Abstract
Objective
We sought to understand how the dietary source of carbohydrates, either high-fructose corn syrup (HFCS) or complex carbohydrates, affects energy expenditure (EE) measures, appetitive sensations, and hormones during 24h of overfeeding.
Methods
Seventeen healthy participants with normal glucose regulation had 24h-EE measures, fasting blood and 24h urine collection during four different one-day diets including an energy balanced diet, fasting, and two 75% carbohydrate diets (5% fat) given at 200% energy requirements with either HFCS or whole-wheat foods as the carbohydrate source. In eight volunteers, hunger was assessed with visual analog scales the morning after the diets.
Results
Compared with energy balance, 24h-EE increased 12.8±6.9% with carbohydrate overfeeding (p<0.0001). No differences in 24h-EE or macronutrient utilization were observed between the two high-carbohydrate diets; however, sleeping metabolic rate was higher after the HFCS diet (Δ=35±48 kcal (146±200 kJ); p=0.01). Insulin, ghrelin, and triglycerides increased the morning after both overfeeding diets. Urinary cortisol concentrations (82.8±35.9 v 107.6±46.9 nmol/24h, p=0.01) and morning-after hunger scores (Δ=2.4±2.0 cm, p=0.01) were higher with HFCS overfeeding.
Conclusions
The dietary carbohydrate source while overeating did not affect 24h-EE, but HFCS overconsumption may predispose to further overeating due to increased glucocorticoid release and increased hunger the following morning.
Keywords: carbohydrates, energy expenditure, cortisol
Introduction
Ingestion of added sugars is prevalent in the United States despite nutritional guidelines recommending against this practice(1). Routine carbohydrate overconsumption is likely secondary to the wide availability of easily prepared food products(2, 3); however, episodes of massive overeating most likely occur only intermittently and for a single day, e.g., a holiday or a celebratory event. Associations between the source of carbohydrate consumed and body weight regulation have been hypothesized(4–6). Increasing the proportion of simple or complex carbohydrates in the diet reportedly does not produce significant weight change or alter metabolic risk profiles(5); however, others have reported a diet with a high proportion of simple sugars causes adverse effects(7–10). Whether overconsumption of simple sugars is more detrimental than overeating more complex carbohydrates is unknown.
In the Lifestyle Heart Trial, participants ate a low-fat, vegetarian diet high in carbohydrates consisting of primarily vegetables and whole grains, and although the experimental group reported the same caloric intake as controls, their weight decreased 10 kg (11). While this effect may be due, in part, to an increase in physical activity, it is possible there is also an effect of diet on energy expenditure (EE). Epidemiologic studies indicate that an increasing percentage of high-fructose corn syrup (HFCS) in diets correlates with higher energy intake, increased body weight, and increased risk of metabolic and cardiovascular disorders(10). However, fructose also is reported to have a higher obligatory cost of metabolism, acutely increasing diet-induced thermogenesis by approximately 2% more than glucose, promoting carbohydrate oxidation, and suppressing lipid oxidation to a greater degree than glucose(12). Most of the studies assessing differences between carbohydrate sources on EE were done over six hours, but a longer observation of at least 24 hours may be needed to assess the full effect upon EE. It is known that overconsuming carbohydrates, especially fructose, can increase triglycerides and uric acid concentrations(7, 8), but acute effects of one day of overeating carbohydrates on appetitive and anabolic hormones are not as clear.
Many studies have investigated differences solely between pure fructose versus glucose(12–16), but studies with direct comparison of common, readily available, similarly prepared diets, only varying in carbohydrate source, are lacking. In addition, the overall effects of carbohydrate overconsumption for 24 hours are not well studied. This study investigated whether dietary source of carbohydrates, HFCS versus not, in readily available, easily prepared foods during 24h of overeating 200% of energy requirements would differentially impact EE, macronutrient utilization, appetitive and anabolic hormones, or appetite.
Methods
Subjects
Seventeen healthy adults greater than 21 years with no evidence of acute or chronic illness, as assessed by history, physical, electrocardiogram and laboratory measures participated in this study between 2011 and 2013, which was a smaller study within a larger study designed to examine the effects of overfeeding on EE (NCT00523627) as previously described(17). All subjects provided informed consent; the study protocol was approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Each subject completed all procedures while residing in the Clinical Research Unit (CRU) of the NIDDK in Phoenix, AZ for approximately 18 days. Normal glucose regulation, as assessed by a 75g oral glucose tolerance test(18), was an inclusion criterion. Body composition was measured by dual-energy x-ray absorptiometry (DXA; GE Lunar, Madison, WI, USA).
Diets
Upon admission, subjects were placed on a weight-maintaining diet (50%, 30%, and 20% of daily energy provided as carbohydrate, fat and protein, respectively) using unit-specific equations as previously described(19). All subjects underwent five separate 24h-EE measurements while residing in a whole-room indirect calorimeter (described below) with four distinct diets: twice with a eucaloric diet to increase measurement precision (20) plus three intervention diets. Energy needs for the first eucaloric measurement were calculated as 80% of the weight-maintaining diet to account for restricted activity in the calorimeter; energy intake for the second eucaloric diet was equal to the individual’s 24hEE measure from the first assessment. The second eucaloric assessment was the baseline comparator, as subjects were in energy balance (EB). Using a cross-over design with intervention diets given in random order, subjects underwent a fasting assessment where only water was provided and two overfeeding diets consisting of 75% carbohydrates (20% protein, 5% fat) with a caloric content equal to twice EE measured during EB. Subjects were not blinded to diet received. Each diet was separated by three days, during which the subject remained on the CRU. The two high-carbohydrate diets consisted of readily available, easily prepared (i.e. frozen, canned or packaged) foods, differing primarily in carbohydrate source, either HFCS or whole-wheat flour (WW) (Table 1). Foods in the HFCS diet had HFCS listed as one of the first three ingredients. Foods in the WW diet contained a long-chained carbohydrate as one of the first three ingredients (i.e., whole-wheat flour) and were more likely to be labeled as “natural” or “multigrain”. Foods in the high-carbohydrate diets were matched except for these ingredients, e.g., buttermilk pancakes and white bread versus multigrain waffles and whole-wheat bread. Selection of WW foods was intended to mimic purchasing healthier options. Macronutrient composition of diets was determined using The Food Processor software (ESHA Research, Salem, OR, USA). All subjects consumed greater than 95% of all diets. There was a 3-day washout period between dietary interventions where subjects consumed the weight-maintaining diet and resided in their room on the CRU. The average CV of the subjects’ body weight taken the mornings before beginning dietary interventions was 0.99±0.59%.
Table 1. Characteristics of the two high (75%) carbohydrate overfeeding diets based on an example diet of 4000 kcal (16,736 kJ).
Macronutrient composition and the energy content of each diet was determined using The Food Processor software (ESHA Research, Salem, OR, USA).
| Diet | Total weight grams | CHO* g | Fiber g | Total Calculated Glycemic Index$ | Energy density kcal/g (kJ/g) | % of food items containing HFCS | % of food served warm | % of protein provided as animal protein | Breakfast kcal (kJ) | Lunch kcal (kJ) | Dinner kcal (kJ) | Snack kcal (kJ) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High-Fructose | 3913 | 733 | 32 | 976 | 1.02 (4.27) |
69 | 19.6 | 72 | 1009 (4222) |
1161 (4858) |
851 (3561) |
1009 (4222) |
| Whole-Wheat | 4766 | 740 | 73 | 935 | 0.84 (3.51) |
9 | 22 | 61.4 | 1134 (4745) |
1158 (4845) |
1036 (4335) |
672 (2812) |
CHO = carbohydrate, HFCS = high-fructose corn syrup
: Gram monosaccharide equivalent weight
: calculated from readily available online resources: http://foodstruct.com/; http://www.glycemicindex.com/index.php; http://file.lacounty.gov/SDSInter/dpr/184917; HealthyPark.pdf; http://recipenutrition.com/Glyo.aspx; and http://www.mendosa.com/gilists.htm
Beginning in 2012, subjects completed a Visual Analog Scale (VAS) the morning after each diet to assess hunger-related characteristics by indicating on a Likert scale (0 to 10 cm) how hungry they felt, their desire to eat, how much food they would like to consume, and how preoccupied they were with thoughts of food (n=8). No EE, anthropometric, or demographic variables differed between these eight subjects and the larger group.
Energy Expenditure
During each dietary intervention, 24h-EE and sleeping measurements were assessed using whole-room indirect calorimetry, as previously described(20). Ambient temperature averaged 24.6±1.1°C. Sleeping metabolic rate (SMR), calculated as the average EE between 2330 and 0530 when movement measured by radar sensors was less than 1.5% (<9 sec/minute), was extrapolated to an 8h time period. Diet-induced thermogenesis (DIT) was calculated as 24hEE during fasting subtracted from 24-hr EE during feeding. The 24h-EE unadjusted for physical activity is presented because there were no differences in activity, assessed by radar measures, between dietary assessments (p=0.8). Non-protein RQ (i.e., the ratio of CO2 to O2 consumption) was calculated after subtracting CO2 and O2 consumption attributable to protein oxidation determined from the 24h urinary nitrogen excretion. Carbohydrate and fat oxidation were calculated from the non-protein RQ as previously described(21).
Assays
Fasting morning plasma was collected both at entry and exit from the calorimeter and stored for batched assessment of hormone concentrations by the Clinical Core Lab of the NIDDK. In addition, fasting blood was immediately sent for assessment of triglyceride, uric acid, aspartate transaminase (AST), and alanine aminotransferase (ALT) using standard clinical assays from the local laboratory and fasting insulin concentrations were assessed using an automated immunoenzymometric assay (Tosoh Bioscience Inc, Tessenderlo, Belgium). Urine was collected for 24 hours during each dietary intervention.
Serum ghrelin, active glucagon-like peptide 1 (GLP-1) and insulin-like growth factor 1 (IGF1) were measured using enzyme-linked immunosorbent assays (ELISA) (R&D Systems, Minneapolis, MN). Adiponectin, C-reactive protein (CRP), fibroblast growth factor 21 (FGF21), leptin, pancreatic polypeptide (PP) and peptide YY (PYY) were measured using Luminex assays (R&D Systems, Minneapolis, MN; Millipore, Billerica, MA). Urinary free cortisol (UFC) concentrations were measured using ELISA (Cayman Chemical Company, Ann Arbor, MI).
Statistical Analysis
Statistical analyses were performed using SAS (SAS version 9.3, SAS Institute, Inc., Cary, NC). Alpha was set as 0.05. Assuming an expected increase in 24h-EE of 288±116 kcal (1205±485 kJ; 14.4±5.8%) when overfeeding a diet with 75% carbohydrate(20), a sample size of 17 provides greater than 80% power to detect an absolute difference of 100 kcal (418 kJ; 4%) between the two dietary interventions using a paired t-test.
All results are presented as mean±SD except for fasting insulin, which was not normally distributed, and is presented as median and interquartile range. EE changes with overfeeding and fasting are expressed as a percentage of the EE during energy balance, i.e., (EEDiet of Interest − EEEB)/EEEB ×100). Student’s t-test or 1-way ANOVA was used for comparisons between groups. Paired t-tests were used to determine differences between the 2 overfeeding diets and differences between variables measured before and after dietary interventions (or Wilcoxon signed-rank tests if data was not normally distributed). Correlations between variables were assessed using Pearson correlations (r). Further analyses to compare EB, fasting, and the two overfeeding diets were done using mixed models to account for repeated measures and, when necessary, to adjust for age, sex, and percentage body fat. A variable to indicate diet order was initially included in the models, but because it was not significant and did alter the results, it was removed.
Results
Subject Characteristics
Characteristics and EE measures during EB are shown in Table 2. No sex differences beyond expected differences in body composition were observed.
Table 2. Characteristics of the Study Population including energy expenditure measures during energy balance.
Data is presented as mean±SD (minimum, maximum).
| Variable | Whole Population (n=17) |
Men (n=13) | Women (n=4) |
|---|---|---|---|
| Ethnicity | 4 Black, 5 White, 8 Native American |
3 Black, 3 White, 7 Native American |
1 Black, 2 White, 1 Native American |
| Age (yrs) | 41.2±9.0 (20.9, 54.1) | 42.4±8.4 (20.9, 54.1) | 37.1±11.0 (20.9, 44.3) |
| Weight (kg) | 77.3±12.3 (51.4, 107.5) | 79.9±19.7 (62.2, 98.3) | 76.5±10.1 (61.4, 107.5) |
| BMI (kg/m2) | 26.3±3.8 (21.1, 39.2) | 25.4±2.1(21.1, 29.3) | 29.3±6.8 (24.9, 39.2) |
| Body Fat (%)* | 30.4±10.2 (11.9, 50.5) | 25.8±6.2 (11.9, 37.9) | 45.2±4.8 (40.4, 50.5) |
| Fat-Free Mass (kg)* | 53.3±8.4 (31.9, 66.3) | 56.4±5.6 (46.9, 66.3) | 43.5±8.8 (31.9, 53.2) |
| Fasting Glucose (mmol/L) | 5.3±0.2 (4.8, 5.5) | 5.3±0.1 (5.0, 5.5) | 5.1±0.2 (4.8, 5.4) |
| Two-hour Glucose (mmol/L) | 6.0±1.2 (3.2, 7.4) | 6.3±1.1 (3.2, 7.4) | 5.5±1.2 (4.5, 7.2) |
| Fasting Insulin (pmol/L) | 54.9±33.3 (22.9, 167.4) | 47.2±16.0 (22.9, 76.4) | 76.4±62.5 (27.8, 167.4) |
| Triglycerides (mmol/L) | 1.3±1.2 (0.3, 4.8) | 1.3±1.3 (0.3, 4.8) | 1.2±0.7 (0.5, 2.1) |
| Energy Expenditure (kcal/24h) [MJ/24h] | 1948±269 (1383, 2328) [8.15±1.13 (5.79, 9.74)] |
1998±240 (1607, 2328) [8.36±1.00 (6.72, 9.74)] |
1787±332 (1383, 2156) [7.48±1.39 (5.79, 9.02)] |
| SMR (kcal/8 h) [MJ/8 h] | 517±65 (358, 579) | 528±53 (404, 575) | 483±96 (358, 579) |
| [MJ/24h] | [2.16±0.27 (1.50, 2.42)] | [2.21±0.22 (1.69, 2.41)] | [2.02±0.40 (1.50, 2.42)] |
| RQ (ratio) | 0.89±0.03 (0.83, 0.93) | 0.89±0.02 (0.86, 0.93) | 0.88±0.04 (0.83, 0.92) |
| Energy Balance (kcal/24h) [MJ/24h] | 63±78 (−29, 130) [0.26±0.33 (−0.12, 0.54)] |
63±86 (−29, 130) [0.26±0.36 (−0.12, 0.54)] |
61±54 (3, 125) [0.26±0.23 (0.01, 0.52)] |
Indicates p<0.05 for the difference between males and females as assessed by Student’s t-test. SMR = sleeping metabolic rate, RQ = respiratory quotient
Energy Expenditure
EE and RQ changes during overfeeding are presented in Table 3 and Figure 1. Compared to EB, EE decreased 7.4% during fasting (p<0.0001). EE increases did not differ between the HFCS and WW diets (13.5±7.6 v 12.2±6.4%; p=0.4); however, percentage increase in SMR was higher following the HFCS diet compared to the WW diet [14.3±7.7 v 7.4±8.1%; p=0.006; absolute difference=35 kcal (146 kJ); p=0.007). This difference was still observed after adjustment for age, sex, body fat, and repeated measures.
Table 3. Energy expenditure measurements during 200% overfeeding with diets containing either primarily whole wheat or high-fructose corn syrup.
All 17 subjects consumed all diets. Data is presented as mean±SD (minimum, maximum). The p-value for ΔHFCS-WW was determined using a paired t-test.
| Variable | Energy Balance (n=17) |
Whole Wheat Diet (n=17) | High-Fructose Corn Syrup Diet (n=17) |
p-value for ΔHFCS-WW |
|---|---|---|---|---|
|
Intake*
(kcal/24h) [MJ/24h] |
2011±252 (1508, 2310) [8.41±1.05 (6.31, 9.67)] |
3799±472 (2719, 4412) [15.90±1.97 (11.38, 18.46)] |
3903±561 (2663, 4656) [16.33±2.35 (11.14, 19.48)] |
0.1 |
|
EE*
(kcal/24h) [MJ/24h] |
1948±269 (1383, 2328) [8.15±1.13 (5.79, 9.74)] |
2187±323 (1388, 2561) [9.15±1.35 (5.81, 10.72)] |
2209±311 (1383, 2608) [9.24±1.30 (5.79, 10.91)] |
0.5 |
| NPRQ* | 0.92±0.04 (0.84, 0.97) | 1.0±0.06 (0.90, 1.10) | 0.99±0.07 (0.84, 1.09) | 0.2 |
|
SMR*
(kcal/8h) [MJ/8h] |
517±65 (358, 579) [2.16±0.27 (1.50, 2.42)] |
560±90 (355, 706) [2.34±0.38 (1.49, 2.95)] |
595±96 (355, 740) [2.49±0.40 (1.49, 3.10)] |
0.007 |
|
DIT*
(kcal/24h) [MJ/24h] |
146±77 (2, 306) [0.61±0.32 (0.01, 1.28)] |
384±146 (134, 567) [1.61±0.61 (0.56, 2.37)] |
406±145 (129, 680) [1.70±0.61 (0.54, 2.85)] |
0.5 |
|
Carbohydrate Oxidation*
(kcal/24h) [MJ/24h] |
1126±240 (721, 1466) [4.71±1.00 (3.02, 6.13)] |
1760±480 (1050, 2447) [7.36±2.01 (4.39, 10.24)] |
1692±470 (681, 2311) [7.04±1.97 (2.85, 9.67)] |
0.4 |
|
Lipid Oxidation*
(kcal/24h) [MJ/24h] |
426±211 (106, 844) [1.78±0.88 (0.44, 3.53)] |
13±339 (−499, 652) [0.05±1.42 (−2.09, 2.73)] |
79±392 (−460, 856) [0.33±1.64 (−1.92, 3.58)] |
0.2 |
|
Protein Oxidation (kcal/24h) [MJ/24h] |
369±94 (42, 441) [1.54±0.38 (0.18, 1.85)] |
384±143 (61, 674) [1.61±0.60 (0.26, 2.82)] |
409±101 (229, 598) [1.71±0.42 (0.96, 2.50)] |
0.5 |
|
Carbohydrate Balance*
(kcal/24h) [MJ/24h] |
−119±202 (−423, 285) [−0.50±0.85 (−1.77, 1.19)] |
1029±340 (408, 1841) [4.31±1.42 (1.71, 7.70)] |
1172±371 (521, 1782) [4.90±1.55 (2.18, 7.46)] |
0.052 |
Indicates p<0.001 for the difference between both overfeeding diets and energy balance as assessed by 1-way ANOVA followed by Dunnett’s test to control for multiple comparisons. DIT = diet-induced thermogenesis, EE = energy expenditure, HFCS = high-fructose corn syrup overfeeding diet, NPRQ = non-protein respiratory quotient, SMR = sleeping metabolic rate, WW = whole wheat overfeeding diet
Figure 1.
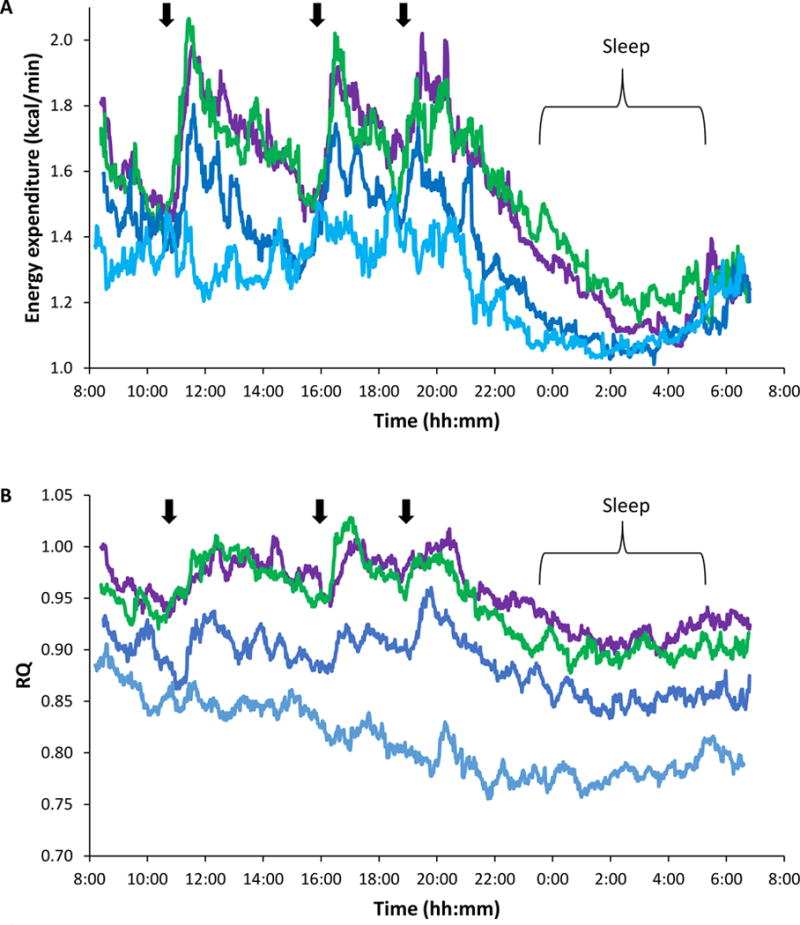
Patterns of energy expenditure (A) and respiratory quotient (B) during 24-h fasting, energy balance, and high-carbohydrate overfeeding. The average energy expenditure (EE) and respiratory quotient (RQ) per minute are shown during 23 hours of energy balance (blue), fasting (turquoise) or 200% overfeeding with a diet containing 75% carbohydrate, the primary source of which was either whole-wheat flour (purple) or high-fructose corn syrup (green). Average sleeping metabolic rate was significantly higher during complex carbohydrate overfeeding (Δ=35 kcal (146 kJ); p=0.007) but there was no difference in overall 24hEE (p=0.5) (A) or 24h RQ (p=0.1) between the overfeeding diets. Participants entered the indirect calorimeter 1h after breakfast, served at 7:00. Arrows indicate meals. Participants were asked to be in bed from the 23:00 to at least the 05:30 in the chamber and to limit unnecessary activity throughout the 24 h period. The overfeeding trajectories differed from both the energy balance and fasting trajectories in analyses using a mixed model to control for repeated measures using a cubic model for time and a compound symmetry covariance structure (EE: p<0.0001; RQ: p<0.001).
After consuming the HFCS and WW diets, subjects were in similar positive energy balance (1612±190 v 1694±308 kcal/24h (6745±795 v 7088±1289 kJ/24h); p=0.6) with no difference in mean DIT or macronutrient oxidation (Table 3). Seven subjects had a non-protein RQ that exceeded 1, consistent with net de novo lipogenesis (DNL), during both the WW and HFCS diets. The other 10 subjects had a non-protein RQ less than one during overfeeding. All seven subjects with net DNL were men; six were of Native American heritage (p=0.01 by Fisher’s exact test). There were no differences in EE measures, age, body adiposity (after adjusting for sex), total energy intake, or any plasma or urinary measures between those with and without net DNL.
Metabolic Consequences
There were no increases in uric acid or liver function tests (AST, ALT) the morning after high-carbohydrate overfeeding. However, insulin, ghrelin (Table 4), and triglycerides increased after both overfeeding diets [Triglycerides: WW: 1.06±0.85 v 1.46±0.88 mmol/L (94±75 v 129±78 mg/dl); p<0.0001; HFCS: 1.18±0.90 v 1.36±0.97 mmol/L (104±80 v 120±86 mg/dl); p=0.006). Degree of insulin and ghrelin increase did not differ between the two overfeeding diets; however, the increase in triglycerides was greater after the WW diet [Δ=0.40±0.26 v 0.20±0.26 mmol/L (35±23 v 18±23 mg/dL); p=0.02).
Table 4. Hormone and Inflammatory Marker Changes during the Dietary Interventions.
Data are presented as mean±SD (minimum, maximum) except for insulin which is presented as median (interquartile range). Percent change was calculated as: (‘After Diet’ measure – ‘Before Diet’ measure) / ‘Before Diet measure’ × 100. Urinary free cortisol was measured from a 24h urine collection taken during the differing diets. All other concentrations are from fasting blood collections taken the morning before and the morning after the diets. CRP = C-reactive protein, FGF21 = fibroblast growth factor 21, GLP-1 = glucagon-like peptide 1, IGF1 = insulin-like growth factor 1, PP = pancreatic polypeptide, PYY = peptide YY.
| Variable | High-Fructose Corn Syrup Overfeeding Diet (n=17) |
Whole Wheat Overfeeding Diet (n=17) |
Fasting (n=17) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Before Diet | After Diet | % Change | Before Diet | After Diet | % Change | Before Diet | After Diet | % Change | |
|
Leptin (pmol/L) |
469±655 (87, 2751) | 457±544 (99, 2162) |
6±21% (−33, 50) | 459±612 (103, 2635) |
409±439 (105, 1884) |
1±15% (−28, 30) | 489±678 (75, 2888) | 193±414* (7, 1721) |
−75±13% (−40, −92)* |
|
Adiponectin (ng/mL) |
7144±2915 (2743, 13769) |
7541±2240 (4486, 12322) |
13±32% (−14, 122) | 7653±2377 (4615, 13086) |
7950±2807 (4588, 13846) |
4±15% (−36, 26) | 6976±2766 (3092, 13080) |
7868±2686* (3769, 14065) |
17±23% (−8, 82)* |
|
CRP (nmol/L) |
21.9±20 (3.8, 84.8) | 27.6±30.5 (3.8, 130.5) | 16±46% (−31, 163) | 32.4±31.4 (3.8, 130.5) |
30.5±26.7 (3.8, 114.3) | 16±95% (−37, 378) |
21.0±13.3 (2.9, 43.8) |
46.7±53.3* (2.8, 228.6) |
107±106% (−33, 420)* |
|
IGF1 (nmol/L)$ |
12.2±3.9 (7.6, 23.3) |
12.1±3.5 (7.9, 21.1) |
−1±6% (−10, 10) | 11.8±3.4 (8.1, 20.6) |
12.3±3.5* (8.1, 21.7) |
5±9% (−7, 31)* |
12.3±3.4 (7.6, 22.5) | 11.4±3.1* (6.9, 18.9) |
−7±11% (−28, 10)* |
|
FGF-21 (pg/mL) |
335±457 (78, 1989) | 372±452 (80, 1940) |
15±33% (−33, 128) | 318±441 (43, 1879) |
343±495 (56, 2148) | 30±124% (−15, 510) |
329±436 (66, 1868) | 376±491* (60, 2044) |
14±27% (−14, 96)* |
|
Insulin (μIU/mL) |
49 (IQR: 35, 70) | 63* (IQR: 49, 90) | 27±44% (−24, 133)* |
49 (IQR: 35, 76) | 76* (IQR: 56, 83) | 58±71% (−29, 200)* | 56 (IQR: 35, 90) | 35* (IQR: 21,63) |
−38±30% (−75, 12)* |
|
Ghrelin (pmol/L) |
6.3±2.2 (3.8, 12.9) | 7.1±2.2* (4.8, 13.9) |
15±15% (−1, 56)* | 6.5±2.8 (4.1, 10.4) | 7.5±2.6* (5.1, 12.5) |
13±34% (−57, 107) |
7.1±3.6 (3.6, 4.4) | 6.3±1.3 (4.3, 8.9) |
−2±19% (−60, 33) |
|
GLP-1 (pmol/L) |
6.5±7.4 (1.7, 30.5) | 5.6±5.5 (2.1, 22.6) | −3±27% (−55, 41) | 7.2±7.6 (1.7, 26.8) |
7.1±7.9 (1.6, 26.5) |
−2±23% (−45, 41) | 4.3±2.3 (1.5, 10.7) | 4.2±2.9 (2.1, 13.6) |
1±33% (−56, 48) |
|
PYY (pmol/L) |
28.5±16.5 (9.8, 57.0) |
28.3±14.0 (11.0, 55.8) |
4±36% (−65, 134) | 29.8±10.3 (12.0, 47.0) |
30.5±14.8 (7.8, 70.8) |
3±45% (−58, 103) | 29.5±13.0 (14.8, 58.0) |
20.3±15.5* (2.0, 47.3) |
21±15% (−98, 147) |
|
PP (pmol/L) |
10.8±12.2 (1.7, 41.8) |
10.8±14.6 (1.7, 58.1) |
51±174% (−73, 510) | 9.1±6.9 (1.2, 22.0) | 11.0±14.3 (0.5, 55.2) |
172±434% (−82, 1558) |
7.2±6.5 (1.0, 19.4) | 20.6±13.6* (3.8, 53.1) |
434±364% (−80, 1160)* |
|
Urinary Free Cortisol (nmol/24h)$ |
108.7±47.2 (41.7, 202.9) | 83.1±35.6 (29.3, 185.5) | 86.4±31.5 (27.6, 142.4) | ||||||
Indicates p<0.05 for the change in fasting plasma concentrations before and after the diet as assessed by a paired t-test and confirmed after adjusting for baseline concentrations in an ANCOVA, or that the percent change is significantly different than 0%. All significant changes with fasting differed from the overfeeding responses with a p<0.001.
Indicates p<0.05 for differences between the hormone response to the high-fructose corn syrup and whole wheat overfeeding diets.
Hormone changes with the dietary interventions are shown in Table 4. No appetitive hormones or inflammatory markers differed between the two overfeeding diets. Of all hormones assessed, only the change in IGF1 and UFC concentrations differed between the two. IGF1 increased only on the morning after the WW diet (Δ=0.55±0.26 v −0.13±0.26 nmol/L; p=0.03). This difference between the IGF1 changes persisted after adjustment for baseline IGF1, age, sex and body fat. UFC concentrations were similar between EB, fasting, and overfeeding with the WW diet, but were higher during HFCS overfeeding (adjusted ΔHFCS-WW: 32.3±43.9 nmol/24h; p=0.01). After adjusting for differences in body fat, no correlations were observed between hormone changes and EE measures with overfeeding. All results were similar if the dataset was limited only to men or to those classified as obese (22).
Visual Analog Scales
The morning after the HFCS overfeeding diet, subjects with VAS data reported greater feelings of hunger (5.2±2.9 v 2.7±2.6 cm; p=0.01), a stronger desire to eat (5.5±3.2 v 3.2±2.4 cm; p=0.02), and were more preoccupied with thoughts of food (3.9±2.5 v 2.0±1.5 cm; p=0.01) compared to the morning after the WW diet (Figure 2). Although there was no difference in how much food subjects felt they could consume (4.7±2.8 v 3.4±2.5 cm; p=0.1), the overall point estimate was in a similar direction as the other hunger variables. In exploratory, bivariate analyses, VAS hunger scores were not correlated with EE, macronutrient oxidation measures, or hormone concentrations with the exception that both the change in FGF21 and the concurrent (morning after) FGF21 concentrations correlated similarly with all VAS scores (e.g., feelings of hunger: r=0.6, p=0.02, Figure 3; r=0.54, p=0.03, respectively).
Figure 2.
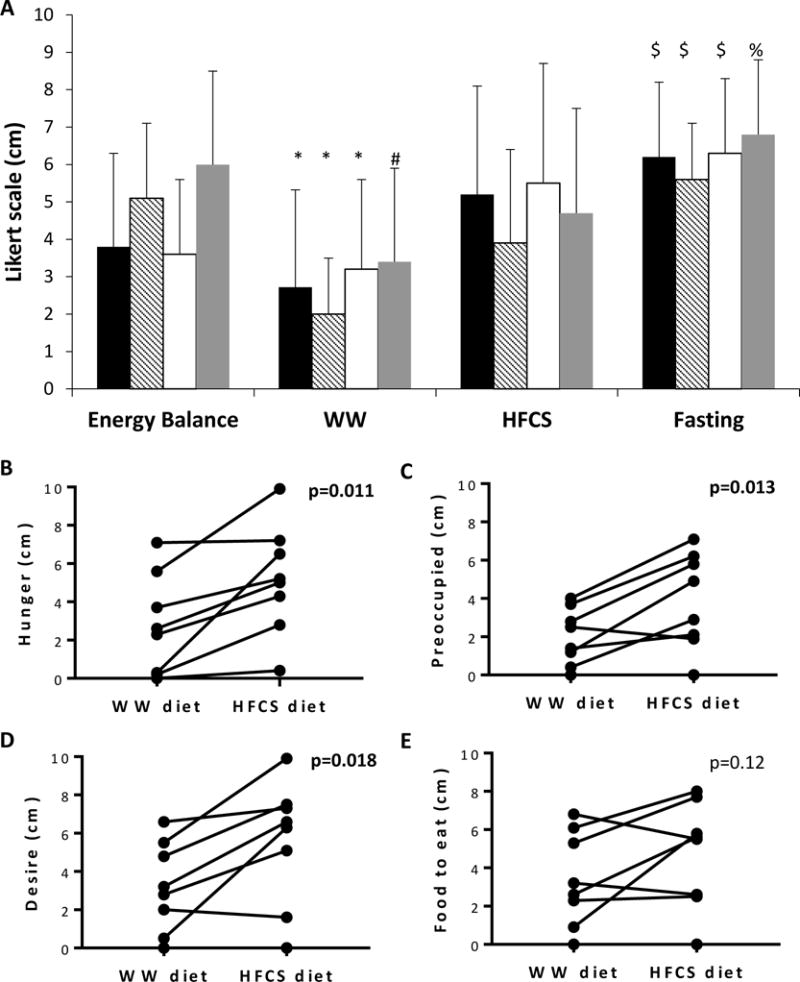
Scores of Visual Analog Scale reporting feelings of hunger. Questions included “How hungry do you feel?” (black bars in Panel A, and Panel B), “How preoccupied are you with thoughts of food?” (striped bars in Panel A, and Panel C), “How strong is your desire to eat?” (white bars in Panel A, and Panel D), and “How much food would you like to eat?” (gray bars in Panel A, and Panel E) (n=8).
$ p<0.05 compared to energy balance and WW, but not different from the HFCS diet. *p<0.05 for changes compared to the other 3 diets. # p<0.05 compared to energy balance and fasting. % p<0.05 compared to WW. The scores the morning after the HFCS and energy balance diets did not differ. HFCS = high-fructose corn syrup overfeeding diet, WW = whole-wheat overfeeding diet.
Figure 3.
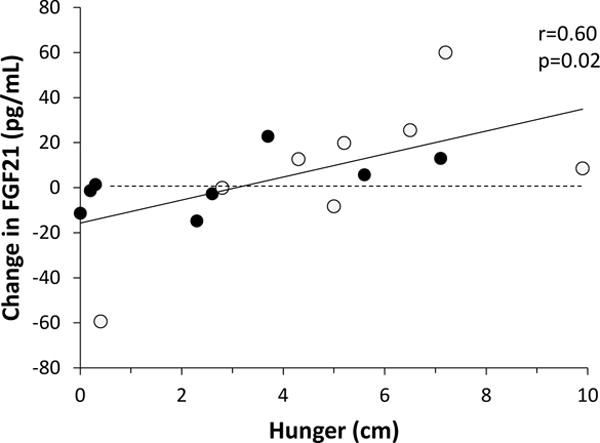
Relationship between changes in FGF21 concentration following overfeeding diets and feelings of hunger. Open circles denote the high-fructose corn syrup overfeeding diet while filled circles denote the whole-wheat flour overfeeding diet; r=0.6, p=0.02, by Pearson’s correlation.
Discussion
The primary purpose of this study was to understand if choosing foods with whole grain carbohydrates (i.e. WW) rather than HFCS would differentially impact EE or macronutrient oxidation during 24 hours of overfeeding in subjects with normal glucose regulation. As secondary, exploratory goals, we evaluated the hormone and hunger changes that occur with carbohydrate overfeeding. We found no differences in overall EE, RQ or DIT between the two overfeeding diets. However, SMR was higher after HFCS consumption. Forty percent of subjects had evidence of net DNL with carbohydrate overconsumption. Insulin, ghrelin, and triglycerides increased with overfeeding regardless of carbohydrate source; however, the HFCS diet was additionally associated with an increase in UFC and greater hunger ratings the following morning. Although overeating in general should be avoided, if overconsumption does occur, choosing HFCS may lead to increased hunger the following morning due either to increased sleeping metabolic rate, increased cortisol, or an increased sensitivity to ghrelin.
In prior studies, fructose increased EE and carbohydrate oxidation more so than glucose, but with a smaller insulin increase (12, 23). However, these studies only assessed EE for a few hours after a single meal, which may not be sufficient to fully assess the effects of differing diets. Also, many past studies used either pure fructose or glucose(23) or the provided mixed meals (12, 24) were not representative of the changes in food preparation that have occurred over past decades. The foods used in our overfeeding diets may be a better indicator of “real-world” differences between choices a consumer might make. In support of our findings, a study assessing the EE effects of overfeeding with a supplemental glucose, sucrose, or fructose drink in women found no differences in carbohydrate oxidation or EE between these three simple sugars(24). Contrary to our findings, a study assessing EE responses to long term consumption of glucose versus fructose sweetened beverages in overweight subjects found that consuming fructose was associated with reductions in resting EE over a 10 week period(25) so it is possible that longer term feeding with our diets may result in EE differences.
There was no difference in total 24h-EE, but we did observe a higher SMR after the HFCS diet. DIT was similar during the two diets despite the increased fiber in the WW diet. When subsumed into the total 24h-EE, the difference in SMR was not enough to lead to a statistically significant difference in total 24h-EE in this small group, as the study was only powered to detect 24h differences of 100 kcal (418 kJ) or greater. Fructose bypasses the rate-limiting step of glycolysis allowing for quicker metabolism(26), but synthesis of glycogen from fructose requires more energy than glycogen synthesis from glucose. In our study, the large amount of carbohydrates consumed may still have been undergoing metabolism and storage overnight such that the increased energy cost of glycogen synthesis from fructose was more evident at that time. The differences in energy content of the final meal may have contributed to the differences in SMR; however, as shown in figure 1, the separation of EE/min between the two diets appears to occur after the diet-induced thermogenesis of the snack is completed, and that difference is sustained through the night. The positive carbohydrate balance likely resulted in glycogen storage, which ranged from 100 to 460g/day, consistent with previous literature indicating that the body can accommodate a gain of up to 500g/day of glycogen during carbohydrate overfeeding(27). The increased SMR was not due to differences in net DNL between diets as the individuals with evidence of net DNL were the same no matter the source of carbohydrates.
For most biomarkers and hormones we assessed, HFCS and the WW overfeeding diets had similar effects. It has been reported that HFCS increases uric acid(28), but in these subjects with normal renal function and normal glucose regulation, we did not observe a change in uric acid the morning after high-carbohydrate overfeeding. However, we did observe the expected(28) increase in triglycerides and insulin concentrations. Surprisingly, the increase in triglycerides was greater after the WW diet. We hypothesize this may indicate that metabolism of the carbohydrates consumed the day before was still ongoing or that lipoprotein lipase activity, a key enzyme involved in the removal of triglycerides from plasma(29), was inhibited to a greater degree with the WW diet. As expected, IGF1 decreased with fasting(30); however, IGF1 only increased with overfeeding of the WW diet and not with HFCS. The IGF1 increase was small, but may reflect growth hormone (GH) responses from the prior day. This would be consistent with a greater inhibition of lipoprotein lipase during the WW diet, as GH inhibits lipoprotein lipase(31). Cortisol has been reported to stimulate lipoprotein lipase activity(32), and UFC was higher with the HFCS diet. Although average UFC was within the physiologic range, this increase may indicate that HFCS induces a small physiologic stress, and theoretically may contribute to the development of insulin resistance that has been reported to occur at higher rates in individuals that increase consumption of sugar-sweetened beverages(33).
Although the sample size was small and the results were primarily hypothesis generating, there was consistently less hunger the morning after the WW diet versus the HFCS diet. The responses following the HFCS diet were similar to those after EB despite the doubling of energy intake. We hypothesize that potential reasons for the increased hunger include the increased UFC or the increased SMR with the HFCS diet, or differences in sensitivity to ghrelin induced by HFCS. At supraphysiologic doses, corticosteroids increase hunger and food intake(34); however, any relationship between physiologic cortisol and hunger or food intake is unclear. Daily EE correlates with both hunger and subsequent food intake(35, 36), and possibly, increased EE overnight could contribute to increased hunger upon awakening. Despite the large number of energy intake the day prior, small increases in ghrelin, a ‘hunger hormone’ known to decrease after meals(37), occurred after both overfeeding diets, but the ghrelin increase was not correlated with hunger scores. It is theoretically possible, however, that HFCS increases sensitivity to ghrelin. Individual differences in FGF21 also may contribute to feelings of hunger, as FGF21 was the only hormone that correlated with individual hunger scores. FGF21 increases with fasting and is associated with increased food intake in rodents after fasting or protein restriction (38). Although the physiologic underpinnings of the increased hunger scores after the HFCS diet are unclear, increased hunger, as opposed to an expected compensatory satiety after eating excess energy, may contribute, in part, to the weight gain that has been reported in individuals with high consumption of sugar-sweetened beverages(33, 39).
We had a small sample size and few women were represented, but we utilized a repeated measures design to increase our power and recruited people representative of most body sizes. Still, with this sample size, our ability to do subgroup analyses was limited. Future studies with diets varying in carbohydrate source are required to confirm that hunger sensations increase after HFCS and further, that the hunger translates to increased food intake. The recommendation to consume a low-fat, high complex carbohydrate diet is based on studies using home cooked whole grains(11, 40). It is possible that we may have observed different results if the WW diet more closely represented these past studies, but the foods in our study may be more representative of the modern-day lifestyle of many who choose convenience foods. The fasting hormone concentrations were measured before and after the diets at the same time each morning; if serum had been collected during the postprandial period, results may have been different.
Conclusion
We investigated differences in EE over 24 hours using common, readily available sources of HFCS versus WW. In general, the overall effects of overeating carbohydrates were similar regardless of the source of the carbohydrates and favored storage of the large majority of excess energy consumed. However, the HFCS diet resulted in a higher SMR, increased UFC and increased hunger the next day. Our results indicate that HFCS may make it difficult to compensate for overeating on the following day by creating a physiologic milieu that may portend a potential for further excess food intake.
What is already known about this subject?
A low-fat, vegetarian diet high in carbohydrates from vegetables and whole grains causes weight loss over one year despite reports of similar caloric intake in the controls; however, the effects of packaged foods containing whole-wheat on energy expenditure is not known.
Fructose has been reported to have a higher obligatory cost of metabolism than glucose and starch; therefore, it acutely increases diet-induced thermogenesis by approximately 2% more than glucose.
The increased consumption of simple sugars over past decades has been implicated in the recent obesity epidemic, but the physiologic response to overeating packaged foods containing high-fructose corn syrup versus those containing ‘whole-wheat’ and labeled as ‘healthy’ are not known.
What does your study add?
When individuals were overfed a diet with 75% of energy sources derived from carbohydrates for 24 hours, similar changes in 24-hour energy expenditure and carbohydrate oxidation were observed regardless of whether the carbohydrate source derived either from high-fructose corn syrup or whole wheat.
Regardless of the source of carbohydrate, a diet with a high proportion of carbohydrates led to increases in triglycerides, insulin, and ghrelin the morning following 24 hours of overeating.
Overconsumption of a diet with a large proportion of high-fructose corn syrup was associated with a higher sleeping metabolic rate, increased 24-hour urinary free cortisol during the diet, and increased hunger the next day.
Acknowledgments
The authors thank the clinical research staff of the Obesity and Diabetes Clinical Research Section of the Phoenix Epidemiology and Clinical Research Branch for their excellent care of the participants.
Funding: This research was supported by the Intramural Research Program of the NIH, The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
Footnotes
ClinicalTrials.gov Identifier: NCT00523627
Disclosure: The authors declare no conflict of interest.
MI analyzed data and wrote the paper, SB conducted experiments and wrote the paper, MS and MW conducted experiments, KLV wrote the paper, PP critically reviewed the paper, CV designed the diets, JK conceived experiments and provided essential materials, MST conceived experiments, conducted experiments, analyzed data, and wrote the paper. All authors edited the paper and had final approval of the submitted and published versions.
References
- 1.Johnson RK, Appel LJ, Brands M, Howard BV, Lefevre M, Lustig RH, et al. Dietary sugars intake and cardiovascular health: a scientific statement from the American Heart Association. Circulation. 2009;120(11):1011–20. doi: 10.1161/CIRCULATIONAHA.109.192627. [DOI] [PubMed] [Google Scholar]
- 2.Ha V, Jayalath VH, Cozma AI, Mirrahimi A, de Souza RJ, Sievenpiper JL. Fructose-containing sugars, blood pressure, and cardiometabolic risk: a critical review. Current hypertension reports. 2013;15(4):281–97. doi: 10.1007/s11906-013-0364-1. [DOI] [PubMed] [Google Scholar]
- 3.Pannacciulli N, Salbe AD, Ortega E, Venti CA, Bogardus C, Krakoff J. The 24-h carbohydrate oxidation rate in a human respiratory chamber predicts ad libitum food intake. The American journal of clinical nutrition. 2007;86(3):625–32. doi: 10.1093/ajcn/86.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma Y, Olendzki B, Chiriboga D, Hebert JR, Li Y, Li W, et al. Association between dietary carbohydrates and body weight. American journal of epidemiology. 2005;161(4):359–67. doi: 10.1093/aje/kwi051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saris WH, Astrup A, Prentice AM, Zunft HJ, Formiguera X, Verboeket-van de Venne WP, et al. Randomized controlled trial of changes in dietary carbohydrate/fat ratio and simple vs complex carbohydrates on body weight and blood lipids: the CARMEN study. The Carbohydrate Ratio Management in European National diets. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2000;24(10):1310–8. doi: 10.1038/sj.ijo.0801451. [DOI] [PubMed] [Google Scholar]
- 6.Melanson KJ, Angelopoulos TJ, Nguyen V, Zukley L, Lowndes J, Rippe JM. High-fructose corn syrup, energy intake, and appetite regulation. The American journal of clinical nutrition. 2008;88(6):1738S–44S. doi: 10.3945/ajcn.2008.25825E. [DOI] [PubMed] [Google Scholar]
- 7.Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. The American journal of clinical nutrition. 2004;79(4):537–43. doi: 10.1093/ajcn/79.4.537. [DOI] [PubMed] [Google Scholar]
- 8.Couchepin C, Le KA, Bortolotti M, da Encarnacao JA, Oboni JB, Tran C, et al. Markedly blunted metabolic effects of fructose in healthy young female subjects compared with male subjects. Diabetes care. 2008;31(6):1254–6. doi: 10.2337/dc07-2001. [DOI] [PubMed] [Google Scholar]
- 9.Stanhope KL, Griffen SC, Bair BR, Swarbrick MM, Keim NL, Havel PJ. Twenty-four-hour endocrine and metabolic profiles following consumption of high-fructose corn syrup-, sucrose-, fructose-, and glucose-sweetened beverages with meals. The American journal of clinical nutrition. 2008;87(5):1194–203. doi: 10.1093/ajcn/87.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stanhope KL, Havel PJ. Endocrine and metabolic effects of consuming beverages sweetened with fructose, glucose, sucrose, or high-fructose corn syrup. The American journal of clinical nutrition. 2008;88(6):1733S–7S. doi: 10.3945/ajcn.2008.25825D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ornish D, Scherwitz LW, Billings JH, Brown SE, Gould KL, Merritt TA, et al. Intensive lifestyle changes for reversal of coronary heart disease. Jama. 1998;280(23):2001–7. doi: 10.1001/jama.280.23.2001. [DOI] [PubMed] [Google Scholar]
- 12.Schwarz JM, Schutz Y, Froidevaux F, Acheson KJ, Jeanpretre N, Schneider H, et al. Thermogenesis in men and women induced by fructose vs glucose added to a meal. The American journal of clinical nutrition. 1989;49(4):667–74. doi: 10.1093/ajcn/49.4.667. [DOI] [PubMed] [Google Scholar]
- 13.Aeberli I, Hochuli M, Gerber PA, Sze L, Murer SB, Tappy L, et al. Moderate amounts of fructose consumption impair insulin sensitivity in healthy young men: a randomized controlled trial. Diabetes care. 2013;36(1):150–6. doi: 10.2337/dc12-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.David Wang D, Sievenpiper JL, de Souza RJ, Cozma AI, Chiavaroli L, Ha V, et al. Effect of fructose on postprandial triglycerides: a systematic review and meta-analysis of controlled feeding trials. Atherosclerosis. 2014;232(1):125–33. doi: 10.1016/j.atherosclerosis.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 15.Lecoultre V, Egli L, Carrel G, Theytaz F, Kreis R, Schneiter P, et al. Effects of fructose and glucose overfeeding on hepatic insulin sensitivity and intrahepatic lipids in healthy humans. Obesity. 2013;21(4):782–5. doi: 10.1002/oby.20377. [DOI] [PubMed] [Google Scholar]
- 16.Luo S, Monterosso JR, Sarpelleh K, Page KA. Differential effects of fructose versus glucose on brain and appetitive responses to food cues and decisions for food rewards. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(20):6509–14. doi: 10.1073/pnas.1503358112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schlogl M, Piaggi P, Pannacciuli N, Bonfiglio SM, Krakoff J, Thearle MS. Energy Expenditure Responses to Fasting and Overfeeding Identify Phenotypes Associated With Weight Change. Diabetes. 2015;64(11):3680–9. doi: 10.2337/db15-0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes care. 2013;36(Suppl 1):S67–74. doi: 10.2337/dc13-S067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferraro R, Boyce VL, Swinburn B, De Gregorio M, Ravussin E. Energy cost of physical activity on a metabolic ward in relationship to obesity. The American journal of clinical nutrition. 1991;53(6):1368–71. doi: 10.1093/ajcn/53.6.1368. [DOI] [PubMed] [Google Scholar]
- 20.Thearle MS, Pannacciulli N, Bonfiglio S, Pacak K, Krakoff J. Extent and determinants of thermogenic responses to 24 hours of fasting, energy balance, and five different overfeeding diets in humans. The Journal of clinical endocrinology and metabolism. 2013;98(7):2791–9. doi: 10.1210/jc.2013-1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. The Journal of clinical investigation. 1986;78(6):1568–78. doi: 10.1172/JCI112749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1–452. [PubMed] [Google Scholar]
- 23.Tappy L, Randin JP, Felber JP, Chiolero R, Simonson DC, Jequier E, et al. Comparison of thermogenic effect of fructose and glucose in normal humans. The American journal of physiology. 1986;250(6 Pt 1):E718–24. doi: 10.1152/ajpendo.1986.250.6.E718. [DOI] [PubMed] [Google Scholar]
- 24.McDevitt RM, Poppitt SD, Murgatroyd PR, Prentice AM. Macronutrient disposal during controlled overfeeding with glucose, fructose, sucrose, or fat in lean and obese women. The American journal of clinical nutrition. 2000;72(2):369–77. doi: 10.1093/ajcn/72.2.369. [DOI] [PubMed] [Google Scholar]
- 25.Cox CL, Stanhope KL, Schwarz JM, Graham JL, Hatcher B, Griffen SC, et al. Consumption of fructose-sweetened beverages for 10 weeks reduces net fat oxidation and energy expenditure in overweight/obese men and women. European journal of clinical nutrition. 2012;66(2):201–8. doi: 10.1038/ejcn.2011.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henry RR, Crapo PA, Thorburn AW. Current issues in fructose metabolism. Annual review of nutrition. 1991;11:21–39. doi: 10.1146/annurev.nu.11.070191.000321. [DOI] [PubMed] [Google Scholar]
- 27.Acheson KJ, Schutz Y, Bessard T, Anantharaman K, Flatt JP, Jequier E. Glycogen storage capacity and de novo lipogenesis during massive carbohydrate overfeeding in man. The American journal of clinical nutrition. 1988;48(2):240–7. doi: 10.1093/ajcn/48.2.240. [DOI] [PubMed] [Google Scholar]
- 28.Angelopoulos TJ, Lowndes J, Zukley L, Melanson KJ, Nguyen V, Huffman A, et al. The effect of high-fructose corn syrup consumption on triglycerides and uric acid. J Nutr. 2009;139(6):1242S–5S. doi: 10.3945/jn.108.098194. [DOI] [PubMed] [Google Scholar]
- 29.Brunzell JD, Hazzard WR, Porte D, Jr, Bierman EL. Evidence for a common, saturable, triglyceride removal mechanism for chylomicrons and very low density lipoproteins in man. The Journal of clinical investigation. 1973;52(7):1578–85. doi: 10.1172/JCI107334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clemmons DR. Metabolic actions of insulin-like growth factor-I in normal physiology and diabetes. Endocrinology and metabolism clinics of North America. 2012;41(2):425–43. vii–viii. doi: 10.1016/j.ecl.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ottosson M, Vikman-Adolfsson K, Enerback S, Elander A, Bjorntorp P, Eden S. Growth hormone inhibits lipoprotein lipase activity in human adipose tissue. The Journal of clinical endocrinology and metabolism. 1995;80(3):936–41. doi: 10.1210/jcem.80.3.7883853. [DOI] [PubMed] [Google Scholar]
- 32.Ottosson M, Vikman-Adolfsson K, Enerback S, Olivecrona G, Bjorntorp P. The effects of cortisol on the regulation of lipoprotein lipase activity in human adipose tissue. The Journal of clinical endocrinology and metabolism. 1994;79(3):820–5. doi: 10.1210/jcem.79.3.8077367. [DOI] [PubMed] [Google Scholar]
- 33.Schulze MB, Manson JE, Ludwig DS, Colditz GA, Stampfer MJ, Willett WC, et al. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. Jama. 2004;292(8):927–34. doi: 10.1001/jama.292.8.927. [DOI] [PubMed] [Google Scholar]
- 34.Tataranni PA, Larson DE, Snitker S, Young JB, Flatt JP, Ravussin E. Effects of glucocorticoids on energy metabolism and food intake in humans. The American journal of physiology. 1996;271(2 Pt 1):E317–25. doi: 10.1152/ajpendo.1996.271.2.E317. [DOI] [PubMed] [Google Scholar]
- 35.Caudwell P, Finlayson G, Gibbons C, Hopkins M, King N, Naslund E, et al. Resting metabolic rate is associated with hunger, self-determined meal size, and daily energy intake and may represent a marker for appetite. The American journal of clinical nutrition. 2013;97(1):7–14. doi: 10.3945/ajcn.111.029975. [DOI] [PubMed] [Google Scholar]
- 36.Piaggi P, Thearle MS, Krakoff J, Votruba SB. Higher Daily Energy Expenditure and Respiratory Quotient, Rather Than Fat-Free Mass, Independently Determine Greater ad Libitum Overeating. The Journal of clinical endocrinology and metabolism. 2015;100(8):3011–20. doi: 10.1210/jc.2015-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benedict C, Axelsson T, Soderberg S, Larsson A, Ingelsson E, Lind L, et al. Fat mass and obesity-associated gene (FTO) is linked to higher plasma levels of the hunger hormone ghrelin and lower serum levels of the satiety hormone leptin in older adults. Diabetes. 2014;63(11):3955–9. doi: 10.2337/db14-0470. [DOI] [PubMed] [Google Scholar]
- 38.Laeger T, Henagan TM, Albarado DC, Redman LM, Bray GA, Noland RC, et al. FGF21 is an endocrine signal of protein restriction. The Journal of clinical investigation. 2014;124(9):3913–22. doi: 10.1172/JCI74915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bundrick SC, Thearle MS, Venti CA, Krakoff J, Votruba SB. Soda consumption during ad libitum food intake predicts weight change. J Acad Nutr Diet. 2014;114(3):444–9. doi: 10.1016/j.jand.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ornish D, Brown SE, Scherwitz LW, Billings JH, Armstrong WT, Ports TA, et al. Can lifestyle changes reverse coronary heart disease? The Lifestyle Heart Trial. Lancet. 1990;336(8708):129–33. doi: 10.1016/0140-6736(90)91656-u. [DOI] [PubMed] [Google Scholar]


