Abstract
Maintaining sinus rhythm in patients with non-paroxysmal AF is an elusive goal. Some suggest that hybrid ablation, combining minimally invasive epicardial surgical ablation with endocardial catheter ablation, may be more effective than either modality alone. However, randomised trials are lacking. We investigated whether hybrid ablation is more effective than epicardial ablation alone at preventing recurrent AF by performing a systematic review and meta-analysis. The review was prospectively registered with PROSPERO (CRD42016043389). MEDLINE and EMBASE were searched for studies of standalone minimally invasive epicardial ablation of AF and/or hybrid ablation, identifying 41 non-overlapping studies comprising 2737 patients. A random-effects meta-analysis, meta-regression and sensitivity analysis were performed. Single-procedure survival free from atrial arrhythmias without antiarrhythmic drugs was similar between epicardial-alone and hybrid approaches at 12 months (epicardial alone 71.5 %; [95 % CI 66.1–76.9], hybrid 63.2 %; [95 % CI 51.5–75.0]) and 24 months (epicardial alone 68.5 %; [95 % CI 57.7–79.3], hybrid 57.0 %; [95 % CI 33.6–80.4]). Freedom from atrial arrhythmias with AADs and rates of unplanned additional catheter ablations were also similar between groups. Major complications occurred more often with hybrid ablation (epicardial alone 2.9 %; [95 % CI 1.9–3.9], hybrid 7.3 %; [95 % CI 4.2–10.5]). Meta-regression suggested that bipolar radiofrequency energy and thoracoscopic access were associated with greater efficacy, but adjusting for these factors did not unmask any difference between epicardial-alone and hybrid ablation. Hybrid and epicardial ablation alone appear to be equally effective treatments for AF, although hybrid ablation may be associated with higher complication rates. These data derived from observational studies should be verified with randomised data.
Keywords: Atrial fibrillation, ablation, minimally invasive, surgical, hybrid, convergent, complications, trans-diaphragmatic, monopolar
The goal of arrhythmia eradication in AF continues to be elusive for cardiac electrophysiologists. Although endocardial radiofrequency (RF) catheter ablation is more effective than pharmacological management at maintaining sinus rhythm,[1] it is far from perfect, especially in patients with non-paroxysmal AF.[2] In an attempt to address this, attention has turned back to the surgical ablation that preceded catheter-based approaches. While the initial open-chest Cox-Maze procedure generated high success rates, the complexity and invasiveness of this procedure limited its widespread adoption. As surgical techniques evolved and the traditional cut-and-sew method of surgical ablation was replaced with device-based lesion formation, the complication rate substantially improved.[3] Aiming to preserve efficacy while reducing complication rates and recovery time, several minimally invasive surgical techniques have been described varying in access site, ablation energy source, and lesion set. These aims may not have been fully met, as a recent review found that that using modern techniques, a full Cox-Maze performed on cardiopulmonary bypass had an equally low complication rate as minimally invasive procedures[3].
Initial reports suggest that minimally invasive epicardial surgical ablation may be more effective than endocardial catheter ablation,[4–6] albeit with a higher risk of complications. Why should ablation from the epicardial surface be more effective? Epicardial ablation using bipolar RF increases the likelihood of fully transmural atrial lesions that reduce the chance of electrical reconnection,[7] although transmural lesions occur less frequently using monopolar RF.[8] Furthermore, the left atrial appendage can be both physically and electrically isolated during surgical ablation, reducing the risk of thromboembolism and possibly recurrent arrhythmias.[9] The disadvantages of surgical ablation relate to difficulties accessing some regions of the atria (the cavotricuspid and mitral isthmuses and the superior aspect of the pulmonary veins with a trans-diaphragmatic approach). However, these regions are easily accessible by endocardial catheter ablation. To exploit the benefits of epicardial and endocardial ablation, some groups therefore advocate a ‘hybrid’[10] or ‘convergent’[11] approach and routinely combine these techniques.
Despite the theoretical advantages of a hybrid approach, it is uncertain whether hybrid ablation is more effective than epicardial ablation alone in practice, as no randomised controlled trials (RCTs) have addressed this question. While a single cohort study suggested that hybrid ablation was more effective,[12] two others did not find a difference,[13,14] potentially stemming from differences in the surgical techniques and lesions sets used.
We asked whether, in patients with AF, the outcomes from hybrid ablation differ from those from minimally invasive surgical epicardial ablation alone with respect to freedom from atrial arrhythmias at 12 and 24 months, major complication rates and the need for additional unplanned catheter ablations. Because of the absence of RCTs, we addressed these questions by performing a systematic review and meta-analysis of all studies reporting the outcomes of these procedures.
Methods
A comprehensive description of the methods used can be found in the supplemental materials (available online at www.aerjournal.com). The Preferred Reporting Items For Systematic Reviews and Meta-analyses[15] and Meta-analysis Of Observational Studies In Epidemiology[16] guidelines for reporting meta-analyses were followed. A protocol for the meta-analysis was prospectively recorded in the PROSPERO registry (CRD42016043389).
Search Strategy
Relevant studies were identified by interrogating the MEDLINE and EMBASE databases including studies from the start of records to 1st November 2016. Additional studies were identified by scanning reference lists of included studies and existing reviews. The search strategy can be found in Figure 1. Shortlisted papers and reasons for exclusion can be found in the Supplemental Materials, available online.
Figure 1: Study Selection Diagram.
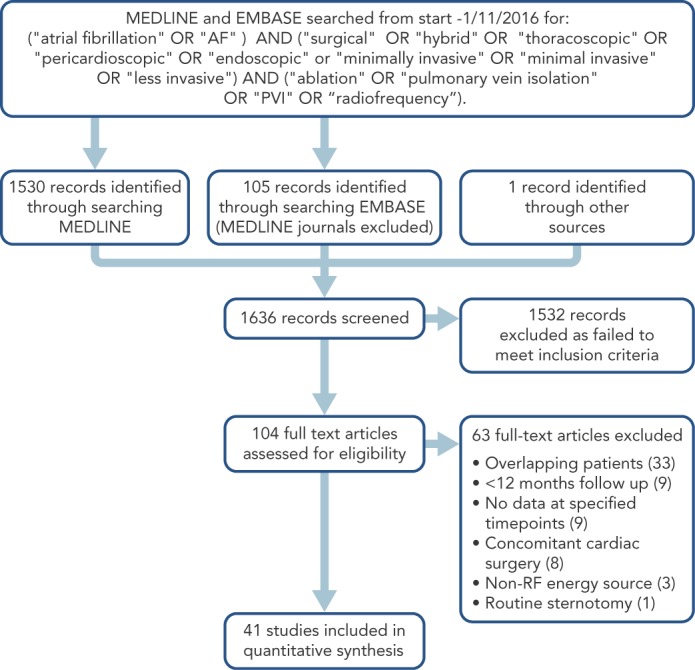
See Supplemental Table 4 (available online) for details of all excluded full text articles.
Outcome Measures
The pre-specified primary outcome was survival free from any atrial arrhythmia (AF, typical or atypical atrial flutter, left or right atrial tachycardia) without antiarrhythmic drugs (AADs) at 12 and 24 months. Secondary outcomes included survival free from any atrial arrhythmias with or without AADs, survival free from AF with or without AADs, need for repeat catheter ablation, and rate of major complications. A list of all data fields extracted can be found in Supplemental Table 1.
Table 1: Demographics of Patients Included in Studies.
| Demographic | Epicardial alone | Hybrid | Difference |
|---|---|---|---|
| Mean (SD) | (p-value) | ||
| (30 series, 2132 patients) | (12 series, 605 patients) | ||
| Age (years) | 59.1 (4.1) | 60.5 (2.9) | 0.22 |
| Gender (male) (%) | 69.6 (11.6) | 76.5 (13.8) | 0.16 |
| LA diameter (mm) | 46.0 (4.2) | 48.7 (2.8) | 0.02 |
| LV ejection fraction (%) | 56.6 (4.4) | 54.4 (4.8) | 0.24 |
| BMI (kg/m2) | 28.6 (3.0) | 29.7 (2.1) | 0.35 |
| CHADS score | 1.2 (1.0) | 1.2 (0.2) | 0.80 |
| Prior catheter ablation (%) | 28.6 (25.9) | 28.7 (33.4) | 0.99 |
| Paroxysmal AF (%) | 43.6 (33.3) | 9.3 (16.1) | <0.001 |
| Persistent AF (%) | 30.0 (28.6) | 35.7 (30.0) | 0.58 |
| Longstanding persistent AF (%) | 26.4 (37.3) | 55.2 (34.7) | 0.03 |
| Duration of AF (years) | 5.0 (2.1) | 5.0 (1.3) | 0.99 |
BMI = body mass index; LA = left atrial; LV = left ventricular. Values in bold denote statistically significant differences
Eligibility and Exclusion Criteria
RCTs, cohort studies and case series published in peer-reviewed journals including at least one cohort of ≥10 patients undergoing minimally invasive epicardial ablation of AF using RF energy were eligible for inclusion. Studies were excluded if patients underwent concomitant cardiac surgery or routine sternotomy, used non-RF energy sources, were not published as full-text articles, or contained patients overlapping an already included study.
Studies in which the planned treatment was a combination of minimally invasive surgical ablation with endocardial catheter ablation and in which both aspects of the ablation were completed in >75 % of patients were included in the ‘hybrid’ group. The endocardial ablation could be performed either simultaneously with the epicardial ablation or as a staged procedure up to 3 months later, as no difference in outcome has been shown between simultaneous and staged hybrid ablation.[17] Studies in which catheter ablation was not performed routinely but was performed ad hoc in cases of recurrent arrhythmia were included in the ‘epicardial only’ group. Studies that fell into neither of these categories were excluded.
Statistical Methods
Data analysis was performed by a statistician (LB). Based on the sampling frame that comprised diverse populations of patients, pooled estimates were obtained for each outcome for epicardial- alone and hybrid ablation via the DerSimonian and Laird method using a random-effects model. Differences between epicardial alone and hybrid ablations were judged to be different if 95 % CIs for the respective pooled estimates did not overlap.[18] Baseline demographics were compared using two-tailed T-tests for normally distributed continuous data and Chi-squared tests for categorical data. Meta-regression was used to examine the impact of several clinical covariates on the effect size of both primary outcomes. Study quality and risk of bias within studies were assessed using a modified version of published criteria[1,19] for measuring the quality of case series (Supplemental Table 3). Risk of publication bias across studies was assessed using Funnel plots and Eggers’ test.[18] Analyses were performed using R version 3.2.3.[20]
Results
The search strategy generated 1636 abstracts (see Figure 1). Forty-one studies comprising a total of 2737 patients met the inclusion criteria (median number of patients per study 52, range 12–240). The commonest reason for excluding studies was overlapping patient cohorts (Supplemental Table 4). Five RCTs and two cohort studies compared minimally invasive surgical ablation against another treatment (catheter ablation[4–6,21] (n=4), medical management[22,23] (n=2), open chest surgical ablation[24] (n=1); in these studies the minimally invasive surgical arm was treated as a single case series. Two RCTs and one cohort study compared surgical ablation techniques (+/- ganglionic plexus ablation[25] (n=1), +/- left atrial appendage exclusion[26] (n=1), immediate versus delayed hybrid ablation[17] (n=1); in these studies the two arms were combined to form a single case series for the primary analysis but treated as separate series for the meta-regression where appropriate. One cohort study compared hybrid with epicardial-alone ablation[13] and in this study the two arms treated as separate case series. Thirty studies were single-arm case series. Forty-one studies therefore yielded 42 case series.
Combined, the demographics of the patients included in the studies of epicardial ablation alone were broadly similar to those included in the studies of hybrid ablation (Table 1, Supplemental Table 5). However, studies of hybrid ablation included patients with greater left atrial diameters, a greater proportion of patients with longstanding persistent AF and correspondingly fewer patients with paroxysmal AF. These discrepancies were addressed and adjusted for in sensitivity analyses.
Clinical Outcomes
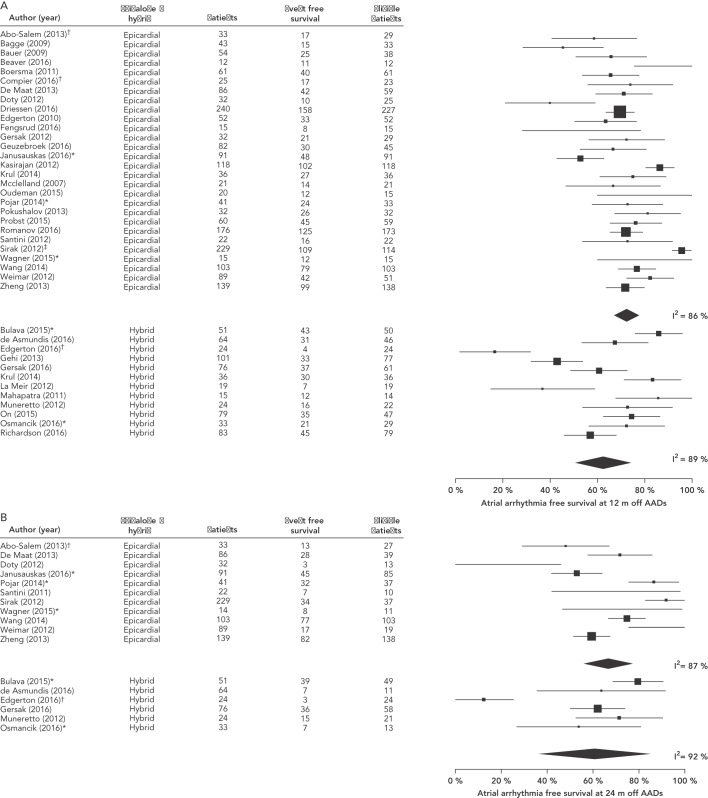
Studies showed heterogeneity across all outcomes and therefore a random-effects model was used. The pooled estimates for the prespecified primary outcome of survival free from any atrial arrhythmias without AADs showed marked heterogeneity (I2), but were similar between epicardial-alone and hybrid approaches at 12 months (epicardial alone 71.5 %; [95 % CI 66.1–76.9]; I2 86 %, hybrid 63.2 %; [95 % CI 51.5–75.0]; I2 89 %, see Figure 2A) and 24 months (epicardial alone 68.5 %; [95 % CI 57.7–79.3]; I2 87 %, hybrid 57.0 %; [95 % CI 33.6–80.4]; I2 92 %, see Figure 2B).
Figure 2: Forest Plot Showing the Primary Outcome of Atrial Arrhythmia-free Survival Without Antiarrhythmic Drugs.
A: 12 months. B: 24 months. Supplemental references64–104; *additional data obtained following personal communication with authors; †outcome adjusted to include deceased patients; ‡reported as outcome at 13 months. AADs = antiarrhythmic drugs.
Survival free from any atrial arrhythmias with or without AADs was also similar at 12 months (epicardial alone 78.4 %; [95 % CI 72.9–83.9]; I2 87 %, hybrid 76.9 %; [95 % CI 66.6–87.3]; I2 90 %, see Supplemental Figure 1) and 24 months (epicardial alone 77.1 %; [95 % CI 67.5–86.6]; I2 82 %, hybrid 65.2 %; [95 % CI 39.0–91.4]; I2 95 %, see Supplemental Figure 2), as was AF-free survival with or without AADs at 12 months (epicardial alone 84.0%; [95 % CI 79.5–88.4]; I2 70 %, hybrid 90 %; [95 % CI 83.0–97.1]; I2 90 %, see Supplemental Figure 3) and 24 months (epicardial alone 87.2 %; [95 % CI 81.2–93.3]; I2 0.0 %, hybrid 93.1 %; [95 % CI 87.2–99]; I2 0.0 %, see Supplemental Figure 4). The rate of major complications defined as a composite of death, stroke/transient ischemic attack, major bleeding, pericardial effusion requiring drainage, atrio-oesophageal fistula, and sternotomy showed somewhat narrower heterogeneity than the primary outcome, and was significantly higher in the hybrid ablation group (epicardial alone 2.8 %; [95 % CI 1.8–3.9]; I2 52 %, hybrid 7.3 %; [95 % CI 4.2–10.5]; I2 54 %, see Supplemental Figure 5 and Supplemental Table 7).
Similar numbers of additional unplanned catheter ablations were performed in each group (epicardial alone 7.2 %; 95 % CI [4.9–9.6]; I2 51 %, hybrid 7.1 %; 95 % CI [2.8–11.4]; I2 81 %, see Supplemental Figure 6).
Meta-Regression
We next explored possible causes for the marked heterogeneity in reported success rates by performing a univariable meta-regression on the primary outcome (Table 2).
Table 2: Meta-regression Analysis.
| 12-month success | Major complications | |||
|---|---|---|---|---|
| OR (95 % CI) | p-value | OR (95 % CI) | p-value | |
|
Procedure Epicardial alone Hybrid |
1 0.92 (0.84–1.02) |
0.14 |
1 1.03 (1.01–1.05) |
0.01 |
|
Access Thoracoscopic Mini-thoracotomy Trans-diaphragmatic Mixed |
1 0.92 (0.82–1.04) 0.72 (0.61–0.86) 0.99 (0.80–1.23) |
0.19 <0.001 0.9 |
1 0.99 (0.97–1.01) 1.05 (1.00–1.11) 1.04 (0.98–1.10) |
0.51 0.04 0.09 |
|
Energy Source Bipolar Monopolar Mixed |
1 0.79 (0.70–0.88) 0.99 (0.74–1.33) |
<0.001 0.95 |
1 1.02 (0.98–1.06) 1.38 (1.11–1.71) |
0.18 <0.001 |
|
Lesion set category PVI only PVI + LA Box lesion PVI + LA Box + RA ablation |
1 1.01 (0.90–1.14) 0.97 (0.86–1.09) |
0.8 0.59 |
1 1 (0.98–1.02) 1.04 (1.02–1.06) |
0.86 <0.001 |
|
Ganglionic plexus ablation False True |
1 1.06 (0.96–1.17) |
0.21 |
1 0.99 (0.97–1.01) |
0.4 |
|
Left atrial appendage exclusion False True |
1 1.15 (1.04–1.27) |
0.01 |
1 1.03 (1.01–1.05) |
0.02 |
|
Conduction block checked False True |
1 1.02 (0.87–1.19) |
0.78 |
1 1 (0.96–1.04) |
0.84 |
|
Ambulatory monitoring duration <48 hours 48 hours–7 days >7 days |
1 0.98 (0.84–1.15) 0.99 (0.85–1.16) |
0.81 0.95 |
1 1.01 (0.97–1.05) 1.02 (0.98–1.06) |
0.43 0.33 |
| Prevalence of paroxysmal AF | 1.03 (0.92–1.16) | 0.63 | 0.98 (0.96–1.00) | 0.1 |
PVI = pulmonary vein isolation; LA = left atrial; RA = right atrial. Values in bold denote statistically significant differences.
Studies using monopolar RF reported lower success rates than those using bipolar RF (OR 0.79; [95 % CI 0.70–0.88] at 12 months). Lower success rates were also seen when trans-diaphragmatic access was compared with thoracoscopic access (OR 0.72; [95 % CI 0.61–0.86] at 12 months).
We investigated the surgical techniques used by grouping studies according to primary lesion set (pulmonary vein isolation [PVI], PVI and box lesion, PVI and box lesion and right atrial [RA] ablation [see Supplemental Table 2), ganglionic plexus ablation, left atrial appendage exclusion, and whether conduction block was verified. Studies in which >50 % of participants underwent left atrial appendage exclusion reported higher success rates (OR 1.15; [95 % CI 1.04–1.27] at 12 months). Neither the proportion of participants with paroxysmal AF nor the duration of ambulatory monitoring in the first 12 months were associated with the primary outcome.
We also performed a meta-regression on the major complication rate (Table 1). Higher rates of major complications were associated with hybrid ablation (OR 1.03; [95 % CI 1.01–1.05]), trans-diaphragmatic access (OR 1.05; [95 % CI 1.00–1.11]), more extensive lesion sets (OR 1.04; [95 % CI 1.02–1.06] for PVI alone versus PVI and box lesion and RA ablation), and left atrial appendage exclusion (OR 1.03; [95 % CI 1.01–1.05]).
Sensitivity Analysis
As our primary analysis suggested that hybrid ablation was no more effective than epicardial ablation alone, we next performed sensitivity analyses to assess the robustness of our conclusions (Supplemental Table 8).
We first addressed the disparity in demographics by performing a sensitivity analysis including only studies in which no more than 20 % of participants had paroxysmal AF, a value which in our experience is representative of the case mix of thoracoscopic ablation in the UK.[27] This analysis removed all demographic differences including the proportion of participants with paroxysmal AF (epicardial alone 1.8 %; [95 % CI 0.0–6.8], hybrid 3.3 %; [95 % CI 0.0–9.1]; p=0.55) and mean left atrial diameter (epicardial alone 49.3 mm; [95 % CI 44.5–54.1], hybrid 49.1 mm; [95 % CI 46.7–51.7]; p=0.88). This analysis did not unmask any differences between groups (epicardial alone 73 %; [95 % CI 62–85], hybrid 63 %; [95 % CI 51–76]). We also used this sensitivity analysis to reassess the meta-regression. Lesion sets more extensive than pulmonary vein isolation were not associated with higher success rates (OR 0.99; [95 % CI 0.77–1.23]) even in studies including predominantly persistent AF, and continued to be associated with higher complication rates (OR 1.05; [95 % CI 1.00–1.11]).
As access site and energy source were each associated with success, we assessed the effect of including only studies that used thoracoscopic access and bipolar RF. Despite this, no difference was seen in the primary outcome at 12 months (epicardial alone 73 %; [95 % CI 67–79], hybrid 75 %; [95 % CI 65–85]).
While many studies defined success according to American College of Cardiology/American Heart Association/European Society of Cardiology (ACC/AHA/ESC) guidelines, some used weaker definitions such as point prevalence of AF. Furthermore, some studies performed prolonged periods of ambulatory monitoring or implantable loop recorders while others performed little or none. Including only studies that explicitly defined success according to ACC/AHA/ESC guidelines[28] did not reveal any differences in the primary outcome (epicardial-alone 74 %; [95 % CI 68–81], hybrid 62 %; [95 % CI 47–78]), neither did including only studies in which participants underwent >7 days of ambulatory monitoring (epicardial alone 75 %; [95 % CI 67–84], hybrid 61 %; [95 % CI 45–77]). Similar results were seen in outcomes at 24 months (Supplemental Table 9).
Having found that a hybrid ablation strategy was associated with higher rates of major complications, we assessed the validity of our statistical methods by adjusting the fixed value added to series with zero events. Hybrid ablation was associated with increased complications rates without overlap of CIs at a range of added fixed values from 0.05 to 1. When we assessed the complication rate including only studies employing the most effective technique of thoracoscopic access and bipolar RF the magnitude of difference in complication rate was similar but the lower statistical power rendered the difference non-significant (epicardial alone 2.8 %; [95 % CI 1.5–4.1], hybrid 6.6 %; [95 % CI 3.2–10.0]).
Study Quality and Risk of Bias
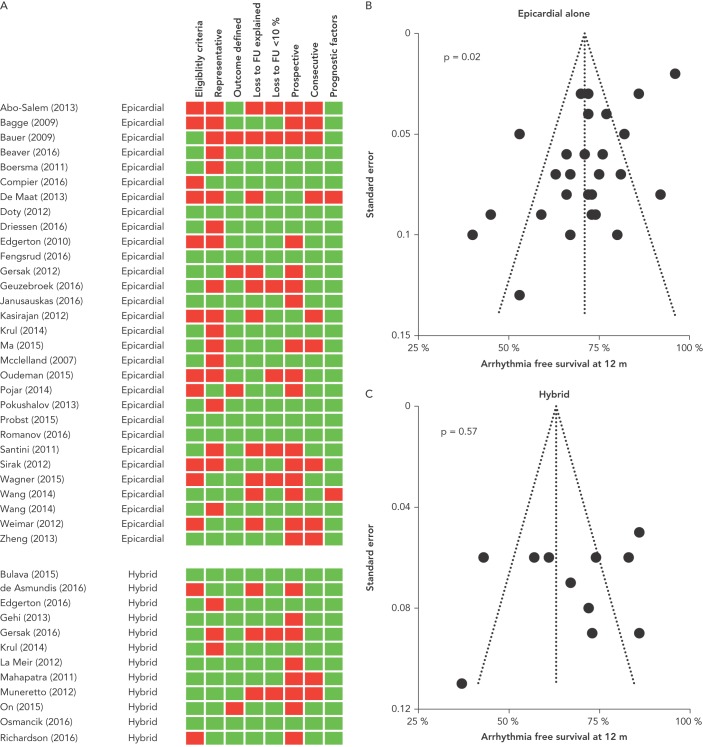
Studies were generally of variable quality (Figure 3A). While six of 41 studies met all eight quality assessment criteria, 23 passed five to seven. Thirteen studies met four or fewer criteria. Thirteen of 41 studies reported detailed inclusion and exclusion criteria. Twenty of 41 studies were unrepresentative of usual practice, including large proportions of participants with paroxysmal AF. Thirty-seven of 41 studies defined outcomes adequately. Twenty-eight of 41 studies reported and explained loss to follow up, and in 8/41 studies >10 % of patients were lost to follow up. Only 17/41 studies recruited prospectively and 30/41 recruited consecutive patients. Thirty-nine of 41 studies reported relevant prognostic factors.
Figure 3: Assessment of Study Quality and Risk of Bias.
A: Study quality assessment. Green boxes indicate that this study passed this criterion and red boxes indicate failure. B: Funnel plot of studies of epicardial ablation alone. C: Funnel plot of studies of hybrid ablation. P-values refer to Egger’s test of likelihood of publication bias.
Funnel plots and Egger’s test were used to assess the risk of publication bias. For studies of epicardial ablation alone, Egger’s test suggested a risk of publication bias, although visual assessment of the Funnel plot was not unduly concerning (Figure 3B). For studies of hybrid ablation, the risk of publication bias was low (Figure 3C).
The numbers of studies meeting all quality inclusion criteria were too small to permit meaningful comparisons between groups.
Discussion
The main findings are that: (1) minimally invasive surgical epicardial ablation and hybrid ablation of AF are both effective; (2) success rates are similar between these groups; (3) hybrid ablation is associated with higher rates of major complications; (4) monopolar RF and trans-diaphragmatic access are associated with lower success rates; and (5) with the exception of left atrial appendage exclusion, lesion sets that were more extensive than pulmonary vein isolation are not associated with higher success rates.
Hybrid and Epicardial-Alone Ablation are Both Effective in Preventing Recurrent AF
Similar to previous reviews of minimally invasive surgical ablation of AF, we found that both hybrid and epicardial-alone approaches are effective,[3,29] although our estimates of success of 72 % and 63 % at 12 months and 69 % and 57 % at 24 months for epicardial-alone and hybrid strategies, respectively, are more conservative than some previous reviews. Different estimates could arise for several reasons. Firstly, many studies report outcomes at last follow up. Although mean follow up in these groups may exceed 12 months, inclusion of patients with shorter follow up is likely to overestimate success rates. We have reported data at specific timepoints to minimise this bias. We took great care to only include non-overlapping studies, reducing the risk of bias arising from including duplicate patients from successful high-volume centres. We also included deceased patients in the follow up denominators for all studies even if these patients were excluded from the headline figure in the original manuscripts.
Hybrid Ablation Offers Little Advantage Over Epicardial Ablation Alone and is Associated with Higher
Complication Rates
Hybrid ablation did not offer higher success rates at 12 or 24 months. No differences were seen when all studies were included or when the disparities in operative technique, patient demographics, definitions of success, or duration of ambulatory monitoring were controlled for in sensitivity analyses. Furthermore, even though all patients receiving hybrid ablation underwent at least one catheter ablation, the need for additional catheter ablations beyond the planned procedure did not decrease with a hybrid strategy. While no significant difference was seen between groups, the numerically lower success rates seen in the hybrid group appear to have been driven by those studies which employed trans-diaphragmatic access and monopolar RF. Meta-regression identified trans-diaphragmatic access and monopolar RF as being predictive of lower success. In addition, the sensitivity analysis excluding studies that used these techniques showed near-identical success rates between hybrid and epicardial-alone ablation.
Whilst failing to provide a clinical benefit, hybrid ablation was associated with more major complications. The difference in complications was largely driven by more patients suffering from major bleeding and pericardial effusions requiring drainage, as expected from the additional vascular access and trans-septal puncture obligated by hybrid ablation. This finding is in keeping with a previous systematic review,[3] which showed that the complication rate from hybrid ablation was higher than that seen from the full Cox-Maze ablation performed on cardiopulmonary bypass. The absolute complication rates reported should be interpreted with caution, as the sensitivity analysis using only series obtained from arms of randomised trials suggested that complication rates could be double that reported amongst the case series as a whole.
Monopolar RF and Trans-diaphragmatic Access are Associated with Lower Success Rates
Corroborating a previous cohort study,[30] monopolar RF was less effective than bipolar RF at preventing recurrent atrial arrhythmias, potentially because transmural ablation lesions are seen less frequently following monopolar than bipolar RF in animal studies.[8] Furthermore, trans-diaphragmatic access was less effective than thoracoscopic access. This may be partly because studies employing trans-diaphragmatic access used monopolar RF. However, as trans-diaphragmatic access limits ablation of the superior aspects of the pulmonary veins, this technique may impair complete pulmonary vein isolation, the cornerstone of successful AF ablation. Relying on one continuous box lesion to encircle all veins means that failure at even a single point along this long ablation line may cause electrical reconnection of all veins.
Despite these shortcomings, trans-diaphragmatic access may have a role for patients with poor respiratory reserve for whom the single lung ventilation required for thoracoscopic ablation may be hazardous.
More Extensive Lesion Sets do Not Increase Success Rates
Pulmonary vein isolation alone was as effective as more extensive ablation. Neither ganglionic plexus ablation, creation of a box lesion set, nor right atrial ablation were associated with higher success rates at 12 or 24 months. This supports randomised data from the Atrial Fibrillation Ablation and Autonomic Modulation via Thoracoscopic Surgery (AFACT) study[25] showing that that the addition of ganglionic plexus ablation to surgical pulmonary vein isolation did not improve outcomes. These findings were consistent across the primary analysis and within a sensitivity analysis minimising the inclusion of patients with paroxysmal AF.
Isolation of the left atrial appendage was associated with greater success at 12 months. Although a similar effect size was found at 24 months, a significant difference was not seen at this timepoint. This uncertainty reflects the controversy in the existing literature. While the Effect of Empirical Left Atrial Appendage Isolation on Long-term Procedure Outcome in Patients With Persistent or Longstanding Persistent Atrial Fibrillation Undergoing Catheter Ablation (BELIEF) trial[9] suggested that left atrial appendage (LAA) electrical isolation decreased AF recurrence following catheter ablation, Romanov et al.[26] found no benefit to LAA ligation during surgical ablation.
Limitations
This meta-analysis is based on case series and therefore is at greater risk of bias than randomised data. Furthermore, the majority of studies were graded only moderate in quality. However, we believe that our approach is valid as randomised data are not currently available to answer this question. Moreover, prior meta-analyses of case series in the field of ablation for AF have given results consistent with RCTs.[19]
As the data were not sourced from RCTs there are inevitable differences in the demographics between study groups. Hybrid studies included more patients with longstanding persistent AF, which may underestimate the relative efficacy of hybrid ablation. However, controlling for these differences using a sensitivity analysis including only studies in which <20 % of patients had paroxysmal AF abolished all differences in baseline demographics without revealing any differences in success rates. Despite this, it is possible that residual unmeasured confounders remain, potentially leaving the hybrid ablation group with a patient cohort with more advanced disease and hiding a true benefit from this procedure.
While the mandate for surgical ablation of AF is greatest in patients with longstanding persistent AF, our pre-specified primary analysis also included studies with a high prevalence of paroxysmal AF, potentially overestimating the success rates of surgical ablation. However, the sensitivity analysis including only studies with <20 % paroxysmal AF showed that success rates were similar to those when all studies were included.
The definition of minimally invasive surgery used here included access via thoracoscopy, trans-diaphragmatic pericardioscopy and mini-thoracotomy. While some argue that mini-thoracotomy is not minimally invasive, lengths of stay and rates of complications were similar between these three methods (Supplemental Table 9) suggesting that mini-thoracotomy is no more invasive than other modes of access.
Complication reporting was highly variable, and underreporting of complications may have been present. To reduce the risk that underreporting might influence a comparison between groups, the definition of major complications used here was relatively narrow, including only the complications most likely to be reported. As a result, the true incidence of complications using a broader definition such as the Ottawa Thoracic Morbidity and Mortality classification system[31] may be higher.
Conclusions
Minimally invasive surgical ablation offers a chance of freedom from the potentially disabling symptoms of AF, at least in the medium term. Epicardial ablation alone appears to be as effective as hybrid ablation and may be associated with fewer complications, although complications may have been underreported in some case series. The highest success rates are associated with thoracoscopic ablation using bipolar RF energy including isolation of the left atrial appendage. These analyses, based upon data predominantly from case series of generally moderate quality, need to be verified with randomised trials.
Clinical Perspective
Maintenance of sinus rhythm is difficult to achieve in patients with persistent or longstanding persistent atrial fibrillation.
Minimally invasive surgical ablation offers a 70 % chance of freedom from atrial arrhythmias at 12 months, at the cost of a complication rate of 2–10 % depending on the technique used.
Hybrid ablation is no more effective than epicardial ablation alone but is associated with a higher rate of major complications.
While left atrial appendage exclusion is associated with higher success rates, lesion sets more extensive than pulmonary vein isolation do not improve outcomes.
Trans-diaphragmatic access and monopolar energy source are associated with inferior outcomes.
References
- 1.Wynn GJ, Das M, Bonnett LJ et al. Efficacy of catheter ablation for persistent atrial fibrillation: a systematic review and meta-analysis of evidence from randomized and nonrandomized controlled trials. Circ Arrhythm Electrophysiol. 2014;7:841–52. doi: 10.1161/CIRCEP.114.001759. [DOI] [PubMed] [Google Scholar]
- 2.Brooks AG, Stiles MK, Laborderie J et al. Outcomes of long-standing persistent atrial fibrillation ablation: a systematic review. Heart Rhythm. 2010;7:835–46. doi: 10.1016/j.hrthm.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 3.Je HG, Shuman DJ, Ad N. A systematic review of minimally invasive surgical treatment for atrial fibrillation: a comparison of the Cox-Maze procedure, beating–heart epicardial ablation, and the hybrid procedure on safety and efficacy. Eur J Cardiothorac Surg. 2015;48:531–540. 540–31. doi: 10.1093/ejcts/ezu536. discussion. [DOI] [PubMed] [Google Scholar]
- 4.Boersma LV, Castella M, van Boven W et al. Atrial fibrillation catheter ablation versus surgical ablation treatment (FAST): a 2-center randomized clinical trial. Circulation. 2012;125((1)):23–30. doi: 10.1161/CIRCULATIONAHA.111.074047. [DOI] [PubMed] [Google Scholar]
- 5.Wang S, Liu L, Zou C. Comparative study of video-assisted thoracoscopic surgery ablation and radiofrequency catheter ablation on treating paroxysmal atrial fibrillation: a randomized, controlled short–term trial. Chin Med J (Engl) 2014;127:2567–70. [PubMed] [Google Scholar]
- 6.Pokushalov E, Romanov A, Elesin D et al. Catheter versus surgical ablation of atrial fibrillation after a failed initial pulmonary vein isolation procedure: a randomized controlled trial. J Cardiovasc Electrophysiol. 2013;24:1338–43. doi: 10.1111/jce.12245. [DOI] [PubMed] [Google Scholar]
- 7.Voeller RK, Zierer A, Lall SC et al. Efficacy of a novel bipolar radiofrequency ablation device on the beating heart for atrial fibrillation ablation: a long-term porcine study. J Thorac Cardiovasc Surg. 2010;140:203–8. doi: 10.1016/j.jtcvs.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schuessler RB, Lee AM, Melby SJ et al. Animal studies of epicardial atrial ablation. Heart Rhythm. 2009;6(12 Suppl):S41–45. doi: 10.1016/j.hrthm.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Biase L, Burkhardt JD, Mohanty P et al. Left Atrial Appendage Isolation in Patients With Longstanding Persistent AF Undergoing Catheter Ablation: BELIEF Trial. J Am Coll Cardiol. 2016;68:1929–40. doi: 10.1016/j.jacc.2016.07.770. [DOI] [PubMed] [Google Scholar]
- 10.Bulava A, Mokracek A, Hanis J et al. Sequential hybrid procedure for persistent atrial fibrillation. J Am Heart Assoc. 2015;4:e001754. doi: 10.1161/JAHA.114.001754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gersak B, Zembala MO, Muller D et al. European experience of the convergent atrial fibrillation procedure: multicenter outcomes in consecutive patients. J Thorac Cardiovasc Surg. 2014;147:1411–6. doi: 10.1016/j.jtcvs.2013.06.057. [DOI] [PubMed] [Google Scholar]
- 12.Kiser AC, Landers MD, Boyce K et al. Simultaneous catheter and epicardial ablations enable a comprehensive atrial fibrillation procedure. Innovations (Phila) 2011;6:243–7. doi: 10.1097/IMI.0b013e31822ca15c. [DOI] [PubMed] [Google Scholar]
- 13.Krul SP, Pison L, La Meir M et al. Epicardial and endocardial electrophysiological guided thoracoscopic surgery for atrial fibrillation: a multidisciplinary approach of atrial fibrillation ablation in challenging patients. Int J Cardiol. 2014;173:229–35. doi: 10.1016/j.ijcard.2014.02.043. [DOI] [PubMed] [Google Scholar]
- 14.La Meir M, Gelsomino S, Luca F et al. Minimally invasive surgical treatment of lone atrial fibrillation: early results of hybrid versus standard minimally invasive approach employing radiofrequency sources. Int J Cardiol. 2013;167:1469–75. doi: 10.1016/j.ijcard.2012.04.044. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta–analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stroup DF, Berlin JA, Morton SC et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/JAMA.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 17.Richardson TD, Shoemaker MB, Whalen SP et al. Staged versus simultaneous thoracoscopic hybrid ablation for persistent atrial fibrillation does not affect time to recurrence of atrial arrhythmia. J Cardiovasc Electrophysiol. 2016;27:428–34. doi: 10.1111/jce.12906. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JPT, Green S. Hoboken, NJ Wiley-Blackwell: 2008. Cochrane Handbook for Systematic Reviews of Interventions. (eds) [DOI] [Google Scholar]
- 19.Chambers D, Rodgers M, Woolacott N. Not only randomized controlled trials, but also case series should be considered in systematic reviews of rapidly developing technologies. J Clin Epidemiol. 2009;62:1253–60. e1254. doi: 10.1016/j.jclinepi.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 20.R Core Team R: A language and environment for statistical computing. 2016. www.R–project.org. www.R–project.org Available at. (accessed 16 November 2016)
- 21.Mahapatra S, LaPar DJ, Kamath S et al. Initial experience of sequential surgical epicardial–catheter endocardial ablation for persistent and long–standing persistent atrial fibrillation with long-term follow-up. Ann Thorac Surg. 2011;91:1890–8. doi: 10.1016/j.athoracsur.2011.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beaver TM, Hedna VS, Khanna AY et al. Thoracoscopic Ablation With Appendage Ligation Versus Medical Therapy for Stroke Prevention: A Proof-of-Concept Randomized Trial. Innovations (Phila) 2016;11:99–105. doi: 10.1097/IMI.0000000000000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fengsrud E, Wickbom A, Almroth H et al. Total endoscopic ablation of patients with long-standing persistent atrial fibrillation: a randomized controlled study. Interact Cardiovasc Thorac Surg. 2016;23:292–8. doi: 10.1093/icvts/ivw088. [DOI] [PubMed] [Google Scholar]
- 24.Gersak B, Kiser AC, Bartus K et al. Importance of evaluating conduction block in radiofrequency ablation for atrial fibrillation. Eur J Cardiothorac Surg. 2012;41:113–118. doi: 10.1016/j.ejcts.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Driessen AH, Berger WR, Krul SP et al. Ganglion Plexus Ablation in Advanced Atrial Fibrillation: The AFACT Study. J Am Coll Cardiol. 2016;68:1155–65. doi: 10.1016/j.jacc.2016.06.036. [DOI] [PubMed] [Google Scholar]
- 26.Romanov A, Pokushalov E, Elesin D et al. Effect of left atrial appendage excision on procedure outcome in patients with persistent atrial fibrillation undergoing surgical ablation. Heart Rhythm. 2016;13:1803–9. doi: 10.1016/j.hrthm.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 27.Pearman CM, Todd D, King R et al. The procedural complication rates and short–term success rates of thoracoscopic AF ablation during the institutional learning curve. EP Europace. 2016;18(Suppl 2):ii13–ii17. [Google Scholar]
- 28.Fuster V, Ryden LE, Cannom DS et al. ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation. J Am Coll Cardiol. 2011;2011;57:e101–98. doi: 10.1016/j.jacc.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 29.Gelsomino S, Van Breugel HN, Pison L et al. Hybrid thoracoscopic and transvenous catheter ablation of atrial fibrillation. Eur J Cardiothorac Surg. 2014;45:401–7. doi: 10.1093/ejcts/ezt385. [DOI] [PubMed] [Google Scholar]
- 30.La Meir M, Gelsomino S, Luca F et al. Minimally invasive thoracoscopic hybrid treatment of lone atrial fibrillation: early results of monopolar versus bipolar radiofrequency source. Interact Cardiovasc Thorac Surg. 2012;14:445–50. doi: 10.1093/icvts/ivr142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seely AJ, Ivanovic J, Threader J et al. Systematic classification of morbidity and mortality after thoracic surgery. Ann Thorac Surg. 2010;90:936–42. doi: 10.1016/j.athoracsur.2010.05.014. discussion 942. [DOI] [PubMed] [Google Scholar]