Abstract
Background
Short-chain fatty acids (SCFA) are microbial fermentation products absorbed by the colon. We recently reported that activation of the SCFA receptor termed free fatty acid receptor 3 (FFA3), expressed on cholinergic nerves, suppresses nicotinic acetylcholine receptor (nAChR)-mediated transepithelial anion secretion. This study aimed to clarify how activation of neurally-expressed FFA3 affects colonic motor function.
Methods
FFA3-expressing myenteric neurons were identified by immunostaining; contractions of isolated circular muscle strips obtained from rat proximal colon were measured by isometric transducers. The effect of FFA3 agonists on defecation in vivo was examined in an exogenous serotonin-induced defecation model.
Key Results
FFA3 immunoreactivity was located in nitrergic and cholinergic neurons in the myenteric plexus. In isolated circular muscle strips without mucosa and submucosa, the addition of nicotine (10 μM) or serotonin transiently relaxed the muscle through nitrergic neurons, whereas high concentrations of nicotine (100 μM) induced large-amplitude contractions that were mediated by cholinergic neurons. Pretreatment with FFA3 agonists inhibited nicotine- or serotonin-induced motility changes but had no effect on bethanechol-induced direct muscle contractions. The Gi/o inhibitor pertussis toxin reversed the inhibitory effect of an FFA3 agonist AR420626 on nicotine-evoked contractions, suggesting that FFA3 activation suppresses nAChR-mediated neural activity in myenteric neurons, consistent with an FFA3-mediated antisecretory effect. In conscious rats, exogenous serotonin increased the volume of fecal output, compared with the vehicle- or AR420626-treated groups. Pretreatment with AR420626 significantly suppressed serotonin-induced fecal output.
Conclusion & Inferences
FFA3 is a promising target for the treatment of neurogenic diarrheal disorders by suppressing nAChR-mediated neural pathways.
Keywords: Free fatty acid receptor 3, enteric neural pathway, proximal colon, circular muscle contraction, defecation
Graphical Abstract
Introduction
The nature and composition of the gut microbiome is altered in a variety of diseases. Supplementation of specific beneficial bacteria termed probiotics or the alteration of gut microflora by non-absorbable antibiotics or by fecal transplantation from healthy subjects significantly improves inflammatory bowel disease, insulin sensitivity, and possibly irritable bowel syndrome (IBS).1-4 Although the pathogenesis of IBS has not been fully identified, gut microbial activity is likely related to symptom severity. Since the colonic mucosa of IBS patients produces excitatory factors for cholinergic motor neurons and nociceptive neurons,5-7 supraphysiologic activation of the enteric nervous system may in part underlie IBS symptoms.
Short-chain fatty acids (SCFA), such as acetate, propionate, and butyrate are key microbial products that interact with host cells. Up to 100 mM SCFA are produced in the colonic lumen of non-ruminants by bacterial fermentation of dietary components that resist foregut digestion and absorption.8,9 In experimental rat IBS models, the supplementation of fermentable dietary fiber increases fecal SCFA content and prevents colonic transit acceleration under stressed conditions.10 IBS patients have variable colonic SCFA content compared with healthy controls, independent of the predominant symptoms of constipation or diarrhea.11,12 Since luminal SCFA are absorbed by colonic epithelial cells into the submucosa and the systemic circulation,13 SCFA-recognizing molecules are expressed in many different organs and cell types. A variety of SCFA signaling pathways are likely involved in acute and long-term physiological responses to luminal bacterial products in the gastrointestinal tract. The recent de-orphanization of SCFA-specific G protein-coupled receptors, principally free fatty acid receptor 2 (FFA2) and FFA3, and olfactory receptor 78,14-16 and SCFA transporters, including the monocarboxylate transporter (MCT) and sodium dependent MCT (SMCT) families,17 has helped to clarify the mechanism of microbiota-related physiological responses, such as the alteration of mucosal ion transport and peristalsis.
Colonic peristalsis is regulated by enteric neural reflexes initiated by luminal pressure and chemicals, including SCFA that are well studied in vivo and in vitro.18-20 When colonic muscle layers, including myenteric plexus, are isolated from the mucosa and submucosa, SCFA had no direct contractile effect on smooth muscle,21 suggesting that the epithelial cells recognize SCFA and transduce signals to motor neurons that stimulate muscle contractions. In the same colonic muscle preparations, neurally-activated muscle contractions are inhibited by SCFA, suggesting that myenteric neurons are directly influenced by SCFA during activation.22
We recently reported that FFA3 is expressed on cholinergic nerves located in the mucosal and submucosal plexus of rat proximal colon, and that FFA3 activation suppressed nAChR-mediated anion secretion, possibly through the inhibition of neural ACh release.23 Myenteric neurons expressed FFA3 more abundantly, suggesting that FFA3 significantly contributes to colonic motility regulation.23 These findings underlie the hypothesis that FFA3-selective agonists have anti-diarrheal effects through the suppression of neurogenic secretion and motility, which might have relevance to neurogenic diarrheal conditions, such as diarrhea-predominant IBS (IBS-D). Therefore, we endeavored to test the effects of FFA3 agonists on colonic motility regulation ex vivo and on an IBS-like experimental defecation model in vivo in order to further test this hypothesis.
Materials and Methods
Animals
Male Sprague–Dawley rats weighing 200–250 g (Harlan, San Diego, CA, USA) were fed a pellet diet and water ad libitum. All studies were performed with approval of the Veterans Affairs Institutional Animal Care and Use Committee (protocol# 05007-16).
Circular muscle contraction measurements
Rats were fasted overnight with free access to water before the experiments. Animals were euthanized by terminal exsanguination under deep isoflurane anesthesia, followed by thoracotomy. Segments of the proximal colon were removed and placed in cold Krebs-Ringer solution (pH 7.4), which consists of (in mmol L–1) 117 NaCl, 4.7 KCl, 1.2 MgCl2, 1.2 NaH2PO4, 25 NaHCO3, 2.5 CaCl2, and 11 glucose. The mesentery and fatty tissues attached to the serosa were removed. The colonic segments were pinned flat to a silicone-filled Petri dish; mucosa and submucosa were gently removed by fine forceps (MB-54-1; Natsume Seisakusho Co., Ltd., Tokyo, Japan) using a stereomicroscope with resultant ∼3 mm wide × 6 mm long circular muscle strips obtained. Each strip was placed in an organ bath and connected to an isometric force transducer (FORT10G; World Precision Instruments [WPI], Sarasota, FL, USA) with silk surgical sutures. The organ bath was filled with 37°C Krebs-Ringer solution and continuously bubbled with 95% O2-5% CO2. A tension of 0.5 g was initially applied to preparations. The strip preparations were equilibrated for >45 min prior to test drug application, while indomethacin (IND), tetrodotoxin (TTX), or pertussis toxin (PTX) were added into the bath immediately after mounting preparations. The viability of preparations was confirmed by the presence of spontaneous contractions (>0.5 g peaks) after the equilibration period and by application of bethanechol (0.1 mM) at the end of experiment. Circular muscle activity was sampled via a Trans-bridge transducer amplifier (TBM4M; WPI), CED Micro 1401 interface, and Spike 2 software (Cambridge Electronic Design, Cambridge, UK) at 50 Hz/16-bit. A custom program of MATLAB quantified contractile data. To determine the effects of test drugs on spontaneous contractions, we quantified the contraction frequency, mean peak amplitude, full-width half maximum (FWHM), and peak time (time between contraction peaks) of 5 peaks. Amplitude and FWHM of peak were determined for high-dose nicotine-induced contraction, and peak time was determined for analyzing the relaxant effect of low-dose nicotine or serotonin. When test drugs suppressed basal contractions, the peak time was determined as the time between the last peak before drug application and the first peak after drug application with >50% extent of prior peak.
Immunohistochemistry
Overnight fasted rats underwent transcardiac perfusion with saline followed by 4% paraformaldehyde in phosphate buffered saline (PBS, pH 7.4) under deep isoflurane anesthesia. Cryostat sections were prepared from rat proximal colon, pre-blocked with normal donkey serum (5% in PBS), and incubated with primary antibodies; goat anti-choline acetyltransferase (ChAT, 1:400; AB144P, Millipore, Temecula, CA, USA), guinea pig anti-vesicular ACh transporter (VAChT, 1:500; AB1588, Millipore), goat anti-neuronal NOS (nNOS, 1:400, ab72428, Abcam, Cambridge, MA, USA), and/or mouse anti-PGP9.5 (1:500; ab8189, Abcam) overnight at 4°C. Rabbit anti-FFA3 antibody (1 μg mL–1) was raised using recombinant C-terminal 28 amino acids of rat FFA3 (RAC VPWTQ EVSLE LKVKN GEEPS KECPS); antibody specificity was confirmed in a previous study.23 The sections were then washed, incubated with fluorescent-conjugated secondary antibodies (1:500, 2 h, room temperature; Molecular Probes, Eugene, OR, USA), and covered with EverBrite mounting medium with 4′,6-diamidino-2-phenylindole (DAPI; Biotium, Hayward, CA, USA). Immunofluorescence was imaged and captured using a confocal laser microscope (LSM710; Carl Zeiss GmbH, Jena, Germany).
Defecation model in vivo
Prior the experimental day, rats were acclimated to wire-mesh floored cages. On the experimental day at 0900, non-fasted rats were given saline-diluted AR420626 (0.1 mg kg–1) or vehicle (DMSO) via intraperitoneal (i.p.) injection. At 0930, vehicle (saline) or serotonin (10 mg kg–1) dissolved in saline was injected i.p.. The treated animals were placed individually in mesh-bottomed cages with free access to food and water; fecal pellet output was counted and weighed for 6 h until 1530.
Chemicals
N-(2-methylphenyl)-4-(furan-3-yl)-2-methyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxamide (MQC)24 was synthesized, purified and verified in Laboratory of Organic Chemistry, School of Pharmaceutical Sciences, University of Shizuoka, Japan. AR420626 was purchased from Cayman Chemical (Ann Arbor, MI, USA). AR420626 is a selective agonist of FFA3 (IC50 = 117 nM on cAMP content) that does not activate the related receptor FFA2 (GPR43) at concentrations up to 100 μM.25 GR113808 and PTX were purchased from Tocris Bioscience (Pittsburgh, PA, USA). Atropine, bethanechol, hexamethonium, serotonin, TTX, IND, nicotine, L-NAME, ondansetron, granisetron, and other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA). IND was dissolved in 100% ethyl alcohol; TTX, nicotine, bethanechol, PTX, and L-NAME were dissolved in distilled water, and other chemicals were dissolved in dimethyl sulfoxide.
Statistical analysis
Values were expressed as mean ± SEM. Statistical analysis was performed using Prism ver. 6 (GraphPad Software Inc., La Jolla, CA, USA). For multiple comparisons of > 3 groups, one-way ANOVA was used for directly measured parameters, whereas the Kruskal-Wallis test was used for results expressed as ratios with P < 0.05 considered statistically significant. All authors had access to the study data and reviewed and approved the final manuscript.
Results
Effect of FFA3 agonists on spontaneous contractions in circular muscle strips of rat proximal colon
In order to examine the effects of FFA3 activation on myenteric neurons, we chose to study isolated muscle preparations including myenteric plexus, which eliminate the influences of the FFA3-expressing submucosal plexus and mucosal enteroendocrine cells.23 Strips of proximal colon circular muscle spontaneously generated constant contractions with 2 large amplitude spikes/min that started within 45 min after mounting the preparation (Fig. 1A). The frequency of spontaneous contractions was increased by treatment with the cyclooxygenase inhibitor IND or the NOS inhibitor L-NAME, but was not altered by TTX, signifying that spontaneous contractions of circular muscle in this experimental system are inhibited by endogenously generated prostaglandins (PGs) and by NO release (Fig. 1B). None of these treatments significantly changed the mean amplitude of spontaneous contractions (Fig. 1C). The time course of repeated waveforms is defined by FWHM and peak time. Mean FWHM was not altered by any drug treatment (Fig. 1D), whereas peak time was decreased by IND, TTX, or L-NAME (Fig. 1E), indicating that the interval of spontaneous contraction was shortened without alteration of each contractile peak form. Although peak time is a reciprocal value of Hz and inversely correlated with frequency, peak time was preferred for statistical analysis among the groups.
Figure 1.
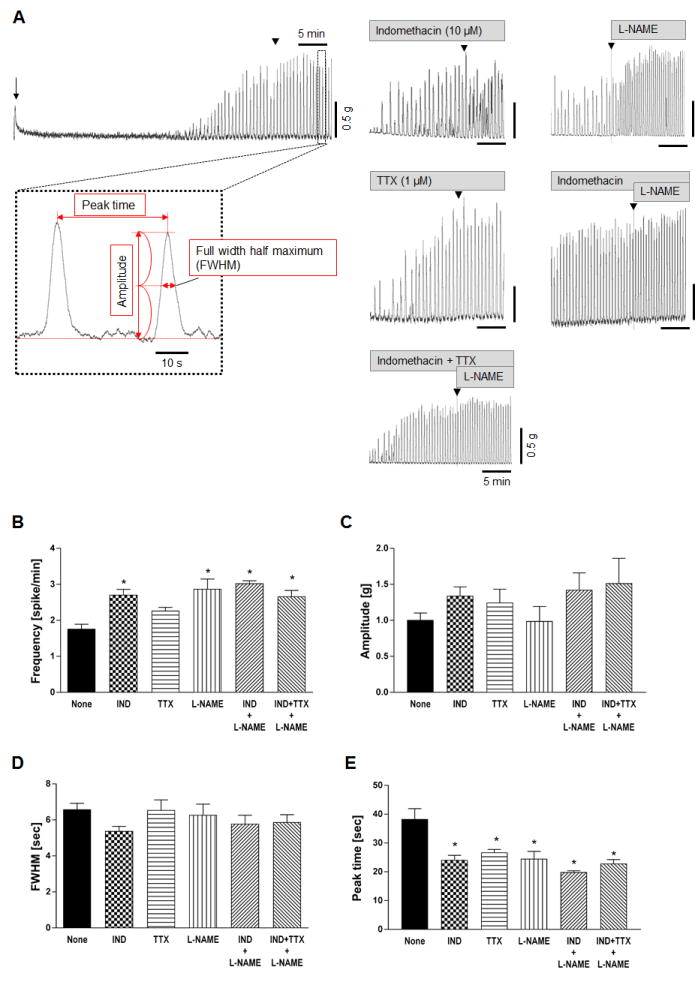
Characterization of spontaneous contractions of circular muscle strips isolated from rat proximal colon. A: Representative traces showing spontaneous mechanical activities of mucosa-free circular muscles. An arrow indicates the initial tension applied after mounting tissues. Arrowheads in each trace indicate at time = 45 min. Calculation method of peak amplitude, peak time, FWHM value were shown in the high-magnification view. B-E: Average of contraction frequency (B), peak amplitude (C), FWHM (D), and peak time (E) were determined in the presence or absence (None) of indomethacin (IND, 10 μM), tetrodotoxin (TTX, 1 μM), and/or L-NAME (0.1 mM). *P < 0.05 vs. None group by ANOVA followed by Dunnett's multiple comparison test. No significant difference was detected in amplitude or FWHM. (N = 6 - 25)
Effect of a FFA3 agonist on high-dose nicotine-induced contractions
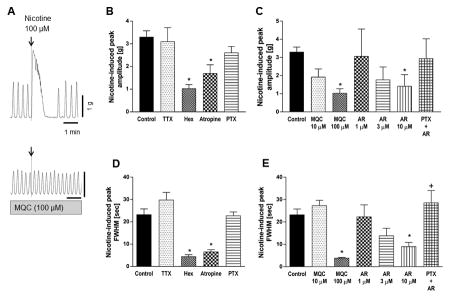
Application of 100 μM nicotine into the organ bath immediately and strongly contracted the circular muscle strip followed by transient inhibition of spontaneous contractions (Fig. 2A). The initial contraction was quantified and analyzed with amplitude and FWHM values in the presence of IND and L-NAME in order to eliminate inhibitory effect of PGs and NO and to compare well-defined contractile responses. FWHM was used as another indicator of contraction strength in order to estimate the average time over which the muscle preparation was contracted by nicotine. FWHM was reproducible for each drug treatment. This response was abolished by pretreatment with the cholinergic antagonist atropine or hexamethonium, but not altered by TTX or PTX, suggesting that TTX-resistant direct ACh release was induced by nicotine (100 μM) through nAChR activation (Fig. 2B and 2D). Nicotine-induced contractions were abolished by the synthetic FFA3 agonists MQC (100 μM) or AR420626 (10 μM) (Fig. 2C and 2E). The effect of AR420626 was more potent than that of MQC, and was reversed by pretreatment with the Gi/o inhibitor PTX (Fig. 2E). These results suggest that FFA3 activation may suppress ACh release through Gi/o activation at the nerve terminals innervating circular muscles.
Figure 2.
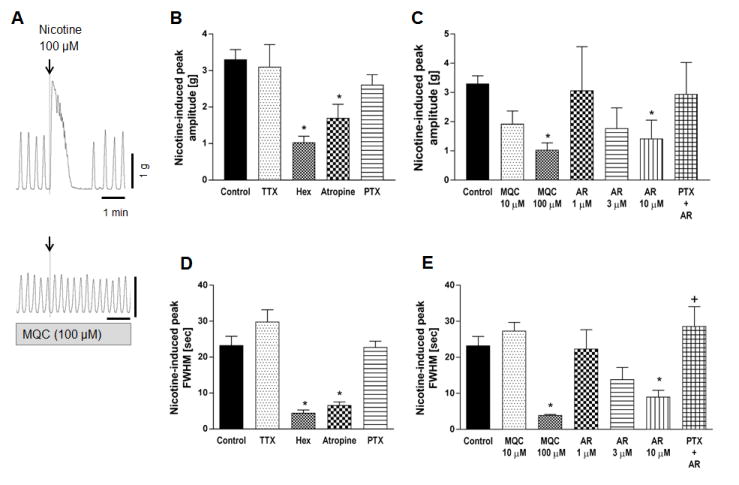
Effect of nicotine (100 μM) on circular muscle contractions of rat proximal colon. A: Representative traces of circular muscle contractions showing the contractile effect of 100 μM nicotine (upper) and the inhibitory effect of FFA3 agonist MQC on nicotine-induced contractions (lower). B-C: Peak amplitude of nicotine-evoked contraction was compared in the presence or absence (Control) of TTX (1 μM), hexamethonium (Hex, 1 mM), atropine (1 μM), PTX (500 ng/ml), or various concentration of MQC or AR420626 (AR). D-E: FWHM values of nicotine-evoked peak were determined. Pretreatment with PTX reversed the inhibitory effect of AR420626 (10 μM). *P < 0.05 vs. Control and †P < 0.05 vs. AR (10 μM) by ANOVA followed by Tukey's multiple comparison test. (N = 4 - 6)
Effect of FFA3 agonists on low-dose nicotine-induced relaxation
The spontaneous contractions of circular muscle were transiently silenced and recovered without alteration of basal tone by the addition of 10 μM nicotine into the organ bath (Fig. 3A). This response was inhibited by TTX or L-NAME, indicating that this concentration of nicotine (10 μM) activated nitrergic neurons to release NO, predominantly reliant on TTX-sensitive voltage-dependent sodium channels (Fig. 3B). AR420626 (10 μM) abolished nicotine-induced relaxation (Fig. 3A, B), suggesting that FFA3 activation may inhibit NO release from nitrergic neurons.
Figure 3.
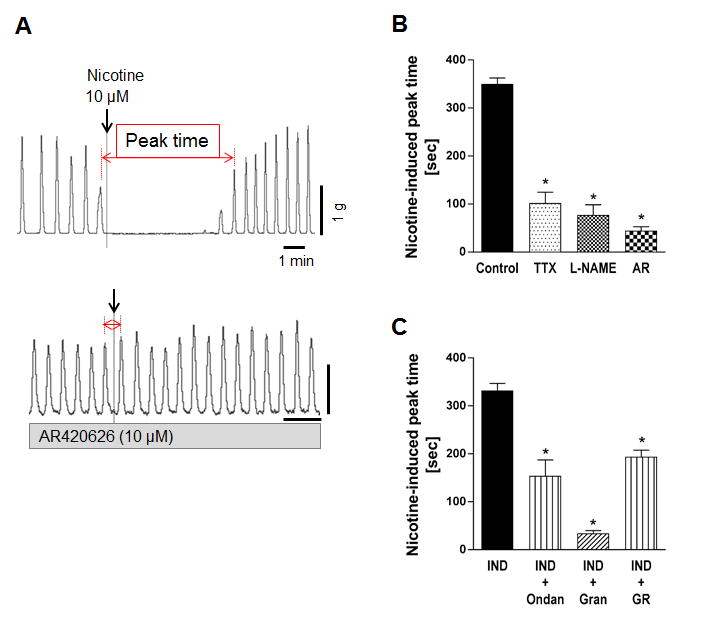
Effect of nicotine (10 μM) on circular muscle contractions of rat proximal colon. A: Representative traces of circular muscle contractions showing that 10 μM nicotine suppressed spontaneous contractions (upper) and pretreatment of AR420626 (10 μM) inhibited the effect of nicotine (lower). B: Nicotine-induced suppression of spontaneous contractions was shown as peak time value in the presence or absence (Control) of TTX (1 μM), L-NAME (0.1 mM), or AR420626 (AR). C: Nicotine-prolonged peak time was determined in the presence or absence of 5-HT3 antagonist ondansetron (Ondan, 10 μM), granisetron (Gran, 10 μM), or the 5-HT4 antagonist GR113808 (GR, 10 μM). All preparations were pretreated with indomethacin. *P < 0.05 vs. Control (B) or IND (C) by ANOVA followed by Dunnett's multiple comparison test. (N = 4 - 6)
Effect of serotonin receptor antagonists on low-dose nicotine-induced relaxation
The involvement of serotonin receptors in nicotine (10 μM)-evoked neurogenic relaxation was examined in the presence of IND in order to avoid PG-mediated relaxation. Nicotine (10 μM) suppressed circular muscle spontaneous contractions to the similar extent in the presence (Fig. 3C) or absence of IND (Fig 3B). Pretreatment with the 5-HT3 antagonist ondansetron (10 μM) or the 5-HT4 antagonist GR113808 (10 μM) significantly inhibited, whereas another potent 5-HT3 antagonist, granisetron (10 μM) abolished the relaxation response to nicotine (Fig. 3C), suggesting that serotonergic neurons and neural 5-HT3 and 5-HT4 receptors 26, 27 are involved in nicotine-induced circular muscle relaxation.
Effect of FFA3 agonists on serotonin-induced relaxation
In order to confirm the involvement of serotonergic pathway in the inhibitory response to low-dose nicotine, the effect of serotonin was examined in the presence of IND. The application of serotonin (100 μM) into the organ bath immediately contracted proximal colon circular muscle strips followed by transient inhibition of spontaneous contractions (Fig. 4A). The first peak after serotonin application was not changed by L-NAME, AR420626 (10 μM), or granisetron (10 μM) (Fig. 4B), whereas the peak time induced by serotonin was significantly reduced by L-NAME, AR420626, or granisetron (Fig. 4C). These data suggest that serotonin stimulates NO release through 5-HT3 activation, inhibiting circular muscle contraction, and that FFA3 activation reverses the serotonin-evoked NO-mediating circular muscle relaxation.
Figure 4.
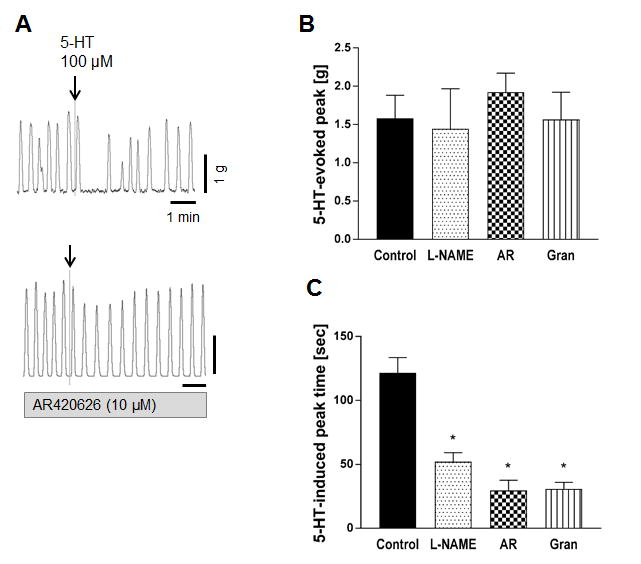
Effect of serotonin (5-HT) on circular muscle contractions of rat proximal colon. A: Representative traces of circular muscle contractions showing that serotonin suppressed spontaneous contractions after initial contraction (upper) and pretreatment of AR420626 inhibited the effect of serotonin (lower). B-C: Serotonin-induced peak (B) and peak time after the initial contraction (C) were determined in the presence or absence (Control) of L-NAME (0.1 mM), AR420626 (10 μM), or granisetron (10 μM). All preparations were pretreated with indomethacin. *P < 0.05 vs. Control by ANOVA followed by Dunnett's multiple comparison test. (N = 4 - 8)
Effect of FFA3 agonists on bethanechol-induced contractions
The muscarinic ACh receptor agonist bethanechol immediately induced large contractions that were abolished by the muscarinic antagonist atropine (Fig. 5). Pretreatment with TTX or hexamethonium did not alter the response to bethanechol likely due to its direct stimulation of smooth muscle cells. Neither AR420626 nor MQC affected bethanechol-induced contractions (Fig. 5), suggesting that the inhibitory effect of FFA3 activation is not via direct action on the muscle cells.
Figure 5.
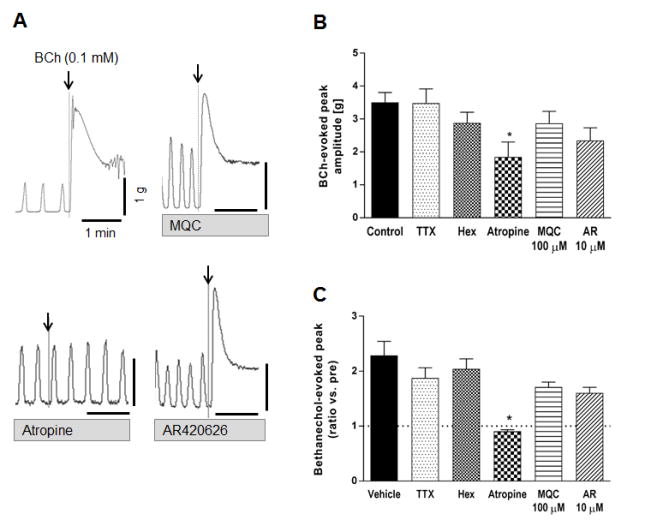
Effect of the muscarinic agonist bethanechol (BCh) on contractions of circular muscle in rat proximal colon. A: Representative traces showing the contractile effect of BCh (0.1 mM) and lack of inhibitory effect of MQC (100 μM) or AR420626 (10 μM). Pretreatment of atropine (1 μM) abolished the response to BCh. B-C: BCh-induced maximal peak amplitude (B) and the ratio vs. spontaneous contraction peak (C) were compared in the presence or absence (Control) of TTX (1 μM), hexamethonium (1 mM), atropine, MQC, or AR420626. *P < 0.05 vs. Control by ANOVA (B) or Kruskal-Wallis (C) test followed by Dunnett's multiple comparison test. (N = 5 - 7)
Effect of FFA3 agonist AR420626 on exogenous serotonin-induced defecation in vivo
Since 5-HT3 receptor antagonists are therapeutic for IBS-D,28 we tested the effect of the potent FFA3 agonist AR420626 on serotonin-induced defecation model.29 Intraperitoneal serotonin injection enhanced fecal pellet output in conscious rats, as determined by pellet number and weight (Fig. 6A, B). Watery diarrhea did not occur; fecal pellet shapes were similar to those in the control group. Although pretreatment with AR420626 alone had no effect on defecation, AR420626 significantly inhibited serotonin-induced fecal output.
Figure 6.
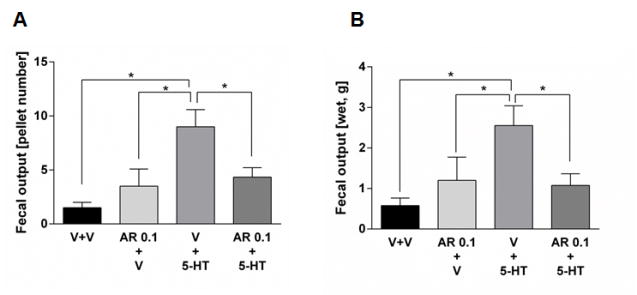
Effect of exogenous serotonin (10 mg/kg) on fecal output in conscious rats. Vehicle (V) or AR420626 (AR, 0.1 mg/kg) were i.p. injected 30 min before the injection of serotonin (5-HT) or vehicle. Fecal number (A) and wet fecal weight (B) were measured for 6 hr. *P < 0.05 by ANOVA followed by Fisher's multiple comparison test. (N = 6)
FFA3 localization in enteric neurons and intramuscular nerves
We previously reported that FFA3 immunoreactivity was localized to a subset of myenteric neurons and nerves in whole mount preparations of rat proximal colon.23 In the present study, we found that intramuscular nerve fibers running along the long axis of smooth muscle cells as well as myenteric neurons co-expressed FFA3 and nNOS (Fig. 7). Almost all PGP9.5-positive intramuscular nerve fibers expressed nNOS, indicating that inhibitory motor neurons innervate the muscle layers and release NO at the connections with smooth muscle cells (Fig. 7). In contrast to the myenteric plexus, nNOS did not typically colocalize with FFA3 in the submucosal plexus (Supplemental Fig. 1), suggesting that nitrergic neurons in the myenteric plexus predominantly innervate muscle layers, but not the submucosa and mucosal layers. FFA3 colocalized with cholinergic marker VAChT in the myenteric plexus enabling identification of intramuscular nerves (Fig. 8), consistent with expression in the mucosal and submucosal plexus.23 Higher magnification of intramuscular nerve fibers in the circular muscle sections revealed colocalization of FFA3-positive dots with cholinergic and nitrergic neural markers, ChAT and nNOS, respectively (Fig. 9).
Figure 7.
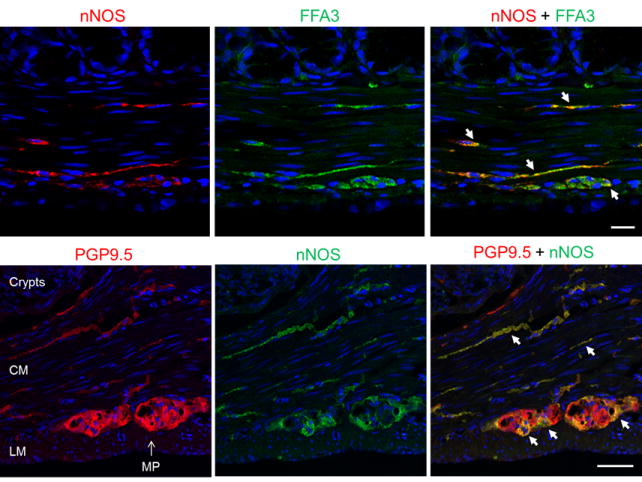
Immunohistochemical study for nitrergic neurons in proximal colonic sections. Upper panels: Immunoreactivities for neural NOS (nNOS, red) and FFA3 (green) were identified in the intramuscular nerves and myenteric plexus. Lower panels: The localization of nNOS (green) in neurons was confirmed by the colocalization with a pan-neuronal marker PGP9.5 (red). Colocalization was indicated by arrows (yellow). Bar: 20 μm.
Figure 8.
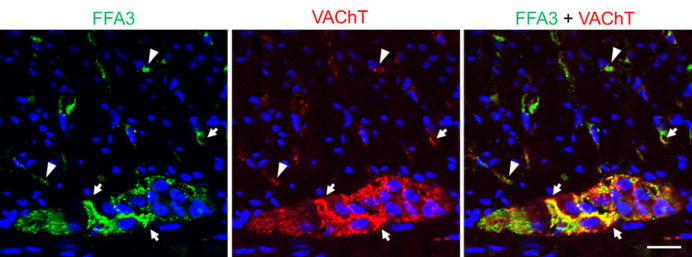
Immunohistochemistry of cholinergic neurons in proximal colonic sections. Immunoreactivities of FFA3 (green) and a cholinergic neural marker VAChT (red) were identified in intramuscular nerves and myenteric plexus. Colocalization was indicated by arrows (yellow). Arrowheads represent the lack of colocalization. Bar: 20 μm.
Figure 9.
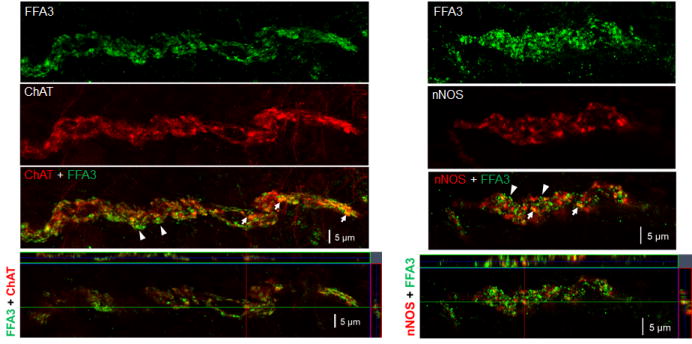
Confocal microscopy with z-stack imaging demonstrating colocalization of FFA3 and cholinergic (ChAT) or nitrergic (nNOS) neural markers in intramuscular nerve fibers in proximal colonic thick sections. The upper three panels depict 3D image whereas the bottom panels depict a confocal image demonstrating the coincidence of green-labeled FFA3 and red-labeled neural marker in varicosities appearing as a yellow color (arrows). Arrowheads indicate lack of colocalization. Bar: 5 μm.
Discussion
We demonstrated that FFA3 activation inhibited nicotine-evoked neural activity in the regulation of colonic motility, extending our prior observations that FFA3 was localized on cholinergic nerves in the mucosal and submucosal plexuses, and that FFA3 activation suppressed nicotine-evoked neurogenic anion secretion via activation of the inhibitory Gi/o pathway.23 In mucosa-free circular muscle strips, both NO-mediated relaxation and ACh-mediated contractions were suppressed by FFA3 agonists, consistent with the immunohistochemical finding that FFA3 is expressed on intramuscular varicosities of nitrergic (inhibitory) and cholinergic nerves and on these neurons. Furthermore, a selective and potent FFA3 agonist prevented exogenous serotonin-induced circular muscle relaxation ex vivo and defecation in vivo, emphasizing the therapeutic potential of FFA3 agonists as anti-diarrheal drugs.
FFA3 localization in myenteric plexus
ACh and NO are primary transmitters of excitatory and inhibitory enteric motor neurons, respectively.30 In the myenteric plexus and intramuscular nerve fibers, FFA3 was expressed in cholinergic and nitrergic nerves, in contrast to the mucosal and submucosal plexuses, where FFA3 was localized mostly in cholinergic nerves.23 As FFA3 is scarcely detected in the submucosal neuronal body, some myenteric FFA3-positive cholinergic neurons likely project nerve fibers to the mucosa and the submucosa. In contrast to the cholinergic neurons, submucosal nitrergic neurons did not express FFA3, suggesting that nitrergic neurons in submucosal and myenteric plexuses may differentially innervate the mucosa and muscle layers, respectively, in order to regulate ion secretion and muscle contractions via distinct pathways.
FFA3 function in nAChR-mediated colonic motility regulation
Vagal excitation and exogenous nicotine activate nAChRs located on numerous postsynaptic neurons, which may release ACh or NO, triggering muscle contraction or relaxation, respectively.31 Consistent with the localization of neural FFA3, FFA3 agonists inhibited both ACh-mediated contraction and NO-mediated relaxation elicited by exogenous nicotine. In neuron-free isolated muscle preparations prepared from guinea pig ileum, TTX-resistant neurotransmitter release elicited by electrical field stimulation and exogenous nicotine contracted smooth muscle.32 In the present study, high dose (100 μM) nicotine induced large-amplitude contractions through a TTX-independent pathway. The anti-muscarinic drug atropine or anti-nicotinic drug hexamethonium blocked the response to nicotine, suggesting that nAChR activation elicited ACh release from varicosities that directly excites circular muscle via muscarinic receptors. The FFA3 agonists MQC or AR420626 abolished nicotine-evoked contraction, whereas they had no effect on direct muscle contraction by the muscarinic ACh receptor agonist bethanechol, suggesting that FFA3 activation specifically inhibits neural nAChR-mediated responses. Low-dose nicotine (10 μM) transiently suppressed spontaneous contractions through the TTX- and L-NAME-sensitive pathways, consistent with nerve transmission-mediated NO release that was reported previously in longitudinal muscle.33, 34 As AR420626 blocked NO-mediated relaxation, which is induced by nicotine or exogenous serotonin, FFA3 activation may also inhibit nitrergic (inhibitory) neurons. Endogenous serotonin signals were involved in the nicotine-induced neurogenic relaxation predominantly through 5-HT3 receptors, implying that intrinsic serotonergic neurons possess nAChR receptors and nitrergic neurons express 5-HT3 receptors. Almost all myenteric neurons express the α3, α5, β2, and β4 subunits of nAChR. The predominant contribution of α3β4 towards the ACh-evoked response is supported by immunohistochemical and electrophysiological experiments with isolated neurons.35 Although future investigation would likely further characterize the nAChR subunits that function in each subtype of myenteric neuron, the present results from tissue-level experiments suggest that a low concentration of nicotine (10 μM) may selectively activate inhibitory pathways, whereas a high concentration (100 μM) likely stimulate varicosities of cholinergic nerve fibers located in colonic circular muscle layers.32 Since Gi/o inhibition blocked the inhibitory effect of FFA3, neuronal Gi/o activation likely underlies the mechanism of FFA3-mediated neural inhibition. For example, the somatostatin receptor, the adrenoceptor α-2a, and the muscarinic ACh receptors 2 and 4 are well-studied Gi/o-coupling membrane receptors, which suppress neuronal action potentials and inhibit neurotransmitter release from synapses.36-39 Similar to ACh receptors, as yet unknown endogenous FFA3 ligands other than bacterially-generated SCFA might additionally provide negative feedback in the enteric nervous system.
Are enteric neurons physiologically suppressed by FFA3 activation?
The present study demonstrated that FFA3 was localized to myenteric neurons and that FFA3 activation appeared to mostly reduce the activity of inhibitory neurons and also reduced the serotonin-evoked peristalsis reflex. FFA3-deficient (KO) mice have accelerated intestinal transit and low plasma concentrations of the anti-motility hormone peptide YY (PYY), released from FFA3-expressing enteroendocrine L cells.40, 41 Together with the present study, intestinal motility in FFA3-KO animals might be enhanced by the lack of inhibitory controls through FFA3-mediated neural and humoral pathways. Due to this lack of inhibition, FFA3-KO mice likely malabsorb SCFA because of rapid peristalsis, limiting intraluminal fermentation and SCFA generation.41 SCFA are the principal metabolites of microbial fermentative activity, serving as a host energy source after active absorption across the intestinal epithelium via the transport proteins SMCT and the MCTs.17 It is reasonable to assume that subepithelial SCFA affect enteric neurons expressing FFA3 with consequent effects on gastrointestinal functions such as ion and water secretion and absorption and peristalsis, in order to maintain host homeostasis and a healthy host-microbial relationship.
Potential of FFA3 agonists for IBS therapy
IBS, which affects > 10% of the global population,42 disrupts quality-of-life with frequent episodes of abdominal discomfort, constipation, and diarrhea. Serotonin and corticotropin-releasing factor receptors have been hypothesized to mediate IBS-like symptoms based on defecation studies in response to physiological stressors;43,44 furthermore, 5-HT3 antagonists improve functional disease symptoms, including IBS and vomiting.28 Peripheral administration of serotonin or its precursor 5-hydroxy-L-tryptophan induce abnormal defecation through the activation of 5-HT3 and 5-HT4 receptors in rats,29,45 implicating that these animal models in some ways resemble IBS-D. In the present study, the potent FFA3 agonist AR420626 prevented serotonin-induced circular muscle relaxation ex vivo and defecation in vivo. Although it is difficult to explain serotonin-evoked peristalsis solely on results derived from ex vivo experiments, circular muscle relaxation occurring together with longitudinal contraction46 in the proximal colon may be a local mechanism by which serotonin evokes the defecatory reflex. In light of these experimental studies, orally-active FFA3 agonists that target enteric nerves may be useful therapeutically for IBS or other neurogenic functional disorders.
The present study and our prior studies documented that FFA3 was localized on enteric nerves and that FFA3 activation inhibits colonic secretion and motility initiated by nAChR activation. From our data obtained from ex vivo and in vivo experiments, FFA3 agonists may have a therapeutic potential for IBS-D treatment, based on the suppression of nAChR-mediated enteric neural pathways.
Supplementary Material
Supplemental Figure 1. Lack of colocalization of nNOS and FFA3 in whole mount submucosal plexus. Double staining for nNOS (red) and FFA3 (green) showed that nitrergic neuron bodies and nerves did not correspond to FFA3 immunoreactivity. Bar: 50 μm.
Key points.
Short-chain fatty acids produced by the gut microbiota contribute to the colonic motility regulation in health and disease although the molecular mechanisms underlying this interaction are not fully understood.
We identified the expression of a short-chain fatty acid receptor, free fatty acid receptor 3 (FFA3), in inhibitory and excitatory myenteric neurons. The activation of FFA3 suppressed nicotine-induced colonic contraction and relaxation ex vivo, and prevented serotonin-induced defecation in vivo.
FFA3 is a promising target for treatment of neurogenic diarrhea.
Acknowledgments
The authors thank Ms. Stacey Jung for manuscript preparation, and Drs. Paul H Guth and Eli Engel for their expert advice. The authors also thank Dr. Daniel Poole in Monash University and Dr. Toshihiko Iwanaga in Hokkaido University for their helpful discussions.
Funding: This study was supported by VA Merit Review funding (to JDK), the National Institutes of Health, USA (R01 DK54221 to JDK), and AGA-Rome Foundation Pilot Research Award for Functional Gastroenterology and Motility Disorders (to IK). Antibody production was supported by a project of Comprehensive Brain Science Network (CBSN) in Japan (to MW).
Abbreviations
- ACh
acetylcholine
- ChAT
choline acetyltransferase
- IBS-D
diarrhea predominant IBS
- FFA
free fatty acid receptor
- IBS
irritable bowel syndrome
- IND
indomethacin
- MCT
monocarboxylate transporter
- SMCT
sodium dependent MCT
- MQC
N-(2-methylphenyl)-4-(furan-3-yl)-2-methyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxamide
- L-NAME
Nω-nitro-L-arginine methyl ester
- nAChR
nicotinic ACh receptor
- NO
nitric oxide
- NOS
NO synthase
- nNOS
neuronal NOS
- PTX
pertussis toxin
- SCFA
short-chain fatty acids
- TTX
tetrodotoxin
- VAChT
vesicular ACh transporter
Footnotes
Disclosure: No conflicts of interest exist.
Author contributions: IK designed, performed, and analyzed the research study, and wrote the paper; YA, AK, MW & JDK supervised the study and edited the manuscript for important intellectual content; FT & DWA contributed essential tools and analyzed data; MW contributed the antibody production; AK & KI contributed essential reagents.
Reference List
- 1.Matsuoka K, Mizuno S, Hayashi A, Hisamatsu T, Naganuma M, Kanai T. Fecal microbiota transplantation for gastrointestinal diseases. Keio J Med. 2014;63:69–74. doi: 10.2302/kjm.2014-0006-RE. [DOI] [PubMed] [Google Scholar]
- 2.Rossen NG, MacDonald JK, de Vries EM, D'Haens GR, de Vos WM, Zoetendal EG, Ponsioen CY. Fecal microbiota transplantation as novel therapy in gastroenterology: A systematic review. World J Gastroenterol. 2015;21:5359–5371. doi: 10.3748/wjg.v21.i17.5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hungin AP, Mulligan C, Pot B, Whorwell P, Agreus L, Fracasso P, Lionis C, Mendive J, Philippart de Foy JM, Rubin G, Winchester C, de WN. Systematic review: probiotics in the management of lower gastrointestinal symptoms in clinical practice -- an evidence-based international guide. Aliment Pharmacol Ther. 2013;38:864–886. doi: 10.1111/apt.12460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmad OF, Akbar A. Microbiome, antibiotics and irritable bowel syndrome. Br Med Bull. 2016;120:91–99. doi: 10.1093/bmb/ldw038. [DOI] [PubMed] [Google Scholar]
- 5.Balestra B, Vicini R, Cremon C, Zecchi L, Dothel G, Vasina V, De GR, Paccapelo A, Pastoris O, Stanghellini V, Corinaldesi R, De PF, Tonini M, Barbara G. Colonic mucosal mediators from patients with irritable bowel syndrome excite enteric cholinergic motor neurons. Neurogastroenterol Motil. 2012;24:1118–e570. doi: 10.1111/nmo.12000. [DOI] [PubMed] [Google Scholar]
- 6.Cenac N, Andrews CN, Holzhausen M, Chapman K, Cottrell G, Andrade-Gordon P, Steinhoff M, Barbara G, Beck P, Bunnett NW, Sharkey KA, Ferraz JG, Shaffer E, Vergnolle N. Role for protease activity in visceral pain in irritable bowel syndrome. J Clin Invest. 2007;117:636–647. doi: 10.1172/JCI29255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buhner S, Li Q, Vignali S, Barbara G, De GR, Stanghellini V, Cremon C, Zeller F, Langer R, Daniel H, Michel K, Schemann M. Activation of human enteric neurons by supernatants of colonic biopsy specimens from patients with irritable bowel syndrome. Gastroenterology. 2009;137:1425–1434. doi: 10.1053/j.gastro.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Cummings JH. Short chain fatty acids in the human colon. Gut. 1981;22:763–779. doi: 10.1136/gut.22.9.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergman EN. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol Rev. 1990;70:567–590. doi: 10.1152/physrev.1990.70.2.567. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi T, Nakade Y, Fukuda H, Tsukamoto K, Mantyh C, Pappas TN. Daily intake of high dietary fiber slows accelerated colonic transit induced by restrain stress in rats. Dig Dis Sci. 2008;53:1271–1277. doi: 10.1007/s10620-008-0228-8. [DOI] [PubMed] [Google Scholar]
- 11.Treem WR, Ahsan N, Kastoff G, Hyams JS. Fecal short-chain fatty acids in patients with diarrhea-predominant irritable bowel syndrome: in vitro studies of carbohydrate fermentation. J Pediatr Gastroenterol Nutr. 1996;23:280–286. doi: 10.1097/00005176-199610000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Mortensen PB, Clausen MR. Short-chain fatty acids in the human colon: relation to gastrointestinal health and disease. Scand J Gastroenterol Suppl. 1996;216:132–148. doi: 10.3109/00365529609094568. [DOI] [PubMed] [Google Scholar]
- 13.Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28:1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ, Pike NB, Strum JC, Steplewski KM, Murdock PR, Holder JC, Marshall FH, Szekeres PG, Wilson S, Ignar DM, Foord SM, Wise A, Dowell SJ. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312–11319. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- 15.Le PE, Loison C, Struyf S, Springael JY, Lannoy V, Decobecq ME, Brezillon S, Dupriez V, Vassart G, Van DJ, Parmentier M, Detheux M. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem. 2003;278:25481–25489. doi: 10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- 16.Pluznick J. A novel SCFA receptor, the microbiota, and blood pressure regulation. Gut Microbes. 2014;5:202–207. doi: 10.4161/gmic.27492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwanaga T, Kishimoto A. Cellular distributions of monocarboxylate transporters: a review. Biomed Res. 2015;36:279–301. doi: 10.2220/biomedres.36.279. [DOI] [PubMed] [Google Scholar]
- 18.Yokokura T, Yajima T, Hashimoto S. Effect of organic acid on gastrointestinal motility of rat in vitro. Life Sci. 1977;21:59–62. doi: 10.1016/0024-3205(77)90424-6. [DOI] [PubMed] [Google Scholar]
- 19.Yajima T. Contractile effect of short-chain fatty acids on the isolated colon of the rat. J Physiol. 1985;368:667–678. doi: 10.1113/jphysiol.1985.sp015882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richardson A, Delbridge AT, Brown NJ, Rumsey RD, Read NW. Short chain fatty acids in the terminal ileum accelerate stomach to caecum transit time in the rat. Gut. 1991;32:266–269. doi: 10.1136/gut.32.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitsui R, Ono S, Karaki S, Kuwahara A. Neural and non-neural mediation of propionate-induced contractile responses in the rat distal colon. Neurogastroenterol Motil. 2005;17:585–594. doi: 10.1111/j.1365-2982.2005.00669.x. [DOI] [PubMed] [Google Scholar]
- 22.Dass NB, John AK, Bassil AK, Crumbley CW, Shehee WR, Maurio FP, Moore GB, Taylor CM, Sanger GJ. The relationship between the effects of short-chain fatty acids on intestinal motility in vitro and GPR43 receptor activation. Neurogastroenterol Motil. 2007;19:66–74. doi: 10.1111/j.1365-2982.2006.00853.x. [DOI] [PubMed] [Google Scholar]
- 23.Kaji I, Akiba Y, Konno K, Watanabe M, Kimura S, Iwanaga T, Kuri A, Iwamoto K, Kuwahara A, Kaunitz JD. Neural FFA3 activation inversely regulates nicotinic ACh receptor-mediated secretion in rat proximal colon. The Journal of physiology. 2016;594:3339–3352. doi: 10.1113/JP271441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ulven T. Short-chain free fatty acid receptors FFA2/GPR43 and FFA3/GPR41 as new potential therapeutic targets. Front Endocrinol (Lausanne ) 2012;3:111. doi: 10.3389/fendo.2012.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engelstoft MS, Park WM, Sakata I, Kristensen LV, Husted AS, Osborne-Lawrence S, Piper PK, Walker AK, Pedersen MH, Nohr MK, Pan J, Sinz CJ, Carrington PE, Akiyama TE, Jones RM, Tang C, Ahmed K, Offermanns S, Egerod KL, Zigman JM, Schwartz TW. Seven transmembrane G protein-coupled receptor repertoire of gastric ghrelin cells. Mol Metab. 2013;2:376–392. doi: 10.1016/j.molmet.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson DS, Heinemann SF. Detection of 5-HT3R-A, a 5-HT3 receptor subunit, in submucosal and myenteric ganglia of rat small intestine using in situ hybridization. Neurosci Lett. 1995;184:67–70. doi: 10.1016/0304-3940(94)11170-n. [DOI] [PubMed] [Google Scholar]
- 27.Poole DP, Xu B, Koh SL, Hunne B, Coupar IM, Irving HR, Shinjo K, Furness JB. Identification of neurons that express 5-hydroxytryptamine4 receptors in intestine. Cell Tissue Res. 2006;325:413–422. doi: 10.1007/s00441-006-0181-9. [DOI] [PubMed] [Google Scholar]
- 28.Prior A, Read NW. Reduction of rectal sensitivity and post-prandial motility by granisetron, a 5 HT3-receptor antagonist, in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 1993;7:175–180. doi: 10.1111/j.1365-2036.1993.tb00087.x. [DOI] [PubMed] [Google Scholar]
- 29.Miyata K, Kamato T, Nishida A, Ito H, Yuki H, Yamano M, Tsutsumi R, Katsuyama Y, Honda K. Role of the serotonin3 receptor in stress-induced defecation. J Pharmacol Exp Ther. 1992;261:297–303. [PubMed] [Google Scholar]
- 30.Furness JB, Poole DP, Cho HJ, Callaghan BP, Rivera LR. The innervation of the gastrointestinal tract Yamada's Textbook of Gastroenterology. Oxford, UK: John Wiley & Sons, Ltd; 2015. pp. 239–258. [Google Scholar]
- 31.Takahashi T, Owyang C. Characterization of vagal pathways mediating gastric accommodation reflex in rats. J Physiol. 1997;504(Pt 2):479–488. doi: 10.1111/j.1469-7793.1997.479be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneider DA, Perrone M, Galligan JJ. Nicotinic acetylcholine receptors at sites of neurotransmitter release to the guinea pig intestinal circular muscle. J Pharmacol Exp Ther. 2000;294:363–369. [PubMed] [Google Scholar]
- 33.Borjesson L, Nordgren S, Delbro DS. DMPP causes relaxation of rat distal colon by a purinergic and a nitrergic mechanism. Eur J Pharmacol. 1997;334:223–231. doi: 10.1016/s0014-2999(97)01173-4. [DOI] [PubMed] [Google Scholar]
- 34.Patel BA, Galligan JJ, Swain GM, Bian X. Electrochemical monitoring of nitric oxide released by myenteric neurons of the guinea pig ileum. Neurogastroenterol Motil. 2008;20:1243–1250. doi: 10.1111/j.1365-2982.2008.01177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou X, Ren J, Brown E, Schneider D, Caraballo-Lopez Y, Galligan JJ. Pharmacological properties of nicotinic acetylcholine receptors expressed by guinea pig small intestinal myenteric neurons. J Pharmacol Exp Ther. 2002;302:889–897. doi: 10.1124/jpet.102.033548. [DOI] [PubMed] [Google Scholar]
- 36.Griffin MT, Ehlert FJ. Specific inhibition of isoproterenol-stimulated cyclic AMP accumulation by M2 muscarinic receptors in rat intestinal smooth muscle. J Pharmacol Exp Ther. 1992;263:221–225. [PubMed] [Google Scholar]
- 37.Tallent M, Liapakis G, O'Carroll AM, Lolait SJ, Dichter M, Reisine T. Somatostatin receptor subtypes SSTR2 and SSTR5 couple negatively to an L-type Ca2+ current in the pituitary cell line AtT-20. Neuroscience. 1996;71:1073–1081. doi: 10.1016/0306-4522(95)00510-2. [DOI] [PubMed] [Google Scholar]
- 38.Hein L, Altman JD, Kobilka BK. Two functionally distinct alpha2-adrenergic receptors regulate sympathetic neurotransmission. Nature. 1999;402:181–184. doi: 10.1038/46040. [DOI] [PubMed] [Google Scholar]
- 39.D'Agostino G, Barbieri A, Chiossa E, Tonini M. M4 muscarinic autoreceptor-mediated inhibition of -3H-acetylcholine release in the rat isolated urinary bladder. J Pharmacol Exp Ther. 1997;283:750–756. [PubMed] [Google Scholar]
- 40.Nohr MK, Pedersen MH, Gille A, Egerod KL, Engelstoft MS, Husted AS, Sichlau RM, Grunddal KV, Seier-Poulsen S, Han S, Jones RM, Offermanns S, Schwartz TW. GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinol. 2013;154:3552–3564. doi: 10.1210/en.2013-1142. [DOI] [PubMed] [Google Scholar]
- 41.Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, Hammer RE, Williams SC, Crowley J, Yanagisawa M, Gordon JI. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci USA. 2008;105:16767–16772. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol. 2014;6:71–80. doi: 10.2147/CLEP.S40245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ozaki A, Yoshidomi M, Sukamoto T. Effect of the 5-hydroxytryptamine3 (5-HT3)-receptor antagonist KB-R6933 on experimental diarrhea models. Jpn J Pharmacol. 1999;80:93–96. doi: 10.1254/jjp.80.93. [DOI] [PubMed] [Google Scholar]
- 44.Taché Y, Million M. Role of Corticotropin-releasing Factor Signaling in Stress-related Alterations of Colonic Motility and Hyperalgesia. J Neurogastroenterol Motil. 2015;21:8–24. doi: 10.5056/jnm14162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang L, Martinez V, Kimura H, Taché Y. 5-Hydroxytryptophan activates colonic myenteric neurons and propulsive motor function through 5-HT4 receptors in conscious mice. Am J Physiol Gastrointest Liver Physiol. 2007;292:G419–G428. doi: 10.1152/ajpgi.00289.2006. [DOI] [PubMed] [Google Scholar]
- 46.Gelal A, Guven H. Characterization of 5-HT receptors in rat proximal colon. Gen Pharmacol. 1998;30:343–346. doi: 10.1016/s0306-3623(97)00096-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Lack of colocalization of nNOS and FFA3 in whole mount submucosal plexus. Double staining for nNOS (red) and FFA3 (green) showed that nitrergic neuron bodies and nerves did not correspond to FFA3 immunoreactivity. Bar: 50 μm.


