Abstract
Background
Better understanding about gastric cancer incidence patterns among Hispanics by birthplace, socioeconomic status (SES), and acculturation can improve preventive strategies and disease models.
Methods
Incidence rates, rate ratios, and estimated annual percent change (EAPC) in rates of anatomic and histologic subtype-specific gastric cancer were calculated by age, sex, and nativity among Hispanics using California Cancer Registry data from 1988 through 2004. Incidence rates in 1998 to 2002 were compared by neighborhood SES and Hispanic enclave status according to 2000 US Census data.
Results
Incidence rates of diffuse gastric cancer increased from 1988 through 2004 among foreign-born Hispanic men (EAPC: 3.5%, 95% CI: 1.5%–5.5%) and U.S.-born Hispanic women (EAPC: 3.0%, 95% CI: 0.7%–5.3%). During the same time period, incidence rates of intestinal gastric cancer declined significantly and both cardia and noncardia gastric cancer were steady or declined among foreign-born and U.S.-born Hispanic men and women. Noncardia and both intestinal and diffuse gastric cancer were more common in foreign-born than U.S.-born Hispanic men and women, and in those from lower SES, higher enclave neighborhoods. By contrast, among younger and middle-aged Hispanic men, cardia tumors were more common in the U.S.-born than the foreign-born, and in higher SES, lower enclave neighborhoods.
Conclusions
Varying gastric cancer risk factors among Hispanic subgroups and increasing rates of diffuse gastric cancer in foreign-born Hispanic men and U.S.-born Hispanic women merit further investigation to identify separate disease etiologies.
Impact
Age, sex, birthplace, SES, and acculturation modify gastric cancer incidence in Hispanics and should be considered when examining disease risk and prevention.
Introduction
Gastric cancer is the seventh leading cause of cancer death in U.S. Hispanic males and females (1). With incidence rates at least 70% higher in Hispanics than non-Hispanic whites (2), gastric cancer is a prominent nationwide ethnic health disparity. Higher rates of gastric cancer in Hispanics than in whites, and in Latin America than in the United States (3), are likely due primarily to differences in the prevalence of Helicobacter pylori infection—the strongest known risk factor for gastric cancer, especially for tumors in the antrum and body of the stomach (4). H. pylori transmission via oral–oral and fecal–oral routes is facilitated by the generally poorer sanitation and more crowded living conditions found in the countries of origin for most Hispanics (4, 5), and these early life environmental conditions contribute to the persistent 2- to 3-fold higher prevalence of H. pylori infection in Hispanics than whites in the United States (6–8). H. pylori plays a lesser etiologic role in cardia gastric cancer (9), however, and may even protect against nonatrophic cardia tumors (10, 11). Instead, obesity and gastroesophageal reflux are stronger risk factors for cancer in the cardia (12–14), the only anatomic subsite of the stomach for which incidence rates are higher in non-Hispanic white males than Hispanic males (15, 16). Other risk factors for both cardia and noncardia gastric cancer include smoking (17–19) and, to a lesser extent, diet (20–22). As with anatomic subsites of gastric cancer, many risk factors also seem to be shared between the 2 main histologic subtypes, intestinal and diffuse gastric cancer, although some etiologic differences have been described (23).
Recently, using Surveillance, Epidemiology, and End Results (SEER) data from the U.S. National Cancer Institute, Anderson and colleagues reported a significant increase in the incidence rate of noncardia gastric cancer from 1977 through 2006 among white (combining Hispanic and non-Hispanic) men and women aged 25 to 39 years, but significant declines among older whites (24). In a sensitivity analysis, Anderson and colleagues noted similar age-specific incidence trends among non-Hispanic whites between 1992 and 2006 (24), and a follow-up study indicated stable rates over time among Hispanics overall (15).
Better knowledge of gastric cancer incidence patterns in Hispanics can inform management of the disease in this fast-growing population and can also provide insight into disease causation and offer guidance for potential preventive efforts such as smoking cessation, dietary modification, and screening and eradication of H. pylori infection. Although gastric cancer incidence is known to vary by country and ethnicity, little is known about how incidence patterns differ by birthplace, acculturation, and socioeconomic status (SES) among U.S. Hispanics, nearly 40% of whom are foreign-born and who span the full spectrum of acculturation and SES (25). For example, gastric cancer risk may vary by place of birth due to differences in early-life exposures, such as H. pylori infection, and other differences in lifestyle and environment between immigrants and nonimmigrants (26). Residential neighborhood characteristics, including SES and degree of ethnic enclave status (as a measure of acculturation) may also affect gastric cancer risk through hygiene practices, housing density, access to health care, diet, and cultural and community attitudes about obesity and smoking (27–31).
To our knowledge, incidence trends of gastric cancer subtypes by anatomic site and histology among Hispanics have not previously been examined by nativity, nor have incidence rate patterns been delineated by neighborhood SES and enclave status. Therefore, to investigate these compelling public health questions about one of the leading causes of cancer death among Hispanics, we examined gastric cancer incidence among Hispanics in California, home to the nation’s largest Hispanic population.
Methods
Cancer patient data
We obtained California Cancer Registry data on all California residents diagnosed with primary invasive gastric cancer (International Classification of Diseases for Oncology, 3rd edition [ICD-O-3] site codes 16.0–16.9, histology codes 8000–8999) from January 1, 1988, through December 31, 2004 (32). We included all 9,001 Hispanics/Latinos (hereafter referred to as "Hispanics," in accordance with U.S. Office of Management and Budget designations) diagnosed with gastric cancer during the study period (5,134 males and 3,867 females). Classification of Hispanic ethnicity was improved by application of the North American Association of Central Cancer Registries Hispanic Identification Algorithm (33). We did not further subclassify Hispanics by country of origin due to a high proportion of missing data, but approximately 84% of California Hispanics are of Mexican origin (34), followed by 9% of Central American origin (35).
Primary gastric tumors were classified according to anatomic location in the cardia (ICD-O-3 site code 16.0), noncardia (site codes 16.1–16.6), or overlapping/unspecified area (site codes 16.8–16.9). Tumors were alternatively classified according to histologic type (36) as intestinal (ICD-O-3 histology codes 8010, 8140, 8144, and 8211), diffuse (histology codes 8490, 8142, and 8145), or other epithelial (all other histology codes excluding 8800–9759 and 8000–8004), using the same codes as in previous studies for comparability (37, 38). Between 1988 and 1992 and 2000 and 2004 in the eligible study population, the percentage of gastric tumors with site classified as "unspecified" (20.8%; 19.8%) or histologic type classified as "not otherwise specified" (1.2%; 1.4%) did not change appreciably.
Classification of nativity
Data on nativity were available in the cancer registry for 84.7% of eligible cases. Because cancer registry data on birthplace are selectively missing (39, 40), we estimated nativity for the minority (15.3%) of patients with unknown birthplace using a statistical imputation method that has minimal bias (41). Based on each patient’s social security number (SSN), which indicates the state and year of issuance (42, 43), we classified patients who received their SSN before age 20 years as U.S.-born and those who received their SSN at or after age 20 years as foreign-born. The cut point of 20 years was determined by comparisons with self-reported nativity from interviews with 1,127 Hispanic cancer patients (39, 40) and maximization of the area under the resultant receiver operating characteristic curve. The optimal positive predictive value of the age cut point was confirmed by using logistic regression models with age at SSN issuance as a continuous predictor of foreign-born status. The selected cut point resulted in immigrant status classifications associated with 81% sensitivity and 80% specificity for detecting foreign-born status in Hispanics. The less than 1% of cases with missing or invalid SSNs were assigned a nativity status based on the known distribution of nativity within matched strata of race/ethnicity, sex, and age in the overall California Cancer Registry patient population.
Classification of neighborhood SES and enclave status
We assigned a neighborhood-level measure of SES based on a previously described index that incorporates 2000 U.S. Census data on education, occupation, unemployment, household income, poverty, rent, and house values (44). Each of the 99.9% of patients with a known residential address at diagnosis was geocoded to a census tract. The remaining cases without a street address or whose address could not be precisely geocoded (0.1%) were randomly assigned to a census tract within their ZIP code of residence. Based on residential census tracts, each patient was assigned to a quintile of neighborhood SES according to the statewide distribution of the SES index across all census tracts in California. For the analysis, we combined quintiles 1 to 2 (lower SES) and quintiles 3 to 5 (higher SES).
We also classified patients according to neighborhood Hispanic enclave status, based on the concept of an ethnic enclave as a geographic unit with higher percentages of foreign-born ethnicity-specific residents and non-English language usage. To characterize residence in a Hispanic enclave, we applied principal components analysis to 2000 U.S. Census block group level data on linguistic isolation, English fluency, Spanish language use, Hispanic ethnicity, immigration history, and nativity; these component values were then combined and averaged across census tracts as the enclave index (45). Again, each case was assigned to a quintile of neighborhood ethnic enclave status based on the distribution of the enclave index across all census tracts in California. We combined quintiles 1 to 3 (lower enclave status) and quintiles 4 to 5 (higher enclave status) for the analysis.
Analyses of neighborhood SES and ethnic enclave status were limited to the pericensal period January 1, 1998, through December 31, 2002, due to data availability, and included 2,954 cases (1,669 males and 1,285 females) diagnosed with gastric cancer during this interval.
Population data
From the 1990 and 2000 U.S. Census Summary File 3 (SF-3), we obtained population counts to estimate incidence rates by sex, race/ethnicity, immigrant status, and 5-year age group for California. For intercensal years, we estimated the foreign-born Hispanic population size using cohort component interpolation and extrapolation methods, adjusting estimates to the populations by age and year provided by the California Department of Finance for years 1988 to 1989 and by the U.S. Census for years 1990 to 2004, based on data availability. For the analyses of neighborhood SES and ethnic enclave status, we used 2000 U.S. Census population estimates by race/ethnicity and sex at the census tract level. Because census data on nativity are not available at the census tract level, the database containing nativity data was separate from the one containing neighborhood SES and ethnic enclave status, and these variables could not be cross-classified.
Statistical analysis
We used SEER* Stat software (46) to compute age-adjusted incidence rates (directly standardized to the 2000 U.S. standard million population) with 95% CIs. We also calculated incidence rate ratios to compare incidence rates between United States and foreign-born populations, and between higher and lower neighborhood SES and/or ethnic enclave status. Due to incidence rate heterogeneity by age group and sex, we conducted separate analyses for men and women aged 25 to 39 years, 40 to 59 years, and 60 years or more. To examine incidence rate trends over time, we combined age groups but stratified analyses by year of diagnosis (1988–1993, 1994–1999, and 2000–2004) and quantified linear trends by the estimated annual percent change (EAPC), calculated by weighted least squares linear regression. For a more detailed analysis of incidence rate trends allowing for varying effects by chronologic age, calendar year of diagnosis, and year of birth, we used age-period-cohort models to compare gastric cancer incidence rate trends by nativity, anatomic subsite, and histology, using previously described methods (47, 48), with age and calendar time recategorized into 4-year intervals. Age-period-cohort modeling was carried out with MATLAB (The Mathworks, Inc.).
Results
Sixty percent of Hispanic males with gastric cancer (N = 3,093) were foreign-born, compared with 45% of the general population. Among Hispanic females, 66% of gastric cancer cases (N = 2,547) were foreign-born, compared with 43% of the general population.
Time trends
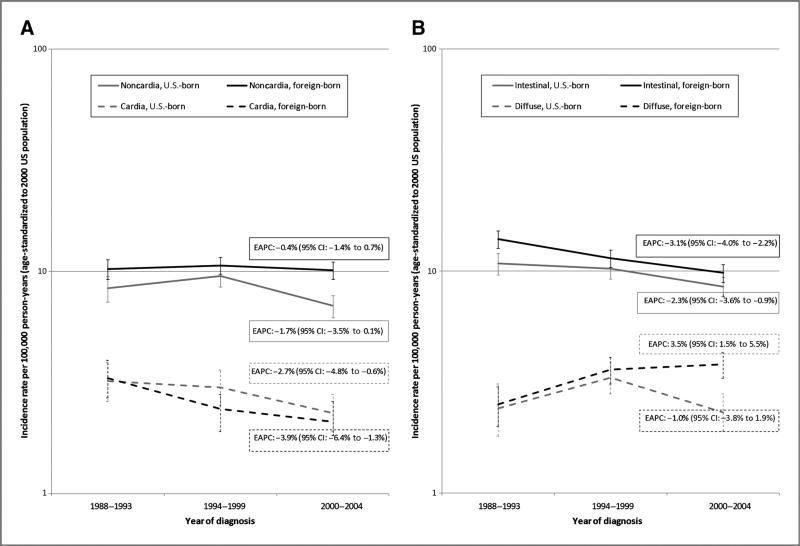
Between 1988 and 1993 and 2000 and 2004, incidence rates of noncardia gastric cancer were stable among both foreign-born (EAPC: −0.4%) and U.S.-born Hispanic men (EAPC: −1.7%), with a slight but statistically nonsignificant decrease in the latter group (Fig. 1A). Incidence rates of cardia gastric cancer, meanwhile, declined significantly in both foreign-born (EAPC: −3.9%) and U.S.-born Hispanic men (EAPC: −2.7%). When gastric cancer was classified according to histologic type instead of anatomic site, however, time trends diverged by nativity (Fig. 1B). Whereas intestinal gastric cancer incidence rates declined significantly among both foreign-born (EAPC: −3.1%) and U.S.-born Hispanic men (EAPC: −2.3%), diffuse gastric cancer incidence rates increased significantly over time among foreign-born Hispanic men (EAPC: 3.5%). By contrast, diffuse gastric cancer incidence rates were stable in U.S.-born Hispanic men (EAPC: −1.0%).
Figure 1.
Time trends in gastric cancer incidence rates among Hispanic men by nativity in California, 1988 to 2004. A, by anatomic site (cardia vs. noncardia). B, by histologic type (intestinal vs. diffuse).
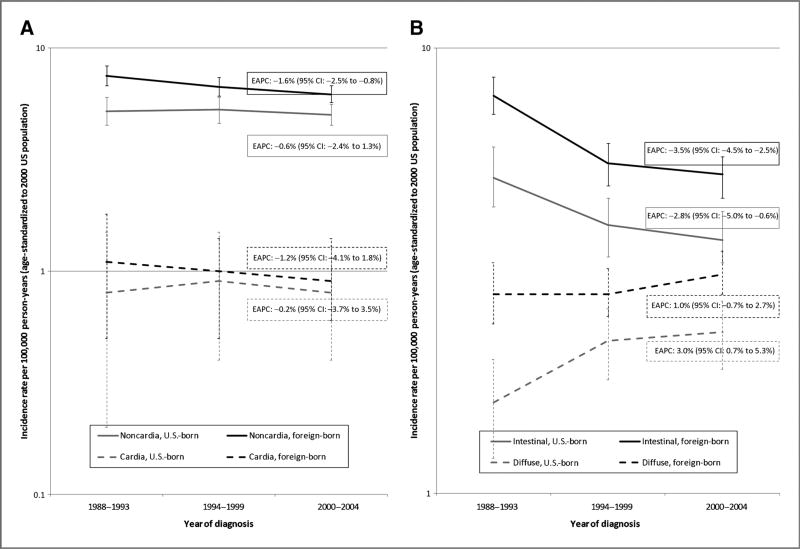
Among Hispanic women, incidence rates of noncardia gastric cancer declined significantly from 1988 to 1993 through 2000 to 2004 among the foreign-born (EAPC: −1.6%), but not the U.S.-born (EAPC: −0.6%; Fig. 2A). In both groups, the incidence rate of cardia gastric cancer did not change over time. Again, when time trends in gastric cancer incidence rates were examined by histologic type, different patterns were observed by nativity (Fig. 2B). As with Hispanic men, intestinal gastric cancer incidence rates decreased significantly over time in both foreign-born (EAPC: −3.5%) and U.S.-born Hispanic women (EAPC: −2.8%). Diffuse gastric cancer incidence rates, meanwhile, increased significantly over time in U.S.-born Hispanic women (EAPC: 3.0%), but not foreign-born Hispanic women (EAPC: 1.0%).
Figure 2.
Time trends in gastric cancer incidence rates among Hispanic women by nativity in California, 1988 to 2004. A, by anatomic site (cardia vs. noncardia). B, by histologic type (intestinal vs. diffuse).
Age-period-cohort analysis detected few differences in age-specific trends between U.S.-born and foreign-born Hispanic men or women, due in part to insufficient sample sizes (data not shown). However, there was evidence of a statistically significant positive secular trend in the incidence rate of diffuse gastric cancer among Hispanic men ages 25 to 36 years, as well as 69 to 76 years, but not other age groups (Pheterogeneity by age = 0.04). By contrast, a statistically significant negative secular trend was observed for intestinal gastric cancer in nearly all age groups of Hispanic men. Suggestions of similar patterns by histologic type were observed among Hispanic women, but differences were not statistically significant.
Patterns by nativity
Incidence rates of noncardia gastric cancer were consistently higher in foreign-born than U.S.-born Hispanic men and women (Table 1). The same pattern by nativity was observed for cardia gastric cancer among older Hispanic men and women. In the younger and middle age groups, however, the opposite pattern was detected among Hispanic men, in whom incidence rates of cardia gastric cancer were higher for the U.S.-born than the foreign-born, and no differences by nativity were found among Hispanic women in these age groups. Incidence rate patterns by nativity for gastric cancer in overlapping or unspecified anatomic locations were similar to those for noncardia cancer (data not shown).
Table 1.
Case counts and age-adjusted incidence rates of invasive first primary gastric cancer by anatomic location, sex, age group, and nativity, and incidence rate ratios for foreign-born versus U.S.-born, in California Hispanics, 1988 to 2004
| Noncardia | Cardia | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Sex | Age group, y |
Nativity | Cases (N) |
Ratea | 95% CI | Rate ratio (95% CI) |
Cases (N) |
Ratea | 95% CI | Rate ratio (95% CI) |
| Male | All | U.S.-born | 1,072 | 8.3 | (7.7–8.8) | Reference | 387 | 2.8 | (2.5–3.1) | Reference |
| Foreign-born | 1,698 | 10.3 | (9.7–10.8) | 1.2 (1.1–1.4) | 411 | 2.5 | (2.3–2.8) | 0.9 (0.8–1.1) | ||
| 25–39 | U.S.-born | 72 | 1.1 | (0.8–1.4) | Reference | 27 | 0.4 | (0.3–0.6) | Reference | |
| Foreign-born | 175 | 1.2 | (1.0–1.4) | 1.1 (0.9–1.5) | 35 | 0.3 | (0.2–0.4) | 0.6 (0.4–1.1) | ||
| 40–59 | U.S.-born | 247 | 5.5 | (4.8–6.2) | Reference | 149 | 3.3 | (2.8–3.9) | Reference | |
| Foreign-born | 493 | 6.3 | (5.8–6.9) | 1.2 (1.0–1.4) | 124 | 1.6 | (1.4–2.0) | 0.5 (0.4–0.6) | ||
| 60+ | U.S.-born | 746 | 39.8 | (36.7–43.1) | Reference | 209 | 10.8 | (9.3–12.5) | Reference | |
| Foreign-born | 1,021 | 50.3 | (47.1–53.6) | 1.3 (1.1–1.4) | 252 | 12.3 | (10.8–14.0) | 1.1 (0.9–1.4) | ||
| Female | All | U.S.-born | 791 | 5.1 | (4.8–5.5) | Reference | 129 | 0.8 | (0.7–1.0) | Reference |
| Foreign-born | 1,474 | 6.8 | (6.4–7.1) | 1.3 (1.2–1.4) | 211 | 1.0 | (0.9–1.2) | 1.2 (1.0–1.6) | ||
| 25–39 | U.S.-born | 48 | 0.7 | (0.5–0.9) | Reference | 9 | 0.1 | (0.1–0.3) | Reference | |
| Foreign-born | 148 | 1.2 | (1.0–1.4) | 1.8 (1.3–2.5) | 16 | 0.1 | (0.1–0.2) | 1.0 (0.4–2.6) | ||
| 40–59 | U.S.-born | 185 | 3.8 | (3.3–4.4) | Reference | 35 | 0.7 | (0.5–1.0) | Reference | |
| Foreign-born | 401 | 4.9 | (4.4–5.4) | 1.3 (1.1–1.5) | 49 | 0.6 | (0.5–0.8) | 0.8 (0.5–1.3) | ||
| 60+ | U.S.-born | 551 | 24.0 | (21.9–26.1) | Reference | 85 | 3.5 | (2.8–4.4) | Reference | |
| Foreign-born | 917 | 31.4 | (29.4–33.5) | 1.3 (1.2–1.5) | 142 | 4.8 | (4.1–5.7) | 1.4 (1.0–1.8) | ||
Rates are per 100,000 person-years and age-adjusted to the 2000 U.S. standard population.
When gastric cancer was classified according to histologic type rather than anatomic location, the incidence rate ratio by nativity was more consistently elevated for both intestinal and diffuse gastric cancer across nearly all age groups of Hispanic men and women (Table 2). Incidence rates of other epithelial gastric cancer were also mostly higher in foreign-born than U.S.-born Hispanic men and women (data not shown). When limited to noncardia anatomic locations (56% of intestinal tumors in men, 62% of intestinal tumors in women, 53% of diffuse tumors in men, 57% of diffuse tumors in women), the incidence rate ratios comparing foreign-born to U.S.-born remained elevated in all age groups of Hispanic men and women (data not shown).
Table 2.
Case counts and age-adjusted incidence rates of invasive first primary gastric cancer by histologic subtype, sex, age group, and nativity, and incidence rate ratios for foreign-born versus U.S.-born, in California Hispanics, 1988 to 2004
| Intestinal type | Diffuse type | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Sex | Age group, y |
Nativity | Cases (N) |
Ratea | 95% CI | Rate ratio (95% CI) |
Cases (N) |
Ratea | 95% CI | Rate ratio (95% CI) |
| Male | All | U.S.-born | 1,232 | 9.8 | (9.2–10.4) | Reference | 378 | 2.7 | (2.4–3.0) | Reference |
| Foreign-born | 1,744 | 11.4 | (10.9–12.0) | 1.2 (1.1–1.3) | 712 | 3.3 | (3.1–3.6) | 1.2 (1.1–1.4) | ||
| 25–39 | U.S.-born | 50 | 0.7 | (0.5–1.0) | Reference | 31 | 0.5 | (0.3–0.6) | Reference | |
| Foreign-born | 125 | 0.9 | (0.7–1.1) | 1.2 (0.9–1.7) | 123 | 0.8 | (0.7–1.0) | 1.8 (1.2–2.9) | ||
| 40–59 | U.S.-born | 290 | 6.5 | (5.8–7.3) | Reference | 129 | 2.8 | (2.4–3.4) | Reference | |
| Foreign-born | 444 | 5.9 | (5.4–6.5) | 0.9 (0.8–1.1) | 299 | 3.7 | (3.3–4.2) | 1.3 (1.1–1.6) | ||
| 60+ | U.S.-born | 889 | 47.8 | (44.4–51.4) | Reference | 215 | 11.0 | (9.5–12.8) | Reference | |
| Foreign-born | 1,174 | 58.5 | (55.1–62.1) | 1.2 (1.1–1.3) | 281 | 13.1 | (11.5–14.8) | 1.2 (1.0–1.4) | ||
| Female | All | U.S.-born | 606 | 4.2 | (3.8–4.5) | Reference | 358 | 2.1 | (1.8–2.3) | Reference |
| Foreign-born | 1,183 | 6.0 | (5.6–6.4) | 1.4 (1.3–1.6) | 785 | 3.0 | (2.7–3.2) | 1.4 (1.3–1.6) | ||
| 25–39 | U.S.-born | 28 | 0.4 | (0.3–0.6) | Reference | 39 | 0.5 | (0.4–0.7) | Reference | |
| Foreign-born | 68 | 0.6 | (0.4–0.7) | 1.4 (0.9–2.2) | 138 | 1.1 | (1.0–1.3) | 2.1 (1.5–3.1) | ||
| 40–59 | U.S.-born | 108 | 2.2 | (1.8–2.7) | Reference | 131 | 2.7 | (2.3–3.2) | Reference | |
| Foreign-born | 228 | 2.8 | (2.5–3.2) | 1.3 (1.0–1.6) | 323 | 3.9 | (3.5–4.3) | 1.4 (1.2–1.8) | ||
| 60+ | U.S.-born | 470 | 21.0 | (19.1–23.1) | Reference | 186 | 7.4 | (6.3–8.6) | Reference | |
| Foreign-born | 885 | 30.9 | (28.9–33.1) | 1.5 (1.3–1.7) | 313 | 10.0 | (8.9–11.2) | 1.4 (1.1–1.6) | ||
Rates are per 100,000 person-years and age-adjusted to the 2000 U.S. standard population.
Patterns by neighborhood SES and enclave status
Given that foreign-born Hispanics are more likely to live in lower SES, higher enclave neighborhoods (49), incidence patterns by neighborhood SES, and enclave status largely mirrored those by nativity for all age groups combined. In particular, incidence rates of noncardia gastric cancer were generally higher among Hispanic men and women living in neighborhoods of lower SES and higher enclave status (Table 3). For cardia gastric cancer, rates were generally lower in Hispanic men living in lower SES, high-enclave neighborhoods, whereas the opposite pattern was observed among Hispanic women, although sample sizes were limited in some strata. Again, incidence rate patterns for gastric cancer in overlapping or unspecified anatomic locations were largely similar to those for noncardia cancer (data not shown).
Table 3.
Case counts and age-adjusted incidence rates of invasive first primary gastric cancer by anatomic location, sex, and neighborhood socioeconomic and/or enclave status, and incidence rate ratios by neighborhood characteristics, in California Hispanics, 1998 to 2002
| Noncardia | Cardia | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Sex | Neighborhood characteristic |
Cases (N) |
Ratea | 95% CI | Rate ratio (95% CI) |
Cases (N) |
Ratea | 95% CI | Rate ratio (95% CI) |
| Male | Higher SES | 309 | 8.3 | (7.3–9.4) | Reference | 100 | 2.7 | (2.2–3.4) | Reference |
| Lower SES | 620 | 10.3 | (9.4–11.3) | 1.2 (1.1–1.5) | 140 | 2.2 | (1.8–2.6) | 0.8 (0.6–1.1) | |
| Lower enclave | 265 | 8.6 | (7.5–9.8) | Reference | 82 | 2.7 | (2.1–3.4) | Reference | |
| Higher enclave | 664 | 10.0 | (9.2–10.9) | 1.2 (1.0–1.4) | 158 | 2.2 | (1.9–2.7) | 0.8 (0.6–1.1) | |
| Higher SES, lower enclave | 196 | 8.1 | (6.8–9.4) | Reference | 70 | 3.0 | (2.3–3.9) | Reference | |
| Higher SES, higher enclave | 113 | 8.8 | (7.1–10.7) | 1.1 (0.8–1.4) | 30 | 2.1 | (1.4–3.1) | 0.7 (0.4–1.1) | |
| Lower SES, lower enclave | 69 | 10.3 | (7.8–13.3) | 1.3 (0.9–1.7) | 12 | 1.5 | (0.7–2.6) | 0.5 (0.2–0.9) | |
| Lower SES, higher enclave | 551 | 10.3 | (9.4–11.3) | 1.3 (1.1–1.5) | 128 | 2.3 | (1.8–2.7) | 0.7 (0.5–1.0) | |
| Female | Higher SES | 261 | 5.4 | (4.7–6.1) | Reference | 37 | 0.8 | (0.5–1.0) | Reference |
| Lower SES | 494 | 6.5 | (5.9–7.2) | 1.2 (1.0–1.4) | 80 | 1.0 | (0.8–1.2) | 1.3 (0.9–2.0) | |
| Lower enclave | 186 | 4.7 | (4.0–5.5) | Reference | 28 | 0.7 | (0.5–1.1) | Reference | |
| Higher enclave | 569 | 6.7 | (6.2–7.3) | 1.4 (1.2–1.7) | 89 | 1.0 | (0.8–1.2) | 1.3 (0.9–2.1) | |
| Higher SES, lower enclave | 153 | 4.8 | (4.1–5.7) | Reference | 23 | 0.7 | (0.5–1.1) | Reference | |
| Higher SES, higher enclave | 108 | 6.4 | (5.2–7.8) | 1.3 (1.0–1.7) | 14 | 0.8 | (0.4–1.3) | 1.1 (0.5–2.2) | |
| Lower SES, lower enclave | 33 | 4.2 | (2.8–5.9) | 0.9 (0.6–1.3) | 5 | 0.8 | (0.2–1.7) | 1.0 (0.3–2.7) | |
| Lower SES, higher enclave | 461 | 6.8 | (6.2–7.5) | 1.4 (1.2–1.7) | 75 | 1.0 | (0.8–1.3) | 1.4 (0.9–2.4) | |
Rates are per 100,000 person-years and age-adjusted to the 2000 U.S. standard population.
Histology-specific incidence patterns by neighborhood SES and enclave status also paralleled those by nativity, with elevated incidence rates of both intestinal and diffuse gastric cancer among Hispanic men and women living in neighborhoods with lower SES and higher enclave status (Table 4). Again, some sample sizes were limited for analyses of diffuse gastric cancer. Incidence rate ratios were also above 1.0 for other epithelial types of gastric cancer and remained elevated when the analysis of intestinal and diffuse types was limited to noncardia tumors (data not shown).
Table 4.
Case counts and age-adjusted incidence rates of invasive first primary gastric cancer by histologic subtype, sex, and neighborhood socioeconomic and/or enclave status, and incidence rate ratios by neighborhood characteristics, in California Hispanics, 1998–2002
| Intestinal | Diffuse | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Sex | Neighborhood characteristic |
Cases (N) |
Ratea | 95% CI | Rate ratio (95% CI) |
Cases (N) |
Ratea | 95% CI | Rate ratio (95% CI) |
| Male | Higher SES | 309 | 8.9 | (7.8–10.1) | Reference | 129 | 3.1 | (2.5–3.7) | Reference |
| Lower SES | 591 | 10.6 | (9.7–11.6) | 1.2 (1.0–1.4) | 274 | 3.6 | (3.1–4.1) | 1.2 (0.9–1.5) | |
| Lower enclave | 263 | 9.0 | (7.9–10.3) | Reference | 109 | 3.0 | (2.4–3.6) | Reference | |
| Higher enclave | 637 | 10.4 | (9.5–11.3) | 1.2 (1.0–1.4) | 294 | 3.6 | (3.1–4.1) | 1.2 (0.9–1.6) | |
| Higher SES, lower enclave | 200 | 8.8 | (7.5–10.3) | Reference | 82 | 3.0 | (2.3–3.8) | Reference | |
| Higher SES, higher enclave | 109 | 9.0 | (7.2–11.0) | 1.0 (0.8–1.3) | 47 | 3.3 | (2.3–4.5) | 1.1 (0.7–1.7) | |
| Lower SES, lower enclave | 63 | 9.7 | (7.3–12.6) | 1.1 (0.8–1.5) | 27 | 3.0 | (1.9–4.5) | 1.0 (0.6–1.7) | |
| Lower SES, higher enclave | 528 | 10.7 | (9.7–11.8) | 1.2 (1.0–1.5) | 247 | 3.6 | (3.1–4.2) | 1.2 (0.9–1.7) | |
| Female | Higher SES | 175 | 3.9 | (3.3–4.5) | Reference | 125 | 2.2 | (1.9–2.7) | Reference |
| Lower SES | 364 | 5.2 | (4.7–5.8) | 1.3 (1.1–1.6) | 279 | 3.1 | (2.7–3.5) | 1.4 (1.1–1.7) | |
| Lower enclave | 138 | 3.8 | (3.2–4.5) | Reference | 105 | 2.3 | (1.9–2.8) | Reference | |
| Higher enclave | 401 | 5.1 | (4.6–5.7) | 1.3 (1.1–1.6) | 299 | 3.0 | (2.6–3.4) | 1.3 (1.0–1.7) | |
| Higher SES, lower enclave | 110 | 3.8 | (3.1–4.6) | Reference | 82 | 2.2 | (1.8–2.8) | Reference | |
| Higher SES, higher enclave | 65 | 4.2 | (3.2–5.3) | 1.1 (0.8–1.5) | 43 | 2.3 | (1.6–3.1) | 1.0 (0.7–1.5) | |
| Lower SES, lower enclave | 28 | 4.1 | (2.7–5.9) | 1.1 (0.7–1.7) | 23 | 2.6 | (1.6–4.0) | 1.2 (0.7–1.9) | |
| Lower SES, higher enclave | 336 | 5.4 | (4.8–6.0) | 1.4 (1.1–1.8) | 256 | 3.2 | (2.8–3.6) | 1.4 (1.1–1.9) | |
Rates are per 100,000 person-years and age-adjusted to the 2000 U.S. standard population.
Discussion
This is the first study to investigate trends in gastric cancer incidence among Hispanics by nativity. Among California Hispanics for the period 1988 through 2004, we observed increasing incidence rates of diffuse gastric cancer in foreign-born Hispanic men and U.S.-born Hispanic women. By contrast, rates of intestinal and both noncardia and cardia gastric cancer mostly were steady or declined over time in foreign-born and U.S.-born Hispanic men and women. These trends bear some resemblance to the declining rates of intestinal, but not diffuse, gastric cancer among whites in the United States and Europe in the 1950s and 1960s (50–52). By considering differences by nativity, sex, and histologic subtype, our results extend the work of Camargo and colleagues, who reported declining incidence rates of gastric cancer in the cardia and some noncardia sites among older U.S. Hispanics from 1999 through 2007. Others previously reported decreasing incidence rates of noncardia and intestinal gastric cancers in U.S. whites, blacks, and other races combined (15, 24, 37, 38). These decreases have been ascribed primarily to the declining prevalence of H. pylori infection due to improved sanitation, economic development, and antibiotic use (53), and secondarily to decreasing tobacco use (54) and perhaps some dietary improvements (55).
Contrary to the findings of Anderson and colleagues, who reported a rising trend in noncardia gastric cancer among young adult whites (24), the only significant incidence rate increases in our study were in certain population subgroups with diffuse gastric cancer, although we had limited power to examine time trends in young adult Hispanics. The trends that we observed could potentially be explained by a population shift away from CagA-positive H. pylori strains, which are preferentially associated with intestinal versus diffuse gastric cancer (56, 57), or the ascendance of other cofactors such as Epstein-Barr virus, which is detected in roughly 8% of gastric tumors and is more strongly associated with diffuse than intestinal gastric cancer (58). We speculate that the rising incidence rates of diffuse gastric cancer in foreign-born Hispanic men and U.S.-born Hispanic women, but not their counterparts, could be due in part to a more rapidly rising prevalence of obesity in the former groups than the latter (47). Increasing incidence rates of diffuse gastric cancer through the end of the last decade have been noted previously in white, black, and other-race men and women (37, 38), but have not previously been reported by nativity among Hispanics.
Besides trends over time, we noted distinct incidence patterns by nativity, with higher rates of noncardia, intestinal, and especially diffuse gastric cancer in foreign-born than U.S.-born Hispanic men and women. By contrast, for cardia gastric cancer, we found the opposite nativity pattern in younger and middle-aged Hispanic men, and no differences by nativity in younger and middle-aged Hispanic women. Patterns by neighborhood SES and enclave status were comparable, with generally higher rates in lower SES, higher enclave neighborhoods for all gastric cancer subtypes except cardia tumors in Hispanic men, who had the opposite pattern. Pinheiro and colleagues reported higher incidence rates of overall gastric cancer among Mexicans, Puerto Ricans, and Cubans in their countries of origin than in Florida in 1999 to 2001 (59), implying higher rates in foreign-born than U.S.-born Hispanics, but they were unable to directly examine incidence patterns of gastric cancer subtypes by birthplace or residential characteristics.
The most probable explanation for the higher incidence rates of noncardia, intestinal, and, to a lesser extent, diffuse gastric cancer in foreign-born than U.S.-born Hispanic men and women, and in those living in lower SES, higher enclave neighborhoods, is variation in the prevalence of H. pylori infection (7, 8). A serologic study of H. pylori infection among Hispanics in the San Francisco Bay Area of California found that the prevalence of infection dropped precipitously from 31.4% in foreign-born Hispanics to 9.1% in first-generation U.S.-born Hispanics and 3.1% in second-generation U.S.-born Hispanics (27). The same study found that after adjustment for immigrant generation, having at least one infected parent and a lower level of education were significant predictors of H. pylori infection, indicating that some of the differences we detected by neighborhood SES and enclave status may have been due to environmental and household factors other than birthplace. These findings echoed earlier results from a seroepidemiologic study of H. pylori in Mexico, where higher household crowding [a common feature of Hispanic enclaves in the United States (60)], lower educational level, and lower SES were independently associated with higher risk of infection (61). Besides H. pylori infection, differences in the prevalence of tobacco smoking, which is more common in foreign-born than U.S.-born Hispanic men [but not women (49)], and infection with Epstein-Barr virus, which usually occurs earlier in foreign-born Hispanics and those living in lower SES, higher enclave neighborhoods (62, 63), may also partially account for some of the observed nativity patterns (17–19).
For gastric cardia cancer, by contrast, the higher incidence rates in U.S.-born than foreign-born younger and middle-aged Hispanic men, and the higher rates among those living in higher SES, lower enclave neighborhoods, may be explained by the greater prevalence of obesity in U.S.-born than foreign-born Hispanic men, and in higher-SES than lower SES Hispanic men, but not women (49). Intake of fruits and vegetables, a moderate risk factor for cardia gastric cancer (20, 22), was also lower in U.S.-born and higher-SES Hispanic men in California in 2001 (but not 2005), and lower in Hispanic men overall than in Hispanic women (49), and could thus have contributed to the observed incidence rate patterns.
The impact of undocumented/unlawful immigration on our results was most likely small. False reporting of birthplace and SSNs by undocumented immigrants, leading to misclassification of some foreign-born Hispanics as U.S.-born, probably affected both numerators and denominators of incidence rates, resulting in minimal bias. Erroneous imputation of U.S.-born nativity based on false SSNs would have affected only some of the 15.3% of cases with missing birthplace data in the cancer registry; 84.7% of our nativity data was derived from patients’ medical records or death certificates, which we have previously shown to be highly accurate for birthplace information (39, 40). Even if the proportion of undocumented immigrants among gastric cases was higher than in the overall Hispanic population of California [estimated at roughly 15% (64, 65)], they still probably represented only a minority of the 15.3% of cases with imputed nativity. Misclassification of nativity as U.S.-born also occurred among the small number of documented immigrants who received an SSN before age 20 and had missing birthplace information in the cancer registry. However, many of those who immigrated in childhood may have more cultural, behavioral, and environmental factors in common with U.S.-born Hispanics, with whom they were grouped in this analysis, than with foreign-born Hispanics who immigrated as adults.
Besides the potential for moderate misclassification of nativity, our study was also limited by the lack of data on individual gastric cancer risk factors, such as H. pylori infection, smoking history, obesity, and diet, to help explain the observed incidence rate patterns. Some comparisons, especially by SES and enclave status, were constrained by small sample sizes and should therefore be interpreted with caution, and we likewise lacked sufficient sample size to examine trends and patterns in young adults. Furthermore, we could not cross-classify nativity and neighborhood factors due to the lack of population data. These limitations are outweighed by our study’s considerable strengths, including its population-based setting with results generalizable across California [28% of all U.S. Hispanics (65)], large overall sample size for evaluating anatomic and histologic subtypes as well as population characteristics, relatively homogeneous study population in terms of national origin (66), and high-quality cancer registry data, including valid data on birthplace (40), residential neighborhood, and Hispanic ethnicity (67, 68), that have not previously been used to investigate gastric cancer incidence patterns.
In summary, we found that incidence rates of most types of gastric cancer by anatomic subsite and histology are declining or remaining steady in foreign-born and U.S.-born Hispanic men and women, but that the incidence rate of diffuse gastric cancer is increasing in specific population segments defined by nativity and sex. Our results thus suggest that diffuse gastric cancer risk factors other than H. pylori may be growing increasingly prominent in foreign-born Hispanic men and U.S.-born Hispanic women. In addition, although most gastric subtypes are more common in foreign-born than U.S.-born Hispanics, and in those living in lower SES, higher enclave neighborhoods, cardia gastric cancer follows the opposite incidence patterns in Hispanic men. These patterns indicate distinct etiologies for anatomic and histologic subtypes of gastric cancer and also highlight the diversity of the growing U.S. Hispanic population (65) in terms of behavioral and environmental risk factors for gastric cancer. Therefore, closer examination of gastric cancer risk factors in distinct Hispanic subgroups may reveal new clues about why certain disease subtypes are more common or increasing in some groups relative to others and offer guidance for potential preventive efforts.
Acknowledgments
The authors thank Drs. William Anderson and Philip Rosenberg (National Cancer Institute) for helpful discussions; Dr. Pamela Kunz (Stanford University School of Medicine) for feedback on the preliminary results; and Ms. Rita Leung (Cancer Prevention Institute of California), Ms. Jane Pham, and Dr. Tim Miller (both formerly of Cancer Prevention Institute of California) for their work on creating the analytic data sets used for this research study.
Grant Support
This work was supported by the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California. The collection of cancer incidence data used in this study was supported by the California Department of Health Services as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement #1U58 DP00080701 awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the authors, and endorsement by the State of California, the California Department of Health Services, the National Cancer Institute, or the Centers for Disease Control and Prevention or their contractors and subcontractors is not intended nor should be inferred.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Authors' Contributions
Conception and design: E.T. Chang, S.L. Gomez, J. Parsonnet, S.L. Glaser.
Development of methodology: S.L. Gomez.
Acquisition of data: S.L. Gomez.
Analysis and interpretation of data:E.T. Chang, S.L. Gomez, K. Fish, C.W. Schupp, M.C. DeRouen, S.L. Glaser.
Writing, review, and/or revision of the manuscript: E.T. Chang, S.L. Gomez, K. Fish, J. Parsonnet, M.C. DeRouen, T.H.M. Keegan, C.A. Clarke, S.L. Glaser.
Administrative, technical, or material support: S.L. Gomez, K. Fish.
Study supervision: S.L. Gomez, C.A. Clarke, S.L. Glaser.
References
- 1.American Cancer Society. Cancer facts & figures for Hispanics/Latinos 2009–2011. Atlanta: American Cancer Society; 2011. [Google Scholar]
- 2.Carozza SE, Howe HL. Patterns of cancer incidence among US Hispanics/Latinos, 1995–2000. Cancer Causes Control. 2006;17:1067–75. doi: 10.1007/s10552-006-0045-3. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 4.Brown LM. Helicobacter pylori: epidemiology and routes of transmission. Epidemiol Rev. 2000;22:283–97. doi: 10.1093/oxfordjournals.epirev.a018040. [DOI] [PubMed] [Google Scholar]
- 5.Pew Hispanic Center. [cited 2011 Oct 7];Data and resources: country of origin profiles. 2011 Available from: http://www.pewhispanic.org/2011/05/26/country-of-origin-profiles/
- 6.Everhart JE, Kruszon-Moran D, Perez-Perez GI, Tralka TS, McQuillan G. Seroprevalence and ethnic differences in Helicobacter pylori infection among adults in the United States. J Infect Dis. 2000;181:1359–63. doi: 10.1086/315384. [DOI] [PubMed] [Google Scholar]
- 7.Staat MA, Kruszon-Moran D, McQuillan GM, Kaslow RA. A population-based serologic survey of Helicobacter pylori infection in children and adolescents in the United States. J Infect Dis. 1996;174:1120–3. doi: 10.1093/infdis/174.5.1120. [DOI] [PubMed] [Google Scholar]
- 8.Grad YH, Lipsitch M, Aiello AE. Secular trends in Helicobacter pylori seroprevalence in adults in the United States: evidence for sustained race/ethnic disparities. Am J Epidemiol. 2012;175:54–9. doi: 10.1093/aje/kwr288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cavaleiro-Pinto M, Peleteiro B, Lunet N, Barros H. Helicobacter pylori infection and gastric cardia cancer: systematic review and meta-analysis. Cancer Causes Control. 2011;22:375–87. doi: 10.1007/s10552-010-9707-2. [DOI] [PubMed] [Google Scholar]
- 10.Derakhshan MH, Malekzadeh R, Watabe H, Yazdanbod A, Fyfe V, Kazemi A, et al. Combination of gastric atrophy, reflux symptoms and histological subtype indicates two distinct aetiologies of gastric cardia cancer. Gut. 2008;57:298–305. doi: 10.1136/gut.2007.137364. [DOI] [PubMed] [Google Scholar]
- 11.Hansen S, Vollset SE, Derakhshan MH, Fyfe V, Melby KK, Aase S, et al. Two distinct aetiologies of cardia cancer; evidence from premorbid serological markers of gastric atrophy and Helicobacter pylori status. Gut. 2007;56:918–25. doi: 10.1136/gut.2006.114504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kubo A, Corley DA. Body mass index and adenocarcinomas of the esophagus or gastric cardia: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:872–8. doi: 10.1158/1055-9965.EPI-05-0860. [DOI] [PubMed] [Google Scholar]
- 13.Merry AH, Schouten LJ, Goldbohm RA, van den Brandt PA. Body mass index, height and risk of adenocarcinoma of the oesophagus and gastric cardia: a prospective cohort study. Gut. 2007;56:1503–11. doi: 10.1136/gut.2006.116665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang P, Zhou Y, Chen B, Wan HW, Jia GQ, Bai HL, et al. Overweight, obesity and gastric cancer risk: results from a meta-analysis of cohort studies. Eur J Cancer. 2009;45:2867–73. doi: 10.1016/j.ejca.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 15.Camargo MC, Anderson WF, King JB, Correa P, Thomas CC, Rosenberg PS, et al. Divergent trends for gastric cancer incidence by anatomical subsite in US adults. Gut. 2011;60:1644–9. doi: 10.1136/gut.2010.236737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu X, Chen VW, Andrews PA, Ruiz B, Correa P. Incidence of esophageal and gastric cancers among Hispanics, non-Hispanic whites and non-Hispanic blacks in the United States: subsite and histology differences. Cancer Causes Control. 2007;18:585–93. doi: 10.1007/s10552-007-9000-1. [DOI] [PubMed] [Google Scholar]
- 17.Freedman ND, Abnet CC, Leitzmann MF, Mouw T, Subar AF, Hollen-beck AR, et al. A prospective study of tobacco, alcohol, and the risk of esophageal and gastric cancer subtypes. Am J Epidemiol. 2007;165:1424–33. doi: 10.1093/aje/kwm051. [DOI] [PubMed] [Google Scholar]
- 18.Ladeiras-Lopes R, Pereira AK, Nogueira A, Pinheiro-Torres T, Pinto I, Santos-Pereira R, et al. Smoking and gastric cancer: systematic review and meta-analysis of cohort studies. Cancer Causes Control. 2008;19:689–701. doi: 10.1007/s10552-008-9132-y. [DOI] [PubMed] [Google Scholar]
- 19.Steevens J, Schouten LJ, Goldbohm RA, van den Brandt PA. Alcohol consumption, cigarette smoking and risk of subtypes of oesophageal and gastric cancer: a prospective cohort study. Gut. 2010;59:39–48. doi: 10.1136/gut.2009.191080. [DOI] [PubMed] [Google Scholar]
- 20.Navarro Silvera SA, Mayne ST, Risch H, Gammon MD, Vaughan TL, Chow WH, et al. Food group intake and risk of subtypes of esophageal and gastric cancer. Int J Cancer. 2008;123:852–60. doi: 10.1002/ijc.23544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang XQ, Terry PD, Yan H. Review of salt consumption and stomach cancer risk: epidemiological and biological evidence. World J Gastroenterol. 2009;15:2204–13. doi: 10.3748/wjg.15.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steevens J, Schouten LJ, Goldbohm RA, van den Brandt PA. Vegetables and fruits consumption and risk of esophageal and gastric cancer subtypes in the Netherlands Cohort Study. Int J Cancer. 2011;129:2681–93. doi: 10.1002/ijc.25928. [DOI] [PubMed] [Google Scholar]
- 23.Sipponen P. Gastric cancer: pathogenesis, risks, and prevention. J Gastroenterol. 2002;37(Suppl 13):39–44. doi: 10.1007/BF02990098. [DOI] [PubMed] [Google Scholar]
- 24.Anderson WF, Camargo MC, Fraumeni JF, Jr, Correa P, Rosenberg PS, Rabkin CS. Age-specific trends in incidence of noncardia gastric cancer in US adults. JAMA. 2010;303:1723–8. doi: 10.1001/jama.2010.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pew Hispanic Center. [cited 2011 Oct 17];Statistical Portrait of Hispanics in the United States, 2008. Available from: http://pewhispanic.org/factsheets/factsheet.php?FactsheetID=58.
- 26.Parkin DM, Khlat M. Studies of cancer in migrants: rationale and methodology. Eur J Cancer. 1996;32A:761–71. doi: 10.1016/0959-8049(96)00062-7. [DOI] [PubMed] [Google Scholar]
- 27.Tsai CJ, Perry S, Sanchez L, Parsonnet J. Helicobacter pylori infection in different generations of Hispanics in the San Francisco Bay Area. Am J Epidemiol. 2005;162:351–7. doi: 10.1093/aje/kwi207. [DOI] [PubMed] [Google Scholar]
- 28.Pickett KE, Pearl M. Multilevel analyses of neighbourhood socioeconomic context and health outcomes: a critical review. J Epidemiol Community Health. 2001;55:111–22. doi: 10.1136/jech.55.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evans GW, Kantrowitz E. Socioeconomic status and health: the potential role of environmental risk exposure. Annu Rev Public Health. 2002;23:303–31. doi: 10.1146/annurev.publhealth.23.112001.112349. [DOI] [PubMed] [Google Scholar]
- 30.Gresenz CR, Rogowski J, Escarce JJ. Community demographics and access to health care among U.S. Hispanics. Health Serv Res. 2009;44:1542–62. doi: 10.1111/j.1475-6773.2009.00997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Osypuk TL, Roux AV, Hadley C, Kandula NR. Are immigrant enclaves healthy places to live? The Multi-ethnic Study of Atherosclerosis. Soc Sci Med. 2009;69:110–20. doi: 10.1016/j.socscimed.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.California Cancer Registry. [cited 2011 Oct 7];California cancer registry F.A.Q. 2009 Available from: http://www.ccrcal.org/Inside_CCR/FAQ.shtml#how%20complete%20is%20ccr%20data.
- 33.NAACCR Latino Research Work Group, editor. NAACCR guideline for enhancing Hispanic-Latino identification: revised NAACCR Hispanic/Latino identification algorithm [NHIA v2] Springfield, IL: North American Association of Central Cancer Registries; 2005. Available from: http://www.naaccr.org/LinkClick.aspx?fileticket=i6MN9F8c1eU%3D&tabid=92. [Google Scholar]
- 34.Pew Hispanic Center. [cited Oct 7 2011];Demographic profile of Hispanics in California, 2009. 2011 Available from: http://pewhispanic.org/states/?stateid=CA.
- 35.Pew Hispanic Center. Survey brief: Latinos in California, Texas, New York, Florida and New Jersey. Washington, D.C.: Pew Hispanic Center; 2004. Available from: http://pewhispanic.org/files/factsheets/10.pdf. [Google Scholar]
- 36.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a Histo-Clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 37.Henson DE, Dittus C, Younes M, Nguyen H, Albores-Saavedra J. Differential trends in the intestinal and diffuse types of gastric carcinoma in the United States, 1973–2000: increase in the signet ring cell type. Arch Pathol Lab Med. 2004;128:765–70. doi: 10.5858/2004-128-765-DTITIA. [DOI] [PubMed] [Google Scholar]
- 38.Wu H, Rusiecki JA, Zhu K, Potter J, Devesa SS. Stomach carcinoma incidence patterns in the United States by histologic type and anatomic site. Cancer Epidemiol Biomarkers Prev. 2009;18:1945–52. doi: 10.1158/1055-9965.EPI-09-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gomez SL, Glaser SL. Quality of birthplace information obtained from death certificates for Hispanics, Asians, and Pacific Islanders. Ethn Dis. 2004;14:292–5. [PubMed] [Google Scholar]
- 40.Gomez SL, Glaser SL. Quality of cancer registry birthplace data for Hispanics living in the United States. Cancer Causes Control. 2005;16:713–23. doi: 10.1007/s10552-005-0694-7. [DOI] [PubMed] [Google Scholar]
- 41.Gomez SL, Quach T, Horn-Ross PL, Pham JT, Cockburn M, Chang ET, et al. Hidden breast cancer disparities in Asian women: disaggregating incidence rates by ethnicity and migrant status. Am J Public Health. 2010;100(Suppl 1):S125–31. doi: 10.2105/AJPH.2009.163931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Block G, Matanoski GM, Seltser RS. A method for estimating year of birth using social security number. Am J Epidemiol. 1983;118:377–95. doi: 10.1093/oxfordjournals.aje.a113645. [DOI] [PubMed] [Google Scholar]
- 43.Shimizu H, Mack TM, Ross RK, Henderson BE. Cancer of the gastrointestinal tract among Japanese and white immigrants in Los Angeles County. J Natl Cancer Inst. 1987;78:223–8. [PubMed] [Google Scholar]
- 44.Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001;12:703–11. doi: 10.1023/a:1011240019516. [DOI] [PubMed] [Google Scholar]
- 45.Chang ET, Yang J, Alfaro-Velcamp T, So SK, Glaser SL, Gomez SL. Disparities in liver cancer incidence by nativity, acculturation, and socioeconomic status in California Hispanics and Asians. Cancer Epidemiol Biomarkers Prev. 2010;19:3106–18. doi: 10.1158/1055-9965.EPI-10-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Surveillance, Epidemiology and End Results Program. [cited Oct 7 2011];SEER*Stat Software version 7.0.5. 2011 Sep 26; Available from: http://seer.cancer.gov/seerstat/
- 47.Rosenberg PS, Anderson WF. Proportional hazards models and age-period-cohort analysis of cancer rates. Stat Med. 2010;29:1228–38. doi: 10.1002/sim.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosenberg PS, Anderson WF. Age-period-cohort models in cancer surveillance research: ready for prime time? Cancer Epidemiol Biomarkers Prev. 2011;20:1263–8. doi: 10.1158/1055-9965.EPI-11-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.UCLA Center for Health Policy Research, California Department of Health Services, Public Health Institute. [cited 2011 Oct 7];California Health Interview Survey (CHIS) 2001, 2003, 2005, 2007, 2009. 2003–2011 Available from: www.chis.ucla.edu.
- 50.Munoz N, Asvall J. Time trends of intestinal and diffuse types of gastric cancer in Norway. Int J Cancer. 1971;8:144–57. doi: 10.1002/ijc.2910080118. [DOI] [PubMed] [Google Scholar]
- 51.Munoz N, Connelly R. Time trends of intestinal and diffuse types of gastric cancer in the United States. Int J Cancer. 1971;8:158–64. doi: 10.1002/ijc.2910080119. [DOI] [PubMed] [Google Scholar]
- 52.Howson CP, Hiyama T, Wynder EL. The decline in gastric cancer: epidemiology of an unplanned triumph. Epidemiol Rev. 1986;8:1–27. doi: 10.1093/oxfordjournals.epirev.a036288. [DOI] [PubMed] [Google Scholar]
- 53.Everhart JE. Recent developments in the epidemiology of Helicobacter pylori. Gastroenterol Clin North Am. 2000;29:559–78. doi: 10.1016/s0889-8553(05)70130-8. [DOI] [PubMed] [Google Scholar]
- 54.Centers for Disease Control and Prevention. State-specific trends in lung cancer incidence and smoking—United States, 1999–2008. MMWR Morb Mortal Wkly Rep. 2011;60:1243–7. [PubMed] [Google Scholar]
- 55.Briefel RR, Johnson CL. Secular trends in dietary intake in the United States. Annu Rev Nutr. 2004;24:401–31. doi: 10.1146/annurev.nutr.23.011702.073349. [DOI] [PubMed] [Google Scholar]
- 56.Blaser MJ, Perez-Perez GI, Kleanthous H, Cover TL, Peek RM, Chyou PH, et al. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111–5. [PubMed] [Google Scholar]
- 57.Shibata A, Parsonnet J, Longacre TA, Garcia MI, Puligandla B, Davis RE, et al. CagA status of Helicobacter pylori infection and p53 gene mutations in gastric adenocarcinoma. Carcinogenesis. 2002;23:419–24. doi: 10.1093/carcin/23.3.419. [DOI] [PubMed] [Google Scholar]
- 58.Camargo MC, Murphy G, Koriyama C, Pfeiffer RM, Kim WH, Herrera-Goepfert R, et al. Determinants of Epstein-Barr virus-positive gastric cancer: an international pooled analysis. Br J Cancer. 2011;105:38–43. doi: 10.1038/bjc.2011.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pinheiro PS, Sherman RL, Trapido EJ, Fleming LE, Huang Y, Gomez-Marin O, et al. Cancer incidence in first generation U.S. Hispanics: Cubans, Mexicans, Puerto Ricans, and new Latinos. Cancer Epidemiol Biomarkers Prev. 2009;18:2162–9. doi: 10.1158/1055-9965.EPI-09-0329. [DOI] [PubMed] [Google Scholar]
- 60.Clark WAV, Deurloo MC, Dieleman FM. Housing consumption and residential crowding in U.S. housing markets. J Urban Affairs. 2000;22:49–63. [Google Scholar]
- 61.Torres J, Leal-Herrera Y, Perez-Perez G, Gomez A, Camorlinga-Ponce M, Cedillo-Rivera R, et al. A community-based seroepidemiologic study of Helicobacter pylori infection in Mexico. J Infect Dis. 1998;178:1089–94. doi: 10.1086/515663. [DOI] [PubMed] [Google Scholar]
- 62.Sumaya CV, Henle W, Henle G, Smith MH, LeBlanc D. Seroepidemiologic study of Epstein-Barr virus infections in a rural community. J Infect Dis. 1975;131:403–8. doi: 10.1093/infdis/131.4.403. [DOI] [PubMed] [Google Scholar]
- 63.Golubjatnikov R, Allen VD, Steadman M, Del Pilar Olmos B, Inhorn SL. Prevalence of antibodies to Epstein-Barr virus, cytomegalovirus and Toxoplasma in a Mexican highland community. Am J Epidemiol. 1973;97:116–24. doi: 10.1093/oxfordjournals.aje.a121488. [DOI] [PubMed] [Google Scholar]
- 64.Public Policy Institute of California. Illegal immigrants. San Francisco: Public Policy Institute of California; 2008. Available from: http://www.ppic.org/content/pubs/jtf/JTF_IllegalImmigrantsJTF.pdf. [Google Scholar]
- 65.United States Census Bureau. Facts for features: Hispanic Heritage Month 2010: Sept. 15—Oct. 15. CB10-FF.17. Washington, D.C.: US Department of Commerce; 2010. [Google Scholar]
- 66.Gonzalez Burchard E, Borrell LN, Choudhry S, Naqvi M, Tsai HJ, Rodriguez-Santana JR, et al. Latino populations: a unique opportunity for the study of race, genetics, and social environment in epidemiological research. Am J Public Health. 95:2161–8. doi: 10.2105/AJPH.2005.068668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gomez SL, Glaser SL. Misclassification of race/ethnicity in a population-based cancer registry (United States) Cancer Causes Control. 2006;17:771–81. doi: 10.1007/s10552-006-0013-y. [DOI] [PubMed] [Google Scholar]
- 68.Clegg LX, Reichman ME, Hankey BF, Miller BA, Lin YD, Johnson NJ, et al. Quality of race, Hispanic ethnicity, and immigrant status in population-based cancer registry data: implications for health disparity studies. Cancer Causes Control. 2007;18:177–87. doi: 10.1007/s10552-006-0089-4. [DOI] [PubMed] [Google Scholar]