Abstract
With improved surgical techniques and medical management for patients with congenital heart diseases, more patients are living longer and well into adulthood. This improved survival comes with a price of increased morbidity, mainly secondary to increased risk of tachyarrhythmias. One of the major arrhythmias commonly encountered in this subset of cardiac patients is AF. Similar to the general population, the risk of AF increases with advancing age, and is mainly secondary to the abnormal anatomy, abnormal pressure and volume parameters in the hearts of these patients and to the increased scarring and inflammation seen in the left atrium following multiple surgical procedures. Catheter ablation for AF has been shown to be a very effective treatment modality in patients with refractory AF. However, data and guidelines regarding catheter ablation in patients with congenital heart disease are not well established. This review will shed light on the procedural techniques, success rates and complications of AF catheter ablation in patients with different types of CHD, including atrial septal defects, tetralogy of Fallot, persistent left superior vena cava, heterotaxy syndrome and atrial isomerism, and Ebstein anomaly.
Keywords: Atrial fibrillation, catheter ablation, congenital heart disease, atrial septal defect, tetralogy of Fallot, persistent left superior vena cava, Ebstein anomaly, atrial isomerism
Although there is no formal database of adults with congenital heart disease (CHD) in the United States, the prevalence and incidence of CHD can be estimated and extrapolated from data in the Canadian providence.[1] As such, the prevalence of CHD in the United States has been estimated in 2010 to be around 2.4 million people (1.4 million adults and 1 million children), with an incidence of between four and 10 per 1,000. Forty-five per cent of these adults have mild disease, 37 % have moderate disease, and 14 % have severe disease.[1–3] Furthermore, the prevalence of patients living with CHD has been increasing secondary to the improvements in surgical techniques and medical management over the past few decades. Mortality secondary to CHD is highest during infancy and childhood declining gradually with age to reach a steady state between 15 and 65 years. It is higher in men than women.[4]
One of the most important causes of morbidity in patients with CHD is the development of cardiac arrhythmias, in particular tachyarrhythmias. These result from multiple surgical scars, haemodynamic abnormalities and structural defects that create arrhythmogenic substrates.[5] In fact, about 11 % of patients with CHD develop atrial arrhythmias (intra-atrial reentrant tachycardia [IART] and AF), with the risk being higher in patients with right-sided heart lesions.[6] The most common arrhythmia in patients with CHD is IART that occurs secondary to reentrant circuits in the right atrium. AF is a less common cause of atrial arrhythmia in CHD, but its prevalence is increasing in these patients because of improved survival to older age.[5]
The 2014 American College of Cardiology/American Heart Association/Heart Rhythm Society guidelines for the management of AF describe medical therapies including rate control, rhythm control and anticoagulation, with radiofrequency catheter ablation mainly reserved for patients who are refractory or intolerant to treatment with antiarrhythmic medication.[7] Here in this review we describe the efficacy, technical limitations and common complications of radiofrequency catheter ablation for AF in patients with different types of CHD.
Atrial Septal Defect
Patients with atrial septal defect (ASD) are prone to developing different types of atrial arrhythmias. These include atrial flutter, atrial tachycardia and – most commonly – AF. Just as in the general population, the incidence of AF in patients with ASD increases with age. Since ASD is usually asymptomatic early on because of the absence of signs and symptoms, many patients may present with AF before correction of the defect. Although correction of the ASD prevents recurrence of most cases of paroxysmal AF, this is not the case in patients with persistent AF.[8,9]
AF Catheter Ablation in Patients with ASD
Catheter ablation has been one of the primary treatment strategies for patients with AF and ASD. Several reports have described the different approaches to perform this procedure, along with success rates and possible complications. Nie et al.[10] described catheter ablation in patients with uncorrected ASD via direct access through the defect when feasible. Success rates were similar to those in matched patients without ASD. The procedure can also be performed in patients who have already had their ASDs closed. This has been described by Lakkireddy et al.[11] and Santangeli et al.[12] In these patients, CT scanning can be useful in defining the anatomy, location of the closure device and any calcifications around the site of closure. Intracardiac echocardiography can also be very helpful in determining a suitable site for transseptal puncture through the native septum. However, in some cases access through the native septum is not feasible. Santangeli et al. have described access into the left atrium (LA) through the closure device.[12] In this study, because the device increases resistance to access directly via the transseptal sheath, the authors reported that extra steps of dilation of the access site were required. The needle was introduced into the LA through the device. Once the needle was in the LA, a dilator (8 Fr in this case) was introduced over the needle. The needle was removed and a guide wire introduced into the left pulmonary vein. Then the dilator was removed and a larger dilator (11 Fr in this case) introduced over the wire. This sequential dilation allowed the transseptal catheter to be advanced through the stiff closure device into the LA.
When transseptal access is done through the native septum, total procedure time, fluoroscopy time and transseptal time are not significantly between patients with corrected ASD compared with those without an ASD.[11] However, when the LA is accessed through the occlusion device, more time is required to puncture and dilate the device to allow the transseptal sheath to pass, and as such total procedure time, fluoroscopy time and transseptal time are significantly increased compared with access through the native septum.[12] The success rate of the procedure is similar between patients with ASD and patients without ASD, and between patients who had LA access through the native septum compared with patients who had access through the device.[11,12] Arrhythmia-free survival rates >14 months after the procedure in patients with ASD range between 56 % and 77 %, and are not significantly different from those seen in patients without ASD undergoing catheter ablation for AF.[10–12]
While Santangeli et al.[12] successfully demonstrated that transseptal access through the occlusion device is feasible, several concerns have been raised. In an accompanying editorial commentary by Demosthenes Katritsis, it was argued that a transoesophageal echocardiogram should have been used after the procedure to confirm there was no residual shunt secondary to the puncture.[13] The issue of device dislodgement was also raised. The concern was mainly that smaller devices and inadequately supported large devices are more likely to dislodge as a result of forces exerted by large sheaths. Therefore, an atrial-septal rim of >5 mm should be present for safe access through the device. Another point that the author made is that Santangeli et al. performed the device puncture only in patients with the Amplatzer device and therefore that the safety of this procedure with other devices (e.g., Cardioseal) is unknown. Finally, it was argued that access through a native septum via a large sheath or via double puncture has been associated with a 20–30 % rate of iatrogenic ASDs. Hence, it was recommended that patients should be carefully selected for this procedure and that the procedure should be performed in symptomatic patients with high AF burden only 6 months or more following the device insertion.[13]
Reported adverse effects of catheter ablation in patients with ASDs in the literature include mild pericardial effusion, haematoma formation, acute heart failure, and transient ischaemic attack. All reported complications resolved and patients achieved full recovery.[11,12] Therefore radiofrequency catheter ablation for AF is a technically feasible procedure in patients with ASD closure devices. Efficacy is similar to that in patients without ASD, and it is a safe procedure with minimal complications. However, special care should be taken when selecting patients with closure devices and AF, especially when access through the device is contemplated.
Tetralogy of Fallot
Tetralogy of Fallot (ToF) is the most common cyanotic congenital heart defect, with an estimated incidence in the range of 0.23–0.63 cases per 1,000 live births.[14] Surgical techniques for the correction of ToF have improved dramatically since the first operation was performed in 1955 and patients are living well into adulthood. With increased survival, increased morbidity secondary to long-term complications of ToF is seen. Atrial and ventricular arrhythmias are major contributors to long-term morbidity from ToF.[15–17] It is estimated that the total arrhythmia burden in patients with ToF ranges from 30–43 %,[14,15] with atrial arrhythmias being more common than ventricular arrhythmias.[15,18] The most common atrial arrhythmia in younger patients (aged <45 years) is IART. However, with age, the incidence of AF increases and exceeds IART making it the most common atrial arrhythmia in patients aged >55 years.[15–17]
AF Catheter Ablation in Tetralogy of Fallot
Unlike in patients with ASDs, limited data exist for radiofrequency catheter ablation for AF in patients with ToF. In a retrospective study by Philip et al.[19] attempted ablation in one patient with ToF and AF failed secondary to difficulties reaching the LA. In a study by Ezzat et al.[17] describing ablation in ToF patients with tachyarrhythmias, the most common arrhythmia was cavo-tricuspid-dependent tachyarrhythmia with only four patients presenting with, or having induced AF during the electrophysiology study. Of those four cases, only two left-sided procedures were performed (wide area circumferential ablation of the pulmonary veins and complex fractionated electrogram ablation). Thus, the effectiveness of radiofrequency ablation of AF in this cohort of ToF patients could not be assessed. More studies are needed to study the efficacy of this treatment modality in ToF patients, particularly those with AF.
Persistent Left Superior Vena Cava
Although persistent left superior vena cava (PLSVC) is the most common congenital anomaly affecting thoracic venous structures, it is quite a rare disorder with a prevalence of 0.1–3 % in the general population.[20–22] PLSVC results when the left cardinal vein fails to obliterate into the ligament of Marshall during the foetal period. The PLSVC drains into the right atrium after joining a dilated coronary sinus. In rare cases, PLSVC may be associated with absence of a right SVC.[20,21],23,24 It is thought that the PLSVC contains remnant pacemaker tissues from foetal development. These tissues can be a source of ectopy that can initiate AF.[25]
AF Catheter Ablation in PLSVC
Because PLSVC is an uncommon condition, studies on AF ablation in patients with PLSVC are mainly case series or case reports. Hsu et al.[25] reported their experience with five patients who had refractory AF and PLSVC. All patients underwent pulmonary vein isolation (PVI), followed by isolation of the PLSVC. On follow-up, three of the five patients were arrhythmia free, one had an unsuccessful PLSVC isolation and AF recurrence and one did not have recurrence of AF but required two further procedures for atrial flutter. In another study by Elayi et al., six patients with refractory AF and PLSVC were studied. After PVI and PLSVC isolation, none of the six patients had recurrence of AF on follow-up. While the above two studies reported PLSVC isolation after the patients had undergone PVI, Anselmino et al.[22] reported success in keeping two patients free of AF with only PLSVC isolation without PVI. Most studies described above showed that there are two sites of connection between the PLSVC and the myocardium.[21,22,25] One connection is located proximally at the junction of the PLSVC and the coronary sinus, and another connection to the LA located higher up along the PLSVC. Ablation of only one of these sites is not sufficient to completely electrically isolate the PLSVC and both sites should be ablated. Complications during this procedure include cardiac tamponade and left phrenic nerve palsy.
Overall, PLSVC isolation as a treatment for refractory AF in this population subset seems to be feasible. However, caution should be practiced during radiofrequency energy delivery to avoid injury to nearby structures such as the circumflex coronary artery and the left phrenic nerve. Figure 1 shows images of the electroanatomical mapping, fluoroscopy images, and electrophysiological tracings in one of our patients with PLSVC who underwent AF catheter ablation.
Figure 1: Patient with PLSVC who Underwent AF Catheter Ablation.
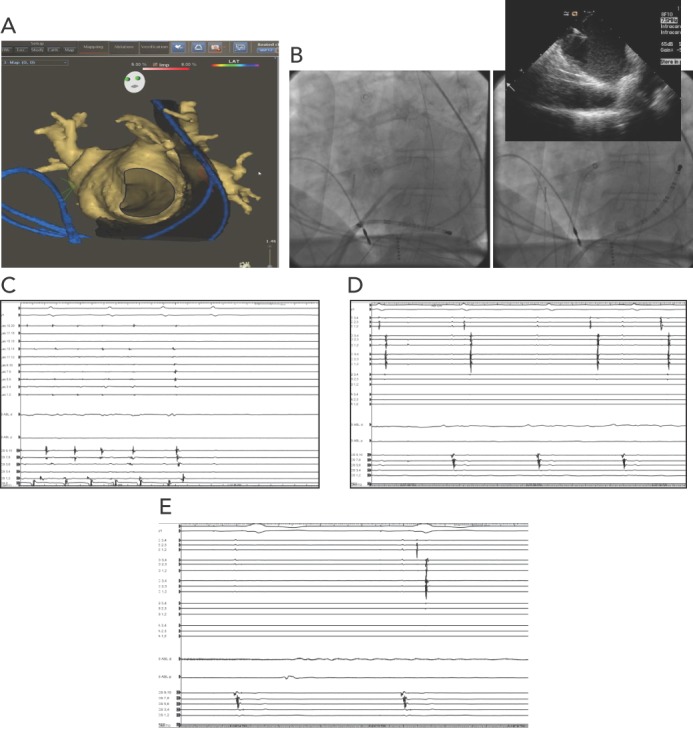
A: CARTO map; B: transseptal puncture; C: normal sinus rhythm during isolation of the right pulmonary vein; D: isolation of the PLSVC; E: no reconnection after administration of adenosine. PLSVC = persistent left superior vena cava.
Heterotaxy and Atrial Isomerism
Atrial isomerism is a rare disorder, but improved surgical procedures have resulted in improved survival and more patients are living into adulthood. Just like in other types of CHD, improved survival into adulthood is associated with an increased burden of arrhythmia. Suman-Horduna et al.[26] reported eight patients with atrial isomerism and atrial tachyarrhythmias. These included twin atrioventricular nodal reentrant tachycardia, atrial tachycardia and AF. Out of the two patients with AF who underwent PVI, one patient remained arrhythmia free on follow-up, while the other developed paroxysmal episodes of atrial tachycardia and AF.
No conclusion can be drawn regarding PVI for AF in patients with heterotaxy syndrome because of the very small number of patients. However, the above mentioned study pointed out that in patients with complex CHD such as atrial isomerism, the use of more advanced techniques and equipment such as magnetic navigation to guide the ablation procedure may provide better dexterity and easier access to the desired locations in the heart, possibly leading to better outcomes.[26]
Ebstein Anomaly
Patients with Ebstein anomaly are predisposed to AF. PVI by catheter ablation can be successfully done in this group of patients. We report a 38 year old with repaired Ebstein anomaly and AF who underwent PVI at another hospital. The patient had recurrent arrhythmia with reconnection of the right inferior pulmonary vein that was isolated (see Figure 2).
Figure 2: Patient with Ebstein Anomaly who Underwent Pulmonary Vein Isolation.
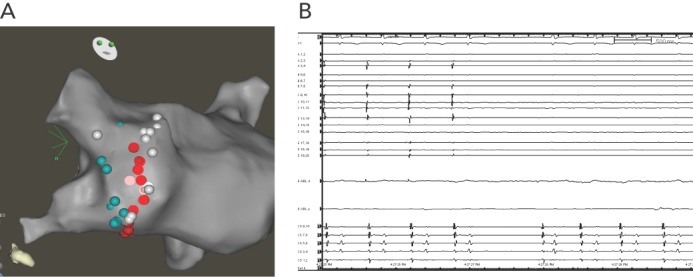
A: CARTO map; B: intracardiac tracings showing pulmonary vein isolation.
Other Congenital Heart Defects
AF is seen in other congenital heart defects that include aortic stenosis and single ventricle after the Fontan operation.[27] For Fontan patients who have AF, the atrial MAZE can be extended to the LA with a variation of the Cox procedure and with a recurrence rate <12 %. Surgical ablation with Fontan has a combined mortality or need for postoperative heart transplant risk that exceeds 5 %.[27]
Conclusion
With improved surgical methods and medical management, patients with CHD are living well into adulthood. This increased survival is associated with increased morbidity secondary to many factors, with arrhythmias being major players in this regard. Atrial arrhythmias including AF are common and tend to become refractory to medical treatment as patients live longer. Catheter ablation for AF can be successfully performed in patients with CHD, and despite the lack of large multicentre controlled trials, available data point to the safety and efficacy of this method in this group of patients. The procedure can be challenging and adjunctive ablation of non-pulmonary vein triggers is often needed in addition to PVI.
Clinical Perspective
Adult CHD puts patients at increased risk of AF and the electrophysiologist in a treatment dilemma.
Catheter ablation for AF is a very appealing modality in patients with ACHD especially because treatment with antiarrhythmic drugs in this group may be ineffective or may carry a higher risk of adverse effects.
Because solid evidence and guidelines are lacking for AF ablation in ACHD, more studies are needed to establish the safety and efficacy of this technique in this group, and patients should undergo careful evaluation and selection.
References
- 1.Bhatt AB, Foster E, Kuehl K et al. Congenital heart disease in the older adult: a scientific statement from the American Heart Association. Circulation. 2015;131:1884–931. doi: 10.1161/CIR.0000000000000204. [DOI] [PubMed] [Google Scholar]
- 2.Gilboa SM, Devine OJ, Kucik JE et al. Congenital Heart Defects in the United States: Estimating the Magnitude of the Affected Population in 2010. Circulation. 2016;134:101–9. doi: 10.1161/CIRCULATIONAHA.115.019307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Blaha MJ, Chiuve SE et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boneva RS, Botto LD, Moore CA et al. Mortality associated with congenital heart defects in the United States: trends and racial disparities, 1979-1997. Circulation. 2001;103:2376–81. doi: 10.1161/01.CIR.103.19.2376. [DOI] [PubMed] [Google Scholar]
- 5.Sherwin ED, Triedman JK, Walsh EP. Update on interventional electrophysiology in congenital heart disease: evolving solutions for complex hearts. Circ Arrhythm Electrophysiol. 2013;6:1032–40. doi: 10.1161/CIRCEP.113.000313. [DOI] [PubMed] [Google Scholar]
- 6.Bernier M, Marelli AJ, Pilote L et al. Atrial arrhythmias in adult patients with right-versus left-sided congenital heart disease anomalies. Am J Cardiol. 2010;106:547–51. doi: 10.1016/j.amjcard.2010.03.068. [DOI] [PubMed] [Google Scholar]
- 7.January CT, Wann LS, Alpert JS et al. 2014/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1–e76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 8.Wi J, Choi J-Y, Shim J-M et al. Fate of Preoperative Atrial Fibrillation After Correction of Atrial Septal Defect. Circ J. 2013;77:109–15. doi: 10.1253/circj.CJ-12-0550. [DOI] [PubMed] [Google Scholar]
- 9.Scaglione M, Caponi D, Ebrille E et al. Very long-term results of electroanatomic-guided radiofrequency ablation of atrial arrhythmias in patients with surgically corrected atrial septal defect. Europace. 2014;16:1800–7. doi: 10.1093/europace/euu076. [DOI] [PubMed] [Google Scholar]
- 10.Nie JG, Dong JZ, Salim M et al. Catheter ablation of atrial fibrillation in patients with atrial septal defect: long-term follow-up results. J Interv Card Electrophysiol. 2015;42:43–9. doi: 10.1007/s10840-014-9958-z. [DOI] [PubMed] [Google Scholar]
- 11.Lakkireddy D, Rangisetty U, Prasad S et al. Intracardiac echo-guided radiofrequency catheter ablation of atrial fibrillation in patients with atrial septal defect or patent foramen ovale repair: a feasibility, safety, and efficacy study. J Cardiovasc Electrophysiol. 2008;19:1137–42. doi: 10.1111/j.1540-8167.2008.01249.x. [DOI] [PubMed] [Google Scholar]
- 12.Santangeli P, Di Biase L, Burkhardt JD et al. Transseptal access and atrial fibrillation ablation guided by intracardiac echocardiography in patients with atrial septal closure devices. Heart Rhythm. 2011;8:1669–75. doi: 10.1016/j.hrthm.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 13.Katritsis DG. Transseptal puncture through atrial septal closure devices. Heart Rhythm. 2011;8:1676–7. doi: 10.1016/j.hrthm.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 14.Wu M-H, Lu C-W, Chen H-C et al. Arrhythmic burdens in patients with tetralogy of Fallot: A national database study. Heart Rhythm. 2015;12:604–9. doi: 10.1016/j.hrthm.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 15.Decker JA, Kim JJ. Management of arrhythmias in patients with a tetralogy of Fallot. Cardiol Young. 2013;23:888–95. doi: 10.1017/S1047951113001789. [DOI] [PubMed] [Google Scholar]
- 16.Khairy P, Aboulhosn J, Gurvitz MZ et al. Arrhythmia burden in adults with surgically repaired tetralogy of Fallot: a multi-institutional study. Circulation. 2010;122:868–75. doi: 10.1161/CIRCULATIONAHA.109.928481. [DOI] [PubMed] [Google Scholar]
- 17.Ezzat VA, Ryan MJ, O’Leary J et al. Radiofrequency ablation of atrial tachyarrhythmias in adults with tetralogy of Fallot - predictors of success and outcome. Cardiol Young. 2017;27:284–93. doi: 10.1017/S1047951116000482. [DOI] [PubMed] [Google Scholar]
- 18.Le Gloan L, Khairy P. Management of arrhythmias in patients with tetralogy of Fallot. Curr Opin Cardiol. 2011;26:60–5. doi: 10.1097/HCO.0b013e328341381a. [DOI] [PubMed] [Google Scholar]
- 19.Philip F, Muhammad KI, Agarwal S, Natale A, Krasuski RA. Pulmonary vein isolation for the treatment of drug-refractory atrial fibrillation in adults with congenital heart disease. Congenit Heart Dis. 2012;7:392–9. doi: 10.1111/j.1747-0803.2012.00649.x. [DOI] [PubMed] [Google Scholar]
- 20.Wissner E, Tilz R, Konstantinidou M et al. Catheter ablation of atrial fibrillation in patients with persistent left superior vena cava is associated with major intraprocedural complications. Heart Rhythm. 2010;7:1755–60. doi: 10.1016/j.hrthm.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Elayi CS, Fahmy TS, Wazni OM et al. Left superior vena cava isolation in patients undergoing pulmonary vein antrum isolation: Impact on atrial fibrillation recurrence. Heart Rhythm. 2006;3:1019–23. doi: 10.1016/j.hrthm.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 22.Anselmino M, Ferraris F, Cerrato N et al. Left persistent superior vena cava and paroxysmal atrial fibrillation: the role of selective radio-frequency transcatheter ablation. J Cardiovasc Med (Hagerstown) 2014;15:647–52. doi: 10.2459/JCM.0000000000000144. [DOI] [PubMed] [Google Scholar]
- 23.Maruyama M, Ino T, Miyamoto S et al. Characteristics of the electrical activity within the persistent left superior vena cava: Comparative view with reference to the ligament of Marshall. J Electrocardiol. 2003;36:53–7. doi: 10.1054/jelc.2003.50004. [DOI] [PubMed] [Google Scholar]
- 24.Liu H, Lim KT, Murray C, Weerasooriya R. Electrogram-guided isolation of the left superior vena cava for treatment of atrial fibrillation. Europace. 2007;9:775–80. doi: 10.1093/europace/eum118. [DOI] [PubMed] [Google Scholar]
- 25.Hsu LF, Jais P, Keane D et al. Atrial fibrillation originating from persistent left superior vena cava. Circulation. 2004;109:828–32. doi: 10.1161/01.CIR.0000116753.56467.BC. [DOI] [PubMed] [Google Scholar]
- 26.Suman-Horduna I, Babu-Narayan SV, Ueda A et al. Magnetic navigation in adults with atrial isomerism (heterotaxy syndrome) and supraventricular arrhythmias. Europace. 2013;15:877–85. doi: 10.1093/europace/eus384. [DOI] [PubMed] [Google Scholar]
- 27.Walsh EP. Interventional electrophysiology in patients with congenital heart disease. Circulation. 2007;115:3224–34. doi: 10.1161/CIRCULATIONAHA.106.655753. [DOI] [PubMed] [Google Scholar]


