Abstract
An extract of a Malleastrum sp. (Meliaceae) collected in Madagascar by the Madagascar International Cooperative Biodiversity Group was found to have antimalarial activity, with an IC50 value between 2.5 and 5 µg/mL. After purification by liquid-liquid partition, chromatography on a Diaion open column, C-18 SPE and C-18 reverse phase HPLC, the new butanolide malleastrumolide A was isolated. The structure of malleastrumolide A was determined by mass spectrometry, NMR and ECD. The double bond position was determined by cross-metathesis and mass spectrometry. The compound has antiproliferative activity against the A2780 ovarian cancer cell line with an IC50 value of 17.4 µM and antiplasmodial activity against the drug-resistant Dd2 strain of Plasmodium falciparum with an IC50 value of 2.74 µM.
Keywords: Malleastrum sp., Antiplasmodial activity, Butanolide
Introduction
Malaria is a very serious disease, causing 429,000 deaths globally in 2015.[1] The first effective class of antimalarial drugs was quinine and its analogs, with chloroquine being a low cost and widely available drug. Regrettably in the 1950’s the Plasmodium parasite developed resistance to chloroquine, and resistant parasites to chloroquine are now widespread.[2] The best antimalarial drug class today is that of artemisinin and its analogs,[3, 4] used in combination with other antimalarials; this is known as artemisinin-based combination therapy (ACT).[5] Unfortunately the malaria parasite P. falciparum has developed significant resistance to artemisinin in Southeast Asia,[6] so there is a continuing need to discover new and effective antimalarial drugs. This paper reports the results of a study of a Malleastrum sp. (Meliaceae) for new antiplasmodial agents. The plant was collected as part of the Madagascar International Cooperative Biodiversity Group (ICBG) program; previous work on antiproliferative compounds from Madagascar ICBG collections of this genus resulted in the discovery of new limonoids and diterpenes.[7, 8]
Results and Discussion
Isolation and Structure Elucidation
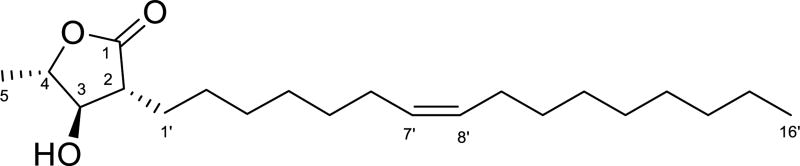
An extract of the wood of a tree of the Malleastrum genus (Meliaceae) was found to have moderate antiplasmodial activity against the Dd2 drug-resistant strain of P. falciparum, and was selected for isolation studies. Liquid-liquid partition between aqueous MeOH and hexanes and then dichloromethane (DCM) indicated that all fractions had some antiplasmodial activity, with IC50 values of about 7.0, 1.3 and 7.0 µg/mL for the hexanes, DCM, and aqueous MeOH fractions, respectively. The major aqueous MeOH fraction was separated by chromatography on a Diaion open column, C-18 SPE, and C-18 reverse phase HPLC to yield the new long-chain butanolide malleastrumolide A (Fig. 1).
Figure 1.
Chemical structure of malleastrumolide A.
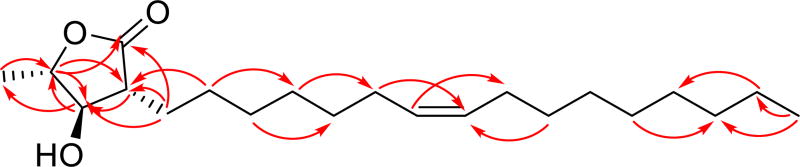
Malleastrumolide A was isolated as a yellow powder. Its positive ion HRESIMS revealed a peak for a protonated molecular ion at m/z 339.2897 and for a sodiated molecular ion at m/z 361.2714, both corresponding to a molecular formula of C21H38O3. Its IR spectrum exhibited bands at 3398 and 1761 cm−1, indicating the presence of hydroxyl and ester carbonyl groups. The presence of a hydroxylated lactone was suggested by 1H NMR signals at δH 2.55 (td, 3J (H,H) = 5.7, 8.1, H-C (2)), 3.84 (dd, 3J (H,H) = 8.1, 6.4, H-C (3)), and 4.20 (dq, 3J (H,H) = 6.4, 6.4, H-C (4)) and by 13C NMR signals at δC 175.8 (C-1), 48.6 (C-2), 79.1 (C-3) and 79.8 (C-4). The hydroxylactone partial structure was confirmed by 2J-HMBC correlations between H-2 and C-3, H-3 and C-4, and 3J-HMBC correlations between H-4 and C-1, H-4 and C-2 (Table 1 and Fig. 2).
Table 1.
1H- (500 MHz) and 13C-NMR (125 MHz) data for compound 1 in CDCl3
| Position | δH (J in Hz) | δC, type |
|---|---|---|
| 1 | 175.8, C | |
| 2 | 2.55 td, 8.1, 5.7 | 48.6, CH |
| 3 | 3.84 dd, 8.1, 6.4 | 79.1, CH |
| 4 | 4.20 dq, 6.4, 6.4 | 79.8, CH |
| 5 | 1.46 d, 6.4 | 18.2, CH3 |
| 1' | 1.82 – 1.91, m | 28.4, CH2 |
| 1.55 – 1.64 m | ||
| 2' | 1.47 – 1.54 m | 26.8, CH2 |
| 1.29 – 1.35 m | ||
| 3' | 1.24 – 1.29, m | 29.4, CH2 |
| 4' | 1.24 – 1.29, m | 29.4, CH2 |
| 5' | 1.31 – 1.35, m | 29.5, CH2 |
| 6' | 1.98 – 2.05, m | 27.2, CH2 |
| 7' | 5.30 – 5.39, m | 129.9, CH |
| 8' | 5.30 – 5.39, m | 130.0, CH |
| 9' | 1.98 – 2.05, m | 27.2, CH2 |
| 10' | 1.31 – 1.35, m | 29.5, CH2 |
| 11' | 1.31 – 1.35, m | 29.5, CH2 |
| 12' | 1.24 – 1.29, m | 29.4, CH2 |
| 13' | 1.24 – 1.29, m | 29.4, CH2 |
| 14' | 1.24 – 1.29, m | 31.9, CH2 |
| 15' | 1.24 – 1.29, m | 22.6, CH2 |
| 16' | 0.88 t, 6.9 | 14.1, CH3 |
Figure 2.
HMBC correlations of malleastrumolide A
The doublet methyl group at 1.46 ppm was connected to C-4 as indicated by 2J-HMBC correlations between H-5 and C-4, and 3J-HMBC correlations between H-5 and C-3 (Fig. 2). The presence of an unsaturated C16 chain, with a terminal methyl group at δH 0.88 (t 6.9, H-16') and δC 14.1 (C-16'), was inferred from the 1H NMR and 13C NMR spectra. The vinyl protons appeared at δH 5.34 (m, 2H) and δC 129.9 and 130.0. The CH2 groups of the side chain resonated at δH 2.01 (m, 4 H), 1.86 (m, 1 H), 1.60 (m, 1 H), 1.48 (m, 1 H), 1.27–1.34 (m, 19 H) and at δC 22.6, 26.8–31.9 (13 C). The long side chain was attached to C-2, based on the 2J-HMBC correlations between H-1' and C-2, and the 3J-HMBC correlations between H-1' and C-1, H-1' and C-3, and H-2' and C-2 (Figure 1).
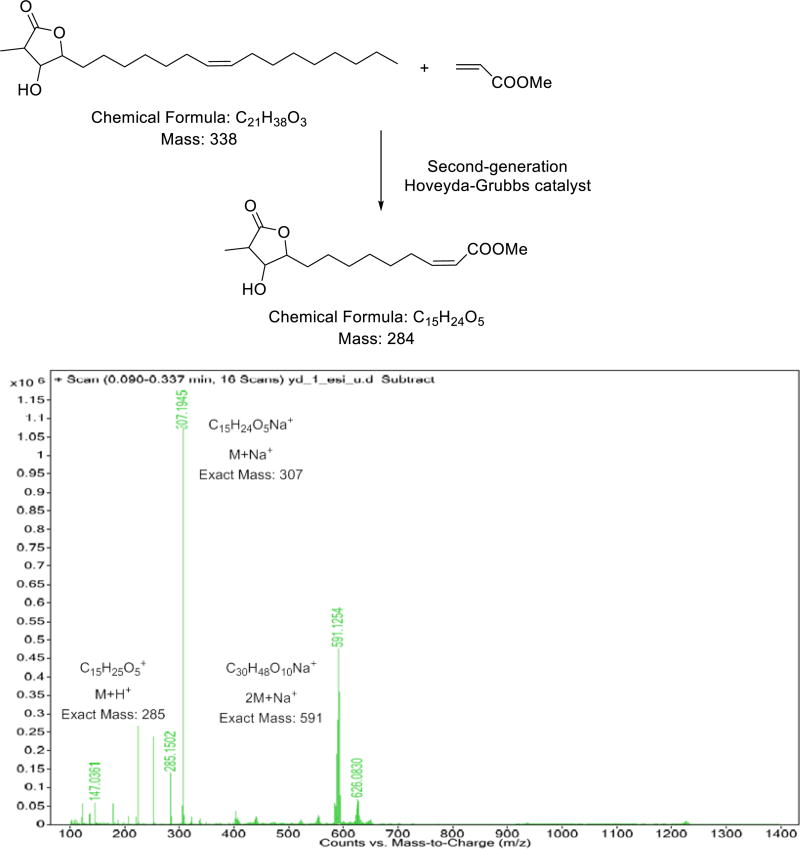
The position of the double bond was determined by the cross-metathesis method.[9] Malleastrumolide A was reacted with methyl acrylate and second-generation Hoveyda-Grubbs catalyst, and the crude product was analyzed by positive ion HRESIMS. The major peak at m/z 307 corresponded to C15H24O5Na+, and peaks at m/z 285 and 591 corresponded to C15H24O5+H+ and to (C15H24O5)2Na+, respectively. This analysis confirmed the position of the double bond as between C-7' and C-8' (Fig. 3).
Figure 3.
Cross-metathesis reaction of 1 and the mass spectrum of the crude product
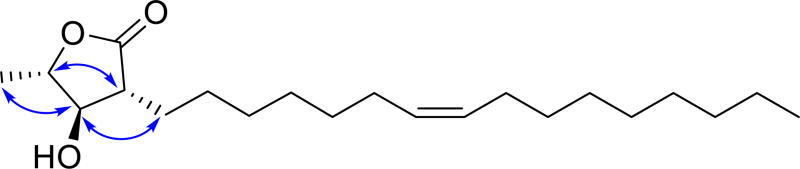
The relative configuration of the double bond was assigned as Z based on the 13C NMR signals at δC 27.2 and 27.2 (C-6' and C-9'), since carbons adjacent to a E double bond in an alkenyl chain normally resonate at 27–28 ppm,[10] while carbons adjacent to a Z double bond normally resonate at 32–34 ppm.[11] The relative configuration of the lactone was assigned as 2R*,3R*,4S* by the 13C NMR signals at δC 79.1 (C-3) and 79.8 (C-4),[12] and by the NOESY crosspeaks between H-1' and H-3, H-2 and H-4, and H-3 and H-5, as shown in Fig. 4.
Figure 4.
NOESY correlations for malleastrumolide A
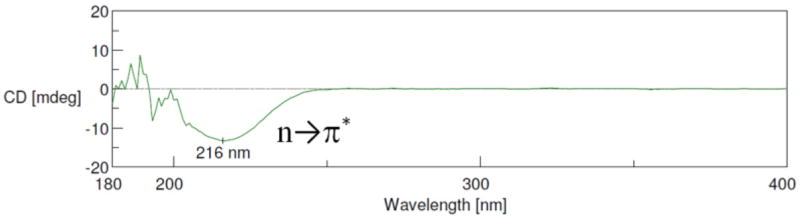
The absolute configuration of malleastrumolide A was assigned by its ECD spectrum (Fig. 5). The n → π* transition band at 216 nm showed a negative Cotton effect, defining the absolute configuration of C-2 as R.[13, 14] The structure and absolute stereochemistry of malleastrumolide A were thus assigned as (2R,3R,4S)-2-((Z)-hexadec-7-en-1-yl)-3-hydroxy-4-methyl-butanolide.[10]
Figure 5.
The ECD spectra of malleastrumolide A
Bioassays
Malleastrumolide A was tested for antiplasmodial activity against the Dd2 strain of P. falciparum and for antiproliferative activity to A2780 ovarian cancer cells. It displayed moderate antiplasmodial activity (Table 2), with an IC50 value of 2.74 µM, and weak antiproliferative activity, with an IC50 value of 17.44 µM.
Table 2.
Bioactivities of malleastrumolide A
| Malleastrumolide A | Artemisinin | Paclitaxel | |
|---|---|---|---|
| P. falciparum Dd2 (µM) | 2.74 ± 0.27 | 0.007 ± 0.002 | NT |
| A2780 (µM) | 17.4 ± 2.1 | NT | 0.028 ± 0.002 |
Experimental Section
General Experimental Procedures
Optical rotations were recorded on a JASCO P-2000 polarimeter. UV and IR spectroscopic data were measured on a Shimadzu UV-1201 spectrophotometer and a MIDAC M-series FTIR spectrophotometer, respectively. CD spectra were obtained on a JASCO J-815 circular dichroism spectrometer. NMR spectra were recorded in CDCl3 on Bruker Avance 500 or 600 spectrometers. The chemical shifts are given in δ (ppm), and coupling constants (J) are reported in Hz. Mass spectra were obtained on an Agilent 6220 LC-TOF-MS in the positive ion mode.
Antiplasmodial Bioassays
The effect of each fraction and pure compound on parasite growth of the P. falciparum Dd2 strain was measured in a 72 h growth assay in the presence of drug as described previously with minor modifications. Briefly, ring stage parasite cultures (100 µL per well, with 1% hematocrit and 1% parasitemia) were then grown for 72 h in the presence of increasing concentrations of the drug in a 5% CO25% O2, and 90% N2 gas mixture at 37 °C. After 72 h in culture, parasite viability was determined by DNA quantitation using SYBR Green I.[15] The half-maximum inhibitory concentration (IC50) calculation was performed with GraphPad Prism software using nonlinear regression curve fitting. IC50 values are the average of three independent determinations with each determination in duplicate and are expressed ± SD and artemisinin was used as positive control.[16]
In vitro antiproliferative activity against A2780 cells
The A2780 ovarian cancer cell line antiproliferative bioassay was performed at Virginia Tech as previously reported.[17, 18] The A2780 cell line is a drug-sensitive ovarian cancer cell line.[19] Paclitaxel was used as the positive control.
Plant Material
Plant specimens of Malleastrum (Baill.) J.-F. Leroy were collected on July 16, 2000 in the Province of Toamasina in the Alaotra-Mangoro region in Zahamena National Park in secondary forest with Lantana camara, on a ridge at an elevation of 880 m at coordinates 17°28'28"S, 048°44'12"E. The plant was a tree 8 m tall with a diameter at breast height of 12 cm. It is unifoliolate, with the petiole winged at the apex; it has a few small flowers and is pubescent on the leaves and inflorescences, but not densely so on the twigs. Voucher specimens are deposited at the herbarium of the Missouri Botanical Garden Herbarium and were collected by L.M. Randrianjanaka, S. Rakotonandrasana, J. Randriamanarivo & L.P. Rakotosoa 564 (MO).
The genus Malleastrum (Baill.) J.-F. Leroy is endemic to Madagascar and comprises 20 currently accepted species. However, there appear to be at least 4 additional species new to science among recently collected specimens, and the plant material in this study is almost certainly from one of those species that is new to science and still waiting to be named and described.
Extraction and Isolation
Dried and powdered the wood of Malleastrum sp. (350 g) was exhaustively extracted with ethanol in two 24-hour percolation steps to yield 10.1 g of extract. For purposes of fractionation and purification, a portion of this extract (1.95 g) designated MG0433 was shipped to Virginia Tech for bioassay and chemical studies. A total of 1.3 g of this sample (IC50 2.5~5 µg/mL) was suspended in aqueous MeOH (MeOH-H2O 9:1, 100 mL), and extracted with hexane (3 × 100 mL portions). The aqueous MeOH layer was diluted to 60% MeOH (v/v) with H2O and extracted with DCM (3 × 100 mL portions). The hexane fraction was evaporated in vacuo to leave 180 mg of material with IC50 7.0 µg/mL. The residue from the DCM fraction (20.7 mg) was the most active fraction with an IC50 value of l.3 µg/mL. The remaining aqueous MeOH fraction was centrifuged to give a supernatant (800 mg) with an IC50 of 7.0 µg/mL.
The aqueous MeOH fraction was directly applied on Diaion open column to yield 40% aqueous MeOH fraction (280 mg) with no activity, 70% aqueous MeOH fraction (188 mg) with no activity, 100% MeOH fraction (115 mg) with an IC50 of ~10 µg/mL and 100% acetone fraction (47 mg) with an IC50 of < 1.25 µg/mL. The DCM fraction and acetone fraction were combined due to the similarity of their TLC patterns. The combined fraction was directly applied on C18 HPLC, and eluted by 60% to 100% acetonitrile in water gradient in 30 min to yield malleastrumolide A (1) (7.0 mg), with retention time of 41.7 minutes.
Malleastrumolide A (1)
yellow powder; [α]D21 −11.3 (c 4×10−4 g/mL, MeOH); UV (c 0.296 mM, MeOH) λmax (ε) 203 nm (1464); IR υmax 3398, 2927, 2858,1781, 1761, 1665, 1461 cm−1; ECD (c 0.296 mM, MeOH) Δε216 −13.4; HRESIMS m/z 361.2714 [M+Na]+ (calcd. for C21H38NaO3+, 361.2713) and 339.2897 [M+H]+ (calcd. for C21H39O3+, 339.2894); 1H-NMR (500 MHz, CDCl3) and 13C-NMR (125 MHz, CDCl3), see Table 1.
Determination of Double-Bond Position
Butanolide 1 (0.5 mg) was dissolved in a 10:1 mixture of CHCl3 and methyl acrylate (0.5 mL), and 50 µg of second generation Hoveyda–Grubbs catalyst was added. The mixture was stirred at room temperature for 3 h, and a 10 µL aliquot was injected into a positive ion HRESIMS (Fig. 3)[9]
Supplementary Material
Acknowledgments
This project was supported by the National Center for Complementary and Integrative Health under award 1 R01 AT008088. The plant collection work was supported by the Fogarty International Center, the National Cancer Institute, the National Institute of Allergy and Infectious Diseases, the National Institute of Mental Health, the National Institute on Drug Abuse, the National Heart Lung and Blood Institute, the National Center for Complementary and Alternative Medicine, the Office of Dietary Supplements, the National Institute of General Medical Sciences, the Biological Sciences Directorate of the National Science Foundation, and the Office of Biological and Environmental Research of the U.S. Department of Energy under Cooperative Agreement U01 TW00313 with the International Cooperative Biodiversity Groups. These supports are gratefully acknowledged. Work at Virginia Tech was supported by the National Science Foundation under Grant No. CHE-0722638 for the purchase of the Agilent 6220 mass spectrometer and Grant No. CHE-0619382 for purchase of the Bruker Avance 500 NMR spectrometer. We thank Mr. B. Bebout and Mr. Mehdi Ashraf-Khorassani for recording the mass spectra, Dr. Narasimhamurthy Shanaiah for assistance with the NMR spectra, and Dr. T. Grove for the use of the JASCO J-815 spectrometer. Fieldwork essential for this project was conducted under a collaborative agreement between the Missouri Botanical Garden and the Parc Botanique et Zoologique de Tsimbazaza and a multilateral agreement between the ICBG partners, including the Centre National d’Application des Recherches Pharmaceutiques. We thank L. M. Randrianjanaka, S. Rakotonandrasana, J. Randriamanarivo, and L. P. Rakotosoa for the plant collection, and we gratefully acknowledge courtesies extended by the Government of Madagascar (Ministère des Eaux et Forêts).
Footnotes
Supporting information for this article is available on the web under https://doi.org/10.1002/cbdv.2017XXXX
Conflict of Interest
The authors declare no conflict of interest.
Author Contributions Statement
David Kingston conceived and designed the experiments, Alexander Abedi isolated the active compound, and Yongle Du determined its structure. Vincent E. Rasamison made the extract of Malleastrum sp. Ana Lisa Valenciano and Maria L. Fernández-Murga carried out the antiplasmodial bioassays under the direction of Maria B. Cassera, and Wendy L. Applequist and James S. Miller provided additional details of the identity of the Malleastrum sp.
References
- 1.Anonymous. World Malaria Report 2016. World Health Organization; Geneva: 2016. [Google Scholar]
- 2.Batista R, Silva AD, de Oliveira AB. Plant-Derived Antimalarial Agents: New Leads and Efficient Phytomedicines. Part II. Non-Alkaloidal Natural Products. Molecules. 2009;14:3037–3072. doi: 10.3390/molecules14083037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kong LY, Tan RX. Artemisinin, a miracle of traditional Chinese medicine. Nat. Prod. Rep. 2015;32:1617–1621. doi: 10.1039/c5np00133a. [DOI] [PubMed] [Google Scholar]
- 4.Balint G. Artemisinin and its Derivatives: an Important New Class of Antimalarial Agents. Pharmacol. Ther. 2001;90:261–265. doi: 10.1016/s0163-7258(01)00140-1. [DOI] [PubMed] [Google Scholar]
- 5.Eastman RT, Fidock DA. Artemisinin-based combination therapies: a vital tool in efforts to eliminate malaria. Nature. 2009;7:864–874. doi: 10.1038/nrmicro2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, Sreng S, Anderson JM, Mao S, Sam B, Sopha C, Chuor CM, Nguon C, Sovannaroth S, Pukrittayakamee S, Jittamala P, Chotivanich K, Chutasmit K, Suchatsoonthorn C, Runcharoen R, Hien TT, Thuy-Nhien NT, Thanh NV, Phu NH, Htut Y, Han K-T, Aye KH, Mokuolu OA, Olaosebikan RR, Folaranmi OO, Mayxay M, Khanthavong M, Hongvanthong B, Newton PN, Onyamboko MA, Fanello CI, Tshefu AK, Mishra N, Valecha N, Phyo AP, Nosten F, Yi P, Tripura R, Borrmann S, Bashraheil M, Peshu J, Faiz MA, Ghose A, Hossain MA, Samad R, Rahman MR, Hasan MM, Islam A, Miotto O, Amato R, MacInnis B, Stalker J, Kwiatkowski DP, Bozdech Z, Jeeyapant A, Cheah PY, Sakulthaew T, Chalk J, Intharabut B, Silamut K, Lee SJ, Vihokhern B, Kunasol C, Imwong M, Tarning J, Taylor WJ, Yeung S, Woodrow CJ, Flegg JA, Das D, Smith J, Venkatesan M, Plowe CV, Stepniewska K, Guerin PJ, Dondorp AM, Day NP, White NJ. Spread of Artemisinin Resistance inPlasmodium falciparum Malaria. New Engl. J. Med. 2014;371:411–423. doi: 10.1056/NEJMoa1314981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y, Wiedle CH, Brodie PJ, Callmander MW, Rakotondrajaona R, Rakotobe E, Rasamison VE, Kingston DGI. Antiproliferative Diterpenes from a Malleastrum sp. from the Madagascar Dry Forest. Nat. Prod. Commun. 2015;10:1509–1512. [PMC free article] [PubMed] [Google Scholar]
- 8.Murphy BT, Brodie P, Slebodnick C, Miller JS, Birkinshaw C, Randrianjanaka LM, Andriantsiferana R, Rasamison VE, TenDyke K, Suh EM, Kingston DGI. Antiproliferative Limonoids of a Malleastrum sp. from the Madagascar Rainforest. J. Nat. Prod. 2008;71:325–329. doi: 10.1021/np070487q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwon Y, Lee S, Oh DC, Kim S. Simple Determination of Double-Bond Positions in Long-Chain Olefins by Cross-Metathesis. Angew. Chem., Int. Ed. 2011;50:8275–8278. doi: 10.1002/anie.201102634. [DOI] [PubMed] [Google Scholar]
- 10.Allouche N, Apel C, Martin MT, Dumontet V, Gueritte F, Litaudon M. Cytotoxic Sesquiterpenoids from Winteraceae of Caledonian Rainforest. Phytochemistry. 2009;70:546–553. doi: 10.1016/j.phytochem.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 11.Zampella A, Giannini C, Debitus C, D'Auriaa MV. Amphiasterins: a New Family of Cytotoxic Metabolites from the Marine Sponge Plakortis quasiamphiaster. Tetrahedron. 2001;57:257–263. [Google Scholar]
- 12.Lorenzo M, Brito I, Cueto M, D'Croz L, Darias J. C-13 NMR-based Empirical Rules to Determine the Configuration of Fatty Acid Butanolides. Novel γ-Dilactones from Pterogorgia spp. Org,. Lett. 2006;8:5001–5004. doi: 10.1021/ol061572c. [DOI] [PubMed] [Google Scholar]
- 13.Beecham AF. Circular Dichroism in Lactones. Tetrahedron Lett. 1968;19:2355–2360. [Google Scholar]
- 14.Polonski T. Optical-Activity of Lactones and Lactams. 1. Conformational Dependence of the Circular-Dichroism of 1,3-Dioxolan-4-Ones. Tetrahedron. 1983;39:3131–3137. [Google Scholar]
- 15.Bennett TN, Paguio M, Gligorijevic B, Seudieu C, Kosar AD, Davidson E, Roepe PD. Novel, Rapid, and Inexpensive Cell-Based Quantification of Antimalarial Drug Efficacy. Antimicrob. Agents Chemother. 2004;48:1807–1810. doi: 10.1128/AAC.48.5.1807-1810.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du Y, Pearce KC, Dai Y, Krai P, Dalal S, Cassera MB, Goetz M, Crawford TD, Kingston DGI. Antiplasmodial Sesquiterpenoid Lactones from Trichospira verticillata: Structure Elucidation by Spectroscopic Methods and Comparison of Experimental and Calculated ECD Data. J. Nat. Prod. 2017;80:1639–1647. doi: 10.1021/acs.jnatprod.7b00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao S, Brodie PJ, Miller JS, Randrianaivo R, Ratovoson F, Birkinshaw C, Andriantsiferana R, Rasamison VE, Kingston DGI. Antiproliferative Xanthones of Terminalia calcicola from the Madagascar Rain Forest. J. Nat. Prod. 2007;70:679–681. doi: 10.1021/np060627g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan ED, Harinantenaina L, Brodie PJ, Miller JS, Callmander MW, Rakotonandrasana S, Rakotobe E, Rasamison VE, Kingston DGI. Four Diphenylpropanes and a Cycloheptadibenzofuran from Bussea sakalava from the Madagascar Dry Forest. J. Nat. Prod. 2010;73:1792–1795. doi: 10.1021/np100411d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Louie KG, Behrens BC, Kinsella TJ, Hamilton TC, Grotzinger KR, McKoy WM, Winker MA, Ozols RF. Radiation Survival Parameters of Antineoplastic Drug-Sensitive and Drug-Resistant Human Ovarian-Cancer Cell-Lines and Their Modification by Buthionine Sulfoximine. Cancer Res. 1985;45:2110–2115. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.