar]
- Goldin AL. Mechanisms of sodium channel inactivation. Curr Opin Neurobiol. 2003;13:284–290. doi: 10.1016/s0959-4388(03)00065-5. [DOI] [PubMed] [Google Scholar]
- Harder HH, Riley SL, McCann SF, Irving SN. DPX-MP062: a novel broad-spectrum, environmentally soft, insect control compound. Proceedings of the Brighton Crop Protection Conference; Brighton, UK. 1996. pp. 449–454. [Google Scholar]
- Hille B. Ion channels of excitable membranes. Sinauer; Sunderland, MA: 2001. [Google Scholar]
- Khakame SK, Wang X, Wu Y. Baseline toxicity of metaflumizone and lack of cross resistance between indoxacarb and metaflumizone in diamondback moth (Lepidoptera: Plutellidae) J Econ Entomol. 2013;106:1423–1429. doi: 10.1603/ec12494. [DOI] [PubMed] [Google Scholar]
- Li HL, Galue A, Meadows L, Ragsdale DS. A molecular basis for the different local anesthetic affinities of resting versus open and inactivated states of the sodium channel. Mol Pharmacol. 1999;55:134–141. doi: 10.1124/mol.55.1.134. [DOI] [PubMed] [Google Scholar]
- Mike A, Lukacs P. The enigmatic drug binding site for sodium channel inhibitors. Curr Mol Pharmacol. 2010;3:129–144. doi: 10.2174/1874467211003030129. [DOI] [PubMed] [Google Scholar]
- Narahashi T. Recent Progress in the Mechanism of Action of Insecticides: Pyrethroids, Fipronil and Indoxacarb. J Pesticide Sci. 2001;26:277–285. [Google Scholar]
- Nau C, Wang SY, Strichartz GR, Wang GK. Point mutations at N434 in D1-S6 of mu1 Na(+) channels modulate binding affinity and stereoselectivity of local anesthetic enantiomers. Mol Pharmacol. 1999;56:404–413. doi: 10.1124/mol.56.2.404. [DOI] [PubMed] [Google Scholar]
- Ragsdale DS, McPhee JC, Scheuer T, Catterall WA. Molecular determinants of state-dependent block of Na+ channels by local anesthetics. Science. 1994;265:1724–1728. doi: 10.1126/science.8085162. [DOI] [PubMed] [Google Scholar]
- Ragsdale DS, McPhee JC, Scheuer T, Catterall WA. Common molecular determinants of local anesthetic, antiarrhythmic, and anticonvulsant block of voltage-gated Na+ channels. Proc Nat Acad Sci. 1996;93:9270–9275. doi: 10.1073/pnas.93.17.9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado VL. Slow voltage-dependent block of sodium channels in crayfish nerve by dihydropyrazole insecticides. Mol Pharmacol. 1992;41:120–126. [PubMed] [Google Scholar]
- Salgado VL, Hayashi JH. Metaflumizone is a novel sodium channel blocker insecticide. Vet Parasitol. 2007;150:182–189. doi: 10.1016/j.vetpar.2007.08.032. [DOI] [PubMed] [Google Scholar]
- Santos VC, de Siqueira HA, da Silva JE, de Farias MJ. Insecticide resistance in populations of the diamondback moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae), from the state of Pernambuco, Brazil. Neotrop Entomol. 2011;40:264–270. doi: 10.1590/s1519-566x2011000200017. [DOI] [PubMed] [Google Scholar]
- Sayyed AH, Wright DJ. Genetics and evidence for an esterase-associated mechanism of resistance to indoxacarb in a field population of diamondback moth (Lepidoptera: Plutellidae) Pest Manag Sci. 2006;62:1045–1051. doi: 10.1002/ps.1270. [DOI] [PubMed] [Google Scholar]
- Shad SA, Sayyed AH, Fazal S, Saleem MA, Zaka SM, Ali M. Field evolved resistance to carbamates, organophosphates, pyrethroids, and new chemistry insecticides in Spodoptera litura Fab.(Lepidoptera: Noctuidae) J Pest Sci. 2012;85:153–162. [Google Scholar]
- Silver KS, Nomura Y, Salgado VL, Dong K. Role of the sixth transmembrane segment of domain IV of the cockroach sodium channel in the action of sodium channel blocker insecticides. Neurotoxicology. 2009;30:613–621. doi: 10.1016/j.neuro.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver KS, Soderlund DM. Action of pyrazoline-type insecticides at neuronal target sites. Pestic Biochem Physiol. 2005;81:136–143. [Google Scholar]
- Silver KS, Soderlund DM. Point mutations at the local anesthetic receptor site modulate the state-dependent block of rat Na v1.4 sodium channels by pyrazoline-type insecticides. Neurotoxicology. 2007;28:655–663. doi: 10.1016/j.neuro.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Silver KS, Song W, Nomura Y, Salgado VL, Dong K. Mechanism of action of sodium channel blocker insecticides (SCBIs) on insect sodium channels. Pestic Biochem Physiol. 2010;97:87–92. doi: 10.1016/j.pestbp.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Silver KS, Du Y, Liu Z, Dong K. Analysis of the action of lidocaine on insect sodium channels. Insect Biochem Mol Biol. 2011;41:36–41. doi: 10.1016/j.ibmb.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Liu Z, Tsai TD, Valles SM, Goldin AL, Dong K. Novel sodium channel gene mutations in Blattella germanica reduce the sensitivity of expressed channels to deltamethrin. Insect Biochem Mol Biol. 2002;32:445–454. doi: 10.1016/s0965-1748(01)00122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatebayashi H, Narahashi T. Differential mechanism of action of the pyrethroid tetramethrin on tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels. J Pharmacol Exp Ther. 1994;270:595–603. [PubMed] [Google Scholar]
- Tikhonov DB, Zhorov BS. Sodium channels: ionic model of slow inactivation and state-dependent drug binding. Biophys J. 2007;93:1557–1570. doi: 10.1529/biophysj.106.100248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong H, Su Q, Zhou X, Bai L. Field resistance of (Lepidoptera: Noctuidae) to organophosphates, pyrethroids, carbamates and four newer chemistry insecticides in Hunan, China. J Pest Sci (2004) 2013;86:599–609. doi: 10.1007/s10340-013-0505-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilin YY, Ruben PC. Slow inactivation in voltage-gated sodium channels: molecular substrates and contributions to channelopathies. Cell Biochem Biophys. 2001;35:171–190. doi: 10.1385/CBB:35:2:171. [DOI] [PubMed] [Google Scholar]
- von Stein RT, Silver KS, Soderlund DM. Indoxacarb, metaflumizone, and other sodium channel inhibitor insecticides: mechanism and site of action on mammalian voltage-gated sodium channels. Pestic Biochem Physiol. 2013;106:101–112. doi: 10.1016/j.pestbp.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GK, Russell C, Wang SY. State-dependent block of voltage-gated Na+ channels by amitriptyline via the local anesthetic receptor and its implication for neuropathic pain. Pain. 2004;110:166–174. doi: 10.1016/j.pain.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Wang SY, Nau C, Wang GK. Residues in Na(+) channel D3-S6 segment modulate both batrachotoxin and local anesthetic affinities. Biophys J. 2000;79:1379–1387. doi: 10.1016/S0006-3495(00)76390-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SY, Wang GK. Point mutations in segment I-S6 render voltage-gated Na+ channels resistant to batrachotoxin. Proc Nat Acad Sci. 1998;95:2653–2658. doi: 10.1073/pnas.95.5.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XL, Su W, Zhang JH, Yang YH, Dong K, Wu YD. Two novel sodium channel mutations associated with resistance to indoxacarb and metaflumizone in the diamondback moth, Plutella xylostella. Insect Science. 2015;00:1–9. doi: 10.1111/1744-7917.12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warmke JW, Reenan RAG, Wang PY, Qian S, Arena JP, Wang JX, Wunderler D, Liu K, Kaczorowski GJ, VanderPloeg LHT, Ganetzky B, Cohen CJ. Functional expression of Drosophila para sodium channels - Modulation by the membrane protein TipE and toxin pharmacology. J Gen Physiol. 1997;110:119–133. doi: 10.1085/jgp.110.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing KD, Andaloro JT, McCann SF, Salgado VL. Indoxacarb and the sodium channel blocker insecticides: chemistry, physiology and biology in insects. Elsevier; New York: 2005. [Google Scholar]
- Wing KD, Sacher M, Kagaya Y, Tsurubuchi Y, Mulderig L, Connair M, Schnee M. Bioactivation and mode of action of the oxadiazine indoxacarb in insects. Crop Protection. 2000;19:537–545. [Google Scholar]
- Wing KD, Schnee ME, Sacher M, Connair M. A novel oxadiazine insecticide is bioactivated in lepidopteran larvae. Arch Insect Biochem Physiol. 1998;37:91–103. [Google Scholar]
- Yarov-Yarovoy V, Brown J, Sharp EM, Clare JJ, Scheuer T, Catterall WA. Molecular determinants of voltage-dependent gating and binding of pore-blocking drugs in transmembrane segment IIIS6 of the Na(+) channel alpha subunit. J Biol Chem. 2001;276:20–27. doi: 10.1074/jbc.M006992200. [DOI] [PubMed] [Google Scholar]
- Yarov-Yarovoy V, McPhee JC, Idsvoog D, Pate C, Scheuer T, Catterall WA. Role of amino acid residues in transmembrane segments IS6 and IIS6 of the Na+ channel alpha subunit in voltage-dependent gating and drug block. J Biol Chem. 2002;277:35393–35401. doi: 10.1074/jbc.M206126200. [DOI] [PubMed] [Google Scholar]
- Zhao JZ, Collins HL, Li YX, Mau RF, Thompson GD, Hertlein M, Andaloro JT, Boykin R, Shelton AM. Monitoring of diamondback moth (Lepidoptera: Plutellidae) resistance to spinosad, indoxacarb, and emamectin benzoate. J Econ Entomol. 2006;99:176–181. doi: 10.1603/0022-0493(2006)099[0176:MODMLP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Zhao X, Ikeda T, Salgado VL, Yeh JZ, Narahashi T. Block of two subtypes of sodium channels in cockroach neurons by indoxacarb insecticides. Neurotoxicology. 2005;26:455–465. doi: 10.1016/j.neuro.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Zhao X, Ikeda T, Yeh JZ, Narahashi T. Voltage-dependent block of sodium channels in mammalian neurons by the oxadiazine insecticide indoxacarb and its metabolite DCJW. Neurotoxicology. 2003;24:83–96. doi: 10.1016/s0161-813x(02)00112-2. [DOI] [PubMed] [Google Scholar]
- Zhorov BS, Tikhonov DB. Potassium, sodium, calcium and glutamate-gated channels: pore architecture and ligand action. J Neurochem. 2004;88:782–799. doi: 10.1111/j.1471-4159.2004.02261.x. [DOI] [PubMed] [Google Scholar]
- Zhou C, Liu Y, Yu W, Deng Z, Gao M, Liu F, Mu W. Resistance of Spodoptera exigua to ten insecticides in Shandong, China. Phytoparasitica. 2011;39:315–324. [Google Scholar]


