Abstract
Acute Heart Failure (AHF) is a “ multi-event disease” and hospitalisation is a critical event in the clinical course of HF. Despite relatively rapid relief of symptoms, hospitalisation for AHF is followed by an increased risk of death and re-hospitalisation. In AHF, risk stratification from clinically available data is increasingly important in evaluating long-term prognosis. From the perspective of patients, information on the risk of mortality and re-hospitalisation would be helpful in providing patients with insight into their disease. From the perspective of care providers, it may facilitate management decisions, such as who needs to be admitted and to what level of care (i.e. floor, step-down, ICU). Furthermore, risk-stratification may help identify patients who need to be evaluated for advanced HF therapies (i.e. left-ventricle assistance device or transplant or palliative care), and patients who need early a post-discharge follow-up plan. Finally, risk stratification will allow for more robust efforts to identify among risk markers the true targets for therapies that may direct treatment strategies to selected high-risk patients. Further clinical research will be needed to evaluate if appropriate risk stratification of patients could improve clinical outcome and resources allocation.
Keywords: Acute heart failure, risk stratification, prognosis models, risk scores
Hospitalisation is a critical event in the clinical course of heart failure (HF) and despite relatively rapid relief of symptoms, hospitalisation is followed by an increased risk of death and re-hospitalisation.[1] While performance measures have been developed in the last few years with the intent of improving post-discharge outcomes, post-discharge mortality rates remain unchanged or have slightly worsened.[2] The mechanisms of these high post-discharge event rates are incompletely understood[3] and, to date, no treatment has improved such outcomes. Although long-term mortality is the result of the continuous deterioration of cardiac substrate, worsening of comorbidities, and progression of HF, there is considerable diversity of both the underlying pathophysiology and the patients involved, which makes it difficult to find an explanation that is suitable for all patients.[4]
Registry data reveal that 20 % of patients are discharged despite persistent signs and symptoms of HF, including minimal decrease or even increase in body weight. These findings suggest failure to relieve clinical congestion during the index hospitalisation may potentially contribute to the high post-discharge mortality rate.[4]
Post-hoc analyses of these clinical trials and international registries have identified several prognostic factors in AHF patients and have attempted to explore their relationship with post-discharge mortality. Knowledge of mortality predictors can be used to generate predictive models that can aid clinicians in their decision-making, in particular by identifying patients who are at high or low risk of death. These models could be used as a framework to discuss prognosis and provide evidence to support rational decision-making.
Even if the phenotypic heterogeneity of AHF patients[5] makes it difficult to find a risk model suitable for all patients, many parameters are common to several of the models. Demographic characteristics, renal function, markers of organ injury, and non-cardiac comorbidities are included in most risk models (see Figure 1). Our goal in the present paper is to review the most important prediction models developed for the risk-stratification of patients with AHF.
Figure 1: 6-axis Risk Model for Post-discharge Mortality in Patients Hospitalized for Acute Heart Failure.
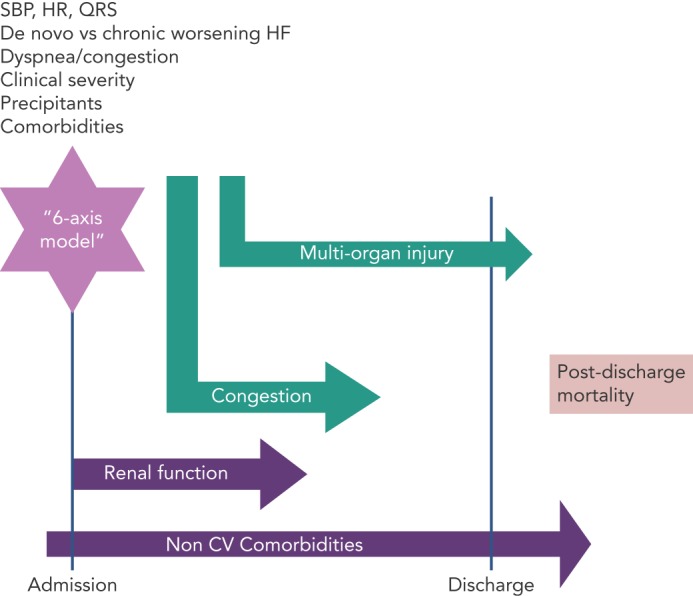
In acute heart failure, multiple entities contribute to post-discharge mortality. Assessment at initial presentation by 6-axis model offers significant prognostic information. Markers reflecting severity of congestion and multi-organ injury are determinants for in-hospital and post-discharge course. Severity of disease, mirrored by alterations in cardiac electromechanical substrate, as well as severity and number of associated non-cardiovascular comorbidities negatively impact post-discharge prognosis. CV = cardiovascular; HR = heart rate; SBP = systolic blood pressure..
Risk Stratification in Acute Heart Failure
Heart failure hospitalisation represents an important opportunity to assess patient prognosis. In the care of patients with HF, estimating and communicating prognosis is recommended by clinical guidelines[6] and is considered to be an important component of high-quality health care. A better understanding of the mechanisms underlying the poor prognosis of patients hospitalised for HF may help provide better care and improve post-discharge mortality.
One of the major goals of AHF risk stratification is to match the risk profile of the patient with the type and intensity of care. AHF is not one distinct pathophysiologic entity, but rather a heterogeneous syndrome with multiple contributors to the progression of the disease and prognosis. A comprehensive assessment in these patients is necessary to identify multiple prognostic characteristics that may become possible therapeutic targets. Moreover, the phenotypic heterogeneity of AHF patients, either at presentation or during the hospital course, suggests that an algorithm is needed to classify these patients. For initial presentation, a “6-axis model” has been proposed to classify AHF patients.[7] While this was designed for the initial assessment, each component of this model has long-term prognostic value (see Figure 1).
Candidate predictors can be obtained from patient demographics, clinical history, physical examination, disease characteristics, laboratory tests, and previous treatment. Studied predictors should be clearly defined, standardised, and reproducible to enhance generalisability and application of study results to practice.[8] Prognostic studies use a multivariable approach in their design and analysis to determine the important predictors of the studied outcomes and to provide outcome probabilities for different combinations of predictors. The aim is to determine whether an outcome can reliably be attributed to a particular risk factor, with adjustment for other causal factors (confounders) using a multivariable approach.
Predictors can be derived from registries (see Table 1) or from randomised clinical trials (RCTs) (see Table 2). Clinical characteristics of patients enrolled in RCTs may differ to those in the general population with HF, and prognostic models obtained from RCT data may have restricted generalisability because of strict eligibility criteria for the trial, low recruitment levels, lower rate of associated comorbidities, or large numbers of patients refusing consent. Registries have increased predictive power due to the large number of patients enrolled, but collection of clinical variables may not be as rigorous and complete as in RCTs.
Table 1: Independent Predictors of Post-discharge Mortality in Registries.
| Registry | Year of publication | Sample size | Prediction period | Independent predictor results from multivariate analysis |
|---|---|---|---|---|
| OPTIMIZE-HF[9] | 2008 | 4400 | 60-day mortality | Creatinine; sodium; age; HR; liver disease; previous CVA/TIA; peripheral vascular disease; race; left ventricular systolic dysfunction; COPD; SBP; previous HF hospitalisation |
| EFICA[11] | 2006 | 599 | 1-month and 12-month mortality | Shock; renal dysfunction; ischaemia; liver dysfunction; previous ADHF episode; comorbidity; SBP; pulmonary oedema |
| MOCA[12]12 | 2013 | 5306 | 1-month and 12-month mortality | Age; sex; SBP and DBP; eGFR; sodium; haemoglobin; heart rate; NT-proBNP; CRP; MR-proADM; sST2 |
| FINN AKVA[13] | 2006 | 620 | age, male gender; lower systolic blood pressure (SBP) on admission; C-reactive protein and serum creatinine >120 mmol/L |
|
| ESC-HF-LT registry[14] | 2016 | 5039 | 1-year mortality | Age; SBP; EF; NYHA III–IV; congestion; aortic stenosis; diabetes; COPD; previous stroke; renal dysfunction; hepatic dysfunction |
| AHEAD[15] | 2011 | 3438 | 1-year mortality | Age; creatinine; valvular disease; LVEF <30 %; previous stroke or TIA; de novo vs worsening chronic HF |
| IN-HF[16] | 2013 | 1855 | 1-year mortality | Age; low SBP; somnolent or confused; Na <136 mEq/l; creatinine >1.5 mg/dl; BUN >50 mg/dl; Hb <12 g/dl; APE; COPD |
ADHF = acute decompensated heart failure; AHEAD = Acute Heart Failure Database; APE = acute pulmonary embolism; BUN = blood urea nitrogen; COPD = chronic obstructive pulmonar disease; CRP = C-reactive protein; CVA/TIA = cerebrovascular accident/transitory ischaemic accident; DBP = diastolic blood pressure; EF = ejection fraction; EFICA = Etude Francaise de l’Insuffisance Cardiaque Aigue; eGFR = estimated glomerular filtration rate; ELAN = European Collaboration on Acute Decompensated Heart Failure; ESC HF LT = European Society of Cardiology Heart Failure Long-Term Registry; FINN AKVA = Finnish Acute Heart Failure Study; Hb = haemoglobin; HF = heart failure; HR = heart rate; IN HF = Italian Network on Heart Failure; LVEF = left ventricular ejection fraction; MOCA = Multinational Observational Cohort on Acute Heart Failure; MR-proADM = mid-regional pro-adrenomedullin; NT-proBNP = N-terminal pro brain natriuretic peptide; NYHA = New York Heart Association; OPTIMIZE-HF = Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure; SBP = systolic blood pressure; sST2 = soluble suppression of tumorigenicity 2.
Table 2: Independent Predictors of Post-discharge Mortality in Randomised Controlled Trials.
| RCT | Year of publication | Sample size | Prediction period | Independent predictor results from multivariate analysis |
|---|---|---|---|---|
| OPTIME-CHF[17]17 | 2004 | 949 | 60-day mortality | Age; NYHA functional class; SBP; BUN; sodium |
| ESCAPE[18] | 2010 | 423 | 6-month mortality | BNP; cardiopulmonary resuscitation or mechanical ventilation during hospitalisation; blood urea nitrogen; serum sodium, age >70 years; daily loop diuretic, furosemide equivalents >240 mg; lack of betablocker; 6-min walk test |
| PROTECT[19] | 2016 | 1990 | 90-day mortality | Age; COPD; SBP; WBC count; serum sodium; bicarbonate; BUN; uric acid |
| CHARM[20] | 2007 | 7572 | 2-year mortality | Age; LVEF; diabetes-insulin treated; low BMI; male; NYHA Class IV; current smoker; cardiomegaly; prior HF hospitalisation within 6 months |
| ASCEND HF[21] | 2015 | 7141 | 30-day and 180-day mortality | Age; BUN; baseline sodium, SBP>140 mmHg; baseline dyspnoea |
ASCEND-HF = Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure; BMI = body mass index; BNP = brain natriuretic peptide; BUN = blood urea nitrogen; CHARM = Candesartan in Heart Failure-Assessment of Reduction in Mortality and Morbidity; COPD = chronic obstructive pulmonary disease; ESCAPE = Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness; HF = heart failure; LVEF = left ventricular ejection fraction; NYHA = New York Heart Association; OPTIME-CHF = Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure; PROTECT = Placebo-controlled Randomized study of the selective A(1) adenosine receptor antagonist rolofylline for patients hospitalized with acute heart failure and volume Overload to assess Treatment Effect on Congestion and renal function; RCT = randomised clincal trial; SBP = systolic blood pressure; WBC = white blood count.
One important consideration when assessing predictors of post-discharge mortality is the time frame of data collection.[22] Variables collected upon admission may be less likely to be linked to 6-month or 1-year prognosis, as changes in clinical status or medical interventions performed during hospitalisation may affect medium or long-term outcomes. However, some variables collected at AHF admission are unmodifiable risk factors, such as age, gender and presence of comorbidities.
In addition, when considering all prognostic factors, it is important to carefully review the selection criteria for the cohort from which the predictor was reported. For example, some RCT enrolled patients with reduced ejection fraction (EF), while other RCTs were inclusive AHF irrespective of EF. Among patients with reduced EF, the Efficacy of Vasopressin Antagonism in Heart Failure: Outcome Study with Tolvaptan (EVEREST) trial database[23] allowed the opportunity for numerous sub-analyses which have provided valuable insights in understanding post-discharge mortality predictors (see Table 3).
Table 3: Main Predictive Factors for Post-discharge Mortality Derived from EVEREST Trial Sub-analyses.
| Baseline | Discharge | 1 week | |
|---|---|---|---|
| Systolic blood pressure | + | + | + |
| Heart rate | No | No | + |
| Congestion score | - | + | + |
| B-type natriuretic peptide | - | + | + |
| Large QRS duration | + | - | - |
| Low cholesterol | + | - | - |
Hepatic function test:
|
No + + |
No + + |
- - - |
| Haematocrit | - | + | - |
| Low osmolality | - | + | - |
| Hyponatraemia | + | - | - |
| Potassium | No | No | - |
| High seric uric acid* | + | ||
| Anaemia | No | + | - |
*Only in patients with normal baseline renal function. + = predictive value; - = no information; ALT = alanine aminotransferase; AST = aspartate aminotransferase.
Variables Predictive of Post-discharge Outcomes in AHF
In RCTs and registries, the predictive factors for post-discharge mortality included age, history of previous hospitalisation, congestion, systolic blood pressure (SBP), heart rate (HR), QRS duration, renal function, markers of organ injury, and non-cardiac comorbidities (such as diabetes, cerebrovascular disease, chronic obstructive pulmonary disease, liver cirrhosis, and anaemia) (see Tables 1–3). Although, there are many candidates that have additional prognostic value, the key variables are SBP and renal function. These two are the best discriminators between patients who survive hospitalisation and those who die or are readmitted post-discharge.
We have highlighted some of these important prognostic markers that are relevant in clinical practice.
Congestion
Clinical trials testing short-term IV therapies have focused on dyspnoea improvement. However, dyspnoea assessments remain imprecise and regardless of how it is measured, the vast majority of resolves or significantly improves in the first 24 to 48 hours of IV standard therapies.[24] In the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure (ASCEND-HF),[25] the relationship between in-hospital dyspnoea improvement and post-discharge outcomes was inconsistent and study medication failed to show any post-discharge outcomes benefit. Furthermore, pathophysiology of congestion is more complex, and the subjective feeling of dyspnoea may poorly correlate with objective measures of decongestion, such as weight change[25] or urine output.[26]
Nonetheless, congestion is the leading cause for AHF readmission, and represents an important therapeutic target of inpatient management, and a major determinant of discharge decision-making. Indeed, a clinical score, including orthopnoea, JVD and pedal oedema was used in the EVEREST trial, and this congestion score was associated with an increased risk of 30-day and 1-year mortality.[27] However, despite the clinical importance of targeting signs and symptoms during hospitalisation, patients with absent or minimal signs and symptoms of congestion may experience a lower, but comparable, post-discharge event rate as compared to the overall cohort. This finding raises the hypothesis that treating beyond resolution of congestion may mediate improvements in post-discharge outcomes.[7] Further research is necessary to prospectively validate the clinical utility of targeting provocative manoeuvres, including an assessment of orthopnoea, orthostatic hypotension, lung ultrasound, and completion of a 6-minute walk test and haemodynamic biomarkers, such as BNP/NT-proBNP, in patients hospitalized for HF.[27]
Natriuretic Peptides
Natriuretic peptides (NPs) represent a sensitive and noninvasive measure of ventricular filling pressures, which correlates with overall cardiac function and informs prognosis, irrespective of ejection fraction.[28] During hospitalisation and the post-discharge period, persistence of elevated levels of NPs after resolution of clinical congestion signifies haemodynamic congestion.
In clinical studies, the absolute level of NPs measured at discharge[29,30] and NPs percentage variation during hospitalisation[31] correlate with post-discharge mortality. Also, BNP level at 1-week post-discharge was associated with the largest increase in prognostic value having the best accuracy of grading patients’ likelihood of death.[32] Additionally, change in natriuretic peptide levels at hospitalisation to 1-month post-discharge carries incremental predictive value above the absolute value of the 1-month measurement alone.[33]
Systolic Blood Pressure
A large number of registries and RCTs have shown that systolic blood pressure (SBP) assessment at admission provides important, independent prognostic information in patients with HF with both reduced and preserved EF. Furthermore, SBP at hospital admission can effectively identify groups of patients that differ with respect to clinical characteristics, prognosis, underlying pathophysiology and therapeutic approach.[34]
In the EVEREST trial, low SBP determined either after the initiation of standard therapy or after the resolution of the “acute” phase of hospitalisation remained an indicator of poor prognosis.[35]
Elevated SBP in the acute setting is a result of high sympathetic tone, termed reactive hypertension, indicating the presence of functional cardiac reserve in the face of an acute physiologic stressor. In contrast, low, or even normal SBP at presentation, which may be the goal of treatment in the ambulatory setting, may be a more ominous finding, reflecting a low cardiac output and suboptimal or inadequate end-organ perfusion.[34]
Heart Rate
At the time of admission for AHF, heart rate (HR) is a reflection of the patient’s haemodynamic status,[36] and changes in HR during hospitalisation have not been associated to short-term outcomes.[37]
A higher HR at both 1 and 4 weeks post-discharge was independently predictive of increased mortality during subsequent follow-up in patients with reduced EF and worsening HF.[38]
QRS Duration
Electrical dys-synchrony, as evidenced by a prolonged QRS duration, remains the currently accepted guidelines indicator to evaluate potential candidates for CRT.[6] Prolonged QRS duration was independently associated with high post-discharge mortality in the EVEREST trial.[39] The presence of a prolonged QRS duration associated with reduced LVEF is not only a marker for significantly increased mortality but becomes a potential therapeutic target. The Cardiac Resynchronisation in Heart Failure (CARE-HF) study demonstrated that CRT improved symptoms and reduced the risk of death in patients with reduced ejection fraction and prolonged QRS duration in the outpatient setting.[40]
Hyponatremia
In the ESC-HF-LT registry,[41] hyponatremia (serum sodium <135 mEq/l) has been reported on admission at 25 % of patients and at discharge at 18 % of patients.
The pathophysiology of hyponatremia in HF has been described as a result of neurohormonal activation, including stimulation of vasopressin, which in complex interactions impairs water excretion (i.e. dilutional hyponatremia).[42]
Also, diuretic agents and several other common non-cardiac comorbidities may decrease serum sodium concentration.[43] Hyponatremia is one of the most constant cited predictors of mortality in clinical trials and registries, and has been associated with a three-fold increase in post-discharge mortality.[44]
A similar finding has been found for serum osmolality and lower discharge serum osmolality was predictive of post-discharge outcomes in a sub-analysis of the EVEREST study.[45]
Although Tolvaptan was successful in reducing hyponatremia and increasing serum osmolality, inducing weight and fluid loss, it has not been shown to improve clinical outcomes.[23] This suggests that hyponatremia and serum osmolality, despite of markers of prognosis, are not targets for drug therapy.
Distinct to hyponatremia, baseline and in-hospital changes in potassium, although may significantly impact in-hospital care and may limit the implementation of evidence-based therapies, they are not associated with all-cause mortality.[46]
Renal function
The majority of registries and RCTs considered baseline renal impairment as a predictor of poor outcome in AHF.[47] Markers of renal function included serum creatinine, BUN, and uric acid.
Baseline creatinine has not been consistently included in prognostic models and a major limitation of this biomarker is that creatinine is not only filtered but is also secreted by the kidney, and its production is dependent on muscle mass.[48]
Although serum blood urea nitrogen (BUN) is considered to be a less specific marker of renal function compared with serum creatinine, it varies independently of changes in creatinine in HF patients because of neurohormonal activation and enhanced tubular reabsorption. Thus, elevated serum BUN in AHF patients may reflect both altered intrinsic renal function and potentially reversible “vasomotor nephropathy” secondary to the haemodynamic and neurohormonal effects of destabilised HF,[49] and its predictive value extends beyond hospitalisation. Prognostic utility of high serum uric acid (sUA) is limited, and sUA is predictive of post-discharge mortality only in patients with preserved admission renal function.[50]
Markers of Organ Injury
Injury or end-organ dysfunction, including myocardial damage, worsening renal function, and hepatic impairment, have been independently associated with mortality in AHF. Although many other organs (e.g. brain, lung, intestine, endothelium, vasculature) are exposed to injury during AHF episodes, organ-specific injury markers for these organs suitable for clinical practice are missing.[51] While data support such markers as prognostic, clinical trial efforts to date with therapies designed to abort end-organ injury during episodes of AHF have been disappointing.
Troponin
An increase in plasma troponin levels is very common in patients hospitalised for HF. The percentage of patients with “elevated” troponin in AHF depends substantially on the severity of HF, the cut-point chosen, as well as the sensitivity of the assay employed.[52] Furthermore, Troponin release in AHF is often persistent. In ASCEND-HF, at 30 days post-discharge, 62 % of patients had detectable values of troponin I and 28 % had elevated values >99 % of URL[53]
Although the exact mechanisms of myocardial injury in HF are uncertain, ischaemia, haemodynamic stress, oxidative stress, inflammation, altered calcium handling and impaired renal clearance have all been proposed as mechanisms of troponin elevation.[54]
Multiple studies have evaluated the association between baseline elevated circulating cTn and post-discharge mortality in various AHF settings. Despite variations in study design, patient populations, and assay characteristics, there has been a mostly consistent association between cTn elevation and worsened post-discharge outcomes.[55–57] Thus, measurement of cTnI in patients hospitalised for AHF is warranted, given the desire to identify patients at high risk for adverse outcomes, as well as to identify patients in whom ischaemia appears to be a trigger of decompensation.[6]
Studies have varied in the type of troponin assay used (I or T), traditional or high sensitivity (hsTn), as well as the cut-off values used to define a positive test. In addition to baseline values and a rise in serum troponin levels during hospitalisation, an index of an event-related myocardial necrosis, is a powerful predictor of outcomes.[53,58] In the Placebo-Controlled Randomized Study of the Selective Adenosine A1 Receptor Antagonist Rolofylline for Patients Hospitalized with Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function (PROTECT) study, positive troponin at baseline, and conversion during hospitalisation from negative to positive levels, were associated with worse outcomes at 60 days.[59] In the Relaxin in Acute Heart Failure (RELAX-AHF) study, an increase in troponin during the hospital stay had an independent relation with 180 days mortality.[60]
Worsening Renal function
A review of the wide body of literature suggests an inconsistent relationship between in-hospital worsening renal function (WRF) and post-discharge outcomes. Data suggest that the clinical context of WRF is essentially in determining its prognostic implication. For example, WRF in the setting of effective decongestion, as exemplified by haemoconcentration, has consistently been associated with favourable long-term prognosis. This is often referred to as “pseudo worsening renal function”.[61] Importantly, longitudinal follow-up of these patients shows a strong tendency for renal function to return to baseline, suggesting that no permanent renal injury takes place despite a change in laboratory values. In contrast, WRF outside the context of active fluid removal may predict worse outcomes.[62] Thus, in patients with AHF, serum creatinine changes during admission are associated with adverse outcome only in the presence of congestion. Persistence of congestion during hospitalisation is the most important prognostic factor and WRF has clinical significance only when occurring in patients with persistent fluid overload.[63] A further decline in eGFR (especially if urinary output decreases or the clinical status of a patient simultaneously deteriorates) may represent true WRF, which is associated with substantially worse long-term outcomes and thus should be avoided.[47] Furthermore, targeting improvement or preservation of renal function did not lead to an improved survival rate in the PROTECT trial.[64]
Liver Injury
Previous studies conducted in patients hospitalised for HF[65–68] have found the association between transaminases and mortality to not be statistically significant after adjusting for natriuretic peptide concentrations[66] or invasive haemodynamic measurements.[67,68] However, in RELAX I, increases in serum transaminases (AST and ALT) were associated with increased 180-day all-cause mortality.[60]
Comorbidities
Two-thirds of readmissions within 30 days from a HF hospitalisation are for non-HF primary issues, regardless of EF. Comorbidities are highly prevalent in this population, and not only do they precipitate rehospitalisation, uncontrolled comorbidities worsen HF over time.[69]
In the ESC-HF-LT-registry,[14] a number of non-cardiac comorbidities, including hepatic or renal dysfunction, previous stroke, diabetes and chronic obstructive pulmonary disease (COPD), were found to be independent predictors for 1-year mortality in patients hospitalised for HF with both preserved and reduced EF.
Although without a graded relationship between baseline glycaemia and outcomes, diabetic status has been found to be one of the most important predictors of 1-year all-cause mortality, independent of EF, eGFR and other comorbidities.[70]
Concurrent COPD independently predicts mortality in patients with reduced and preserved ejection fraction,[71–73] and greater airflow obstruction is associated with worsening survival.[74]
In EVEREST, anaemia at discharge, but not admission, was independently associated with increased all-cause mortality.[75] However, it is unclear whether anaemia is truly a prognostic marker or a mediator of risk. It has been postulated that anaemia is a marker of disease severity for HF, or that other factors associated with anaemia are responsible for the increased events. Multiple RCTs have investigated the impact of anaemia treatment on mortality, including the recent RED-HF trial,[76] but have failed to demonstrate significant benefit.
Since the aetiology of anaemia in AHF is multifactorial, targeting anaemia in the broad population with AHF may not be a viable strategy until there is an improved understanding of how anaemia or different components of anaemia directly affect post-discharge outcomes.
Risk Scores
Post-hoc analyses have combined various risk markers from multi-variable models in risk scores, in order to better stratify patients and thus identify the highest-risk patients.[23] Probability as an individual patient who experienced an event had a higher risk score than a patient who had not experienced the event is evaluated by C-statistic.[77]
Given the complexity and heterogeneity of AHF, prior attempts for risk modelling have not been easily adapted to clinical practice and generally have not had high discriminatory capacity. Most models were derived from demographic, clinical and biological data collected at admission (see Table 4) and only a few have used discharge data. Also, including a variable in a risk model will be negatively impacted by the quantity of missing data, and the rate of collection has varied among registries. Also, the current available models do not include the use of devices, which may carry a strong impact on prognosis in the long term.[78] Actually, risk stratification by scoring methods remains only informative in the clinical decision-making process.
Table 4: Risk Stratification in Acute Heart Failure and Clinical Relevant Risk Scores.
| Study | Sample size | Prediction period | Risk score variables | C-statistic |
|---|---|---|---|---|
| OPTIMIZE-HF[9] | 4400 | 60-day mortality | Age; HR; SBP; serum creatinine; serum sodium; primary cause of admission (heart failure or other); and LVEF | 0.74 |
| ELAN[10] | 1301 | 180-day mortality | NT-proBNP at discharge; NT-proBNP reduction; age; peripheral oedema; SBP; low sodium; serum urea; NYAH III and IV | 0.78 |
| OPTIME-CHF[17] | 949 | 60-day mortality | Age; lower; SBP; NYHA class IV; symptoms; elevated BUN; decreased sodium | 0.77 |
| ESCAPE[18] | 423 | 6-month mortality | Age >70 years; BUN >40; BUN >90 mg/dl; 6MWT; NA <130 mEq/l; cardiopulmonary esuscitation or mechanical ventilation during hospitalisation; furosemide equivalents >240 mg; lack of betablocker; BNP >500 and BNP >1300 pg/ml | 0.74 |
| ASCEND-HF[21] | 7141 | 6-month mortality | Age; low SBP; low sodium; high BUN; dyspnoea at rest | 0.70 |
| MOCA[12] | 5306 | 12-month mortality | Age; SBP; eGFR <60 ml/min; sodium; haemoglobin; heart rate | 0.73 |
6MWT = 6-minute walking test; ASCEND-HF = Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure; BNP = brain natriuretic peptide; BUN = blood urea nitrogen; COPD = chronic obstructive pulmonary disease; eGFR = estimated glomerular filtration rate; ELAN = European collaboration on acute decompensated Heart Failure; ESCAPE = Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness; HF = heart failure; HR = heart rate; LVEF = left ventricular ejection fraction; MOCA = Multinational Observational Cohort on Acute Heart Failure; MR-proADM = mid-regional pro-adrenomedullin; NT-proBNP = N-terminal pro brain natriuretic peptide; NYHA = New York Heart Association; OPTIME-CHF = Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure; OPTIMIZE-HF = Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure; SBP = systolic blood pressure.
Limitation of Prognostic Models
Despite numerous clinical and biological variables showing in registries and RCTs independent predictive value for post-discharge mortality, very few represent true targets for in-hospital therapies such as congestion, QRS duration, and non-cardiac comorbidities. In addition, heart rate measured at 1-week and 1-month post-discharge could be considered a target for If antagonist Ivabradine. Although in the RELAX-AHF I trial markers of organ injury were associated with 180 days mortality and decreased as result of Serelaxin treatment, the RELAX II study did not confirm these beneficial effects.
Another major limitation of prognostic models in AHF is the absence of prospective validation and lack of the impact studies.
The main ways to evaluate or validate the performance of a prognostic model on a new dataset are to compare observed and predicted event rates for groups of patients (calibration) and to quantify the model’s ability to distinguish between patients who do or do not experience the event of interest (discrimination).[77]
Furthermore, studies to evaluate the effect of using a prognostic model on current medical practice and on patient outcome would be informative and could lead to clinical implementation of such a model.[79] An impact analysis can determine whether use of the model is better than usual care.[80] This remains an unmet need.
Conclusion
In AHF, the attempts to develop risk models are justified by the evidence that the risk of post-discharge mortality and rehospitalisation remains high. Furthermore, developing risk models would aid in targeting limiting resources to the appropriate patients. Even if the phenotypic heterogeneity of AHF patients makes it difficult to find a risk model suitable for all patients, some parameters recur in many models. However, in spite of limitations, prognostic models add to our understanding of the determinants of the course and outcome of patients with AHF. The impact of stratification of AHF patients on current clinical practice should be further evaluated in prospective studies.
Acknowledgement
All of the authors acknowledge the important contribution of Professor Mihai Gheorghiade in this manuscript, which represents only one of his hundreds of research contributions. Mihai Gheorghiade was one of the most respected, quoted and liked cardiologists. Committed to excellence from early on in his career he was determined to excel in patient care, research and teaching. He felt that his highest calling was to mentor students, trainees, and junior colleagues. He was never more enthusiastic than when supporting new collaborations to take place between great minds in different countries. He passed away on 24 August 2017. The world of cardiology has lost an iconic figure, but his legacy of challenging concepts will live on for many years to come.
References
- 1.Gheorghiade M, Shah AN, Vaduganathan M et al. Recognizing hospitalized heart failure as an entity and developing new therapies to improve outcomes: academics’, clinicians’, industry’s, regulators’, and payers’ perspectives. Heart Fail Clin. 2013;9:285–90. doi: 10.1016/j.hfc.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Gheorghiade M, Peterson ED. Improving postdischarge outcomes in patients hospitalized for acute heart failure syndromes. JAMA. 2011;305:2456–7. doi: 10.1001/jama.2011.836. [DOI] [PubMed] [Google Scholar]
- 3.Butler J, Fonarow GC, Gheorghiade M. Need for increased awareness and evidence-based therapies for patients hospitalized for heart failure. JAMA. 2013;310:2035–6. doi: 10.1001/jama.2013.282815. [DOI] [PubMed] [Google Scholar]
- 4.Ambrosy A, Fonarow GC, Butler J et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63:1123–33. doi: 10.1016/j.jacc.2013.11.053. [DOI] [PubMed] [Google Scholar]
- 5.Ambrosy A, Gheorghiade M. Clinical profiles in acute heart failure: one size fits all or not at all? Eur J Heart Fail. 2017;19:1255–7. doi: 10.1002/ejhf.907. [DOI] [PubMed] [Google Scholar]
- 6.Ponikowski P, Voors AA, Anker SD et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 7.Gheorghiade M, Braunwald E. A proposed model for initial assessment and management of acute heart failure syndromes. JAMA. 2011;305:1702–3. doi: 10.1001/jama.2011.515. [DOI] [PubMed] [Google Scholar]
- 8.Moons MG, Royston P, Vergouwe Y et al. Prognosis and prognostic research: what, why, and how? BMJ. 2009;338:b375. doi: 10.1136/bmj.b375. [DOI] [PubMed] [Google Scholar]
- 9.O’Connor CM, Abraham WT, Albert NM et al. Predictors of mortality after discharge in patients hospitalized with heart failure: an analysis from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF). Am Heart J. 2008;156:662–73. doi: 10.1016/j.ahj.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 10.Salah K, Kok WE, Eurlings LW et al. A novel discharge risk model for patients hospitalised for acute decompensated heart failure incorporating N-terminal pro-B-type natriuretic peptide levels: a European coLlaboration on Acute decompeNsated Heart Failure: ELAN-HF Score. Heart. 2014;100:115–125. doi: 10.1136/heartjnl-2013-303632. [DOI] [PubMed] [Google Scholar]
- 11.Zannad F, Mebazaa A, Juilliere Y et al. Clinical profile, contemporary management and one-year mortality in patients with severe acute heart failure syndromes: The EFICA study. Eur J Heart Fail. 2006;8:697–705. doi: 10.1016/j.ejheart.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Lassus J, Gayat E, Mueller C et al. Incremental value of biomarkers to clinical variables for mortality prediction in acutely decompensated heart failure: the Multinational Observational Cohort on Acute Heart Failure (MOCA) study. Int J Cardiol. 2013;168:2186–94. doi: 10.1016/j.ijcard.2013.01.228. [DOI] [PubMed] [Google Scholar]
- 13.Siirilä-Waris K, Lassus J, Melin J et al. FINN-AKVA Study Group. Characteristics, outcomes, and predictors of 1-year mortality in patients hospitalized for acute heart failure. Eur Heart J. 2006 Dec;27((24)):3011–7. doi: 10.1093/eurheartj/ehl407. [DOI] [PubMed] [Google Scholar]
- 14.Crespo-Leiro M, Anker S, Maggioni A et al. European Society of Cardiology Heart Failure Long-Term Registry (ESC-HF-LT): 1-year follow-up outcomes and differences across regions. Eur J Heart Fail. 2016;18:613–25. doi: 10.1002/ejhf.566. [DOI] [PubMed] [Google Scholar]
- 15.Parenica J, Spinar J, Vitovec J et al. Long-term survival following acute heart failure: The Acute Heart Failure Database Main registry (AHEAD Main). Eur J Int Med. 2013;24:151–60. doi: 10.1016/j.ejim.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Tavazzi L, Senni M, Metra M et al. Multicenter prospective observational study on acute and chronic heart failure: one-year follow-up results of IN-HF (Italian Network on Heart Failure) outcome registry. Circ Heart Fail. 2013;6:473–81. doi: 10.1161/CIRCHEARTFAILURE.112.000161. [DOI] [PubMed] [Google Scholar]
- 17.Felker GM, Leimberger JD, Califf RM et al. Risk stratification after hospitalization for decompensated heart failure. J Card Fail. 2004;10:460–6. doi: 10.1016/j.cardfail.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 18.O’Connor C, Hasselblad V, Mehta RH et al. Triage after hospitalization with advanced heart failure: the ESCAPE (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness) risk model and discharge score. J Am Coll Cardiol. 2010;55:872–8. doi: 10.1016/j.jacc.2009.08.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davison BA, Metra M, Senger S et al. Patient journey after admission for acute heart failure: length of stay, 30-day readmission and 90-day mortality. Eur J Heart Fail. 2016;18:1041–50. doi: 10.1002/ejhf.540. [DOI] [PubMed] [Google Scholar]
- 20.Solomon SD, Dobson J, Pocock S et al. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation. 2007;116:1482–7. doi: 10.1161/CIRCULATIONAHA.107.696906. [DOI] [PubMed] [Google Scholar]
- 21.Khazanie P, Heizer GM, Hasselblad V et al. Predictors of clinical outcomes in acute decompensated heart failure: Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure outcome models. Am Heart J. 2015;170:290–7. doi: 10.1016/j.ahj.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Cohen-Solal A, Laribi S, Ishihara S et al. Prognostic markers of acute decompensated heart failure: The emerging roles of cardiac biomarkers and prognostic scores. Arch Cardiovasc Dis. 2015;108:64–74. doi: 10.1016/j.acvd.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Konstam MA, Gheorghiade M, Burnett JC et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: The EVEREST Outcome Trial. JAMA. 2007;297:1319–31. doi: 10.1001/jama.297.12.1319. [DOI] [PubMed] [Google Scholar]
- 24.Hamo CE, Butler J, Gheorghiade M et al. The bumpy road to drug development for acute heart failure. Eur Heart J. 2016;18:G19–G32. doi: 10.1093/eurheartj/suw045. [DOI] [Google Scholar]
- 25.Ambrosy AP, Cerbin LP, Armstrong PW et al. Body Weight Change During and After Hospitalization for Acute Heart Failure: Patient Characteristics, Markers of Congestion, and Outcomes: Findings From the ASCEND-HF Trial. JACC Heart Fail. 2017;5:1–13. doi: 10.1016/j.jchf.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Felker GM, Mentz RJ, Cole RT et al. Efficacy and safety of tolvaptan in patients hospitalized with acute heart failure. J Am Coll Cardiol. 2017;69:1399–1406. doi: 10.1016/j.jacc.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Ambrosy AP, Pang PS, Khan S et al. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: findings from the EVEREST trial. Eur Heart J. 2013;34:835–43. doi: 10.1093/eurheartj/ehs444. [DOI] [PubMed] [Google Scholar]
- 28.Chioncel O, Collins SP, Greene SJ et al. Natriuretic peptide-guided management in heart failure. J Cardiovasc Med. 2016;17:556–68. doi: 10.2459/JCM.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 29.Lassus J, Gayat E, Mueller C et al. Incremental value of biomarkers to clinical variables for mortality prediction in acutely decompensated heart failure: the Multinational Observational Cohort on Acute Heart Failure (MOCA) study. Int J Cardiol. 2013;168:2186–94. doi: 10.1016/j.ijcard.2013.01.228. [DOI] [PubMed] [Google Scholar]
- 30.Bettencourt P, Azevedo A, Pimenta J et al. N-terminal-pro-brain natriuretic peptide predicts outcome after hospital discharge in heart failure patients. Circulation. 2004;110:2168–74. doi: 10.1161/01.CIR.0000144310.04433.BE. [DOI] [PubMed] [Google Scholar]
- 31.Salah K, Kok WE, Eurlings LW et al. A novel discharge risk model for patients hospitalised for acute decompensated heart failure incorporating N-terminal pro-B-type natriuretic peptide levels: a European coLlaboration on Acute decompeNsated Heart Failure: ELAN-HF Score. Heart. 2014;100:115–25. doi: 10.1136/heartjnl-2013-303632. [DOI] [PubMed] [Google Scholar]
- 32.Dunlay SM, Gheorghiade M, Reid KJ et al. Critical elements of clinical follow-up after hospital discharge for heart failure: insights from the EVEREST trial. Eur J Heart Fail. 2010;12:367–74. doi: 10.1093/eurjhf/hfq019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greene SJ, Maggioni AP, Fonarow GC et al. Clinical profile and prognostic significance of natriuretic peptide trajectory following hospitalization for worsening chronic heart failure: findings from the ASTRONAUT trial. Eur J Heart Fail. 2015;17:98–108. doi: 10.1002/ejhf.201. [DOI] [PubMed] [Google Scholar]
- 34.Gheorghiade M, Abraham WT, Albert NM et al. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. JAMA. 2006;296:2217–26. doi: 10.1001/jama.296.18.2217. [DOI] [PubMed] [Google Scholar]
- 35.Ambrosy AP, Vaduganathan M, Mentz R et al. Clinical profile and prognostic value of low systolic blood pressure in patients hospitalized for heart failure with reduced ejection fraction: insights from the Efficacy of Vasopressin Antagonism in Heart Failure: Outcome Study with Tolvaptan (EVEREST) trial. Am Heart J. 2013;165:216–25. doi: 10.1016/j.ahj.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 36.Reil JC, Custodis F, Swedberg K et al. Heart rate reduction in cardiovascular disease and therapy. Clin Res Cardiol. 2011;100:11–9. doi: 10.1007/s00392-010-0207-x. [DOI] [PubMed] [Google Scholar]
- 37.Bui AL, Grau-Sepulveda MV, Hernandez AF et al. Admission heart rate and in-hospital outcomes in patients hospitalized for heart failure in sinus rhythm and in atrial fibrillation. Am Heart J. 2013;165:567–74.e6. doi: 10.1016/j.ahj.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 38.Greene SJ, Vaduganathan M, Wilcox JE et al. The prognostic significance of heart rate in patients hospitalized for heart failure with reduced ejection fraction in sinus rhythm: insights from the EVEREST (Efficacy of Vasopressin Antagonism in Heart Failure: Outcome Study With Tolvaptan) trial. J Am Coll Cardiol. 2013;1:488–96. doi: 10.1016/j.jchf.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 39.Cleland JG, Daubert JC, Erdmann E et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–49. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 40.Wang NC, Maggioni AP, Konstam MA et al. Clinical implications of QRS duration in patients hospitalized with worsening heart failure and reduced left ventricular ejection fraction. JAMA. 2008;299:2656–66. doi: 10.1001/jama.299.22.2656. [DOI] [PubMed] [Google Scholar]
- 41.Chioncel O, Mebazaa A, Harjola VP et al. Clinical phenotypes and outcome of patients hospitalized for acute heart failure: the ESC Heart Failure Long-Term Registry. European Journal of Heart Failure. 2017;19:1242–54. doi: 10.1002/ejhf.890. [DOI] [PubMed] [Google Scholar]
- 42.Goldsmith SR, Gheorghiade M. Vasopressin antagonism in heart failure. J Am Coll Cardiol. 2005;46:1785–91. doi: 10.1016/j.jacc.2005.02.095. [DOI] [PubMed] [Google Scholar]
- 43.Lee CT, Guo HR, Chen JB. Hyponatremia in the emergency department. Am J Emerg Med. 2000;18:264–8. doi: 10.1016/s0735-6757(00)90118-9. [DOI] [PubMed] [Google Scholar]
- 44.Gheorghiade M, Abraham WT, Albert NM et al. OPTIMIZE-HF Investigators and Coordinators. Relationship between admission serum sodium concentration and clinical outcomes in patients hospitalized for heart failure: an analysis from the OPTIMIZE-HF registry. Eur Heart J. 2007;28:980–8. doi: 10.1093/eurheartj/ehl542. [DOI] [PubMed] [Google Scholar]
- 45.Vaduganathan M, Marti CN, Mentz RJ et al. EVEREST trial investigators. Serum osmolality and postdischarge outcomes after hospitalization for heart failure. Am J Cardiol. 2016;117:1144–50. doi: 10.1016/j.amjcard.2015.12.059. [DOI] [PubMed] [Google Scholar]
- 46.Khan SS, Campia U, Chioncel O et al. EVEREST Trial Investigators. Changes in serum potassium levels during hospitalization in patients with worsening heart failure and reduced ejection fraction (from the EVEREST trial). Am J Cardiol. 2015;115:790–6. doi: 10.1016/j.amjcard.2014.12.045. [DOI] [PubMed] [Google Scholar]
- 47.Damman K, Valente MA, Voors AA et al. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur Heart J. 2014;35:455–69. doi: 10.1093/eurheartj/eht386. [DOI] [PubMed] [Google Scholar]
- 48.Larsson A, Akerstedt T, Hansson LO, Axelsson J. Circadian variability of cystatin C, creatinine, and glomerular filtration rate (Gfr) in healthy men during normal sleep and after an acute shift of sleep. Chronobiol Int. 2008;25:1047–61. doi: 10.1080/07420520802553614. [DOI] [PubMed] [Google Scholar]
- 49.Klein L, Massie BM, Leimberger JD et al. Admission or changes in renal function during hospitalization for worsening heart failure predict postdischarge survival: results from the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF). Circ Heart Fail. 2008;1:25–33. doi: 10.1161/CIRCHEARTFAILURE.107.746933. [DOI] [PubMed] [Google Scholar]
- 50.Vaduganathan M, Greene SJ, Ambrosy AP et al. EVEREST trial investigators. Relation of serum uric acid levels and outcomes among patients hospitalized for worsening heart failure with reduced ejection fraction (from the efficacy of vasopressin antagonism in heart failure outcome study with tolvaptan trial). Am J Cardiol. 2014;114:1713–21. doi: 10.1016/j.amjcard.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 51.Harjola VP, Mullens W, Banaszewski M et al. Organ dysfunction, injury and failure in acute heart failure: from pathophysiology to diagnosis and management. A review on behalf of the Acute Heart Failure Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur J Heart Fail. 2017;19:821–36. doi: 10.1002/ejhf.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Januzzi J, Filippatos G, Nieminen M et al. Troponin elevation in patients with heart failure: on behalf of the third Universal Definition of Myocardial Infarction Global Task Force: Heart Failure Section. Eur Heart J. 2012;33:2265–71. doi: 10.1093/eurheartj/ehs191. [DOI] [PubMed] [Google Scholar]
- 53.Felker M, Hasselblad V, Tang W et al. Troponin I in acute decompensated heart failure: insights from the ASCEND-HF study. Eur J Heart Fail. 2012;11:1257–64. doi: 10.1093/eurjhf/hfs110. [DOI] [PubMed] [Google Scholar]
- 54.Kociol RD, Pang PS, Gheorghiade M et al. Troponin elevation in heart failure prevalence, mechanisms, and clinical implications. J Am Coll Cardiol. 2010;56:1071–8. doi: 10.1016/j.jacc.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 55.You JJ, Austin PC, Alter DA et al. Relation between cardiac troponin I and mortality in acute decompensated heart failure. Am Heart J. 2007;153:462–70. doi: 10.1016/j.ahj.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 56.Parenti N, Bartolacci S, Carle F et al. Cardiac troponin I as prognostic marker in heart failure patients discharged from emergency department. Intern Emerg Med. 2008;3:43–7. doi: 10.1007/s11739-008-0092-8. [DOI] [PubMed] [Google Scholar]
- 57.La Vecchia L, Mezzena G, Zanolla L et al. Cardiac troponin I as diagnostic and prognostic marker in severe heart failure. J Heart Lung Transplant. 2000;19:644–52. doi: 10.1016/s1053-2498(00)00120-0. [DOI] [PubMed] [Google Scholar]
- 58.Wettersten N, Maisel J. Role of cardiac troponin levels in acute heart failure. Cardiac Fail Rev. 2015;1:102–6. doi: 10.15420/cfr.2015.1.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O’Connor CM, Fiuzat M, Lombardi C et al. Impact of serial troponin release on outcomes in patients with acute heart failure analysis from the PROTECT pilot study. Circ Heart Fail. 2011;4:724–32. doi: 10.1161/CIRCHEARTFAILURE.111.961581. [DOI] [PubMed] [Google Scholar]
- 60.Metra M, Cotter G, Davison BA et al. Effect of serelaxin on cardiac, renal, and hepatic biomarkers in the Relaxin in Acute Heart Failure (RELAX-AHF) development program: correlation with outcomes. J Am Coll Cardiol. 2013;61:196–206. doi: 10.1016/j.jacc.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 61.Metra M, Cotter G, Gheorghiade M et al. The role of the kidney in heart failure. Eur Heart J. 2012;33:2135–42. doi: 10.1093/eurheartj/ehs205. [DOI] [PubMed] [Google Scholar]
- 62.Greene SJ, Gheorghiade M, Vaduganathan M et al. Haemoconcentration, renal function, and post-discharge outcomes among patients hospitalized for heart failure with reduced ejection fraction: insights from the EVEREST trial. Eur J Heart Fail. 2013;15:1401–11. doi: 10.1093/eurjhf/hft110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Metra M, Davison B, Bettari L et al. Is worsening renal function an ominous prognostic sign in patients with acute heart failure? The role of congestion and its interaction with renal function. Circ Heart Fail. 2012;5:54–62. doi: 10.1161/CIRCHEARTFAILURE.111.963413. [DOI] [PubMed] [Google Scholar]
- 64.Voors AA, Dittrich HC, Massie BM et al. Effects of the adenosine A1 receptor antagonist rolofylline on renal function in patients with acute heart failure and renal dysfunction: results from PROTECT (Placebo-Controlled Randomized Study of the Selective Adenosine A1 Receptor Antagonist Rolofylline for Patients Hospitalized with Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function). J Am Coll Cardiol. 2011;57:1899–1907. doi: 10.1016/j.jacc.2010.11.057. [DOI] [PubMed] [Google Scholar]
- 65.Ambrosy AP, Gheorghiade M, Bubenek S et al. The predictive value of transaminases at admission in patients hospitalized for heart failure: findings from the RO-AHFS registry. Eur Heart J Acute Cardiovasc Care. 2013;2:99–108. doi: 10.1177/2048872612474906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ambrosy AP, Vaduganathan M, Huffman MD et al. Clinical course and predictive value of liver function tests in patients hospitalized for worsening heart failure with reduced ejection fraction: an analysis of the EVEREST trial. Eur J Heart Fail. 2012;14:302–11. doi: 10.1093/eurjhf/hfs007. [DOI] [PubMed] [Google Scholar]
- 67.van Deursen VM, Damman K, Hillege HL et al. Abnormal liver function in relation to hemodynamic profile in heart failure patients. J Card Fail. 2010;16:84–90. doi: 10.1016/j.cardfail.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 68.Shinagawa H, Inomata T, Koitabashi T et al. Prognostic significance of increased serum bilirubin levels coincident with cardiac decompesantion in chronic heart failure. Circ J. 2008;72:364–9. doi: 10.1253/circj.72.364. [DOI] [PubMed] [Google Scholar]
- 69.Fonarow GC, Abraham WT, Albert NM et al. Factors identified as precipitating hospital admissions for heart failure and clinical outcomes: findings from OPTIMIZE-HF. Arch Intern Med. 2008;168:847–54. doi: 10.1001/archinte.168.8.847. [DOI] [PubMed] [Google Scholar]
- 70.Targher G, Dauriz M, Laroche C et al. In-hospital and 1-year mortality associated with diabetes in patients with acute heart failure: results from the ESC-HFA Heart Failure Long-Term Registry. Eur J Heart Fail. 2017;19:54–65. doi: 10.1002/ejhf.679. [DOI] [PubMed] [Google Scholar]
- 71.Nathaniel M, Virani HS, Ceconi C et al. Heart failure and chronic obstructive pulmonary disease: the challenges facing physicians and health services. Eur Heart J. 2013;34:2795–803. doi: 10.1093/eurheartj/eht192. [DOI] [PubMed] [Google Scholar]
- 72.De Blois J, Simard S, Atar D et al. Norwegian Heart Failure Registry COPD predicts mortality in HF: the Norwegian Heart Failure Registry. J Card Fail. 2010;16:225–9. doi: 10.1016/j.cardfail.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 73.Mentz RJ, Schmidt PH, Kwasny MJ et al. The impact of chronic obstructive pulmonary disease in patients hospitalized for worsening heart failure with reduced ejection fraction: an analysis of the Everest trial. J Card Fail. 2012;18:515–23. doi: 10.1016/j.cardfail.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 74.Arnaudis B, Lairez O, Escamilla R et al. Impact of chronic obstructive pulmonary disease severity on symptoms and prognosis in patients with systolic heart failure. Clin Res Cardiol. 2012;101:717–26. doi: 10.1007/s00392-012-0450-4. [DOI] [PubMed] [Google Scholar]
- 75.Mentz RJ, Greene SJ1, Ambrosy AP et al. Clinical profile and prognostic value of anemia at the time of admission and discharge among patients hospitalized for heart failure with reduced ejection fraction: findings from the EVEREST trial. Circ Heart Fail. 2014;7:401–8. doi: 10.1161/CIRCHEARTFAILURE.113.000840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Swedberg K, Young JB, Anand IS et al. Treatment of anemia with darbepoetin alfa in systolic heart failure. N Engl J Med. 2013;368:1210–9. doi: 10.1056/NEJMoa1214865. [DOI] [PubMed] [Google Scholar]
- 77.Altman DC, Vergouwe Y, Royston P et al. Prognosis and prognostic research: validating a prognostic model. BMJ. 2009;338:b605. doi: 10.1136/bmj.b605. [DOI] [PubMed] [Google Scholar]
- 78.Giamouzis G, Kalogeropoulos A, Georgiopoulou V et al. Hospitalization epidemic in patients with heart failure: risk factors, risk prediction, knowledge gaps, and future directions. J Card Fail. 2011;17:54–75. doi: 10.1016/j.cardfail.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 79.Moons KGM, Altman DG, Vergouwe Y et al. Prognosis and prognostic research: application and impact of prognostic models in clinical practice. BMJ. 2009;338:b606. doi: 10.1136/bmj.b606. [DOI] [PubMed] [Google Scholar]
- 80.Passantino A, Monitillo F, Iacoviello M et al. Predicting mortality in patients with acute heart failure: Role of risk scores. World J Cardiol. 2015;26(7):902–11. doi: 10.4330/wjc.v7.i12.902. [DOI] [PMC free article] [PubMed] [Google Scholar]


