Abstract
Introduction
We examined whether metabolic health status increases the risk of cancer mortality, and whether this association varied by body mass index (BMI) category.
Methods
We performed a prospective study of 22,514 participants from the REasons for Geographic and Racial Differences in Stroke (REGARDS) cohort. We defined metabolic unhealthy status as having 3+ of the following: 1) elevated fasting glucose, 2) high triglycerides, 3) dyslipidemia, 4) hypertension, and 5) elevated waist circumference. We categorized participants into normal weight (BMI: 18.5-24.9 kg/m2), overweight (BMI 25.0-29.9 kg/m2) and obese (BMI ≥ 30 kg/m2) groups. We performed Cox proportional hazards regression to estimate hazards ratios (HRs) and 95% confidence intervals (CIs) for cancer mortality during follow-up.
Results
Among participants with normal weight, those who were metabolically unhealthy had increased risk of cancer mortality (HR: 1.57; 95% CI: 1.15 – 2.16) compared with metabolically healthy participants. Overall mortality risk for participants who were metabolically unhealthy and normal-weight was stronger for obesity-related cancers (HR: 2.31, 95% CI: 1.13 – 4.73). Compared with participants with normal weight, those who were metabolically healthy overweight were at reduced risk of any cancer mortality (adjusted HR: 0.79, 95% CI: 0.63-0.98).
Conclusion
There was an increased risk of overall- and obesity-related- cancer mortality among metabolically unhealthy normal weight participants.
Keywords: obesity, metabolic syndrome, cancer, race
INTRODUCTION
Obesity has long been understood to increase the risk of cancer mortality1-5, and is also an established risk factor for multiple chronic diseases, including type 2 diabetes, cardiovascular disease, metabolic syndrome and cancer. The prevalence of obesity in the United States has reached epidemic proportions, with up to two-thirds of US adults currently in overweight or obese categories6. Concurrently, the prevalence of metabolic syndrome, a cluster of cardio-metabolic risk factors that include high fasting blood glucose, low HDL cholesterol, high blood pressure and high triglycerides, has also increased rapidly7. However, a recent study based on data from the US National Health and Nutrition Examination Survey showed that 32% of US adults with obesity were metabolically healthy, i.e., had none or one of the cardio-metabolic risk factors typically associated with obesity 8. Clinical research studies suggest that this sub-group of metabolically healthy individuals with obesity display favorable insulin, inflammatory and lipid profiles and may be at lower risk for obesity-related chronic conditions compared with their metabolic unhealthy counterparts 9. This raises the intriguing question of whether there is a universal association between metabolic health status and risk of adverse health outcomes in US adults, of if the association varies by body mass index (BMI) category 10.
Recent studies provide some evidence for differences in the biological response to obesity and the risk of cancer based on metabolic health status. For instance, a recent study observed a 200% increased risk of cancer among overweight adults with elevated blood glucose, compared with a 50% increased risk among overweight adults with normal glucose11. Two other recent studies examined metabolic health status and obesity in relation to cancer mortality among Korean adults; both studies observed significantly lower risk of cancer mortality among metabolically healthy adults with obesity compared with those who were metabolically unhealthy with obesity 12,13. This is in line with prior studies showing that metabolically healthy individuals with obesity may be at lower or similar risk of cardiovascular diseases compared with adults without obesity, and thus may be protected against the cardio-metabolic complications of obesity9. The evidence on whether metabolically health obesity is similarly associated with cancer mortality among US adults remains sparse. If we find consistent scientific evidence that the influence of metabolic health status on cancer outcomes varies by BMI, this information will add to the growing literature regarding the importance of metabolic risk factors in cancer prognosis. In this study, we examine the association between metabolic health and obesity (as categorized by BMI) on cancer mortality in a national US population of Blacks and Whites.
METHODS
Data Source
The REasons for Geographic And Racial Differences in Stroke (REGARDS) is one of the largest ongoing national longitudinal cohorts of community-dwelling adults in the United States 14. Designed to identify contributors to racial and geographic differences in stroke mortality, the cohort included 30,239 participants aged ≥ 45 years at baseline; 45% were male, 41% were Black, and 69% were >60 years old. Participants were recruited between January 2003 and October 2007, and detailed information about demographics, health behaviors, chronic medical conditions, physical status, diet, and medications were collected 14. During the course of prospective follow-up, participants are contacted by telephone every 6-months to identify any medical event or hospitalizations experienced since the prior contact. For statistical analysis, we excluded participants who were missing data on metabolic components (4846), had a prior history of cancer diagnosis at baseline (2175), were missing data on follow-up time (236), or had a BMI lower than 18.5 kg/m2 (349), leaving a total of 22,514 participants for analysis.
Cancer Mortality Outcome
The primary outcome in this study was death due to any cancer. Cancer mortality was ascertained using death certificates, medical records, interviewed proxies, linkages with the Social Security Death Index (SSDI) as well as the National Death Index (NDI). Date of death was confirmed using death certificates, SSDI, and/or NDI, and cause of death was adjudicated by a committee of experts using all available information as recommended by national guidelines 15. As a secondary outcome, we examined obesity-related cancer deaths defined as cancers of the breast, colorectal, kidney, pancreas, endometria, and esophagus 16. Follow-up time for each participant was calculated from the enrollment date through date of cancer death, death, or last telephone follow-up through December 31, 2012.
Main Exposure Variables
The main variables of interest were metabolic health and BMI category. First, we defined metabolic health using the criteria for metabolic syndrome proposed by international consensus in 2009 17. These criteria define metabolic syndrome based on presence of three or more of the following components: 1) elevated waist circumference (WC): >102 cm for males or >88 cm for females; 2) elevated triglycerides: ≥150 mg/dL or reported use of medication for elevated triglycerides; 3) reduced high-density lipoprotein cholesterol (HDL-C): <40 mg/dL for males and <50 mg/dL for females, or use of lipid lowering medications; 4) elevated blood pressure: systolic blood pressure ≥130 mm Hg, diastolic blood pressure ≥80 mm Hg, or the reported use of antihypertensive agents; and 5) elevated glucose: fasting glucose ≥100 mg/L or the use of insulin or oral hypoglycemic agents. For this analysis, metabolic unhealthy participants were those with at least three of the five metabolic syndrome components, while metabolic healthy participants were those with less than three. We further defined BMI category based on interviewer-measured participant height and weight during the baseline in-home examination. We classified BMI category into three categories as recommended by the Centers for Disease Control and Prevention (CDC): 1) normal weight - BMI < 25.0 kg/m2, 2) overweight - BMI between 25.0 and 29.9 kg/m2, and 3) obese - BMI ≥ 30 kg/m2.18 As a sensitivity analysis, we further classified metabolic unhealthy as those with at least two of the five metabolic syndrome components, and conducted subgroup analysis focusing only on mortality due to obesity-associated cancers.
Participant Characteristics
Baseline demographic variables used in the analysis included age, race, sex, household income, education, and geographic region. Health behaviors included tobacco, alcohol use, and physical activity. Baseline chronic medical conditions included atrial fibrillation, chronic kidney disease, deep vein thrombosis (DVT), myocardial infarction, peripheral artery disease (PAD, and stroke. Excluding components of metabolic syndrome, an individual level comorbidity score was created based on the sum of total number comorbidities at baseline for each participant (i.e., atrial fibrillation, chronic kidney disease, coronary artery disease, DVT, MI, peripheral artery disease, and stroke). We presented the distribution of comorbidity scores as means and standard deviations.
Statistical Analysis
We compared baseline characteristics by metabolic health and BMI category using Chi-square tests for categorical characteristics, Analysis of Variance (ANOVA) for normal continuous variables, and Wilcoxon test for non-normal continuous variables. We estimated any cancer and obesity-related cancer mortality rates per 1000 person-years using Poisson regression for each weight status category. To estimate the hazards of cancer mortality, we fit three series (for each BMI category group) of Cox proportional hazard models examining the association between metabolic health status and time to cancer death. We examined the effect of each metabolic syndrome component on cancer mortality, and stratified by BMI category using Cox regression. We examined the association between the total number of metabolic syndrome components and risk of cancer mortality using Cox regression. In secondary analysis, we performed additional Cox proportional hazard models to estimate the hazards of obesity-related cancer mortality by metabolic health status. To account for all-cause mortality as a competing risk, we employed the Fine and Gray method of Cox regression to calculate the sub-distribution hazard of cancer mortality and obesity related cancer mortality 19. We adjusted all models for age, race, sex, education, income, tobacco use, alcohol use, physical inactivity, LDL cholesterol, and comorbidity score. We assessed for multicollinearity among our study covariates by calculating the variance inflation factors (VIF). To obtain the VIF, we regressed each explanatory variable onto all other covariates. There was no evidence of multicollinearity between the covariates, and all VIF were less than 1.50. In sensitivity analyses, we examined the effect of BMI category on cancer mortality using Cox regression, and additionally stratified by metabolic health status. We additionally examined the main effect of obesity on obesity-related cancer mortality using Cox regression, and additionally stratified by metabolic health status. Models examining the main effect of BMI on any/obesity-related cancer mortality were additionally adjusted for metabolic health status when not stratified by metabolic health status. We conducted sensitivity analyses excluding all cancer deaths within the first two years of entry into the REGARDS cohort, as well as excluding all participants with comorbidities at baseline. The results of all models were expressed as adjusted hazard ratios (HR) and the corresponding 95% confidence intervals (CI). Participants were censored at the time of death, loss to follow-up, or the end of cancer mortality ascertainment (December 31, 2012). SAS version 9.4 and STATA version 13 were used for all statistical analysis. Two-sided p values <0.05 were considered statistically significant.
Ethical Statement
The institutional review board of all participating universities approved the study and all participants provided written informed consent.
RESULTS
Among 30,239 REGARDS participants, 7,725 were excluded, resulting in 22,514 for analysis. Of the included participants, 5,377 (23.9%) were categorized as normal weight, 8,351 (37.1%) overweight, and 8,786 (39.0%) with obesity. In general, metabolically unhealthy participants were older, had lower education and income, and were more likely to be current tobacco users (Table 1; P values <0.01) compared with metabolically healthy participants in all weight categories. In addition, metabolically unhealthy normal weight participants had greater prevalence of atrial fibrillation, coronary artery disease, DVT, diabetes, dyslipidemia, hypertension, myocardial infarction, PAD, and stroke (P values < 0.01). Similar patterns were observed among participants with overweight and obesity, although the prevalence of diabetes was much higher among metabolically unhealthy participants with overweight and obesity.
Table 1.
Participant characteristics by metabolic health status, stratified by BMI category. Among 22,514 REGARDS participants.
| Normal Weight BMI 18.5 – 24.9 kg/m2 (N = 5377) | Overweight BMI 25.0 – 29.9 kg/m2 (N = 8351) | Obese BMI ≥ 30 kg/m2 (N = 8786) | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| MH (N = 4720) | MU (N = 657) | P value* | MH (N = 5503) | MU (N = 2848) | P value* | MH (N = 2872) | MU (N = 5914) | P value* | |
| Age† | 65.5 (10.2) | 68.4 (9.2) | <0.01 | 64.2 (9.3) | 66.4 (9.1) | <0.01 | 62.1 (8.7) | 63.0 (8.5) | <0.01 |
| Race (%) | |||||||||
| Black | 1365 (28.9) | 204 (31.0) | 0.3 | 1963 (35.7) | 1045 (36.7) | 0.4 | 1545 (53.8) | 2907 (49.2) | <0.01 |
| White | 3355 (71.1) | 453 (69.0) | 3540 (64.3) | 1803 (63.3) | 1327 (46.2) | 3007 (50.8) | |||
| Sex (%) | |||||||||
| Male | 2020 (42.8) | 285 (43.4) | 0.8 | 2980 (54.2) | 1353 (47.5) | <0.01 | 1029 (35.8) | 2250 (38.1) | 0.04 |
| Female | 2700 (57.2) | 372 (56.6) | 2523 (45.9) | 1495 (52.5) | 1843 (64.2) | 3664 (61.9) | |||
| Education ≤ High School (%) | 415 (8.8) | 107 (16.3) | <0.01 | 483 (8.8) | 412 (14.5) | <0.01 | 319 (11.1) | 888 (15.0) | <0.01 |
| Income ≤ $20,000 (%) | 683 (14.5) | 162 (24.7) | <0.01 | 628 (11.4) | 582 (20.4) | <0.01 | 488 (17.0) | 1278 (21.6) | <0.01 |
| Current Tobacco Use (%) | 865 (18.4) | 186 (28.4) | <0.01 | 646 (11.8) | 491 (17.3) | <0.01 | 278 (9.7) | 780 (13.2) | <0.01 |
| Heavy Alcohol Use (%) | 280 (6.0) | 32 (5.0) | <0.01 | 258 (4.8) | 104 (3.7) | <0.01 | 86 (3.1) | 158 (2.7) | <0.01 |
| No Physical Activity (%) | 1332 (28.7) | 287 (44.4) | <0.01 | 1427 (26.3) | 1039 (37.1) | <0.01 | 1028 (36.2) | 2406 (41.3) | <0.01 |
| Chronic Medical Conditions (%) | |||||||||
| Atrial fibrillation | 375 (8.1) | 75 (11.7) | <0.01 | 369 (6.8) | 273 (9.8) | <0.01 | 204 (7.3) | 542 (9.4) | <0.01 |
| Chronic lung disease | 403 (8.6) | 55 (8.4) | 0.9 | 433 (7.9) | 255 (9.0) | 0.09 | 289 (10.1) | 658 (11.1) | 0.1 |
| Coronary artery disease | 689 (14.9) | 157 (24.5) | <0.01 | 788 (14.6) | 646 (23.0) | <0.01 | 337 (12.0) | 1179 (20.3) | <0.01 |
| Deep vein thrombosis | 187 (4.0) | 40 (6.2) | 0.01 | 201 (3.7) | 151 (5.3) | <0.01 | 157 (5.5) | 394 (6.7) | 0.03 |
| Myocardial infarction | 497 (10.7) | 111 (17.3) | <0.01 | 546 (10.1) | 449 (16.0) | <0.01 | 240 (8.5) | 842 (14.5) | <0.01 |
| Peripheral artery disease | 105 (2.2) | 27 (4.1) | <0.01 | 89 (1.6) | 81 (2.9) | <0.01 | 27 (0.9) | 139 (2.4) | <0.01 |
| Stroke | 246 (5.2) | 73 (11.2) | <0.01 | 221 (4.0) | 236 (8.3) | <0.01 | 101 (3.5) | 385 (6.5) | <0.01 |
| Comorbidity score†** | 0.52 (0.92) | 0.80 (1.10) | <0.01 | 0.48 (0.86) | 0.73 (1.05) | <0.01 | 0.47 (0.85) | 0.69 (1.02) | <0.01 |
| Follow-up years‡ | 6.9 (5.5 - 8.2) | 6.5 (4.6 - 8.2) | 0.01 | 7.1 (5.6 - 8.4) | 6.9 (5.4 - 8.4) | <0.01 | 6.8 (5.6 - 8.2) | 6.8 (5.4 - 8.2) | 0.12 |
| BMI† | 23.3 (1.4) | 22.7 (1.6) | <0.01 | 27.3 (1.4) | 27.8 (1.4) | <0.01 | 34.3 (4.4) | 36.0 (5.2) | <0.01 |
N (%) – Presented as count and column percentages.
Mean (Standard deviation)
Median (Interquartile Range)
Significance determined using ANOVA (normal continuous), Wilcoxon (non-parametric continuous), and chi-square test.
Comorbidity score presented as means and standard deviations of the sum total of comorbidities.
MH – Metabolically healthy
MU – Metabolically unhealthy defined as the presence of three or more metabolic components including 1) elevated fasting glucose, 2) high triglycerides, 3) dyslipidemia, 4) hypertension, and 5) high waist circumference.
There were 766 (3.4%) cancer deaths observed among 22,750 study participants over a mean follow up time of 6.5 years. The most common cancer types were lung (28.9%), gastro-intestinal (19.6%) and hematological (10.8%) cancers (Appendix I). Among normal weight participants, metabolically unhealthy participants were at a 65% increased risk of cancer mortality (adjusted HR: 1.65; 95% CI: 1.20 – 2.26) compared with metabolically healthy participants (Table 2), and metabolically unhealthy normal weight participants had the lowest survival probability over the follow-up period (Figure 1). Even when considering all-cause mortality as a competing risk (Table 2), the increased risk of cancer mortality among normal weight participants persisted when comparing metabolically unhealthy to metabolically healthy participants (adjusted SHR 1.67; 95% CI: 1.21 – 2.30). However, there was no statistically significant increased risk of cancer mortality for metabolically unhealthy participants with overweight (adjusted HR: 0.89, 95% CI: 0.67 – 1.17) or obesity (adjusted HR: 1.12, 95% CI: 0.84 - 1.49).
Table 2.
Hazard Ratios (HRs)1 and associated 95% Confidence Intervals for the association between metabolic health status and time to any cancer death, and obesity-related cancer death, stratified by BMI category.
| Any Cancer Deaths2 | Obesity-Related Cancer Deaths3 | |
|---|---|---|
| Normal Weight (BMI 18.5 – 24.9 kg/m2) | ||
|
| ||
| MH (# cancer deaths / # at risk) | 199 / 4720 | 30 / 4720 |
| MU (# cancer deaths / # at risk) | 57 / 657 | 12 / 657 |
| MH Mortality Per 1,000 Person-Years (95% CI) | 6.45 (5.62 – 7.42) | 0.97 (0.68 – 1.39) |
| MU Mortality Per 1,000 Person-Years (95% CI) | 13.94 (10.75 – 18.10) | 2.93 (1.66 – 5.17) |
| Hazard Ratio (95% CI) – Referent group MH | 1.65 (1.20 – 2.26) | 2.40 (1.17 – 4.91) |
| Subdistribution Hazard Ratio (95% CI)4 – Referent group MH | 1.67 (1.21 – 2.30) | 2.37 (1.13 – 4.98) |
|
| ||
| Overweight (BMI 25.0 – 29.9 kg/m2) | ||
|
| ||
| MH (# cancer deaths / # at risk) | 167 / 5503 | 41 / 5503 |
| MU (# cancer deaths / # at risk) | 88 / 2848 | 13 / 2848 |
| MH Mortality Per 1,000 Person-Years (95% CI) | 4.50 (3.87 – 5.24) | 1.11 (0.81 – 1.50) |
| MU Mortality Per 1,000 Person-Years (95% CI) | 4.74 (3.85 – 5.85) | 0.70 (0.41 – 1.21) |
| Hazard Ratio (95% CI) – Referent group MH | 0.89 (0.67 – 1.17) | 0.58 (0.30 – 1.12) |
| Subdistribution Hazard Ratio (95% CI)4 – Referent group MH | 0.85 (0.64 – 1.13) | 0.57 (0.30 – 1.09) |
|
| ||
| Obese (BMI ≥ 30 kg/m2) | ||
|
| ||
| MH (# cancer deaths / # at risk) | 71 / 2872 | 20 / 2872 |
| MU (# cancer deaths / # at risk) | 184 / 5914 | 49 / 5914 |
| MH Mortality Per 1,000 Person-Years (95% CI) | 3.76 (2.98 – 4.75) | 1.06 (0.68 – 1.64) |
| MU Mortality Per 1,000 Person-Years (95% CI) | 4.83 (4.18 – 5.58) | 1.29 (0.97 – 1.70) |
| Hazard Ratio (95% CI) – Referent group MH | 1.12 (0.84 – 1.49) | 1.17 (0.68 – 2.02) |
| Subdistribution Hazard Ratio (95% CI)4 – Referent group MH | 1.09 (0.81 – 1.46) | 1.15 (0.67 – 1.97) |
Models adjusted for age, race, sex, education, income, tobacco use, alcohol use, physical inactivity, LDL cholesterol, and comorbidity score.
766 total any cancer deaths.
165 total obesity-related cancer deaths. Included: breast, colorectal, kidney, pancreatic, endometrial, and esophageal cancers.
Estimated using Fine & Gray method accounting for 2971 all-cause deaths as competing risk, models adjusted for age, race, sex, education, income, tobacco use, alcohol use, physical inactivity, LDL cholesterol, and comorbidity score.
MH – Metabolically healthy. MU – Metabolically unhealthy defined as the presence of three or more metabolic components including 1) elevated fasting glucose, 2) high triglycerides, 3) dyslipidemia, 4) hypertension, and 5) high waist circumference.
Bold indicates statistically significant association at 0.05 alpha level.
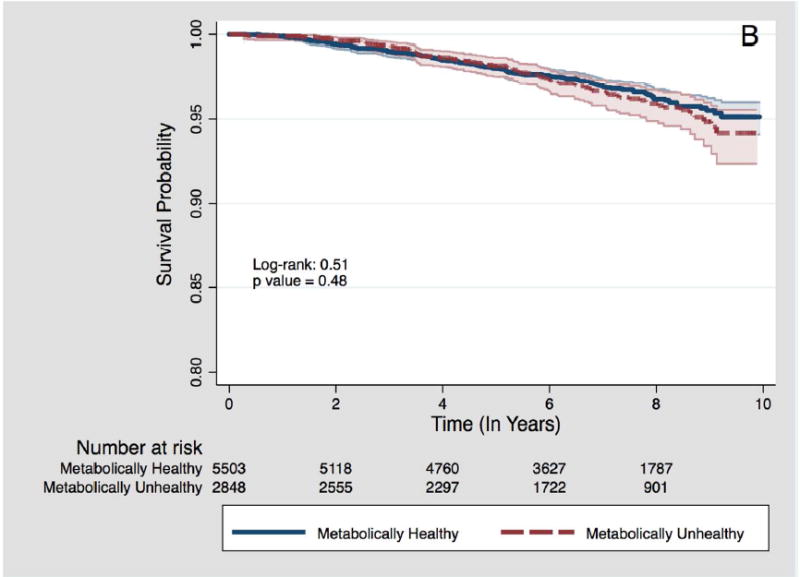
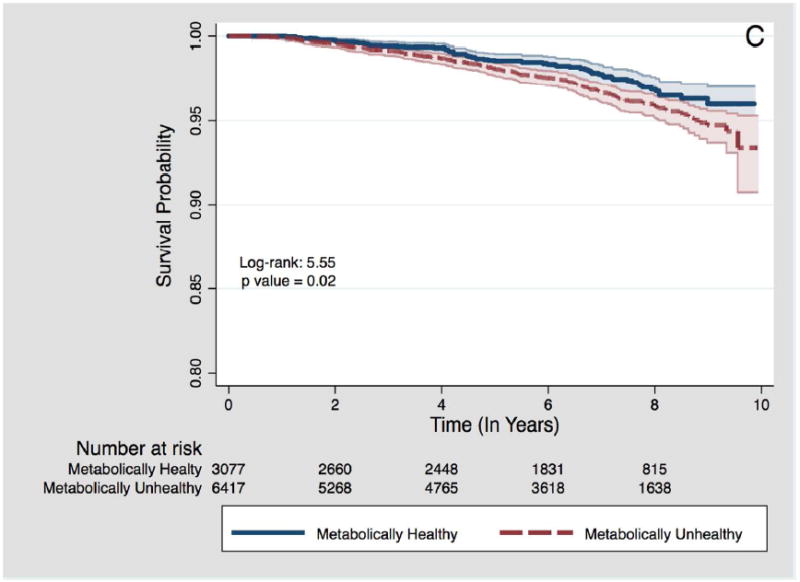
Figure 1.
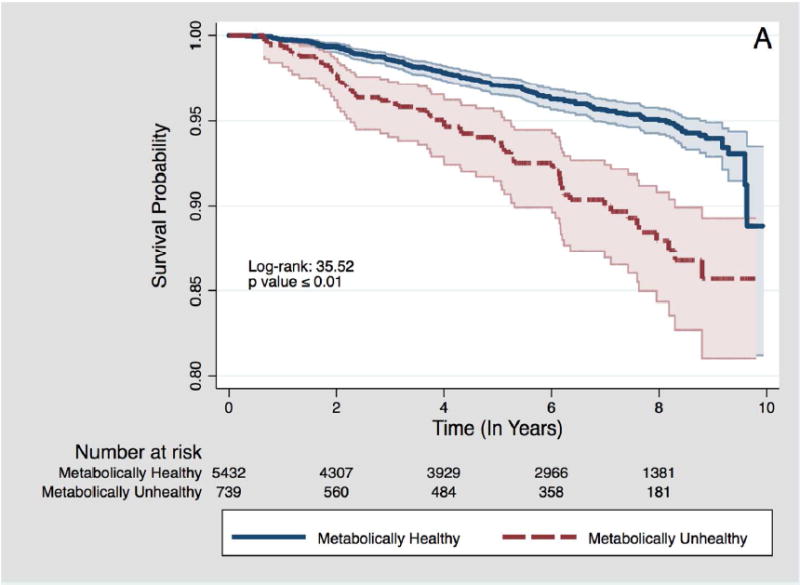
Kaplan-Meier survival plots for time to cancer death by metabolic health status, stratified by BMI category.
A – Among participants with normal weight BMI category
B – Among participants with overweight BMI category
C – Among participants with obesity BMI category
When focused on mortality due to obesity-related cancers, there were 165 total events over the observation period (Table 2). Among normal weight participants, metabolically unhealthy participants were at more than a 2-fold increased risk of an obesity-related cancer death (adjusted HR: 2.40; 95% CI: 1.17 – 4.91) compared with metabolically healthy participants. There was no statistically significant risk of obesity-related cancer mortality for metabolically unhealthy participants with overweight (adjusted HR: 0.58; 95% CI: 0.30 – 1.12) or obesity (adjusted HR: 1.17; 95% CI: 0.68 – 2.02). Similar results were obtained in analyses unadjusted for comorbidities (data not shown). In both unadjusted (p = 0.0002) and adjusted models (p = 0.0232), the interaction terms between metabolic health status and obesity category were significant (data not shown).
In sensitivity analysis excluding participants with comorbidities at baseline (n=8,333, 49% of cancer deaths), metabolic unhealthy status was associated with a statistically non-significant 42% increased risk of any cancer mortality among normal weight participants, however the association with obesity-related cancer mortality became stronger (HR: 3.31, 95% CI: 1.38 – 7.95). In separate sensitivity analysis excluding participants who experienced a cancer death within the first 2 years of entry into the REGARDS cohort (n=111), the association between metabolic unhealthy status and any cancer mortality among normal weight participants became slightly attenuated but remained statistically significant (HR: 1.52, 95% CI: 1.07 – 2.16).
We further analyzed the association between BMI categories and any- and obesity-related cancer mortality stratified by metabolic health status (Tables 3 and 4). Participants with overweight (adjusted HR: 0.65; 95% CI: 0.54 – 0.79) or obesity (adjusted HR: 0.76; 95% CI: 0.61 – 0.94) had reduced risk of any cancer mortality after adjusting for metabolic health status (Table 3). Metabolically healthy participants with overweight, but not obesity, were at reduced risk of any cancer mortality compared with participants with normal weight (adjusted HR: 0.79, 95% CI: 0.63-0.99), while metabolically unhealthy participants with overweight (adjusted HR: 0.39; 95% CI: 0.27 – 0.55) or obesity (adjusted HR: 0.51; 95% CI: 0.36 – 0.70) were at significantly reduced risk of any cancer mortality. The associations for obesity-related cancer mortality were mostly non-significant (Table 4).
Table 3.
Hazard Ratios (HRs) and 95% Confidence Intervals for the association between obesity status and time to any cancer death, stratified by BMI category. Among 766 total cancer deaths, and 2971 competing all-cause deaths.
| Any Cancer Deaths
| ||||||
|---|---|---|---|---|---|---|
| Overall | (# Deaths /# At risk) | Mortality Rate1 (95% CI) | Model 12 HR (95% CI) | Model 1 Subdistribution HR (95% CI)4 | Model 23 HR (95% CI) | Model 2 Subdistribution HR (95% CI)4 |
| Normal Weight (Referent) | 256 / 5377 | 7.33 (6.49 – 8.29) | - | - | - | - |
| Overweight | 255 / 8351 | 4.58 (4.05 – 5.18) | 0.67 (0.55 – 0.81) | 0.69 (0.57 – 0.83) | 0.65 (0.54 – 0.79) | 0.67 (0.55 – 0.82) |
| Obese | 255 / 8786 | 4.48 (3.96 – 5.06) | 0.81 (0.67 – 0.98) | 0.82 (0.68 – 0.99) | 0.76 (0.61 – 0.94) | 0.78 (0.62 – 0.97) |
|
| ||||||
| Metabolically Healthy | ||||||
|
| ||||||
| Normal Weight (Referent) | 199 / 4720 | 6.45 (5.62 – 7.42) | - | - | - | - |
| Overweight | 167 / 5503 | 4.50 (3.87 – 5.24) | 0.79 (0.63 – 0.99) | 0.83 (0.66 – 1.03) | - | - |
| Obese | 71 / 2872 | 3.76 (2.98 – 4.75) | 0.89 (0.66 – 1.20) | 0.91 (0.68 – 1.22) | - | - |
|
| ||||||
| Metabolically Unhealthy | ||||||
|
| ||||||
| Normal Weight (Referent) | 57 / 657 | 13.94 (10.75 – 18.07) | - | - | - | - |
| Overweight | 88 / 2848 | 4.74 (3.85 – 5.85) | 0.39 (0.27 – 0.55) | 0.39 (0.28 – 0.56) | - | - |
| Obese | 184 / 5914 | 4.83 (4.18 – 5.58) | 0.51 (0.36 – 0.70) | 0.51 (0.36 – 0.71) | - | - |
Rate per 1,000 Person-Years
Adjusted for age, race, sex, education, income, tobacco use, alcohol use, physical inactivity, LDL cholesterol, and comorbidity score.
Adjusted for age, race, sex, education, income, tobacco use, alcohol use, physical inactivity, LDL cholesterol, comorbidity score, and metabolic health status.
Estimated using Fine & Gray method accounting for all-cause mortality as competing risk, models adjusted for age, race, sex, education, income, tobacco use, alcohol use, physical inactivity, LDL cholesterol, and comorbidity score.
Metabolically unhealthy defined as the presence of three or more metabolic components including 1) elevated fasting glucose, 2) high triglycerides, 3) dyslipidemia, 4) hypertension, and 5) high waist circumference. Normal weight: BMI 18.5 – 24.9 kg/m2; Overweight: BMI 25.0 – 29.9 kg/m2; Obese: BMI = 30 kg/m2
Bold indicates statistically significant association at 0.05 alpha level.
Table 4.
Hazard Ratios (HRs) and 95% Confidence Intervals for the association between obesity status and time to obesity-related cancer death, stratified by BMI category. Among 165 total obesity-related cancer deaths, and 3,572 competing all-cause deaths.
| Obesity-Related Cancer Deaths
| ||||||
|---|---|---|---|---|---|---|
| Overall | (# Deaths / # At risk) | Mortality Rate1 (95% CI) | Model 12 HR (95% CI) | Model 1 Subdistribution HR (95% CI)4 | Model 23 HR (95% CI) | Model 2 Subdistribution HR (95% CI)4 |
| Normal Weight (Referent) | 42 / 5377 | 1.20 (0.19 – 1.63) | - | - | - | - |
| Overweight | 54 / 8351 | 0.97 (0.74 – 1.27) | 0.86 (0.57 – 1.31) | 0.89 (0.58 – 1.35) | 0.85 (0.56 – 1.31) | 0.88 (0.56 – 1.37) |
| Obese | 69 / 8786 | 1.21 (0.96 – 1.53) | 1.32 (0.87 – 2.01) | 1.32 (0.87 – 2.01) | 1.29 (0.82 – 2.05) | 1.29 (0.79 – 2.09) |
|
| ||||||
| Metabolically Healthy | ||||||
|
| ||||||
| Normal Weight (Referent) | 30 / 4720 | 0.97 (0.68 – 1.39) | - | - | - | - |
| Overweight | 41 / 5503 | 1.11 (0.81 – 1.50) | 1.29 (0.79 – 2.12) | 1.33 (0.82 – 2.17) | - | - |
| Obese | 20 / 2872 | 1.06 (0.68 – 1.64) | 1.50 (0.82 – 2.77) | 1.52 (0.83 – 2.80) | - | - |
|
| ||||||
|
Metabolically Unhealthy
| ||||||
| Normal Weight (Referent) | 12 / 657 | 2.93 (1.67 – 5.17) | - | - | - | - |
| Overweight | 13 / 2848 | 0.70 (0.41 – 1.21) | 0.27 (0.12 – 0.63) | 0.27 (0.12 – 0.64) | - | - |
| Obese | 49 / 5914 | 1.29 (0.97 – 1.70) | 0.65 (0.33 – 1.31) | 0.61 (0.30 – 1.25) | - | - |
Rate per 1,000 Person-Years
Adjusted for age, race, sex, education, income, tobacco use, alcohol use, physical inactivity, and comorbidity score.
Adjusted for age, race, sex, education, income, tobacco use, alcohol use, physical inactivity, comorbidity score, metabolic health status.
Estimated using Fine & Gray method accounting for all-cause mortality as competing risk, models adjusted for age, race, sex, education, income, tobacco use, alcohol use, physical inactivity, LDL cholesterol, and comorbidity score.
Metabolically unhealthy defined as the presence of three or more metabolic components including 1) elevated fasting glucose, 2) high triglycerides, 3) dyslipidemia, 4) hypertension, and 5) high waist circumference.
Normal weight: BMI 18.5 – 24.9 kg/m2; Overweight: BMI 25.0 – 29.9 kg/m2; Obese: BMI ≥ 30 kg/m2
Bold indicates statistically significant association at 0.05 alpha level.
The cardio-metabolic component that was most associated with any cancer mortality was reduced HDL cholesterol (overall adjusted HR: 1.20; 95% CI: 1.03 – 1.40), with the highest risk observed among normal weight (adjusted HR: 1.89, 95% CI: 1.43 – 2.49) participants (Table 5). The cardio-metabolic component most associated with obesity-related cancer mortality were reduced HDL cholesterol (adjusted HR: 2.45, 95% CI: 1.25 – 4.79) and elevated fasting glucose (adjusted HR: 2.00, 95% CI: 1.04 – 3.85). In addition, normal weight participants with one (adjusted HR: 1.67; 95% CI: 1.08 – 2.57) and three or more (adjusted HR: 2.31; 95% CI: 1.44 – 3.72) cardio-metabolic components were at significantly increased risk of cancer mortality when compared to participants with no components. Normal weight participants with at least three metabolic unhealthy components were at nearly a 4-fold increased risk of obesity-related cancer mortality (adjusted HR: 3.78, 95% CI: 1.16 – 12.31) compared to those with none (Table 5).
Table 5.
Hazard Ratiosa (HRs) and 95% confidence intervals for the association between components of metabolic health status and time to cancer death and time to obesity-relatedc cancer death stratified by BMI category.
| Obesity Status
|
||||
|---|---|---|---|---|
| All | Normal Weight (BMI 18.5 – 24.9 kg/m2) | Overweight (BMI 25.0 – 29.9 kg/m2) | Obese (BMI ≥ 30 kg/m2) | |
| Cancer Mortality | ||||
|
| ||||
| Componentsb | ||||
| High WC | 1.00 (0.86 – 1.17) | 1.21 (0.74 – 1.97) | 1.05 (0.79 – 1.39) | 1.37 (0.86 – 2.18) |
| Elevated Triglycerides | 0.95 (0.80 – 1.13) | 1.10 (0.80 – 1.52) | 1.17 (0.88 – 1.56) | 0.75 (0.55 – 1.01) |
| Reduced HDL Cholesterol | 1.20 (1.03 – 1.40) | 1.89 (1.43 – 2.49) | 0.84 (0.64 – 1.11) | 1.26 (0.97 – 1.65) |
| Elevated blood pressure | 1.14 (0.95 – 1.38) | 1.21 (0.90 – 1.62) | 1.03 (0.76 – 1.41) | 1.35 (0.89 – 2.05) |
| Elevated fasting glucose | 1.01 (0.87 – 1.18) | 1.01 (0.75 – 1.36) | 0.99 (0.76 – 1.30) | 1.14 (0.88 – 1.49) |
| Overalla | Ref | 0.67 (0.55 – 0.80) | 0.82 (0.68 – 0.99) | |
|
| ||||
| Obesity-Related Cancer Mortalityc | ||||
|
| ||||
| Componentsb | ||||
| High WC | 1.29 (0.92 – 1.79) | 0.71 (0.17 – 3.06) | 0.86 (0.45 – 1.61)3 | 4.22 (1.02 – 17.40) |
| Elevated Triglycerides | 0.87 (0.60 – 1.28) | 1.34 (0.62 – 2.88) | 0.89 (0.46 – 1.73)3 | 0.61 (0.33 – 1.10) |
| Reduced HDL Cholesterol | 1.21 (0.87 – 1.68) | 2.45 (1.25 – 4.79) | 0.70 (0.38 – 1.31)3 | 1.15 (0.70 – 1.90) |
| Elevated blood pressure | 1.32 (0.88 – 1.98) | 1.17 (0.57 – 2.37) | 1.28 (0.64 – 2.55)3 | 1.36 (0.64 – 2.89) |
| Elevated fasting glucose | 1.20 (0.87 – 1.66) | 2.00 (1.04 – 3.85) | 1.04 (0.59 – 1.83) | 0.94 (0.58 – 1.54) |
| Overalla | Ref | 0.86 (0.57 – 1.31) | 1.32 (0.87 – 2.01) | |
|
| ||||
| Cancer Mortality | ||||
|
| ||||
| # Metabolic Unhealthy Components | ||||
| 0 (Referent) | - | - | - | - |
| 1 | 1.31 (0.94 – 1.82) | 1.67 (1.08 – 2.57) | 1.07 (0.62 – 1.84) | 0.78 (0.17 – 3.56) |
| 2 | 1.28 (0.92 – 1.77) | 1.48 (0.93 – 2.36) | 1.13 (0.67 – 1.93) | 1.13 (0.27 – 4.65) |
| 3+ | 1.29 (0.95 – 1.77) | 2.31 (1.44 – 3.72) | 0.95 (0.56 – 1.62) | 1.18 (0.29 – 4.77) |
| p value for trend | 0.41 | <0.01 | 0.74 | 0.65 |
|
| ||||
| Obesity-Related Cancer Mortalityc | ||||
|
| ||||
| # Metabolic Unhealthy Components | ||||
| 0 (Referent) | - | - | - | - |
| 1 | 2.25 (0.94 – 5.40) | 1.74 (0.56 – 5.40) | 2.85 (0.66 – 12.36) | Undefined |
| 2 | 2.72 (1.15 – 6.39) | 1.92 (0.59 – 6.28) | 2.68 (0.62 – 11.62) | Undefined |
| 3+ | 2.65 (1.14 – 6.15) | 3.78 (1.16 – 12.31) | 1.41 (0.31 – 6.45) | Undefined |
| p value for trend | 0.12 | 0.10 | 0.15 | 0.46 |
766 cancer deaths
Models adjusted for age, sex, race, education, income, tobacco use, alcohol use, physical inactivity, and comorbidity score.
Referent groups for each hazard ratio is the absence of metabolic component.
165 obesity-related cancer deaths included: breast, colorectal, kidney, pancreatic, endometrial, and esophageal cancers.
Bold indicates statistically significant association at 0.05 alpha level.
DISCUSSION
In this large, prospective cohort of Black and White adults, we observed that metabolic health status was a strong predictor of cancer mortality among participants with normal weight. Metabolically unhealthy participants experienced significantly higher risk of cancer mortality during follow-up compared with metabolically healthy participants in the normal weight category, with an observed 65% increased risk. In addition, metabolically unhealthy participants with normal weight were at a 2-fold increased risk of obesity-related cancer mortality, compared with those who were metabolically healthy. Among participants with overweight and obesity, there were no significantly increased risk of cancer mortality due to metabolic unhealthy status observed after adjusting for age, sex, income tobacco use, alcohol and physical activity at baseline. When restricted to the major obesity-related cancers (breast, colorectal, kidney, pancreatic, endometrial and esophageal cancers), there was a two-fold increased risk of mortality observed among metabolically unhealthy versus metabolically healthy participants with normal weight. Participants with normal weight and at least 3 altered cardio-metabolic components were at almost two-fold (all cancers) to over three-fold (obesity-related cancers) increased risk of cancer mortality compared with participants with none. Low HDL-cholesterol was the cardio-metabolic risk factor most consistently associated with risk of cancer mortality, while elevated fasting glucose was also important for obesity-associated cancer mortality, suggesting a likely biological mechanism linking metabolic health status and cancer mortality.
There is increasing recognition of the importance of metabolic factors in influencing health risks and mortality outcomes 9,20-22. Metabolic dysregulation, in addition to chronic inflammation, genomic alterations and immune system dysfunction, have been shown to influence cancer etiology and mortality, and an increasing number of studies have begun to directly assess the mechanisms through which metabolic dysfunction affects cancer outcomes independent of body weight, with mixed results12,13. A recent study of over 22,000 adults in England and Scotland observed that metabolic healthy adults with obesity were not at increased risk of cancer mortality compared with metabolically healthy adults without obesity, while all adults (regardless of obesity status) with two or more metabolic risk factors (i.e. metabolically unhealthy) had a 59-64% increased risk of mortality23. Similarly, a recent study among Korean adults observed that regardless of BMI category, metabolically unhealthy participants were at significantly higher risk for cancer mortality compared with metabolically healthy adults12. In contrast, a recent systematic review and meta-analysis concluded that adults with obesity were at increased risk for mortality compared with normal-weight adults, even in the absence of metabolic abnormalities.22
We observed a significant association between reduced HDL-cholesterol and cancer mortality among participants. This suggests that the biological mechanism linking obesity, metabolic dysregulation and cancer mortality may involve dyslipidemia, and clinical strategies focused on cholesterol may provide added benefits to cancer patients, in addition to other strategies such as exercise and dietary recommendations to reduce metabolic dysfunction. Other studies have observed reduced risk of cancer with increased HDL-cholesterol32,33, while some studies suggest that the HDL-apolipoprotein ratio may be a stronger indicator of risk of cancer mortality32. Future work in this area is warranted as very few studies have directly examined HDL-cholesterol levels and cancer-specific mortality prospectively. Another potential biological mechanism for this association may be through the relationship between insulin resistance and liver fat content, which may act as an independent risk factor for cancer mortality separate from the well-established association with visceral fat mass. Several studies have observed that higher liver fat content and non-alcoholic fatty liver disease increase the risk of cardiovascular diseases 34 and type 2 diabetes 35,36, via insulin resistance, independent of visceral fat mass. This may be an important underlying mechanism for the higher risk of cancer mortality among normal weight, metabolically unhealthy individuals. Research studies focused on assessing liver fat content in addition to visceral fat among metabolically unhealthy adults may further shed light on this mechanism. Other potential mechanisms include inflammatory profile imbalance due to increased adiposity, and genetic or epigenetic changes that are important consequences of altered metabolic components such as obesity, dyslipidemia and high blood pressure, and may play an important role in the function of genes responsible for tumor angiogenesis, apoptosis or metastasis.
While still the subject of much debate, there is compelling evidence that obesity status itself may be a less informative risk or prognostic factor for many diseases, including cancer. In fact, our findings as well as others24,29 indicate that metabolic health status may be a more important cancer prognostic factor that should be evaluated in addition to obesity. We observed consistently higher risk of cancer mortality among normal-weight, metabolically unhealthy participants with associations that were significantly higher when focused on obesity-related cancers. In addition, regardless of metabolic health status, we observed lower risk of cancer death among participants overweight and obesity. There is a large body of literature regarding the role of inflammation in cancer prognosis30,31, and our observation that the metabolic health component most associated with mortality were reduced HDL cholesterol and elevated fasting glucose supports the hypothesis that tissue inflammation and insulin resistance, rather than obesity per se, is associated with cancer prognosis. However, considerable debate remains regarding the direct effects of obesity, versus mediating factors such as inflammation and/or metabolic dysregulation on cancer mortality. Future prospective studies of cancer will be needed to definitively identify which risk factor(s) are most strongly associated with cancer mortality and can be valuable targets of clinical interventions for cancer patients.
There are some limitations to this research. First, there are currently no established criteria for defining metabolically healthy obesity, and this analysis was based on the definition proposed in the joint harmonized criteria for metabolic syndrome. It is impossible to assess whether our definition overestimated or underestimated the prevalence of metabolic health. However, by using a strict criteria of 3 out of 5 altered metabolic components, we reduced the likelihood of overestimation, and our results did not change significantly after a sensitivity analysis using 2 out of 5 components to define metabolic health. Second, metabolically health and BMI category were assessed at baseline, and while this reduced the likelihood of reverse causality bias, it is likely that metabolic health and/or BMI category changed over the course of follow up. However, by excluding participants with a history of cancer at baseline we further reduced the likelihood of reverse causality. There are likely some participants who were classified as ‘cancer-free’ at baseline, but who had undiagnosed cancer, this may lead to biased estimates especially if those participants are more likely to be normal-weight. To address this possibility, we conducted sensitivity analysis excluding all cancer deaths occurring in the first 2 years after entry into the cohort, and our results remained consistent. We expect that these changes will be non-differential in relation to baseline metabolic health or BMI status, and that our observed estimates will likely underestimate the true association between metabolic health status and cancer mortality within the REGARDS cohort. In addition, we observed stronger associations between metabolic health status and cancer mortality when focused on obesity related cancers, and this association became stronger when excluding participants with comorbidities at baseline. Third, our cohort had only up to ten years of follow-up time, limiting our availability to observe cancer-specific deaths that will occur as the cohort ages. Fourth, even though REGARDS is a large prospective cohort study with over 30,000 participants, we were limited by the number of cancer mortality events observed which inhibited our ability to conduct race- and sex- stratified analysis. The strengths of this analysis include the use of a large prospective cohort study, and cancer mortality outcomes obtained using standardized adjudication techniques that minimized the chances of outcome misclassification. Baseline measures of obesity and metabolic health components were directly measured by highly trained interviewers during in-home visits, and therefore less vulnerable to misclassification or recall bias.
In conclusion, we provide evidence that metabolic health status is an important prognostic factor for cancer among normal weight adults. Longer follow-up times in the REGARDS cohort will enable us to better characterize this association by cancer type, in different racial and sex groups, and to better evaluate the biological mechanisms underlying this association in order to inform specific strategies to reduce the risk. Meanwhile, clinical and public health strategies to reduce obesity and improve metabolic health status among metabolic unhealthy adults, especially normal-weight adults, may go a long way in improving cancer outcomes specifically, and health outcomes in general, among US patients.
Supplementary Material
What is already known about this subject?
Obesity has long been understood to increase the risk of cancer mortality.
The prevalence of obesity and metabolic syndrome, a cluster of cardio-metabolic risk factors, have concurrently increased in the US over the past few decades.
What does this study add?
Metabolic health status is an important predictor of cancer mortality among normal-weight participants.
Among participants who were normal weight, being metabolically unhealthy increased the risk for mortality from obesity-related cancers.
Acknowledgments
FINANCIAL SUPPORT
This study was supported by award R01-NR012726 from the National Institute for Nursing Research, UL1-RR025777 from the National Center for Research Resources, K08HL096841 from the National Heart, Lung, and Blood Institute, as well as by grants from the Center for Clinical and Translational Science and the Lister Hill Center for Health Policy of the University of Alabama at Birmingham. The REGARDS study was supported by cooperative agreement U01-NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service. Dr. Akinyemiju was supported by grants U54 CA118948 and K01 TW010271-01A1 from the NIH. Dr. Moore received grant support from grants R25 CA47888 and T32CA190194 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
CONFLICTS OF INTERESTS
Dr. Safford reports the following potential conflicts of interest: Amgen - salary support to study patterns of statin use in Medicare and other large databases; diaDexus - salary support for a research grant on lipids and CHD outcomes; diaDexus - consulting to help with FDA application; NIH, AHRQ - salary support for research grants. Dr. Akinyemiju, Dr. Moore, Dr. Pisu, Dr. Judd, Dr. Goodman, Dr. Shikany, Dr. Howard, and Dr. Gilchrist do not report any related conflicts of interest.
AUTHORS CONTRIBUTIONS
TA led the design, analysis, interpretation of data and writing of the manuscript. TA and JM conducted statistical analysis and drafting of the manuscript. TA, JM, MP, SJ, MG, JS, VH, MS, SG contributed to the interpretation of data and writing and reviewing of the manuscript. All authors have read and approved the final version of the manuscript.
References
- 1.Akinyemiju T, Sakhuja S, Vin-Raviv N. Racial and socio-economic disparities in breast cancer hospitalization outcomes by insurance status. Cancer epidemiology. 2016;43:63–69. doi: 10.1016/j.canep.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doleman B, Mills KT, Lim S, Zelhart MD, Gagliardi G. Body mass index and colorectal cancer prognosis: a systematic review and meta-analysis. Tech Coloproctol. 2016;20(8):517–535. doi: 10.1007/s10151-016-1498-3. [DOI] [PubMed] [Google Scholar]
- 3.Nagle CM, Dixon SC, Jensen A, et al. Obesity and survival among women with ovarian cancer: results from the Ovarian Cancer Association Consortium. Br J Cancer. 2015;113(5):817–826. doi: 10.1038/bjc.2015.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang X, Zhou G, Sun B, et al. Impact of obesity upon prostate cancer-associated mortality: A meta-analysis of 17 cohort studies. Oncol Lett. 2015;9(3):1307–1312. doi: 10.3892/ol.2014.2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan DS, Vieira AR, Aune D, et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014;25(10):1901–1914. doi: 10.1093/annonc/mdu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill JL, You W, Zoellner JM. Disparities in obesity among rural and urban residents in a health disparate region. BMC Public Health. 2014;14:1051. doi: 10.1186/1471-2458-14-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003-2012. JAMA. 2015;313(19):1973–1974. doi: 10.1001/jama.2015.4260. [DOI] [PubMed] [Google Scholar]
- 8.Wildman RP, Muntner P, Reynolds K, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999-2004) Arch Intern Med. 2008;168(15):1617–1624. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 9.Stefan N, Haring HU, Hu FB, Schulze MB. Metabolically healthy obesity: epidemiology, mechanisms, and clinical implications. Lancet Diabetes Endocrinol. 2013;1(2):152–162. doi: 10.1016/S2213-8587(13)70062-7. [DOI] [PubMed] [Google Scholar]
- 10.Shea JL, Randell EW, Sun G. The prevalence of metabolically healthy obese subjects defined by BMI and dual-energy X-ray absorptiometry. Obesity (Silver Spring) 2011;19(3):624–630. doi: 10.1038/oby.2010.174. [DOI] [PubMed] [Google Scholar]
- 11.Moore LL, Chadid S, Singer MR, Kreger BE, Denis GV. Metabolic health reduces risk of obesity-related cancer in framingham study adults. Cancer Epidemiol Biomarkers Prev. 2014;23(10):2057–2065. doi: 10.1158/1055-9965.EPI-14-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oh CM, Jun JK, Suh M. Risk of cancer mortality according to the metabolic health status and degree of obesity. Asian Pac J Cancer Prev. 2014;15(22):10027–10031. doi: 10.7314/apjcp.2014.15.22.10027. [DOI] [PubMed] [Google Scholar]
- 13.Yang HK, Han K, Kwon HS, et al. Obesity, metabolic health, and mortality in adults: a nationwide population-based study in Korea. Sci Rep. 2016;6:30329. doi: 10.1038/srep30329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howard VJ, Cushman M, Pulley L, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25(3):135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 15.Safford MM, Brown TM, Muntner PM, et al. Association of race and sex with risk of incident acute coronary heart disease events. JAMA. 2012;308(17):1768–1774. doi: 10.1001/jama.2012.14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ligibel JA, Alfano CM, Courneya KS, et al. American Society of Clinical Oncology position statement on obesity and cancer. J Clin Oncol. 2014;32(31):3568–3574. doi: 10.1200/JCO.2014.58.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 18.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307(5):491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 19.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94(446):496–509. [Google Scholar]
- 20.Primeau V, Coderre L, Karelis AD, et al. Characterizing the profile of obese patients who are metabolically healthy. Int J Obes (Lond) 2011;35(7):971–981. doi: 10.1038/ijo.2010.216. [DOI] [PubMed] [Google Scholar]
- 21.Kaur A, Johnston DG, Godsland IF. Does metabolic health in overweight and obesity persist? - Individual variation and cardiovascular mortality over two decades. Eur J Endocrinol. 2016;175(2):133–143. doi: 10.1530/EJE-16-0095. [DOI] [PubMed] [Google Scholar]
- 22.Kramer CK, Zinman B, Retnakaran R. Are metabolically healthy overweight and obesity benign conditions?: A systematic review and meta-analysis. Ann Intern Med. 2013;159(11):758–769. doi: 10.7326/0003-4819-159-11-201312030-00008. [DOI] [PubMed] [Google Scholar]
- 23.Hamer M, Stamatakis E. Metabolically healthy obesity and risk of all-cause and cardiovascular disease mortality. J Clin Endocrinol Metab. 2012;97(7):2482–2488. doi: 10.1210/jc.2011-3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka A, Perlick A, Miller CC, et al. Metabolic Syndrome but not Obesity Adversely Affects Outcomes after Open Aortoiliac Bypass Surgery. Ann Vasc Surg. 2017 doi: 10.1016/j.avsg.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 25.Shen N, Fu P, Cui B, Bu CY, Bi JW. Associations between body mass index and the risk of mortality from lung cancer: A dose-response PRISMA-compliant meta-analysis of prospective cohort studies. Medicine (Baltimore) 2017;96(34):e7721. doi: 10.1097/MD.0000000000007721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X, Liu Y, Shao H, Zheng X. Obesity paradox in lung cancer prognosis: evolving biological insights and clinical implications. J Thorac Oncol. 2017 doi: 10.1016/j.jtho.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 27.Vadstrup S, Pedersen TE, Weywadt L, Wandrup J. Correlation between severity of septic conditions and circulating levels of ionized calcium. Intensive Care Med. 1989;15(5):329–330. [PubMed] [Google Scholar]
- 28.Banack HR, Stokes A. The ’obesity paradox’ may not be a paradox at all. Int J Obes (Lond) 2017;41(8):1162–1163. doi: 10.1038/ijo.2017.99. [DOI] [PubMed] [Google Scholar]
- 29.Carreras-Torres R, Johansson M, Haycock PC, et al. Obesity, metabolic factors and risk of different histological types of lung cancer: A Mendelian randomization study. PLoS One. 2017;12(6):e0177875. doi: 10.1371/journal.pone.0177875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rose DP, Gracheck PJ, Vona-Davis L. The Interactions of Obesity, Inflammation and Insulin Resistance in Breast Cancer. Cancers (Basel) 2015;7(4):2147–2168. doi: 10.3390/cancers7040883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perez-Hernandez AI, Catalan V, Gomez-Ambrosi J, Rodriguez A, Fruhbeck G. Mechanisms linking excess adiposity and carcinogenesis promotion. Front Endocrinol (Lausanne) 2014;5:65. doi: 10.3389/fendo.2014.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chandler PD, Song Y, Lin J, et al. Lipid biomarkers and long-term risk of cancer in the Women’s Health Study. Am J Clin Nutr. 2016;103(6):1397–1407. doi: 10.3945/ajcn.115.124321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tamura T, Inagawa S, Hisakura K, Enomoto T, Ohkohchi N. Evaluation of serum high-density lipoprotein cholesterol levels as a prognostic factor in gastric cancer patients. J Gastroenterol Hepatol. 2012;27(10):1635–1640. doi: 10.1111/j.1440-1746.2012.07189.x. [DOI] [PubMed] [Google Scholar]
- 34.Liu J, Musani SK, Bidulescu A, et al. Fatty liver, abdominal adipose tissue and atherosclerotic calcification in African Americans: the Jackson Heart Study. Atherosclerosis. 2012;224(2):521–525. doi: 10.1016/j.atherosclerosis.2012.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sung KC, Jeong WS, Wild SH, Byrne CD. Combined influence of insulin resistance, overweight/obesity, and fatty liver as risk factors for type 2 diabetes. Diabetes Care. 2012;35(4):717–722. doi: 10.2337/dc11-1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jung CH, Lee WJ, Hwang JY, et al. Assessment of the fatty liver index as an indicator of hepatic steatosis for predicting incident diabetes independently of insulin resistance in a Korean population. Diabet Med. 2013;30(4):428–435. doi: 10.1111/dme.12104. [DOI] [PubMed] [Google Scholar]
- 37.Bandera EV, Maskarinec G, Romieu I, John EM. Racial and ethnic disparities in the impact of obesity on breast cancer risk and survival: a global perspective. Adv Nutr. 2015;6(6):803–819. doi: 10.3945/an.115.009647. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


