Abstract
Brains of females are more sensitive to the acute catabolic actions of leptin. However, sex differences in the long-term physiological responses to central leptin receptor modulation are unknown. To this end, we centrally delivered a viral vector to overexpress leptin (Leptin), a neutral leptin receptor antagonist (Leptin-Antagonist), or a green fluorescence protein (Control). We examined chronic changes in body weight and composition in male and female rats. Females displayed greater and sustained responses to Leptin whereas males rapidly lost physiological effects and developed leptin resistance confirmed by lower acute leptin-mediated phosphorylation of STAT3 (P-STAT3). Surprisingly, despite persistent physiological responses, Leptin-females also exhibited reduced acute leptin-mediated P-STAT3, suggesting an onset of leptin resistance near time of death. In line with this interpretation, Leptin-females and Control-females consumed the same amount of food on the last day of the experiment. Both Leptin-Antagonist groups gained similar percentages of their initial body weight and fat mass, whereas only Leptin-Antagonist-females gained lean body mass. Consequently, lean/fat mass ratio with Leptin-Antagonist was preserved in females and decreased in males, suggesting a deterioration of body composition in males. In summary, this study establishes that females are more responsive to long-term central leptin overexpression than males and that leptin antagonism has a greater physiological impact in males. The hormone environment may have played a role in these processes; however, future studies are needed to establish whether physiological responses are mediated by female or male sex hormones.
Keywords: leptin resistance, sex differences, leptin sensitivity, body composition, body mass, STAT3 Phosphorylation
Introduction
A complex and multifaceted physiological system has evolved to adjust fuel stores and maintain energy balance at an optimum level. The adipocyte-derived hormone leptin is a fundamental component of this system (1). The main function of leptin consists in providing catabolic signals to the central nervous system (CNS) upon body fat enlargement and/or overnutrition. When stimulated, putative leptin receptors localized in the hypothalamus activate a neural network promoting satiety and energy expenditure, thus resulting in weight loss (2). While leptin appears to be a promising anti-obesity therapeutic target, most overweight individuals display high levels of circulating leptin, and are resistant to the effects of leptin. Because leptin therapy in obese humans with elevated leptin levels has proven to be ineffective, enthusiasm for this therapeutic strategy has been abandoned (3). Leptin resistance is caused by either reduced/insufficient transport across the blood-brain-barrier (BBB) (4), or defective leptin receptor signaling in the hypothalamus (5). The latter more specifically is defined as cellular leptin resistance. Chronic overexpression of leptin in the CNS induces a leptin resistance comparable to diet-induced or adult-onset obesity that results in reduced leptin receptors, diminished signaling, and impaired responsiveness to exogenous leptin (6, 7). Furthermore, central leptin overexpression increases susceptibility to diet-induced obesity (8). These findings suggest a role of elevated leptin levels in the development of leptin resistance.
Accumulating evidence points toward a sexual dimorphism in the onset of leptin resistance (9, 10). When submitted to a high fat diet, males tend to develop leptin resistance at an earlier stage than females. For instance, after 8-week exposure to a high fat diet, female mice remained responsive to intraperitoneal injections of leptin (11) whereas males were leptin resistant (12). A sex bias in the onset of leptin resistance was also observed in mice that ectopically express agouti protein (10). Female gonadal hormones may be the primary contributor to the sex bias in the onset of leptin resistance. Estradiol is known to enhance leptin synthesis and to induce leptin gene expression in subcutaneous, perirenal and parametrial rat adipocytes (13). Another potential mechanism explaining sex differences in leptin responsiveness is the relatively greater binding to the soluble form of the leptin receptor in males relative to females, that results in lower leptin transport across the BBB (14). Although leptin is secreted principally by adipocytes, Wiesner et al. have identified the brain as a producer of leptin by measuring of transcerebral leptin flux. Remarkably, they found that the female brain synthetizes more leptin than the male brain (15). Leptin produced in the brain might exert a paracrine effect in the hypothalamus; hence establishing another mechanism for sex bias in leptin sensitivity. When weight or age matched, females exhibit hypophagia and lose body weight at lower doses of leptin than males (16). Interestingly, altering the hormonal environment in males by the addition of estradiol enhances anorexic response to central leptin injection, suggesting a role for sex steroid milieu on central leptin sensitivity (17). The modulation of central leptin sensitivity by female gonadal hormones might be due to the fact that estradiol enhances hypothalamic expression of the long form of the leptin receptor (18).
In attempt to further document sex-specific energy homeostasis regulation, we examined chronic leptin activation and chronic leptin receptor partial blockade (19, 20) on the long-term regulation of body weight in male and female rats. To this end, we employed recombinant adeno-associated virus 1 (rAAV1)-mediated gene delivery to overexpress leptin or a mutant of rat leptin that produces a protein acting as a neutral leptin receptor antagonist, and examined the long-term sex-specific changes in feeding, body weight, and body composition. We hypothesized greater weight loss following leptin gene delivery and enhanced weight gain upon partial leptin receptor blockade in females compared with males.
Subjects and methods
Animals
Three-month old male and female Sprague Dawley rats (n = 54), were obtained from Taconic (Germantown, NY). Upon arrival, animals were housed individually on a 12-h light and 12-h dark cycle. All rats were allowed at least one week to acclimate to their new environment and daily handling before beginning any experiment. Rats were fed a standard rodent chow (18% kcal from fat, no sucrose, 3.1 kcal/g, Envigo Teklad Global diet 2918). Health status, body weight, and food intake were monitored daily throughout the study. However, in order to treat all statistical analyses the same, only a few time points were reported and those time point match other body composition analyses described below. All experimental protocols were approved by the University of Florida’s Animal Care and Use Committee, and in compliance with the “Guide for the Care and Use of Laboratory Animals”.
Surgeries and group
Rats were anaesthetized with isoflurane (2–3%) and administered the analgesics: buprenorphine (0.025 mg/kg; SC) and carprofen (5 mg/kg; SC) every 24 hours for 72 hours starting immediately prior the surgery. The day before surgeries, rats from each sex were pseudo-randomized into three weight-matched groups: Green Fluorescent Protein (Control), Leptin, or dominant negative leptin mutant (Leptin-Antagonist). Each rat received 1 μL of rAAV1 (5 × 1012 vg/mL) into the third ventricle using the following coordinates: 1.3 mm anterior to Bregma, 0.0 mm from midline to depth of 9.6 mm ventral from surface of skull, at an angle of 20° (21). These coordinates were validated by injecting a bromothymol blue dye. The pTR(2)ObW construct encoding leptin transgene was packaged as previously described (22). To generate the Leptin-Antagonist vector, rat DNA wild type amino acid sequence Leucine (L39), Aspartic acid (D40) and Phenylalanine (F41), was mutated to Alanine, Alanine, Alanine, and Aspartic acid (D23) was mutated to Leucine and was pharmacologically characterized as dominant negative leptin mutant (22). The viral vectors Control and Leptin were packaged by the University of Florida vector core, and the Leptin-Antagonist vector was packaged by Vectorbiolabs (Philadelphia, PA, USA).
Determination of body composition using time-domain nuclear magnetic resonance
Body composition was determined at day 0, 7, 14, and 26 using time-domain nuclear magnetic resonance (TD-NMR; Minispec, Bruker Optics, The Woodlands, TX). The MiniSpec quantifies three components of body composition: fat mass (FM), lean body mass (LBM), and free fluid. The TD-NMR acquires and analyzes signals from all protons in the sample area and the manufacturer software generates values reported in grams and % of total body mass. Scans were acquired in conscious animals restrained in a cylindrical device inserted into the analyzer. The final values comprised the average of two scans for each measurement. Absolute levels were analyzed and reported in Fig. S1. Given the substantial differences in baseline values between males and females, data in the manuscript are presented as percent change from initial values in order to limit data interpretation bias. For example, the formula used to calculate delta fat mass (ΔFM) was: (FM− initial FM)/initial FM.
Acute central leptin administration
At day 26, following the final body composition assessment, rats were anesthetized using a cocktail of ketamine (60 mg/kg; IP) and xylazine (8 mg/kg; IP). Leptin peptide was injected using the same coordinates as the initial vector delivery for assessment of leptin-stimulated STAT3 phosphorylation. Leptin peptide (Amgen, Thousand Oaks, CA) was diluted in artificial cerebrospinal fluid (aCSF; NaCl 148 mM, KCl 3mM, CaCl2-2H2O 1.5 mM, MgCl2-6H2O 1.4 mM, Na2HPO4 1.5 mM, NaH2PO4 0.2mM) at a final concentration of 0.2 μg/μl and was administered at a rate of 1 μl/min over 5 min, for a total 1 μg leptin.
Tissue Collection, Harvesting and, Preparation
After leptin delivery, rats remained anesthetized for one hour on a heat pad. Thereafter, 30 mL cold 0.9% saline were perfused in the circulatory system to remove blood from the brain. The hypothalamus was dissected from the whole brain by a medial incision to the piriform lobes, caudal to the optic chiasm and anterior to the cerebral crus to a depth of 2.5 mm and immediately snap frozen in liquid nitrogen. Several other organs and tissues were removed and weighed (Mettler AE 163). Five fat depots were collected: mesenteric (mWAT), perirenal (pWAT), epididymal for males (eWAT) or parameterial for females (paWAT), retroperitoneal (rtWAT), and interscapular brown adipose tissue (iBAT). Several lean tissues were collected: gastrocnemius, soleus, plantaris, tibialis anterior (TA), and extensor digitorum longus (EDL), heart, liver, and kidneys. The hypothalamus samples were stored at −80 °C until analyses were performed.
Western analyses
The hypothalami were sonicated in 270 μL homogenization buffer (Tris-HCL 10 mM; pH 6.9, and 2% SDS in the presence of phosphatase/protease inhibitors; Thermo Scientific, Rockford, IL). Protein lysates were separated on a SDS-PAGE gel and transferred to nitrocellulose membranes. Immunoreactivity was detected with ECL prime (GE Healthcare, Piscataway NJ), scanned with a ChemiDoc XRS+ (BioRad, Hercules, CA) and quantified using ImageJ software. All values, were normalized to the mean of the Control group for each sex and reported as a percentage. Phospho-STAT3 content was determined by comparing the signals obtained using antibodies specific to the phosphorylated protein (P-STAT3; Cell Signaling, Danvers, MA) relative to those obtained using antibodies that bind both phosphorylated and unphosphorylated STAT3 (STAT3; Cell Signaling, Danvers, MA). A ratio of P-STAT3 over STAT3 was generated for each animal. The signal ratio (P-STAT3/STAT3) was then normalized to the mean ratio of the control group, established at 100%.
Serum leptin
Enzyme immunoassays were used to measure serum leptin levels (rat leptin ELISA kit, EZRL-83K; Milipore, Waltham, MA). Leptin was assayed with the blood (fed state) that was collected during euthanasia.
Reverse transcription polymerase chain reaction
Total RNA was extracted from the hypothalamus using TRI reagent (Sigma Aldrich, St. Louis MO), according to the manufacturer’s protocol. Total RNA (2 μg) was reverse-transcribed into complementary DNA using high-capacity complementary DNA reverse transcription kits (Applied Biosystems; Waltham MA). The gene expression of leptin was determined with SYBR Green Supermix using primer sets designed to specifically amplify leptin generated from the vector (forward 5′ GGCAACGTGCTGGTTATTGT 3′ and 5′ ATATCCATCACACTGGCGGC 3′). Gapdh (forward 5′ TCTCTGCTCCTCCCTGTTCT 3′ and reverse 5′ TACGGCCAAATCCGTTCACA 3′) was used as the housekeeping gene. The IQ (Bio-Rad, Hercules CA) was used to detect the amplification level and programmed with an initial step of 3 min at 95°C, followed by forty cycles for 5 s at 95°C and 15 s at 60°C. All reactions were run in duplicate, and the average of threshold cycle (C T) was used for quantification. The relative quantification of the target genes was determined using the ΔΔC T method. Briefly, the C T values of the target genes were normalized (ΔC T= C T target– C T Gapdh) and compared with a calibrator (ΔΔC T= ΔC T Sample– ΔC T Calibrator). Relative expression (RQ) was calculated using the IQ software (Bio-Rad; Hercules CA).
Statistical analyses
Results are expressed as means and standard errors. For endpoint analyses, differences between means were tested for statistical significance (P < 0.05) using a one-way ANOVA, and Tukey’s multiple comparison tests were applied in the event of a significant effect (P < 0.05). For longitudinal analyses, we employed repeated measures (mixed model) ANOVA using time and viral vectors as main factors and performed a Bonferroni posttest in the case of significant interaction between variables.
Results
Males are less responsive to central Leptin gene delivery than females and exhibit accelerated onset of leptin resistance
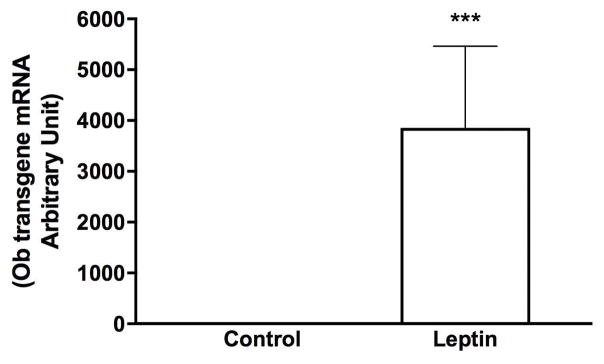
Leptin was over-expressed in a manner that would maximize the chance to activate leptin receptors throughout the brain. Gene transduction utilized rAAV serotype one which readily infects the cells lining the 3rd ventricle but is less specific for neuronal cells. This was previously confirmed by immunohistochemistry in the brain of rats injected with the Control vector (GFP) (23). The leptin construct contains a secretory sequence and thus the leptin protein is secreted into the 3rd ventricle and potentially can reach leptin receptors throughout the brain. We have previously demonstrated that leptin gene delivered using this serotype increases the level of leptin by 75% in cerebrospinal fluid (24). In the present study, expression of the leptin transgene, leptin mRNA was examined in the hypothalamus by RT-PCR using a sense primer specific to a region of the vector that is not present in native rat leptin and an antisense primer specific for leptin. This method allowed detection of leptin mRNA in Leptin but not in Control animals (Fig. 1).
Fig. 1. Confirmation of leptin overexpression in the brain.
The hypothalamus from separate male rats (same age and strain) that underwent the same treatment were used to confirm expression of leptin transgene. Values are reported as Fold expression levels based on levels recorded in animals injected with the Control vector (n=10/group). *** Leptin significantly different from Control group (P < 0.001).
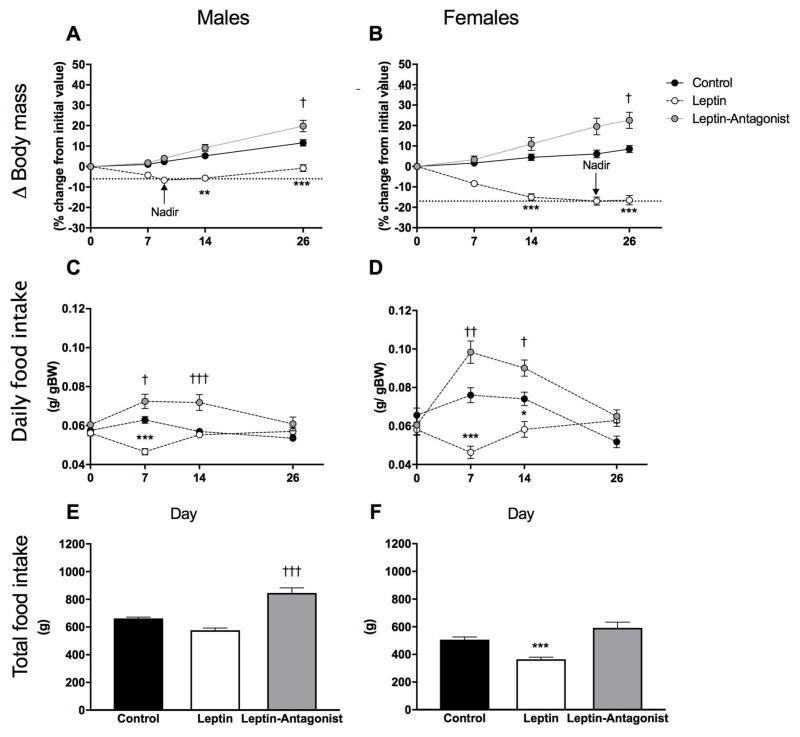
Six weeks after vector delivery, GFP+ cells were distributed in mid-line structures along the site of injection in the 3rd ventricle, extending from the anterior commissure to the posterior hypothalamus (23). Both males and females lost body mass in response to central leptin gene delivery and changes in body mass in Leptin animals were significantly different than those recorded in Control animals at day 14 and 26 (Fig. 2A and B; Control vs Leptin: P < 0.01). An important sexual dimorphism was observed in the extent and duration of body weight loss in response to Leptin vector delivery. Males reached a nadir of weight loss at day 9 (−6 %; Fig. 2A), and weight loss was maintained through day 14, followed by regain of body weight until reaching initial body weight by day 26 (−1%; Fig. 2A). In contrast, by day 9, females lost 12% of their initial body weight (data not shown) with a maximal loss of −17% by day 22 that was maintained until the end of the experiment (Fig. 2B). Leptin gene transfer significantly reduced daily food intake (g per g of BW) at day 7 in both males and females (Fig. 2C and D; Control vs Leptin:P < 0.001). However, at day 14, daily food intake was normalized in Leptin-males and remained significantly reduced in Leptin-females (P < 0.05). In contrast, Leptin-Antagonist treated groups had increased food intake. Both males and females had significantly higher daily food intake at day 7 and day 14 (Fig. 2C and D; Control vs Leptin-Antagonist: Ps < 0.05). While leptin gene transfer did not significantly affect cumulative food in males (Fig. 2E), the same treatment significantly reduced total feeding in females (Fig. 2F; Control vs Leptin: P < 0.01). Following gene delivery of the Leptin-Antagonist, body mass was significantly increased in both sexes (Fig. 2A and B; Control vs Leptin-Antagonist: P < 0.05). Leptin-Antagonist treated males consumed a significantly higher cumulative amount of food than Control-males (Fig. 2E; Control vs Leptin-Antagonist: P < 0.001), but this effect was not observed in female rats (Fig. 2F).
Fig. 2. Longitudinal changes in body mass and cumulative food intake.
A and B delta (Δ) body mass in males and females, respectively. Values represent individual change (%) in body mass from their respective initial body mass recorded the day of vector delivery (day 0). C and D relative daily food intake in males and females, respectively. E and F cumulative food consumed from day 0 to day 26 in grams in males and females, respectively. Body mass and food intake were recorded daily. Values at days 0, 7, 14 and 26 were used for statistical analyses. Nadir values of body weight loss in each sex are also displayed in panels A and B for clarity but were not included in statistical analyses. Values are means ± standard errors ** Leptin significantly different from Control group (P < 0.01), *** (P < 0.001), † Leptin-Antagonist significantly different from Control group (P < 0.05), ††† (P < 0.001). n=8–10/group
Central leptin gene delivery induced higher fat and lean mass catabolism in females
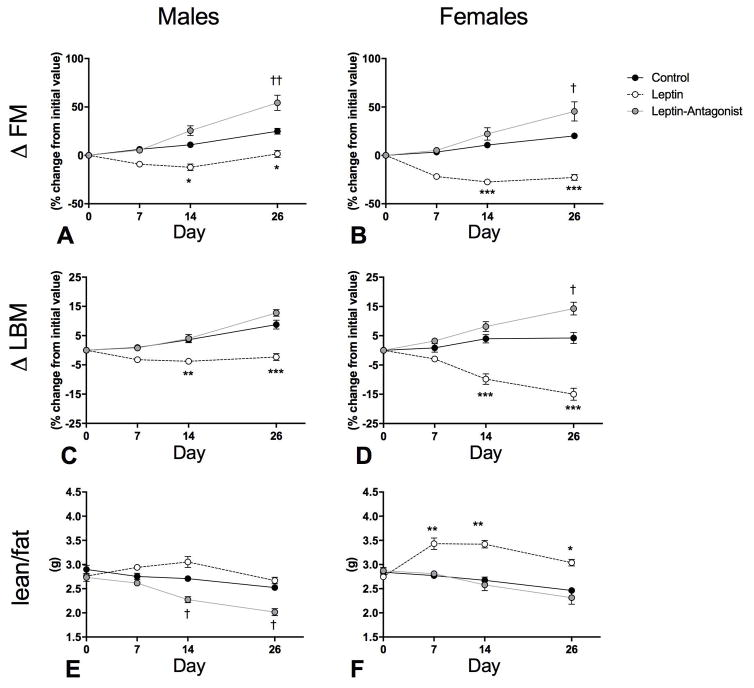
Body composition analyses indicated that both Leptin groups lost FM, when expressed as either absolute FM or percentage of initial FM at days 14 and 26, (Fig. 3A and B; Control vs Leptin: Ps < 0.05 and Fig. S1 A and B; Control vs Leptin: Ps < 0.05). FM percentage (FM%) was reduced in Leptin-females (Fig. S1 D; Control vs Leptin: Ps < 0.05), but not in Leptin-males (Fig. S1 C). Similar to body mass, we found that Leptin-females lost a considerably higher percentage of their initial absolute FM throughout the study than Leptin-males. Body composition analyses also indicated that, by day 26, both Leptin groups lost LBM, when expressed as either absolute LBM or percentage of initial LBM (Fig. 3C and D; Control vs Leptin; P < 0.001 and Fig. S1 E and F; Control vs Leptin: P < 0.05). An intriguing observation was that LBM was preserved in males, but not in females. When overall body composition was examined, the lean/fat mass ratio was not changed in Leptin-males (Fig. 3E) but significantly increased in Leptin-females from day 7 through 26 (Fig. 3F; Control vs Leptin: P < 0.05).
Fig. 3. Longitudinal changes in body composition.
A and B delta fat mass (Δ FM) in males and females, respectively. Values represent individual change (%) in FM from their respective initial absolute FM recorded by TD-NMR the day of vector delivery. Values at days 0, 7, 14 and 26 are displayed. C and D delta lean body mass (Δ LBM) in males and females, respectively. Values represent individual change (%) in LBM from their respective initial absolute LBM recorded the day of vector delivery. Absolute values are displayed in Fig. S1. Values are means ± standard errors * Leptin significantly different from Control group (P < 0.05), ** (P < 0.01), *** (P < 0.001), † Leptin-Antagonist significantly different from Control group (P < 0.05), †† (P < 0.01), ††† (P < 0.001). n=8–10/group
Central leptin blockade appears to have greater physiological effect in males than females
Body composition analyses also indicated that both Leptin-Antagonist groups gained FM by day 26 (Fig. 3A and B; Control vs Leptin-Antagonist: P < 0.05). When absolute FM or FM percentage (FM%) were examined, Leptin-Antagonist-males displayed significantly higher levels than Control-males at day 26 (Fig. S1 A; Control vs Leptin-Antagonist; P < 0.01), however there was no difference between respective female groups. Conversely, there was no change in LBM in Leptin-Antagonist-males (Fig. 3C), but Leptin-Antagonist-females gained a significant amount of LBM by day 26 (Fig. 3D; Control vs Leptin-Antagonist; P < 0.05). LBM percentage (LBM%) was significantly lower in Leptin-Antagonist-males than in Control-males at day 14 and 26 (Fig. S1 G; Control vs Leptin-Antagonist: P < 0.01), but not in respective female groups (Fig. S1 H). In males, lean/fat mass ratio was significantly reduced in the Leptin-Antagonist group at day 14 and 26 (Fig. 3E; Control vs Leptin-Antagonist:P < 0.01) but was preserved in Leptin-Antagonist-females.
Central leptin gene overexpression differentially affects lean tissue weight in males and females
Consistent with TD-NMR data, Leptin groups displayed lower abdominal fat pad mass than respective Control groups (Table 1; Control vs Leptin: P < 0.05). In attempt to identify the source of LBM differences across groups, mass of several lean tissues were weighed. Mass of heart, kidneys, liver, TA, gastrocnemius, plantaris, soleus, and EDL were significantly lower in Leptin relative to Control-females (Table 1; Control vs Leptin: P < 0.05). In males, only liver mass was determined to be lower in the Leptin group (Table 1; Control vs Leptin: P < 0.05). Consistent with previous studies (24), iBAT mass was lower in Leptin than in Control rats (Table 1; Control vs Leptin: P < 0.05). Leptin-Antagonist-males accumulated significantly more fat in all depots relative to Control-males (Table 1; Control vs Leptin-Antagonist: P < 0.01). However, in females, only pWAT and mWAT were significantly enlarged (Table 1; Control vs Leptin-Antagonist: P < 0.05), suggesting that females were slightly less responsive to Leptin antagonism than males. In both sexes, Leptin-Antagonist animals exhibited higher iBAT mass than Control animals (Table 1; Control vs Leptin-Antagonist: P < 0.01).
Table 1.
Tissue weights in grams
| Males | Females | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Control | Leptin | Leptin-Antagonist | Control | Leptin | Leptin-Antagonist | |
| Heart | 1.43 ± 0.06 | 1.35 ± 0.04 | 1.47 ± 0.08 | 0.94 ± 0.03 | 0.83 ± 0.03 * | 1.00 ± 0.03 |
| Kidneys | 3.31 ± 0.12 | 3.05 ± 0.11 | 3.52 ± 0.13 | 1.94 ± 0.09 | 1.54 ± 0.06 *** | 2.03 ± 0.04 |
| Liver | 17.67 ± 0.67 | 15.22 ± 0.60 * | 20.66 ± 0.65 † | 9.82 ± 0.44 | 6.33 ± 0.56 *** | 11.26 ± 0.43 |
| pWAT | 1.78 ± 0.10 | 0.81 ± 0.16* | 3.31 ± 0.42 †† | 1.28 ± 0.18 | 0.20 ± 0.03 *** | 1.84 ± 0.20 † |
| epi/paWAT | 7.24 ± 0.47 | 3.86 ± 0.55 *** | 12.83 ± 0.59 ††† | 6.41 ± 0.79 | 0.21 ± 0.11 *** | 9.30 ± 1.70 |
| rtWAT | 5.40 ± 0.63 | 1.93 ± 0.48 ** | 9.99 ± 0.82 ††† | 2.33 ± 0.34 | 0.06 ± 0.05 ** | 3.73 ± 0.70 |
| mWAT | 4.07 ± 0.29 | 2.01 ± 0.35 * | 7.81 ± 0.77 ††† | 2.61 ± 0.22 | 0.34 ± 0.12 ** | 4.82 ± 0.80 †† |
| iBAT | 0.35 ± 0.03 | 0.22 ± 0.02* | 0.55 ± 0.05 ††† | 0.33 ± 0.02 | 0.13 ± 0.01 *** | 0.47 ± 0.05 †† |
| TA | 0.82 ± 0.11 | 0.86 ± 0.02 | 0.89 ± 0.02 | 0.56 ± 0.02 | 0.49 ± 0.02 * | 0.54 ± 0.03 |
| Gastrocnemius | 2.50 ± 0.06 | 2.31 ± 0.06 | 2.34 ± 0.07 | 1.54 ± 0.04 | 1.28 ± 0.04 *** | 1.61 ± 0.03 |
| Plantaris | 0.50 ± 0.01 | 0.49 ± 0.02 | 0.48 ± 0.02 | 0.33 ± 0.01 | 0.27 ± 0.01 *** | 0.34 ± 0.01 |
| Soleus | 0.20 ± 0.01 | 0.20 ± 0.01 | 0.21 ± 0.01 | 0.13 ± 0.00 | 0.14 ± 0.00 ** | 0.11 ± 0.00 |
| EDL | 0.21 ± 0.01 | 0.20 ± 0.01 | 0.22 ± 0.01 | 0.13 ± 0.00 | 0.11 ± 0.00 * | 0.14 ± 0.00 |
Leptin vs Control group: P < 0.05,
P < 0.01,
P < 0.001
Leptin-Antagonist vs Control group: P < 0.05,
P < 0.01,
P < 0.001; n=8–12/group
pWAT: perirenal white adipose tissue, epi/paWAT: epidydimal/parametrial white adipose tissue, rtWAT: retroperitoneal white adipose tissue, mWAT: mesenteric white adipose tissue, iBAT: interscapular brown adipose tissue, EDL: extensor digitorum longus, TA: tibialis anterior.
Despite differences in physiological responses, central leptin gene overexpression induced cellular leptin resistance in both sexes despite no change in serum leptin
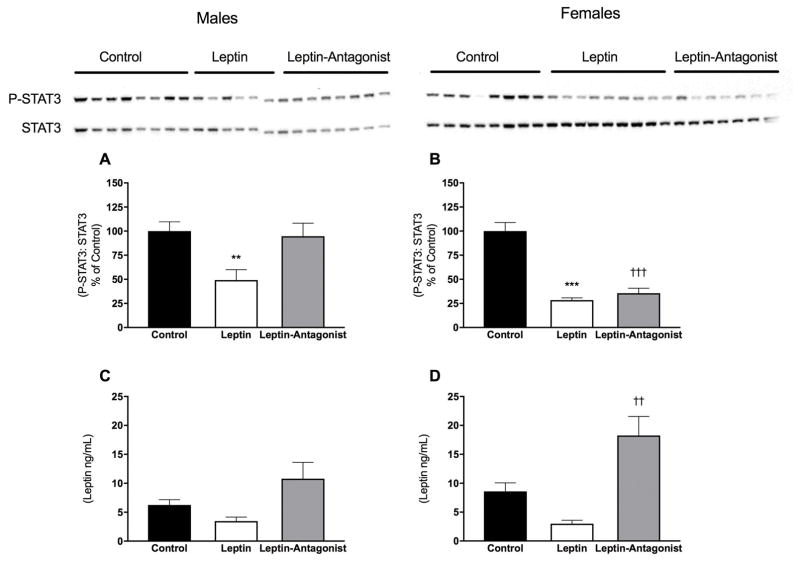
Consistent with attenuated physiological responses recorded in males, leptin gene delivery was associated with lower leptin-mediated phosphorylation of STAT3, indicative of lower leptin receptor activity and onset of leptin resistance (Fig. 4A; Control vs Leptin: P < 0.05). Unexpectedly, in contrast to their persistent physiological responses, acute Leptin-induced P-STAT3 was also attenuated in Leptin-females, suggestive of onset of leptin resistance near time of death (Fig. 4B; Control vs Leptin: P < 0.001). Even though the increases in body weight in response to the Leptin-Antagonist were similar in both sexes when compared to their respective controls, there was a sexual dimorphism in leptin-mediated P-STAT3. Males that received the Leptin-Antagonist vector displayed similar P-STAT3 response to Leptin as males that received the Control vector (Fig. 4A) whereas Leptin-Antagonist-females displayed lower leptin receptor activity than Control-females (Fig. 4B; Control vs Leptin-Antagonist: P < 0.001). The absence of change in serum leptin levels in Leptin treated groups (Fig. 4C and D: Control vs Leptin: N.S.) confirms that leptin effects were centrally mediated. Unexpectedly, serum leptin levels were significantly elevated in Leptin-Antagonist females (Fig. 4D: Control vs Leptin-Antagonist: P < 0.01), however, this effect was not observed in males.
Fig. 4. Hypothalamic P-STAT3 content and serum leptin.
A and B P-STAT3 protein level in males and females, respectively. Values represent densitometry of P-STAT3 analysis divided by densitometry obtained from total STAT3 analysis and respective images. C and D endpoint serum leptin in males and females, respectively. * Leptin significantly different from Control group (P < 0.05), *** (P < 0.001), ††† Leptin-Antagonist significantly different from Control group (P < 0.001). n=8–10/group
Discussion
Although sexual dimorphisms in the field of obesity have been clearly established more than a decade ago (25), limited attention has been paid to sex differences in homeostatic control of body weight. Leptin resistance is considered the main constraint to pharmacological leptin treatment for obesity, and examinations of sex differences in this process are scarce. While female sex and hormonal milieu are clear modulators of acute leptin sensitivity (16, 17), their long-term roles on leptin response and the onset of leptin resistance have yet to be established. Without further understanding sex-specific onset of leptin resistance, development of effective therapies to combat obesity will remain challenging.
Leptin resistance is characterized as a defect in central leptin receptor-mediated signaling (5), a feature that pharmacologically resembles submaximal central leptin receptor blockade (20). Leptin resistance can be triggered by high and persistent leptin receptor signaling (19). We previously demonstrated that central leptin gene delivery rapidly induces leptin resistance in high fat fed and/or aged male rodents (19). In addition, our laboratory has also developed a unique tool to achieve partial blockade of endogenous leptin receptor activity using virus-mediated gene delivery of a leptin antagonist (20). This approach enabled us to explore the influence of lower leptin receptor activity, a model for cellular leptin resistance, on body weight homeostasis without disturbing peripheral responses to leptin. However, our models lack an effective validation system that could determine more accurate sex differences on how leptin and leptin antagonist vector affects leptin receptor biology. Future works should localize brain regions affected by the vectors in each sex. Furthermore, developping tools to track antagonist binding to the leptin receptor and observe receptor trafficking or conformational changes would help better understand sex difference in the modulation of leptin receptor activity.
The present study aimed to examine the long-term sex-specific physiological responses to central leptin receptor activation or blockade. We observed a clear sexual dimorphism in the long-term regulation of body weight and body composition by central leptin receptor activation. Our findings extend those of Clegg et al. by showing that, in addition to enhanced acute leptin sensitivity (16, 17) females display greater and more persistent physiological responses to central leptin receptor activation. In fact, females lost a higher percentage of their initial body weight than males (females vs males: 17 ± 1.5% vs 7 ± 1%) and maximal weight/fat mass loss was maintained until the end of the experiment whereas males essentially rebounded to their initial body weight (−1%) by day 26. This sex bias is attributable to, at least in part, the effect of leptin on long-term caloric consumption. Indeed, cumulative food intake in the Leptin group was significantly lower than the Control group in females, but not in males. It cannot be excluded that differential responses to leptin were due to sexual dimorphism in energy expenditure. In support of this hypothesis, a recent study demonstrated sex differences in the function of a POMC neuron subpopulation with regard to energy expenditure (26). Given that POMC neurons are one of the main targets of leptin receptor, sex differences in leptin response may be due to a sexual dimorphism in energy expenditure in response to leptin. In line with this interpretation, it was observed in humans that serum leptin levels correlated with resting energy expenditure in females, but not in males (27), suggesting that leptin may exert a greater role on energy expenditure in females than in males. Our study has limitations that need to be taken into account. For instance, our experiments were conducted in only one rat strain and we did not perform any direct measurements of energy expenditure. Reproduding similar experiments in other rat strains, and other model organisms as well as examining oxygen consumption, and physical activity upon central leptin gene delivery would help build stronger overall evidence of a sexual dimorphism in leptin-induced energy expenditure.
On the other hand, the results from this experiment do not support our initial hypothesis that females would develop more severe obesity than males in response to central leptin receptor blockade. Unlike with the Leptin vector, few sex differences were observed with Leptin-Antagonist treatment. Males and females displayed similar body weight and fat mass gain. We found that only two fat pads out of four were enlarged in Leptin-Antagonist-females, whereas Leptin-Antagonist-males accumulated significantly more fat in all depots collected compared to Control counterparts. Unlike females, Leptin-Antagonist-males did not display significantly higher serum leptin levels than control males. This is surprising given that serum leptin levels are positively associated with adiposity. This sex bias may simply due to the female hormone environment, more specifically the higher estradiol levels in females, known to enhance leptin secretion in adipose tissues (13). Leptin-Antagonist-females gained significantly more LBM than Control-females, and that phenomenon was not observed in respective male groups. Consequently, lean/fat mass ratio was lower in Leptin-Antagonist-males compared to Control-males. These observations are puzzling considering the partial antagonist blockade was apparently less effective in the males than females based on leptin receptor signaling as described in detail below.
Consistent with leptin-induced cellular leptin resistance, leptin gene delivery was associated with both reduced physiological responses, lower acute leptin-mediated STAT3 phosphorylation in males. Surprisingly, acute increase in P-STAT3/STAT3 ratio was also less pronounced in Leptin-females compared to Control-females despite persistent physiological responses. One potential explanation is that the onset of leptin resistance occurred just prior to the end of the experiment at day 26. In line with this hypothesis, Leptin-females and Control-females consumed the same amount of food on the last day of the experiment. Normalization of food intake likely would have required several days to translate into body weight gain. Future long-term studies are necessary to evaluate whether females would eventually regain the lost weight. Another potential explanation for discord between maximal leptin receptor signaling and physiological responses is that these functions are not tightly coupled. Our previous studies utilizing a leptin receptor antagonist indicated leptin mediated P-STAT3 signaling and metabolic responses are uncoupled (28). Additionally, others found that leptin also controls metabolism via mechanisms that are independent of STAT3 phosphorylation (29, 30). It should be noted that other signaling cascades also induces phosphorylation of STAT3. Although P-STAT3 is not specific to leptin signaling, high affinity and specificity antibodies to assess leptin receptor activation (Phospho Leptin Receptor) are currently not commercially available and therefore, P-STAT3 antibodies remain valuable tools to compare leptin receptor activation across groups that underwent similar treatments.
In the present study, body weight in response to Leptin-Antagonist were similar in both sexes when compared to their respective Control group, however, there was a sexual dimorphism in leptin-mediated P-STAT3. Treatment with the Leptin-Antagonist vector in males did not attenuate leptin-induced P-STAT3 to the same extent as in females. One speculative explanation is that there is a greater number of leptin receptors or greater reserve capacity in the males and the level of antagonist expression was unable to sufficiently saturate the receptors. To confirm this hypothesis, future studies should compare P-STAT3/STAT3 ratio in a dose-response to the Leptin-Antagonist vector between sexes. Again, replicating those experiment in different rat strains and in other model organisms would help establish stronger conclusion of a sex bias.
Collectively, these data suggest that the female hormonal milieu enhances long-term leptin responsiveness. However, it is unclear whether the nature of physiological responses is a function of high female or low male sex hormone levels. Acute injections of leptin into the 3rd ventricle of rats inhibits eating to a greater extent in females than males and those differential responses are likely mediated by female sex hormones because estradiol treatment in males enhances leptin responsiveness (16, 17). However, androgens appear to affect leptin potency in the opposite manner as estrogens and could potentially contribute to sex difference in leptin physiology. The androgen 5α-dihydrotestosterone (DHT) reduced leptin sensitivity in Ovariectomized female rats (31) and exacerbated obesity in castrated male mice (32). Besides, the primary male sex hormone testosterone interacts with the negative feedback signal of the leptin receptor (Suppressor of cytokine signaling 3; SOCS3). Testosterone enhances this negative feedback mechanism, hence could contribute to sex differences in leptin sensitivity. Future studies aiming at evaluating the roles of male hormones in long-term leptin responsiveness would be necessary to confirm this hypothesis.
In summary, this report established a clear sex bias in long-term central leptin responsiveness. We found differential physiological responses to central leptin gene delivery in male and female rats. Females displayed greater and more sustained physiological responses to central leptin gene delivery than males. Additionally, lean/fat mass ratio was lower in Leptin-Antagonist-males than Control-males, but not in respective female groups. It is likely that the hormonal milieu contributed to sex differences; however, future studies are needed to establish whether physiological responses are mediated by female or male sex hormones.
Supplementary Material
A and B absolute fat mass (FM) in males and females, respectively. C and D FM percentage in males and females, respectively. E and F lean body mass (LBM) in males and females, respectively. G and H LBM percentage in males and females, respectively. Values at days 0, 7, 14 and 26 are displayed. Values are means ± standard errors * Leptin significantly different from Control group (P < 0.05), ** (P < 0.01), †† Leptin-Antagonist significantly different from Control group (P < 0.01), ††† (P < 0.001). n=8–10/group
Acknowledgments
Funding
This work was supported by a grant from the National Institutes of Health, USA (DK091710).
Footnotes
Declaration of interest
None to declare.
Author contribution
IC, DM, and PJS conceived and designed the study. SMG and IC collected the data. IC, DM, CSC, NT, and PJS analyzed and interpreted the data. IC, DM, and PJS wrote and edited the manuscript.
All authors had access to data and had final responsibility for the manuscript content
References
- 1.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 2.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395(6704):763–70. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 3.Proietto J, Thorburn AW. The therapeutic potential of leptin. Expert Opin Investig Drugs. 2003;12(3):373–8. doi: 10.1517/13543784.12.3.373. [DOI] [PubMed] [Google Scholar]
- 4.Banks WA, Clever CM, Farrell CL. Partial saturation and regional variation in the blood-to-brain transport of leptin in normal weight mice. Am J Physiol Endocrinol Metab. 2000;278(6):E1158–65. doi: 10.1152/ajpendo.2000.278.6.E1158. [DOI] [PubMed] [Google Scholar]
- 5.El-Haschimi K, Pierroz DD, Hileman SM, Bjorbaek C, Flier JS. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J Clin Invest. 2000;105(12):1827–32. doi: 10.1172/JCI9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scarpace PJ, Matheny M, Zolotukhin S, Tumer N, Zhang Y. Leptin-induced leptin resistant rats exhibit enhanced responses to the melanocortin agonist MT II. Neuropharmacology. 2003;45(2):211–9. doi: 10.1016/s0028-3908(03)00158-8. [DOI] [PubMed] [Google Scholar]
- 7.Scarpace PJ, Matheny M, Zhang Y, Shek EW, Prima V, Zolotukhin S, Tumer N. Leptin-induced leptin resistance reveals separate roles for the anorexic and thermogenic responses in weight maintenance. Endocrinology. 2002;143(8):3026–35. doi: 10.1210/endo.143.8.8966. [DOI] [PubMed] [Google Scholar]
- 8.Scarpace PJ, Matheny M, Tumer N, Cheng KY, Zhang Y. Leptin resistance exacerbates diet-induced obesity and is associated with diminished maximal leptin signalling capacity in rats. Diabetologia. 2005;48(6):1075–83. doi: 10.1007/s00125-005-1763-x. [DOI] [PubMed] [Google Scholar]
- 9.Harris RB, Bowen HM, Mitchell TD. Leptin resistance in mice is determined by gender and duration of exposure to high-fat diet. Physiol Behav. 2003;78(4–5):543–55. doi: 10.1016/s0031-9384(03)00035-0. [DOI] [PubMed] [Google Scholar]
- 10.Harris RB, Mitchell TD, Mynatt RL. Leptin responsiveness in mice that ectopically express agouti protein. Physiol Behav. 2002;75(1–2):159–67. doi: 10.1016/s0031-9384(01)00653-9. [DOI] [PubMed] [Google Scholar]
- 11.Harris RB, Mitchell TD, Hebert S. Leptin-induced changes in body composition in high fat-fed mice. Exp Biol Med (Maywood) 2003;228(1):24–32. doi: 10.1177/153537020322800103. [DOI] [PubMed] [Google Scholar]
- 12.Lin S, Thomas TC, Storlien LH, Huang XF. Development of high fat diet-induced obesity and leptin resistance in C57Bl/6J mice. Int J Obes Relat Metab Disord. 2000;24(5):639–46. doi: 10.1038/sj.ijo.0801209. [DOI] [PubMed] [Google Scholar]
- 13.Machinal F, Dieudonne MN, Leneveu MC, Pecquery R, Giudicelli Y. In vivo and in vitro ob gene expression and leptin secretion in rat adipocytes: evidence for a regional specific regulation by sex steroid hormones. Endocrinology. 1999;140(4):1567–74. doi: 10.1210/endo.140.4.6617. [DOI] [PubMed] [Google Scholar]
- 14.Smith JT, Mark PJ, Waddell BJ. Developmental increases in plasma leptin binding activity and tissue Ob-Re mRNA expression in the rat. J Endocrinol. 2005;184(3):535–41. doi: 10.1677/joe.1.06045. [DOI] [PubMed] [Google Scholar]
- 15.Wiesner G, Vaz M, Collier G, Seals D, Kaye D, Jennings G, Lambert G, Wilkinson D, Esler M. Leptin is released from the human brain: influence of adiposity and gender. J Clin Endocrinol Metab. 1999;84(7):2270–4. doi: 10.1210/jcem.84.7.5854. [DOI] [PubMed] [Google Scholar]
- 16.Clegg DJ, Riedy CA, Smith KA, Benoit SC, Woods SC. Differential sensitivity to central leptin and insulin in male and female rats. Diabetes. 2003;52(3):682–7. doi: 10.2337/diabetes.52.3.682. [DOI] [PubMed] [Google Scholar]
- 17.Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes. 2006;55(4):978–87. doi: 10.2337/diabetes.55.04.06.db05-1339. [DOI] [PubMed] [Google Scholar]
- 18.Rocha M, Bing C, Williams G, Puerta M. Physiologic estradiol levels enhance hypothalamic expression of the long form of the leptin receptor in intact rats. J Nutr Biochem. 2004;15(6):328–34. doi: 10.1016/j.jnutbio.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Scarpace PJ, Matheny M, Zhang Y, Shek EW, Prima V, Zolotukhin S, Tu Mer N. Leptin-Induced Leptin Resistance Reveals Separate Roles for the Anorexic and Thermogenic Responses in Weight Maintenance. Endocrinology. 2002;143(8):3026–35. doi: 10.1210/endo.143.8.8966. [DOI] [PubMed] [Google Scholar]
- 20.Matheny M, Zhang Y, Shapiro A, Tumer N, Scarpace PJ. Central overexpression of leptin antagonist reduces wheel running and underscores importance of endogenous leptin receptor activity in energy homeostasis. Am J Physiol Regul Integr Comp Physiol. 2009;297(5):R1254–61. doi: 10.1152/ajpregu.90449.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paxinos G, Watson CR, Emson PC. AChE-stained horizontal sections of the rat brain in stereotaxic coordinates. J Neurosci Methods. 1980;3(2):129–49. doi: 10.1016/0165-0270(80)90021-7. [DOI] [PubMed] [Google Scholar]
- 22.Strehler KY, Matheny M, Kirichenko N, Sakarya Y, Bruce E, Toklu HZ, Carter CS, Morgan D, Tumer N, Scarpace PJ. Onset of leptin resistance shows temporal differences related to dose or pulsed treatment. Eur J Pharmacol. 2016:779177–85. doi: 10.1016/j.ejphar.2016.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dhillon H, Kalra SP, Prima V, Zolotukhin S, Scarpace PJ, Moldawer LL, Muzyczka N, Kalra PS. Central leptin gene therapy suppresses body weight gain, adiposity and serum insulin without affecting food consumption in normal rats: a long-term study. Regul Pept. 2001;99(2–3):69–77. doi: 10.1016/s0167-0115(01)00237-3. [DOI] [PubMed] [Google Scholar]
- 24.Scarpace PJ, Matheny M, Zhang Y, Tumer N, Frase CD, Shek EW, Hong B, Prima V, Zolotukhin S. Central leptin gene delivery evokes persistent leptin signal transduction in young and aged-obese rats but physiological responses become attenuated over time in aged-obese rats. Neuropharmacology. 2002;42(4):548–61. doi: 10.1016/s0028-3908(02)00003-5. [DOI] [PubMed] [Google Scholar]
- 25.Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146(4):1650–73. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- 26.Burke LK, Doslikova B, D’Agostino G, Greenwald-Yarnell M, Georgescu T, Chianese R, Martinez de Morentin PB, Ogunnowo-Bada E, Cansell C, Valencia-Torres L, Garfield AS, Apergis-Schoute J, Lam DD, Speakman JR, Rubinstein M, Low MJ, Rochford JJ, Myers MG, Evans ML, Heisler LK. Sex difference in physical activity, energy expenditure and obesity driven by a subpopulation of hypothalamic POMC neurons. Mol Metab. 2016;5(3):245–52. doi: 10.1016/j.molmet.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicklas BJ, Toth MJ, Poehlman ET. Daily energy expenditure is related to plasma leptin concentrations in older African-American women but not men. Diabetes. 1997;46(9):1389–92. doi: 10.2337/diab.46.9.1389. [DOI] [PubMed] [Google Scholar]
- 28.Scarpace PJ, Matheny M, Zhang Y, Cheng KY, Tumer N. Leptin antagonist reveals an uncoupling between leptin receptor signal transducer and activator of transcription 3 signaling and metabolic responses with central leptin resistance. J Pharmacol Exp Ther. 2007;320(2):706–12. doi: 10.1124/jpet.106.112813. [DOI] [PubMed] [Google Scholar]
- 29.Buettner C, Muse ED, Cheng A, Chen L, Scherer T, Pocai A, Su K, Cheng B, Li X, Harvey-White J, Schwartz GJ, Kunos G, Rossetti L, Buettner C. Leptin controls adipose tissue lipogenesis via central, STAT3-independent mechanisms. Nat Med. 2008;14(6):667–75. doi: 10.1038/nm1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bates SH, Kulkarni RN, Seifert M, Myers MG., Jr Roles for leptin receptor/STAT3-dependent and -independent signals in the regulation of glucose homeostasis. Cell Metab. 2005;1(3):169–78. doi: 10.1016/j.cmet.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Kanaya N, Vonderfecht S, Chen S. Androgen (dihydrotestosterone)-mediated regulation of food intake and obesity in female mice. J Steroid Biochem Mol Biol. 2013:138100–6. doi: 10.1016/j.jsbmb.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moverare-Skrtic S, Venken K, Andersson N, Lindberg MK, Svensson J, Swanson C, Vanderschueren D, Oscarsson J, Gustafsson JA, Ohlsson C. Dihydrotestosterone treatment results in obesity and altered lipid metabolism in orchidectomized mice. Obesity (Silver Spring) 2006;14(4):662–72. doi: 10.1038/oby.2006.75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A and B absolute fat mass (FM) in males and females, respectively. C and D FM percentage in males and females, respectively. E and F lean body mass (LBM) in males and females, respectively. G and H LBM percentage in males and females, respectively. Values at days 0, 7, 14 and 26 are displayed. Values are means ± standard errors * Leptin significantly different from Control group (P < 0.05), ** (P < 0.01), †† Leptin-Antagonist significantly different from Control group (P < 0.01), ††† (P < 0.001). n=8–10/group