Abstract
Immunological requirements for rejection and tolerance induction differ between various organs. While memory CD8+ T cells are considered a barrier to immunosuppression-mediated acceptance of most tissues and organs, tolerance induction after lung transplantation is critically dependent on central memory CD8+ T lymphocytes. Here we demonstrate that costimulation blockade-mediated tolerance after lung transplantation is dependent on PD-1 expression on CD8+ T cells. In the absence of PD-1 expression, CD8+ T cells form prolonged interactions with graft-infiltrating CD11c+ cells, their differentiation is skewed towards an effector memory phenotype and grafts are rejected acutely. These findings extend the notion that requirements for tolerance induction after lung transplantation differ from other organs. Thus, immunosuppressive strategies for lung transplant recipients need to be tailored based on the unique immunological properties of this organ.
Introduction
Immune responses after lung transplantation differ from those observed after transplantation of other organs (1, 2). Our previous work has established that interactions between innate and adaptive immune cell populations within lung allografts regulate both rejection and tolerance (1) (3). This sets the lung apart from other organs and tissues such as hearts and pancreatic islets, where graft rejection and tolerance depend on cell trafficking to graft-draining secondary lymphoid organs (4) (5). Based on this unique local environment lung grafts have been referred to as “lymph nodes with alveoli.” (6). The capacity to generate alloimmune responses locally may provide an explanation why conditions such as primary graft dysfunction and respiratory infections, which are associated with infiltration of immune cells into lung grafts, can predispose to immune-mediated graft failure. Nevertheless, despite these unique immunological features of lungs pulmonary transplant recipients continue to receive immunosuppressive regimens that have been established for recipients of other organs. Such treatment strategies may actually be deleterious. For example, our laboratory has shown that a subset of CD8+ T cells are critical to induce tolerance to pulmonary allografts in immunosuppressed recipients (2). Therefore, targeting T cells indiscriminately during the peri-operative period, a practice that is the current standard of care at many centers, may adversely impact outcomes. Thus, the use of inadequate immunosuppressive strategies may contribute to comparatively poor long-term outcomes after lung transplantation. Clearly, a better mechanistic understanding of immunological requirements for tolerance induction after pulmonary transplantation will aid with the development of therapies that are tailored for lung transplant recipients.
We have recently made the surprising observation that central memory CD8+ T cells, a population that contributes to rejection and immunosuppression resistance of other organs, promotes tolerance induction to lung allografts through IFN-gamma-mediated production of nitric oxide (2). Interestingly, tolerance induction was dependent on the expression of CCR7 by CD8+ T cells, which facilitated their interaction with antigen presenting cells in the graft and promoted the production of IFN-gamma. In the current study we set out to further define mechanisms that regulate tolerance induction after lung transplantation. We describe that PD-1 expression on recipient CD8+ T cells is critically important for tolerance induction after lung transplantation. Mechanistically, when CD8+ T cells do not express PD-1 they form prolonged synapses with antigen presenting cells in the graft and undergo differentiation towards effector memory T cells. Thus, this study extends the concept that the fate of transplanted lungs is controlled by immune cell encounters within pulmonary allografts at early time points after transplantation.
Methods
Mice
C57BL/6J (B6), Balb/cJ (BALB/c), B6. SJL-Ptprca Pepcb/BoyJ (CD45.1+) and B6.PL-Thy1a/CyJ (Thy1.1+) mice were purchased from The Jackson Laboratories (Bar Harbor, ME). B6 mice expressing enhanced yellow fluorescent protein driven by a CD11c promoter (CD11c-EYFP+) were a gift from M Nussenzweig (Rockefeller University, New York, NY) and bred at our facility. PD-1-deficient mice were obtained from A. Ayala (Brown University, Providence, RI), who had originally obtained a breeding colony from T. Honjo (Kyoto University Graduate School of Medicine, Kyoto, Japan). All procedures were approved by the Institutional Animal Studies Committee.
Lung transplantation
Left orthotopic vascularized lung transplants were performed as previously described (7). Mice were treated with co-stimulatory blockade consisting of MR1 (250 μg intraperitoneally) and CTLA4-Ig (200 μg intraperitoneally), on days 0 and 2, respectively (Bio X Cell, West Lebanon, NH). For select experiments, lung recipients were additionally treated with anti-PD-1 antibody (clone RMP1-14: 250 μg intraperitoneally, days −2, 2) (Bio X Cell, West Lebanon, NH). For some experiments, CD8+ T cells isolated from spleens of either B6 wildtype (CD45.2+Thy1.2+ or CD45.2+Thy1.1+) or PD-1-deficient (CD45.2+Thy1.2+) mice were injected into B6 CD45.1+Thy1.2+ recipients of BALB/c lungs at the time of transplantation.
Histology
Portions of transplanted lungs were fixed in formaldehyde, sectioned, and stained with hematoxylin and eosin. Grading for acute cellular rejection was performed in a blinded manner by a pathologist (JHR) using standard criteria (8).
Flow cytometry
Single cell suspensions were prepared from lung allografts, draining mediastinal lymph nodes and spleens as previously described and incubated with CD16/32 antibody 30 minutes prior to the staining with fluorochrome-labeled antibodies specific for CD90.2 (clone 30-H12), CD90.1 (clone HIS51), CD62L (clone MEL-14), CD44 (clone IM7), CD8a (clone 53-6.7), Foxp3 (clone FJK-16s), CD4 (clone RM4-5), CD11c (clone N418), CD45.2 (clone 104), CD45.1 (clone A20) (all from ebioscience, San Diego, CA) PD-L1 (clone MIH5) and PD-1 (clone J43) (BD Pharmingen, San Jose, CA) (2).
ELISA
CD8+ T cells, positively selected from lung allografts seven days after transplantation into PD-1 antibody-treated or control recipients, were cultured with T cell-depleted donor Balb/c splenocytes at a 1:1 ratio (3 × 105 cells each) (Miltenyi Biotec, San Diego, CA). Supernatants were collected at 24 and 48 hours and analyzed for IFN-γ by ELISA according to manufacturer’s protocol (R&D System; Minneapolis, MN).
Measurement of serum alloantibody titers
200 μL aliquots of PBA (PBS with 0.5% bovine serum albumin and 0.02% sodium azide) containing 2 × 106 BALB/c thymocytes were added to 200 μL of diluted serum in PBA (1/4, 1/16, 1/64 and 1/256 dilutions) for 1 hour at 4°C with frequent agitation. After three washes with PBA, cells were stained for 30 min at 4°C with 100 μL of PBA containing 1 μL of polyclonal PE-conjugated goat anti-mouse IgM, μ chain specific (Jackson ImmunoResearch, West Grove, PA). Cells were analyzed on a FACScan (BD Biosciences, San Jose, CA) and FlowJo software (FlowJo, Ashland, OR) was used to calculate the median fluorescence intensity.
In vitro cell cultures
3 × 105 T cell–depleted BALB/c splenocyte (as stimulators) were cultured with 3 × 105 CD8+ T cell responders isolated with CD90 magnetic beads (Miltenyi Biotec, Auburn, CA) from spleens of wildtype or PD-1-deficient mice in complete medium in round bottom 96-well plates for 48 hours. CD90.2+CD45+CD8+ were sorted in Trizol RNA extraction reagent (Thermo Fisher Scientific, Carlsbad, CA) using a Sony Synergy cell sorter SY3200 (SONY, San Jose, CA). Quantitative realtime PCR was performed as described previously (9). Primer sequences for Perforin (pfr-1) are: 5′-AGCACAAGTTCGTGCCAGG −3′ (forward) and 5′-GCGTCTCTCATTAGGGAGTTTTT −3′ (reverse).
Intravital two-photon microscopy
BALB/c lungs were transplanted into immunosuppressed B6 CD11c-EFYP recipients and on day 3, received an injection of 107 CD8+ T cells isolated from wild-type B6 mice (labeled with CMTMR) and PD-1-deficient mice (labeled with CellTrace Violet, Molecular Probes, Eugene, OR). Twenty-four hours after cell injection, time-lapse imaging was performed with a custom-built two-photon microscope running ImageWarp version 2.1 acquisition software (A&B Software, New London, CT).
Statistics
Data are reported as mean ± SEM. The Mann-Whitney U test was performed using GraphPad Prism version 7.0 (GraphPad Software, La Jolla, CA). p < 0.05 was considered significant.
Results
PD-1 blockade prevents induction of lung transplant tolerance
Based on previous reports that inhibition of PD-1/PD-L1 can accelerate rejection of hearts and pancreatic islets we set out to evaluate the role of this inhibitory pathway in tolerance after lung transplantation (10, 11). We transplanted BALB/c lungs into B6 recipients that were treated with peri-operative co-stimulatory blockade. When these recipients were treated with anti-PD-1 antibody two days prior and two days after transplantation, pulmonary allografts were acutely rejected (Figures 1A, B). Consistent with previous findings graft rejection was associated with a significant increase in the ratio of CD8+ T cells to CD4+ T cells within lung allografts (Figure 1C) (12). While we observed an increase in the percentage of proliferating cells for both CD8+ and CD4+ T lymphocytes after injection of anti-PD-1 antibody, this increase was more pronounced for CD8+ T cells (Figure 1 D). We also found that a higher proportion of graft-infiltrating CD4+ T cells expressed Foxp3 when PD-1 is inhibited (Figure 1E). Moreover, treatment with anti-PD-1 antibody resulted in a relative increase in the abundance of effector memory CD4+CD44hiCD62Llow and CD8+ CD44hiCD62Llow T cells with a decrease in the abundance of naïve CD4+CD44lowCD62Lhi and CD8+ CD44lowCD62Lhi T lymphocytes within the allografts (Figures 1 F, G). An expansion of effector memory CD8+ T cells was not observed in graft-draining lymph nodes or spleen of lung recipients that were injected with anti-PD-1 antibody (Figure S1). Graft-infiltrating CD8+ T cells isolated from recipients that were treated with anti-PD-1 antibody produced higher levels of IFN-γ after stimulation with donor antigen presenting cells (Figure 1H). Finally, blockade of PD-1 resulted in higher serum titers of alloantibodies (Figure 1I). Thus, prevention of immunosuppression-mediated acceptance of lung allografts after blockade of PD-1 is associated with differentiation of graft-infiltrating T lymphocytes towards an effector memory phenotype as well as enhanced B and T cell alloresponses.
Figure 1. PD-1 blockade prevents costimulation blockade-mediated lung allograft acceptance.
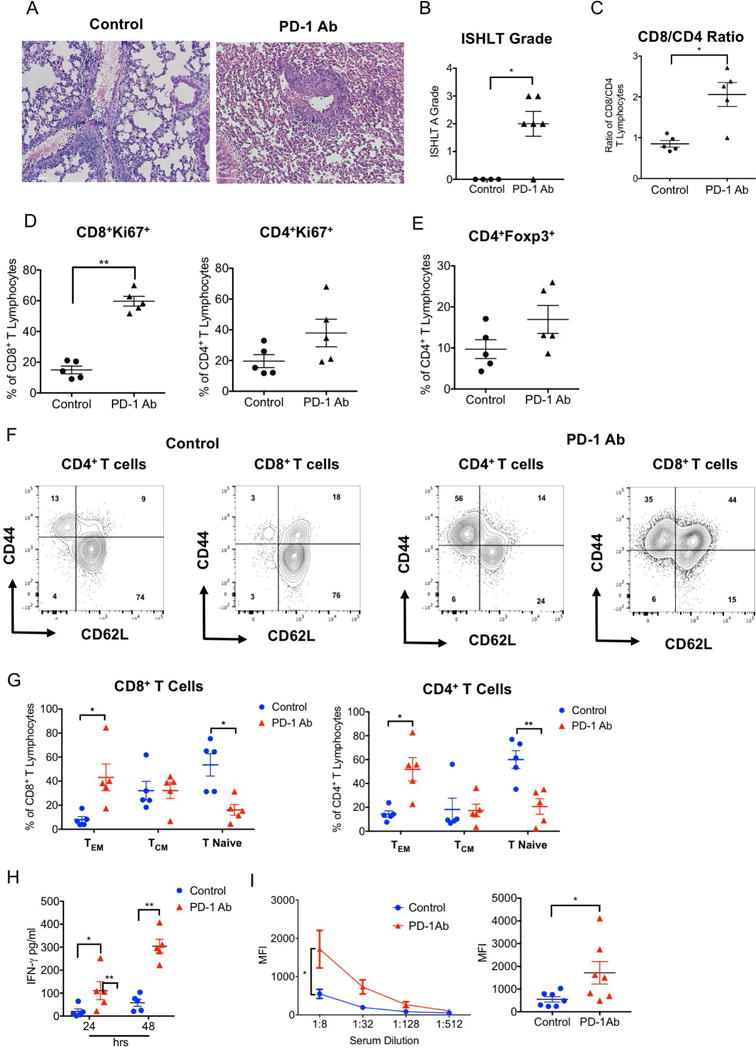
(A) Representative histology, (B) numerical ISHLT rejection grades, (C) intragraft CD8/CD4 T cell ratio, (D) CD8+ and CD4+ T cell proliferative responses and (E) percentage of intragraft CD4+ T cells that express Foxp3 for BALB/c lung allografts seven days after transplantation into control and anti-PD-1-antibody-treated immunosuppressed B6 recipients. Data represent mean ± SEM, *p<0.05, **p<0.01, ***p<0.001 by unpaired T test. (F) Representative contour plot and (G) graphic representation of relative abundance of naïve (CD62LhiCD44low), central memory (CM) (CD62LhiCD44hi) and effector memory (EM) (CD62LlowCD44hi) CD4+ and CD8+ T cells in BALB/c lung allografts seven days after transplantation into control and anti-PD-1-antibody-treated immunosuppressed B6 recipients. (H) IFN-γ levels in supernatants after 24- and 48-hour co-cultures of T cell-depleted Balb/c splenocytes with CD8+ T cells, isolated from Balb/c lung grafts seven days after transplantation into control and anti-PD-1-antibody-treated immunosuppressed B6 recipients. (I) Representative plot (left) and graphic representation (right) of serum IgM alloreactive antibody titers in control and anti-PD-1-antibody-treated immunosuppressed B6 recipients of Balb/c lungs seven days after transplantation. Results are expressed as mean fluorescent intensities (MFI). Data represent mean ± SEM. *p<0.05, **p<0.01 by Mann-Whitney U test.
PD-1 expression on CD8+ T cells is critical for lung transplant tolerance induction
Of note, the vast majority of CD11c+ antigen presenting cells within accepted lung allografts expresses PD-L1 and a portion of graft-infiltrating CD8+ T cells expresses PD-1 (Figure 2A). We next transplanted Balb/c lungs into immunosuppressed B6 PD-1-deficient recipients. Seven days after engraftment the transplanted lungs had histological evidence of allograft rejection and the vast majority of graft-infiltrating CD4+ and CD8+ T cells had an effector memory phenotype (Figure S2). As cell populations other than T cells can express PD-1 and we have previously shown that recipient central memory CD8+ T cells are critical to induce tolerance after lung transplantation we next wanted to determine whether PD-1 expression on recipient CD8+ T cells regulates tolerance induction (2, 13). To this end, we transplanted BALB/c lungs into B6 CD45.1+ hosts that were treated with peri-operative costimulatory blockade. At the time of transplantation, we injected 107 B6 CD45.2+ wild-type or B6 CD45.2+ PD-1-deficient CD8+ T cells into the recipients. While minimal inflammation was observed after injection of wild-type CD8+ T cells, grafts demonstrated severe acute rejection after adoptive transfer of PD-1-deficient CD8+ T cells (Figure 2B, C). Similar to our observations after antibody blockade, the ratio of endogenous recipient CD8+ to CD4+ T lymphocytes was significantly increased after adoptive transfer of PD-1-deficient CD8+ T cells (Figure 2D). After injection of CD8+ T cells that lacked expression of PD-1 both adoptively transferred CD8+ T lymphocytes as well as native CD4+ and CD8+ T cells had higher rates of proliferation (Figure 2E). Furthermore, we observed a decrease in the abundance of graft-infiltrating naïve (CD44lowCD62Lhi) and an increase in the abundance of effector memory (CD44hiCD62Llow) T cells in both native (Figure 2F) and adoptively transferred T lymphocytes (Figure 2G) when we injected CD8+ T lymphocytes that lacked expression of PD-1.
Figure 2. PD-1 expression on CD8+ T cells is critical to induce tolerance after lung transplantation.
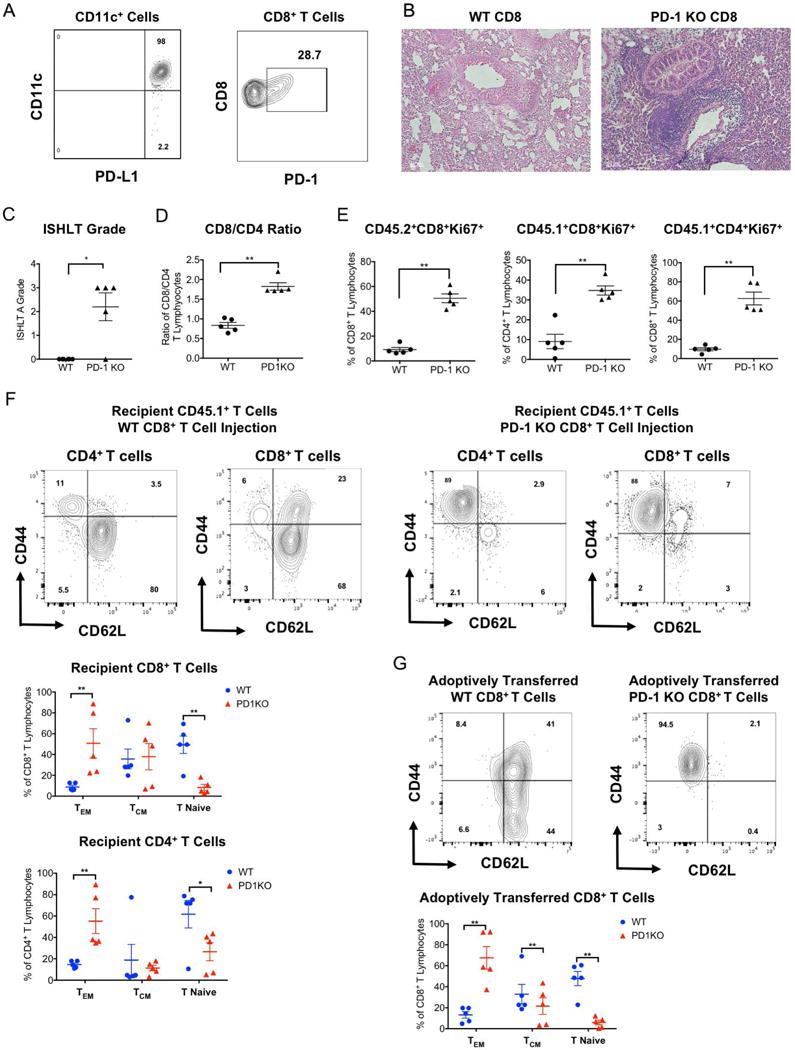
(A) Representative contour plot depicting PD-L1 expression on CD11c+ antigen presenting cells and PD-1 expression on CD8+ T cells within BALB/c lung allografts seven days after transplantation into immunosuppressed B6 recipients. (B) Representative histology, (C) numerical ISHLT rejection grades, (D) intragraft CD8+/CD4+ T cell ratio of native (CD45.1+) recipient CD8+ T lymphocytes and (E) proliferative responses of adoptively transferred (CD45.2+) as well as native (CD45.1+) T cells within BALB/c lung allografts seven days after transplantation into immunosuppressed B6 CD45.1+ recipients that received either wildtype or PD-1 deficient B6 CD45.2+ CD8+ T lymphocytes at the time of transplantation. Data represent mean ± SEM. *p<0.05, **p<0.01, ***p<0.001 by unpaired T test. Representative contour plots and graphic representation of relative abundance of naïve (CD62LhiCD44low), central memory (CM) (CD62LhiCD44hi) and effector memory (EM) (CD62LlowCD44hi) for (F) native CD45.1+ CD4+ and CD8+ T cells and for (G) adoptively transferred wildtype and PD-1-deficient B6 CD45.2+ CD8+ T cells in BALB/c lung allografts seven days after transplantation into immunosuppressed B6 recipients. Data represent mean ± SEM. *p < 0.05, **p < 0.01 by Mann-Whitney U test.
We next set out to examine how PD-1 expression impacted CD8+ T cell differentiation after activation via direct allorecognition in vitro. We isolated CD8+ T cells from the spleens of wildtype and PD-1-deficient B6 mice. The relative abundance of naïve (CD44lowCD62Lhi), central memory (CD44hiCD62Lhi) and effector memory (CD44hiCD62Llow) T cells was comparable between the two strains at baseline (Figure 3A). After two days of co-culture with allogeneic BALB/c splenocytes we observed an increased differentiation towards an effector memory phenotype when CD8+ T cells lacked expression of PD-1 (Figure 3B). Furthermore, compared to central memory T cells, both wildtype and PD-1-deficient effector memory T cells expressed higher levels of perforin suggesting higher cytotoxic potential (Figure 3C).
Figure 3. PD-1 expression regulates CD8+ T cell differentiation in response to stimulation with allogeneic antigen presenting cells.
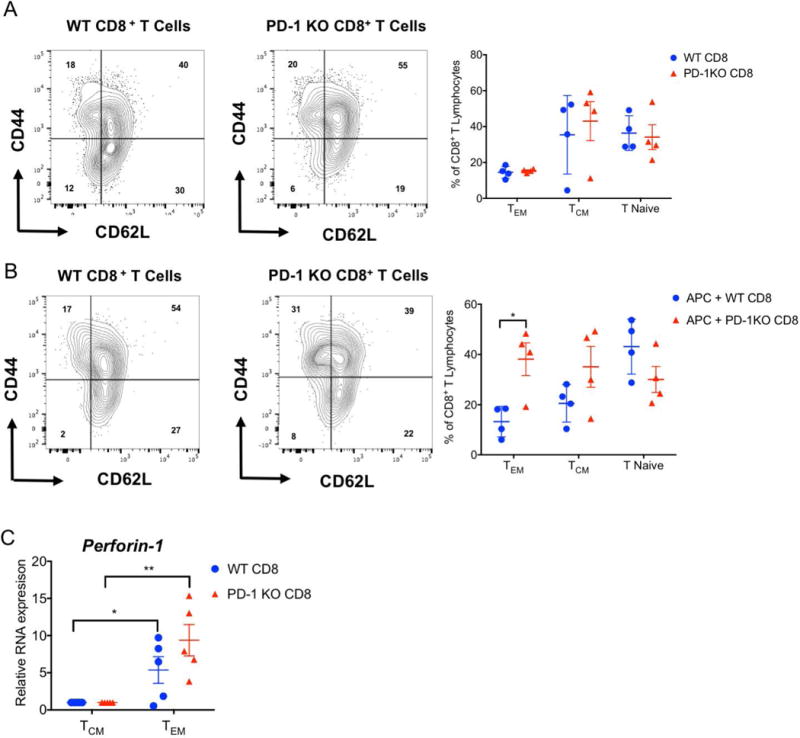
Representative contour plots and graphic representation of phenotype of wildtype and PD-1-deficient B6 CD8+ T cells (A) at baseline and (B) after 48 hours of co-culture with T cell-depleted BALB/c splenocytes. Data represent mean ± SEM for 3 biological replicates. *p<0.05, **p<0.01, ***p<0.001 by 2-Way ANOVA. (C) Perforin expression in central memory (CM) (CD62LhiCD44hi) and effector memory (EM) (CD62LlowCD44hi) wildtype and PD-1-deficient B6 CD8+ T cells after 48 hours of co-culture with T cell-depleted BALB/c splenocytes. Results were normalized to 18s RNA and compared with central memory T cells in each group. Data represent the mean ± SEM. *p < 0.05, **p < 0.01 by Mann-Whitney U test.
PD-1 expression regulates the interaction between CD8+ T cells and CD11c+ antigen presenting cells in lung allografts
We have previously shown that the duration of interactions between CD8+ T cells and CD11c+ antigen presenting cells within lung allografts impacts outcomes after lung transplantation (2). As PD-1 expression on T cells has been previously shown to regulate the interaction between T cells and antigen presenting cells we wanted to evaluate whether PD-1 regulates the dynamic behavior of CD8+ T cells within lung allografts (14, 15). To this end, we transplanted BALB/c lungs into immunosuppressed B6 CD11c-EYFP mice that express enhanced yellow fluorescent protein under a CD11c promoter. We have previously shown that graft-infiltrating CD11c+ cells express both recipient and donor MHC molecules on their surface and prime allogeneic T cells (2) (16). Four days after transplantation we injected fluorochrome-labeled B6 WT and PD-1-deficient CD8+ T cells into the B6 CD11c-EYFP hosts and imaged the lung by intravital two-photon microscopy 24 hours later (Figures 4 A, B; Video S1). Compared to wildtype CD8+ T cells, we observed significantly longer interactions between CD11c+ antigen presenting cells and CD8+ T cells that were deficient in PD-1.
Figure 4. PD-1 expression on graft-infiltrating CD8+ T cells regulates their interaction with CD11c+ antigen presenting cells.
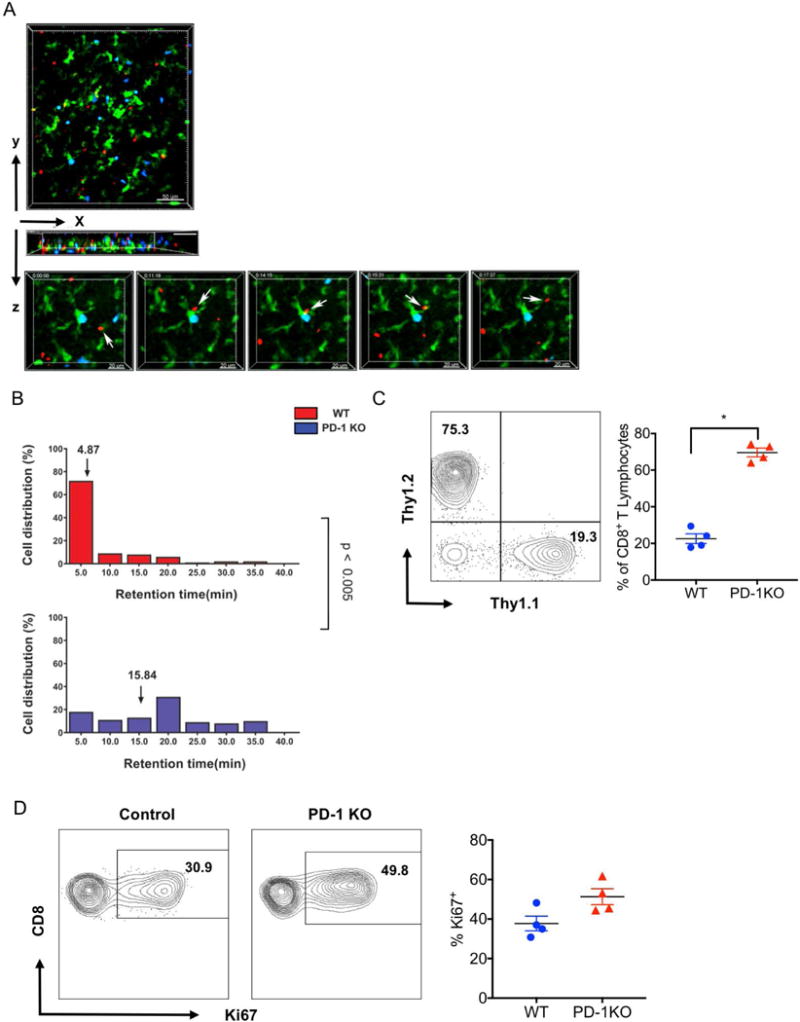
(A) Intravital two-photon microscopy showing wild-type B6 CD8+ T cells (red), PD-1-deficient CD8+ T cells (blue) and CD11c+ cells (green) in BALB/c lung allografts 4 days after transplantation into immunosuppressed B6-EYFP recipients. Cells were injected 24 hours prior to imaging. Scale bars 50 μm (top) and 20 μm (bottom). Images in bottom panel are individual frames from a continuous time-lapse recording (Video S1). Relative time displayed in minutes:seconds. (B) PD-1-deficient CD8+ T cells (blue) have longer retention times (mostly associated with EYFP+ CD11c+ T cells) than wildtype CD8+ T cells (red) (15.8 vs. 4.9 minutes, p<0.005). Representative data are shown from two independent experiments with similar results. (C) Representative contour plot (left) and graphic representation (right) of relative abundance of adoptively transferred wildtype (CD45.2+Thy1.1+) and PD-1-deficient (CD45.2+Thy1.2+) CD8+ T cells in Balb/c lung allografts seven days after transplantation into CD45.1+Thy.1.2+ hosts. Contour plots are gated on live CD45.2+CD8+ cells. (D) Representative contour plot (left) and graphic representation (right) of Ki67 expression in adoptively transferred wildtype (CD45.2+Thy1.1+) and PD-1-deficient (CD45.2+Thy1.2+) CD8+ T cells in Balb/c lung allografts seven days after transplantation into CD45.1+Thy.1.2+ hosts. Data represent the mean ± SEM. *p < 0.05 by Mann-Whitney U test.
Having demonstrated that PD-1 expression on CD8+ T cells regulates their dwell time with CD11c+ antigen presenting cells within lung allografts we next set out to examine how PD-1 expression impacts proliferative responses and accumulation of CD8+ T lymphocytes within allografts. We injected equal numbers of PD-1-deficient (CD45.2+Thy1.2+) and wildtype (CD45.2+Thy.1.1+) B6 CD8+ T cells into B6 CD45.1+Thy1.2+ recipients of Balb/c lungs at the time of pulmonary transplantation and evaluated the adoptively transferred cells seven days later. We found a significantly higher abundance of PD-1 deficient cells and a trend towards higher expression of Ki67 at this time point.
Discussion
Previous studies have examined the role of the PD-1 pathway in the survival of allografts. Long term survival of allogeneic murine hearts could not be induced by anti-CD154 antibodies and donor splenocyte transfusion when the PD-1/PD-L1 pathway was inhibited (17). Here, PD-1 expression on CD4+ T cells was found to blunt their proliferative responses, inhibit their production of proinflammatory cytokines and promote their induction of anergy (17). Similarly, blockade of PD-1/PD-L1 abrogated cardiac allograft tolerance induced by treatment with CTLA4-Ig (10). Graft rejection was associated with an expansion of effector memory CD8+ T cells in the recipients’ spleens and – unlike our observations in lung allografts- a decrease in the abundance of CD4+ Foxp3+ regulatory T cells in heart allografts. Blockade of PD-1/PD-L1 triggered rejection of long term surviving skin grafts in recipients that had been treated perioperatively with anti-CD28 and anti-CD40L (18). Mechanistically, residual alloreactive T cells, which express PD-1, exhibited enhanced proliferation and acquired effector function such as production of inflammatory cytokines. Spontaneous acceptance of mouse liver allografts can be abrogated by inhibiting PD-L1 (19). Rejected livers were infiltrated with CD8+ cytotoxic T lymphocytes and expression levels of genes associated with these cells such perforin and granzyme B were increased in the grafts. Our studies extend these previous observations and add new mechanistic insight into the role of the PD-1 pathway in lung allograft survival.
We have previously shown that central memory CD8+ T cells mediate pulmonary graft acceptance in recipients that are treated with peri-operative costimulatory blockade, while effector memory CD8+ T cells trigger rejection, which may in part be due to higher expression levels of inflammatory and cytotoxic molecules (2). A requirement for CD8+ T cells for tolerance induction after pulmonary transplantation may depend on the immunosuppressive regimen. For example, long-term acceptance of lung allografts can be achieved in miniature swine through preoperative nonmyeloablative irradiation, perioperative donor splenocyte infusion and short-term postoperative treatment with steroids and tacrolimus (20). CD8+ T cell depletion may facilitate the establishment of mixed hematopoietic chimerism and graft acceptance in this model. Our results suggest that PD-1 expression maintains a central memory phenotype in CD8+ T cells that infiltrate lung allografts where tolerance is induced through peri-operative blockade of the B7-CD28 and CD40-CD40L pathways. When they lack PD-1 expression CD8+ T cells form prolonged interactions with donor antigen-bearing CD11c+ cells within pulmonary grafts and preferentially differentiate towards effector memory cells. It is well established that central memory can develop into effector memory CD8+ T cells (21) (22) (23). Our observation is consistent with the notion that expression of costimulatory and co-inhibitory molecules can regulate the differentiation of T cells by modulating the signal strength of the T cell receptor (24). In particular, our results are in agreement with a previous study that defined PD-1 as a checkpoint in the differentiation of CD8+ T cells from a central memory to an effector memory phenotype (25). Several studies have identified various CD8+ T cell subsets that have immunoregulatory functions (26). Of note, CD8+CD122+PD-1+ cells have been described that suppress alloimmune responses through production of IL-10 (27). These CD8+ T cells differ from the central memory CD8+ T lymphocytes that mediate the induction of lung transplantation tolerance through production of IFN-γ (2).
Engagement of the T cell receptor by its cognate ligand on the surface of antigen presenting cells results in motility decreases of T lymphocytes and the formation of durable synapses between T lymphocytes and antigen presenting cells. Previous studies have shown that T cells fail to undergo efficient activation if their interactions with antigen presenting cells are terminated prematurely (28). The duration of interactions between T cells and antigen presenting cells can be regulated by expression of molecules on their respective surfaces. For example, expression of ICAM-1 by mature dendritic cells mediates long-lasting dendritic cell - T lymphocyte interactions and regulates survival and memory formation of CD8+ T cells (29). Our previous work has shown that synapses between CD8+ T cells and CD11c+ antigen presenting cells are short lived in lung allografts when CD8+ T lymphocytes do not express CCR7 (2). CCR7 deficiency in recipient CD8+ T cells results in graft rejection, presumably secondary to their insufficient activation and low level of production of cytokines that mediate tolerance. Conversely, we now demonstrate that interactions between CD11+ antigen presenting cells and CD8+ T cells are significantly prolonged when CD8+ T cells are deficient in PD-1, which results in their differentiation towards an effector memory phenotype and an inability to induce tolerance. Previous kinetic studies have shown a dynamic course of PD-1 expression on T cells with the highest expression levels and a higher proportion of T cells expressing PD-1 early after activation followed by a subsequent decline (30). Two previous studies have examined how PD-1 regulates T cell motility and the formation of immune synapses. Similar to our findings, Fife observed in a model of autoimmunity that PD-L1 blockade was associated with prolonged interactions between CD4+ T lymphocytes and dendritic cells in antigen-bearing lymph nodes resulting in the activation of T cells and their production of proinflammatory cytokines (15). In apparent contrast to our results, Zinselmeyer demonstrated that the PD-L1-PD-1 pathway mediates a motility arrest of CD4+ and CD8+ T cells in spleens of mice during chronic viral infections (14). We speculate that the nature of the inflammatory stimuli may in part account for differences in the observations in these studies. Collectively, our findings extend the notion that - in contrast to other transplanted organs and tissues, where T cells encounter antigen presenting cells in draining lymph nodes - interactions between immune cells within lung allografts can shape their fate (4) (5). Thus, local targeting of immune pathways within lungs may be a promising avenue to ameliorate rejection.
Supplementary Material
Figure S1: Phenotype of CD8+ T cells in draining lymph nodes and spleen of lung allograft recipients. Graphic representation of relative abundance of naïve (CD62LhiCD44low), central memory (CM) (CD62LhiCD44hi) and effector memory (EM) (CD62LlowCD44hi) CD8+ T cells in draining mediastinal lymph nodes and spleen in control and anti-PD-1-antibody-treated immunosuppressed B6 recipients seven days after transplantation of BALB/c lung allografts. Data represent the mean ± SEM. Statistical analysis by Mann-Whitney U test.
Figure S2: (A) Representative histology, (B) numerical ISHLT rejection grades, representative contour plot and graphic representation of relative abundance of naïve (CD62LhiCD44low), central memory (CM) (CD62LhiCD44hi) and effector memory (EM) (CD62LlowCD44hi) (C) CD8+ and (D) CD4+ T cells in BALB/c lung allografts seven days after transplantation into PD-1-deficient immunosuppressed B6 recipients.
Video S1: Time-lapse two-photon intravital imaging of costimulatory blockade-treated BALB/c → B6 CD11c-EYFP lung allograft four days after transplantation. Compared to wildtype CD8+ T cells (red), PD-1-deficient CD8+ T cells (blue) make more durable contacts with CD11c+ antigen presenting cells (green).
Acknowledgments
Daniel Kreisel is supported by National Institutes of Health grants 1P01AI116501, R01 HL113931, R01 HL094601 and Veterans Administration Merit Review grant 1I01BX002730.
Footnotes
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Gelman AE, Li W, Richardson SB, Zinselmeyer BH, Lai J, Okazaki M, Kornfeld CG, Kreisel FH, Sugimoto S, Tietjens JR, et al. Cutting edge: Acute lung allograft rejection is independent of secondary lymphoid organs. J Immunol. 2009;182(7):3969–73. doi: 10.4049/jimmunol.0803514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krupnick AS, Lin X, Li W, Higashikubo R, Zinselmeyer BH, Hartzler H, Toth K, Ritter JH, Berezin MY, Wang ST, et al. Central memory CD8+ T lymphocytes mediate lung allograft acceptance. J Clin Invest. 2014;124(3):1130–43. doi: 10.1172/JCI71359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li W, Bribriesco AC, Nava RG, Brescia AA, Ibricevic A, Spahn JH, Brody SL, Ritter JH, Gelman AE, Krupnick AS, et al. Lung transplant acceptance is facilitated by early events in the graft and is associated with lymphoid neogenesis. Mucosal Immunol. 2012;5(5):544–54. doi: 10.1038/mi.2012.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lakkis FG, Arakelov A, Konieczny BT, Inoue Y. Immunologic ‘ignorance’ of vascularized organ transplants in the absence of secondary lymphoid tissue. Nat Med. 2000;6(6):686–8. doi: 10.1038/76267. [DOI] [PubMed] [Google Scholar]
- 5.Zhang N, Schroppel B, Lal G, Jakubzick C, Mao X, Chen D, Yin N, Jessberger R, Ochando JC, Ding Y, et al. Regulatory T cells sequentially migrate from inflamed tissues to draining lymph nodes to suppress the alloimmune response. Immunity. 2009;30(3):458–69. doi: 10.1016/j.immuni.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shilling RA, Wilkes DS. Immunobiology of chronic lung allograft dysfunction: new insights from the bench and beyond. Am J Transplant. 2009;9(8):1714–8. doi: 10.1111/j.1600-6143.2009.02690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okazaki M, Krupnick AS, Kornfeld CG, Lai JM, Ritter JH, Richardson SB, Huang HJ, Das NA, Patterson GA, Gelman AE, et al. A mouse model of orthotopic vascularized aerated lung transplantation. Am J Transplant. 2007;7(6):1672–9. doi: 10.1111/j.1600-6143.2007.01819.x. [DOI] [PubMed] [Google Scholar]
- 8.Yousem SA, Berry GJ, Cagle PT, Chamberlain D, Husain AN, Hruban RH, Marchevsky A, Ohori NP, Ritter J, Stewart S, et al. Revision of the 1990 working formulation for the classification of pulmonary allograft rejection: Lung Rejection Study Group. J Heart Lung Transplant. 1996;15(1 Pt 1):1–15. [PubMed] [Google Scholar]
- 9.Spahn JH, Li W, Bribriesco AC, Liu J, Shen H, Ibricevic A, Pan JH, Zinselmeyer BH, Brody SL, Goldstein DR, et al. DAP12 expression in lung macrophages mediates ischemia/reperfusion injury by promoting neutrophil extravasation. J Immunol. 2015;194(8):4039–48. doi: 10.4049/jimmunol.1401415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka K, Albin MJ, Yuan X, Yamaura K, Habicht A, Murayama T, Grimm M, Waaga AM, Ueno T, Padera RF, et al. PDL1 is required for peripheral transplantation tolerance and protection from chronic allograft rejection. J Immunol. 2007;179(8):5204–10. doi: 10.4049/jimmunol.179.8.5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baas M, Besancon A, Goncalves T, Valette F, Yagita H, Sawitzki B, Volk HD, Waeckel-Enee E, Rocha B, Chatenoud L, et al. TGFbeta-dependent expression of PD-1 and PD-L1 controls CD8(+) T cell anergy in transplant tolerance. Elife. 2016;5:e08133. doi: 10.7554/eLife.08133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gelman AE, Okazaki M, Lai J, Kornfeld CG, Kreisel FH, Richardson SB, Sugimoto S, Tietjens JR, Patterson GA, Krupnick AS, et al. CD4+ T lymphocytes are not necessary for the acute rejection of vascularized mouse lung transplants. J Immunol. 2008;180(7):4754–62. doi: 10.4049/jimmunol.180.7.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang X, Venet F, Wang YL, Lepape A, Yuan Z, Chen Y, Swan R, Kherouf H, Monneret G, Chung CS, et al. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc Natl Acad Sci U S A. 2009;106(15):6303–8. doi: 10.1073/pnas.0809422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zinselmeyer BH, Heydari S, Sacristan C, Nayak D, Cammer M, Herz J, Cheng X, Davis SJ, Dustin ML, McGavern DB. PD-1 promotes immune exhaustion by inducing antiviral T cell motility paralysis. J Exp Med. 2013;210(4):757–74. doi: 10.1084/jem.20121416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fife BT, Pauken KE, Eagar TN, Obu T, Wu J, Tang Q, Azuma M, Krummel MF, Bluestone JA. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol. 2009;10(11):1185–92. doi: 10.1038/ni.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gelman AE, Okazaki M, Sugimoto S, Li W, Kornfeld CG, Lai J, Richardson SB, Kreisel FH, Huang HJ, Tietjens JR, et al. CCR2 regulates monocyte recruitment as well as CD4 T1 allorecognition after lung transplantation. Am J Transplant. 2010;10(5):1189–99. doi: 10.1111/j.1600-6143.2010.03101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L, Han R, Hancock WW. Programmed cell death 1 (PD-1) and its ligand PD-L1 are required for allograft tolerance. Eur J Immunol. 2007;37(10):2983–90. doi: 10.1002/eji.200737583. [DOI] [PubMed] [Google Scholar]
- 18.Koehn BH, Ford ML, Ferrer IR, Borom K, Gangappa S, Kirk AD, Larsen CP. PD-1-dependent mechanisms maintain peripheral tolerance of donor-reactive CD8+ T cells to transplanted tissue. J Immunol. 2008;181(8):5313–22. doi: 10.4049/jimmunol.181.8.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morita M, Fujino M, Jiang G, Kitazawa Y, Xie L, Azuma M, Yagita H, Nagao S, Sugioka A, Kurosawa Y, et al. PD-1/B7-H1 interaction contribute to the spontaneous acceptance of mouse liver allograft. Am J Transplant. 2010;10(1):40–6. doi: 10.1111/j.1600-6143.2009.02859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Avsar M, Jansson K, Sommer W, Kruse B, Thissen S, Dreckmann K, Knoefel AK, Salman J, Hafer C, Hecker J, et al. Augmentation of Transient Donor Cell Chimerism and Alloantigen-Specific Regulation of Lung Transplants in Miniature Swine. Am J Transplant. 2016;16(5):1371–82. doi: 10.1111/ajt.13629. [DOI] [PubMed] [Google Scholar]
- 21.Marzo AL, Yagita H, Lefrancois L. Cutting edge: migration to nonlymphoid tissues results in functional conversion of central to effector memory CD8 T cells. J Immunol. 2007;179(1):36–40. doi: 10.4049/jimmunol.179.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huster KM, Koffler M, Stemberger C, Schiemann M, Wagner H, Busch DH. Unidirectional development of CD8+ central memory T cells into protective Listeria-specific effector memory T cells. Eur J Immunol. 2006;36(6):1453–64. doi: 10.1002/eji.200635874. [DOI] [PubMed] [Google Scholar]
- 23.Bouneaud C, Garcia Z, Kourilsky P, Pannetier C. Lineage relationships, homeostasis, and recall capacities of central- and effector-memory CD8 T cells in vivo. J Exp Med. 2005;201(4):579–90. doi: 10.1084/jem.20040876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mousavi SF, Soroosh P, Takahashi T, Yoshikai Y, Shen H, Lefrancois L, Borst J, Sugamura K, Ishii N. OX40 costimulatory signals potentiate the memory commitment of effector CD8+ T cells. J Immunol. 2008;181(9):5990–6001. doi: 10.4049/jimmunol.181.9.5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charlton JJ, Chatzidakis I, Tsoukatou D, Boumpas DT, Garinis GA, Mamalaki C. Programmed death-1 shapes memory phenotype CD8 T cell subsets in a cell-intrinsic manner. J Immunol. 2013;190(12):6104–14. doi: 10.4049/jimmunol.1201617. [DOI] [PubMed] [Google Scholar]
- 26.Wan N, Dai H, Wang T, Moore Y, Zheng XX, Dai Z. Bystander central memory but not effector memory CD8+ T cells suppress allograft rejection. J Immunol. 2008;180(1):113–21. doi: 10.4049/jimmunol.180.1.113. [DOI] [PubMed] [Google Scholar]
- 27.Dai H, Wan N, Zhang S, Moore Y, Wan F, Dai Z. Cutting edge: programmed death-1 defines CD8+CD122+ T cells as regulatory versus memory T cells. J Immunol. 2010;185(2):803–7. doi: 10.4049/jimmunol.1000661. [DOI] [PubMed] [Google Scholar]
- 28.Benvenuti F, Lagaudriere-Gesbert C, Grandjean I, Jancic C, Hivroz C, Trautmann A, Lantz O, Amigorena S. Dendritic cell maturation controls adhesion, synapse formation, and the duration of the interactions with naive T lymphocytes. J Immunol. 2004;172(1):292–301. doi: 10.4049/jimmunol.172.1.292. [DOI] [PubMed] [Google Scholar]
- 29.Scholer A, Hugues S, Boissonnas A, Fetler L, Amigorena S. Intercellular adhesion molecule-1-dependent stable interactions between T cells and dendritic cells determine CD8+ T cell memory. Immunity. 2008;28(2):258–70. doi: 10.1016/j.immuni.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 30.Xing K, Gu B, Zhang P, Wu X. Dexamethasone enhances programmed cell death 1 (PD-1) expression during T cell activation: an insight into the optimum application of glucocorticoids in anti-cancer therapy. BMC Immunol. 2015;16:39. doi: 10.1186/s12865-015-0103-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Phenotype of CD8+ T cells in draining lymph nodes and spleen of lung allograft recipients. Graphic representation of relative abundance of naïve (CD62LhiCD44low), central memory (CM) (CD62LhiCD44hi) and effector memory (EM) (CD62LlowCD44hi) CD8+ T cells in draining mediastinal lymph nodes and spleen in control and anti-PD-1-antibody-treated immunosuppressed B6 recipients seven days after transplantation of BALB/c lung allografts. Data represent the mean ± SEM. Statistical analysis by Mann-Whitney U test.
Figure S2: (A) Representative histology, (B) numerical ISHLT rejection grades, representative contour plot and graphic representation of relative abundance of naïve (CD62LhiCD44low), central memory (CM) (CD62LhiCD44hi) and effector memory (EM) (CD62LlowCD44hi) (C) CD8+ and (D) CD4+ T cells in BALB/c lung allografts seven days after transplantation into PD-1-deficient immunosuppressed B6 recipients.
Video S1: Time-lapse two-photon intravital imaging of costimulatory blockade-treated BALB/c → B6 CD11c-EYFP lung allograft four days after transplantation. Compared to wildtype CD8+ T cells (red), PD-1-deficient CD8+ T cells (blue) make more durable contacts with CD11c+ antigen presenting cells (green).


