Abstract
AIM
To determine the prevalence of work disability in inflammatory bowel disease (IBD), and to assess risk factors associated with work disability.
METHODS
For this retrospective cohort study, we retrieved clinical data from the Dutch IBD Biobank on July 2014, containing electronic patient records of 3388 IBD patients treated in the eight University Medical Centers in the Netherlands. Prevalence of work disability was assessed in 2794 IBD patients and compared with the general Dutch population. Multivariate analyses were performed for work disability (sick leave, partial and full disability) and long-term full work disability (> 80% work disability for > 2 years).
RESULTS
Prevalence of work disability was higher in Crohn’s disease (CD) (29%) and ulcerative colitis (UC) (19%) patients compared to the general Dutch population (7%). In all IBD patients, female sex, a lower education level, and extra-intestinal manifestations, were associated with work disability. In CD patients, an age > 40 years at diagnosis, disease duration > 15 years, smoking, surgical interventions, and anti-TNFα use were associated with work disability. In UC patients, an age > 55 years, and immunomodulator use were associated with work disability. In CD patients, a lower education level (OR = 1.62, 95%CI: 1.02-2.58), and in UC patients, disease complications (OR = 3.39, 95%CI: 1.09-10.58) were associated with long-term full work disability.
CONCLUSION
The prevalence of work disability in IBD patients is higher than in the general Dutch population. Early assessment of risk factors for work disability is necessary, as work disability is substantial among IBD patients.
Keywords: Inflammatory bowel disease, Crohn’s disease, Ulcerative colitis, Work disability, Health care costs
Core tip: Our study shows that a lower education is patients with Crohn’s disease, and disease complications (osteopenia, thromboembolic event) in patients with ulcerative colitis are associated with long-term full work disability (> 80% work disability for > 2 years). This highlights the need for early assessment of risk factors for work disability, as work disability is substantial among inflammatory bowel disease patients and associated with high societal health care costs.
INTRODUCTION
Inflammatory bowel disease (IBD), consisting of Crohn’s disease (CD) and ulcerative colitis (UC) is a heterogeneous/multifaceted disease. Patients suffer from symptoms like diarrhoea, abdominal pain, fatigue, and weight loss. The disease course is unpredictable, complicated by flares, need for chronic medication use and need for surgery. This leads to increasing intestinal damage with a high disease burden in many patients with IBD[1]. It has been established that, depending on disease activity, patients with IBD experience a lower quality of life[2,3]. IBD generally makes its debut during the second or third decade of life. Therefore, patients with IBD can encounter major problems during their economically productive life, which can lead to work disability, sometimes at young age. Work disability in patients with IBD is associated with a further decrease in quality of life, and high societal costs, especially if work disability is long-term and arises at a young age[4,5]. Preventing work disability is therefore an important goal in IBD management.
Work disability rates have been reported before, but reported rates are heterogeneous, ranging between 5% and 33% depending on sample size, methodology, and study population[6-12]. Several clinical factors have been suggested to play a role in work disability, such as female sex, age, disease duration, disease severity, and surgical interventions[10-16]. However, in most study designs, risk factors for any form of work disability are the focus of interest, neglecting risk factors associated specifically with long-term full work disability (> 80% work disability for > 2 years).
Therefore, our aims were: (1) to assess the prevalence of work disability in patients with IBD and to compare this with the general Dutch population; and (2) to identify risk factors for work disability, especially risk factors for long-term full work disability.
MATERIALS AND METHODS
Study design and study population
For this study, we used data from the Dutch IBD Biobank, which is part of the Parelsnoer Institute (www.parelsnoer.org). Since 2007, every patient with IBD that is treated in one of the eight Dutch University Medical Centers (UMCs) is invited to participate. The data is collected prospectively and patients with IBD are being enrolled continuously. Clinical data is retrieved from medical records by using a standardized information model containing 225 IBD related items. Items used for this study are; demographic items, diagnosis, smoking status, employment status, disease location, disease behaviour, education level, surgery-related items, medication use, extra-intestinal manifestations and complications. Definitions of these items can be found in Table 1. At the moment of data freeze (the 17th of July 2014), the Dutch IBD Biobank contained 3388 patients with IBD. For this study, patients for whom employment status was missing were excluded. We only included adult patients with IBD within working age, and thus excluded patients over the age of 65 years.
Table 1.
Definition of used parelsnoer information model items
| Diagnosis of IBD | |
| Crohn’s disease Ulcerative colitis IBD-unclassified IBD-Indeterminate | Diagnosis of IBD was defined by clinical symptoms of the disease and confirmed by endoscopy, radiology or histology. If differentiation between CD and UC was not possible, patient were classified as Inflammatory Bowel Disease Unclassified (IBD-U). In the case that a pathologist was not able to differentiate between CD or UC following colectomy, the patient was classified as Inflammatory Bowel Disease Indeterminate (IBD-I). |
| Family history of IBD | |
| Family history of IBD was registered up to 3rd degree relatives | |
| Smoking status | |
| Smoking status at diagnosis was documented. Patients were classified as never smokers, current smokers or former smokers | |
| Education level | |
| Patients were classified in two groups; low education (Lower general education; Lower vocational education; General secondary education; Vocational secondary education; Did not finish primary school) and high education (Pre-university secondary education; Vocational post-secondary education; University) | |
| Disease behavior | Disease behavior is reported if it occurred at any point in the disease course up to baseline. |
| Fistulising disease | Fistulising disease is confirmed by physical examination, radiological or endoscopy assessment. |
| Stricturing disease | A stricture had to be symptomatic. An anal stenosis had to be symptomatic and confirmed by physical examination. |
| Penetrating disease | Penetrating disease is defined as an intestinal abscess or intestinal perforation due to disease activity. |
| Prescribed medication | |
| Anti-tumor necrosis factor alpha | Prescribed medication included anti-tumor necrosis factor alpha (TNF-alpha) agents (infliximab, adalimumab or certolizumab). Data on medication use was available for the entire disease course; medication use was therefore defined as medication ‘ever used’ |
| Immunomodulators | immunomodulators (mercaptopurine, azathioprine, thioguanine or methotrexate). Data on medication use was available for the entire disease course; medication use was therefore defined as medication ‘ever used’ |
| IBD-related surgical interventions | |
| IBD-related surgical interventions included small bowel resection, ileocecal resection, colon resection, resection other, strictureplasty, ileostomy/colostomy, surgery for abscesses or fistula, stoma and pouch | |
| Extra-intestinal manifestations | |
| Skin manifestations | Skin manifestations unrelated to the use of IBD medication such as pyoderma gangrenosum, erythema nodosum, hidradenitis suppurativa, psoriasis or palmoplantar psoriasiform pustolosis, and metastatic CD. |
| Musculoskeletal manifestations | Arthritis was defined as red and swollen joints, dactylitis, reactive arthritis and gout. Arthropathy was defined as painful joints, without any swelling or redness, with an inflammatory pattern: pain at night or at rest (e.g., sacroiliitis, ankylosing spondylitis, enthesitis, and inflammatory back pain). |
| Ocular manifestations | Ocular manifestations included uveitis and episcleritis. |
| Complications | |
| Osteopenia | Osteopenia was defined as a bone mineral density T-score lower than -1. |
| Thromboembolic event | A thromboembolic event was confirmed by additional tests (radiology, endoscopy or histology). |
IBD: Inflammatory bowel disease; CD: Crohn’s disease; UC: Ulcerative colitis.
Definition of work disability
Patients were asked about their employment status at inclusion, which was defined using the following categories; (1) working full time (80%-100%, or working 30 h or more a week); (2) working part time (< 80%, or working less than 30 h a week); (3) partial work disability (35%-80% work disability for > 2 years); (4) full work disability (> 80% work disability for > 2 years); (5) sick leave (work disability for < 2 years); (6) retired; and (7) “Other”.
A Dutch law (“Wet werk en inkomen naar arbeidsvermogen” or WIA) prescribes that from the moment Dutch citizens are disabled to work they are entitled to receive a maximum of 170% of their wages during a period of 104 mo (generally 100% for 52 wk, followed by 70% for 52 wk). If after 104 mo the person is still on sick leave the same law prescribes whether patients receive a disability pension. There are two types of disability pension; partial disability (35%-80% work disability) and full disability (more than 80% work disability). A specialized physician determines the percentage of work disability based on questionnaires and physical examination.
Due to the nature of our data we could not distinguish whether work disability was solely attributable to IBD or whether there was an additional cause.
To compare the currently reported work disability rates with the general Dutch population we retrieved information from Statistics Netherlands (CBS), a national institute gathering statistical information about the Dutch population, including employment rates[17,18]. Employment rates were collected for 2014 and matched for sex and age.
Statistical analysis
In all analyses, patients with UC were grouped with patients with IBD-Unclassified (IBD-U) and IBD-Indeterminate (IBD-I). Dichotomous variables were compared using the Pearson χ2 test. Continuous variables were compared using the Mann-Whitney-U test. The Student t-test was used to compare overall work disability in our cohort to the general Dutch population. The variable “age” represented the age at inclusion at which employment status was assessed. To assess clinical predictors of work disability in patients with IBD, we categorized the variable employment status into two groups; employed (both full time and part time) and disabled for work (including partial disability, full disability and sick leave). Retirement and “other” were left out of the final analyses after we determined that “other” was unlikely to contain undeclared differential work disability, such as pregnancy leave. We performed a multivariate analysis with employment status as outcome and included demographic and clinical items that had a P < 0.10 in the univariate analysis for employment status. Analyses were repeated in patients with CD and patients with UC, separately. To assess risk factors for long-term full work disability, patients with full disability were compared to patients with partial disability. Statistical analyses were performed with Stata Software V.13.1[19].
RESULTS
Patient population and demographic characteristics
A total of 2794 patients with IBD were within working age and were included in the analysis; 1740 patients with CD and 1054 patients with UC. Demographic and clinical characteristics are depicted in Table 2. In our cohort, more patients with CD were female compared to patients with UC (63% vs 53%, P < 0.01). Age at diagnosis was lower in patients with CD compared to patients with UC (24 years old vs 29 years old, P < 0.01). The prevalence of a family history positive for IBD (30% vs 25%, P = 0.01) as well as the appendectomy rate (15% vs 7%, P < 0.01) were higher in patients with CD. Patients with CD were more often smokers than patients with UC (27% vs 12%, P < 0.01). According to the Montreal classification, most patients with CD had ileocolonic disease [47% (L3)], whereas most patients with UC had an extensive colitis [58% (E3)] (Table 2).
Table 2.
Demographic and clinical characteristics in patients with Crohn’s disease and ulcerative colitis n (%)
| CD | UC | P value | |
| n | 1740 | 1054 | |
| Sex (female) | 1103 (63) | 561 (53) | < 0.01 |
| Median age, yr, (IQR 25-75) | 39.9 (30-51) | 43.4 (33-53) | < 0.01 |
| Median age diagnosis, yr, (IQR 25-75) | 24.1 (19-32) | 29.0 (22-39) | < 0.01 |
| Median age disease duration, yr, (IQR 25-75) | 11.7 (6-21) | 10.3 (5-18) | < 0.01 |
| Family history of IBD1 | 518 (30) | 266 (25) | 0.01 |
| Appendectomy1 | 266 (15) | 70 (7) | < 0.01 |
| Smoking | 1620 (100) | 984 (100) | |
| Yes | 439 (27) | 117 (12) | < 0.01 |
| Disease location | |||
| L1: ileal disease2 | 324 (22) | ||
| L2: colon disease2 | 450 (31) | ||
| L3: ileocolon disease2 | 696 (47) | ||
| L4: upper GI disease1 | 156 (9) | ||
| P: peri-anal disease1 | 485 (28) | ||
| E1: proctitis3 | 77 (8) | ||
| E2: left-sided colitis3 | 315 (34) | ||
| E3: extensive colitis3 | 541 (58) | ||
| Disease behavior CD | |||
| Fistulising disease1 | 289 (17) | ||
| Penetrating disease1 | 232 (13) | ||
| Stricturing disease1 | 445 (26) | ||
| Education | 1708 (100) | 1036 (100) | |
| Low | 932 (55) | 517 (50) | 0.02 |
| Employment status | |||
| Full-time | 607 (35) | 440 (42) | < 0.01 |
| Part-time | 359 (21) | 224 (21) | |
| Partially disabled to work | 129 (7) | 54 (5) | |
| Fully disabled to work | 342 (20) | 124 (12) | |
| Sick leave | 77 (4) | 36 (3) | |
| Retired | 22 (1.3) | 21 (2) | |
| Other | 204 (12) | 155 (15) |
Missing values were scored as non-present;
These percentages are calculated for 1470 patients with CD;
These percentages are calculated for 993 patients with UC. CD: Crohn’s disease; UC: Ulcerative colitis.
Employment status in patients with IBD compared to the general Dutch population
Figure 1 shows the percentages of work disability in patients with CD, patients with UC and the general Dutch population per age category in females (Figure 1A) and males (Figure 1B). Overall, work disability was significantly higher in IBD patients compared to the general Dutch population, independent of sex (P < 0.05).
Figure 1.
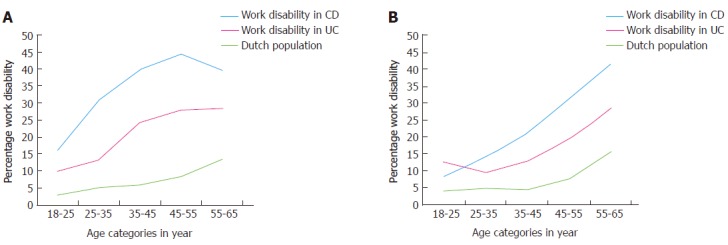
Percentage of work disability per age category in females and males. Comparing work disability rates in patients with CD and patients with UC with the general Dutch population. A: Work disability in females; B: Work disability in males. CD: Crohn’s disease; UC: Ulcerative colitis.
Clinical risk factors of (long-term full) work disability in patients with CD
Table 3 depicts the (clinical) risk factors that were significantly associated with work disability in CD (548 patients with CD were fully disabled and 966 patients with CD were employed). In the multivariate model, female sex (OR = 2.03, 95%CI: 1.53-2.69), an age > 40 years at diagnosis (OR = 3.69, 95%CI: 1.83-7.42), a disease duration > 15 years (OR = 1.67, 95%CI: 1.15-2.43), a lower education level (OR = 2.02, 95%CI: 1.55-2.64), smoking (OR = 1.45, 95%CI: 1.09-1.92), surgical interventions (OR = 1.48, 95%CI: 1.10-2.00), anti- Tumor necrosis factor alpha (TNFα) use (OR = 1.86, 95%CI: 1.43-2.43), and extra-intestinal manifestations (OR = 1.36, 95%CI: 1.05-1.77) were all associated with an increased odds ratio for work disability in CD.
Table 3.
Univariate and multivariate regression analyses of work disability in patients with Crohn’s disease n (%)
| Work disability | Employed | Unadj. OR (95%CI) | Adj. OR (95%CI) | |
| n | 548 (100) | 966 (100) | ||
| Sex (female) | 397 (72) | 556 (58) | 1.94 (1.55-2.43) | 2.03 (1.53-2.69) |
| Age, yr | ||||
| < 40 | 215 (39) | 537 (55) | 1.00 | 1.00 |
| 40-55 | 220 (40) | 335 (35) | 1.64 (1.30-2.07) | 0.94 (0.65-1.35) |
| > 55 | 113 (21) | 94 (10) | 3.00 (2.19-4.12) | 1.63 (0.95-2.77) |
| Age at diagnosis, yr | ||||
| A1: diagnosis ≤ 16 | 47 (9) | 156 (16) | 1.00 | 1.00 |
| A2: diagnosis 17-40 | 413 (75) | 719 (75) | 1.91 (1.35-2.70) | 2.02 (1.30-3.13) |
| A3: diagnosis > 40 | 88 (16) | 91 (9) | 3.21 (2.07-4.98) | 3.69 (1.83-7.42) |
| Disease duration, yr | ||||
| ≤ 15 | 285 (52) | 618 (64) | 1.00 | 1.00 |
| > 15 | 263 (48) | 348 (36) | 1.64 (1.32-2.03) | 1.67 (1.15-2.43) |
| Education | 537 (100) | 950 (100) | ||
| Low | 366 (68) | 446 (47) | 2.42 (1.94-3.02) | 2.02 (1.55-2.64) |
| Smoking | 508 (100) | 904 (100) | ||
| Yes | 180 (35) | 203 (22) | 1.90 (1.49-2.41) | 1.45 (1.09-1.92) |
| Disease location | ||||
| L1: ileal disease1 | 88 (19) | 184 (23) | 1.00 | 1.00 |
| L2: colon disease1 | 138 (30) | 263 (33) | 1.10 (0.79-1.52) | 1.10 (0.75-1.61) |
| L3: ileocolon disease1 | 237 (51) | 359 (44) | 1.38 (1.02-1.87) | 1.29 (0.91-1.83) |
| L4: upper GI disease2 | 52 (9) | 82 (8) | 1.13 (0.79-1.63) | - |
| P: peri-anal disease2 | 170 (31) | 256 (26) | 1.25 (0.99-1.57) | 1.03 (0.77-1.37) |
| Disease behavior CD | ||||
| Fistulising disease2 | 95 (17) | 156 (16) | 1.09 (0.82-1.44) | - |
| Stricturing disease2 | 145 (26) | 248 (26) | 1.04 (0.82-1.32) | - |
| Penetrating disease2 | 72 (13) | 128 (13) | 0.99 (0.73-1.35) | - |
| Pouch2 | 12 (2) | 17 (1.8) | 1.25 (0.59-2.64) | - |
| Stoma2 | 96 (18) | 108 (11) | 1.69 (1.25-2.27) | 1.15 (0.78-1.69) |
| Surgery2 | 288 (53) | 406 (42) | 1.53 (1.24-1.89) | 1.48 (1.10-2.00) |
| Medication | 537 (100) | 949 (100) | ||
| Anti-TNFα | 309 (58) | 435 (46) | 1.60 (1.29-1.98) | 1.86 (1.43-2.43) |
| Immunomodulators | 395 (74) | 698 (74) | 1.00 (0.79-1.27) | - |
| Extra-intestinal manifestations2 | 229 (42) | 294 (30) | 1.64 (1.32-2.04) | 1.36 (1.05-1.77) |
| PSC2 | 7 (1.3) | 14 (1.5) | 0.88 (0.35-2.19) | - |
| Complications2 | 155 (28) | 223 (23) | 1.31 (1.04-1.67) | 1.18 (0.88-1.59) |
These percentages are calculated for 463 patients with CD that were disabled for work and 806 patients with CD that were employed;
Missing values were scored as non-present. CD: Crohn’s disease; Unadj: Unadjusted; TNFα: Tumor necrosis factor alpha; PSC: Primary sclerosing cholangitis.
In the second analysis, partial disability was compared to long-term full work disability for CD. In the multivariate model, an age > 55 years (OR = 3.06, 95%CI: 1.54-6.07), and a lower education level (OR = 1.62, 95%CI: 1.02-2.58) were associated with long-term full work disability in CD (Table 4).
Table 4.
Univariate and multivariate regression analyses of full and partial work disability in patients with Crohn’s disease n (%)
| Full disability | Partial disability | Unadj. OR (95%CI) | Adj. OR (95%CI) | |
| n | 342 (100) | 129 (100) | ||
| Sex (female) | 251 (73) | 100 (78) | 0.80 (0.50-1.29) | - |
| Age | ||||
| < 40 yr | 110 (32) | 54 (42) | 1.00 | 1.00 |
| 40-55 yr | 137 (40) | 61 (47) | 1.10 (0.71-1.72) | 1.05 (0.65-1.70) |
| > 55 yr | 95 (28) | 14 (11) | 3.33 (1.74-6.37) | 3.06 (1.54-6.07) |
| Age at diagnosis | ||||
| A1: diagnosis ≤ 16 yr | 28 (8) | 13 (10) | 1.00 | - |
| A2: diagnosis 17-40 yr | 256 (75) | 103 (80) | 1.15 (0.58-2.32) | |
| A3: diagnosis > 40 yr | 58 (17) | 13 (10) | 2.07 (0.85-5.05) | |
| Disease duration | ||||
| ≤ 15 yr | 151 (44) | 65 (50) | 1.00 | - |
| > 15 yr | 191 (56) | 64 (50) | 1.28 (0.86-1.93) | |
| Education | 332 (100) | 128 (100) | ||
| Low | 236 (71) | 73 (57) | 1.85 (1.21-2.83) | 1.62 (1.02-2.58) |
| Smoking | 323 (100) | 115 (100) | ||
| Yes | 125 (39) | 33 (29) | 1.57 (0.99-2.49) | 1.53 (0.93-2.50) |
| Disease location | ||||
| L1: ileal disease1 | 56 (19) | 21 (19) | 1.00 | - |
| L2: colon disease1 | 80 (28) | 38 (35) | 0.79 (0.42-1.49) | |
| L3: ileocolon disease1 | 155 (53) | 50 (46) | 1.16 (0.64-2.11) | |
| L4: upper GI disease2 | 38 (11) | 8 (6) | 1.89 (0.86-4.17) | - |
| P: peri-anal disease2 | 109 (32) | 45 (35) | 0.87 (0.57-1.34) | - |
| Disease behavior CD | ||||
| Fistulising disease2 | 64 (19) | 23 (18) | 1.06 (0.63-1.80) | - |
| Stricturing disease2 | 92 (27) | 33 (26) | 1.07 (0.67-1.70) | - |
| Penetrating disease2 | 47 (14) | 19 (15) | 0.92 (0.52-1.64) | - |
| Pouch2 | 6 (1.8) | 4 (3) | 0.56 (0.15-2.01) | - |
| Stoma2 | 69 (20) | 20 (16) | 1.38 (0.80-2.38) | - |
| Surgery2 | 192 (56) | 64 (50) | 1.30 (0.87-1.95) | - |
| Medication | 335 (100) | 125 (100) | ||
| Anti-TNFα | 193 (58) | 68 (54) | 1.14 (0.75-1.72) | - |
| Immunomodulators | 249 (74) | 87 (70) | 1.26 (0.80-1.99) | - |
| Extra-intestinal manifestations2 | 152 (44) | 50 (39) | 1.26 (0.84-1.91) | - |
| PSC2 | 2 (0.6) | 4 (3) | 0.18 (0.03-1.02) | 0.19 (0.03-1.10) |
| Complications2 | 106 (31) | 34 (26) | 1.25 (0.80-1.98) | - |
These percentages are calculated for 291 patients with CD that were fully disabled and 109 patients with CD that were partially disabled;
Missing values were scored as non-present. CD: Crohn’s disease; Unadj: Unadjusted; TNFα: Tumor necrosis factor alpha; PSC: Primary sclerosing cholangitis.
Clinical risk factors of (long-term full) work disability in patients with UC
In Table 5, (clinical) risk factors that were significantly associated with work disability in UC (214 patients with UC were fully disabled and 664 patients with UC were employed) have been shown. In the multivariate model, female sex (OR = 1.76, 95%CI: 1.23-2.53), an age > 55 years (OR = 2.93, 95%CI: 1.68-5.14), a lower education level (OR = 2.59, 95%CI: 1.81-3.70), immunomodulator use (OR = 1.58, 95%CI: 1.09-2.28), and extra-intestinal manifestations (OR = 2.13, 95%CI: 1.47-3.09) were all associated with an increased odds ratio for work disability in UC.
Table 5.
Univariate and multivariate regression analyses of work disability in patients with ulcerative colitis n (%)
| Work disability | Employed | Unadj. OR (95%CI) | Adj. OR (95%CI) | |
| n | 214 (100) | 664 (100) | ||
| Sex (female) | 124 (58) | 333 (50) | 1.37 (1.00-1.87) | 1.76 (1.23-2.53) |
| Age | ||||
| < 40 yr | 58 (27) | 287 (43) | 1.00 | 1.00 |
| 40-55 yr | 94 (44) | 277 (42) | 1.68 (1.16-2.42) | 1.52 (0.97-2.40) |
| > 55 yr | 62 (29) | 100 (15) | 3.07 (2.01-4.69) | 2.93 (1.68-5.14) |
| Age at diagnosis | ||||
| A1: diagnosis ≤ 16 yr | 18 (8) | 64 (10) | 1.00 | 1.00 |
| A2: diagnosis 17-40 yr | 124 (58) | 474 (71) | 0.93 (0.53-1.63) | 1.06 (0.55-2.04) |
| A3: diagnosis > 40 yr | 72 (34) | 126 (19) | 2.03 (1.12-3.69) | 1.99 (0.92-4.28) |
| Disease duration | ||||
| ≤ 15 yr | 129 (60) | 439 (66) | 1.00 | - |
| > 15 yr | 85 (40) | 225 (34) | 1.29 (0.94-1.77) | |
| Education | 208 (100) | 656 (100) | ||
| Low | 144 (69) | 282 (43) | 2.98 (2.14-4.16) | 2.59 (1.81-3.70) |
| Smoking | 206 (100) | 621 (100) | ||
| Yes | 30 (15) | 71 (11) | 1.32 (0.83-2.09) | - |
| Disease location | ||||
| E1: proctitis1 | 14 (7) | 54 (9) | 1.00 | - |
| E2: left-sided colitis1 | 54 (27) | 215 (37) | 97 (0.50-1.87) | |
| E3: extensive colitis1 | 130 (66) | 317 (54) | 1.58 (0.85-2.95) | |
| Pouch2 | 37 (17) | 54 (8) | 2.36 (1.50-3.71) | 1.72 (0.81-3.65) |
| Stoma2 | 35 (16) | 54 (8) | 2.21 (1.40-3.49) | 1.13 (0.54-2.38) |
| Surgery2 | 60 (28) | 97 (15) | 2.28 (1.58-3.29) | 1.68 (0.75-3.75) |
| Medication | 211 (100) | 649 (100) | ||
| Anti-TNFα | 53 (25) | 120 (18) | 1.48 (1.02-2.14) | 1.33 (0.86-2.06) |
| Immunomodulators | 139 (66) | 350 (54) | 1.65 (1.19-2.28) | 1.58 (1.09-2.28) |
| Extra-intestinal manifestations2 | 78 (36) | 130 (20) | 2.36 (1.68-3.30) | 2.13 (1.47-3.09) |
| PSC2 | 8 (4) | 21 (3) | 1.19 (0.52-2.73) | - |
| Complications2 | 37 (17) | 89 (13) | 1.35 (0.89-2.05) | - |
These percentages are calculated for 198 patients with UC that were disabled for work and 586 patients with UC that were employed;
Missing values were scored as non-present. UC: Ulcerative colitis; Unadj: Unadjusted; Adj: Adjusted; TNFα: Tumor necrosis factor alpha; PSC: Primary sclerosing cholangitis.
In the second analysis, partial disability was compared to long-term full work disability for UC. In the multivariate model, an age > 55 years (OR = 3.49, 95%CI: 1.23-9.92), and complications (OR = 3.39, 95%CI: 1.09-10.58) were associated with long-term full work disability in UC (Table 6).
Table 6.
Univariate and multivariate regression analyses of full and partial work disability in patients with ulcerative colitis n (%)
| Full disability | Partial disability | Unadj. OR (95%CI) | Adj. OR (95%CI) | |
| n | 124 (100) | 54 (100) | ||
| Sex (female) | 75 (60) | 26 (48) | 1.65 (0.87-3.14) | - |
| Age | ||||
| < 40 yr | 25 (20) | 15 (28) | 1.00 | 1.00 |
| 40-55 yr | 52 (42) | 32 (59) | 0.98 (0.45-2.12) | 0.90 (0.40-2.03) |
| > 55 yr | 47 (38) | 7 (13) | 4.03 (1.45-11.17) | 3.49 (1.23-9.92) |
| Age at diagnosis | ||||
| A1: diagnosis ≤ 16 yr | 7 (6) | 5 (9) | 1.00 | - |
| A2: diagnosis 17-40 yr | 70 (56) | 34 (63) | 1.47 (0.43-4.97) | |
| A3: diagnosis > 40 yr | 47 (38) | 15 (28) | 2.24 (0.62-8.10) | |
| Disease duration | ||||
| ≤ 15 yr | 65 (52) | 36 (67) | 1.00 | 1.00 |
| > 15 yr | 59 (48) | 18 (33) | 1.82 (0.93-3.54) | 1.78 (0.87-3.62) |
| Education | 122 (100) | 50 (100) | ||
| Low | 84 (69) | 37 (74) | 0.78 (0.37-1.63) | - |
| Smoking | 121 (100) | 52 (100) | ||
| Yes | 18 (15) | 7 (13) | 1.12 (0.44-2.88) | - |
| Disease location | ||||
| E1: proctitis1 | 9 (8) | 4 (8) | 1.00 | - |
| E2: left-sided colitis1 | 39 (33) | 9 (18) | 1.93 (0.48-7.68) | - |
| E3: extensive colitis1 | 70 (59) | 38 (74) | 0.82 (0.24-2.84) | - |
| Pouch2 | 24 (19) | 9 (17) | 1.20 (0.52-2.79) | - |
| Stoma2 | 23 (19) | 8 (15) | 1.31 (0.54-3.15) | - |
| Surgery2 | 37 (30) | 14 (26) | 1.22 (0.59-2.50) | - |
| Medication | 122 (100) | 53 (100) | ||
| Anti-TNFα | 30 (25) | 10 (19) | 1.40 (0.63-3.13) | - |
| Immunomodulators | 77 (63) | 37 (70) | 0.74 (0.37-1.48) | - |
| Extra-intestinal manifestations2 | 51 (41) | 19 (35) | 1.29 (0.66-2.50) | - |
| PSC2 | 3 (2) | 4 (7) | 0.31 (0.07-1.44) | - |
| Complications2 | 28 (23) | 4 (7) | 3.65 (1.21-10.97) | 3.39 (1.09-10.58) |
These percentages are calculated for 118 patients with UC that were fully disabled and 51 patients with UC that were partially disabled;
Missing values were scored as non-present. UC: Ulcerative colitis; Unadj: Unadjusted; Adj: Adjusted; TNFα: Tumor necrosis factor alpha; PSC: Primary sclerosing cholangitis.
DISCUSSION
In this nationwide clinical database and biobank study we assessed work disability rates in patients with IBD. We found higher rates of work disability in patients with IBD compared to the general Dutch population. Furthermore, in patients with IBD female sex, a lower education level, and extra-intestinal manifestations were significantly associated with work disability. In patients with CD, an age > 40 years at diagnosis, disease duration > 15 years, smoking, surgical interventions, and anti-TNFα use were significantly associated with higher work disability. In patients with UC, an age > 55 years, and immunomodulator use were significantly associated with higher work disability. In patients with CD a lower education level was associated with long-term full work disability, whereas in patients with UC disease complications were associated with long-term full work disability.
High work disability rates in patients with IBD
In patients with IBD, the overall proportion of work disability was 29% in CD, and 19% in UC. These work disability rates were higher than those in the age-adjusted general Dutch population (7%). Suffering from (severe) IBD may in itself be explanatory for this higher percentage. Furthermore, all patients in the Dutch IBD Biobank were treated in tertiary referral centers with a severe disease course signature: 47% ileocolonic disease (L3 Montreal CD) and 58% extensive colitis (E3 Montreal UC). Indeed, disease in remission was associated with increased employment in CD[5] whereas disease severity was a predictor of work disability in IBD[16].
Criteria for disability pension differ between countries due to political and socioeconomic factors. Comparison of work disability rates between different countries is therefore difficult. In addition, differences in selection criteria, study methodology, sample size, and definitions of work disability result in highly variable disability rates reported (from 5% to 33%)[6-14]. In two comparable Dutch IBD-population studies it has been concluded by the authors that disability rates in CD and in UC were higher compared to the general Dutch population, which is in line with the current results. However, work disability rates of 33% in CD, 24% in UC, and 11% in the control population were reported in the first study that was conducted in 2002[8], which is higher than the disability rates we found. This may be due to changes in the welfare system since 2002, when patients got a disability pension after one year of sick leave, instead of after two years as it is now. Disability rates in a second, more recent Dutch study, by van der Valk et al[10], were more in line with our findings but lower (18% in CD, 10% in UC, and 7% in the control population). Although there seems to be a wide range in work disability rates in IBD, it may be concluded that the rate of work disability is substantial among patients with IBD.
Clinical risk factor for work disability
Female sex[10,11,13,15,16], an age > 40 years at diagnosis[11,13], older age at inclusion[10,12,13,15,16], a lower education level[10,13], extra-intestinal manifestations[10,12,16], a disease duration > 15 years[12,15,16], surgical interventions[11-13], and immunomodulator use[16] have been reported to be associated with work disability in patients with IBD.
In UC, an age > 55 years was associated with work disability, which might not be directly disease related. Indeed, it has been reported that disability pensions were not caused by UC but often by other age-related comorbidities[11,12]. On the other hand, in CD it has been found that patients with CD receive their disability pension because of IBD or other IBD related comorbidity[11], rather than other age related comorbidities.
Surgical interventions were a risk factor for work disability in patients with CD, but not in UC. As surgery is an indicator of severe disease these data seemed to corroborate the hypothesis that severity of disease predicts work disability. In line with this, higher work disability rates have been reported after colectomy in patients with UC[14]. A recent review has shown that surgical rates have declined since the introduction of biologicals, indicating a beneficial effect of treatment with biologicals on disease outcome[20]. Our study is not well suited to study this effect, since our most reliable clinical information has been collected after the introduction of biologicals. Other studies report no differences in disability between patients receiving anti-TNFα treatment or patient that underwent surgery[21,22]. At the moment, a clinical trail (LIRIC) assesses differences in outcome comparing anti-TNFα treatment with Ileocecal resection in patients with CD not responsive to prednisolone[23].
While it has been established that anti-TNFα and/or immunomodulators are generally used to maintain remission in patients with IBD, our study found an association between use of anti-TNFα use and (full) work disability. This could be due to selection bias, as all patients were treated in tertiary referral centers, with most of them having extensive disease involvement (Montreal classification; 47% L3 and 58% E3). Therefore, it is likely that patients receiving anti-TNFα and immunomodulator therapy are patients with more severe disease, receiving “rescue therapy” rather than “top-down therapy”. Smoking was found to be a risk factor for work disability in CD, but not in UC. In this study, smoking status was scored at moment of inclusion, as it has been known that smoking can increase frequency and severity of flares in CD[24], while it can ameliorate disease in UC[25]. It has been reported that smoking at diagnosis was associated with work disability in UC[13], whereas others reported on an association between numbers of sick leave days and smoking at the onset of CD[16], corroborating the current findings.
Clinical risk factor for long-term full work disability
We replicated most known, clinical risk factors for work disability and evaluated risk factors for long-term full work disability (> 80% work disability) comparing it to partial work disability (> 35% work disability). A lower education level was the only factor, which remained statistically significantly associated with long-term full work disability in CD. In only a few studies, educational level has been taken into account showing conflicting results. A higher education level seemed to be associated to work disability and sick leave in patients with CD in previous studies[8,13]. On the other hand, a study by Vester-Andersen et al. reported no patients with CD with a higher education level that were receiving a disability pension[13]. Hence, the relationship between education level and work disability remains unclear, and more population specific studies are needed before hard conclusions can be drawn[26,27].
Risk factors identified in this report should be interpreted with caution, as the confidence intervals were quite large and data were derived from third-line referral centres. Furthermore, due to the nature of our data we could not distinguish between work disability solely attributable to IBD, or work disability due to a different cause. However, the median age in our study was 40 years for CD patients and 43 years for UC patients, ages at which age-related comorbidities are generally low. Furthermore, we did not score how physically heavy the patients’ jobs were, known to contribute to work disability. Moreover, the reason for work disability was unknown. It could have been that sick leave (< 2 years), was caused by another reason than IBD, for example pregnancy leave or psychological health problems. When comparing our data from the Dutch social security system with the more widely used Work Productivity and Activity Impairment Questionnaire (WPAI), the main difference lies within the objectivity of the data from the Dutch social security system, since it is assessed by a physician as opposed to the more subjective WPAI, which is patient reported. A main disadvantage of the data from the Dutch social security system is that it makes no distinction between work disability due to IBD or due to a different cause. Furthermore, the WPAI assesses activity, not solely work related, on a weekly basis, providing more insight in the disease course.
The strength of our study is the extended disease and patient documentation by the clinician.
In conclusion, in this study we show a higher prevalence of work disability in IBD compared to the general Dutch population. Furthermore, we identified risk factors associated with work disability, with a lower level of education being a risk factor for long-term full work disability in CD, and disease complications being a risk factor for long-term full work disability in UC. In future studies web-based follow-up of Patient-Reported Outcome Measurements (PROMs) including clinical disease activity scores, could be promising tool for detecting a decline in work activity due to disease activity.
COMMENTS
Background
Inflammatory bowel disease (IBD), consisting of Crohn’s disease (CD) and ulcerative colitis (UC) is a heterogeneous/multifaceted disease. Patients suffer from symptoms like diarrhoea, abdominal pain, fatigue, and weight loss. IBD generally makes its debut during the second or third decade of life. Therefore, patients with IBD can encounter major problems during their economically productive life, which can lead to work disability, sometimes at young age. Preventing work disability is therefore an important goal in IBD management.
Research frontiers
Work disability rates have been reported before, but reported rates are heterogeneous, ranging between 5% and 33% depending on sample size, methodology, and study population. However, in most study designs, risk factors for any form of work disability are the focus of interest, neglecting risk factors associated specifically with long-term full work disability (> 80% work disability for > 2 years). This study assesses the prevalence of work disability in patients with IBD and to compare this with the general Dutch population, and secondly this study tries to identify risk factors for work disability, especially risk factors for long-term full work disability.
Innovations and breakthroughs
In patients with IBD, the overall proportion of work disability was 29% in CD, and 19% in UC. These work disability rates were higher than those in the age-adjusted general Dutch population (7%). Suffering from (severe) IBD may in itself be explanatory for this higher percentage. Furthermore, all patients in the Dutch IBD Biobank were treated in tertiary referral centers with a severe disease course signature: 47% ileocolonic disease (L3 Montreal CD) and 58% extensive colitis (E3 Montreal UC). The authors replicated most known, clinical risk factors (female sex, an age > 40 years at diagnosis, older age at inclusion, a lower education level, extra-intestinal manifestations, a disease duration > 15 years surgical interventions, and immunomodulator use) for work disability. A lower education level was the only factor, which remained statistically significantly associated with long-term full work disability in CD. In patients with UC disease complications were associated with long-term full work disability.
Applications
This study shows that a lower education is patients with CD, and disease complications (osteopenia, thromboembolic event) in patients with UC are associated with long-term full work disability (> 80% work disability for > 2 years). This highlights the need for early assessment of risk factors for work disability, as work disability is substantial among IBD patients and associated with high societal health care costs and lower quality of life. In future studies web-based follow-up of Patient-Reported Outcome Measurements (PROMs) including clinical disease activity scores, could be a promising tool for detecting a decline in work activity due to disease activity.
Terminology
To identify risk factors for work disability we categorized the variable employment status into two groups; employed (both full time and part time) versus disabled for work (including partial disability, full disability and sick leave). To evaluate risk factors for long-term full work disability, we compared risk factors for long-term full work disability (> 80% work disability) to risk factors for partial work disability (> 35% work disability).
Peer-review
In this paper, the authors investigate the prevalence of and risk factors for partial and full work disability in Dutch patients with IBD. The manuscript is complete and well written, and it is expected to improve our knowledge of the health economics of IBD. This is a well-written paper exploring a rapidly evolving area of IBD research. The inherent limitations of this study are somewhat understandable and provide further direction to research in this important area.
ACKNOWLEDGMENTS
We would like to thank all the IBD patients who participate in the Dutch IBD Biobank. This nationwide PSI project is part of and funded by the Netherlands Federation of University Medical Centers and has received initial funding from the Dutch Government (from 2007-2011). The PSI currently facilitates the uniform nationwide collection of information on and biomaterials of thirteen other diseases.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: the Netherlands
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B, B, B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: This nationwide Parelsnoer Institute project is part of and funded by the Netherlands Federation of University Medical Centers and has received initial funding from the Dutch Government (from 2007-2011). The Parelsnoer Institute currently facilitates the uniform nationwide collection of information on and biomaterials of thirteen other diseases.
Informed consent statement: All patients included in this study gave informed consent prior to study inclusion.
Conflict-of-interest statement: All authors have no conflict of interest related to the manuscript.
Data sharing statement: Festen EAM is accepting full responsibility for the conduct of the study. This author has had access to the data and had control of the decision to publish.
Peer-review started: June 28, 2017
First decision: July 27, 2017
Article in press: September 13, 2017
P- Reviewer: Ahluwalia NK, Annese V, Blonski W, Brzozowski T, Jadallah KA, Limdi JKK, Sergi CM, Lakatos PL S- Editor: Ma YJ L- Editor: A E- Editor: Ma YJ
Contributor Information
Lieke M Spekhorst, Department of Gastroenterology and Hepatology, University of Groningen and University Medical Centre Groningen, Groningen, 9700 RB Groningen, the Netherlands; Department of Genetics, University of Groningen and University Medical Centre Groningen, Groningen, 9700 RB Groningen, the Netherlands.
Bas Oldenburg, Department of Gastroenterology and Hepatology, University Medical Center Utrecht, Utrecht, 3584 CX Utrecht, the Netherlands.
Ad A van Bodegraven, Department of Gastroenterology and Hepatology, VU University Medical Center, Amsterdam, 1081 HV Amsterdam, the Netherlands.
Dirk J de Jong, Department of Gastroenterology and Hepatology, University Medical Center St. Radboud, Nijmegen, 6525 GA Nijmegen, the Netherlands.
Floris Imhann, Department of Gastroenterology and Hepatology, University of Groningen and University Medical Centre Groningen, Groningen, 9700 RB Groningen, the Netherlands; Department of Genetics, University of Groningen and University Medical Centre Groningen, Groningen, 9700 RB Groningen, the Netherlands.
Andrea E van der Meulen-de Jong, Department of Gastroenterology and Hepatology, Leiden University Medical Center, Leiden, 2333 ZA Leiden, the Netherlands.
Marieke J Pierik, Department of Gastroenterology and Hepatology, University Medical Center Maastricht, Maastricht, 6229 HX Maastricht, the Netherlands.
Janneke C van der Woude, Department of Gastroenterology and Hepatology, Erasmus Medical Center, the Netherlands, Rotterdam, 3015 CE Rotterdam, the Netherlands.
Gerard Dijkstra, Department of Gastroenterology and Hepatology, University of Groningen and University Medical Centre Groningen, Groningen, 9700 RB Groningen, the Netherlands.
Geert D’Haens, Department of Gastroenterology and Hepatology, Amsterdam Medical Center, Amsterdam, 1105 AZ Amsterdam-Zuidoost, the Netherlands.
Mark Löwenberg, Department of Gastroenterology and Hepatology, Amsterdam Medical Center, Amsterdam, 1105 AZ Amsterdam-Zuidoost, the Netherlands.
Rinse K Weersma, Department of Gastroenterology and Hepatology, University of Groningen and University Medical Centre Groningen, Groningen, 9700 RB Groningen, the Netherlands.
Eleonora A M Festen, Department of Gastroenterology and Hepatology, University of Groningen and University Medical Centre Groningen, Groningen, 9700 RB Groningen, the Netherlands. e.a.m.festen@umcg.nl; Department of Genetics, University of Groningen and University Medical Centre Groningen, Groningen, 9700 RB Groningen, the Netherlands.
References
- 1.Pariente B, Cosnes J, Danese S, Sandborn WJ, Lewin M, Fletcher JG, Chowers Y, D’Haens G, Feagan BG, Hibi T, et al. Development of the Crohn’s disease digestive damage score, the Lémann score. Inflamm Bowel Dis. 2011;17:1415–1422. doi: 10.1002/ibd.21506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casellas F, Arenas JI, Baudet JS, Fábregas S, García N, Gelabert J, Medina C, Ochotorena I, Papo M, Rodrigo L, et al. Impairment of health-related quality of life in patients with inflammatory bowel disease: a Spanish multicenter study. Inflamm Bowel Dis. 2005;11:488–496. doi: 10.1097/01.mib.0000159661.55028.56. [DOI] [PubMed] [Google Scholar]
- 3.Russel MG, Pastoor CJ, Brandon S, Rijken J, Engels LG, van der Heijde DM, Stockbrügger RW. Validation of the Dutch translation of the Inflammatory Bowel Disease Questionnaire (IBDQ): a health-related quality of life questionnaire in inflammatory bowel disease. Digestion. 1997;58:282–288. doi: 10.1159/000201455. [DOI] [PubMed] [Google Scholar]
- 4.Stark R, König HH, Leidl R. Costs of inflammatory bowel disease in Germany. Pharmacoeconomics. 2006;24:797–814. doi: 10.2165/00019053-200624080-00006. [DOI] [PubMed] [Google Scholar]
- 5.Lichtenstein GR, Yan S, Bala M, Hanauer S. Remission in patients with Crohn’s disease is associated with improvement in employment and quality of life and a decrease in hospitalizations and surgeries. Am J Gastroenterol. 2004;99:91–96. doi: 10.1046/j.1572-0241.2003.04010.x. [DOI] [PubMed] [Google Scholar]
- 6.Feagan BG, Bala M, Yan S, Olson A, Hanauer S. Unemployment and disability in patients with moderately to severely active Crohn’s disease. J Clin Gastroenterol. 2005;39:390–395. doi: 10.1097/01.mcg.0000159220.70290.41. [DOI] [PubMed] [Google Scholar]
- 7.Bernklev T, Jahnsen J, Henriksen M, Lygren I, Aadland E, Sauar J, Schulz T, Stray N, Vatn M, Moum B. Relationship between sick leave, unemployment, disability, and health-related quality of life in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:402–412. doi: 10.1097/01.MIB.0000218762.61217.4a. [DOI] [PubMed] [Google Scholar]
- 8.Boonen A, Dagnelie PC, Feleus A, Hesselink MA, Muris JW, Stockbrügger RW, Russel MG. The impact of inflammatory bowel disease on labor force participation: results of a population sampled case-control study. Inflamm Bowel Dis. 2002;8:382–389. doi: 10.1097/00054725-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 9.De Boer AG, Bennebroek Evertsz’ F, Stokkers PC, Bockting CL, Sanderman R, Hommes DW, Sprangers MA, Frings-Dresen MH. Employment status, difficulties at work and quality of life in inflammatory bowel disease patients. Eur J Gastroenterol Hepatol. 2016;28:1130–1136. doi: 10.1097/MEG.0000000000000685. [DOI] [PubMed] [Google Scholar]
- 10.van der Valk ME, Mangen MJ, Leenders M, Dijkstra G, van Bodegraven AA, Fidder HH, de Jong DJ, Pierik M, van der Woude CJ, Romberg-Camps MJ, et al. Risk factors of work disability in patients with inflammatory bowel disease--a Dutch nationwide web-based survey: work disability in inflammatory bowel disease. J Crohns Colitis. 2014;8:590–597. doi: 10.1016/j.crohns.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 11.Høivik ML, Moum B, Solberg IC, Henriksen M, Cvancarova M, Bernklev T; IBSEN Group. Work disability in inflammatory bowel disease patients 10 years after disease onset: results from the IBSEN Study. Gut. 2013;62:368–375. doi: 10.1136/gutjnl-2012-302311. [DOI] [PubMed] [Google Scholar]
- 12.Mandel MD, Bálint A, Lovász BD, Gulácsi L, Strbák B, Golovics PA, Farkas K, Kürti Z, Szilágyi BK, Mohás A, et al. Work disability and productivity loss in patients with inflammatory bowel diseases in Hungary in the era of biologics. Eur J Health Econ. 2014;15 Suppl 1:S121–S128. doi: 10.1007/s10198-014-0603-7. [DOI] [PubMed] [Google Scholar]
- 13.Vester-Andersen MK, Prosberg MV, Vind I, Andersson M, Jess T, Bendtsen F. Low Risk of Unemployment, Sick Leave, and Work Disability Among Patients with Inflammatory Bowel Disease: A 7-year Follow-up Study of a Danish Inception Cohort. Inflamm Bowel Dis. 2015;21:2296–2303. doi: 10.1097/MIB.0000000000000493. [DOI] [PubMed] [Google Scholar]
- 14.Neovius M, Arkema EV, Blomqvist P, Ekbom A, Smedby KE. Patients with ulcerative colitis miss more days of work than the general population, even following colectomy. Gastroenterology. 2013;144:536–543. doi: 10.1053/j.gastro.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Netjes JE, Rijken M. Labor participation among patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:81–91. doi: 10.1002/ibd.22921. [DOI] [PubMed] [Google Scholar]
- 16.Siebert U, Wurm J, Gothe RM, Arvandi M, Vavricka SR, von Känel R, Begré S, Sulz MC, Meyenberger C, Sagmeister M; Swiss IBD Cohort Study Group. Predictors of temporary and permanent work disability in patients with inflammatory bowel disease: results of the swiss inflammatory bowel disease cohort study. Inflamm Bowel Dis. 2013;19:847–855. doi: 10.1097/MIB.0b013e31827f278e. [DOI] [PubMed] [Google Scholar]
- 17.CBS StatLine - Arbeidsongeschiktheidsuitkering per wet; kenmerken uitkeringsontvanger. [cited 2017 Feb 14] Available from: http://statline.cbs.nl/Statweb/publication/?DM=SLNLPA=80904NEDD1=0D2=1-2D3=1-5D4=0D5=0D6=4HDR=TSTB=G1, G2,G3,G4,G5VW=T.
- 18.CBS StatLine - Arbeidsdeelname; kerncijfers. [cited 2017 Feb 14] Available from: http://statline.cbs.nl/Statweb/publication/?DM=SLNLPA=82309NEDD1=0D2=1-2D3=1, 4-6,9D4=0D5=59HDR=G4STB=G1,G2,G3,TVW=T.
- 19.Data Analysis and Statistical Software | Stata. [cited 2017 Feb 14] Available from: http://www.stata.com/
- 20.Olivera P, Spinelli A, Gower-Rousseau C, Danese S, Peyrin-Biroulet L. Surgical rates in the era of biological therapy: up, down or unchanged? Curr Opin Gastroenterol. 2017;33:246–253. doi: 10.1097/MOG.0000000000000361. [DOI] [PubMed] [Google Scholar]
- 21.Meijs S, Gardenbroek TJ, Sprangers MA, Bemelman WA, Buskens CJ, D’Haens GR, Löwenberg M. Health-related quality of life and disability in patients with ulcerative colitis and proctocolectomy with ileoanal pouch versus treatment with anti-TNF agents. J Crohns Colitis. 2014;8:686–692. doi: 10.1016/j.crohns.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 22.van Gennep S, Sahami S, Buskens CJ, van den Brink GR, Ponsioen CY, D’Hoore A, de Buck van Overstraeten A, van Assche G, Ferrante M, Vermeire S, et al. Comparison of health-related quality of life and disability in ulcerative colitis patients following restorative proctocolectomy with ileal pouch-anal anastomosis versus anti-tumor necrosis factor therapy. Eur J Gastroenterol Hepatol. 2017;29:338–344. doi: 10.1097/MEG.0000000000000798. [DOI] [PubMed] [Google Scholar]
- 23.LIRIC-trial [Internet] 2016 [cited 2017 May 27] Available from: https://www.crohn-colitis.nl/wp-content/uploads/2016/09/LIRIC-trial.pdf.
- 24.Cosnes J. Smoking, physical activity, nutrition and lifestyle: environmental factors and their impact on IBD. Dig Dis. 2010;28:411–417. doi: 10.1159/000320395. [DOI] [PubMed] [Google Scholar]
- 25.Bastida G, Beltrán B. Ulcerative colitis in smokers, non-smokers and ex-smokers. World J Gastroenterol. 2011;17:2740–2747. doi: 10.3748/wjg.v17.i22.2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stjernman H, Tysk C, Almer S, Ström M, Hjortswang H. Unfavourable outcome for women in a study of health-related quality of life, social factors and work disability in Crohn’s disease. Eur J Gastroenterol Hepatol. 2011;23:671–679. doi: 10.1097/MEG.0b013e328346f622. [DOI] [PubMed] [Google Scholar]
- 27.Longobardi T, Jacobs P, Bernstein CN. Work losses related to inflammatory bowel disease in the United States: results from the National Health Interview Survey. Am J Gastroenterol. 2003;98:1064–1072. doi: 10.1111/j.1572-0241.2003.07285.x. [DOI] [PubMed] [Google Scholar]


