Abstract
Classical microtubule associated proteins (MAPs) were originally identified based on their co-purification with microtubules assembled from mammalian brain lysate. They have since been found to perform a range of functions involved in regulating the dynamics of the microtubule cytoskeleton. Most of these MAPs play integral roles in microtubule organization during neuronal development, microtubule remodeling during neuronal activity, and microtubule stabilization during neuronal maintenance. As a result, mutations in MAPs contribute to neurodevelopmental disorders, psychiatric conditions, and neurodegenerative diseases. MAPs are post-translationally regulated by phosphorylation depending on developmental time point and cellular context. Phosphorylation can affect the microtubule affinity, cellular localization, or overall function of a particular MAP and can thus have profound implications for neuronal health. Here we review MAP1, MAP2, MAP4, MAP6, MAP7, MAP9, Tau and DCX, and how each is regulated by phosphorylation in neuronal physiology and disease.
Keywords: Kinases, cytoskeleton, neuronal development, neurodegeneration
INTRODUCTION
Neuronal development and function is governed by the underlying architecture of the cytoskeleton, which includes the microtubule, actin and intermediate filament networks. The microtubule cytoskeletal network is organized into stable and dynamic arrays that provide structural support, serve as tracks for molecular motor transport, and function as signaling platforms during neuronal development and plasticity (Keating and Borisy, 1999; Bartolini and Gundersen, 2006; Witte and Bradke, 2008; Hoogenraad and Bradke, 2009; Stiess et al., 2010; Dent and Baas, 2014). It is therefore essential to understand the regulatory mechanisms of microtubule cytoskeleton organization as a neuron develops, changes, or maintains its internal structure, because altering these processes can disrupt neuronal function and ultimately lead to pathological conditions (Kaufmann and Moser, 2000; Jan and Jan, 2010). For example, mutations in proteins affecting the assembly of microtubules can lead to neurodevelopmental disorders such as Lissencephaly (Pilz et al., 1998; Viot et al., 2004; Tsai et al., 2016), mutations in proteins involved in the remodeling of the microtubule cytoskeleton are correlated with neuropsychiatric disorders such as schizophrenia (Arnold et al., 1991; Cotter et al., 2000; Somenarain and Jones, 2010), and mutations in proteins important for microtubule stability or microtubule-based transport can cause neurodegenerative pathologies, such as motor neuron disease and Alzheimer’s disease (Tolnay and Probst, 1999; Lewczuk et al., 2004; Harms et al., 2012). Therefore, the precise regulation of the microtubule cytoskeleton is critical for neuronal health at all stages of life.
Microtubules are composed of alpha- and beta-tubulin heterodimers that assemble into protofilaments, which then form lateral contacts with one another to form a tubule (Borisy et al., 1974; Kirschner et al., 1974; Olmsted and Borisy, 1975; Downing and Nogales, 1998). Both alpha and beta-tubulin bind, but only beta-tubulin hydrolyzes, GTP. Beta-tubulin must be in the GTP-bound state in order for the heterodimer to assemble onto a protofilament. Once assembled, beta-tubulin is exposed at the “plus end” and alpha-tubulin is exposed at the “minus end”. This structural polarity results in growth rate differences of each end, and it has been observed that the plus end grows much more rapidly than the minus end both in vivo and in vitro (Howard and Hyman, 1993; Howard and Hyman, 2003; Bieling et al., 2007; Bieling et al., 2010). Microtubules can be remodeled within the cell by switching between states of assembly and disassembly in a process known as dynamic instability (Mitchison and Kirschner, 1984). There are numerous microtubule associated proteins (MAPs) that can bind the microtubule lattice, tubulin heterodimers, or both, and regulate these dynamics to properly organize and remodel the microtubule cytoskeleton during neuronal development and activity (Papasozomenos et al., 1985; Caceres and Kosik, 1990; Bulinski et al., 1997; Gleeson et al., 1999a; Gordon-Weeks and Fischer, 2000; Volle et al., 2013; Tymanskyj et al., 2017).
Different types of MAPs perform various functions. Proteins that associate with the growing plus end of the microtubule, or plus-end tracking proteins (+TIP s), regulate microtubule growth and catastrophe (reviewed in (Akhmanova and Steinmetz, 2010)). There are also proteins that associate with the minus ends of the microtubule and protect them from depolymerization (Goodwin and Vale, 2010; Jiang et al., 2014). Many MAPs are molecular motors that utilize the energy of nucleotide hydrolysis to produce force along microtubule, including the long-distance transport motors, cytoplasmic dynein and kinesin-1, the microtubule sliding motors, kinesin-5 and −12, and microtubule depolymerizing or severing proteins, such as mitotic centrosome-associated kinesin (MCAK), spastin, and katanin (Andersen and Wittmann, 2002; Vale, 2003; Walczak, 2003; Myers and Baas, 2007; Roll-Mecak and McNally, 2010; Vallee et al., 2012). There are also tubulin-modifying enzymes that exert control over many aspects of microtubule biology through post-translational modification of alpha and beta-tubulin (reviewed in (Verhey and Gaertig, 2007; Janke and Bulinski, 2011; Yu et al., 2015)). Finally, there are structural or classical MAPs that bind along the microtubule lattice and have various effects on microtubule polymerization, stability, and bundling (Figure 1 and Figure 2) (Vallee and Borisy, 1978). Many of these MAPs are abundantly expressed in the nervous system and some may have overlapping roles within neurons in order to maintain the microtubule cytoskeletal architecture (Baas et al., 2016).
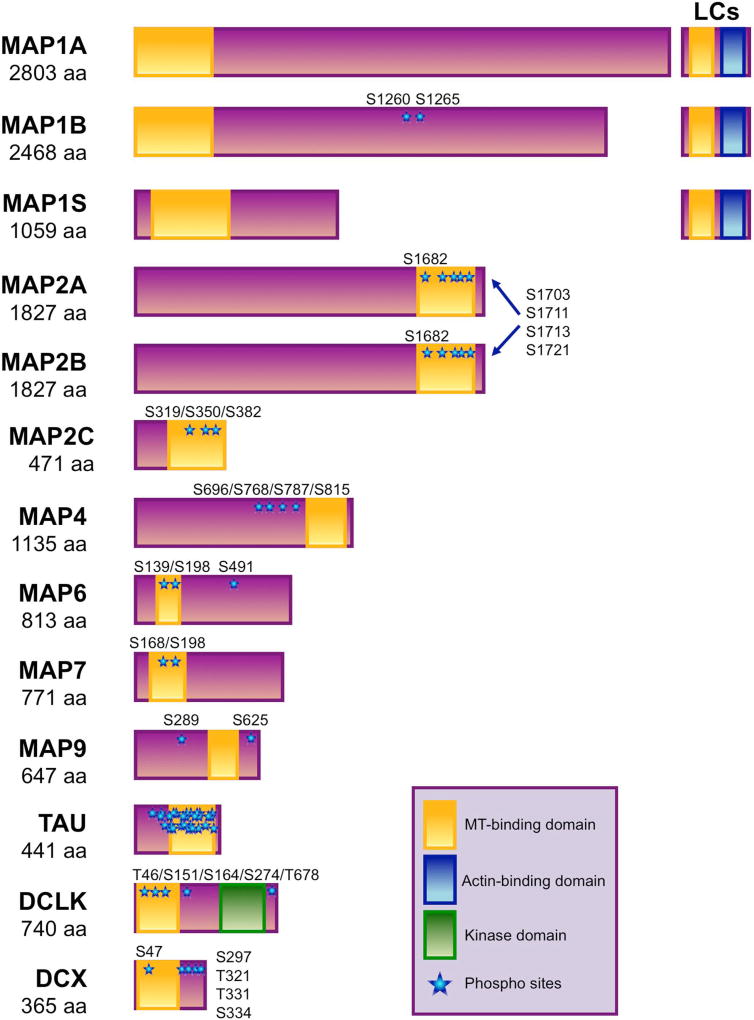
Figure 1. Domain organization of microtubule-associated proteins (MAPs).
The microtubule-binding domains (yellow), actin-binding domains (blue), kinase domains (green), and phosphorylation sites (blue stars) are illustrated for each MAP. Note that the phosphorylation sites of DCLK1 are proposed autophosphorylation sites. The following accession numbers for the protein sequences were used for this schematic: NP_002364.5 for MAP1A, NP_005900.2 for MAP1B, NP_060644.4 for MAP1S, NP_002365.3 for MAP2A/B, NP_114033.2 for MAP2C, NP_001127836.1 for MAP4, NP_149052.1 for MAP6, NP_001185537.1 for MAP7, NP_001034669.1 for MAP9, NP_005901.2 for tau, NP_001317001.1 for DCLK, and NP_835365.1 for DCX.
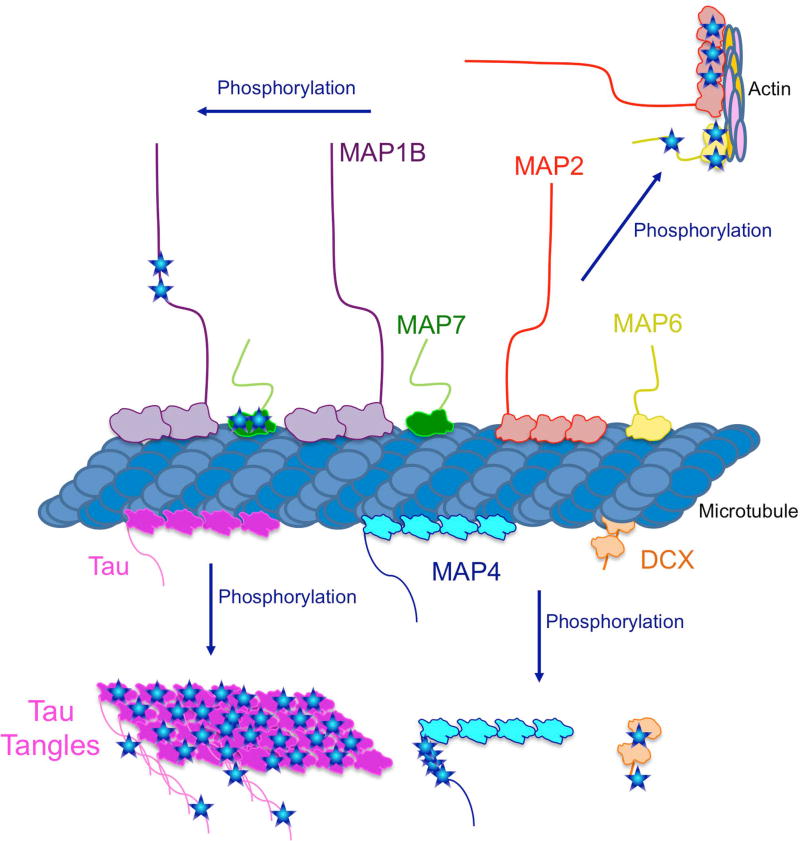
Figure 2. Model for the effect of phosphorylation on each MAP.
Based on the literature reviewed here, there are different classes of phosphorylation effects on MAP activity. Phosphorylation of MAP1B and MAP7 do not seem to affect their ability to bind the microtubule, while phosphorylation of DCX, MAP4, Tau, MAP2, and MAP6 all release from the microtubule upon phosphorylation. MAP2 and MAP6 re-localize to actin-rich regions with the cell, but how these MAPs bind actin is unknown.
Microtubule organization within cells can be governed by the association and dissociation of different MAPs that can have opposing or synergistic activities (Heins et al., 1991; Vandecandelaere et al., 1996; McNally et al., 2002; Szebenyi et al., 2005). It is well documented that the interaction of MAPs with the microtubule cytoskeleton is affected by posttranslational modifications (Drewes et al., 1997). In particular, the phosphorylation state of MAPs can have a pronounced effect on microtubule dynamics, and subtle changes in MAP phosphorylation patterns accompany major rearrangements of the microtubule network during neuronal outgrowth, differentiation, and plasticity (Table 1 and Figure 2) (Ikegami et al., 2000; Biernat et al., 2002; Szebenyi et al., 2005). This review focuses on the known kinase regulators of classical MAPs and the downstream effect these modifications have on the microtubule cytoskeleton during neuronal development and maintenance. We also review how phosphorylation of tubulin heterodimers can directly affect microtubule dynamics.
Table 1.
Effects of phosphorylation on each MAP.
| MAP | Phospho-Site(s) | Kinase(s) | Phospho- Effects |
References |
|---|---|---|---|---|
| MAP1A | Unknown | DYRKIa | Release from clathrin coated vesicles | Szebenyi et al., 2005; Murakami et al., 2012 |
| MAP1B | S1260, T1265 | GSKIIIB,JNK1, CKII, cdc2, MAPK, DYRKIa | Maintains dynamic MT pool and localizes to growing axons | Trivedi et al., 2005; Chang et al., 2003; Scales et al., 2009 |
| MAP1S | Unknown | Unknown | Unknown | N.A. |
| MAP2 | MAP2C:S319,S350, S382 MAP2A/B: S1682, S1703, S1711, S1713, S1721 | PKA,PKC,MARK, cdc2,CaMKII,GSK IIIB, JNK1, ERK, CDKs | Dissociation from MTs and association with actin | Ozer&Halpain, 2000; Rubino et al., 1999; Ebneth et al., 1999; Drewes et al., 1997; Satillaro, 1986 |
| MAP4 | S696,S768,S787,S815 | PKC, MAPK, MARK, cyclin-B-cdc2 | Dissociation from MTs | Ookata et al., 1997; Mori et al., 1991; Hoshi et al., 1992; Illenberger et al. 1996 |
| MAP6 | S139,S198,S491 | CaMKII | Dissociation from MTs and localization to actin-rich synapses | Baratier et al., 2006 Lefevre et al., 2013 Bosc et al., 2001 Bosc et al., 2003 |
| MAP7 | S168,S198 | MARK | Phosphorylation affects localization and/or MT binding | Masson&Kreis, 1993; Faire et al., 1999; Sung et al., 2008 |
| MAP9 | S625 S289 | Aurora A Plk1 | Spindle localization and function | Venoux et al., 2008; Eot-Houllier et al., 2010 |
| TAU | ≥40 residues including: T69,T181,S184,S198, S199,S202,T212,S214,T231 | MARK, MAPK, JNK1, CDK5, GSKIIIβ, ERK DYRKIa, CKI, PKA | Dissociation from MTs or EB1 | Hanger et al., 2009; Wang et al., 2013; Duka et al., 2013 |
| DCX | S47,S297,T321,T331, S334 | CDK5, PKA, MARK, JNK1 | Dissociates from MTs; altered localization | Tanaka et al., 2006; Schaar et al., 2004; Gdalyahu et al., 2004 |
MAP1 FAMILY (MAP1A, MAP1B, MAP1S)
The MAP1 family members were originally identified as high molecular weight protein complexes, consisting at least one heavy chain and one light chain, that co-purified with microtubules prepared from mammalian brain lysate, and were predominantly enriched in brain white matter (Kuznetsov et al., 1981; Vallee, 1982; Bonifacino et al., 1985). All MAP1 subtypes are expressed in the nervous system, where they function in neuronal development and overall maintenance (Hanley et al., 1999; Meixner et al., 2000). Each of the MAP1 complexes are composed of a heavy chain and at least one light chain, which can modulate their interactions with microtubules and actin, among other intracellular partners (Noiges et al., 2002).
MAPlA is expressed abundantly throughout development and into adulthood, where it is enriched specifically in dendrites (Fink et al., 1996). MAP1B is highly expressed during neuronal development where it is localized to axons, then expression decreases after neuronal differentiation, but increases again in adulthood where MAP1B has both dendritic and axonal functions (Kutschera et al., 1998; Gonzalez-Billault et al., 2001; Bodaleo et al., 2016). The shortest and least studied MAP1 member, MAP1S, is expressed in a variety of tissues including the brain, liver, spleen, heart and other organs (Orban-Nemeth et al., 2005), and recent studies indicate that it is involved in cell division, autophagy and phagocytosis (Eriksson et al., 2007; Xie et al., 2011a; Xie et al., 2011b; Liu et al., 2012c; Tegha-Dunghu et al., 2014).
All three MAP1 proteins, MAP1A, MAP1B and MAP1S, associate with both microtubules and actin (Noiges et al., 2002; Tegha-Dunghu et al., 2014). The heavy chains of MAP1A and MAP1B have either one or two microtubule binding domains, respectively. The light chains for MAP1A, MAP1B and MAP1S contain non-overlapping microtubule and actin binding domains, and it is speculated that MAP1 proteins can simultaneously bind and crosslink microtubules and actin (Noiges et al., 2002; Halpain and Dehmelt, 2006). All MAP1 proteins bind the microtubule lattice and are thought to stabilize microtubules (Vandecandelaere et al., 1996; Bondallaz et al., 2006; Tegha-Dunghu et al., 2014; Liu et al., 2015).
The role of MAP1A in dendrites during neuronal development and maintenance has been studied extensively. Not only is MAP1A important for dendritic outgrowth and morphology, but it is also essential in remodeling the dendritic arbor in response to neuronal activity (Szebenyi et al., 2005; Liu et al., 2015). MAP1A localizes to excitatory synapses where it transiently interacts with post-synaptic density proteins, PSD-93 and PSD95, and is important for the localization of NMDA receptors (Brenman et al., 1998; Ikeda et al., 2002; Takei et al., 2015). As a result of its role at synapses, MAP1A knockout mice exhibit learning and memory defects with impaired long-term potentiation and long-term depression (Takei et al., 2015). A mouse model with MAP1A mutations develops ataxia, tremors, and late-onset degeneration of Purkinje neurons (Liu et al., 2015). MAP1A has a number of other interacting partners that it may tether to the microtubule or actin cytoskeletons, including calcium and potassium channels, indicating that loss of MAP1A could cause a number of neurological abnormalities depending on neuronal cell type. For a comprehensive review of MAP1A, MAP1B and MAP1S function and interacting partners, please refer to Halpain and Delnert (2006).
As expected based on its expression and localization patterns, knockdown of MAP1B inhibits both axon outgrowth and dendritic spine formation (Riederer, 2007; Tortosa et al., 2011). Intriguingly, deletion of the MAP1B gene region results in the absence of corpus callosum, along with other defects in neuronal migration and neuronal activity (Meixner et al., 2000). In regenerating adult mouse dorsal root ganglion neurons, knockdown of MAP1B results in enhanced axonal branching, but impaired axonal turning behavior (Bodaleo et al., 2016). MAP1B therefore has distinct roles in microtubule-based processes, such as neuronal migration and growth cone turning, and actin-based processes, such as dendritic spine maturation. Considering MAP1B interacts either directly or indirectly with numerous neurotransmitter receptors, including GABAc, NMDA, AMPA, and mGluR, receptor regulatory proteins, and various channels, it is unsurprising that it has been implicated in a variety of neurological diseases including Parkinson’s disease (Chan et al., 2014; Shah and Lahiri, 2017), Alzheimer’s Disease (Ulloa et al., 1994; Good et al., 2004; Gevorkian et al., 2008), and schizophrenia (Davidkova and Carroll, 2007; Lebeau et al., 2011; Tortosa et al., 2011; Benoist et al., 2013). Whether MAP1B acts an anchor for these proteins to either the actin or the microtubule cytoskeletons, or whether it is involved in the proper transport and localization of these proteins remains to be more fully elucidated.
MAP1S appears to have roles during mitosis and autophagy. MAP1S decorates the mitotic spindle and knockdown of MAP1S results in the formation of an unstable metaphase plate, leading to improper chromatid separation and mitotic defects (Dallol et al., 2007; Liu et al., 2012c; Tegha-Dunghu et al., 2014). As a result, although MAP1S knockout mice do not manifest any developmental or behavioral phenotypes, they are at a higher risk to develop cancer (Xie et al., 2011b). Furthermore, MAP1S plays a role in suppressing tumorigenesis in mouse cancer models and in pancreatic cancer patients (Liu et al., 2012c). It has been shown that MAP1S also positively regulates autophagy from biogenesis to degradation via its interaction with LC3 (Liu et al., 2012c), and may facilitate the interaction between autophagosomes and damaged mitochondria as well (Xie et al., 2011a).
MAP1A and MAP1B are regulated by phosphorylation, but there is little known about the post-translational regulation of MAP1S (Halpain and Dehmelt, 2006). It has been shown that MAP1A can be activated through the mitogen-activated protein kinase (MAPK) pathway during dendritic remodeling, but whether MAP1A is phosphorylated during this process is unknown (Szebenyi et al., 2005). Dual-specificity tyrosine phosphorylated-regulated kinase-1a (DYRK1a) phosphorylates MAP1A and MAP2 and causes their release from clathrin-coated vesicles, but the effect on microtubule binding has not been assessed (Murakami et al., 2012). In addition, it is unknown why MAP1A and MAP2 are associated with clathrin-coated vesicles. There are three potential DYRK1a phosphorylation sites in MAP1A (Thr2059, Ser2221, and Ser2546) based on the consensus sequence for DYRK1a, but none have been confirmed as direct targets of DYRK1a.
MAP1B is a target for phosphorylation by a number of different kinases (Tymanskyj et al., 2010). Phosphorylated MAP1B is highly enriched in growing axons while non-phosphorylated MAP1B is present in the somatodendritic compartment (Sato-Yoshitake et al., 1989; Riederer et al., 1993; Gordon-Weeks and Fischer, 2000; Riederer, 2007). Glycogen synthase kinase III β (GSKIIIβ) has been shown to phosphorylate MAP1B at Ser1260 and Thr1265 in growing axons, especially in the distal growth cone (Goold et al., 1999; Goold and Gordon-Weeks, 2003; Trivedi et al., 2005). DYRK1a phosphorylates MAP1B at one residue, Ser1392, which primes MAP1B for phosphorylation by GSKIIIβ at Ser1388 as well (Scales et al., 2009). These phosphorylation events enhance the ability of MAP1B to maintain a dynamic microtubule population. The role of MAP1B in facilitating dynamic microtubules is counter to the original hypothesis that MAP1B stabilizes microtubules, but indicates that MAP1B could have multiple roles in regulating the microtubule cytoskeleton depending on its phosphorylation state, and may maintain a balance between microtubule stability and catastrophe. Jun N-terminal kinase 1 (JNK1) has also been shown to phosphorylate MAP1B in the growing axons to promote microtubule dynamicity, and when JNK1 is knocked down, MAP1B is hypophosphorylated and microtubules become highly stable in the axons (Chang et al., 2003; Kawauchi et al., 2005). MAP1B can be phosphorylated in vitro by other kinases such as cdc2, MARK, and casein-kinase II, and dephosphorylated by protein phosphatase 2B (calcineurin) and protein phosphatase 2A (Ulloa et al., 1993; Ulloa et al., 1994). Phosphorylation of MAP1B is likely to be highly regulated, because it dictates the functions of MAP1B depending on neuronal context. Rather than regulating the microtubule binding affinity of MAP1B, phosphorylation appears to affect the function of MAP1B in manipulating cytoskeletal dynamics, which are remodeled extensively during neuronal outgrowth and after neuronal activity.
MAP2
Similar to the MAP1 family, MAP2 proteins were also identified as high affinity microtubule associated proteins in preparations from mammalian brain. In contrast to MAP1, MAP2 proteins were found to be enriched in grey brain matter, suggesting a specific localization to neuronal dendrites (Murphy et al., 1977; Vallee and Borisy, 1978; Bulinski and Borisy, 1980; Hernandez et al., 1986). Since its discovery, MAP2 has become a widely used marker for dendrites both in vivo and ex vivo due to its consistent expression in neurons and localization to dendrites (Cumming et al., 1984; Vouyiouklis and Brophy, 1995). There are four isoforms of MAP2: the high molecular weight proteins (~1830 kDa), MAP2A and MAP2B, and the low molecular weight proteins (~470 kDa), MAP2C and MAP2D (Hernandez et al., 1986). MAP2D is predominantly expressed during development, while MAP2C and MAP2B are expressed throughout development and into adulthood, and MAP2A is expressed in adulthood (Cumming et al., 1984; Nunez, 1988; Doll et al., 1993; Ferhat et al., 1998). In addition, MAP2A appears to have compensatory roles, and has been shown to be upregulated when MAP2C levels are low (Chung et al., 1996).
There have been numerous functions ascribed to the MAP2 family of proteins. They have been shown to stabilize microtubules by rescuing catastrophes and increasing microtubule stiffness (Gamblin et al., 1996), as well as to bundle microtubules and provide regular spacing between microtubules within dendrites (Chen et al., 1992; Dominguez et al., 1994; Cunningham et al., 1997; Itoh et al., 1997). Knockdown of MAP2 or deletion of the MAP2 microtubule-binding domain results in decreased microtubule density and impaired dendrite elongation, while overexpression of MAP2 leads to increased dendrite number and length (Harada et al., 2002; Tang et al., 2014). MAP2 can also bind actin and neurofilaments and may be able to mediate interactions between the three cytoskeletal filaments (Bloom and Vallee, 1983; Selden and Pollard, 1983; Papasozomenos et al., 1985; Pedrotti et al., 1994; Roger et al., 2004). The ability of MAP2 to crosslink microtubules with actin/neurofilaments has been shown to be important during neuronal morphogenesis and dendrite formation, during which the cytoskeletal networks are grossly reorganized (Dehmelt et al., 2011).
MAP2 also helps direct microtubule motor transport within dendrites. Not only is MAP2 important for proper localization of certain proteins and channels to the somatodendritic compartment (Theurkauf and Vallee, 1982; Obar et al., 1989; Rubino et al., 1989; Davare et al., 1999), but it is also known to be involved in the transport of endoplasmic reticulum membranes along microtubules (Heins et al., 1991; Farah et al., 2005). MAP2 may indirectly affect the transport of molecular motors such as kinesins and dynein by competing for the same binding sites on the microtubule lattice, or directly affect these motors by sterically hindering their progress with its projection domain (Heins et al., 1991; Lopez and Sheetz, 1993; Hagiwara et al., 1994). Interestingly, the levels of MAP2 are decreased in postmortem brains from patients with schizophrenia (Arnold et al., 1991; Cotter et al., 2000; Broadbelt et al., 2002; Rioux et al., 2003; Somenarain and Jones, 2010; Shelton et al., 2015), highlighting the importance of MAP2 function in dendrites, whether that is maintaining microtubule stability or directing motor transport of essential cargo. A recent study suggests that the two isoforms: MAP2C and MAP2B have very different roles in directing kinesin-based transport (Gumy et al., 2017). Gumy et al., (2017) shows that unlike MAP2C, MAP2B is not bound to the microtubule lattice, but is free in the cytoplasm where it binds and sequesters kinesin-1. This observed function allows for kinesin-3 motors to transport important cargo into the axons of neurons. These data provide a new mechanism for how MAP2 contributes to neuronal development and maintenance.
All isoforms of MAP2 are highly phosphorylated at different stages of development by a number of kinases, but in most cases phosphorylation causes MAP2 to dissociate from the microtubule. There are a number of KXGS motifs within the microtubule binding repeats of MAP2, which have been shown to be targets of phosphorylation by cAMP-dependent protein kinase (PKA), protein kinase C (PKC), and MARK. PKA phosphorylates MAP2C at three serine residues, Ser319, Ser350, and Ser382, which causes MAP2C to release from the microtubule and associate with actin in peripheral membrane ruffles in vivo (Rubino et al., 1989; Ozer and Halpain, 2000). Non-phosphorylated MAP2 is able to bundle actin filaments, but phosphorylated MAP2 remains competent to interact with actin, though it is unable to generate actin bundles (Sattilaro, 1986). Therefore, the role of phosphorylated MAP2 in actin organization is complex and remains unclear. Phosphorylation by both MARK at Ser1713 and Ser1682 and PKC at Ser1703, Ser1711, Ser1728 causes MAP2 to dissociate from the microtubule (Ainsztein and Purich, 1994; Illenberger et al., 1996; Drewes et al., 1997; Ebneth et al., 1999). JNK1 phosphorylation of both MAP2 and MAP1B occurs during neuronal development and is thought to regulate microtubule assembly for proper dendrite outgrowth (Chang et al., 2003). Based on the functions of MAP2 during development, phosphorylation and release from the lattice could have profound implications not only for microtubule dynamics, but also for motor transport of essential dendritic cargo (Heins et al., 1991). Many other protein kinases can phosphorylate MAP2 proteins in vitro such as Ca2+/calmodulin-dependent protein kinase (CaMKII), extracellular signal-regulated kinase-1 (ERK), GSKIIIβ, and cdc2 kinase, but the targeted residues and the functional consequences of phosphorylation have yet to be determined (Brugg and Matus, 1991; Itoh et al., 1997). The phosphorylation state of MAP2 is also determined by neuronal activity, which correlates with changes in cytoskeletal architecture within dendrites (Halpain and Greengard, 1990; Diaz-Nido et al., 1993; Quinlan and Halpain, 1996; Philpot et al., 1997). Therefore, MAP2 function is regulated by phosphorylation not only during neuronal development, but also after differentiation during synaptic activity for maintenance of neuronal health (Diaz-Nido et al., 1993; Diez-Guerra and Avila, 1993; Dhamodharan and Wadsworth, 1995; Riederer et al., 1995).
MAP4/MAP3
Microtubule-associated protein 4 (MAP4, a.k.a. MAP3) is expressed in a variety of different cell types, where it has been shown to promote microtubule assembly, inhibit the binding of other MAPs, and potentially cross-link microtubules and actin/neurofilaments (Huber et al., 1985; Matsushima et al., 2012). During neuronal development, MAP4 is lowly expressed in the nervous system, but is subsequently upregulated in both neurons and glia in the adult brain (Bernhardt et al., 1985; Huber et al., 1985; Matsushima et al., 2012). Immunofluorescence studies on primary neurons reveal that MAP4 is mainly restricted to the axons (Matsushima et al., 2012), where it is concentrated at branching points (Tokuraku et al., 2010). Both in vivo and in vitro studies have revealed that MAP4 enhances tubulin polymerization and overall microtubule stability (Nguyen et al., 1997; Nguyen et al., 1998; Xiao et al., 2012). In Xenopus melanophores, muscle cells, and in vitro motility assays, MAP4 has been shown to inhibit dynein motility, but there are conflicting results as to its effects on kinesin (Bulinski et al., 1997; Tokuraku et al., 2010; Samora et al., 2011; Semenova et al., 2014; Mogessie et al., 2015). In Xenopus melanophores, MAP4 enhances kinesin-2-based transport and inhibits dynein-based transport (Semenova et al., 2014). In muscle myoblast cells, a MAP4 isoform, termed oMAP4, aligns microtubules into antiparallel bundles that withstand motor force, and prevents dynein-and kinesin-driven microtubule sliding (Mogessie et al., 2015). This newly identified oMAP4 isoform is necessary to establish and maintain microtubule bundles during polarization and elongation of myotubes. The presence of MAP4 on the microtubule also inhibits the association of the depolymerizing kinesin, MCAK (Holmfeldt et al., 2002), and the microtubule severing enzyme, katanin (McNally et al., 2002). Therefore, the regulation of MAP4 association with the microtubule lattice should have profound consequences for microtubule stability.
Based on the above studies, MAP4 could contribute to microtubule stability in two ways: 1) by bundling microtubules and promoting resistance to external forces, and 2) by binding the microtubule and sterically blocking proteins that actively disassemble the microtubule.
MAP4 can be regulated by a number of kinases, including PKC, MAPK, MARK, and cyclin-B-cdc2 (Mori et al., 1991; Hoshi et al., 1992; Illenberger et al., 1996; Ookata et al., 1997; Ebneth et al., 1999). Phosphorylation by all four of these kinases leads to the detachment of MAP4 from microtubules and subsequent destabilization of the microtubule cytoskeleton (Ebneth et al., 1999; Mandelkow et al., 2004), but they each exert their effects on MAP4 in different cellular contexts. The MAP4 phosphorylation sites, Ser696, Ser768, Ser787, and Ser815, located within the proline-rich region of its microtubule-binding domain are proposed to be the key residues regulating microtubule affinity (Mori et al., 1991; Ookata et al., 1997; Srsen et al., 1999). p38/MAPK phosphorylation of MAP4 is induced in cardiomyocytes under hypoxic conditions (Hu et al., 2010; Hu et al., 2014). Furthermore, p38/MAPK is an essential signal required for the cytoskeletal rearrangement that precedes endothelial barrier disruption (Li et al., 2015). Therefore, it is conceivable that a potential regulatory balance between MAP4 phosphorylation and dephosphorylation controlled by p38/MAPK may exist to maintain microtubule organization under high stress conditions.
Cyclin B-cdc2 phosphorylates MAP4 at Ser696 and Ser787 in vivo and in vitro, and causes microtubules to become more dynamic by decreasing the frequency of rescue (Ookata et al., 1995; Ookata et al., 1997). Phosphorylation of MAP4 by PKC and MARK has also been shown to disrupt microtubule stability and lead to cell death (Mori et al., 1991; Illenberger et al., 1996; Drewes et al., 1997; Ebneth et al., 1999). Taken together, phosphorylation and subsequent release of MAP4 from the microtubule affects microtubule stability in two ways: 1) by removing a stabilizing protein from the lattice that would otherwise prevent catastrophe, or 2) by allowing catastrophe factors, such as MCAK and katanin, to more easily access the microtubule surface and induce disassembly. Further studies will be necessary to determine the molecular basis for MAP4 inhibition of other MAPs and the functional output on microtubule growth and stability.
MAP6/STOP
MAP6, or STOP (Stable-Tubule-Only Polypeptide), is expressed in a range of tissues in vertebrate animals, and is known to protect microtubules from depolymerization in conditions such as low temperatures or treatment with depolymerizing drugs (Bosc et al., 2003; Arama et al., 2012; Delphin et al., 2012; Gory-Faure et al., 2014). There are three major MAP6 isoforms that have been described in the mouse nervous system: MAP6-E (E-STOP), which is expressed throughout neuronal development, as well as in the adult brain, and MAP6-N (N-STOP), and MAP6d1 (SL21), both of which are expressed in the brain and other tissues postnatally (Gory-Faure et al., 2014; Deloulme et al., 2015). Mice deficient in MAP6 have lower synaptic vesicle pools, defects in synaptic plasticity, and altered neurotransmission, all of which are associated with behavioral disorders such as schizophrenia (Andrieux et al., 2002; Eastwood et al., 2007; Bouvrais-Veret et al., 2008; Fournet et al., 2012b; Volle et al., 2013; Daoust et al., 2014). In cultured neurons, MAP6 proteins are present in both axons and dendrites, where they are thought to interact with the microtubule cytoskeleton, but MAP6 proteins also show transient localization within both pre- and post-synaptic compartments, where they are thought to interact with the actin cytoskeleton (Andrieux et al., 2002; Baratier et al., 2006). There are several proteins that interact with both the actin and microtubule cytoskeletons to enable cross-talk between the two cytoskeletal networks (Bartolini and Gundersen, 2010). However, the interactions between MAP6 and microtubules or actin may be mutually exclusive and appear tightly regulated by phosphorylation (Baratier et al., 2006; Lefevre et al., 2013).
Many studies have focused on the phosphorylation of MAP6 by CaMKII (Bosc et al., 2001; Bosc et al., 2003; Baratier et al., 2006). MAP6 is phosphorylated by CaMKII in vitro on at least three of the four potential CaMKII consensus sites found within its sequence: Ser139, Ser198, and Ser491 (Baratier et al., 2006; Lefevre et al., 2013). Phosphorylation of MAP6 by CaMKII causes MAP6 to dissociate from the microtubule (Lefevre et al., 2013). In vivo, phosphorylation is normally preceded by synaptic activation and causes a redistribution of MAP6 from primary branches to synaptic compartments (Baratier et al., 2006). It has also been shown that during synaptic activation, microtubule binding by MAP6 can be inhibited by Ca2+-bound calmodulin, which binds and competes with the microtubule-binding domain of MAP6 to prevent microtubule association (Lefevre et al., 2013). These data suggest a model in which the microtubule association of MAP6 is regulated in two ways: 1) by binding Ca2+-calmodulin in response to transient Ca2+ influxes induced by synaptic activation, or 2) through phosphorylation by active CaMKII. The latter is a proposed mechanism for MAP6 regulation during short or long term synaptic potentiation, which both involve active CaMKII after Ca2+ levels return to basal levels (Baratier et al., 2006; Lefevre et al., 2013).
Phosphorylation by CaMKII could also be an important regulatory switch for MAP6 function. In vitro, phosphorylated MAP6 is unable to bind to microtubules, though it can still bind to polymerized actin (Baratier et al., 2006). In cultured neurons, phosphorylated MAP6 co-localizes with actin in pre- and post-synaptic compartments (Baratier et al., 2006). Actin is important for the localization and regulation of synaptic vesicle pools and MAP6 binding to actin may be important to maintain the vesicular pool (Hannah et al., 1999). It has been suggested that during synapse formation, there is increased actin polymerization events in both pre- and post-synaptic compartments (Fischer et al., 1998). It will be interesting to examine how MAP6 actually affects actin dynamics both in vivo and in vitro to further understand its role in these compartments during synaptic transmission.
As mentioned above, a hallmark of MAP6 deficiency is a dramatic depletion of synaptic vesicle pools in glutamatergic synapses, which could underlie the behavioral abnormalities observed in MAP6 mutant mice (Eastwood et al., 2007; Fournet et al., 2012b; Volle et al., 2013; Daoust et al., 2014). Taking all of this data together, the current model is that dephosphorylated MAP6 binds and stabilizes microtubules throughout the neuron, but upon synaptic activation, CaMKII phosphorylation of MAP6 facilitates its dissociation from the microtubule and relocalization to pools of actin within synapses. There is a controversy as to whether the behavioral alterations observed in the MAP6 null mice are attributed to the downstream effects on the microtubule or the actin cytoskeleton. Surprisingly, treatment of the MAP6 null mice with either antipsychotics or microtubule stabilizing drugs improves the observed behavioral phenotypes (Andrieux et al., 2002; Merenlender-Wagner et al., 2010; Fournet et al., 2012a), indicating that the role of MAP6 in stabilizing the microtubule cytoskeleton is essential during neuronal function. It will be interesting to further investigate the independent vs. concurrent roles of MAP6 in regulating the microtubule and actin cytoskeletons, and determine if MAP6 can facilitate cross talk between these two networks.
MAP7/Ensconsin/E-MAP-115
MAP7, also known as Ensconsin or E-MAP-115, was originally identified based on its tenacious association with microtubules (Bulinski and Borisy, 1980; Masson and Kreis, 1993; Bulinski and Bossler, 1994; Masson and Kreis, 1995; Fabre-Jonca et al., 1998; Bulinski et al., 1999; Faire et al., 1999). In vivo, MAP7 interacts with kinesin-1 to regulate cell polarity in Drosophila oocytes, organelle transport in S2 cells, and nuclear positioning in both Drosophila and mammalian muscle cells (Sung et al., 2008; Metzger et al., 2012; Barlan et al., 2013). In vitro, MAP7 competes with another MAP, tau, and directly enhances kinesin-1 binding to the microtubule, but inhibits kinesin-3 from accessing the microtubule (Monroy et al., 2017). Recently, MAP7 was found to be upregulated during collateral branch formation in dorsal root ganglion (DRG) neurons, and overexpression of MAP7 led to a dramatic increase in the number of collateral branches (Tymanskyj et al., 2017). Together these results suggest that MAP7 is involved both in kinesin-1-based transport and microtubule organization in a variety of cell types.
As with most MAPs, MAP7 is regulated by phosphorylation, but the extent to which phosphorylation regulates its function is less understood. Initial studies in HeLa cells reported that MAP7 was differentially localized depending on the stage of mitosis (Masson and Kreis, 1993). MAP7 is absent from microtubules during early prophase, but as mitosis progresses, MAP7 intensity increases at the spindle poles, then spreads to coat the mitotic spindle (Masson and Kreis, 1995; Gallaud et al., 2014). This localization pattern is consistent in the syncytial divisions of the Drosophila embryo (Gallaud et al., 2014). Interestingly, MAP7 was found to be hyperphosphorylated in mitotic cells and in microtubule co-pelleting assays from mitotic cell extract, MAP7 was enriched in the supernatant, suggesting that the hyperphosphorylated protein could not bind microtubules (Masson and Kreis, 1993). Fluorescent speckle microscopy used to analyze MAP7-microtubule binding dynamics also revealed an effect of phosphorylation. Treatment with a general kinase inhibitor, staurosporine, showed a decrease in MAP7 dynamics compared to untreated cells, indicating phosphorylation may regulate microtubule-binding dynamics by affecting MAP7 binding or dissociation rates (Faire et al., 1999; Bulinski et al., 2001).
In Drosophila oocytes, polarity is established by the kinase, MARK (a.k.a. Par-1), and in its absence, MAP7 is no longer spatially restricted to the anterior region of the oocyte (Sung et al., 2008). MARK was found to phosphorylate MAP7 within both the N-terminal and the C-terminal regions (Sung et al., 2008). Deletion or alanine-mutations of six predicted phosphorylation sites within the MAP7 N-terminus did not affect the association of MAP7 with the microtubule, but did affect is localization pattern similar to loss of MARK. Mutating the only two residues conserved in mammalian MAP7, Ser168 and Ser198, reduced the ability of MARK to phosphorylate MAP7 and was sufficient to produce the same mislocalization phenotypes in Drosophila oocytes (Sung et al., 2008). This data suggests that the phosphorylation of MAP7 by MARK is required for its localization pattern, but does not affect microtubule affinity. Although this is in contrast to previous hypotheses about the regulation of MAP7 by phosphorylation, other kinases may phosphorylate MAP7 and differentially affect its function or localization. To date, MARK is the only kinase that has been shown to phosphorylate MAP7, and it could regulate the localization pattern of MAP7 during other cellular processes such as mitosis. There are three paralogs for the MAP7 gene: MAP7D1, MAP7D2 and MAP7D3. MAP7D1 exhibits the highest conservation with MAP7, and was recently identified as a phosphorylation substrate of DCLK1 in cortical neurons (Koizumi et al., 2017). DCLK1-mediated phosphorylation of MAP7D1 at Ser315 was shown to promote the elongation of growing axons within cortical neurons, but it is unknown how phosphorylation at this site modulates MAP7D1 activities (Koizumi et al., 2017). It is possible based on the original studies of MAP7 that other kinases could regulate the microtubule binding affinity of MAP7 to spatially or functionally control this protein during mitotic progression. Further investigation is necessary to determine how context dependent phosphorylation of MAP7 regulates its microtubule affinity versus its subcellular localization pattern.
MAP9/ASAP
MAP9, also termed ASAP (ASter-Associated Protein), is a microtubule-associated protein that localizes to the mitotic spindle and has important roles in bipolar spindle assembly and centrosome integrity (Saffin et al., 2005; Venoux et al., 2008b; Somenarain and Jones, 2010). MAP9 is expressed in the vertebrate nervous system throughout development, and depletion of MAP9 in the zebrafish embryo leads to a number of developmental defects that lead to early embryonic lethality (Fontenille et al., 2014). In cultured cells, MAP9 decorates the mitotic spindle and overexpression causes monopolar spindles, while knockdown leads to multipolar spindles and defects in cytokinesis (Saffin et al., 2005). MAP9 is also downregulated in certain types of cancers, such as colorectal cancer, and could be used as a valuable disease marker (Rouquier et al., 2014). These findings indicate that MAP9 has a crucial role in the organization of the bipolar mitotic spindle and in mitotic progression.
To date, studies have shown that MAP9 is phosphorylated by two mitotic kinases: Aurora A and Polo-like kinase 1 (PLK1) (Venoux et al., 2008a; Eot-Houllier et al., 2010). Aurora A is an important regulator of cell division and phosphorylates a number of MAPs throughout mitosis (Glotzer, 2009). MAP9 is phosphorylated by Aurora A on Ser625, and this one residue is necessary and sufficient to dictate the localization pattern of MAP9 within the mitotic spindle (Venoux et al., 2008a). Phosphorylated MAP9 localizes to the centrosome from G2 to telophase, then to the midbody during cytokinesis, and perturbing the phosphorylation of this residue leads to abnormal spindles and defects in mitotic progression (Venoux et al., 2008a). In the absence of Aurora A, MAP9 is degraded, highlighting the importance of phosphorylation in MAP9 protein stability. It is thought that MAP9 function, but not localization, is dependent upon Plk1 kinase (Eot-Houllier et al., 2010). Plk1 phosphorylates MAP9 at Ser289 after MAP9 has already been recruited to the centrosomes by the NEDD1-γ-tubulin pathway. This event may be important for subsequent microtubule nucleation from the centrosome, but further studies are necessary to dissect the functional interaction between MAP9 and the NEDD1-γ-tubulin pathway (Eot-Houllier et al., 2010). Taken together, Aurora A seems to be required to localize and stabilize MAP9 specifically to centrosomes during spindle formation, while Plk1 seems to be required for subsequent functions of MAP9 within the spindle pole, suggesting specific contributions of both kinases to MAP9 regulation.
TAU
Tau is one of the most widely studied MAPs due to its association with neurological diseases such as fronto-temporal dementia (FTD) and Alzheimer’s disease (AD) (Tolnay and Probst, 1999; Del Carmen Alonso, 2010; Irwin et al., 2013; Ghetti et al., 2015; Gao et al., 2017; Kocahan and Dogan, 2017). Tau is now also a marker of brain damage following traumatic brain injury (TBI)(Zemlan et al., 1999; Zemlan et al., 2002; Franz et al., 2003; Smith et al., 2003; Tran et al., 2011a; Tran et al., 2011b; Hawkins et al., 2013; Kondo et al., 2015), highlighting the deleterious role of tau in neurodegeneration. Although tau was originally discovered to enhance microtubule stability and polymerization, it has subsequently been ascribed a number of other functions, which include controlling microtubule modifications, altering the stiffness and mechanical properties of the microtubule polymer, spacing microtubules at certain distances within the axon, and regulating microtubule motor transport (Cleveland et al., 1977; Drubin and Kirschner, 1986a; Drubin and Kirschner, 1986b; Fellous et al., 1986; Bre and Karsenti, 1990; Drechsel et al., 1992; Ebneth et al., 1998; Samsonov et al., 2004; Peck et al., 2011; Duan et al., 2017). Tau is expressed in the brain throughout development and into adulthood, and multiple isoforms of tau exist in the central nervous system due to alternative splicing of exons 2, 3, and 10 of the tau pre-mRNA (Kosik and Caceres, 1991; Goedert, 2015). There is one tau isoform expressed in the neonatal brain, and six tau isoforms expressed in the human adult brain. Of these six, three contain four microtubule-binding repeats (4R) and three contain three microtubule-binding repeats (3R) (Goedert et al., 1989). Within the brain, tau is predominantly expressed in neurons (Drubin and Kirschner, 1986b; Caceres and Kosik, 1990; Kosik and Caceres, 1991; Goode et al., 1997). Non-phosphorylated tau is restricted to the axon and is used as an axonal marker; however, antibodies against total tau have revealed that it is in both dendritic and axonal compartments (Mandell and Banker, 1996). During neuronal development, 3R-tau plays clear roles in neurite outgrowth, and in mature neurons, both 3R- and 4R-tau aids in the assembly of microtubules (Drubin and Kirschner, 1986a; Caceres and Kosik, 1990; Kosik and Caceres, 1991; Goode et al., 1997). Tau also contributes to the stability of microtubules by inhibiting katanin severing within the axon (Qiang et al., 2006). It has also been shown both in vivo and in vitro that tau inhibits kinesin-1 motility, but has less of an effect on dynein motility along microtubules (Ebneth et al., 1998; Seitz et al., 2002; Terwel et al., 2002; Vershinin et al., 2007; Dixit et al., 2008). Therefore, tau also directly impacts microtubule-based transport.
Tau has been reported to have a number of interacting partners, under normal and pathogenic conditions, which are comprehensively reviewed elsewhere (Mandelkow and Mandelkow, 2012; Bakota et al., 2017). Based on numerous studies, tau is an important MAP necessary for the development and maintenance of a neuron; however, tau null mice are viable and display increasing cognitive impairments with age (Harada et al., 1994; Ikegami et al., 2000; Fujio et al., 2007; Lei et al., 2012). This could be due to the compensatory effects of MAP1A, which is upregulated in the brains of tau null mice (Dawson et al., 2001), or MAP1B. Tau/MAP1B double knockout mice display more severe phenotypes in axonal elongation and neuronal migration compared to the single knockouts (Takei et al., 2000). The functional redundancy between MAPs that is observed in these studies highlights the importance of these classical MAPs during neuronal development.
Phosphorylation of tau contributes to a number of neuronal pathologies. Tau can be posttranslationally modified in a number of different ways: phosphorylation, glycosylation, ubiquitination, O-GlcNAcylation, and oxidation, among others (Avila et al., 2004; Avila, 2006; Avila et al., 2006; Avila, 2008). Phosphorylation of tau has been extensively studied, because hyperphosphorylated tau forms large aggregates called neurofibrillary tangles that contribute to the pathologies of FTD, AD, and neurodegeneration resulting from TBI (Mandelkow and Mandelkow, 1994; Trojanowski and Lee, 1995; Mandelkow and Mandelkow, 1998). Tau contains 80 potential serine/threonine and 5 potential tyrosine phosphorylation sites (Wang et al., 2007; Wang et al., 2013). In the brains of AD patients, tau is hyperphosphorylated at 20–40 sites (Duka et al., 2013). The kinases that have been shown to phosphorylate tau include: GSKIIIβ, MARK, MAPK, JNK1, PKA, ERKs, DYRK1a, casein kinase I, and cyclin-dependent kinase-5 (CDK5) (Avila, 2008; Hanger et al., 2009). Similar to other MAPs reviewed here, it has been demonstrated that tau phosphorylation reduces its affinity for microtubules or its ability to promote microtubule polymerization, resulting in overall microtubule instability (Avila et al., 2006). Phosphorylation at specific sites, such as Y18, regulates the dynamicity of tau on the microtubule and has significant effects on kinesin-1 motility (Stern et al., 2017). Whether neuronal cell death is a consequence of microtubule instability, transport, or tau neurofibrillary tangles is controversial (Dawson et al., 2010). However, it has been established that tau tangles are highly pathogenic and can cause neurodegeneration when introduced into healthy mice (Clavaguera et al., 2009; Zetterberg et al., 2013; Clavaguera et al., 2015; Wu et al., 2016; Fu et al., 2017). In addition, tau aggregates can transfer between neurons across synapses and can therefore spread and infect healthy neurons, causing a sweep of neurodegeneration (Liu et al., 2012b; Iba et al., 2013; Wu et al., 2016).
Most studies focus on the phosphorylation sites that cause tau to release from the microtubule and form aggregates, but thus far, there is one phosphorylation site on tau that does not affect microtubule affinity. Phosphorylation at Ser262 within tau does not cause tau to dissociate from the microtubule, but affects its ability to interact with and inhibit End-binding protein-1 (EB1) from tracking the plus end of the microtubule (Sayas et al., 2015; Ramirez-Rios et al., 2016). Therefore, phosphorylation of tau does not always cause tau to dissociate from the microtubule, but can affect other functions of this protein, which could contribute to neuronal alterations observed in neurodegenerative diseases.
DOUBLECORTIN FAMILY (DCX & DCLK)
The doublecortin (DCX) and doublecortin-like kinase (DCLK) proteins are part of the doublecortin-domain containing MAP family that have two tandem microtubule binding domains (DC1 and DC2)(Coquelle et al., 2006; Reiner et al., 2006). DCLK proteins contain an additional serine/threonine kinase domain at their C-terminal end that shares homology with the kinase domain of CaMKs (Kim et al., 2003; Dijkmans et al., 2010). DCX is expressed during the early stages of neuronal development and is predominantly localized to migrating neurons, followed by downregulation in differentiated neurons (Francis et al., 1999; Gleeson et al., 1999a; Liu et al., 2012a). The DCX gene has two paralogs: doublecortin-like kinase 1 and 2 (DCLK1 and DCLK2) (Reiner et al., 2006). In contrast to DCX, DCLK1 and DCLK2 are expressed in both developing and mature neurons (Burgess and Reiner, 2000; Tanaka et al., 2006; Liu et al., 2012a). DCLK1 preferentially localizes to the distal ends of dendrites and like DCX, DCLK1 is speculated to promote growth of dendrites. DCX and DCLK1-deficient neurons do not show dramatic defects in microtubule organization; however, they do display vesicular trafficking defects (Deuel et al., 2006). Both DCX and DCLK1 directly interact with kinesin-3 family members (KIF1A and KIF1C) to facilitate kinesin-3 transport of cargo within dendrites (Liu et al., 2012a; Lipka et al., 2016). This conserved function highlights the importance for DCX/DCLK1 in neuronal transport processes not only during development but also during neuronal maintenance.
Accumulating evidence suggests that DCLK1 is involved in a variety of cancers. DCLK1 is highly expressed in pancreatic cancer cells, where it promotes cell division and cell migration; however, its role during these two processes is unclear (Ito et al., 2016). In addition, a range of mutations throughout DCLK1 causes breast, colorectal, pancreatic and other cancers (Patel et al., 2016). A recent study has also identified a short isoform of DCLK1, termed DCLK1-S, which lacks the DCX microtubule-binding domains, but retains the entire kinase domain (Sarkar et al., 2017). This isoform is highly expressed in human colorectal tumors and colon cancer cells, and is currently being investigated as a potential biomarker for the detection cancer stem cells (Sarkar et al., 2017).
The doublecortin family is distinct from other MAPs in terms of its binding to microtubules. Classical MAPs such as MAP2 and Tau bind the ridges of the microtubule protofilament, while the DCX/DCLK proteins attach to the valleys between protofilaments (Al-Bassam et al., 2002; Moores et al., 2004; Bechstedt and Brouhard, 2012). Such binding between the grooves of the protofilaments is thought to provide both lateral and longitudinal stability to the microtubule filament and may regulate the 13-protofilament microtubules in vivo (Moores et al., 2004; Moores et al., 2006; Bechstedt and Brouhard, 2012; Bechstedt et al., 2014; Ettinger et al., 2016). Both DCX and DCLK1 potently stimulate microtubule polymerization in vitro, and it is proposed that they are required to specifically nucleate 13-protofilament microtubules (Bechstedt and Brouhard, 2012).
DCX and DCLK1 are important during neurogenesis and neuronal migration (Mizuguchi et al., 1999; Mizuguchi et al., 2002; Shu et al., 2006; Jean et al., 2012; Liu et al., 2012a; Saaltink et al., 2012; Shin et al., 2013; Verissimo et al., 2013; Lipka et al., 2016). Certain mutations that cause the neuronal migration disorder, Lissencephaly, have been mapped to the dcx gene (Gleeson et al., 1998; Pilz et al., 1998; Gleeson et al., 1999b; Viot et al., 2004). Most patient missense mutations are located within the tandem microtubule binding domains of DCX, suggesting that the microtubule binding, polymerization and stabilizing activities of DCX may be important for neuronal migration (Gleeson et al., 1999b; Sapir et al., 2000; Taylor et al., 2000).
DCX is phosphorylated by a number of different kinases in the developing brain. CDK5 phosphorylates DCX at Ser297 (Tanaka et al., 2006), PKA and MARK phosphorylate Ser47 (Schaar et al., 2004), and JNK1 phosphorylates three different sites: Thr321, Thr331 and Ser334 (Gdalyahu et al., 2004). All of these sites are located within the tandem microtubule binding domains. DCLK1 autophosphorylates itself at a number of different sites both inside and outside the microtubule-binding domain (Patel et al., 2016). In general, phosphorylation of DCX/DCLK1 lowers their microtubule binding affinities, thereby reducing their effects on microtubule polymerization (Tanaka et al., 2006; Patel et al., 2016). CDK5-mediated phosphorylation alters the localization pattern of DCX within cultured migrating neurons from perinuclear regions to distal microtubule bundles (Tanaka et al., 2006). JNK1-mediated phosphorylation of DCX is critical for its role in growth cones and perturbation of this phosphorylation event affects neurite outgrowth and the velocity of migrating neurons (Gdalyahu et al., 2004). PKA and MARK phosphorylation of DCX are also necessary for DCX localization to the leading processes during neuronal migration, but negatively regulate the association of DCX with microtubules, highlighting the importance of MAP association and dissociation in controlling microtubule dynamics (Schaar et al., 2004). Interestingly, the PKA/MARK phospho-site on DCX, Ser47, is mutated in two individuals with Lissencephaly (Schaar et al., 2004). Collectively, these results underscore the importance of phosphorylation in regulating DCX during neuronal development.
TUBULIN
Most studies focus on how MAPs control microtubule dynamics and stability, but tubulin itself can be post-translationally modified and these modifications can have profound effects on microtubule dynamics and MAP association. For the purpose of this review, we will focus on what is known about the effects of phosphorylation on tubulin (Table 2). For comprehensive reviews on other tubulin post-translational modifications, please see: (Bulinski, 2009; Janke and Bulinski, 2011; Yu et al., 2015).
Table 2.
Effects of phosphorylation on the tubulin heterodimer.
| Tubulin Subunit |
Phospho- Site(s) |
Kinase(s) | Phospho- Effects on Function |
References |
|---|---|---|---|---|
|
| ||||
| Beta-tubulin | S444/446 | cAMP casein kinase II | No effect on MT assembly | Gard&Kirschner, 1985; Luduena et al., 1988; Serrano et al., 1987 |
| S172 | CDK1, DYRKIa | Inhibits MT assembly | Fourest-Lieuvin et al., 2006; Ori-McKenney et al., 2016 | |
|
| ||||
| Alpha-tubulin | Tyrosine Residues | Syk and others | May or may not affect MT assembly | Wandosell et al., 1987; Peters et al., 1996; Faruki et al., 2000 |
| T349 | MAPK Phosphatase | Inhibits MT assembly | Ban et al., 2013; Fujita et al., 2013 | |
|
| ||||
| Not Determined | Tyrosine residues | Pp60c-src | Unknown | Matten et al., 1990 |
| Jak2 | Stimulates MT assembly | Ma&Sayeski, 2007 | ||
| c-Fes | Unknown | Laurent et al., 2004 | ||
Phosphorylation of tubulin was reported by a number of groups over 30 years ago using total brain tubulin from either bovine or rat (Goodman et al., 1970; Murray and Froscio, 1971; Eipper, 1974). Brain tubulin was initially found to be phosphorylated by cAMP (Goodman et al., 1970; Murray and Froscio, 1971), then Eipper (1974) performed further experiments to show that beta-tubulin, in particular, was phosphorylated within its C-terminal tail. Gard and Kirschner (1985) also found that beta-tubulin was phosphorylated in neuroblastoma cell lines and that treatment with microtubule stabilizing drugs such as taxol induced tubulin phosphorylation, while treatment with depolymerizing drugs such as nocodazole decreased tubulin phosphorylation. Further work both in vivo in neuroblastoma lines and in vitro with purified components revealed that casein kinase II could phosphorylate beta-tubulin within the C-terminal tail at Ser444 or Ser446, and that this phosphorylation event did not inhibit microtubule assembly (Gard and Kirschner, 1985; Serrano et al., 1987; Luduena et al., 1988). It is unknown whether phosphorylation of this residue occurs on the tubulin heterodimer or within the lattice, or both. How phosphorylation of this particular residue affects other aspects of microtubule dynamics besides assembly, or the binding of MAPs, will be an interesting area of investigation. Beta-tubulin can also be phosphorylated at Ser172, and phosphorylation at this residue has been shown to inhibit microtubule assembly (Fourest-Lieuvin et al., 2006; Ori-McKenney et al., 2016). Ser172 is located near the GTP binding pocket of beta-tubulin and may prevent nucleotide exchange (Figure 3A). Only beta-tubulin in the GTP state is able to assemble onto the microtubule protofilament, thus if GTP binding is blocked, then microtubule polymerization will likely be inhibited. To date, CDK1 and DYRK1a have been shown to phosphorylate Ser172 to regulate microtubule growth during the cell cycle and during neuronal morphogenesis, respectively (Fourest-Lieuvin et al., 2006; Ori-McKenney et al., 2016).
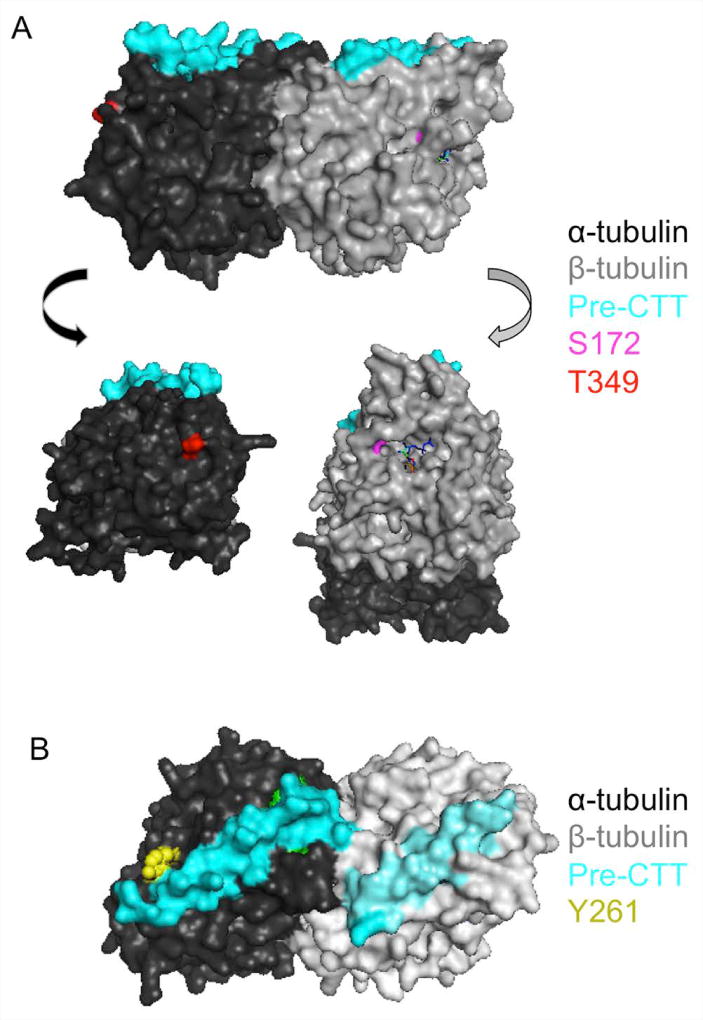
Figure 3. Phosphorylated residues in tubulin using the PDB structure, 1TUB.
(A) The structure of the tubulin heterodimer highlighting the two residues that, upon phosphorylation, inhibit microtubule polymerization. S172 (in pink) is located near the GTP binding pocket of beta-tubulin (in gray). T349 (in red) is located within alpha-tubulin at the binding interface. The sequence that precedes the C-terminal tails (CTTs) is colored in cyan. (B) The structure of the tubulin heterodimer highlighting one potential tyrosine residue that is exposed on alpha-tubulin that could be a target of phosphorylation.
Like beta-tubulin, phosphorylation of alpha-tubulin can also result in a range of effects within the cell. Unlike beta-tubulin, which is predominantly phosphorylated at serine and threonine residues, alpha-tubulin is phosphorylated at threonine and tyrosine residues, indicating that different types of kinases could act upon this subunit. In the presence of insulin, insulin receptor kinase phosphorylates alpha-tubulin at multiple tyrosine residues (Wandosell et al., 1987). The effect on microtubule growth varies depending on which tyrosine residue is phosphorylated. Phosphorylation of a tyrosine residue in the C-terminal tail of alpha-tubulin inhibits microtubule assembly, but phosphorylation of tyrosine residues in other regions of alpha-tubulin does not affect microtubule assembly (Wandosell et al., 1987). Upon T-cell activation, alpha-tubulin can be phosphorylated at a tyrosine residue, which prevents heterodimer assembly into microtubules (Ley et al., 1994). Similarly, after B-cell activation, the tyrosine kinase, Syk, phosphorylates alpha-tubulin on a tyrosine present near, but not within its C-terminal tail (Peters et al., 1996). Faruki et al. (2000) followed up on this work and showed that phosphorylation of alpha-tubulin at this tyrosine can occur on the heterodimer and on the microtubule polymer indicating that it does not affect microtubule assembly (Faruki et al., 2000). Interestingly, phosphorylation at one conserved site within alpha-tubulin inhibits the assembly of the heterodimer into the microtubule polymer: Thr349. An atypical MAPK phosphatase phosphorylates Thr349 within alpha-tubulin in response to osmotic stress in rice and Arabidopsis (Ban et al., 2013; Fujita et al., 2013). Thr349 is located at the exposed surface of alpha-tubulin that interacts with the beta-tubulin of another heterodimer (Figure 3A), and phosphorylation of this residue likely impairs this interaction and prevents microtubule assembly (Ban et al., 2013; Fujita et al., 2013).
Other studies have identified tyrosine phosphorylation of tubulin, but were unable to determine which subunit was specifically phosphorylated. The kinase, pp60c-src phosphorylates tubulin on tyrosine residues in neuronal growth cones (Matten et al., 1990). C-Fes tyrosine kinase phosphorylates tubulin on tyrosine residues, which actually stimulates microtubule assembly in vitro (Laurent et al., 2004). Finally, Jak2 phosphorylates tubulin on tyrosine residues, but the functional output of this modification is unknown (Ma and Sayeski, 2007). Considering that tyrosine phosphorylation has not been detected on beta-tubulin by previous groups, it is likely that these tyrosine phosphorylation sites are occurring on alpha-tubulin (Serrano et al., 1987; Luduena et al., 1988). By examining the PDB structure (1TUB) of the tubulin heterodimer, one potential tyrosine residue that may be exposed is Tyr261 (Figure 3B). Future studies are necessary to determine not only the precise residues targeted for phosphorylation by the tyrosine kinases, but their functional consequences on both microtubule assembly and MAP binding.
CONCLUDING PERSPECTIVE
Phosphorylation of MAPs was originally shown to affect association with the microtubule, but advances in this field have revealed that phosphorylation can dictate intracellular MAP patterning and function. For the MAPs reviewed above, we find three general classes of phosphorylation effects (Table 1 and Figure 2): 1) MAP dissociation from the microtubule (MAP2, MAP4, MAP6, Tau, DCX), 2) MAP relocalization within the cell (MAP2, MAP6, MAP7, MAP9, DCX), or 3) altered MAP function (MAP1A, MAP1B, MAP9, Tau). The same MAPs can be classified into multiple groups indicating they can be modulated by phosphorylation in different ways. It is also apparent that a relatively few number of kinases are responsible for MAP phosphorylation (Table 1). The observations that the same kinases regulate multiple MAPs underscores the importance of these kinases during neuronal development and maintenance, but also raises an interesting question of how these kinases coordinate MAP binding and localization along the microtubule surface. MAPs bound to a particular microtubule could determine the growth rate or stability of the microtubule polymer, direct motor transport and/or dictate the binding of other MAPs. Many of these MAPs exhibit similar spatial and temporal patterns (Gumy et al., 2017). Some are dendrite-specific, such as MAP1A, MAP2, and DCX, or axon-specific, such as Tau, at certain developmental timepoints, but others localize to both the dendrites and the axons throughout development and into adulthood. MAP2 and Tau compete for the same binding site on the microtubule, but they are compartmentalized into dendrites and axons, respectively (Al-Bassam et al., 2002). Few studies have focused on how other MAPs compete for microtubule binding, but phosphorylation could be an important layer of regulation in coordinating MAPs that are localized to the same neuronal regions at the same time. Investigating the role of phosphorylation in directing MAP behavior will be essential in understanding the functions of MAPs, individually and as ensembles, on the organization of the microtubule cytoskeleton. This exciting research area will also provide greater insight into how MAPs are coordinated at the molecular and cellular level to ensure proper development and maintenance of neurons.
Acknowledgments
This work was supported by grant R00HD080981 from the National Institutes of Health (K.M.O.M.) and the March of Dimes Basil O’Connor Award (K.M.O.M.). We thank Richard McKenney and Francis McNally for helpful discussions and critical reading of the manuscript.
References
- Ainsztein AM, Purich DL. Stimulation of tubulin polymerization by MAP-2. Control by protein kinase C-mediated phosphorylation at specific sites in the microtubule-binding region. J Biol Chem. 1994;269:28465–28471. [PubMed] [Google Scholar]
- Akhmanova A, Steinmetz MO. Microtubule +TIPs at a glance. J Cell Sci. 2010;123:3415–3419. doi: 10.1242/jcs.062414. [DOI] [PubMed] [Google Scholar]
- Al-Bassam J, Ozer RS, Safer D, Halpain S, Milligan RA. MAP2 and tau bind longitudinally along the outer ridges of microtubule protofilaments. J Cell Biol. 2002;157:1187–1196. doi: 10.1083/jcb.200201048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SS, Wittmann T. Toward reconstitution of in vivo microtubule dynamics in vitro. Bioessays. 2002;24:305–307. doi: 10.1002/bies.10084. [DOI] [PubMed] [Google Scholar]
- Andrieux A, Salin PA, Vernet M, Kujala P, Baratier J, Gory-Faure S, Bosc C, Pointu H, Proietto D, Schweitzer A, Denarier E, Klumperman J, Job D. The suppression of brain cold-stable microtubules in mice induces synaptic defects associated with neuroleptic-sensitive behavioral disorders. Genes Dev. 2002;16:2350–2364. doi: 10.1101/gad.223302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arama J, Boulay AC, Bosc C, Delphin C, Loew D, Rostaing P, Amigou E, Ezan P, Wingertsmann L, Guillaud L, Andrieux A, Giaume C, Cohen-Salmon M. Bmcc1s, a novel brain-isoform of Bmcc1, affects cell morphology by regulating MAP6/STOP functions. PLoS One. 2012;7:e35488. doi: 10.1371/journal.pone.0035488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold SE, Lee VM, Gur RE, Trojanowski JQ. Abnormal expression of two microtubule-associated proteins (MAP2 and MAP5) in specific subfields of the hippocampal formation in schizophrenia. Proc Natl Acad Sci U S A. 1991;88:10850–10854. doi: 10.1073/pnas.88.23.10850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila J. Tau phosphorylation and aggregation in Alzheimer's disease pathology. FEBS Lett. 2006;580:2922–2927. doi: 10.1016/j.febslet.2006.02.067. [DOI] [PubMed] [Google Scholar]
- Avila J. Tau kinases and phosphatases. J Cell Mol Med. 2008;12:258–259. doi: 10.1111/j.1582-4934.2007.00214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila J, Perez M, Lim F, Gomez-Ramos A, Hernandez F, Lucas JJ. Tau in neurodegenerative diseases: tau phosphorylation and assembly. Neurotox Res. 2004;6:477–482. doi: 10.1007/BF03033284. [DOI] [PubMed] [Google Scholar]
- Avila J, Santa-Maria I, Perez M, Hernandez F, Moreno F. Tau phosphorylation, aggregation, and cell toxicity. J Biomed Biotechnol. 2006;2006:74539. doi: 10.1155/JBB/2006/74539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Rao AN, Matamoros AJ, Leo L. Stability properties of neuronal microtubules. Cytoskeleton (Hoboken) 2016;73:442–460. doi: 10.1002/cm.21286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakota L, Ussif A, Jeserich G, Brandt R. Systemic and network functions of the microtubule-associated protein tau: Implications for tau-based therapies. Mol Cell Neurosci. 2017 doi: 10.1016/j.mcn.2017.03.003. [DOI] [PubMed] [Google Scholar]
- Ban Y, Kobayashi Y, Hara T, Hamada T, Hashimoto T, Takeda S, Hattori T. alpha-tubulin is rapidly phosphorylated in response to hyperosmotic stress in rice and Arabidopsis. Plant Cell Physiol. 2013;54:848–858. doi: 10.1093/pcp/pct065. [DOI] [PubMed] [Google Scholar]
- Baratier J, Peris L, Brocard J, Gory-Faure S, Dufour F, Bosc C, Fourest-Lieuvin A, Blanchoin L, Salin P, Job D, Andrieux A. Phosphorylation of microtubule-associated protein STOP by calmodulin kinase II. J Biol Chem. 2006;281:19561–19569. doi: 10.1074/jbc.M509602200. [DOI] [PubMed] [Google Scholar]
- Barlan K, Lu W, Gelfand VI. The microtubule-binding protein ensconsin is an essential cofactor of kinesin-1. Curr Biol. 2013;23:317–322. doi: 10.1016/j.cub.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolini F, Gundersen GG. Generation of noncentrosomal microtubule arrays. J Cell Sci. 2006;119:4155–4163. doi: 10.1242/jcs.03227. [DOI] [PubMed] [Google Scholar]
- Bartolini F, Gundersen GG. Formins and microtubules. Biochim Biophys Acta. 2010;1803:164–173. doi: 10.1016/j.bbamcr.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechstedt S, Brouhard GJ. Doublecortin recognizes the 13-protofilament microtubule cooperatively and tracks microtubule ends. Dev Cell. 2012;23:181–192. doi: 10.1016/j.devcel.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechstedt S, Lu K, Brouhard GJ. Doublecortin recognizes the longitudinal curvature of the microtubule end and lattice. Curr Biol. 2014;24:2366–2375. doi: 10.1016/j.cub.2014.08.039. [DOI] [PubMed] [Google Scholar]
- Benoist M, Palenzuela R, Rozas C, Rojas P, Tortosa E, Morales B, Gonzalez-Billault C, Avila J, Esteban JA. MAP1B-dependent Rac activation is required for AMPA receptor endocytosis during long-term depression. EMBO J. 2013;32:2287–2299. doi: 10.1038/emboj.2013.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt R, Huber G, Matus A. Differences in the developmental patterns of three microtubule-associated proteins in the rat cerebellum. J Neurosci. 1985;5:977–991. doi: 10.1523/JNEUROSCI.05-04-00977.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieling P, Laan L, Schek H, Munteanu EL, Sandblad L, Dogterom M, Brunner D, Surrey T. Reconstitution of a microtubule plus-end tracking system in vitro. Nature. 2007;450:1100–1105. doi: 10.1038/nature06386. [DOI] [PubMed] [Google Scholar]
- Bieling P, Telley IA, Hentrich C, Piehler J, Surrey T. Fluorescence microscopy assays on chemically functionalized surfaces for quantitative imaging of microtubule, motor, and +TIP dynamics. Methods Cell Biol. 2010;95:555–580. doi: 10.1016/S0091-679X(10)95028-0. [DOI] [PubMed] [Google Scholar]
- Biernat J, Wu YZ, Timm T, Zheng-Fischhofer Q, Mandelkow E, Meijer L, Mandelkow EM. Protein kinase MARK/PAR-1 is required for neurite outgrowth and establishment of neuronal polarity. Mol Biol Cell. 2002;13:4013–4028. doi: 10.1091/mbc.02-03-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom GS, Vallee RB. Association of microtubule-associated protein 2 (MAP 2) with microtubules and intermediate filaments in cultured brain cells. J Cell Biol. 1983;96:1523–1531. doi: 10.1083/jcb.96.6.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodaleo FJ, Montenegro-Venegas C, Henriquez DR, Court FA, Gonzalez-Billault C. Microtubule-associated protein 1B (MAP1B)-deficient neurons show structural presynaptic deficiencies in vitro and altered presynaptic physiology. Sci Rep. 2016;6:30069. doi: 10.1038/srep30069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondallaz P, Barbier A, Soehrman S, Grenningloh G, Riederer BM. The control of microtubule stability in vitro and in transfected cells by MAP1B and SCG10. Cell Motil Cytoskeleton. 2006;63:681–695. doi: 10.1002/cm.20154. [DOI] [PubMed] [Google Scholar]
- Bonifacino JS, Klausner RD, Sandoval IV. A widely distributed nuclear protein immunologically related to the microtubule-associated protein MAP1 is associated with the mitotic spindle. Proc Natl Acad Sci U S A. 1985;82:1146–1150. doi: 10.1073/pnas.82.4.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisy GG, Olmsted JB, Marcum JM, Allen C. Microtubule assembly in vitro. Fed Proc. 1974;33:167–174. [PubMed] [Google Scholar]
- Bosc C, Andrieux A, Job D. STOP proteins. Biochemistry. 2003;42:12125–12132. doi: 10.1021/bi0352163. [DOI] [PubMed] [Google Scholar]
- Bosc C, Frank R, Denarier E, Ronjat M, Schweitzer A, Wehland J, Job D. Identification of novel bifunctional calmodulin-binding and microtubule-stabilizing motifs in STOP proteins. J Biol Chem. 2001;276:30904–30913. doi: 10.1074/jbc.M011614200. [DOI] [PubMed] [Google Scholar]
- Bouvrais-Veret C, Weiss S, Hanoun N, Andrieux A, Schweitzer A, Job D, Hamon M, Giros B, Martres MP. Microtubule-associated STOP protein deletion triggers restricted changes in dopaminergic neurotransmission. J Neurochem. 2008;104:745–756. doi: 10.1111/j.1471-4159.2007.05025.x. [DOI] [PubMed] [Google Scholar]
- Bre MH, Karsenti E. Effects of brain microtubule-associated proteins on microtubule dynamics and the nucleating activity of centrosomes. Cell Motil Cytoskeleton. 1990;15:88–98. doi: 10.1002/cm.970150205. [DOI] [PubMed] [Google Scholar]
- Brenman JE, Topinka JR, Cooper EC, McGee AW, Rosen J, Milroy T, Ralston HJ, Bredt DS. Localization of postsynaptic density-93 to dendritic microtubules and interaction with microtubule-associated protein 1A. J Neurosci. 1998;18:8805–8813. doi: 10.1523/JNEUROSCI.18-21-08805.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbelt K, Byne W, Jones LB. Evidence for a decrease in basilar dendrites of pyramidal cells in schizophrenic medial prefrontal cortex. Schizophr Res. 2002;58:75–81. doi: 10.1016/s0920-9964(02)00201-3. [DOI] [PubMed] [Google Scholar]
- Brugg B, Matus A. Phosphorylation determines the binding of microtubule-associated protein 2 (MAP2) to microtubules in living cells. J Cell Biol. 1991;114:735–743. doi: 10.1083/jcb.114.4.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulinski JC. Tubulin posttranslational modifications: a Pushmi-Pullyu at work? Dev Cell. 2009;16:773–774. doi: 10.1016/j.devcel.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Bulinski JC, Borisy GG. Microtubule-associated proteins from cultured HeLa cells. Analysis of molecular properties and effects on microtubule polymerization. J Biol Chem. 1980;255:11570–11576. [PubMed] [Google Scholar]
- Bulinski JC, Bossler A. Purification and characterization of ensconsin, a novel microtubule stabilizing protein. J Cell Sci. 1994;107(Pt 10):2839–2849. doi: 10.1242/jcs.107.10.2839. [DOI] [PubMed] [Google Scholar]
- Bulinski JC, Gruber D, Faire K, Prasad P, Chang W. GFP chimeras of E-MAP-115 (ensconsin) domains mimic behavior of the endogenous protein in vitro and in vivo. Cell Struct Funct. 1999;24:313–320. doi: 10.1247/csf.24.313. [DOI] [PubMed] [Google Scholar]
- Bulinski JC, McGraw TE, Gruber D, Nguyen HL, Sheetz MP. Overexpression of MAP4 inhibits organelle motility and trafficking in vivo. J Cell Sci. 1997;110(Pt 24):3055–3064. doi: 10.1242/jcs.110.24.3055. [DOI] [PubMed] [Google Scholar]
- Bulinski JC, Odde DJ, Howell BJ, Salmon TD, Waterman-Storer CM. Rapid dynamics of the microtubule binding of ensconsin in vivo. J Cell Sci. 2001;114:3885–3897. doi: 10.1242/jcs.114.21.3885. [DOI] [PubMed] [Google Scholar]
- Burgess HA, Reiner O. Doublecortin-like kinase is associated with microtubules in neuronal growth cones. Mol Cell Neurosci. 2000;16:529–541. doi: 10.1006/mcne.2000.0891. [DOI] [PubMed] [Google Scholar]
- Caceres A, Kosik KS. Inhibition of neurite polarity by tau antisense oligonucleotides in primary cerebellar neurons. Nature. 1990;343:461–463. doi: 10.1038/343461a0. [DOI] [PubMed] [Google Scholar]
- Chan SL, Chua LL, Angeles DC, Tan EK. MAP1B rescues LRRK2 mutant-mediated cytotoxicity. Mol Brain. 2014;7:29. doi: 10.1186/1756-6606-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Jones Y, Ellisman MH, Goldstein LS, Karin M. JNK1 is required for maintenance of neuronal microtubules and controls phosphorylation of microtubule-associated proteins. Dev Cell. 2003;4:521–533. doi: 10.1016/s1534-5807(03)00094-7. [DOI] [PubMed] [Google Scholar]
- Chen J, Kanai Y, Cowan NJ, Hirokawa N. Projection domains of MAP2 and tau determine spacings between microtubules in dendrites and axons. Nature. 1992;360:674–677. doi: 10.1038/360674a0. [DOI] [PubMed] [Google Scholar]
- Chung WJ, Kindler S, Seidenbecher C, Garner CC. MAP2a, an alternatively spliced variant of microtubule-associated protein 2. J Neurochem. 1996;66:1273–1281. doi: 10.1046/j.1471-4159.1996.66031273.x. [DOI] [PubMed] [Google Scholar]
- Clavaguera F, Bolmont T, Crowther RA, Abramowski D, Frank S, Probst A, Fraser G, Stalder AK, Beibel M, Staufenbiel M, Jucker M, Goedert M, Tolnay M. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol. 2009;11:909–913. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavaguera F, Hench J, Goedert M, Tolnay M. Invited review: Prion-like transmission and spreading of tau pathology. Neuropathol Appl Neurobiol. 2015;41:47–58. doi: 10.1111/nan.12197. [DOI] [PubMed] [Google Scholar]
- Cleveland DW, Hwo SY, Kirschner MW. Purification of tau, a microtubule-associated protein that induces assembly of microtubules from purified tubulin. J Mol Biol. 1977;116:207–225. doi: 10.1016/0022-2836(77)90213-3. [DOI] [PubMed] [Google Scholar]
- Coquelle FM, Levy T, Bergmann S, Wolf SG, Bar-El D, Sapir T, Brody Y, Orr I, Barkai N, Eichele G, Reiner O. Common and divergent roles for members of the mouse DCX superfamily. Cell Cycle. 2006;5:976–983. doi: 10.4161/cc.5.9.2715. [DOI] [PubMed] [Google Scholar]
- Cotter D, Wilson S, Roberts E, Kerwin R, Everall IP. Increased dendritic MAP2 expression in the hippocampus in schizophrenia. Schizophr Res. 2000;41:313–323. doi: 10.1016/s0920-9964(99)00068-7. [DOI] [PubMed] [Google Scholar]
- Cumming R, Burgoyne RD, Lytton NA. Immunofluorescence distribution of alpha tubulin, beta tubulin and microtubule-associated protein 2 during in vitro maturation of cerebellar granule cell neurones. Neuroscience. 1984;12:775–782. doi: 10.1016/0306-4522(84)90169-6. [DOI] [PubMed] [Google Scholar]
- Cunningham CC, Leclerc N, Flanagan LA, Lu M, Janmey PA, Kosik KS. Microtubule-associated protein 2c reorganizes both microtubules and microfilaments into distinct cytological structures in an actin-binding protein-280-deficient melanoma cell line. J Cell Biol. 1997;136:845–857. doi: 10.1083/jcb.136.4.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallol A, Cooper WN, Al-Mulla F, Agathanggelou A, Maher ER, Latif F. Depletion of the Ras association domain family 1, isoform A-associated novel microtubule-associated protein, C19ORF5/MAP1S, causes mitotic abnormalities. Cancer Res. 2007;67:492–500. doi: 10.1158/0008-5472.CAN-06-3604. [DOI] [PubMed] [Google Scholar]
- Daoust A, Bohic S, Saoudi Y, Debacker C, Gory-Faure S, Andrieux A, Barbier EL, Deloulme JC. Neuronal transport defects of the MAP6 KO mouse - a model of schizophrenia - and alleviation by Epothilone D treatment, as observed using MEMRI. Neuroimage. 2014;96:133–142. doi: 10.1016/j.neuroimage.2014.03.071. [DOI] [PubMed] [Google Scholar]
- Davare MA, Dong F, Rubin CS, Hell JW. The A-kinase anchor protein MAP2B and cAMP-dependent protein kinase are associated with class C L-type calcium channels in neurons. J Biol Chem. 1999;274:30280–30287. doi: 10.1074/jbc.274.42.30280. [DOI] [PubMed] [Google Scholar]
- Davidkova G, Carroll RC. Characterization of the role of microtubule-associated protein 1B in metabotropic glutamate receptor-mediated endocytosis of AMPA receptors in hippocampus. J Neurosci. 2007;27:13273–13278. doi: 10.1523/JNEUROSCI.3334-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson HN, Cantillana V, Jansen M, Wang H, Vitek MP, Wilcock DM, Lynch JR, Laskowitz DT. Loss of tau elicits axonal degeneration in a mouse model of Alzheimer's disease. Neuroscience. 2010;169:516–531. doi: 10.1016/j.neuroscience.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson HN, Ferreira A, Eyster MV, Ghoshal N, Binder LI, Vitek MP. Inhibition of neuronal maturation in primary hippocampal neurons from tau deficient mice. J Cell Sci. 2001;114:1179–1187. doi: 10.1242/jcs.114.6.1179. [DOI] [PubMed] [Google Scholar]
- Dehmelt L, Poplawski G, Hwang E, Halpain S. NeuriteQuant: an open source toolkit for high content screens of neuronal morphogenesis. BMC Neurosci. 2011;12:100. doi: 10.1186/1471-2202-12-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Carmen Alonso A. Tau, neurodegeneration and Alzheimer's disease. Curr Alzheimer Res. 2010;7:653–655. doi: 10.2174/156720510793611574. [DOI] [PubMed] [Google Scholar]
- Deloulme JC, Gory-Faure S, Mauconduit F, Chauvet S, Jonckheere J, Boulan B, Mire E, Xue J, Jany M, Maucler C, Deparis AA, Montigon O, Daoust A, Barbier EL, Bosc C, Deglon N, Brocard J, Denarier E, Le Brun I, Pernet-Gallay K, Vilgrain I, Robinson PJ, Lahrech H, Mann F, Andrieux A. Microtubule-associated protein 6 mediates neuronal connectivity through Semaphorin 3E–dependent signalling for axonal growth. Nat Commun. 2015;6:7246. doi: 10.1038/ncomms8246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delphin C, Bouvier D, Seggio M, Couriol E, Saoudi Y, Denarier E, Bosc C, Valiron O, Bisbal M, Arnal I, Andrieux A. MAP6-F is a temperature sensor that directly binds to and protects microtubules from cold-induced depolymerization. J Biol Chem. 2012;287:35127–35138. doi: 10.1074/jbc.M112.398339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent EW, Baas PW. Microtubules in neurons as information carriers. J Neurochem. 2014;129:235–239. doi: 10.1111/jnc.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuel TA, Liu JS, Corbo JC, Yoo SY, Rorke-Adams LB, Walsh CA. Genetic interactions between doublecortin and doublecortin-like kinase in neuronal migration and axon outgrowth. Neuron. 2006;49:41–53. doi: 10.1016/j.neuron.2005.10.038. [DOI] [PubMed] [Google Scholar]
- Dhamodharan R, Wadsworth P. Modulation of microtubule dynamic instability in vivo by brain microtubule associated proteins. J Cell Sci. 1995;108(Pt 4):1679–1689. doi: 10.1242/jcs.108.4.1679. [DOI] [PubMed] [Google Scholar]
- Diaz-Nido J, Montoro RJ, Lopez-Barneo J, Avila J. High external potassium induces an increase in the phosphorylation of the cytoskeletal protein MAP2 in rat hippocampal slices. Eur J Neurosci. 1993;5:818–824. doi: 10.1111/j.1460-9568.1993.tb00933.x. [DOI] [PubMed] [Google Scholar]
- Diez-Guerra FJ, Avila J. MAP2 phosphorylation parallels dendrite arborization in hippocampal neurones in culture. Neuroreport. 1993;4:419–422. doi: 10.1097/00001756-199304000-00020. [DOI] [PubMed] [Google Scholar]
- Dijkmans TF, van Hooijdonk LW, Fitzsimons CP, Vreugdenhil E. The doublecortin gene family and disorders of neuronal structure. Cent Nerv Syst Agents Med Chem. 2010;10:32–46. doi: 10.2174/187152410790780118. [DOI] [PubMed] [Google Scholar]
- Dixit R, Ross JL, Goldman YE, Holzbaur EL. Differential regulation of dynein and kinesin motor proteins by tau. Science. 2008;319:1086–1089. doi: 10.1126/science.1152993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll T, Meichsner M, Riederer BM, Honegger P, Matus A. An isoform of microtubule-associated protein 2 (MAP2) containing four repeats of the tubulin-binding motif. J Cell Sci. 1993;106(Pt 2):633–639. doi: 10.1242/jcs.106.2.633. [DOI] [PubMed] [Google Scholar]
- Dominguez JE, Buendia B, Lopez-Otin C, Antony C, Karsenti E, Avila J. A protein related to brain microtubule-associated protein MAP1B is a component of the mammalian centrosome. J Cell Sci. 1994;107(Pt 2):601–611. [PubMed] [Google Scholar]
- Downing KH, Nogales E. Tubulin structure: insights into microtubule properties and functions. Curr Opin Struct Biol. 1998;8:785–791. doi: 10.1016/s0959-440x(98)80099-7. [DOI] [PubMed] [Google Scholar]
- Drechsel DN, Hyman AA, Cobb MH, Kirschner MW. Modulation of the dynamic instability of tubulin assembly by the microtubule-associated protein tau. Mol Biol Cell. 1992;3:1141–1154. doi: 10.1091/mbc.3.10.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewes G, Ebneth A, Preuss U, Mandelkow EM, Mandelkow E. MARK, a novel family of protein kinases that phosphorylate microtubule-associated proteins and trigger microtubule disruption. Cell. 1997;89:297–308. doi: 10.1016/s0092-8674(00)80208-1. [DOI] [PubMed] [Google Scholar]
- Drubin D, Kirschner M. Purification of tau protein from brain. Methods Enzymol. 1986a;134:156–160. doi: 10.1016/0076-6879(86)34084-9. [DOI] [PubMed] [Google Scholar]
- Drubin DG, Kirschner MW. Tau protein function in living cells. J Cell Biol. 1986b;103:2739–2746. doi: 10.1083/jcb.103.6.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan AR, Jonasson EM, Alberico EO, Li C, Scripture JP, Miller RA, Alber MS, Goodson HV. Interactions between Tau and Different Conformations of Tubulin: Implications for Tau Function and Mechanism. J Mol Biol. 2017;429:1424–1438. doi: 10.1016/j.jmb.2017.03.018. [DOI] [PubMed] [Google Scholar]
- Duka V, Lee JH, Credle J, Wills J, Oaks A, Smolinsky C, Shah K, Mash DC, Masliah E, Sidhu A. Identification of the sites of tau hyperphosphorylation and activation of tau kinases in synucleinopathies and Alzheimer's diseases. PLoS One. 2013;8:e75025. doi: 10.1371/journal.pone.0075025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood SL, Lyon L, George L, Andrieux A, Job D, Harrison PJ. Altered expression of synaptic protein mRNAs in STOP (MAP6) mutant mice. J Psychopharmacol. 2007;21:635–644. doi: 10.1177/0269881106068825. [DOI] [PubMed] [Google Scholar]
- Ebneth A, Drewes G, Mandelkow EM, Mandelkow E. Phosphorylation of MAP2c and MAP4 by MARK kinases leads to the destabilization of microtubules in cells. Cell Motil Cytoskeleton. 1999;44:209–224. doi: 10.1002/(SICI)1097-0169(199911)44:3<209::AID-CM6>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Ebneth A, Godemann R, Stamer K, Illenberger S, Trinczek B, Mandelkow E. Overexpression of tau protein inhibits kinesin-dependent trafficking of vesicles, mitochondria, and endoplasmic reticulum: implications for Alzheimer's disease. J Cell Biol. 1998;143:777–794. doi: 10.1083/jcb.143.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eipper BA. Rat brain tubulin and protein kinase activity. J Biol Chem. 1974;249:1398–1406. [PubMed] [Google Scholar]
- Eot-Houllier G, Venoux M, Vidal-Eychenie S, Hoang MT, Giorgi D, Rouquier S. Plk1 regulates both ASAP localization and its role in spindle pole integrity. J Biol Chem. 2010;285:29556–29568. doi: 10.1074/jbc.M110.144220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson M, Samuelsson H, Samuelsson EB, Liu L, McKeehan WL, Benedikz E, Sundstrom E. The NMDAR subunit NR3A interacts with microtubule-associated protein 1S in the brain. Biochem Biophys Res Commun. 2007;361:127–132. doi: 10.1016/j.bbrc.2007.06.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger A, van Haren J, Ribeiro SA, Wittmann T. Doublecortin Is Excluded from Growing Microtubule Ends and Recognizes the GDP-Microtubule Lattice. Curr Biol. 2016;26:1549–1555. doi: 10.1016/j.cub.2016.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre-Jonca N, Allaman JM, Radlgruber G, Meda P, Kiss JZ, French LE, Masson D. The distribution of murine 115-kDa epithelial microtubule-associated protein (E-MAP-115) during embryogenesis and in adult organs suggests a role in epithelial polarization and differentiation. Differentiation. 1998;63:169–180. doi: 10.1111/j.1432-0436.1998.00169.x. [DOI] [PubMed] [Google Scholar]
- Faire K, Waterman-Storer CM, Gruber D, Masson D, Salmon ED, Bulinski JC. E-MAP-115 (ensconsin) associates dynamically with microtubules in vivo and is not a physiological modulator of microtubule dynamics. J Cell Sci. 1999;112(Pt 23):4243–4255. doi: 10.1242/jcs.112.23.4243. [DOI] [PubMed] [Google Scholar]
- Farah CA, Liazoghli D, Perreault S, Desjardins M, Guimont A, Anton A, Lauzon M, Kreibich G, Paiement J, Leclerc N. Interaction of microtubule-associated protein-2 and p63: a new link between microtubules and rough endoplasmic reticulum membranes in neurons. J Biol Chem. 2005;280:9439–9449. doi: 10.1074/jbc.M412304200. [DOI] [PubMed] [Google Scholar]
- Faruki S, Geahlen RL, Asai DJ. Syk-dependent phosphorylation of microtubules in activated B-lymphocytes. J Cell Sci. 2000;113(Pt 14):2557–2565. doi: 10.1242/jcs.113.14.2557. [DOI] [PubMed] [Google Scholar]
- Fellous A, Ohayon R, Mazie JC, Rosa F, Luduena RF, Prasad V. Tau microheterogeneity: an immunological approach with monoclonal antibodies. Ann N Y Acad Sci. 1986;466:240–256. doi: 10.1111/j.1749-6632.1986.tb38397.x. [DOI] [PubMed] [Google Scholar]
- Ferhat L, Represa A, Ferhat W, Ben-Ari Y, Khrestchatisky M. MAP2d mRNA is expressed in identified neuronal populations in the developing and adult rat brain and its subcellular distribution differs from that of MAP2b in hippocampal neurones. Eur J Neurosci. 1998;10:161–171. doi: 10.1046/j.1460-9568.1998.00044.x. [DOI] [PubMed] [Google Scholar]
- Fink JK, Jones SM, Esposito C, Wilkowski J. Human microtubule-associated protein 1a (MAP1A) gene: genomic organization, cDNA sequence, and developmental- and tissue-specific expression. Genomics. 1996;35:577–585. doi: 10.1006/geno.1996.0400. [DOI] [PubMed] [Google Scholar]
- Fischer M, Kaech S, Knutti D, Matus A. Rapid actin-based plasticity in dendritic spines. Neuron. 1998;20:847–854. doi: 10.1016/s0896-6273(00)80467-5. [DOI] [PubMed] [Google Scholar]
- Fontenille L, Rouquier S, Lutfalla G, Giorgi D. Microtubule-associated protein 9 (Map9/Asap) is required for the early steps of zebrafish development. Cell Cycle. 2014;13:1101–1114. doi: 10.4161/cc.27944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourest-Lieuvin A, Peris L, Gache V, Garcia-Saez I, Juillan-Binard C, Lantez V, Job D. Microtubule regulation in mitosis: tubulin phosphorylation by the cyclin-dependent kinase Cdk1. Mol Biol Cell. 2006;17:1041–1050. doi: 10.1091/mbc.E05-07-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournet V, de Lavilleon G, Schweitzer A, Giros B, Andrieux A, Martres MP. Both chronic treatments by epothilone D and fluoxetine increase the short-term memory and differentially alter the mood status of STOP/MAP6 KO mice. J Neurochem. 2012a;123:982–996. doi: 10.1111/jnc.12027. [DOI] [PubMed] [Google Scholar]
- Fournet V, Schweitzer A, Chevarin C, Deloulme JC, Hamon M, Giros B, Andrieux A, Martres MP. The deletion of STOP/MAP6 protein in mice triggers highly altered mood and impaired cognitive performances. J Neurochem. 2012b;121:99–114. doi: 10.1111/j.1471-4159.2011.07615.x. [DOI] [PubMed] [Google Scholar]
- Francis F, Koulakoff A, Boucher D, Chafey P, Schaar B, Vinet MC, Friocourt G, McDonnell N, Reiner O, Kahn A, McConnell SK, Berwald-Netter Y, Denoulet P, Chelly J. Doublecortin is a developmentally regulated, microtubule-associated protein expressed in migrating and differentiating neurons. Neuron. 1999;23:247–256. doi: 10.1016/s0896-6273(00)80777-1. [DOI] [PubMed] [Google Scholar]
- Franz G, Beer R, Kampfl A, Engelhardt K, Schmutzhard E, Ulmer H, Deisenhammer F. Amyloid beta 1–42 and tau in cerebrospinal fluid after severe traumatic brain injury. Neurology. 2003;60:1457–1461. doi: 10.1212/01.wnl.0000063313.57292.00. [DOI] [PubMed] [Google Scholar]
- Fu H, Rodriguez GA, Herman M, Emrani S, Nahmani E, Barrett G, Figueroa HY, Goldberg E, Hussaini SA, Duff KE. Tau Pathology Induces Excitatory Neuron Loss, Grid Cell Dysfunction, and Spatial Memory Deficits Reminiscent of Early Alzheimer's Disease. Neuron. 2017;93:533–541. e535. doi: 10.1016/j.neuron.2016.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujio J, Hosono H, Ishiguro K, Ikegami S, Fujita SC. Tau phosphorylation in the mouse brain during aversive conditioning. Neurochem Int. 2007;51:200–208. doi: 10.1016/j.neuint.2007.04.024. [DOI] [PubMed] [Google Scholar]
- Fujita S, Pytela J, Hotta T, Kato T, Hamada T, Akamatsu R, Ishida Y, Kutsuna N, Hasezawa S, Nomura Y, Nakagami H, Hashimoto T. An atypical tubulin kinase mediates stress-induced microtubule depolymerization in Arabidopsis. Curr Biol. 2013;23:1969–1978. doi: 10.1016/j.cub.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Gallaud E, Caous R, Pascal A, Bazile F, Gagne JP, Huet S, Poirier GG, Chretien D, Richard-Parpaillon L, Giet R. Ensconsin/Map7 promotes microtubule growth and centrosome separation in Drosophila neural stem cells. J Cell Biol. 2014;204:1111–1121. doi: 10.1083/jcb.201311094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamblin TC, Nachmanoff K, Halpain S, Williams RC., Jr Recombinant microtubule-associated protein 2c reduces the dynamic instability of individual microtubules. Biochemistry. 1996;35:12576–12586. doi: 10.1021/bi961135d. [DOI] [PubMed] [Google Scholar]
- Gao Y, Tan L, Yu JT, Tan L. Tau in Alzheimer's disease: Mechanisms and therapeutic strategies. Curr Alzheimer Res. 2017 doi: 10.2174/1567205014666170417111859. [DOI] [PubMed] [Google Scholar]
- Gard DL, Kirschner MW. A polymer-dependent increase in phosphorylation of beta-tubulin accompanies differentiation of a mouse neuroblastoma cell line. J Cell Biol. 1985;100:764–774. doi: 10.1083/jcb.100.3.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gdalyahu A, Ghosh I, Levy T, Sapir T, Sapoznik S, Fishler Y, Azoulai D, Reiner O. DCX, a new mediator of the JNK pathway. EMBO J. 2004;23:823–832. doi: 10.1038/sj.emboj.7600079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevorkian G, Gonzalez-Noriega A, Acero G, Ordonez J, Michalak C, Munguia ME, Govezensky T, Cribbs DH, Manoutcharian K. Amyloid-beta peptide binds to microtubule-associated protein 1B (MAP1B) Neurochem Int. 2008;52:1030–1036. doi: 10.1016/j.neuint.2007.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghetti B, Oblak AL, Boeve BF, Johnson KA, Dickerson BC, Goedert M. Invited review: Frontotemporal dementia caused by microtubule-associated protein tau gene (MAPT) mutations: a chameleon for neuropathology and neuroimaging. Neuropathol Appl Neurobiol. 2015;41:24–46. doi: 10.1111/nan.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson JG, Allen KM, Fox JW, Lamperti ED, Berkovic S, Scheffer I, Cooper EC, Dobyns WB, Minnerath SR, Ross ME, Walsh CA. Doublecortin, a brain-specific gene mutated in human X-linked lissencephaly and double cortex syndrome, encodes a putative signaling protein. Cell. 1998;92:63–72. doi: 10.1016/s0092-8674(00)80899-5. [DOI] [PubMed] [Google Scholar]
- Gleeson JG, Lin PT, Flanagan LA, Walsh CA. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron. 1999a;23:257–271. doi: 10.1016/s0896-6273(00)80778-3. [DOI] [PubMed] [Google Scholar]
- Gleeson JG, Minnerath SR, Fox JW, Allen KM, Luo RF, Hong SE, Berg MJ, Kuzniecky R, Reitnauer PJ, Borgatti R, Mira AP, Guerrini R, Holmes GL, Rooney CM, Berkovic S, Scheffer I, Cooper EC, Ricci S, Cusmai R, Crawford TO, Leroy R, Andermann E, Wheless JW, Dobyns WB, Walsh CA, et al. Characterization of mutations in the gene doublecortin in patients with double cortex syndrome. Ann Neurol. 1999b;45:146–153. doi: 10.1002/1531-8249(199902)45:2<146::aid-ana3>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Glotzer M. The 3Ms of central spindle assembly: microtubules, motors and MAPs. Nat Rev Mol Cell Biol. 2009;10:9–20. doi: 10.1038/nrm2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M. NEURODEGENERATION. Alzheimer's and Parkinson's diseases: The prion concept in relation to assembled Abeta, tau, and alpha-synuclein. Science. 2015;349:1255555. doi: 10.1126/science.1255555. [DOI] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer's disease. Neuron. 1989;3:519–526. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Billault C, Avila J, Caceres A. Evidence for the role of MAP1B in axon formation. Mol Biol Cell. 2001;12:2087–2098. doi: 10.1091/mbc.12.7.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good PF, Alapat D, Hsu A, Chu C, Perl D, Wen X, Burstein DE, Kohtz DS. A role for semaphorin 3A signaling in the degeneration of hippocampal neurons during Alzheimer's disease. J Neurochem. 2004;91:716–736. doi: 10.1111/j.1471-4159.2004.02766.x. [DOI] [PubMed] [Google Scholar]
- Goode BL, Denis PE, Panda D, Radeke MJ, Miller HP, Wilson L, Feinstein SC. Functional interactions between the proline-rich and repeat regions of tau enhance microtubule binding and assembly. Mol Biol Cell. 1997;8:353–365. doi: 10.1091/mbc.8.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman DB, Rasmussen H, DiBella F, Guthrow CE., Jr Cyclic adenosine 3':5'-monophosphate-stimulated phosphorylation of isolated neurotubule subunits. Proc Natl Acad Sci U S A. 1970;67:652–659. doi: 10.1073/pnas.67.2.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin SS, Vale RD. Patronin regulates the microtubule network by protecting microtubule minus ends. Cell. 2010;143:263–274. doi: 10.1016/j.cell.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goold RG, Gordon-Weeks PR. NGF activates the phosphorylation of MAP1B by GSK3beta through the TrkA receptor and not the p75(NTR) receptor. J Neurochem. 2003;87:935–946. doi: 10.1046/j.1471-4159.2003.02062.x. [DOI] [PubMed] [Google Scholar]
- Goold RG, Owen R, Gordon-Weeks PR. Glycogen synthase kinase 3beta phosphorylation of microtubule-associated protein 1B regulates the stability of microtubules in growth cones. J Cell Sci. 1999;112(Pt 19):3373–3384. doi: 10.1242/jcs.112.19.3373. [DOI] [PubMed] [Google Scholar]
- Gordon-Weeks PR, Fischer I. MAP1B expression and microtubule stability in growing and regenerating axons. Microsc Res Tech. 2000;48:63–74. doi: 10.1002/(SICI)1097-0029(20000115)48:2<63::AID-JEMT2>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Gory-Faure S, Windscheid V, Brocard J, Montessuit S, Tsutsumi R, Denarier E, Fukata Y, Bosc C, Delaroche J, Collomb N, Fukata M, Martinou JC, Pernet-Gallay K, Andrieux A. Non-microtubular localizations of microtubule-associated protein 6 (MAP6) PLoS One. 2014;9:e114905. doi: 10.1371/journal.pone.0114905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumy LF, Katrukha EA, Grigoriev I, Jaarsma D, Kapitein LC, Akhmanova A, Hoogenraad CC. MAP2 Defines a Pre-axonal Filtering Zone to Regulate KIF1- versus KIF5-Dependent Cargo Transport in Sensory Neurons. Neuron. 2017;94:347–362. e347. doi: 10.1016/j.neuron.2017.03.046. [DOI] [PubMed] [Google Scholar]
- Hagiwara H, Yorifuji H, Sato-Yoshitake R, Hirokawa N. Competition between motor molecules (kinesin and cytoplasmic dynein) and fibrous microtubule-associated proteins in binding to microtubules. J Biol Chem. 1994;269:3581–3589. [PubMed] [Google Scholar]
- Halpain S, Dehmelt L. The MAP1 family of microtubule-associated proteins. Genome Biol. 2006;7:224. doi: 10.1186/gb-2006-7-6-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpain S, Greengard P. Activation of NMDA receptors induces rapid dephosphorylation of the cytoskeletal protein MAP2. Neuron. 1990;5:237–246. doi: 10.1016/0896-6273(90)90161-8. [DOI] [PubMed] [Google Scholar]
- Hanger DP, Seereeram A, Noble W. Mediators of tau phosphorylation in the pathogenesis of Alzheimer's disease. Expert Rev Neurother. 2009;9:1647–1666. doi: 10.1586/ern.09.104. [DOI] [PubMed] [Google Scholar]
- Hanley JG, Koulen P, Bedford F, Gordon-Weeks PR, Moss SJ. The protein MAP-1B links GABA(C) receptors to the cytoskeleton at retinal synapses. Nature. 1999;397:66–69. doi: 10.1038/16258. [DOI] [PubMed] [Google Scholar]
- Hannah MJ, Schmidt AA, Huttner WB. Synaptic vesicle biogenesis. Annu Rev Cell Dev Biol. 1999;15:733–798. doi: 10.1146/annurev.cellbio.15.1.733. [DOI] [PubMed] [Google Scholar]
- Harada A, Oguchi K, Okabe S, Kuno J, Terada S, Ohshima T, Sato-Yoshitake R, Takei Y, Noda T, Hirokawa N. Altered microtubule organization in small-calibre axons of mice lacking tau protein. Nature. 1994;369:488–491. doi: 10.1038/369488a0. [DOI] [PubMed] [Google Scholar]
- Harada A, Teng J, Takei Y, Oguchi K, Hirokawa N. MAP2 is required for dendrite elongation, PKA anchoring in dendrites, and proper PKA signal transduction. J Cell Biol. 2002;158:541–549. doi: 10.1083/jcb.200110134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms MB, Ori-McKenney KM, Scoto M, Tuck EP, Bell S, Ma D, Masi S, Allred P, Al-Lozi M, Reilly MM, Miller LJ, Jani-Acsadi A, Pestronk A, Shy ME, Muntoni F, Vallee RB, Baloh RH. Mutations in the tail domain of DYNC1H1 cause dominant spinal muscular atrophy. Neurology. 2012;78:1714–1720. doi: 10.1212/WNL.0b013e3182556c05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins BE, Krishnamurthy S, Castillo-Carranza DL, Sengupta U, Prough DS, Jackson GR, DeWitt DS, Kayed R. Rapid accumulation of endogenous tau oligomers in a rat model of traumatic brain injury: possible link between traumatic brain injury and sporadic tauopathies. J Biol Chem. 2013;288:17042–17050. doi: 10.1074/jbc.M113.472746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heins S, Song YH, Wille H, Mandelkow E, Mandelkow EM. Effect of MAP2, MAP2c, and tau on kinesin-dependent microtubule motility. J Cell Sci Suppl. 1991;14:121–124. doi: 10.1242/jcs.1991.supplement_14.24. [DOI] [PubMed] [Google Scholar]
- Hernandez MA, Avila J, Andreu JM. Physicochemical characterization of the heat-stable microtubule-associated protein MAP2. Eur J Biochem. 1986;154:41–48. doi: 10.1111/j.1432-1033.1986.tb09356.x. [DOI] [PubMed] [Google Scholar]
- Holmfeldt P, Brattsand G, Gullberg M. MAP4 counteracts microtubule catastrophe promotion but not tubulin-sequestering activity in intact cells. Curr Biol. 2002;12:1034–1039. doi: 10.1016/s0960-9822(02)00897-7. [DOI] [PubMed] [Google Scholar]
- Hoogenraad CC, Bradke F. Control of neuronal polarity and plasticity--a renaissance for microtubules? Trends Cell Biol. 2009;19:669–676. doi: 10.1016/j.tcb.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Hoshi M, Ohta K, Gotoh Y, Mori A, Murofushi H, Sakai H, Nishida E. Mitogen-activated-protein-kinase-catalyzed phosphorylation of microtubule-associated proteins, microtubule-associated protein 2 and microtubule-associated protein 4, induces an alteration in their function. Eur J Biochem. 1992;203:43–52. doi: 10.1111/j.1432-1033.1992.tb19825.x. [DOI] [PubMed] [Google Scholar]
- Howard J, Hyman AA. Preparation of marked microtubules for the assay of the polarity of microtubule-based motors by fluorescence microscopy. Methods Cell Biol. 1993;39:105–113. doi: 10.1016/s0091-679x(08)60164-8. [DOI] [PubMed] [Google Scholar]
- Howard J, Hyman AA. Dynamics and mechanics of the microtubule plus end. Nature. 2003;422:753–758. doi: 10.1038/nature01600. [DOI] [PubMed] [Google Scholar]
- Hu J, Chu Z, Han J, Zhang Q, Zhang D, Dang Y, Ren J, Chan HC, Zhang J, Huang Y. Phosphorylation-dependent mitochondrial translocation of MAP4 is an early step in hypoxia-induced apoptosis in cardiomyocytes. Cell Death Dis. 2014;5:e1424. doi: 10.1038/cddis.2014.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JY, Chu ZG, Han J, Dang YM, Yan H, Zhang Q, Liang GP, Huang YS. The p38/MAPK pathway regulates microtubule polymerization through phosphorylation of MAP4 and Op18 in hypoxic cells. Cell Mol Life Sci. 2010;67:321–333. doi: 10.1007/s00018-009-0187-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber G, Alaimo-Beuret D, Matus A. MAP3: characterization of a novel microtubule-associated protein. J Cell Biol. 1985;100:496–507. doi: 10.1083/jcb.100.2.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iba M, Guo JL, McBride JD, Zhang B, Trojanowski JQ, Lee VM. Synthetic tau fibrils mediate transmission of neurofibrillary tangles in a transgenic mouse model of Alzheimer's-like tauopathy. J Neurosci. 2013;33:1024–1037. doi: 10.1523/JNEUROSCI.2642-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda A, Zheng QY, Zuberi AR, Johnson KR, Naggert JK, Nishina PM. Microtubule-associated protein 1A is a modifier of tubby hearing (moth1) Nat Genet. 2002;30:401–405. doi: 10.1038/ng838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami S, Harada A, Hirokawa N. Muscle weakness, hyperactivity, and impairment in fear conditioning in tau-deficient mice. Neurosci Lett. 2000;279:129–132. doi: 10.1016/s0304-3940(99)00964-7. [DOI] [PubMed] [Google Scholar]
- Illenberger S, Drewes G, Trinczek B, Biernat J, Meyer HE, Olmsted JB, Mandelkow EM, Mandelkow E. Phosphorylation of microtubule-associated proteins MAP2 and MAP4 by the protein kinase p110mark. Phosphorylation sites and regulation of microtubule dynamics. J Biol Chem. 1996;271:10834–10843. doi: 10.1074/jbc.271.18.10834. [DOI] [PubMed] [Google Scholar]
- Irwin DJ, Lee VM, Trojanowski JQ. Parkinson's disease dementia: convergence of alpha-synuclein, tau and amyloid-beta pathologies. Nat Rev Neurosci. 2013;14:626–636. doi: 10.1038/nrn3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Tanaka S, Akiyama Y, Shimada S, Adikrisna R, Matsumura S, Aihara A, Mitsunori Y, Ban D, Ochiai T, Kudo A, Arii S, Yamaoka S, Tanabe M. Dominant Expression of DCLK1 in Human Pancreatic Cancer Stem Cells Accelerates Tumor Invasion and Metastasis. PLoS One. 2016;11:e0146564. doi: 10.1371/journal.pone.0146564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh TJ, Hisanaga S, Hosoi T, Kishimoto T, Hotani H. Phosphorylation states of microtubule-associated protein 2 (MAP2) determine the regulatory role of MAP2 in microtubule dynamics. Biochemistry. 1997;36:12574–12582. doi: 10.1021/bi962606z. [DOI] [PubMed] [Google Scholar]
- Jan YN, Jan LY. Branching out: mechanisms of dendritic arborization. Nat Rev Neurosci. 2010;11:316–328. doi: 10.1038/nrn2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janke C, Bulinski JC. Post-translational regulation of the microtubule cytoskeleton: mechanisms and functions. Nat Rev Mol Cell Biol. 2011;12:773–786. doi: 10.1038/nrm3227. [DOI] [PubMed] [Google Scholar]
- Jean DC, Baas PW, Black MM. A novel role for doublecortin and doublecortin-like kinase in regulating growth cone microtubules. Hum Mol Genet. 2012;21:5511–5527. doi: 10.1093/hmg/dds395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang K, Hua S, Mohan R, Grigoriev I, Yau KW, Liu Q, Katrukha EA, Altelaar AF, Heck AJ, Hoogenraad CC, Akhmanova A. Microtubule minus-end stabilization by polymerization-driven CAMSAP deposition. Dev Cell. 2014;28:295–309. doi: 10.1016/j.devcel.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Moser HW. Dendritic anomalies in disorders associated with mental retardation. Cereb Cortex. 2000;10:981–991. doi: 10.1093/cercor/10.10.981. [DOI] [PubMed] [Google Scholar]
- Kawauchi T, Chihama K, Nishimura YV, Nabeshima Y, Hoshino M. MAP1B phosphorylation is differentially regulated by Cdk5/p35, Cdk5/p25, and JNK. Biochem Biophys Res Commun. 2005;331:50–55. doi: 10.1016/j.bbrc.2005.03.132. [DOI] [PubMed] [Google Scholar]
- Keating TJ, Borisy GG. Centrosomal and non-centrosomal microtubules. Biol Cell. 1999;91:321–329. [PubMed] [Google Scholar]
- Kim MH, Cierpicki T, Derewenda U, Krowarsch D, Feng Y, Devedjiev Y, Dauter Z, Walsh CA, Otlewski J, Bushweller JH, Derewenda ZS. The DCX-domain tandems of doublecortin and doublecortin-like kinase. Nat Struct Biol. 2003;10:324–333. doi: 10.1038/nsb918. [DOI] [PubMed] [Google Scholar]
- Kirschner MW, Williams RC, Weingarten M, Gerhart JC. Microtubules from mammalian brain: some properties of their depolymerization products and a proposed mechanism of assembly and disassembly. Proc Natl Acad Sci U S A. 1974;71:1159–1163. doi: 10.1073/pnas.71.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocahan S, Dogan Z. Mechanisms of Alzheimer's Disease Pathogenesis and Prevention: The Brain, Neural Pathology, N-methyl-D-aspartate Receptors, Tau Protein and Other Risk Factors. Clin Psychopharmacol Neurosci. 2017;15:1–8. doi: 10.9758/cpn.2017.15.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi H, Fujioka H, Togashi K, Thompson J, Yates JR, 3rd, Gleeson JG, Emoto K. DCLK1 phosphorylates the microtubule-associated protein MAP7D1 to promote axon elongation in cortical neurons. Dev Neurobiol. 2017;77:493–510. doi: 10.1002/dneu.22428. [DOI] [PubMed] [Google Scholar]
- Kondo A, Shahpasand K, Mannix R, Qiu J, Moncaster J, Chen CH, Yao Y, Lin YM, Driver JA, Sun Y, Wei S, Luo ML, Albayram O, Huang P, Rotenberg A, Ryo A, Goldstein LE, Pascual-Leone A, McKee AC, Meehan W, Zhou XZ, Lu KP. Antibody against early driver of neurodegeneration cis P-tau blocks brain injury and tauopathy. Nature. 2015;523:431–436. doi: 10.1038/nature14658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik KS, Caceres A. Tau protein and the establishment of an axonal morphology. J Cell Sci Suppl. 1991;15:69–74. doi: 10.1242/jcs.1991.supplement_15.10. [DOI] [PubMed] [Google Scholar]
- Kutschera W, Zauner W, Wiche G, Propst F. The mouse and rat MAP1B genes: genomic organization and alternative transcription. Genomics. 1998;49:430–436. doi: 10.1006/geno.1998.5294. [DOI] [PubMed] [Google Scholar]
- Kuznetsov SA, Rodionov VI, Gelfand VI, Rosenblat VA. Purification of high-Mr microtubule proteins MAP1 and MAP2. FEBS Lett. 1981;135:237–240. doi: 10.1016/0014-5793(81)80790-9. [DOI] [PubMed] [Google Scholar]
- Laurent CE, Delfino FJ, Cheng HY, Smithgall TE. The human c-Fes tyrosine kinase binds tubulin and microtubules through separate domains and promotes microtubule assembly. Mol Cell Biol. 2004;24:9351–9358. doi: 10.1128/MCB.24.21.9351-9358.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeau G, Miller LC, Tartas M, McAdam R, Laplante I, Badeaux F, DesGroseillers L, Sossin WS, Lacaille JC. Staufen 2 regulates mGluR long-term depression and Map1b mRNA distribution in hippocampal neurons. Learn Mem. 2011;18:314–326. doi: 10.1101/lm.2100611. [DOI] [PubMed] [Google Scholar]
- Lefevre J, Savarin P, Gans P, Hamon L, Clement MJ, David MO, Bosc C, Andrieux A, Curmi PA. Structural basis for the association of MAP6 protein with microtubules and its regulation by calmodulin. J Biol Chem. 2013;288:24910–24922. doi: 10.1074/jbc.M113.457267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei P, Ayton S, Finkelstein DI, Spoerri L, Ciccotosto GD, Wright DK, Wong BX, Adlard PA, Cherny RA, Lam LQ, Roberts BR, Volitakis I, Egan GF, McLean CA, Cappai R, Duce JA, Bush AI. Tau deficiency induces parkinsonism with dementia by impairing APP-mediated iron export. Nat Med. 2012;18:291–295. doi: 10.1038/nm.2613. [DOI] [PubMed] [Google Scholar]
- Lewczuk P, Esselmann H, Bibl M, Beck G, Maler JM, Otto M, Kornhuber J, Wiltfang J. Tau protein phosphorylated at threonine 181 in CSF as a neurochemical biomarker in Alzheimer's disease: original data and review of the literature. J Mol Neurosci. 2004;23:115–122. doi: 10.1385/JMN:23:1-2:115. [DOI] [PubMed] [Google Scholar]
- Ley SC, Verbi W, Pappin DJ, Druker B, Davies AA, Crumpton MJ. Tyrosine phosphorylation of alpha tubulin in human T lymphocytes. Eur J Immunol. 1994;24:99–106. doi: 10.1002/eji.1830240116. [DOI] [PubMed] [Google Scholar]
- Li L, Hu J, He T, Zhang Q, Yang X, Lan X, Zhang D, Mei H, Chen B, Huang Y. P38/MAPK contributes to endothelial barrier dysfunction via MAP4 phosphorylation-dependent microtubule disassembly in inflammation-induced acute lung injury. Sci Rep. 2015;5:8895. doi: 10.1038/srep08895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipka J, Kapitein LC, Jaworski J, Hoogenraad CC. Microtubule-binding protein doublecortin-like kinase 1 (DCLK1) guides kinesin-3-mediated cargo transport to dendrites. EMBO J. 2016;35:302–318. doi: 10.15252/embj.201592929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JS, Schubert CR, Fu X, Fourniol FJ, Jaiswal JK, Houdusse A, Stultz CM, Moores CA, Walsh CA. Molecular basis for specific regulation of neuronal kinesin-3 motors by doublecortin family proteins. Mol Cell. 2012a;47:707–721. doi: 10.1016/j.molcel.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Drouet V, Wu JW, Witter MP, Small SA, Clelland C, Duff K. Trans-synaptic spread of tau pathology in vivo. PLoS One. 2012b;7:e31302. doi: 10.1371/journal.pone.0031302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, McKeehan WL, Wang F, Xie R. MAP1S enhances autophagy to suppress tumorigenesis. Autophagy. 2012c;8:278–280. doi: 10.4161/auto.8.2.18939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Lee JW, Ackerman SL. Mutations in the microtubule-associated protein 1A (Map1a) gene cause Purkinje cell degeneration. J Neurosci. 2015;35:4587–4598. doi: 10.1523/JNEUROSCI.2757-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez LA, Sheetz MP. Steric inhibition of cytoplasmic dynein and kinesin motility by MAP2. Cell Motil Cytoskeleton. 1993;24:1–16. doi: 10.1002/cm.970240102. [DOI] [PubMed] [Google Scholar]
- Luduena RF, Zimmermann HP, Little M. Identification of the phosphorylated beta-tubulin isotype in differentiated neuroblastoma cells. FEBS Lett. 1988;230:142–146. doi: 10.1016/0014-5793(88)80658-6. [DOI] [PubMed] [Google Scholar]
- Ma X, Sayeski PP. Identification of tubulin as a substrate of Jak2 tyrosine kinase and its role in Jak2-dependent signaling. Biochemistry. 2007;46:7153–7162. doi: 10.1021/bi700101n. [DOI] [PubMed] [Google Scholar]
- Mandelkow EM, Mandelkow E. Tau protein and Alzheimer's disease. Neurobiol Aging. 1994;15(Suppl 2):S85–86. doi: 10.1016/0197-4580(94)90178-3. [DOI] [PubMed] [Google Scholar]
- Mandelkow EM, Mandelkow E. Tau in Alzheimer's disease. Trends Cell Biol. 1998;8:425–427. doi: 10.1016/s0962-8924(98)01368-3. [DOI] [PubMed] [Google Scholar]
- Mandelkow EM, Mandelkow E. Biochemistry and cell biology of tau protein in neurofibrillary degeneration. Cold Spring Harb Perspect Med. 2012;2:a006247. doi: 10.1101/cshperspect.a006247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelkow EM, Thies E, Trinczek B, Biernat J, Mandelkow E. MARK/PAR1 kinase is a regulator of microtubule-dependent transport in axons. J Cell Biol. 2004;167:99–110. doi: 10.1083/jcb.200401085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell JW, Banker GA. A spatial gradient of tau protein phosphorylation in nascent axons. J Neurosci. 1996;16:5727–5740. doi: 10.1523/JNEUROSCI.16-18-05727.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson D, Kreis TE. Identification and molecular characterization of E-MAP-115, a novel microtubule-associated protein predominantly expressed in epithelial cells. J Cell Biol. 1993;123:357–371. doi: 10.1083/jcb.123.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson D, Kreis TE. Binding of E-MAP-115 to microtubules is regulated by cell cycle-dependent phosphorylation. J Cell Biol. 1995;131:1015–1024. doi: 10.1083/jcb.131.4.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima K, Tokuraku K, Hasan MR, Kotani S. Microtubule-associated protein 4 binds to actin filaments and modulates their properties. J Biochem. 2012;151:99–108. doi: 10.1093/jb/mvr119. [DOI] [PubMed] [Google Scholar]
- Matten WT, Aubry M, West J, Maness PF. Tubulin is phosphorylated at tyrosine by pp60c-src in nerve growth cone membranes. J Cell Biol. 1990;111:1959–1970. doi: 10.1083/jcb.111.5.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally KP, Buster D, McNally FJ. Katanin-mediated microtubule severing can be regulated by multiple mechanisms. Cell Motil Cytoskeleton. 2002;53:337–349. doi: 10.1002/cm.10080. [DOI] [PubMed] [Google Scholar]
- Meixner A, Haverkamp S, Wassle H, Fuhrer S, Thalhammer J, Kropf N, Bittner RE, Lassmann H, Wiche G, Propst F. MAP1B is required for axon guidance and Is involved in the development of the central and peripheral nervous system. J Cell Biol. 2000;151:1169–1178. doi: 10.1083/jcb.151.6.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merenlender-Wagner A, Pikman R, Giladi E, Andrieux A, Gozes I. NAP (davunetide) enhances cognitive behavior in the STOP heterozygous mouse--a microtubule-deficient model of schizophrenia. Peptides. 2010;31:1368–1373. doi: 10.1016/j.peptides.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Metzger T, Gache V, Xu M, Cadot B, Folker ES, Richardson BE, Gomes ER, Baylies MK. MAP and kinesin-dependent nuclear positioning is required for skeletal muscle function. Nature. 2012;484:120–124. doi: 10.1038/nature10914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison T, Kirschner M. Dynamic instability of microtubule growth. Nature. 1984;312:237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- Mizuguchi M, Qin J, Yamada M, Ikeda K, Takashima S. High expression of doublecortin and KIAA0369 protein in fetal brain suggests their specific role in neuronal migration. Am J Pathol. 1999;155:1713–1721. doi: 10.1016/S0002-9440(10)65486-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi M, Takashima S, Ikeda K, Kato M, Hori A. Loss of doublecortin in heterotopic gray matter of a fetus with subcortical laminar heterotopia. Neurology. 2002;59:143–144. doi: 10.1212/wnl.59.1.143. [DOI] [PubMed] [Google Scholar]
- Mogessie B, Roth D, Rahil Z, Straube A. A novel isoform of MAP4 organises the paraxial microtubule array required for muscle cell differentiation. Elife. 2015;4:e05697. doi: 10.7554/eLife.05697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroy BY, Sawyer DL, Ackermann BE, Borden MM, Tan TC, Ori-McKenney KM. Competition between microtubule-associated proteins directs motor transport. 2017 doi: 10.1038/s41467-018-03909-2. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moores CA, Perderiset M, Francis F, Chelly J, Houdusse A, Milligan RA. Mechanism of microtubule stabilization by doublecortin. Mol Cell. 2004;14:833–839. doi: 10.1016/j.molcel.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Moores CA, Perderiset M, Kappeler C, Kain S, Drummond D, Perkins SJ, Chelly J, Cross R, Houdusse A, Francis F. Distinct roles of doublecortin modulating the microtubule cytoskeleton. EMBO J. 2006;25:4448–4457. doi: 10.1038/sj.emboj.7601335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori A, Aizawa H, Saido TC, Kawasaki H, Mizuno K, Murofushi H, Suzuki K, Sakai H. Site-specific phosphorylation by protein kinase C inhibits assembly-promoting activity of microtubule-associated protein 4. Biochemistry. 1991;30:9341–9346. doi: 10.1021/bi00102a029. [DOI] [PubMed] [Google Scholar]
- Murakami N, Bolton DC, Kida E, Xie W, Hwang YW. Phosphorylation by Dyrk1A of clathrin coated vesicle-associated proteins: identification of the substrate proteins and the effects of phosphorylation. PLoS One. 2012;7:e34845. doi: 10.1371/journal.pone.0034845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DB, Vallee RB, Borisy GG. Identity and polymerization-stimulatory activity of the nontubulin proteins associated with microtubules. Biochemistry. 1977;16:2598–2605. doi: 10.1021/bi00631a004. [DOI] [PubMed] [Google Scholar]
- Murray AW, Froscio M. Cyclic adenosine 3':5'-monophosphate and microtubule function: specific interaction of the phosphorylated protein subunits with a soluble brain component. Biochem Biophys Res Commun. 1971;44:1089–1095. doi: 10.1016/s0006-291x(71)80197-3. [DOI] [PubMed] [Google Scholar]
- Myers KA, Baas PW. Kinesin-5 regulates the growth of the axon by acting as a brake on its microtubule array. J Cell Biol. 2007;178:1081–1091. doi: 10.1083/jcb.200702074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HL, Chari S, Gruber D, Lue CM, Chapin SJ, Bulinski JC. Overexpression of full-or partial-length MAP4 stabilizes microtubules and alters cell growth. J Cell Sci. 1997;110(Pt 2):281–294. doi: 10.1242/jcs.110.2.281. [DOI] [PubMed] [Google Scholar]
- Nguyen HL, Gruber D, McGraw T, Sheetz MP, Bulinski JC. Stabilization and functional modulation of microtubules by microtubule-associated protein 4. Biol Bull. 1998;194:354–357. doi: 10.2307/1543111. [DOI] [PubMed] [Google Scholar]
- Noiges R, Eichinger R, Kutschera W, Fischer I, Nemeth Z, Wiche G, Propst F. Microtubule-associated protein 1A (MAP1A) and MAP1B: light chains determine distinct functional properties. J Neurosci. 2002;22:2106–2114. doi: 10.1523/JNEUROSCI.22-06-02106.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez J. Immature and mature variants of MAP2 and tau proteins and neuronal plasticity. Trends Neurosci. 1988;11:477–479. doi: 10.1016/0166-2236(88)90004-5. [DOI] [PubMed] [Google Scholar]
- Obar RA, Dingus J, Bayley H, Vallee RB. The RII subunit of cAMP-dependent protein kinase binds to a common amino-terminal domain in microtubule-associated proteins 2A, 2B, and 2C. Neuron. 1989;3:639–645. doi: 10.1016/0896-6273(89)90274-2. [DOI] [PubMed] [Google Scholar]
- Olmsted JB, Borisy GG. Ionic and nucleotide requirements for microtubule polymerization in vitro. Biochemistry. 1975;14:2996–3005. doi: 10.1021/bi00684a032. [DOI] [PubMed] [Google Scholar]
- Ookata K, Hisanaga S, Bulinski JC, Murofushi H, Aizawa H, Itoh TJ, Hotani H, Okumura E, Tachibana K, Kishimoto T. Cyclin B interaction with microtubule-associated protein 4 (MAP4) targets p34cdc2 kinase to microtubules and is a potential regulator of M-phase microtubule dynamics. J Cell Biol. 1995;128:849–862. doi: 10.1083/jcb.128.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ookata K, Hisanaga S, Sugita M, Okuyama A, Murofushi H, Kitazawa H, Chari S, Bulinski JC, Kishimoto T. MAP4 is the in vivo substrate for CDC2 kinase in HeLa cells: identification of an M-phase specific and a cell cycle-independent phosphorylation site in MAP4. Biochemistry. 1997;36:15873–15883. doi: 10.1021/bi971251w. [DOI] [PubMed] [Google Scholar]
- Orban-Nemeth Z, Simader H, Badurek S, Trancikova A, Propst F. Microtubule-associated protein 1S, a short and ubiquitously expressed member of the microtubule-associated protein 1 family. J Biol Chem. 2005;280:2257–2265. doi: 10.1074/jbc.M408984200. [DOI] [PubMed] [Google Scholar]
- Ori-McKenney KM, McKenney RJ, Huang HH, Li T, Meltzer S, Jan LY, Vale RD, Wiita AP, Jan YN. Phosphorylation of beta-Tubulin by the Down Syndrome Kinase, Minibrain/DYRK1a, Regulates Microtubule Dynamics and Dendrite Morphogenesis. Neuron. 2016;90:551–563. doi: 10.1016/j.neuron.2016.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozer RS, Halpain S. Phosphorylation-dependent localization of microtubule-associated protein MAP2c to the actin cytoskeleton. Mol Biol Cell. 2000;11:3573–3587. doi: 10.1091/mbc.11.10.3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papasozomenos SC, Binder LI, Bender PK, Payne MR. Microtubule-associated protein 2 within axons of spinal motor neurons: associations with microtubules and neurofilaments in normal and beta,beta'-iminodipropionitrile-treated axons. J Cell Biol. 1985;100:74–85. doi: 10.1083/jcb.100.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel O, Dai W, Mentzel M, Griffin MD, Serindoux J, Gay Y, Fischer S, Sterle S, Kropp A, Burns CJ, Ernst M, Buchert M, Lucet IS. Biochemical and Structural Insights into Doublecortin-like Kinase Domain 1. Structure. 2016;24:1550–1561. doi: 10.1016/j.str.2016.07.008. [DOI] [PubMed] [Google Scholar]
- Peck A, Sargin ME, LaPointe NE, Rose K, Manjunath BS, Feinstein SC, Wilson L. Tau isoform-specific modulation of kinesin-driven microtubule gliding rates and trajectories as determined with tau-stabilized microtubules. Cytoskeleton (Hoboken) 2011;68:44–55. doi: 10.1002/cm.20494. [DOI] [PubMed] [Google Scholar]
- Pedrotti B, Colombo R, Islam K. Interactions of microtubule-associated protein MAP2 with unpolymerized and polymerized tubulin and actin using a 96-well microtiter plate solid-phase immunoassay. Biochemistry. 1994;33:8798–8806. doi: 10.1021/bi00195a023. [DOI] [PubMed] [Google Scholar]
- Peters JD, Furlong MT, Asai DJ, Harrison ML, Geahlen RL. Syk, activated by cross-linking the B-cell antigen receptor, localizes to the cytosol where it interacts with and phosphorylates alpha-tubulin on tyrosine. J Biol Chem. 1996;271:4755–4762. doi: 10.1074/jbc.271.9.4755. [DOI] [PubMed] [Google Scholar]
- Philpot BD, Lim JH, Halpain S, Brunjes PC. Experience-dependent modifications in MAP2 phosphorylation in rat olfactory bulb. J Neurosci. 1997;17:9596–9604. doi: 10.1523/JNEUROSCI.17-24-09596.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilz DT, Matsumoto N, Minnerath S, Mills P, Gleeson JG, Allen KM, Walsh CA, Barkovich AJ, Dobyns WB, Ledbetter DH, Ross ME. LIS1 and XLIS (DCX) mutations cause most classical lissencephaly, but different patterns of malformation. Hum Mol Genet. 1998;7:2029–2037. doi: 10.1093/hmg/7.13.2029. [DOI] [PubMed] [Google Scholar]
- Qiang L, Yu W, Andreadis A, Luo M, Baas PW. Tau protects microtubules in the axon from severing by katanin. J Neurosci. 2006;26:3120–3129. doi: 10.1523/JNEUROSCI.5392-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan EM, Halpain S. Postsynaptic mechanisms for bidirectional control of MAP2 phosphorylation by glutamate receptors. Neuron. 1996;16:357–368. doi: 10.1016/s0896-6273(00)80053-7. [DOI] [PubMed] [Google Scholar]
- Ramirez-Rios S, Denarier E, Prezel E, Vinit A, Stoppin-Mellet V, Devred F, Barbier P, Peyrot V, Sayas CL, Avila J, Peris L, Andrieux A, Serre L, Fourest-Lieuvin A, Arnal I. Tau antagonizes end-binding protein tracking at microtubule ends through a phosphorylation-dependent mechanism. Mol Biol Cell. 2016;27:2924–2934. doi: 10.1091/mbc.E16-01-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner O, Coquelle FM, Peter B, Levy T, Kaplan A, Sapir T, Orr I, Barkai N, Eichele G, Bergmann S. The evolving doublecortin (DCX) superfamily. BMC Genomics. 2006;7:188. doi: 10.1186/1471-2164-7-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riederer BM. Microtubule-associated protein 1B, a growth-associated and phosphorylated scaffold protein. Brain Res Bull. 2007;71:541–558. doi: 10.1016/j.brainresbull.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Riederer BM, Draberova E, Viklicky V, Draber P. Changes of MAP2 phosphorylation during brain development. J Histochem Cytochem. 1995;43:1269–1284. doi: 10.1177/43.12.8537643. [DOI] [PubMed] [Google Scholar]
- Riederer BM, Moya F, Calvert R. Phosphorylated MAP1b, alias MAP5 and MAP1x, is involved in axonal growth and neuronal mitosis. Neuroreport. 1993;4:771–774. doi: 10.1097/00001756-199306000-00044. [DOI] [PubMed] [Google Scholar]
- Rioux L, Nissanov J, Lauber K, Bilker WB, Arnold SE. Distribution of microtubule-associated protein MAP2-immunoreactive interstitial neurons in the parahippocampal white matter in subjects with schizophrenia. Am J Psychiatry. 2003;160:149–155. doi: 10.1176/appi.ajp.160.1.149. [DOI] [PubMed] [Google Scholar]
- Roger B, Al-Bassam J, Dehmelt L, Milligan RA, Halpain S. MAP2c, but not tau, binds and bundles F-actin via its microtubule binding domain. Curr Biol. 2004;14:363–371. doi: 10.1016/j.cub.2004.01.058. [DOI] [PubMed] [Google Scholar]
- Roll-Mecak A, McNally FJ. Microtubule-severing enzymes. Curr Opin Cell Biol. 2010;22:96–103. doi: 10.1016/j.ceb.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouquier S, Pillaire MJ, Cazaux C, Giorgi D. Expression of the microtubule-associated protein MAP9/ASAP and its partners AURKA and PLK1 in colorectal and breast cancers. Dis Markers. 2014;2014:798170. doi: 10.1155/2014/798170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino HM, Dammerman M, Shafit-Zagardo B, Erlichman J. Localization and characterization of the binding site for the regulatory subunit of type II cAMP-dependent protein kinase on MAP2. Neuron. 1989;3:631–638. doi: 10.1016/0896-6273(89)90273-0. [DOI] [PubMed] [Google Scholar]
- Saaltink DJ, Havik B, Verissimo CS, Lucassen PJ, Vreugdenhil E. Doublecortin and doublecortin-like are expressed in overlapping and non-overlapping neuronal cell population: implications for neurogenesis. J Comp Neurol. 2012;520:2805–2823. doi: 10.1002/cne.23144. [DOI] [PubMed] [Google Scholar]
- Saffin JM, Venoux M, Prigent C, Espeut J, Poulat F, Giorgi D, Abrieu A, Rouquier S. ASAP, a human microtubule-associated protein required for bipolar spindle assembly and cytokinesis. Proc Natl Acad Sci U S A. 2005;102:11302–11307. doi: 10.1073/pnas.0500964102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samora CP, Mogessie B, Conway L, Ross JL, Straube A, McAinsh AD. MAP4 and CLASP1 operate as a safety mechanism to maintain a stable spindle position in mitosis. Nat Cell Biol. 2011;13:1040–1050. doi: 10.1038/ncb2297. [DOI] [PubMed] [Google Scholar]
- Samsonov A, Yu JZ, Rasenick M, Popov SV. Tau interaction with microtubules in vivo. J Cell Sci. 2004;117:6129–6141. doi: 10.1242/jcs.01531. [DOI] [PubMed] [Google Scholar]
- Sapir T, Horesh D, Caspi M, Atlas R, Burgess HA, Wolf SG, Francis F, Chelly J, Elbaum M, Pietrokovski S, Reiner O. Doublecortin mutations cluster in evolutionarily conserved functional domains. Hum Mol Genet. 2000;9:703–712. doi: 10.1093/hmg/9.5.703. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Popov VL, O'Connell MR, Stevenson HL, Lee BS, Obeid RA, Luthra GK, Singh P. A novel antibody against cancer stem cell biomarker, DCLK1-S, is potentially useful for assessing colon cancer risk after screening colonoscopy. Lab Invest. 2017 doi: 10.1038/labinvest.2017.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato-Yoshitake R, Shiomura Y, Miyasaka H, Hirokawa N. Microtubule-associated protein 1B: molecular structure, localization, and phosphorylation-dependent expression in developing neurons. Neuron. 1989;3:229–238. doi: 10.1016/0896-6273(89)90036-6. [DOI] [PubMed] [Google Scholar]
- Sattilaro RF. Interaction of microtubule-associated protein 2 with actin filaments. Biochemistry. 1986;25:2003–2009. doi: 10.1021/bi00356a025. [DOI] [PubMed] [Google Scholar]
- Sayas CL, Tortosa E, Bollati F, Ramirez-Rios S, Arnal I, Avila J. Tau regulates the localization and function of End-binding proteins 1 and 3 in developing neuronal cells. J Neurochem. 2015;133:653–667. doi: 10.1111/jnc.13091. [DOI] [PubMed] [Google Scholar]
- Scales TM, Lin S, Kraus M, Goold RG, Gordon-Weeks PR. Nonprimed and DYRK1A–primed GSK3 beta-phosphorylation sites on MAP1B regulate microtubule dynamics in growing axons. J Cell Sci. 2009;122:2424–2435. doi: 10.1242/jcs.040162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaar BT, Kinoshita K, McConnell SK. Doublecortin microtubule affinity is regulated by a balance of kinase and phosphatase activity at the leading edge of migrating neurons. Neuron. 2004;41:203–213. doi: 10.1016/s0896-6273(03)00843-2. [DOI] [PubMed] [Google Scholar]
- Seitz A, Kojima H, Oiwa K, Mandelkow EM, Song YH, Mandelkow E. Single-molecule investigation of the interference between kinesin, tau and MAP2c. EMBO J. 2002;21:4896–4905. doi: 10.1093/emboj/cdf503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selden SC, Pollard TD. Phosphorylation of microtubule-associated proteins regulates their interaction with actin filaments. J Biol Chem. 1983;258:7064–7071. [PubMed] [Google Scholar]
- Semenova I, Ikeda K, Resaul K, Kraikivski P, Aguiar M, Gygi S, Zaliapin I, Cowan A, Rodionov V. Regulation of microtubule-based transport by MAP4. Mol Biol Cell. 2014;25:3119–3132. doi: 10.1091/mbc.E14-01-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano L, Diaz-Nido J, Wandosell F, Avila J. Tubulin phosphorylation by casein kinase II is similar to that found in vivo. J Cell Biol. 1987;105:1731–1739. doi: 10.1083/jcb.105.4.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah K, Lahiri DK. A Tale of the Good and Bad: Remodeling of the Microtubule Network in the Brain by Cdk5. Mol Neurobiol. 2017;54:2255–2268. doi: 10.1007/s12035-016-9792-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton MA, Newman JT, Gu H, Sampson AR, Fish KN, MacDonald ML, Moyer CE, DiBitetto JV, Dorph-Petersen KA, Penzes P, Lewis DA, Sweet RA. Loss of Microtubule-Associated Protein 2 Immunoreactivity Linked to Dendritic Spine Loss in Schizophrenia. Biol Psychiatry. 2015;78:374–385. doi: 10.1016/j.biopsych.2014.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin E, Kashiwagi Y, Kuriu T, Iwasaki H, Tanaka T, Koizumi H, Gleeson JG, Okabe S. Doublecortin-like kinase enhances dendritic remodelling and negatively regulates synapse maturation. Nat Commun. 2013;4:1440. doi: 10.1038/ncomms2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu T, Tseng HC, Sapir T, Stern P, Zhou Y, Sanada K, Fischer A, Coquelle FM, Reiner O, Tsai LH. Doublecortin-like kinase controls neurogenesis by regulating mitotic spindles and M phase progression. Neuron. 2006;49:25–39. doi: 10.1016/j.neuron.2005.10.039. [DOI] [PubMed] [Google Scholar]
- Smith C, Graham DI, Murray LS, Nicoll JA. Tau immunohistochemistry in acute brain injury. Neuropathol Appl Neurobiol. 2003;29:496–502. doi: 10.1046/j.1365-2990.2003.00488.x. [DOI] [PubMed] [Google Scholar]
- Somenarain L, Jones LB. A comparative study of MAP2 immunostaining in areas 9 and 17 in schizophrenia and Huntington chorea. J Psychiatr Res. 2010;44:694–699. doi: 10.1016/j.jpsychires.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Srsen V, Kitazawa H, Sugita M, Murofushi H, Bulinski JC, Kishimoto T, Hisanaga S. Serum-dependent phosphorylation of human MAP4 at Ser696 in cultured mammalian cells. Cell Struct Funct. 1999;24:321–327. doi: 10.1247/csf.24.321. [DOI] [PubMed] [Google Scholar]
- Stern JL, Lessard DV, Hoeprich GJ, Morfini GA, Berger CL. Phosphoregulation of Tau modulates inhibition of kinesin-1 motility. Mol Biol Cell. 2017;28:1079–1087. doi: 10.1091/mbc.E16-10-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiess M, Maghelli N, Kapitein LC, Gomis-Ruth S, Wilsch-Brauninger M, Hoogenraad CC, Tolic-Norrelykke IM, Bradke F. Axon extension occurs independently of centrosomal microtubule nucleation. Science. 2010;327:704–707. doi: 10.1126/science.1182179. [DOI] [PubMed] [Google Scholar]
- Sung HH, Telley IA, Papadaki P, Ephrussi A, Surrey T, Rorth P. Drosophila ensconsin promotes productive recruitment of Kinesin-1 to microtubules. Dev Cell. 2008;15:866–876. doi: 10.1016/j.devcel.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Szebenyi G, Bollati F, Bisbal M, Sheridan S, Faas L, Wray R, Haferkamp S, Nguyen S, Caceres A, Brady ST. Activity-driven dendritic remodeling requires microtubule-associated protein 1A. Curr Biol. 2005;15:1820–1826. doi: 10.1016/j.cub.2005.08.069. [DOI] [PubMed] [Google Scholar]
- Takei Y, Kikkawa YS, Atapour N, Hensch TK, Hirokawa N. Defects in Synaptic Plasticity, Reduced NMDA-Receptor Transport, and Instability of Postsynaptic Density Proteins in Mice Lacking Microtubule-Associated Protein 1A. J Neurosci. 2015;35:15539–15554. doi: 10.1523/JNEUROSCI.2671-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei Y, Teng J, Harada A, Hirokawa N. Defects in axonal elongation and neuronal migration in mice with disrupted tau and map1b genes. J Cell Biol. 2000;150:989–1000. doi: 10.1083/jcb.150.5.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Koizumi H, Gleeson JG. The doublecortin and doublecortin-like kinase 1 genes cooperate in murine hippocampal development. Cereb Cortex. 2006;16(Suppl 1):i69–73. doi: 10.1093/cercor/bhk005. [DOI] [PubMed] [Google Scholar]
- Tang L, Lu Y, Zheng W, Li Y. Overexpression of MAP-2 via formation of microtubules plays an important role in the sprouting of mossy fibers in epileptic rats. J Mol Neurosci. 2014;53:103–108. doi: 10.1007/s12031-013-0204-4. [DOI] [PubMed] [Google Scholar]
- Taylor KR, Holzer AK, Bazan JF, Walsh CA, Gleeson JG. Patient mutations in doublecortin define a repeated tubulin-binding domain. J Biol Chem. 2000;275:34442–34450. doi: 10.1074/jbc.M007078200. [DOI] [PubMed] [Google Scholar]
- Tegha-Dunghu J, Bausch E, Neumann B, Wuensche A, Walter T, Ellenberg J, Gruss OJ. MAP1S controls microtubule stability throughout the cell cycle in human cells. J Cell Sci. 2014;127:5007–5013. doi: 10.1242/jcs.136457. [DOI] [PubMed] [Google Scholar]
- Terwel D, Dewachter I, Van Leuven F. Axonal transport, tau protein, and neurodegeneration in Alzheimer's disease. Neuromolecular Med. 2002;2:151–165. doi: 10.1385/NMM:2:2:151. [DOI] [PubMed] [Google Scholar]
- Theurkauf WE, Vallee RB. Molecular characterization of the cAMP-dependent protein kinase bound to microtubule-associated protein 2. J Biol Chem. 1982;257:3284–3290. [PubMed] [Google Scholar]
- Tokuraku K, Okuyama S, Matsushima K, Ikezu T, Kotani S. Distinct neuronal localization of microtubule-associated protein 4 in the mammalian brain. Neurosci Lett. 2010;484:143–147. doi: 10.1016/j.neulet.2010.08.038. [DOI] [PubMed] [Google Scholar]
- Tolnay M, Probst A. REVIEW: tau protein pathology in Alzheimer's disease and related disorders. Neuropathol Appl Neurobiol. 1999;25:171–187. doi: 10.1046/j.1365-2990.1999.00182.x. [DOI] [PubMed] [Google Scholar]
- Tortosa E, Montenegro-Venegas C, Benoist M, Hartel S, Gonzalez-Billault C, Esteban JA, Avila J. Microtubule-associated protein 1B (MAP1B) is required for dendritic spine development and synaptic maturation. J Biol Chem. 2011;286:40638–40648. doi: 10.1074/jbc.M111.271320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran HT, LaFerla FM, Holtzman DM, Brody DL. Controlled cortical impact traumatic brain injury in 3xTg-AD mice causes acute intra-axonal amyloid-beta accumulation and independently accelerates the development of tau abnormalities. J Neurosci. 2011a;31:9513–9525. doi: 10.1523/JNEUROSCI.0858-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran HT, Sanchez L, Esparza TJ, Brody DL. Distinct temporal and anatomical distributions of amyloid-beta and tau abnormalities following controlled cortical impact in transgenic mice. PLoS One. 2011b;6:e25475. doi: 10.1371/journal.pone.0025475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi N, Marsh P, Goold RG, Wood-Kaczmar A, Gordon-Weeks PR. Glycogen synthase kinase-3beta phosphorylation of MAP1B at Ser1260 and Thr1265 is spatially restricted to growing axons. J Cell Sci. 2005;118:993–1005. doi: 10.1242/jcs.01697. [DOI] [PubMed] [Google Scholar]
- Trojanowski JQ, Lee VM. Phosphorylation of paired helical filament tau in Alzheimer's disease neurofibrillary lesions: focusing on phosphatases. FASEB J. 1995;9:1570–1576. doi: 10.1096/fasebj.9.15.8529836. [DOI] [PubMed] [Google Scholar]
- Tsai MH, Kuo PW, Myers CT, Li SW, Lin WC, Fu TY, Chang HY, Mefford HC, Chang YC, Tsai JW. A novel DCX missense mutation in a family with X-linked lissencephaly and subcortical band heterotopia syndrome inherited from a low-level somatic mosaic mother: Genetic and functional studies. Eur J Paediatr Neurol. 2016;20:788–794. doi: 10.1016/j.ejpn.2016.05.010. [DOI] [PubMed] [Google Scholar]
- Tymanskyj SR, Lin S, Gordon-Weeks PR. Evolution of the spatial distribution of MAP1B phosphorylation sites in vertebrate neurons. J Anat. 2010;216:692–704. doi: 10.1111/j.1469-7580.2010.01228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tymanskyj SR, Yang B, Falnikar A, Lepore AC, Ma L. MAP7 Regulates Axon Collateral Branch Development in Dorsal Root Ganglion Neurons. J Neurosci. 2017;37:1648–1661. doi: 10.1523/JNEUROSCI.3260-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulloa L, Dombradi V, Diaz-Nido J, Szucs K, Gergely P, Friedrich P, Avila J. Dephosphorylation of distinct sites on microtubule-associated protein MAP1B by protein phosphatases 1, 2A and 2B. FEBS Lett. 1993;330:85–89. doi: 10.1016/0014-5793(93)80925-k. [DOI] [PubMed] [Google Scholar]
- Ulloa L, Montejo de Garcini E, Gomez-Ramos P, Moran MA, Avila J. Microtubule-associated protein MAP1B showing a fetal phosphorylation pattern is present in sites of neurofibrillary degeneration in brains of Alzheimer's disease patients. Brain Res Mol Brain Res. 1994;26:113–122. doi: 10.1016/0169-328x(94)90081-7. [DOI] [PubMed] [Google Scholar]
- Vale RD. The molecular motor toolbox for intracellular transport. Cell. 2003;112:467–480. doi: 10.1016/s0092-8674(03)00111-9. [DOI] [PubMed] [Google Scholar]
- Vallee RB. A taxol-dependent procedure for the isolation of microtubules and microtubule-associated proteins (MAPs) J Cell Biol. 1982;92:435–442. doi: 10.1083/jcb.92.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee RB, Borisy GG. The non-tubulin component of microtubule protein oligomers. Effect on self-association and hydrodynamic properties. J Biol Chem. 1978;253:2834–2845. [PubMed] [Google Scholar]
- Vallee RB, McKenney RJ, Ori-McKenney KM. Multiple modes of cytoplasmic dynein regulation. Nat Cell Biol. 2012;14:224–230. doi: 10.1038/ncb2420. [DOI] [PubMed] [Google Scholar]
- Vandecandelaere A, Pedrotti B, Utton MA, Calvert RA, Bayley PM. Differences in the regulation of microtubule dynamics by microtubule-associated proteins MAP1B and MAP2. Cell Motil Cytoskeleton. 1996;35:134–146. doi: 10.1002/(SICI)1097-0169(1996)35:2<134::AID-CM6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Venoux M, Basbous J, Berthenet C, Prigent C, Fernandez A, Lamb NJ, Rouquier S. ASAP is a novel substrate of the oncogenic mitotic kinase Aurora-A: phosphorylation on Ser625 is essential to spindle formation and mitosis. Hum Mol Genet. 2008a;17:215–224. doi: 10.1093/hmg/ddm298. [DOI] [PubMed] [Google Scholar]
- Venoux M, Delmouly K, Milhavet O, Vidal-Eychenie S, Giorgi D, Rouquier S. Gene organization, evolution and expression of the microtubule-associated protein ASAP (MAP9) BMC Genomics. 2008b;9:406. doi: 10.1186/1471-2164-9-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhey KJ, Gaertig J. The tubulin code. Cell Cycle. 2007;6:2152–2160. doi: 10.4161/cc.6.17.4633. [DOI] [PubMed] [Google Scholar]
- Verissimo CS, Elands R, Cheng S, Saaltink DJ, ter Horst JP, Alme MN, Pont C, van de Water B, Havik B, Fitzsimons CP, Vreugdenhil E. Silencing of doublecortin-like (DCL) results in decreased mitochondrial activity and delayed neuroblastoma tumor growth. PLoS One. 2013;8:e75752. doi: 10.1371/journal.pone.0075752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vershinin M, Carter BC, Razafsky DS, King SJ, Gross SP. Multiple-motor based transport and its regulation by Tau. Proc Natl Acad Sci U S A. 2007;104:87–92. doi: 10.1073/pnas.0607919104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viot G, Sonigo P, Simon I, Simon-Bouy B, Chadeyron F, Beldjord C, Tantau J, Martinovic J, Esculpavit C, Brunelle F, Munnich A, Vekemans M, Encha-Razavi F. Neocortical neuronal arrangement in LIS1 and DCX lissencephaly may be different. Am J Med Genet A. 2004;126A:123–128. doi: 10.1002/ajmg.a.20569. [DOI] [PubMed] [Google Scholar]
- Volle J, Brocard J, Saoud M, Gory-Faure S, Brunelin J, Andrieux A, Suaud-Chagny MF. Reduced expression of STOP/MAP6 in mice leads to cognitive deficits. Schizophr Bull. 2013;39:969–978. doi: 10.1093/schbul/sbs113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vouyiouklis DA, Brophy PJ. Microtubule-associated proteins in developing oligodendrocytes: transient expression of a MAP2c isoform in oligodendrocyte precursors. J Neurosci Res. 1995;42:803–817. doi: 10.1002/jnr.490420609. [DOI] [PubMed] [Google Scholar]
- Walczak CE. The Kin I kinesins are microtubule end-stimulated ATPases. Mol Cell. 2003;11:286–288. doi: 10.1016/s1097-2765(03)00067-4. [DOI] [PubMed] [Google Scholar]
- Wandosell F, Serrano L, Avila J. Phosphorylation of alpha-tubulin carboxyl-terminal tyrosine prevents its incorporation into microtubules. J Biol Chem. 1987;262:8268–8273. [PubMed] [Google Scholar]
- Wang JZ, Grundke-Iqbal I, Iqbal K. Kinases and phosphatases and tau sites involved in Alzheimer neurofibrillary degeneration. Eur J Neurosci. 2007;25:59–68. doi: 10.1111/j.1460-9568.2006.05226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JZ, Xia YY, Grundke-Iqbal I, Iqbal K. Abnormal hyperphosphorylation of tau: sites, regulation, and molecular mechanism of neurofibrillary degeneration. J Alzheimers Dis. 2013;33(Suppl 1):S123–139. doi: 10.3233/JAD-2012-129031. [DOI] [PubMed] [Google Scholar]
- Witte H, Bradke F. The role of the cytoskeleton during neuronal polarization. Curr Opin Neurobiol. 2008;18:479–487. doi: 10.1016/j.conb.2008.09.019. [DOI] [PubMed] [Google Scholar]
- Wu JW, Hussaini SA, Bastille IM, Rodriguez GA, Mrejeru A, Rilett K, Sanders DW, Cook C, Fu H, Boonen RA, Herman M, Nahmani E, Emrani S, Figueroa YH, Diamond MI, Clelland CL, Wray S, Duff KE. Neuronal activity enhances tau propagation and tau pathology in vivo. Nat Neurosci. 2016;19:1085–1092. doi: 10.1038/nn.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H, Wang H, Zhang X, Tu Z, Bulinski C, Khrapunovich-Baine M, Hogue Angeletti R, Horwitz SB. Structural evidence for cooperative microtubule stabilization by Taxol and the endogenous dynamics regulator MAP4. ACS Chem Biol. 2012;7:744–752. doi: 10.1021/cb200403x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie R, Nguyen S, McKeehan K, Wang F, McKeehan WL, Liu L. Microtubule-associated protein 1S (MAP1S) bridges autophagic components with microtubules and mitochondria to affect autophagosomal biogenesis and degradation. J Biol Chem. 2011a;286:10367–10377. doi: 10.1074/jbc.M110.206532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie R, Wang F, McKeehan WL, Liu L. Autophagy enhanced by microtubule- and mitochondrion-associated MAP1S suppresses genome instability and hepatocarcinogenesis. Cancer Res. 2011b;71:7537–7546. doi: 10.1158/0008-5472.CAN-11-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu I, Garnham CP, Roll-Mecak A. Writing and Reading the Tubulin Code. J Biol Chem. 2015;290:17163–17172. doi: 10.1074/jbc.R115.637447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemlan FP, Jauch EC, Mulchahey JJ, Gabbita SP, Rosenberg WS, Speciale SG, Zuccarello M. C-tau biomarker of neuronal damage in severe brain injured patients: association with elevated intracranial pressure and clinical outcome. Brain Res. 2002;947:131–139. doi: 10.1016/s0006-8993(02)02920-7. [DOI] [PubMed] [Google Scholar]
- Zemlan FP, Rosenberg WS, Luebbe PA, Campbell TA, Dean GE, Weiner NE, Cohen JA, Rudick RA, Woo D. Quantification of axonal damage in traumatic brain injury: affinity purification and characterization of cerebrospinal fluid tau proteins. J Neurochem. 1999;72:741–750. doi: 10.1046/j.1471-4159.1999.0720741.x. [DOI] [PubMed] [Google Scholar]
- Zetterberg H, Wilson D, Andreasson U, Minthon L, Blennow K, Randall J, Hansson O. Plasma tau levels in Alzheimer's disease. Alzheimers Res Ther. 2013;5:9. doi: 10.1186/alzrt163. [DOI] [PMC free article] [PubMed] [Google Scholar]