Abstract
Background
Integrating molecularly targeted agents with cytotoxic drugs used in curative treatment of pediatric cancers is complex. An evaluation was undertaken with the ERBB3/Her3 specific antibody patritumab (P) either alone or with the ERBB1/EGFR inhibitor erlotinib (E) in combination with standard cytotoxic agents, cisplatin, vincristine and cyclophosphamide in pediatric sarcoma xenograft models that express receptors and ligands targeted by these agents.
Procedures
Tumor models were selected based upon ERBB3 expression and phosphorylation, and ligand (heregulin) expression. Patritumab, E or these agents combined was evaluated without or with concomitant cytotoxic agents using procedures developed by the Pediatric Preclinical Testing Program (PPTP).
Results
Full doses of cytotoxic agents were tolerated when combined with P, whereas dose reductions of 25% (vincristine, cisplatin) or 50% (cyclophosphamide) were required when combined with P+E. Patritumab, and E alone or in combination did not significantly inhibit growth of any tumor model, except for Rh18 xenografts (E alone). Patritumab had no single agent activity and marginally enhanced the activity of vincristine and cisplatin only in Ewing sarcoma ES-4. P+E did not increase the antitumor activity of vincristine or cisplatin, whereas dose-reduced cyclophosphamide was significantly less active tan cyclophosphamide administered at its Maximum Tolerated Dose (MTD) when combined with P+E.
Conclusions
P had no single agent activity although it marginally potentiated the activity of vincristine and cisplatin in 1 of 3 models studied. However, addition of E necessitated dose reduction of each cytotoxic agent, abrogating the enhancement observed with P alone.
Introduction
The role of epidermal growth factor receptor (EGFR) family of receptors (ERBB1-4) has been extensively characterized in adult cancers, but less so in pediatric cancers, the exception being in brain tumors [1–3]. In medulloblastoma, co-expression of ERBB2 and ERBB4 occurs in 10–50% of high-grade glial cells [3] and co-expression demonstrated independent prognostic significance [4]. ERBB1 is expressed in glioma, neuroblastoma, rhabdomyosarcoma and osteosarcoma [2,3,5,6] while ERBB2 is expressed in rhabdomyosarcoma medulloblastoma/ATRT Wilms tumor and osteosarcoma [7], and some acute leukemias [8]. ERBB3 is expressed in rhabdomyosarcoma, particularly the alveolar subtype, and has been proposed as a therapeutic target [9] as RNAi mediated knockdown of ERBB3 in RD embryonal rhabdomyosarcoma cells suppresses proliferation. However, as noted by Gilbertson [9], overexpression of a drug target does not necessarily predict for therapeutic efficacy. For example, Norberg et al [9] proposed overexpression of the EphA2 receptor as a possible target in sarcoma. To validate EphA2 as a target, these authors cited the activity of dasatinib (which has inhibitory activity against EphA2) in osteosarcoma xenografts. Yet the tumor inhibitory activity was marginal in these models [10]. In adult cancers, therapeutic efficacy of targeted drugs such as lapatinib or trastuzumab occurs when the target gene is amplified (as in ERBB2/Her2 amplified breast cancer [11]), and where there exists an activating mutation (imatinib in treatment of chronic myelogenous leukemia; [12]). In pediatric cancers certain mutations in ALK in neuroblastoma [13] and activation of ALK through t(2;5)(p23;q35) chromosomal translocation, amplification or mutation [14] confer sensitivity to crizotinib
Although ERBB3 does not signal directly [15,16] it heterodimerizes with and allosterically activates ERBB1 and ERBB2. Expression of ERBB3 has a negative prognostic impact on survival of patients with breast or lung carcinoma [17]. Thus, ERBB3 has become a focus for targeted therapy [18]. Additionally, anti-ERBB3 therapy was also been reported to sensitize erlotinib refractory non-small cell lung cancer [19]. Further, the expression level of the ERBB3 ligand, heregulin, appears to be a biomarker for response to therapy with patritumab, an anti-ERBB3 monoclonal antibody, in NSCLC patients [20]. However, these results were based upon a small subgroup analysis and were not confirmed in the HER3-Lung study [21]
In previous studies using antibodies directed against the Type I insulin-like growth factor receptor (IGF1R), we showed several sarcoma xenografts regressed following treatment, however the majority of sarcoma (and other tumor types) were intrinsically resistant to IGF1R-targeted therapy [22,23]. While resistance to IGF1R-targeted antibodies was associated with expression of the insulin receptor [24], this did not explain the intrinsic resistance present in many tumor models. In vitro, intrinsic resistance, and acquired resistance to IGF-1R-targeted antibodies was associated with constitutive expression of ERBB-family receptors, or rapid induction of alternative growth factor receptors including ERBB1, ERBB2 and ERBB3 [25]. Using the PPTP cell line and xenograft gene expression database, we probed expression of ERBB-family members and observed high level expression of ERBB3 predominantly in alveolar rhabdomyosarcoma models and ERBB1 in embryonal rhabdomyosarcoma. Using phosphotyrosine arrays, we observed expression of activated ERBB1 and ERBB3 in many Ewing sarcoma cell lines [25]. Thus, it was of interest to evaluate the ERBB3 targeted antibody patritumab alone or combined with erlotinib, a small molecule inhibitor of ERBB1/2 [26], and to evaluate if either drug or these drugs in combination had significant antitumor activity. As development of such targeted therapeutics will involve integration with cytotoxic agents, we asked whether these agents could enhance the activity of standard of care cytotoxic therapy used in the treatment of childhood sarcomas.
Materials and Methods
Gene Expression Profiling
Relative expression of ERBB3/Her3, ERBB1 (EGFR), and Heregulin mRNA was determined on PPTP tumors and cell lines using data derived from Agilent Sureprint 3 arrays. Data was processed by selecting the median for each foreground signal. The differences caused by batch effects were addressed by quantile normalization across all arrays. For each tumor line the average of all replicates was calculated as a gene expression measure.
Pharmacodynamics Studies
In vitro, cell lines were screened for expression of phosphorylated-ERBB3, ERBB3, and Heregulin. For ES-4 cells, that showed the highest levels of phospho-ERBB3, the effect of patritumab was studied after 24 Hr exposure to 5–20 μg/mL antibody. The effect of patritumab, erlotinib or the combination was studied in vivo using ES-4 xenografts. Mice bearing ES-4 xenografts were untreated or treated with patritumab (60 mg/kg day 0, and day 3), erlotinib (100 mg/kg days 1–3) or the combination. Tumors were harvested 24 Hr after the last drug administration (i.e. 96 Hr after starting therapy).
Western Blotting
Tumor tissues and cell lines were snap frozen and immunoblotting was performed as previously described [27]. Antibodies used were against ERBB3 (D22C5 Rabbit mAb), phospho-ERBB3 (Y1289; D1B5 Rabbit mAb), Heregulin (#2573 Rabbit Ab) and GAPDH (D16H11 rabbit Mab; loading control), purchased from Cell Signaling Tecnology (Danvers, MA). Antibodies were used at 1:1000 dilutions except for GAPDH (1:5000). The protein bands were quantified using Image Studio software for western analysis. The signal was determined for each band, and GAPDH was used to normalize (band signal/GAPDH signal).
Toxicity Testing
The maximum tolerated dose (MTD) for vincristine and cyclophosphamide administered weekly for four consecutive weeks was 1 mg/kg and 150 mg/kg, respectively. The MTD for cisplatin was 4 mg/kg administered one time. Cohorts of non-tumor bearing mice (n=5) were dosed with single agents or combinations of Patritumab plus cytotoxic agent, or patritumab combined with erlotinib plus the individual cytotoxic agent. Mice were weighed daily. The dose resulting in no deaths and a maximum of 10% body weight loss was selected for further testing.
In Vivo Evaluation
Single agents and combinations were evaluated in three sarcoma models, ES-2 and ES-4 Ewing sarcomas and Rh18 embryonal rhabdomyosarcoma. Tumors were propagated as subcutaneous xenografts in female CB17 scid mice as described previously [28]. All procedures and data analysis were as described in [28]. Ten mice per treatment group were used. All experiments were conducted under protocols approved by the IACUC at UTHSCSA.
Statistical Methods
Treatment groups were compared with regard to time to event with log rank tests. Treatment groups were compared with regard to T/C assessment day with Wilcoxon tests. All statistical testing was two sided and corrected for multiple comparisons with the Bonferroni method and an experimentwise significance level of 5%. SAS Version 9.4 for Windows (SAS Institute, Cary NC) was used throughout. For all experiments tumor volume at start of treatment was similar for all treatment and control groups (mean ± SE = 0.264 ± 0.00644 Cm3; n=536).
Drug Formulation and Administration
Lyophilized patritumab was formulated in phosphate buffered saline and administered intravenously (IV) at 25 mg/kg twice weekly for 4 consecutive weeks. Erlotinib was formulated in 6% filter sterilized Captisol in water for administration and stored at 4°C for 7 days. Erlotinib was administered at 75 mg/kg orally for 28 consecutive days. Both patritumab and erlotinib were provided by Daiichi Sankyo. Cisplatin was dissolved in 0.9% NaCl and administered at 4 mg/kg within 24 hours. Vincristine and cyclophosphamide were formulated in sterile water for injection and administered at 1 mg/kg and 150 mg/kg, respectively, weekly for four consecutive weeks when administered alone or with patritumab. Doses of cisplatin, vincristine and cyclophosphamide were dose adjusted when administered in combination with patritumab combined with erlotinib.
Results
Selection of Tumor Models
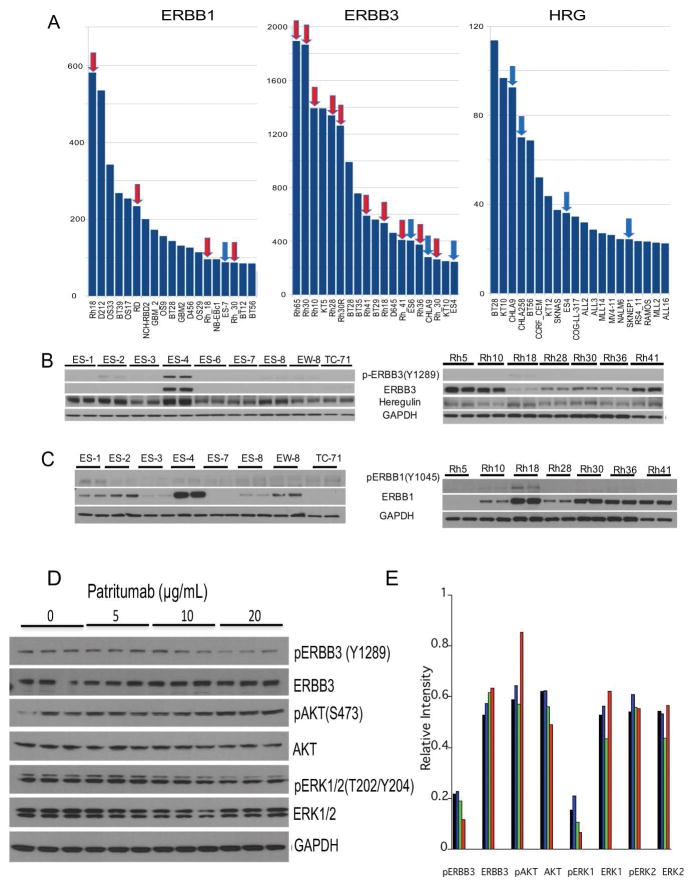
mRNA expression of ERBB3, ERBB1 and Heregulin (the ligand for ERBB3) were determined across the PPTP panels of cell lines and xenografts including solid tumors, brain tumors and acute lymphoblastic leukemias (the highest expression in 19 lines of 96 is shown), Figure 1A. Of note, of the 10 highest lines expressing ERBB3, 6 are derived from alveolar RMS xenografts. ERBB3 protein was relatively abundant in alveolar RMS lines (Rh5, Rh10, Rh41), and was detected at lower levels in other alveolar and embryonal RMS cell lines, Figure 1B. While ERBB3 was expressed at the mRNA level in several Ewing sarcoma models, ERBB3 protein was detected only in ES-4, Ewing sarcoma cells, and this line also expressed the highest level of phospho-ERBB3 (Y1289). Phospho-ERBB3 (Y1289) was detected at low levels in ES-2 and also in Rh18 although the level of ERBB3 was lower than in other rhabdomyosarcoma models. Heregulin was detected in all of the sarcoma lines examined. ERBB3 protein was barely detected in Ewing sarcoma lines other than ES-4. In contrast, RMS models Rh5, Rh10 and Rh41 expressed ERBB3 at high levels, but there was no detectable phospho-ERBB3 despite detectable levels of heregulin. ERBB1 protein was detected in 5 of 8 Ewing sarcoma cell lines, with highest expression in ES-4, and was detected in all of the RMS lines except Rh5, Figure 1C. Rh18 embryonal RMS had the highest expression of ERBB1 of all models. The effect of increasing concentrations (5–20 μg/mL) of patritumab was examined in ES-4 cells. Cells were exposed to patritumab for 24 Hr, and levels of pERBB3, pAKT, pERK1/2 and total proteins determined. As shown in Figure 1D (and quantified in Figure 1E) increasing concentrations of patritumab resulted in decreased pERBB3 (~46% decrease), pERK1 (~57%), and an increase in pAKT at the 20 μg/mL patritumab concentration (~45%).
Figure 1.
Expression of ERBB1, ERBB3 and heregulin (HRG).
A, mRNA expression levels (Y axis, arbitrary units, Agilent Sureprint G3 array) showing the 20% highest expressing lines (19/96 PPTP models) for each gene. Data derived from xenograft tissue (e.g. designated Rh30) are distinguished from cell line derived expression data (e.g. designated Rh_30). Rhabdomyosarcomas (red arrows), Ewing sarcomas (blue arrows).
B, Western blot analysis of phospho-ERBB3, ERBB3 and HRG in Ewing sarcoma and rhabdomyosarcoma cell lines. GAPDH is used as a loading control.
C, Western blot analysis of phospho-ERBB1, and ERBB1 in Ewing sarcoma and rhabdomyosarcoma cell lines. GAPDH is used as a loading control.
D, ES-4 cells were exposed to increasing concentrations of patritumab for 24 Hr. Cells were harvested and lysates probed for phospho-ERBB3, ERBB3, phospho-AKT, AKT, phospho-ERK1/2, ERK1/2. GAPDH is used as a loading control.
E, Densitometric quantitation of western blot results in C. Bands were normalized to GAPDH. Control (black), patritumab 5 μg/mL (blue), 10 μg/mL (green), 20 μg/mL (red).
Toxicity of Combinations
Patritumab was tolerated at 25 mg/kg twice weekly for 4 consecutive weeks with minimal weight loss (maximum ~2%), whereas patritumab combined with erlotinib (75 mg/kg/day) resulted in a maximum of 6% weight loss. The MTD for vincristine and cyclophosphamide administered weekly for four consecutive weeks was 1 mg/kg and 150 mg/kg, respectively. The MTD for cisplatin was 4 mg/kg administered one time. Addition of patritumab did not necessitate dose reduction for any cytotoxic agent. In contrast, when patritumab was combined with erlotinib, the doses of vincristine and cisplatin had to be reduced to 75% of the MTD, and the dose of cyclophosphamide had to be reduced to 75 mg/kg/week (50% of the MTD), Supplemental Figure S1.
Evaluation of patritumab, erlotinib or both agents combined
Based on the potential ‘activation’ of ERBB3, determined by phospho-ERBB3(Y1289), and levels of ERBB1 expression, we selected ES-4, ES-2 and Rh18 xenografts against which to evaluate patritumab and erlotinib. Statistical analysis for all studies is presented in Table 1. Additional details of testing are provided including total numbers of mice, number of mice that died (or were otherwise excluded), numbers of mice with events and average times to event, tumor growth delay, as well as numbers of responses and T/C values (Supplemental Table S1). Patritumab did not induce statistically significant growth delay in any tumor model, whereas erlotinib had slight activity reaching significance only against Rh18 embryonal rhabdomyosarcoma (P=0.016). Patritumab combined with erlotinib had very modest activity and did not delay tumor progression significantly in any model, Table 1. Statistical comparisons between patritumab and erlotinib as single agents compared to their activity in combination are also shown in Supplemental Table S2.
Table 1.
Summary of in vivo Activity of Patritumab, Erlotinib alone or in combination with Cytotoxic Agents
| Tumor Line | Treatment Group | Median Time to Event | P-value | EFS T/C | Tumor Volume T/C | EFS Activity | Response |
|---|---|---|---|---|---|---|---|
| Stage 1 | |||||||
| ES-2 | Patritumab | 20.6 | 0.525 | 0.7 | 0.97 | Low | PD1 |
| ES-2 | Erlotinib | 21.3 | 0.350 | 0.7 | 1.25 | Low | PD1 |
| ES-2 | P+E1 | 29.7 | 1.000 | 0.90 | 0.97 | Low | PD1 |
| ES-4 | Patritumab | 11.2 | 0.547 | 1.9 | 1.21 | Low | PD1 |
| ES-4 | Erlotinib | 10.8 | 0.112 | 0.9 | 0.48. | Low | PD1 |
| ES-4 | P+E | 12.3 | 1.000 | 1.0 | 0.93 | Low | PD1 |
| Rh18 | Patritumab | 12.3 | 1.000 | 1.3 | 0.84 | Low | PD1 |
| Rh18 | Erlotinib | 17.7 | 0.031 | 1.9 | 0.65 | Low | PD1 |
| Rh18 | P+E | 15.0 | 0.388 | 1.6 | 0.73 | Low | PD1 |
| Stage 2 | |||||||
| ES-2 | Cisplatin | 33.3 | 1.000 | 1.1 | 1.26 | Low | PD1 |
| ES-2 | P + Cisplatin | 38.2 | 1.000 | 1.2 | 0.95 | Low | PD1 |
| ES-2 | Vincristine | 46.0 | 1.000 | 1.5 | 0.57 | Low | PD1 |
| ES-2 | P + Vincristine | 49.3 | 1.000 | 1.6 | 1.62 | Low | PD2 |
| ES-2 | Cyclophos | . | <0.001 | >2.9 | 0.03 | High | MCR |
| ES-2 | P + Cyclophosp | . | <0.001 | >2.7 | 0.02 | High | MCR |
| ES-4 | Cisplatin | 9.6 | 1.000 | 1.1 | 0.64 | Low | PD1 |
| ES-4 | P + Cisplatin | 17.2 | 1.000 | 1.9 | 0.59 | Low | PD2 |
| ES-4 | Vincristine | 44.2 | 0.006 | 5.0 | 0.41 | High | CR |
| ES-4 | P + Vincristine | 62.3 | <0.001 | 7.0 | 0.31 | High | CR |
| ES-4 | Cyclophos | 42.0 | 0.067 | 4.7 | 0.60 | High | CR |
| ES-4 | P + Cyclophosp | 52.2 | <0.001 | 5.9 | 0.06 | High | CR |
| Rh18 | Cisplatin | 35.9 | 1.000 | 1.4 | 1.05 | Low | PD1 |
| Rh18 | P + Cisplatin | 34.8 | 1.000 | 1.4 | 1.32 | Low | PD1 |
| Rh18 | Vincristine | 40.0 | 0.247 | 1.6 | 1.07 | Low | PD2 |
| Rh18 | P + Vincristine | 39.6 | 0.080 | 1.6 | 1.22 | Low | PD2 |
| Rh18 | Cyclophos | 50.3 | <0.001 | 2.0 | 0.89 | Low | PD2 |
| Rh18 | P + Cyclophos | 44.5 | 0.001 | 1.8 | 0.96 | Low | PD2 |
| Stage 3 | |||||||
| ES-4 | Erlotinib | 9.9 | 1.000 | 1.3 | 2.2 | Low | PD1 |
| ES-4 | Vincristine | 44.2 | <0.001 | 5.6 | 0.98 | High | CR |
| ES-4 | Cyclophosphamide | 42.4 | 0.001 | 5.4 | 0.14 | High | CR |
| ES-4 | Cisplatin | 12.7 | 0.978 | 1.6 | 1.31 | Low | PD2 |
| ES-4 | P+E + Vincristine | 40.9 | 0.012 | 5.2 | 0.18 | High | CR |
| ES-4 | P+E + Cyclophos | 32.3 | 0.429 | 4.1 | 1.4 | Int | PD2 |
| ES-4 | P+E + Cisplatin | 15.6 | 1.000 | 2.0 | 0.98 | Int | PD2 |
| Rh18 | P+E | 11.5 | 1.000 | 1.1 | 0.82 | Low | PD1 |
| Rh18 | Vincristine | 31.5 | 0.022 | 3.0 | 0.44 | Int | PD2 |
| Rh18 | Cyclophosphamide | 61.4 | <0.001 | 5.8 | 0.06 | High | CR |
| Rh18 | Cisplatin | 11.3 | 1.000 | 1.1 | 0.99 | Low | PD1 |
| Rh18 | P+E + Vincristine | 53.4 | 0.006 | 5.1 | 0.37 | Int | PD2 |
| Rh18 | P+E + Cyclophos | 21.1 | 1.000 | 2.0 | 0.47 | Int | PD2 |
| Rh18 | P+E + Cisplatin | 23.0 | 0.977 | 2.2 | 0.49 | Int | SD |
P, patritumab; E, Erlotinib; cyclophos, cyclophosphamide.
Evaluation of patritumab with cytotoxic agents
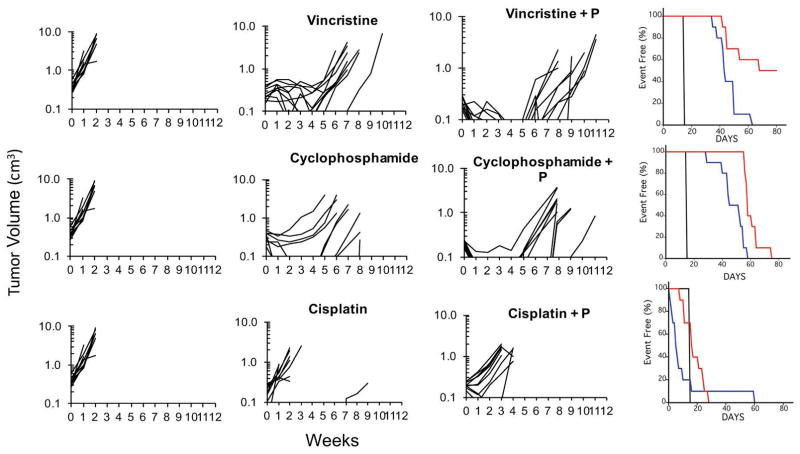
As a second phase for testing, we combined patritumab with vincristine, cisplatin or cyclophosphamide. Full doses of each cytotoxic agent were tolerated in combination with patritumab. For ES-2 xenografts only cyclophosphamide or cyclophosphamide + P significantly inhibited tumor growth (P<0.001). Tumor control for patritumab combined with vincristine was not significantly different compared to control tumor growth (Supplemental Table S2). ES-4 xenografts were more sensitive to cytotoxic agents, particularly cyclophosphamide and vincristine, Table 1. Patritumab significantly potentiated the antitumor activity of vincristine (p=0.037), and cisplatin (P= 0.008) over these agents administered at their respective MTDs (Supplemental Table S2). Patritumab also potentiated the activity for cyclophosphamide, however this did not meet significance (P=0.154). However, the effect of patritumab potentiating the activity of these cytotoxic agents was observed only in ES-4 xenografts, Figure 2.
Figure 2.
Patritumab enhances the activity of vincristine and cyclophosphamide. Mice bearing ES-4 Ewing sarcoma xenografts were treated as described in Materials and Methods. Growth of individual tumors is presented. Tumor volumes (cm3) are plotted against time (weeks) (P, patritimab). Right panels show Kaplan-Maier Event Free survival (%); control (black), cytotoxic drug (blue), cytotoxic drug + patritumab (P; red).
Evaluation of patritumab combined with erlotinib and cytotoxic agents
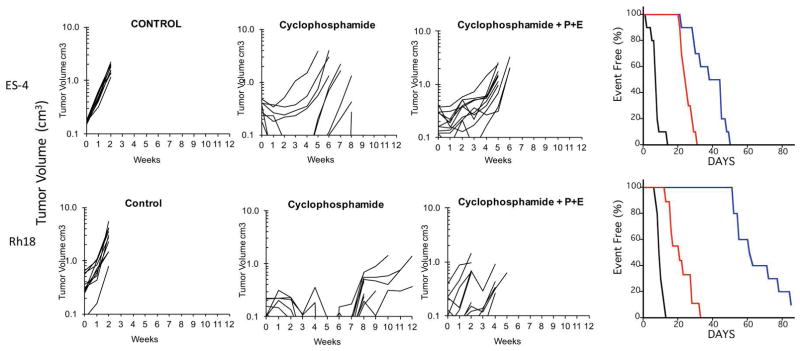
The third phase of testing combined patritumab with erlotinib (P+E) with each of the three cytotoxic agents. This required dose reduction of vincristine from 1 mg/kg/dose to 0.75 mg/kg/dose, cisplatin from 4 mg/kg to 3 mg/kg and cyclophosphamide from 150 mg/kg/dose to 75 mg/kg/dose. In ES-4 xenografts there was no significant difference between vincristine or cisplatin combined with P+E compared to the MTD for each cytotoxic agent alone (Supplemental Table S2). Similar results were obtained in the Rh18 xenograft model. However, in both ES-4 and Rh18 models the combination of P+E with dosed-reduced cyclophosphamide was significantly inferior (P=0.002, and <0.001, respectively) to cyclophosphamide administered at its MTD, Figure 3. For ES-4 xenografts cyclophosphamide administered at the MTD extended event-free survival by 5.4-fold over control (EFS 7.9 days vs 42.4 days), whereas the combination with P+E was less effective (EFS 32.3 days). Similarly, the activity of cyclophosphamide was reduced in Rh18 xenografts (EFS T/C = 5.8 for cyclophosphamide vs EFS T/C= 2.0 for P+E = cyclophosphamide; Supplemental Table S1).
Figure 3.
The antitumor activity of cyclophosphamide is compromised when combined with patritumab and erlotinib (P+E). Mice bearing ES-4 or Rh18 rhabdomyosarcoma xenografts were treated as described in Materials and Methods. Growth of individual tumors is presented Tumor volumes (cm3) are plotted against time (weeks). Right panels show Kaplan-Maier Event Free survival (%); control (black), cyclophosphamide (blue), cytotoxic drug + patritumab + erlotinib (P; red).
Pharmacodymanic Studies
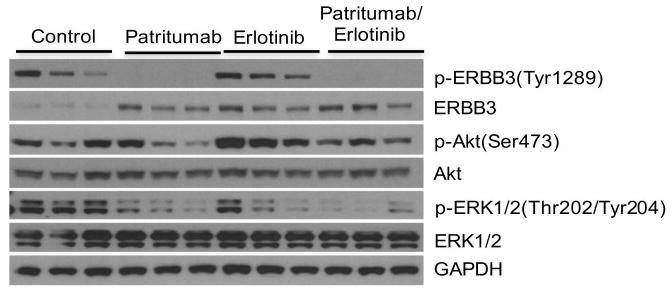
To determine whether patritumab and erlotinib, at the doses and schedules used, inhibited their molecular targets, we chose to study their effects on ES-4 xenografts as this line in vitro had the most robust phosphorylation of ERBB3. Tumor-bearing mice received patritumab (60 mg/kg) on day 0 and day 3, whereas erlotinib (75 mg/kg) was administered daily for 3 days as single agents, or the same doses and schedules for combined treatment. Tumors were excised 24 Hr after the last drug dose. As shown in Figure 4, phospho-ERBB3(Tyr1289) was detected in control tumors (n=3), with some variability, but was completely suppressed by patritumab treatment. Patritumab also decreased phospho-ERK1/2(Thr202/Tyr204), and possibly decreased phospho-Akt(Ser473) in 2 of 3 xenografts. In contrast, erlotinib did not decrease phospho-ERBB3, but decreased phosphor-ERK1/2, but also appeared to increase phosphorylation of Akt. The combination of patritumab with erlotinib completely suppressed phosphorylation of ERBB3, and dramatically reduced phosphorylation of ERK1/2. Moreover, combination with patritumab suppressed induction of phospho-Akt by erlotinib.
Figure 4.
Both patritumab and erlotinib inhibit their molecular targets in ES-4 xenografts. Mice bearing ES-4 tumors were treated with patritumab, or erlotinib, or the combination as described in Materials and Methods. Tumors (n=3) were harvested 24 Hr after the last drug administration, and processed for western blotting as described. GAPDH is used as a loading control.
Discussion
Development of targeted therapies for childhood sarcoma presents significant challenges as the genomic landscape is relatively silent with few mutations predicted to activate potential drug targets such as tyrosine kinases ([29–33], and most molecularly targeted agents are being developed for treatment of adult cancers, where the ‘target’ may not be represented in the pediatric cancer population. Further, it is unlikely that when administered as single agents, molecular targeted therapeutics will have curative activity, hence combinations of targeted agents are anticipated to have greater therapeutic value. For example combination of dabrafenib with trametinib in treatment of melanoma results in increased progression-free survival [34], and in a preclinical model trametinib-resistant astrocytoma, combination with a STAT3 inhibitor results in re-sensitization to trametinib [35]. However, combining molecularly-targeted agents with little or no single agent activity is likely to result in minimal combination activity, unless based upon some underlying synthetic lethal interaction. Development is also challenging because for many patients current multimodality treatment protocols are curative. Thus, clinical development of a novel targeted therapeutic will have to integrate with current cytotoxic drug protocols. Our prior studies in the PPTP also suggest that in the absence of a known activating event (e.g. BRAF in astrocytoma or ALK activation in neuroblastoma) the response rate for signaling inhibitors is low (reviewed in [36]). For example, lapatinib an inhibitor of ERBB2 had very modest antitumor activity with no tumor regressions in 41 xenograft models tested at dose levels of drug giving clinically relevant exposures [37]. The results of our current study further emphasize that high-level expression, or activation of a signaling pathway does not necessarily constitute a valid target for therapy, in the absence of a known activating mutation or translocation.
ERBB3 is unique to the ERB family of receptors in that evolutionary divergence within the kinase domain has led to the protein being in a constitutively inactive conformation [38–40]. Recent evidence has shown that the ERBB3 kinase domain is a highly specialized allosteric activator of its ERBB partners [16]. The rationale for combining an ERBB3 inhibitor with an ERBB1/2 inhibitor (erlotinib) is that this may inhibit dimerization and signaling through hybrid receptors and may prevent resistance to ERBB1/2 directed therapy [16]. Our studies showed that relative to other pediatric xenografts and cell lines developed in the PPTP, ERBB1 mRNA was high in embryonal rhabdomyosarcoma lines (Rh18 and RD) whereas ERBB3 mRNA was highly expressed in alveolar rhabdomyosarcoma xenografts (Rh10, Rh28, Rh30, Rh41 and Rh65), consistent with clinical samples [9]. ERBB3 protein was elevated in Rh5, Rh10 and Rh41 alveolar rhabdomyosarcoma cell lines, although phosphorylation was not detected. Based on expression of ERBB3 with or without phosphorylation, ES-4, ES-2 and Rh18 xenograft models were selected for testing. In ES-4 cells patritumab reduced pERBB3, and reduced pERK, suggesting that it decreased signaling in this cell line.
Patritumab has been shown to inhibit ERBB3 at doses similar or lower than used in this study [41]. We selected ES-4 xenografts, as this cell line showed the highest phosphorylation of ERBB3. In ES-4 xenografts, patritumab completely suppressed ERBB3 phosphorylation, confirming that at the dose and schedule used this antibody effectively inhibited its target. Erlotinib, a small molecule inhibitor of ERBB1/2, had no effect on phospho-ERBB3, as anticipated. However, evidence of target inhibition was shown by suppression of ERK1/2 downstream of the receptors targeted by this agent. Of note, erlotinib induced an increase in phospho-Akt, suggesting compensation for ERBB1/2 inhibition. Importantly, the combination of patritumab with erlotinib completely suppressed phospho-ERBB3 and ~90% decrease in phospho-ERK1/2 and prevented erlotinib induced phosphorylation of Akt. However, as discussed below, the combination had very modest effect on growth of ES-4 xenografts.
Patritumab combined with erlotinib showed promising efficacy in early phase clinical trials for treatment of NSCLC [42], and when combined with trastuzumab and paclitaxel in ERBB2 overexpressing metastatic breast cancer [17]. When tested against our pediatric sarcoma xenografts patritumab, as a single agent, was devoid of significant antitumor activity, whereas erlotinib induced marginal, although statistically significant, inhibition of Rh18 tumor growth. Erlotinib treatment induced statistically significant growth delay in only one of three models. However, the magnitude of these effects was quite marginal, and the combination of patritumab with erlotinib did not significantly retard tumor growth. Development of this combination in NSCLC as the first part of the phase 3 study did not meet pre-defined efficacy criteria, hence further development was terminated [21].
Because of the potential for erlotinib to alter the clearance of drugs such as vincristine, we chose to evaluate patritumab with cytotoxic agents before combining this agent with erlotinib and cytotoxic agents. Of interest, patritumab significantly potentiated the antitumor activity of vincristine and cisplatin in the ES-4 model. This is the only xenograft model to demonstrate robust phosphorylation of ERBB3, although whether this relates to the increased activity of these cytotoxic agents by patritumab would have to be confirmed in other models showing ERBB3 phosphorylation. In contrast, there was no potentiation for any of the drugs in ES-2 and Rh18 tumors. Combining erlotinib with patritumab necessitated reduction in doses of cisplatin, cyclophosphamide and vincristine. Erlotinib is a substrate and inhibitor of ABCB1 (P-glycoporotein) and other drug transporters [43], hence likely decreasing clearance of vincristine [44,45]. The cause of the interaction between patritumab-erlotinib and cisplatin or cyclophosphamide is less clear, but may be related to the ERB pathway regulating DNA damage repair [46,47]. Of note, addition of erlotinib to patritumab resulted in less antitumor activity than observed when these cytotoxic agents were combined with patritumab alone. This was particularly apparent for cyclophosphamide, probably as a consequence of the reduced cyclophosphamide dose tolerated, where the combination with patritumab and erlotinib was significantly less active than cyclophosphamide administered at the MTD.
Our study demonstrates the potential, but also the challenges of integrating targeted therapeutics with conventional cytotoxic therapy. While patritumab modestly potentiated the activity of each cytotoxic agent in one of three xenograft models, the addition of erlotinib necessitated dose reductions that abrogated the modest gains by combining the cytotoxic drugs with patritumab. It is of note that many ATP-competitive tyrosine kinase inhibitors are potent substrate-inhibitors of ABC drug transporters [47], hence may necessitate dose reductions in cytotoxic agents such as vincristine, doxorubicin, topotecan and irinotecan used frequently in treatment of sarcoma. Based on the results presented, patritumab with or without erlotinib has marginal activity in three sarcoma models, and the increase in activity of the cytotoxic drugs when combined with patritumab was modest and limited to the one tumor model where robust phosphorylation of ERBB3 was detected. In our preclinical study, levels of heregulin were similar in each tumor model, and did not associate with better response to patritumab alone or in combination with erlotinib, hence these preclinical results are consistent with the NSCLC clinical trials [21]. In our study, including erlotinib with patritumab led to a decrease in the therapeutic activity of cyclophosphamide when compared to single agent activity when administered at full dose levels (i.e. MTD). These data emphasize the need for a clear understanding the interaction of chemotherapy and targeted therapy combinations prior to clinical evaluation in pediatric cancer patients.
Supplementary Material
Supplemental Figure S1. Animal body weight measurements (Body weight day X/body weight day 0%) × 100 where day 0 = pretreatment weight) for cytotoxic agents combined with patritumab and erlotinib.
Supplemental Appendix I Response and Event Definitions for Solid Tumor Xenograft Models
Supplemental Table S1. Efficacy of patritumab (P), erlotinib (E) alone or in combination with vincristine, cyclophosphamide or cisplatin against sarcoma models.
Supplemental Table S2. Summary of statistical differences between treatment groups.
Acknowledgments
This work was supported by PHS awards PO1 CA165995, cancer center support grant P30 CA054174 from the National Cancer Institute and by the Daiichi Sankyo Company Limited.
Abbreviations
- ERBB1
Epidermal growth factor receptor (EGFR)
- ERBB2
Her2
- ERBB3
Her3
- HRG
Heregulin
- P
Patritumab
- E
Erlotinib
- NSCLC
Non-small cell lung cancer
- MTD
Maximum Tolerated Dose
Footnotes
Conflict of Interest: Kenji Hirotani MD, and Ling Zhang MD are employees of Daiichi Sankyo Company Limited.
References
- 1.Bodey B, Kaiser HE, Siegel SE. Epidermal growth factor receptor (EGFR) expression in childhood brain tumors. In vivo. 2005;19(5):931–941. [PubMed] [Google Scholar]
- 2.Bredel M, Pollack IF, Hamilton RL, James CD. Epidermal growth factor receptor expression and gene amplification in high-grade non-brainstem gliomas of childhood. Clinical cancer research : an official journal of the American Association for Cancer Research. 1999;5(7):1786–1792. [PubMed] [Google Scholar]
- 3.Gilbertson RJ, Bentley L, Hernan R, Junttila TT, Frank AJ, Haapasalo H, Connelly M, Wetmore C, Curran T, Elenius K, Ellison DW. ERBB receptor signaling promotes ependymoma cell proliferation and represents a potential novel therapeutic target for this disease. Clinical cancer research : an official journal of the American Association for Cancer Research. 2002;8(10):3054–3064. [PubMed] [Google Scholar]
- 4.Gilbertson RJ, Perry RH, Kelly PJ, Pearson AD, Lunec J. Prognostic significance of HER2 and HER4 coexpression in childhood medulloblastoma. Cancer research. 1997;57(15):3272–3280. [PubMed] [Google Scholar]
- 5.Hughes DP, Thomas DG, Giordano TJ, Baker LH, McDonagh KT. Cell surface expression of epidermal growth factor receptor and Her-2 with nuclear expression of Her-4 in primary osteosarcoma. Cancer research. 2004;64(6):2047–2053. doi: 10.1158/0008-5472.can-03-3096. [DOI] [PubMed] [Google Scholar]
- 6.Tamura S, Hosoi H, Kuwahara Y, Kikuchi K, Otabe O, Izumi M, Tsuchiya K, Iehara T, Gotoh T, Sugimoto T. Induction of apoptosis by an inhibitor of EGFR in neuroblastoma cells. Biochemical and biophysical research communications. 2007;358(1):226–232. doi: 10.1016/j.bbrc.2007.04.124. [DOI] [PubMed] [Google Scholar]
- 7.Saletta F, Wadham C, Ziegler DS, Marshall GM, Haber M, McCowage G, Norris MD, Byrne JA. Molecular profiling of childhood cancer: Biomarkers and novel therapies. BBA clinical. 2014;1:59–77. doi: 10.1016/j.bbacli.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilbertson RJ. ERBB2 in pediatric cancer: innocent until proven guilty. The oncologist. 2005;10(7):508–517. doi: 10.1634/theoncologist.10-7-508. [DOI] [PubMed] [Google Scholar]
- 9.Nordberg J, Mpindi JP, Iljin K, Pulliainen AT, Kallajoki M, Kallioniemi O, Elenius K, Elenius V. Systemic analysis of gene expression profiles identifies ErbB3 as a potential drug target in pediatric alveolar rhabdomyosarcoma. PloS one. 2012;7(12):e50819. doi: 10.1371/journal.pone.0050819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kolb EA, Gorlick R, Houghton PJ, Morton CL, Lock RB, Tajbakhsh M, Reynolds CP, Maris JM, Keir ST, Billups CA, Smith MA. Initial testing of dasatinib by the pediatric preclinical testing program. Pediatric blood & cancer. 2008;50(6):1198–1206. doi: 10.1002/pbc.21368. [DOI] [PubMed] [Google Scholar]
- 11.Seidman AD, Fornier MN, Esteva FJ, Tan L, Kaptain S, Bach A, Panageas KS, Arroyo C, Valero V, Currie V, Gilewski T, Theodoulou M, Moynahan ME, Moasser M, Sklarin N, Dickler M, D’Andrea G, Cristofanilli M, Rivera E, Hortobagyi GN, et al. Weekly trastuzumab and paclitaxel therapy for metastatic breast cancer with analysis of efficacy by HER2 immunophenotype and gene amplification. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001;19(10):2587–2595. doi: 10.1200/JCO.2001.19.10.2587. [DOI] [PubMed] [Google Scholar]
- 12.Mahon FX. Discontinuation of TKI therapy and ‘functional’ cure for CML. Best practice & research Clinical haematology. 2016;29(3):308–313. doi: 10.1016/j.beha.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 13.Bresler SC, Wood AC, Haglund EA, Courtright J, Belcastro LT, Plegaria JS, Cole K, Toporovskaya Y, Zhao H, Carpenter EL, Christensen JG, Maris JM, Lemmon MA, Mosse YP. Differential inhibitor sensitivity of anaplastic lymphoma kinase variants found in neuroblastoma. Science translational medicine. 2011;3(108):108ra114. doi: 10.1126/scitranslmed.3002950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christensen JG, Zou HY, Arango ME, Li Q, Lee JH, McDonnell SR, Yamazaki S, Alton GR, Mroczkowski B, Los G. Cytoreductive antitumor activity of PF-2341066, a novel inhibitor of anaplastic lymphoma kinase and c-Met, in experimental models of anaplastic large-cell lymphoma. Molecular cancer therapeutics. 2007;6(12 Pt 1):3314–3322. doi: 10.1158/1535-7163.MCT-07-0365. [DOI] [PubMed] [Google Scholar]
- 15.Amin DN, Campbell MR, Moasser MM. The role of HER3, the unpretentious member of the HER family, in cancer biology and cancer therapeutics. Seminars in cell & developmental biology. 2010;21(9):944–950. doi: 10.1016/j.semcdb.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell MR, Amin D, Moasser MM. HER3 comes of age: new insights into its functions and role in signaling, tumor biology, and cancer therapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16(5):1373–1383. doi: 10.1158/1078-0432.CCR-09-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukai H, Saeki T, Aogi K, Naito Y, Matsubara N, Shigekawa T, Ueda S, Takashima S, Hara F, Yamashita T, Ohwada S, Sasaki Y. Patritumab plus trastuzumab and paclitaxel in human epidermal growth factor receptor 2-overexpressing metastatic breast cancer. Cancer science. 2016;107(10):1465–1470. doi: 10.1111/cas.13017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horinouchi H. The prospect of patritumab for treating non-small cell lung cancer. Expert opinion on biological therapy. 2016;16(12):1549–1555. doi: 10.1080/14712598.2016.1249846. [DOI] [PubMed] [Google Scholar]
- 19.Yonesaka K, Hirotani K, Kawakami H, Takeda M, Kaneda H, Sakai K, Okamoto I, Nishio K, Janne PA, Nakagawa K. Anti-HER3 monoclonal antibody patritumab sensitizes refractory non-small cell lung cancer to the epidermal growth factor receptor inhibitor erlotinib. Oncogene. 2016;35(7):878–886. doi: 10.1038/onc.2015.142. [DOI] [PubMed] [Google Scholar]
- 20.Mendell J, Freeman DJ, Feng W, Hettmann T, Schneider M, Blum S, Ruhe J, Bange J, Nakamaru K, Chen S, Tsuchihashi Z, von Pawel J, Copigneaux C, Beckman RA. Clinical Translation and Validation of a Predictive Biomarker for Patritumab, an Anti-human Epidermal Growth Factor Receptor 3 (HER3) Monoclonal Antibody, in Patients With Advanced Non-small Cell Lung Cancer. EBioMedicine. 2015;2(3):264–271. doi: 10.1016/j.ebiom.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paz-Ares L, Serwatowski P, Szczesna A, von Pawel J, Toschi L, Csoszi T, Morabito A, Zhang L, Shuster D, Chen S, Copigneaux C, WLA Patritumab Plus Erlotinib in EGFR Wild-type Advanced Non–Small Cell Lung Cancer (NSCLC): Part A Results of HER3-Lung Study. Journal of Thoracic Oncology. 2016;12(1):S1214–S1215. [Google Scholar]
- 22.Houghton PJ, Morton CL, Gorlick R, Kolb EA, Keir ST, Reynolds CP, Kang MH, Maris JM, Wu J, Smith MA. Initial testing of a monoclonal antibody (IMC-A12) against IGF-1R by the Pediatric Preclinical Testing Program. Pediatric blood & cancer. 2010;54(7):921–926. doi: 10.1002/pbc.22367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolb EA, Gorlick R, Houghton PJ, Morton CL, Lock R, Carol H, Reynolds CP, Maris JM, Keir ST, Billups CA, Smith MA. Initial testing (stage 1) of a monoclonal antibody (SCH 717454) against the IGF-1 receptor by the pediatric preclinical testing program. Pediatric blood & cancer. 2008;50(6):1190–1197. doi: 10.1002/pbc.21450. [DOI] [PubMed] [Google Scholar]
- 24.Forest A, Amatulli M, Ludwig DL, Damoci CB, Wang Y, Burns CA, Donoho GP, Zanella N, Fiebig HH, Prewett MC, Surguladze D, DeLigio JT, Houghton PJ, Smith MA, Novosiadly R. Intrinsic Resistance to Cixutumumab Is Conferred by Distinct Isoforms of the Insulin Receptor. Molecular cancer research : MCR. 2015;13(12):1615–1626. doi: 10.1158/1541-7786.MCR-15-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shackleford TJ, Hariharan S, Bid HK, Houghton PJ. Mechanisms of resistance to IGFR-targeted therapy in pediatric sarcomas. Proceedings AACR. 2016;107 Abstr 2472. [Google Scholar]
- 26.Singh D, Attri BK, Gill RK, Bariwal J. Review on EGFR Inhibitors: Critical Updates. Mini Rev Med Chem. 2016;16(14):1134–1166. doi: 10.2174/1389557516666160321114917. [DOI] [PubMed] [Google Scholar]
- 27.Studebaker A, Bondra K, Seum S, Shen C, Phelps DA, Chronowski C, Leasure J, Smith PD, Kurmasheva RT, Mo X, Fouladi M, Houghton PJ. Inhibition of MEK confers hypersensitivity to X-radiation in the context of BRAF mutation in a model of childhood astrocytoma. Pediatric blood & cancer. 2015;62(10):1768–1774. doi: 10.1002/pbc.25579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Houghton PJ, Morton CL, Tucker C, Payne D, Favours E, Cole C, Gorlick R, Kolb EA, Zhang W, Lock R, Carol H, Tajbakhsh M, Reynolds CP, Maris JM, Courtright J, Keir ST, Friedman HS, Stopford C, Zeidner J, Wu J, et al. The pediatric preclinical testing program: description of models and early testing results. Pediatric blood & cancer. 2007;49(7):928–940. doi: 10.1002/pbc.21078. [DOI] [PubMed] [Google Scholar]
- 29.Shern JF, Chen L, Chmielecki J, Wei JS, Patidar R, Rosenberg M, Ambrogio L, Auclair D, Wang J, Song YK, Tolman C, Hurd L, Liao H, Zhang S, Bogen D, Brohl AS, Sindiri S, Catchpoole D, Badgett T, Getz G, et al. Comprehensive genomic analysis of rhabdomyosarcoma reveals a landscape of alterations affecting a common genetic axis in fusion-positive and fusion-negative tumors. Cancer Discov. 2014;4(2):216–231. doi: 10.1158/2159-8290.CD-13-0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brohl AS, Solomon DA, Chang W, Wang J, Song Y, Sindiri S, Patidar R, Hurd L, Chen L, Shern JF, Liao H, Wen X, Gerard J, Kim JS, Lopez Guerrero JA, Machado I, Wai DH, Picci P, Triche T, Horvai AE, et al. The genomic landscape of the Ewing Sarcoma family of tumors reveals recurrent STAG2 mutation. PLoS Genet. 2014;10(7):e1004475. doi: 10.1371/journal.pgen.1004475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hingorani P, Janeway K, Crompton BD, Kadoch C, Mackall CL, Khan J, Shern JF, Schiffman J, Mirabello L, Savage SA, Ladanyi M, Meltzer P, Bult CJ, Adamson PC, Lupo PJ, Mody R, DuBois SG, Parsons DW, Khanna C, Lau C, et al. Current state of pediatric sarcoma biology and opportunities for future discovery: A report from the sarcoma translational research workshop. Cancer Genet. 2016;209(5):182–194. doi: 10.1016/j.cancergen.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kovac M, Blattmann C, Ribi S, Smida J, Mueller NS, Engert F, Castro-Giner F, Weischenfeldt J, Kovacova M, Krieg A, Andreou D, Tunn PU, Durr HR, Rechl H, Schaser KD, Melcher I, Burdach S, Kulozik A, Specht K, Heinimann K, et al. Exome sequencing of osteosarcoma reveals mutation signatures reminiscent of BRCA deficiency. Nat Commun. 2015;6:8940. doi: 10.1038/ncomms9940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crompton BD, Stewart C, Taylor-Weiner A, Alexe G, Kurek KC, Calicchio ML, Kiezun A, Carter SL, Shukla SA, Mehta SS, Thorner AR, de Torres C, Lavarino C, Sunol M, McKenna A, Sivachenko A, Cibulskis K, Lawrence MS, Stojanov P, Rosenberg M, et al. The genomic landscape of pediatric Ewing sarcoma. Cancer Discov. 2014;4(11):1326–1341. doi: 10.1158/2159-8290.CD-13-1037. [DOI] [PubMed] [Google Scholar]
- 34.Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, Lichinitser M, Dummer R, Grange F, Mortier L, Chiarion-Sileni V, Drucis K, Krajsova I, Hauschild A, Lorigan P, Wolter P, Long GV, Flaherty K, Nathan P, Ribas A, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372(1):30–39. doi: 10.1056/NEJMoa1412690. [DOI] [PubMed] [Google Scholar]
- 35.Bid HK, Kibler A, Phelps DA, Manap S, Xiao L, Lin J, Capper D, Oswald D, Geier B, DeWire M, Smith PD, Kurmasheva RT, Mo X, Fernandez S, Houghton PJ. Development, characterization, and reversal of acquired resistance to the MEK1 inhibitor selumetinib (AZD6244) in an in vivo model of childhood astrocytoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19(24):6716–6729. doi: 10.1158/1078-0432.CCR-13-0842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murphy B, Yin H, Maris JM, Kolb EA, Gorlick R, Reynolds CP, Kang MH, Keir ST, Kurmasheva RT, Dvorchik I, Wu J, Billups CA, Boateng N, Smith MA, Lock RB, Houghton PJ. Evaluation of Alternative In Vivo Drug Screening Methodology: A Single Mouse Analysis. Cancer research. 2016;76(19):5798–5809. doi: 10.1158/0008-5472.CAN-16-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gorlick R, Kolb EA, Houghton PJ, Morton CL, Phelps D, Schaiquevich P, Stewart C, Keir ST, Lock R, Carol H, Reynolds CP, Maris JM, Wu J, Smith MA. Initial testing (stage 1) of lapatinib by the pediatric preclinical testing program. Pediatric blood & cancer. 2009;53(4):594–598. doi: 10.1002/pbc.21989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guy PM, Platko JV, Cantley LC, Cerione RA, Carraway KL., 3rd Insect cell-expressed p180erbB3 possesses an impaired tyrosine kinase activity. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(17):8132–8136. doi: 10.1073/pnas.91.17.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sierke SL, Cheng K, Kim HH, Koland JG. Biochemical characterization of the protein tyrosine kinase homology domain of the ErbB3 (HER3) receptor protein. The Biochemical journal. 1997;322( Pt 3):757–763. doi: 10.1042/bj3220757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jura N, Shan Y, Cao X, Shaw DE, Kuriyan J. Structural analysis of the catalytically inactive kinase domain of the human EGF receptor 3. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(51):21608–21613. doi: 10.1073/pnas.0912101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li C, Brand TM, Iida M, Huang S, Armstrong EA, van der Kogel A, Wheeler DL. Human epidermal growth factor receptor 3 (HER3) blockade with U3-1287/AMG888 enhances the efficacy of radiation therapy in lung and head and neck carcinoma. Discov Med. 2013;16(87):79–92. [PMC free article] [PubMed] [Google Scholar]
- 42.Nishio M, Horiike A, Murakami H, Yamamoto N, Kaneda H, Nakagawa K, Horinouchi H, Nagashima M, Sekiguchi M, Tamura T. Phase I study of the HER3-targeted antibody patritumab (U3-1287) combined with erlotinib in Japanese patients with non-small cell lung cancer. Lung cancer. 2015;88(3):275–281. doi: 10.1016/j.lungcan.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 43.Duckett DR, Cameron MD. Metabolism considerations for kinase inhibitors in cancer treatment. Expert opinion on drug metabolism & toxicology. 2010;6(10):1175–1193. doi: 10.1517/17425255.2010.506873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harmsen S, Meijerman I, Beijnen JH, Schellens JH. The role of nuclear receptors in pharmacokinetic drug-drug interactions in oncology. Cancer treatment reviews. 2007;33(4):369–380. doi: 10.1016/j.ctrv.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 45.Noguchi K, Kawahara H, Kaji A, Katayama K, Mitsuhashi J, Sugimoto Y. Substrate-dependent bidirectional modulation of P-glycoprotein-mediated drug resistance by erlotinib. Cancer science. 2009;100(9):1701–1707. doi: 10.1111/j.1349-7006.2009.01213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li L, Wang H, Yang ES, Arteaga CL, Xia F. Erlotinib attenuates homologous recombinational repair of chromosomal breaks in human breast cancer cells. Cancer research. 2008;68(22):9141–9146. doi: 10.1158/0008-5472.CAN-08-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mahajan K, Mahajan NP. Cross talk of tyrosine kinases with the DNA damage signaling pathways. Nucleic acids research. 2015;43(22):10588–10601. doi: 10.1093/nar/gkv1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1. Animal body weight measurements (Body weight day X/body weight day 0%) × 100 where day 0 = pretreatment weight) for cytotoxic agents combined with patritumab and erlotinib.
Supplemental Appendix I Response and Event Definitions for Solid Tumor Xenograft Models
Supplemental Table S1. Efficacy of patritumab (P), erlotinib (E) alone or in combination with vincristine, cyclophosphamide or cisplatin against sarcoma models.
Supplemental Table S2. Summary of statistical differences between treatment groups.