Abstract
Background
Allergy and allergic asthma are significant health burdens in developed countries and are increasing in prevalence. Dendritic cells (DCs) initiate immune responses to common aeroallergens and ADAM10 has been demonstrated to be important for the development of adaptive responses. This study’s objective was to understand the role of ADAM10 on DCs in the development of allergic and anaphylactic responses.
Methods
In this study we used mouse models of allergic airway inflammation (house dust mice and Alternaria alternata) and OVA-induced models of active anaphylaxis to determine the DC-specific function of ADAM10 and Notch signaling. To examine TH1 and TH17 immunity infection with Anaplasma phagocytophilum and Citrobacter rodentium were used.
Results
Mice, which have ADAM10 deleted from DCs, have dramatic reductions in IgE production and do not develop significant TH2 immune responses. Further, ADAM10DC−/− mice are resistant to IgE-mediated anaphylaxis. This response is selective for TH2 immunity as TH1 and TH17 immunity are largely unaffected. Notch1, a known ADAM10 substrate, when knocked out of DCs (Notch1DC−/−) demonstrated a similar reduction in anaphylaxis and IgE. Without ADAM10 and Notch1 signaling, DCs were unable to make cytokines that stimulate TH2 cells and cytokines. Anaphylaxis and allergic lung inflammation were restored in ADAM10DC−/− with the overexpression of the Notch1-intracellular domain, confirming the role of Notch signaling.
Conclusions
Targeting ADAM10 and Notch1 on DCs represent a novel strategy for modulating TH2 immune responses and IgE production.
Introduction
Allergic asthma has become a major disease of the developing world with yearly increases in incidence. Worldwide, the number of people with asthma is estimated to grow by 100 million by the year 20241. Innate and adaptive immune responses lead to the production of T helper 2 (TH2) cytokines, IL-4, IL-5, and IL-13, which in turn cause many of the clinical symptoms seen in asthma including eosinophil infiltration, mucus overproduction, and airway constriction2. IgE plays a role in many TH2-mediated allergic diseases, including airway inflammation and type 1 hypersensitivity anaphylaxis. Specific control of IgE production has not been successful to date. We examined the role of the metalloproteinase ADAM10 on dendritic cells (DCs) in TH2 immunity using models of allergic airway inflammation and anaphylaxis.
In prototypic antigen (ovalbumin (OVA) and aeroallergen (house dust mite (HDM)) models of allergic airway inflammation in mice, DCs have been extensively studied as initiators of TH2 immunity. Specific cytokine milieus generated by DCs are important for initiating TH2 responses, particularly expression of IL-6 and lack of IL-12p70. Recently, the transcription factor KLF4 has also been shown to be important for TH2 immunity3. Further, certain costimulatory molecules, OX40L, CD86, PDL2, and cell surface proteins, CD301b (MGL2), jagged 1, and jagged 2, on DCs also influence the priming of T cells toward TH24.
We examined how DC ADAM10, a member of the A disintegrin and metalloproteinase (ADAM) family which mediate ectodomain shedding, influenced the generation of TH2 immune responses. This has not previously been explored though ADAM10 has been studied in many physiological and disease processes5,6. Ligands for ADAMs include growth factors and cytokines as well as their receptors, including Notch receptors. Upon Notch receptor–ligand engagement a conformational shift occurs revealing the S2 cleavage site for ADAMs. This step is followed by cleavage by γ-secretase, releasing the Notch-intracellular domain (N-ICD), which translocates to the nucleus and with binding partners initiates transcription of Notch target genes7. ADAM10 is required for the cleavage of Notch as is evident in the global ADAM10 knockout, which displays typical loss of function Notch defects8. Within the immune system, lack of ADAM10 in T cells ablates T cell development9. ADAM10 deletion in B cells leads to inhibition of humoral immunity10,11 and absence of marginal zone B cells12.
Herein we show new evidence for the role of Notch1 and ADAM10 in DCs in the development of murine TH2 airway inflammation and IgE production. Phenotype analysis indicated that Notch1 expression was particularly critical. Deletion of ADAM10 on DCs led to changes in the costimulatory molecule OX40L and cytokine IL-6, both of which are critical for the generation of TH2 responses. To determine the T helper type specificity of ADAM10DC−/−, infection with Anaplasma phagocytophilum an obligate intracellular pathogen, clearance of which requires CD4+ T cells and IFNγ13,14, Alternaria alternate, a common fungal aeroallergen, which stimulates both TH2 and TH17 immune responses15,16 and Citrobacter rodentium, an attaching and effacing enteric pathogen that elicits innate lymphocyte type 3 and TH17 responses17 were used. Our data demonstrates that TH2 responses were consistently reduced and other T helper types were less affected.
Results
ADAM10DC−/− mice have reduced high affinity IgG1 and recall responses
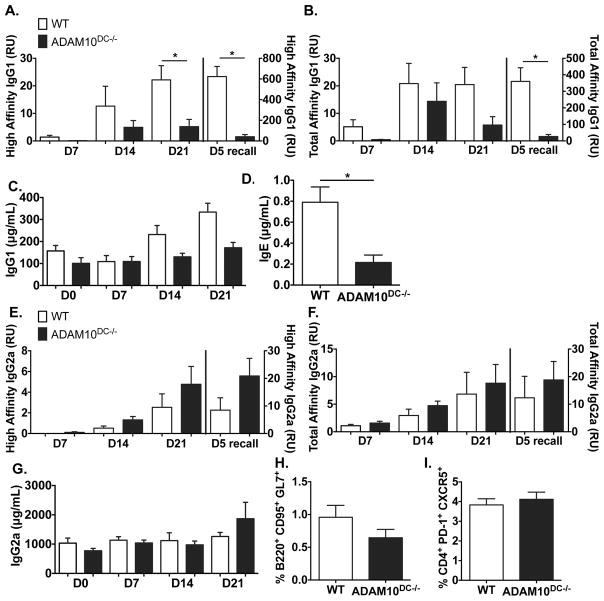
To determine if ADAM10 had a role in DC function, we generated ADAM10DC−/− mice (ADAM10 is absent in CD11c+ cells). As an initial study of DC stimulation of T cells, WT and ADAM10DC−/− were immunized with NP31-KLH in alum and IgG1 anti-DNP determined (Fig 1A, 1B). Total affinity NP-specific IgG1 was only significantly different in the recall, while ADAM10DC−/− had significantly less high affinity NP-specific IgG1 at day 21 and more dramatically 5 days after boost (Fig 1A, 1B). Total serum levels (Fig 1C) were not different. Antigen-specific IgE is not detectable even in WT mice, however total IgE levels were lower in the ADAM10DC−/− mice (Fig 1D). The differences in response between WT and ADAM10DC−/− were not seen when NP-specific IgG2a was measured (Fig 1E–G), indicating the defect may specifically affect TH2 immunity. Examination of 14 day draining popliteal lymph nodes revealed no significant differences in germinal center (GC) B cells and T follicular helper (TFH) cells (Fig 1H, 1I).
Figure 1.
ADAM10DC−/− mice have reduced high affinity IgG1 and recall responses. Mice were immunized with NP31-KLH in alum and bled on indicated days. NP4-BSA and NP25-BSA coated ELISAs were used to measure high affinity (A) and total affinity (B) NP-specific IgG1 in serum. Total IgG1 was measured in the serum (C). D. Total IgE was measured in the serum 7 days after boost NP-KLH injection. High (E.) and total (F.) affinity NP-specific IgG2a were measured in serum. G. Total IgG2a was measured in serum. Data are combined from two independent experiments with n = 6 mice per group. GC B cells (B220+ CD95+ GL7+) and TFH cells (CD4+ PD-1+ CXCR5+) were analyzed by flow cytometry on day 14 (H and I) draining popliteal LNs from NP-KLH footpad injections. Data are presented as mean ± SEM. *p<0.05 unpaired Student’s t test.
ADAM10DC−/− mice have reduced TH2 immune response to house dust mite (HDM) extract
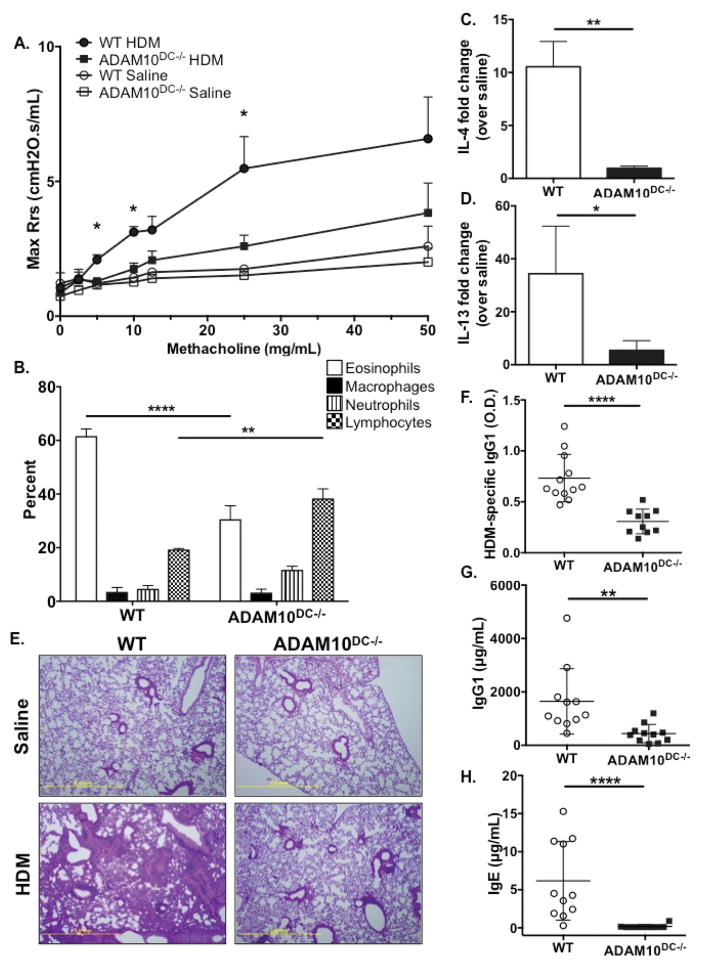
We then examine the effects of ADAM10 deletion from DCs on TH2 immunity using a 14 day model of allergic airway inflammation with HDM extract18. ADAM10DC−/− mice had significantly less airway hyperresponsiveness, indeed resistance was almost at saline levels (Fig 2A). Further, ADAM10DC−/− had fewer eosinophils in the bronchoalveolar lavage fluid (BALF) (Fig 2B) and less Il4 and Il13 mRNA expression in the lung tissue compared (Fig 2C, 2D). By H&E staining, ADAM10DC−/− lungs had dramatically less inflammatory infiltrate (Fig 2E). Serum HDM-specific IgG1 (Fig 2F) and total IgG1 (Fig 2G) were reduced in ADAM10DC−/− mice and most strikingly, serum total IgE was almost completely absent (Fig 2H). These results indicate that ADAM10DC−/− mice have a diminished TH2 response to HDM, particularly with respect to IgE production.
Figure 2.
ADAM10DC−/− mice have diminished TH2 responses to HDM. ADAM10DC−/− and WT mice were subjected to HDM or saline sensitization and challenge. A. Airway hyperresponsivness was assessed by nebulizing increasing doses of methacholine and measuring resistance. B. Bronchoalveolar lavage fluid (BALF) was harvested and analyzed by flow cytometry. C. and D. Il4 and Il13 mRNA expression was measured in lung tissue relative to Gapdh. E. Formalin fixed lung sections were stained with H&E. F. – H. HDM-specific IgG1, total IgG1, and total IgE were measured in serum by ELISA. Symbols represent individual mice. Data are combined from three independent experiments with n = 10 per HDM group and n = 3 per saline group. Data are presented as mean ± SEM. ****p<0.0001, ***p<0.001, **p<0.01, *p<0.05, ANOVA with Tukey’s post hoc test (A, B), unpaired Student’s t test (C, D, F, G), and Mann-Whitney test (H).
ADAM10DC−/− mice are resistant to active systemic anaphylaxis
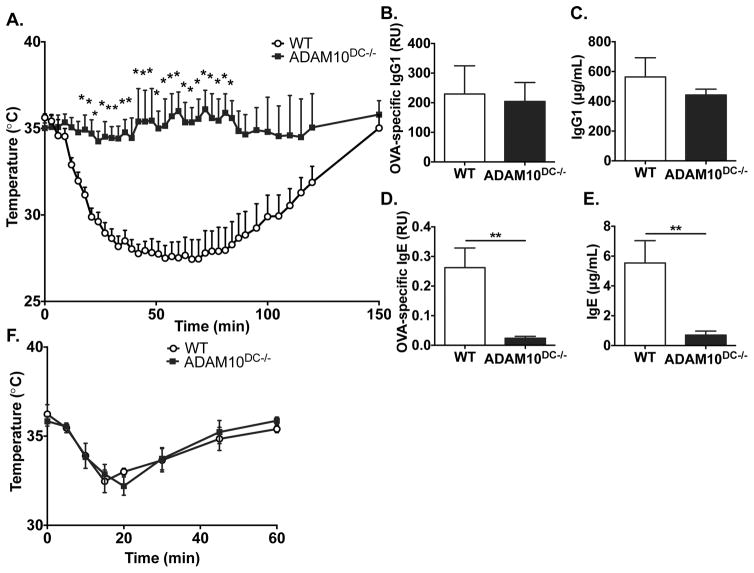
Given the remarkable reduction in IgE in the HDM model, we turned to an OVA model of active systemic anaphylaxis (ASA), which is exquisitely sensitive for the presence of antigen-specific IgE. Upon challenge with the sensitizing antigen, OVA, WT mice displayed a severe temperature drop, typical of anaphylaxis. Remarkably, ADAM10DC−/− mice were completely resistant, exhibiting little temperature change after challenge (Fig 3A). Total and OVA-specific IgG1 levels were similar between WT and ADAM10DC−/− (Fig 3B, 3C). However, ADAM10DC−/− mice had significantly less total IgE and almost absent OVA-specific IgE (Fig 3D, 3E) consistent with the lack of anaphylaxis. To demonstrate that mast cells were functioning properly in the ADAM10DC−/− mice, they were subjected to passive systemic anaphylaxis, in which antigen-specific IgE (IgE anti-DNP) is i.p. injected into mice, and 24h later, the mice are challenged with the antigen (DNP-BSA). WT and ADAM10DC−/− mice displayed a similar degree of temperature drop after challenge indicating no defect in mast cell function (Fig 3F).
Figure 3.
ADAM10DC−/− mice are resistant to active systemic anaphylaxis. WT and ADAM10DC−/− mice were immunized for the ASA protocol and then challenged with OVA on day 25 after immunization. A. After challenge, core body temperature was measured. B. – E. Mice were bled on day 21 after immunization. OVA-specific and total IgG1 and IgE were measured in the serum by ELISA. F. Mice were subjected to passive systemic anaphylaxis. IgE anti-DNP was injected i.p. into mice and then 24h later mice were challenged i.p. with DNP-BSA and core body temperature measured. Data are from three independent experiments with n = 11 mice per group. Data are presented as mean ± SEM. **p<0.01, *p<0.05, unpaired Student’s t test.
Our previous studies with ADAM10B−/− mice demonstrated a reduction in HDM induced allergy airway inflammation19 similar to the results generated with ADAM10DC−/−. To determine if ADAM10 deficiency on B cells contributes to the findings here, we first examined the expression of the low affinity IgE receptor, CD23, on B cells in ADAM10DC−/−, ADAM10B−/−, and WT mice. ADAM10 is the primary sheddase of CD23, and ADAM10-deficient B cells express higher levels of CD23 than WT B cells12. Splenic B220+ cells from ADAM10DC−/− mice expressed a similar level of CD23 on the surface as WT, which was less than ADAM10B−/− mice (Fig S1A, S1B). We further demonstrate the difference between knocking out ADAM10 from DCs compared to B cells in the ASA model used in Figure 3. ADAM10B−/− mice exhibit anaphylaxis upon OVA challenge similar to WT and have to be euthanized due to severe temperature drop, whereas ADAM10DC−/− mice are resistant to anaphylaxis (Fig S1C). These results strongly suggest that ADAM10 deficiency on B cells does not occur in ADAM10DC−/− mice and is not involved in the phenotype of these mice.
ADAM10DC−/− mice have intact TH1 and TH17 responses
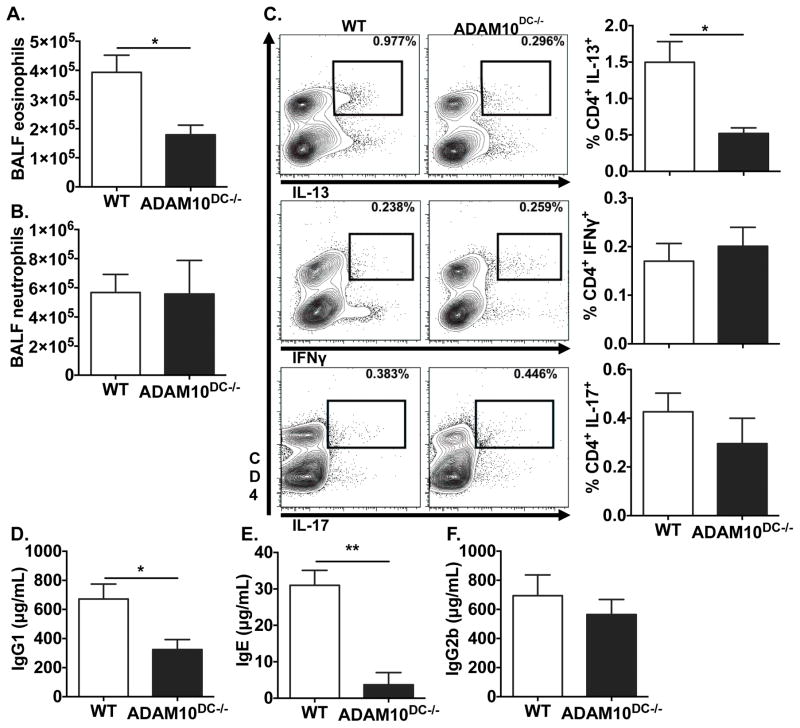
To examine TH1 immunity, we infected ADAM10DC−/− and WT mice with A. phagocytophilum and found equivalent bacterial burden in the blood of WT and ADAM10DC−/− mice at all time points. Both groups had cleared the infection by day 28 (Fig S2A). To further demonstrate the specificity of the TH2 defect in ADAM10DC−/− mice, responses to a fungal aeroallergen, A. alternata, and infections with A. phagocytophilum and C. rodentium were tested. After intranasal sensitization and challenge with A. alternata, we found that ADAM10DC−/− mice had reduced eosinophils in the BALF compared to WT, consistent with the HDM model (Fig 4A). Interestingly, they had equivalent levels of neutrophils (Fig 4B). ADAM10DC−/− mice had fewer CD4+ IL-13+ cells, but similar levels of CD4+ IFNγ+ and CD4+ IL-17+ cells to WT (Fig 4C). Reduced amount of IgG1 and IgE were found in the serum of ADAM10DC−/− mice, but we did not see any reduction in IgG2b (Fig 4D–F). Antibody, BALF, and intracellular cytokine data reinforce the selective defect in TH2 immunity present in ADAM10DC−/− mice. After infection with the extracellular bacterium Citrobacter rodentium ADAM10DC−/− mice lost more body weight over the course of infection (Fig S2B) and were more likely to succumb (Fig S2C) than WT. However, no differences were seen in total and CR-specific IgG2b (Fig S2D, S2E), similar to the response to fungal allergen. While colony counts indicated a similar infection level in the colon (Fig S2F), ADAM10DC−/− mice had more disseminated infection as measured by CFUs in the spleen (Fig S2G). These data suggest that ADAM10DC−/− mice were less able to control the C. rodentium infection despite equivalent antibody levels.
Figure 4.
ADAM10DC−/− mice have impaired TH2, but intact TH17 responses. WT and ADAM10DC−/− mice were sensitized and challenged with intranasal administration of A. alternata extract. A. and B. BALF eosinophils and neutrophils were analyzed by flow cytometry. C. Total medLN cells were stimulated with plate bound anti-CD3ε for 4h with monensin. Intracellular cytokine expression in CD4+ T cells was assessed by flow cytometry. Representative contour plots and combined results are shown. D. – F. Total IgG1, IgE, and IgG2b were measured in the serum by ELISA. Data shown is from two independent experiments with n = 5 mice per group. Data are presented as mean ± SEM. **p<0.01, *p<0.05, unpaired Student’s t test.
Role of Notch1 and Notch2 in the immune defects of ADAM10DC−/− mice
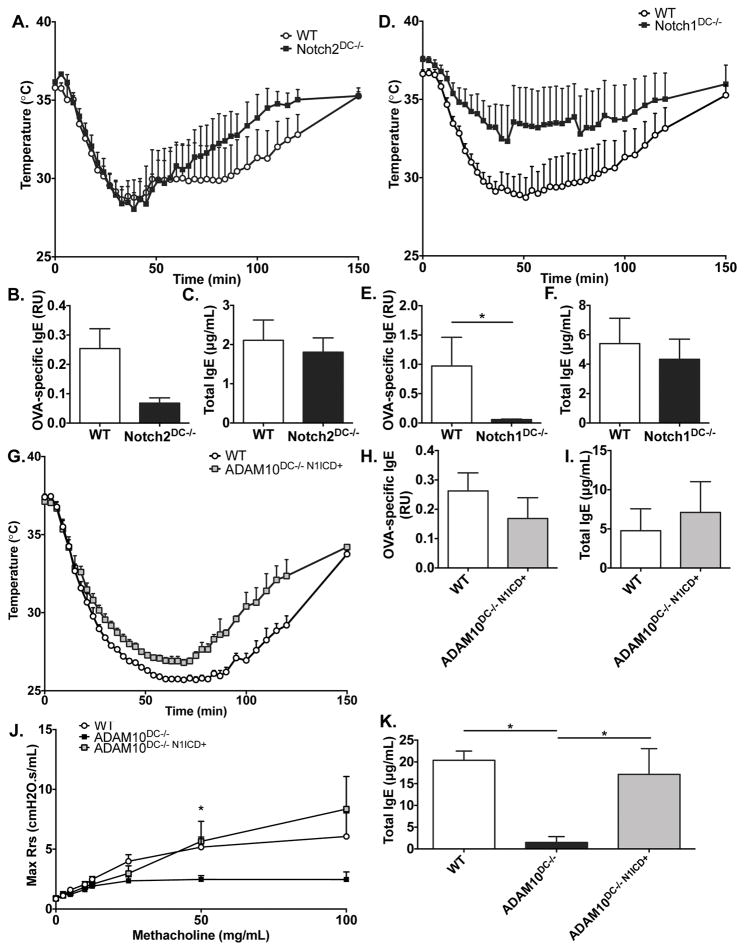
One of the most well-known ADAM10 substrates are Notch receptors5, and Notch2 at least is known to play an important role in DC function. To determine if the anaphylaxis phenomenon in the ADAM10DC−/− is due to lack of Notch signaling, we subjected Notch2DC−/− mice to the OVA ASA protocol as in Figure 3. Notch2DC−/− produced a similar level of temperature drop as the WT controls (Fig 5A). Serum total and OVA-specific IgE were not significantly different than WT control (Fig 5B, 5C), although the IgE trended down. We then tested the other DC relevant Notch, Notch1 (Notch1DC−/−). A total of nine mice were utilized in the experiment; six of nine had essentially no anaphylaxis while three had similar temperature drops as the controls. The combined temperature data are shown in Fig 5D. Due to the aforementioned variability, there was not a significant difference in temperature drop, but Notch1DC−/− had less OVA-specific IgE levels compared to WT (Fig 5E, 5F). Fig S3 shows the comparison between the 6 mice (Fig S3A–C), which exhibited anaphylaxis versus three (Fig S3D–F) that responded like WT. The six non-responders are clearly quite similar to ADAM10DC−/− mice. To confirm that specificity of our results, we examined Notch target gene expression in B cells, T cells, and DCs. We found that B and T cells from Notch1DC−/− and WT mice had similar levels of Hes1 expression, whereas CD11c+ BMDCs from Notch1DC−/− mice had significantly less expression of Hes1 compared to WT mice (Fig S4).
Figure 5.
Notch signaling is critical for anaphylaxis responses. Notch2DC−/− (A), Notch1DC−/− (D), and ADAM10DC−/− N1-ICD+ (G) mice were subjected to the active systemic anaphylaxis protocol and core body temperature was measured after challenged with OVA. OVA-specific and total IgE were measured in the serum of Notch2DC−/− (B, C), Notch1DC−/− (E, F), and ADAM10DC−/− N1-ICD+ (H, I) mice on day 21 after immunization. J. WT, ADAM10DC−/−, and ADAM10DC−/− N1-ICD+ mice were examined in the HDM model of allergic airway inflammation. K. Total serum IgE after HDM protocol. Data are from two independent experiments with n = 6 – 9 mice per group. Data are presented as mean ± SEM. *p<0.05, unpaired Student’s t test.
To confirm involvement of Notch signaling in the ADAM10DC−/− mice, we crossed the ADAM10DC−/− mice to the ROSAN1-ICD mice, which have a lox-stop-lox before the Notch1-ICD (N1-ICD) inserted into the ROSA locus20. ASA with these mice were quite similar to WT mice (Fig 5G) and levels of total and OVA-specific IgE were restored to WT levels (Fig 5H, 5I). Further, we found that N1-ICD expression rescued the airway hyperresponsiveness and total serum IgE in the HDM model in ADAM10DC−/− mice (Fig 5J, 5K). Overall the data demonstrate the importance of Notch signaling in the anaphylaxis response and allergic airway inflammation as well as in the production of total and OVA-specific IgE.
ADAM10DC−/− DCs stimulate less TH2 cytokine expression in T cells
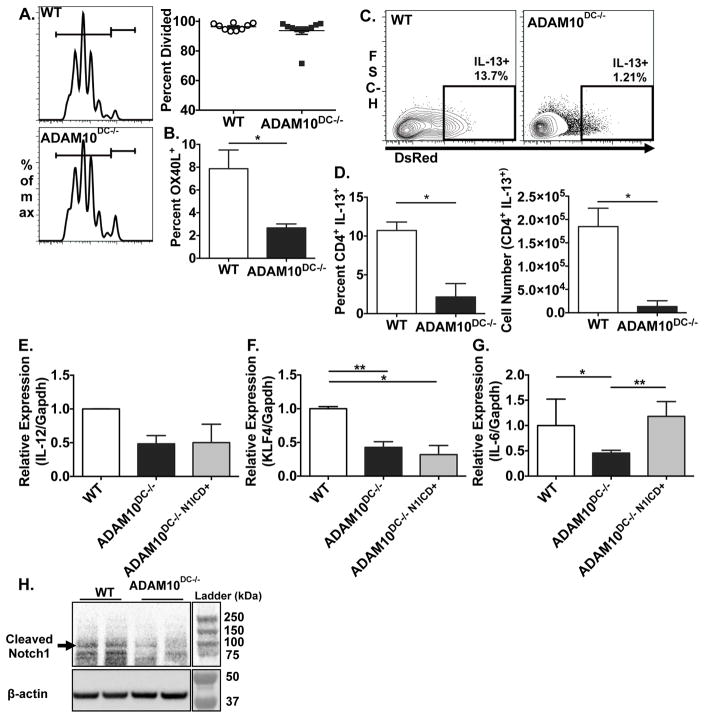
To understand how ADAM10-deficient DCs were modulating T helper responses, we examined the ability of ADAM10-deficient and WT DCs to present antigen to stimulate T helper cytokines in vivo. Labeled CD4+ OT-II T cells in the medLN proliferated to similar extent in WT and ADAM10DC−/− mice after i.n. administration of OVA/HDM (Fig 6A). Interestingly, CD172+ DCs, which stimulate CD4+ T cells, in the medLN of ADAM10DC−/− mice expressed less OX40L, which is a costimulatory molecule associated with TH2 responses (Fig 6B)21,22. 4C13R OT-II CD4+ T cells, which express AmCyan with IL-4 and DsRed with IL-13 expression, were utilized in the same system as above. While IL-4 levels were not detectable at this time point, ADAM10DC−/− mice had dramatically fewer CD4+ IL-13+ T cells in the medLN than WT (Fig 6C, D). Overall these data indicate that ADAM10-deficient DCs are not able to stimulate CD4+ T cells to make the TH2 cytokine, IL-13.
Figure 6.
ADAM10-deficient DCs stimulate fewer TH2 cells. A. In vivo antigen presentation assay with violet tracer labeled CD4+ OT-II T cells i.v. injected into WT (upper panel) and ADAM10DC−/− (lower panel) mice followed by OVA/HDM. Combined data from three independent experiments with n = 9 mice per group. B. OX40L expression on CD172+ DCs (cDC2s) in medLN was examined by flow cytometry and shown as percent OX40L+. C. and D. Labeled CD4+ 4C13RTg OT-II T cells were used as in (A) CFSE+ CD4+ IL-13+ cells were assessed by flow cytometry. Data are from two independent experiments with n = 6 mice per group. Data are presented as mean ± SEM. *p<0.05, unpaired Student’s t test. E.–G. CD172+ BMDCs were sorted from WT, ADAM10DC−/−, and ADAM10DC−/− N1-ICD+ mice. mRNA expression of Il12, Klf4, and Il6 relative to Gapdh was measured. Data shown is from three independent experiments with n = 5 mice per group. Data are presented as mean ± SEM. H. WT and ADAM10DC−/− CD172+ BMDCs were examined by western blot for cleaved Notch1 and β-actin. Data shown is representative of two experiment with n = 5 mice per group. **p<0.01, *p<0.05, ANOVA with Tukey’s post hoc test.
ADAM10DC−/− DCs have reduced Klf4 and Il6 expression
Several DC-produced cytokines and transcription factors have been implicated in skewing T helper responses toward TH2. Lack of IL-12 and high IL-6 expression have been cited as promoting TH2 cell development4 as well as the expression of Kruppel-like factor 4 (Klf4)3. While no difference in Il12 message was seen (Fig 6E), expression of Klf4 and Il6 mRNA were significantly reduced in CD172+ DCs isolated from BMDCs from ADAM10DC−/− mice (Fig 6F,G). However, only Il6 expression was restored in ADAM10-deficient CD172+ BMDCs when Notch signaling was recovered (ADAM10DC−/−N1-ICD+) (Fig 6E–G). As a control, we also examined the expression of the Notch target gene Hes1 and indeed found that ADAM10DC−/− BMDCs had less expression of Hes1 than WT, which was restored with constitutive N1-ICD expression (Fig S5). We also analyzed ADAM10 deficient and WT CD172+ BMDCs for the presence of cleaved Notch1 by western blot. ADAM10DC−/− BMDCs had less cleaved Notch1 than WT BMDCs (Fig 6H), indicating less activation of Notch1. These findings further underscore the importance in Notch signaling in the ability of DCs to stimulate TH2 responses.
Discussion
While the role of DCs in the initiation of TH2 immune responses is well recognized, the function of ADAM10 in DCs has not previously been investigated. Here we demonstrate that deletion of ADAM10 from DCs results in diminished TH2/IgE-mediated pathology using several TH2 models. Firstly, the main antibody defect was in IgE production in all models tested. Airway resistance in the HDM model was reduced to essentially the saline control and eosinophil levels were decreased by 50% (Fig 2B). These results are further supported by the reduction in lung TH2 cytokines (Fig 2C, 2D). A TH2 selective defect is supported by the A. alternata (Fig 4), which stimulates TH2 and TH17 responses16. Fewer eosinophils, IL-13+ CD4+ T cells, and decreased IgE levels were seen (Fig 4A, 4C, 4E). In contrast, levels IL-17+ or IFNγ+ cells, while relatively rare, were similar between WT and ADAM10DC−/− mice (Fig 4C). Further, IgG2b and neutrophils in the BALF were also equivalent. In the other model of TH17 immunity, infection with C. rodentium, we did find differences, specifically that ADAM10DC−/− lost more weight during the infection and had more disseminated bacteria than WT. Despite these findings, we found similar levels of total and CR-specific antibody (S2B–G). Notch2DC−/− have been studied in this infection model and exhibit severely impaired immunity compared to WT due to the loss of the CD11b+ CD103+ DC population in the mesenteric LN and lamina propria23. ADAM10DC−/− mice have a mild reduction in this subset (data not shown), which may explain the disparity in severity of infection between ADAM10DC−/− and Notch2DC−/− mice. For examination of TH1 defects, ADAM10DC−/− and WT mice with the A. phagocytophilum, where T cell production of IFNγ is crucial for pathogen elimination13,14. ADAM10DC−/− cleared this infection with similar efficiency as WT (Fig S2A). Overall, the primary defect in the ADAM10DC−/− animals relates to TH2 function.
To further examine the role of DC Notch expression in the TH2 impairment seen, two DC-specific Notch knockouts were used. Notch2 expression on DCs was not responsible for the loss of TH2 function as Notch2DC−/− mice responded similarly to WT in the ASA model. Data from Notch1DC−/− mice fell into two groups; 6 of 9 showing no evidence of anaphylaxis while 3 of 9 responded the same as WT controls (Fig S3A, S3B). This suggests that the level of specific IgE is near the threshold for anaphylaxis, whereas ADAM10DC−/− mice are clearly under that threshold with 10 of 11 exhibiting no temperature drop. Perhaps a small amount of compensation by Notch2 is occurring in the Notch1DC−/− mice, as ADAM10 deletion would cause a defect in both Notch1 and 2. Notch signaling is undoubtedly implicated in our results since the overexpression of N1-ICD restored the temperature drop in the ADAM10DC−/− animals (Fig 5H). Overall the results obtained indicate that the ADAM10 substrate that is important for mediating TH2 immunity is indeed Notch, with Notch1 exhibiting a higher level of importance than Notch2. The activation of Notch signaling requires the Notch receptor to bind to a Notch ligand, typically on adjacent cells. As both T cells and DCs can express the Notch ligands24, either interaction could work. Several Notch ligands on DCs have been studied in the context of TH2 immunity. Jagged 1 and 2 on DCs have been shown to promote TH2 response4, but we did not find alterations in either of these on ADAM10-deficient DCs (data not shown). While the role of Notch1 has previously been studied in DCs, the studies found no alterations in DC subsets25, which is consistent with our findings (data not shown), however our results demonstrate an important role for DC Notch1 expression and indeed, this is the first demonstration of Notch1’s importance on DCs.
We found that the TH2 promoting co-stimulatory molecule OX40L21,22 on DCs in the medLN from ADAM10DC−/− mice is greatly reduced compared to WT (Fig 6B). OX40L has been demonstrated to be upregulated by thymic stromal lymphopoietin (TSLP) in the airways after HDM exposure acting by transcription factor PU.1 binding to the proximal promoter and activation of NFkB26. Notch signaling increased PU.1 levels in hematopoietic progenitor cell line and lead to NFkB activation. Another study demonstrated that Notch1-Delta-like 1 signaling led to increased OX40L and IL-6 expression27. OX40L on DCs mediates priming and maintenance of memory for TH2 responses21. The cytokine IL-6 has also been shown to be critical for the development of TH2 responses as DCs from IL-6KO mice are unable to stimulate TH2 cytokine and Der p-specific IgG128. ADAM10DC−/− mice have reduced IL-6, HDM-specific IgG1, and IgE (Fig 6G, 2F, 2H). The transcription factor KLF4 is able to bind to and activate the Il6 promoter in DCs29. We show that both Klf4 and Il6 expression is reduced in ADAM10-deficient BMDCs (Fig 6F, 6G). However, only Il6 expression was restored with N1-ICD expression, indicating that it may be responsible for the phenotype observed in ADAM10DC−/− mice. Further support of this finding is that IL-6 has been demonstrated to be upregulated by non-canonical Notch signaling and is dependent on N1-ICD30.
Ever since IgE was discovered in the 1960s and the recognition of its importance in Type I hypersensitivity, specific reduction in IgE synthesis has been a long sought mechanism to control these diseases. This has proven to be a difficult task, as this needs to be accomplished without significantly reducing protective immunoglobulin. This work demonstrates a clear reduction in TH2-mediated disease and IgE production in ADAM10DC−/− mice. In an earlier paper, we also demonstrated that the OVA31 or HDM19 allergy model was reduced when ADAM10 was deleted from B cells. However the B cell knockout has an overall reduction in all antibody classes10, while the ADAM10DC−/− mice had primarily a TH2 defect through Notch1. While the C. rodentium data indicate Notch2 defects as well, the results presented here combined with fact that relatively specific delivery of drugs to lungs indicate that inhibition of ADAM10 on DCs in the lungs would represent a method to control the antigen specific IgE production that plays a role in Type I airway hypersensitivity. Current studies are examining this possibility using mouse models. If successful, this would have the potential of being a general therapy for TH2-mediated airway diseases.
Experimental Procedures
Mice
All animal experiments were performed with the approval of the VCU Institutional Animal Care and Use Committee. Mice were maintained in VCU animal facility in accordance with guidelines for the humane treatment of laboratory animals set forth by the NIH and AAALAC. ADAM10flox mice12 were bred to B6.Cg-Tg(Itgax-cre)1-1Reiz/J (CD11c-cre, Stock No. 008068) to generate ADAM10DC−/− mice. Notch1flox (Stock No. 006951), Notch2flox (Stock No. 010525), and N1-ICD Gt(ROSA)26Sortm1(Notch1)Dam/J (Stock No. 8159) were bred to the CD11c-cre mouse (all from The Jackson Laboratory, Bar Harbor, ME. 4C13RTg reporter mice, were a kind gift from Bill Paul’s lab at NIH/NIAID32. The 4C13RTg mice were bred to OT-II mice to generate 4C13R-OT-II mice. All mice were on the C57Bl/6 background, and healthy male and female 6–12 week old mice were used for experiments.
Antibodies
Antibodies used were: anti-mouse CD45R/B220 (clone RA3-6B2), PE anti-mouse CD95 (clone SA367H8), and APC anti-GL7 (clone GL7), (FITC anti-mouse CD4 (clone GK1.5), BV421 anti-mouse PD-1 (clone 29F.1A12), biotin anti-mouse CXCR5 (clone L138D7), and PE-Cy7 Streptavidin), APC anti-mouse CD45R/B220 (clone RA3-6B2), APC anti-mouse CD3 (clone 17A2), BV421 anti-mouse I-A/I-E (clone M5/114.15.2), PE anti-mouse CCR3 (clone J073E5) and PE-Cy7 anti-mouse/human CD11b (clone M1/70)), anti-CD3ε (1μg/mL, clone 145-2C11, for 4h. PE-Cy7 anti-mouse IL-13 (clone eBio13A, eBioscience), AlexaFluor647 anti-mouse IFNγ (clone XMG1.2, BV650 anti-mouse IL-17A (clone TC11-18H10.1, BD Biosciences), and BV421 anti-mouse CD4 (clone GK1.5,). Unless indicated, all labeled antibodies were from Biolegend. All flow experiments utilized a BD Fortessa X-20 and FlowJo software for analysis, with a BD Aria II for sorting.
NP-KLH immunization
10μg NP31-KLH (Biosearch Technologies) in 4mg alum (Imject, Sigma) was injected i.p. or into footpads as previously described10,11. At indicated days mice serum was collected and NP-specific ELISAs10 were done. Alternatively, flow cytometry was performed on cells from popliteal lymph nodes. GC B cells and TFH were detected by flow cytometry.
HDM and Alternaria alternata models
Mice were immunized with HDM extract (Stallergenes Greer, Lenoir, NC) as previously described18. After last i.n. administration, mice were subjected to forced oscillations using the Flexivent apparatus (Scireq Inc., Montreal, Canada) with increasing doses of methacholine. Bronchoalveolar lavage fluid (BALF) was collected, and stained cell types determined by flow cytometry33. Lung lobes were snap frozen in liquid N2 for RNA isolation or fixed in 10% formalin for hematoxylin and eosin staining. Serum was collected for total IgG1 and IgE and HDM-specific IgG1 ELISAs34. 50μg Alternaria alternata extract was as previously described15,16. 24h after the last administration, BALF was collected and analyzed by flow cytometry. Single cell suspension from medLNs were re-stimulated with plate bound anti-CD3ε and monensin (Biolegend) for 4h and then examined for IL-13, IFNγ and IL-17 intracellular expression. Serum IgG1, IgE, and IgG2b were measured by ELISA.
qPCR
RNA was isolated using TRIzol Reagent (LifeTech) according to manufacturer’s instructions. RNA was reverse transcribed into cDNA using SuperScript IV and oligo dTs (LifeTech). Taqman probes and gene expression master mix (Applied Biotech) or primers (LifeTech) and PowerUp SYBR green master mix (LifeTech) were used with a QuantStudio3 system (LifeTech) (Supplemental Table 1)
Active systemic anaphylaxis (ASA)
Mice were immunized with 100μg ovalbumin (OVA), 10μg pertussis toxin, and 10mg Alum in saline35. At day 21, serum total and OVA-specific IgG1 and IgE were determined. Anaphylaxis induced by ip injecting 500μg OVA and analyzed by rectal temperature measurement. OVA-specific IgE was measured by coating Immunolon 4HBX plates (Thermo Scientific) with rat anti-mouse IgE (clone R1E4). After adding samples and blocking, OVA labeled with the DNP-X-Biocytin SE Kit (Thermo Fisher) was added, followed by detection with Streptavidin-AP. Passive systemic anaphylaxis was conducted by i.v. injecting 20μg anti-DNP IgE, 24h later i.p. injecting 500μg DNP-BSA, and measuring rectal temperatures36.
Antigen presentation
For in vivo antigen presentation, 5×106 labeled CD4+ OT-II T cells were i.v. injected into mice. After 24h 25μg OVA and 10μg HDM extract were administered i.n. to mice. 72h later medLN were analyzed by flow cytometry. For IL-4/IL-13 detection, CD4+ T cells isolated from 4C13R-OT-II mice and labeled with carboxyfluorescein succinimidyl ester (CFSE, Biolegend) were used. IL-4 and IL-13 expression was determined by CD4+ T expression of AmCyan and DsRed.
BMDC Cultures
Bone marrow cells were cultured at 2×106 cell/mL with 160ng/mL Flt3L (Peprotech) for 6–8 days. After culture, cells were Fc blocked with anti-mouse CD16/32 (clone 93, Biolegend) and stained for sorting. CD11c+ MHCII+ CD24− CD172+ BMDCs were sorted on a FACS Aria II.
Statistical Analyses
All statistical analyses were performed using Prism6 (GraphPad Software Inc., La Jolla, CA). Statistical significance was assessed by two-tailed, unpaired Student’s t test (two groups), Mann-Whitney test, or one-way ANOVA for multiple groups with a Tukey’s post hoc test. Unless otherwise indicated differences are not significant. ****p<0.0001, *** p<0.001, ** p<0.01, * p<0.05. Samples that were below the limits of detection were assigned a value that represented the lower limit of detection.
Supplementary Material
Fig S1. A. Splenocytes from ADAM10B−/−, ADAM10DC−/−, and WT mice were examined for CD23 expression on B220+ cells (mean fluorescence intensity (MFI)) by flow cytometry. B. Quantification of (A). n = 3–4 mice per group. C. ADAM10B−/−, ADAM10DC−/−, and WT mice were subjected to the ASA protocol and core body temperatures were recorded. n = 6 – 11 mice per group, at least 2 independent repeats.
Fig S2. A. ADAM10DC−/− and WT were inoculated i.p. with 107 A. phagocytophilum bacteria. Mice were bled on days 3, 7, 10, 14, 17, 21, and 28. DNA was isolated and qPCR run with primers for A. phagocytophilum 16s DNA and mouse Actb (β-actin). Data are from two independent experiments with n = 6 mice per group. B. WT and ADAM10DC−/− mice were infected by oral gavage with 1010 CFU of C. rodentium suspension. Body weight was measured over the course of infection and reported as change from initial body weight. C. Kaplan-Meier survival analysis. D. and E. Total and CR-specific IgG2b was measured in the serum by ELISA. F. and G. Colon and spleen were homogenized and bacterial load were determined. Data are from two independent experiments with n = 7 mice per group. ***p<0.001, *p<0.05, unpaired Student’s t test.
Fig S3. Active systemic anaphylaxis (ASA) in Notch1DC−/− and WT mice. A. 6 of 9 Notch1DC−/− were resistant to ASA. B. and C. OVA-specific and total IgE for Notch1DC−/− mice resistant to anaphylaxis. D. 3 of 9 Notch1DC−/− mice exhibited temperature change after challenge similar to WT. E. and F. OVA-specific and total IgE in Notch1DC−/− mice which underwent anaphylaxis.
Fig S4. B220+ and CD4+ cells were magnetically isolated from total splenocytes from WT and Notch1DC−/− mice. Notch1DC−/− and WT BMDCs were generated. cDNA from B220+, CD4+, and CD11c+ cells was assessed for Notch1 target gene Hes1 expression relative to Gapdh. n = 4 mice per group. *p<0.05, Student’s unpaired t test.
Fig S5. Hes1 expression relative to Gapdh in BMDCs from WT, ADAM10DC−/− and ADAM10DC−/− N1-ICD+ mice.
Acknowledgments
We would like to thank Andrea Luker and Matthew Zellner for their technical assistance. This study was funded, in part by and NIH/NIAID RO1AI18697A1-33-38 (to D.H.C.). Flow cytometry was supported, in part, by Massey Cancer Center Core NIH Grant P30 CA16059. Microscopy was performed at the VCU—Department of Anatomy and Neurobiology Microscopy Facility, supported, in part, by funding from NIH-NINDS Center Core Grant 5 P30 NS047463 and, in part, by funding from NIH-NCI Cancer Center Support Grant P30 CA016059.
Footnotes
Conflicts of Interest
Authors declare that they have no conflicts of interest.
Author Contributions
Conceptualization, S.R.D., R.K.M., D.H.C.; Methodology, S.R.D., R.K.M.; Investigation, S.R.D., R.K.M., A.D.S.; Resources, C.L.C., J.A.C., A.D.S., D.H.C.; Funding Sources, S.R.D., D.H.C.; Writing – Original Draft, S.R.D., R.K.M., D.H.C., Writing – Review & Editing, S.R.D, R.K.M, J.C.L., D.H.C.
References
- 1.World Health Organization. Chron Respir Dis. 2007. World Health Organization. Global surveillance, prevention and control of chronic respiratory diseases: a comprehensive approach. Geneva, Switzerland; pp. 1–146. [Google Scholar]
- 2.Wynn Ta. Type 2 cytokines: mechanisms and therapeutic strategies. Nat Rev Immunol. 2015;15:271–82. doi: 10.1038/nri3831. [DOI] [PubMed] [Google Scholar]
- 3.Tussiwand R, Everts B, Grajales-Reyes GE, Kretzer NM, Iwata A, Bagaitkar J, et al. Klf4 Expression in Conventional Dendritic Cells Is Required for T Helper 2 Cell Responses. Immunity. 2015;42:916–28. doi: 10.1016/j.immuni.2015.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lambrecht BN, Galli SJ. SnapShot: Integrated Type 2 Immune Responses. Immunity. 2015;43:408–408. doi: 10.1016/j.immuni.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 5.Saftig P, Reiss K. The “A Disintegrin And Metalloproteases” ADAM10 and ADAM17: novel drug targets with therapeutic potential? Eur J Cell Biol. 2011;90:527–35. doi: 10.1016/j.ejcb.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Conrad DH, Ford JW, Sturgill JL, Gibb DR. CD23: an overlooked regulator of allergic disease. Curr Allergy Asthma Rep. 2007;7:331–7. doi: 10.1007/s11882-007-0050-y. [DOI] [PubMed] [Google Scholar]
- 7.Bray SJ. Notch signalling in context. Nat Rev Mol Cell Biol. 2016;9:722–35. doi: 10.1038/nrm.2016.94. [DOI] [PubMed] [Google Scholar]
- 8.Hartmann D, de Strooper B, Serneels L, Craessaerts K, Herreman A, Annaert W, et al. The disintegrin/metalloprotease ADAM 10 is essential for Notch signalling but not for alpha-secretase activity in fibroblasts. Hum Mol Genet. 2002;11:2615–24. doi: 10.1093/hmg/11.21.2615. [DOI] [PubMed] [Google Scholar]
- 9.Tian L, Wu X, Chi C, Han M, Xu T, Zhuang Y. ADAM10 is essential for proteolytic activation of Notch during thymocyte development. Int Immunol. 2008;20:1181–7. doi: 10.1093/intimm/dxn076. [DOI] [PubMed] [Google Scholar]
- 10.Chaimowitz NS, Martin RK, Cichy J, Gibb DR, Patil P, Kang D-J, et al. A disintegrin and metalloproteinase 10 regulates antibody production and maintenance of lymphoid architecture. J Immunol. 2011;187:5114–22. doi: 10.4049/jimmunol.1102172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Folgosa L, Zellner HB, El Shikh ME, Conrad DH. Disturbed follicular architecture in B cell A disintegrin and metalloproteinase (ADAM)10 knockouts is mediated by compensatory increases in ADAM17 and TNF-α shedding. J Immunol. 2013;191:5951–8. doi: 10.4049/jimmunol.1302042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibb DR, El Shikh M, Kang D-J, Rowe WJ, El Sayed R, Cichy J, et al. ADAM10 is essential for Notch2-dependent marginal zone B cell development and CD23 cleavage in vivo. J Exp Med. 2010;207:623–35. doi: 10.1084/jem.20091990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akkoyunlu M, Fikrig E. Gamma interferon dominates the murine cytokine response to the agent of human granulocytic ehrlichiosis and helps to control the degree of early rickettsemia. Infect Immun. 2000;68:1827–33. doi: 10.1128/iai.68.4.1827-1833.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pedra JHF, Tao J, Sutterwala FS, Sukumaran B, Berliner N, Bockenstedt LK, et al. IL-12/23p40-dependent clearance of Anaplasma phagocytophilum in the murine model of human anaplasmosis. FEMS Immunol Med Microbiol. 2007;50:401–10. doi: 10.1111/j.1574-695X.2007.00270.x. [DOI] [PubMed] [Google Scholar]
- 15.Denis O, Vincent M, Havaux X, De Prins S, Treutens G, Huygen K. Induction of the specific allergic immune response is independent of proteases from the fungus Alternaria alternata. Eur J Immunol. 2013;43:907–17. doi: 10.1002/eji.201242630. [DOI] [PubMed] [Google Scholar]
- 16.Valladao AC, Frevert CW, Koch LK, Campbell DJ, Ziegler SF. STAT6 Regulates the Development of Eosinophilic versus Neutrophilic Asthma in Response to Alternaria alternata. J Immunol. 2016 doi: 10.4049/jimmunol.1600007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collins JW, Keeney KM, Crepin VF, Rathinam VaK, Fitzgerald Ka, Finlay BB, et al. Citrobacter rodentium: infection, inflammation and the microbiota. Nat Rev Microbiol. 2014;12:612–23. doi: 10.1038/nrmicro3315. [DOI] [PubMed] [Google Scholar]
- 18.Hirota Ja, Budelsky a, Smith D, Lipsky B, Ellis R, Xiang Y-Y, et al. The role of interleukin-4Ralpha in the induction of glutamic acid decarboxylase in airway epithelium following acute house dust mite exposure. Clin Exp Allergy. 2010;40:820–30. doi: 10.1111/j.1365-2222.2010.03458.x. [DOI] [PubMed] [Google Scholar]
- 19.Cooley LF, Martin RK, Zellner HB, Irani AM, Uram-Tuculescu C, El Shikh ME, et al. Increased B cell ADAM10 in allergic patients and Th2 prone mice. PLoS One. 2015;10:1–16. doi: 10.1371/journal.pone.0124331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murtaugh LC, Stanger BZ, Kwan KM, Melton DA. Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci U S A. 2003;100:14920–5. doi: 10.1073/pnas.2436557100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenkins SJ, Perona-Wright G, Worsley AGF, Ishii N, MacDonald AS. Dendritic cell expression of OX40 ligand acts as a costimulatory, not polarizing, signal for optimal Th2 priming and memory induction in vivo. J Immunol. 2007;179:3515–23. doi: 10.4049/jimmunol.179.6.3515. [DOI] [PubMed] [Google Scholar]
- 22.Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202:1213–23. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Satpathy AT, Briseño CG, Lee JS, Ng D, Nicholas a, Kc W, et al. Immunity Against Attaching and Effacing Bacterial Pathogens. 2014;14:937–48. doi: 10.1038/ni.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koyanagi A, Sekine C, Yagita H. Expression of Notch receptors and ligands on immature and mature T cells. Biochem Biophys Res Commun. 2012;418:799–805. doi: 10.1016/j.bbrc.2012.01.106. [DOI] [PubMed] [Google Scholar]
- 25.Lewis KL, Caton ML, Bogunovic M, Greter M, Grajkowska LT, Ng D, et al. Notch2 receptor signaling controls functional differentiation of dendritic cells in the spleen and intestine. Immunity. 2011;35:780–91. doi: 10.1016/j.immuni.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yashiro T, Hara M, Ogawa H, Okumura K, Nishiyama C. Critical Role of Transcription Factor PU. 1 in the Function of the OX40L/TNFSF4 Promoter in Dendritic. Cells Sci Rep. 2016;6:34825. doi: 10.1038/srep34825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakano N, Nishiyama C, Yagita H, Koyanagi A, Akiba H, Chiba S, et al. Notch signaling confers antigen-presenting cell functions on mast cells. J Allergy Clin Immunol. 2009;123:74–81.e1. doi: 10.1016/j.jaci.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 28.Lin YL, Chen SH, Wang JY. Critical role of IL-6 in dendritic cell-induced allergic inflammation of asthma. J Mol Med. 2016;94:51–9. doi: 10.1007/s00109-015-1325-8. [DOI] [PubMed] [Google Scholar]
- 29.Rosenzweig JM, Glenn JD, Calabresi PA, Whartenby KA. KLF4 modulates expression of IL-6 in dendritic cells via both promoter activation and epigenetic modification. J Biol Chem. 2013;288:23868–74. doi: 10.1074/jbc.M113.479576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin S, Mutvei aP, Chivukula IV, Andersson ER, Ramsköld D, Sandberg R, et al. Non-canonical Notch signaling activates IL-6/JAK/STAT signaling in breast tumor cells and is controlled by p53 and IKKα/IKKβ. Oncogene. 2013;32:4892–902. doi: 10.1038/onc.2012.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mathews Ja, Ford J, Norton S, Kang D, Dellinger a, Gibb DR, et al. A potential new target for asthma therapy: a disintegrin and metalloprotease 10 (ADAM10) involvement in murine experimental asthma. Allergy. 2011;66:1193–200. doi: 10.1111/j.1398-9995.2011.02614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo L, Huang Y, Chen X, Hu-Li J, Urban JF, Paul WE. Innate immunological function of TH2 cells in vivo. Nat Immunol. 2015;16:1051–9. doi: 10.1038/ni.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Rijt LS, Kuipers H, Vos N, Hijdra D, Hoogsteden HC, Lambrecht BN. A rapid flow cytometric method for determining the cellular composition of bronchoalveolar lavage fluid cells in mouse models of asthma. J Immunol Methods. 2004;288:111–21. doi: 10.1016/j.jim.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 34.Cates EC, Fattouh R, Wattie J, Inman MD, Goncharova S, Coyle AJ, et al. Intranasal Exposure of Mice to House Dust Mite Elicits Allergic Airway Inflammation via a GM-CSF-Mediated Mechanism. J Immunol. 2004;173:6384–92. doi: 10.4049/jimmunol.173.10.6384. [DOI] [PubMed] [Google Scholar]
- 35.Allen IC, editor. Mouse Models of Allergic Disease. Totowa, NJ: Humana Press; 2013. [Google Scholar]
- 36.Metz M, Schäfer B, Tsai M, Maurer M, Galli SJ. Evidence that the endothelin A receptor can enhance IgE-dependent anaphylaxis in mice. J Allergy Clin Immunol. 2011;128:424–426.e1. doi: 10.1016/j.jaci.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang B, Troese MJ, Howe D, Ye S, Sims JT, Heinzen RA, et al. Anaplasma phagocytophilum APH_0032 is expressed late during infection and localizes to the pathogen-occupied vacuolar membrane. Microb Pathog. 2010;49:273–84. doi: 10.1016/j.micpath.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Troese MJ, Carlyon JA. Anaplasma phagocytophilum dense-cored organisms mediate cellular adherence through recognition of human P-selectin glycoprotein ligand 1. Infect Immun. 2009;77:4018–27. doi: 10.1128/IAI.00527-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. A. Splenocytes from ADAM10B−/−, ADAM10DC−/−, and WT mice were examined for CD23 expression on B220+ cells (mean fluorescence intensity (MFI)) by flow cytometry. B. Quantification of (A). n = 3–4 mice per group. C. ADAM10B−/−, ADAM10DC−/−, and WT mice were subjected to the ASA protocol and core body temperatures were recorded. n = 6 – 11 mice per group, at least 2 independent repeats.
Fig S2. A. ADAM10DC−/− and WT were inoculated i.p. with 107 A. phagocytophilum bacteria. Mice were bled on days 3, 7, 10, 14, 17, 21, and 28. DNA was isolated and qPCR run with primers for A. phagocytophilum 16s DNA and mouse Actb (β-actin). Data are from two independent experiments with n = 6 mice per group. B. WT and ADAM10DC−/− mice were infected by oral gavage with 1010 CFU of C. rodentium suspension. Body weight was measured over the course of infection and reported as change from initial body weight. C. Kaplan-Meier survival analysis. D. and E. Total and CR-specific IgG2b was measured in the serum by ELISA. F. and G. Colon and spleen were homogenized and bacterial load were determined. Data are from two independent experiments with n = 7 mice per group. ***p<0.001, *p<0.05, unpaired Student’s t test.
Fig S3. Active systemic anaphylaxis (ASA) in Notch1DC−/− and WT mice. A. 6 of 9 Notch1DC−/− were resistant to ASA. B. and C. OVA-specific and total IgE for Notch1DC−/− mice resistant to anaphylaxis. D. 3 of 9 Notch1DC−/− mice exhibited temperature change after challenge similar to WT. E. and F. OVA-specific and total IgE in Notch1DC−/− mice which underwent anaphylaxis.
Fig S4. B220+ and CD4+ cells were magnetically isolated from total splenocytes from WT and Notch1DC−/− mice. Notch1DC−/− and WT BMDCs were generated. cDNA from B220+, CD4+, and CD11c+ cells was assessed for Notch1 target gene Hes1 expression relative to Gapdh. n = 4 mice per group. *p<0.05, Student’s unpaired t test.
Fig S5. Hes1 expression relative to Gapdh in BMDCs from WT, ADAM10DC−/− and ADAM10DC−/− N1-ICD+ mice.