Abstract
Objective
While it has been shown that prophylactic vaccination can induce genital immunity, there is inadequate information on HPV vaccine-induced oral immunity, which is of particular interest due to HPV-associated oropharyngeal malignancies and Recurrent Respiratory Papillomatosis. Therefore, we assessed the efficacy of various HPV vaccines against oral HPV pseudovirus infection in mice.
Study Design
Pre-clinical scientific investigation
Methods
C57BL/6 mice were vaccinated three times at 2-week intervals with either Gardasil (50 μl i.m.) or a candidate pan-HPV L2 vaccine with alum adjuvant (25 μg s.c.). Additional mice were immunized with passive transfer of either Gardasil human antisera or non-immunized sera (100 μL i.p.). All vaccinated and naïve control mice were then challenged with HPV16 E6E7 Luciferase pseudovirus in the oral mucosa. Visualization of HPV pseudovirus infection was monitored through luciferase activity using the IVIS Spectrum Imaging System.
Results
Oral Luciferase-expressing HPV16 pseudovirus infection was not detected in Gardasil, L2 vaccine and Gardasil antisera-immunized mice whereas robust Luciferase expression was observed in all control mice. An in vitro neutralization assay from sera of Gardasil-vaccinated mice confirmed vaccine efficacy was due to neutralizing antibodies.
Conclusions
Oral HPV16 pseudovirus infection in mice was completely prevented with all methods of prophylactic HPV immunization. These findings provide preliminary evidence that human vaccines induce protection against oral HPV infection, which has significant public health implications for HPV-associated oropharyngeal malignancies.
Keywords: Human Papillomavirus, HPV, vaccines, oral HPV, oral immunity
Introduction
Although human papillomavirus (HPV) is most commonly associated with cervical cancer, HPV is now recognized as an important etiologic factor in a subset of oropharyngeal squamous cell carcinomas (OPSCC)1–3, independent of alcohol or tobacco exposure, which are additional well-established risk factors4. The rates of these HPV-associated OPSCC have been increasing over time and now predominate in newly diagnosed OPSCC in the U.S.5. HPV is also the cause of Recurrent Respiratory Papillomatosis (RRP), which affects children and adults through infection of the aerodigestive tract with low-risk HPV.
Preventative vaccines against HPV became available in 2006, and were approved for use based on the remarkable protection they induce against cervical and genital HPV infection6,7. These vaccines are constructed from the viral major capsid protein L1 assembled into empty virus-like particles (VLPs) which induce a robust neutralizing antibody response. Because the protection induced by L1 VLP vaccines is predominantly type-restricted, the commercial vaccines are multivalent; for example quadrivalent Gardasil contains L1 VLP derived from the two most common low-risk types found in genital warts and RRP (HPV 6 and 11), and the two most common high-risk types in cervical cancer and oropharyngeal cancer (HPV 16 and 18). Now approved for boys and girls aged 9 – 26, the more recently available 9-valent Gardasil formulation targets five additional high risk types (HPV 31, 33, 45, 52, and 58). Immunity against low-risk and high-risk HPV strains can be induced by active vaccination or by passive transfer of the neutralizing antibodies after vaccination8. Prophylactic pan-HPV vaccines are also in development, which induce immunity against L2, a viral capsid protein containing broadly cross reactive protective epitopes9, but the effect of these L2 vaccines on genital immunity has not been established in human trials. Furthermore, there is a lack of research on HPV vaccine-induced oral immunity, which is of particular interest for the prevention of OPSCC.
We hypothesized that oral immunity in the mice would be established after immunization, and the goal of our study was to determine if murine oral HPV16 infection could be prevented with prophylactic vaccines targeting either L1 or L2.
Materials and Methods
Pseudovirus production
HPV16 pseudovirus (PsV) infection is an established method for evaluating immunologic responses to vaccination by producing a virus with the HPV capsid proteins, but containing a reporter plasmid instead of the viral genome10. HPV16 was chosen for this study as it is the dominant (~90%) genotype associated with OPSCC3. HPV16 pseudovirus containing an HPV16 E6E7 Luciferase reporter was produced by co-transfection of 293TT cells with a p16sheLL, a plasmid encoding HPV16 L1 and L2, and a second reporter plasmid simultaneously expressing HPV E6 and E7 and the reporter protein firefly luciferase. Transfection occurred in the presence of Opti-MEM I and Lipofectamine 2000. Cells were lysed using a Triton X-100 detergent solution. Following pseudovirus purification using an Optiprep gradient with iodixanol, fractions were collected and tested on 293TT cultures to evaluate reporter gene expression.
Animals
C57BL/6 female mice, 4–6 week old, were purchased for animal studies (Charles River, Maryland). Animal protocols were performed in accordance with the recommendations for proper ethical use and care of laboratory animals.
Immunization
Mice were divided into vaccination groups (n=5): Gardasil, Gardasil human antisera, pan-HPV L2 vaccine, and naïve control. The first group of mice received three doses i.m. of 50 μl Gardasil at two week intervals. The third vaccination group received three doses at 2-week intervals of pan-HPV L2 vaccine (L2 α11–88×5) with alum adjuvant (25 μg s.c.). The control group was not immunized, and a separate control group for each experimental arm was performed using the same batch of PsV to control for bioluminescent variance in PsV production. All vaccinated and control groups were sedated with ketamine and challenged with 50 μl diluted HPV16 E6E7 Luciferase PsV in the oral mucosa by traumatizing oral mucosa with a 25 gauge needle followed by submucosal injection. Immunization and challenge timelines are shown in Figures 1A and 2A.
Figure 1. Gardasil vaccination.
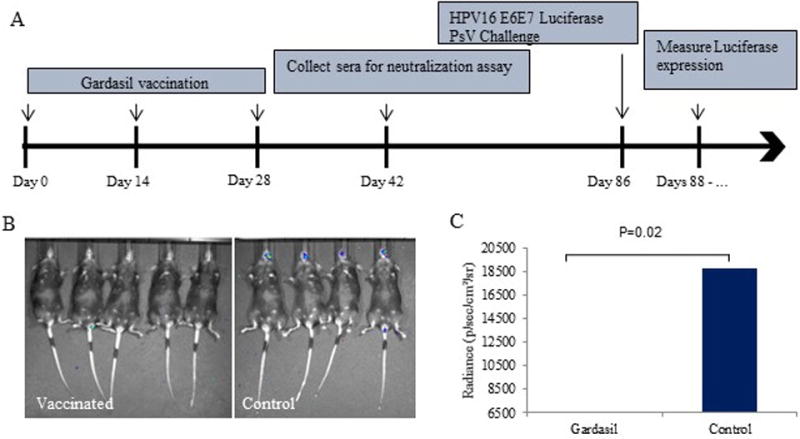
Mice were vaccinated with three doses of Gardasil at 2-week intervals (50 μl i.m.). A) Vaccination and PsV challenge timeline. B) Representative image of luciferase activity in vaccinated v. control mice. C) Average radiance (p/sec/cm2/sr) in vaccinated v. control mice with t-test results. The y-scale minimum was set to 6500 to account for background signal.
Figure 2. Pan-HPV L2 with alum adjuvant vaccination.
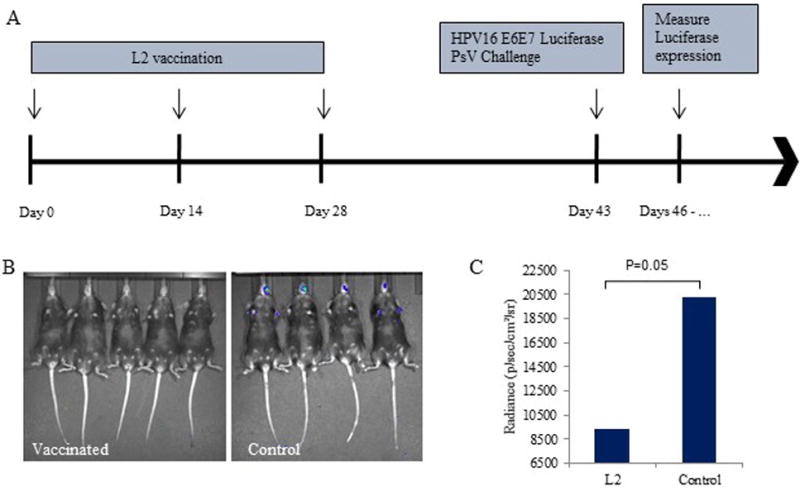
Mice were vaccinated three times at 2-week intervals with a pan-HPV L2 vaccine with alum adjuvant (25 μg s.c.). A) Vaccination and PsV challenge timeline. B) Representative image of luciferase activity in vaccinated v. control mice. C) Average radiance (p/sec/cm2/sr) in vaccinated v. control mice with t-test results. The y-scale minimum was set to 6500 to account for background signal.
Passive transfer studies
The second group of naïve mice was administered a single dose of 100 μl i.p. of either human Gardasil antisera or human non-immunized sera. Immunization and challenge timeline is shown in Figure 3A.
Figure 3. Passive immunization with human Gardasil antisera.
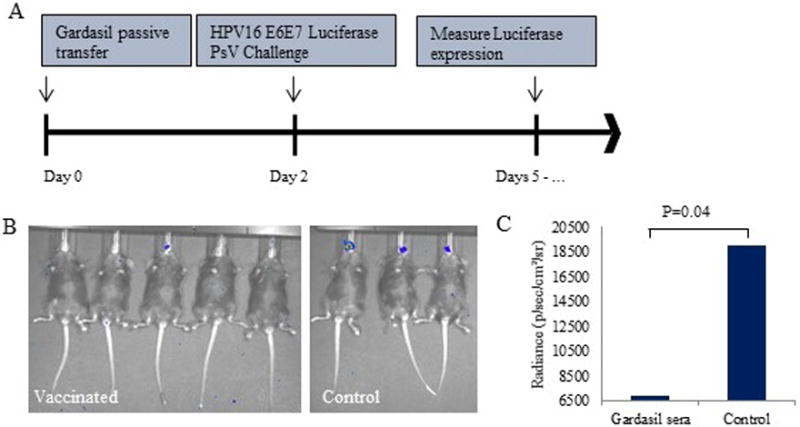
Mice were vaccinated with a single dose of Gardasil immunized human sera or non-immunized sera (100 μl i.p.). A) Vaccination and PsV challenge timeline. B) Representative image of luciferase activity in vaccinated v. control mice. C) Average radiance (p/sec/cm2/sr) in vaccinated v. control mice with t-test results. The y-scale minimum was set to 6500 to account for background signal.
Luciferase activity
To measure the luciferase activity in vivo, mice were anesthetized with ketamine and injected with 3.9 mg/ml Luciferin (200 μl i.p.). Ten minutes after injection, luciferase activity was visualized in all mice using the IVIS Spectrum Imaging System. Bioluminescent levels were extracted from resulting images using image analysis software provided as part of the IVIS Imaging System.
Neutralization assay
A luciferase-based pseudovirus neutralization assay was performed with mouse sera collected two weeks after final Gardasil vaccination to assess vaccine efficacy. Two-fold dilutions of titrated mouse sera (starting dilution 1:25) were incubated with diluted HPV16 Luciferase PsV for two hours. This mixture was then added to a 96 well plate with 293TT cells and incubated at 37°C for 72 hours. The cells were then lysed at room temperature with Cell Culture Lysis Reagent (Promega, Madison WI) for 15 minutes. After lysis, luciferase activity was measured using a luminometer.
Statistics
A heteroscedastic student’s t-test was performed with a two-tailed distribution to analyze the mean bioluminescence between the experimental mice and their associated control group. P < 0.05 was considered to be statistically significant.
Results
Visualization and quantitation of oral HPV16 infection in naïve and immunized mice
After completion of the 3 dose vaccine regimen, the control mice, and those vaccinated with Gardasil or L2 multimer were all challenged with HPV16 E6E7 Luciferase PsV in the oral mucosa. To evaluate the protective immune response of each vaccination method in vivo, luciferase activity was measured following PsV challenge. Luciferase expression was at its peak in control mice at approximately 3 days post infection. Bioluminescent images were taken on the same days for the vaccinated mice. As shown in Figure 1B and Figure 2B, the control mice displayed significantly higher Luciferase activity compared to the vaccinated groups. Gardasil and the pan-HPV L2 vaccines were able to completely prevent HPV infection in the immunized mice. The average radiance in the Gardasil vaccinated mice was 6054.2 p/sec/cm2/sr compared to 18728 p/sec/cm2/sr in the control group (p=0.02) (Figure 1C). The L2 vaccinated mice also displayed significantly lower Luciferase expression, 9378.6 p/sec/cm2/sr, compared to controls, 20242.8 p/sec/cm2/sr (p=0.05) (Figure 2C). Multiple images were taken over the next week after PsV challenge, and no signal was detected at any time point for any vaccinated mice (results not shown).
Passive transfer of neutralizing immune serum
A passive transfer experiment was performed to confirm that Gardasil vaccination in humans produced neutralizing antibodies capable of preventing the oral HPV16 PsV infection. As shown in Figure 3B, passive transfer of human Gardasil antisera into mice prevented Luciferase expression in the oral cavity after PsV challenge. Passive transfer of Gardasil human antisera resulted in an average radiance of 6869.8 p/sec/cm2/sr versus 18937.5 p/sec/cm2/sr in the non-immunized mice (p=0.04) (Figure 3C).
Serum neutralization of PsV activity
Finally, to confirm that the humoral immunity induced by vaccination was responsible for the prevention of HPV16 oral PsV infection a serum neutralization assay was performed. Serial dilution of two representative mouse sera collected two weeks after final Gardasil vaccination demonstrated increased virus neutralizing activity against in vitro HPV16 PsV infection of 283TT cells compared to non-vaccinated sera (Supplemental Figure 1).
Discussion
In this study we demonstrate the prevention of oral HPV16 pseudovirus infection in mice with prophylactic HPV vaccines, including Gardasil, which targets L1, and an HPV L2 vaccine. C57BL/6 mice vaccinated with L1 and L2 vaccines generated significant in vivo immune responses against HPV16, as shown by the prevention of bioluminescent Luciferase activity using the HPV16 PsV challenge. All control naïve mice that were challenged with the pseudovirus displayed significantly greater bioluminescent activity compared to immunized mice. Bioluminescent images were taken over the course of several days after PsV challenge, and complete protection was achieved in all vaccinated mice. This prevention of infection is due to neutralizing antibodies produced by vaccination, confirmed by both the passive transfer of human Gardasil antisera and direct measurement of virus neutralizing activity in vaccinated mouse sera.
Prophylactic vaccination studies on HPV have been mainly focused on the prevention of anogenital and cervical HPV-associated diseases6,7. However, in developed countries OPSCC may overtake cervical disease in incidence11. Therefore, the question of whether prophylactic vaccination protects against oral HPV infection is of critical import. The lack of precursor lesions in the oropharynx or effective screening tools (such as pap smears) means the direct evidence of decreased oral HPV infection in response to vaccination humans is challenging to obtain. In this study however, we are able to show effective prevention of oral HPV16 by using human L1 and L2 vaccines in a murine model.
Gardasil is a commercially available HPV L1 VLP vaccine, currently available in a 9-valent format, as the quadrivalent vaccine utilized in this study has been discontinued (although it has been widely used globally). Vaccination with L1 VLP vaccines induce a durable antibody response12, and neutralizing antibody titres8. In agreement with previous studies examining protective mechanisms active against vaginal challenge, the results from the present study demonstrated significant immune responses against the HPV16 L1 capsid protein in Gardasil immunized mice using the in vitro neutralization assay and that Gardasil-specific neutralizing antiserum was in turn able directly confer oral immunity, both by primary vaccination as well as by transfer of murine anti-serum from humans previously vaccinated with Gardasil. Taken together, this provides strong evidence that the systemic immune response to vaccination is sufficient to provide mucosal protection in the oral cavity as well as in the female reproductive tract and anogenital sites demonstrated in clinical studies.
A pan-HPV L2 vaccine with an alum adjuvant was chosen for the third vaccination group due to the low antigenic variation of L2 in HPV. L2 is therefore the target for the development of a broad spectrum prophylactic HPV vaccine that could be administered to the same target demographic as the currently approved vaccines and provide broad protection against all HPV subtypes. Previous studies have shown that the amino terminus of L2 is protective in various animal models, such as rabbits13 as well as cattle14. Passive immunization in naïve mice with L2 immunized human sera is also able to elicit an immune response against vaginal HPV infection8 and pan-HPV vaccine candidates are able to elicit L2-specific antibody titers in immunized mice9. Although L2-specific antibody titers were not measured in this study, protection against oral HPV16 infection was visualized in the mice following vaccination with the L2-based pan-HPV vaccine. L2-based vaccines therefore offer an alternate method to elicit both cervical and non-cervical immunity against HPV.
Evidence that oral immunity is induced by HPV vaccination in humans is beginning to emerge now that widespread vaccination has been implemented. HPV antibody responses can be detected in oral secretions after vaccination in a very high percentage of patients15,16. These oral antibody levels correlate closely with the serum levels obtained after vaccination, and the normalized IgG levels are not substantially different than serum levels15. Post-hoc analysis of a vaccinated population in Costa Rica demonstrated decreased oral HPV infection in females four years after vaccination17. However, due to the difficulty in evaluating rates of actual HPV-associated disease in the oropharynx without precursor lesions, human studies demonstrating changes in rates of OPSCC due to vaccination will likely take many years to complete.
All methods of vaccination in this study were able to provide robust protection against HPV16 PsV infection via oral challenge. While we did not directly measure oral antibody responses, our in vivo challenge results, in conjunction with studies that show oral antibodies after vaccination in humans, together provide strong evidence that the systemic and local immune response generated by vaccination is sufficient to prevent oral injection.
Conclusion
Vaccination with HPV vaccines, including Gardasil, pan HPV L2 vaccine, and Gardasil antiserum induced protection against HPV 16 infection after challenge at oral mucosa sites in mice. Both in vivo and in vitro findings in this study suggest the merit of studying the effect of human vaccines on oral immunity and suggest that long-term population control of OPSCC and RRP may be achievable through widespread vaccination.
Supplementary Material
Supplemental Figure 1. Neutralization assay. Two-fold dilutions of titrated control (pink) and Gardasil-treated (purple) mouse sera (starting dilution 1:25) were incubated with diluted HPV16 Luciferase PsV for two hours. Luciferase activity was measured after incubation with 293TT cells for 72 hours. Gardasil-treated mice display increased viral neutralization capacity up to 1:800 dilution compared to control sera from non-vaccinated mice.
Acknowledgments
Financial Support: Financial Support for this study was provided by the Triological Society Career Development Award (S. Best) and the NIDCD Mentored Patient-Oriented Research Career Development Award 1K23DC014758 (S. Best).
Footnotes
Conflict of Interest: Richard Roden is an inventor of L2-related patents licensed to Shantha Biotechnics Ltd., GlaxoSmithKline, PaxVax, Inc. and Acambis, Inc. Richard Roden has received research funding from Sanofi Pasteur, Shantha Biotechnic and GlaxoSmithKline and is a co-founder of, and has an equity ownership interest in Papivax LLC. Richard Roden owns Papivax Biotech Inc. stock options and is a member of Papivax Biotech Inc.’s Scientific Advisory Board. Richard Roden owns Pathovax LLC stock options and is a member of Pathovax’s Scientific Advisory Board. These arrangements have been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies. The other authors have no funding, financial relationships, or conflicts of interest to disclose.
Level of Evidence: N/A (Animal Study and basic research)
Authors’ Note
This study was performed in accordance with the Public Health Service Policy on Humane Care and Use of Laboratory Animals, the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the Animal Welfare Act (7 U.S.C. et seq.); the animal use protocol was approved by the Institutional Animal Care and Use Committee of Johns Hopkins Medical Institution.
References
- 1.Gillison ML, D’Souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100(6):407–420. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 2.Herrero R, Castellsague X, Pawlita M, et al. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst. 2003;95(23):1772–1783. doi: 10.1093/jnci/djg107. [DOI] [PubMed] [Google Scholar]
- 3.Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14(2):467–475. doi: 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- 4.D’Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356(19):1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 5.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356(19):1928–1943. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 7.Group FIS. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356(19):1915–1927. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 8.Wang JW, Jagu S, Wang C, et al. Measurement of neutralizing serum antibodies of patients vaccinated with human papillomavirus L1 or L2-based immunogens using furin-cleaved HPV Pseudovirions. PLoS One. 2014;9(7):e101576. doi: 10.1371/journal.pone.0101576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang D, Li Z, Xiao J, et al. Identification of Broad-Genotype HPV L2 Neutralization Site for Pan-HPV Vaccine Development by a Cross-Neutralizing Antibody. PLoS One. 2015;10(4):e0123944. doi: 10.1371/journal.pone.0123944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Longet S, Schiller JT, Bobst M, Jichlinski P, Nardelli-Haefliger D. A murine genital-challenge model is a sensitive measure of protective antibodies against human papillomavirus infection. J Virol. 2011;85(24):13253–13259. doi: 10.1128/JVI.06093-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lowy DR, Schiller JT. Reducing HPV-associated cancer globally. Cancer Prev Res (Phila) 2012;5(1):18–23. doi: 10.1158/1940-6207.CAPR-11-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.GlaxoSmithKline Vaccine HPVSG. Romanowski B, de Borba PC, et al. Sustained efficacy and immunogenicity of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 6.4 years. Lancet. 2009;374(9706):1975–1985. doi: 10.1016/S0140-6736(09)61567-1. [DOI] [PubMed] [Google Scholar]
- 13.Embers ME, Budgeon LR, Pickel M, Christensen ND. Protective immunity to rabbit oral and cutaneous papillomaviruses by immunization with short peptides of L2, the minor capsid protein. J Virol. 2002;76(19):9798–9805. doi: 10.1128/JVI.76.19.9798-9805.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawana K, Yoshikawa H, Taketani Y, Yoshiike K, Kanda T. Common neutralization epitope in minor capsid protein L2 of human papillomavirus types 16 and 6. J Virol. 1999;73(7):6188–6190. doi: 10.1128/jvi.73.7.6188-6190.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinto LA, Kemp TJ, Torres BN, et al. Quadrivalent Human Papillomavirus (HPV) Vaccine Induces HPV-Specific Antibodies in the Oral Cavity: Results From the Mid-Adult Male Vaccine Trial. J Infect Dis. 2016;214(8):1276–1283. doi: 10.1093/infdis/jiw359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rowhani-Rahbar A, Carter JJ, Hawes SE, et al. Antibody responses in oral fluid after administration of prophylactic human papillomavirus vaccines. J Infect Dis. 2009;200(9):1452–1455. doi: 10.1086/606026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herrero R, Quint W, Hildesheim A, et al. Reduced prevalence of oral human papillomavirus (HPV) 4 years after bivalent HPV vaccination in a randomized clinical trial in Costa Rica. PLoS One. 2013;8(7):e68329. doi: 10.1371/journal.pone.0068329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Neutralization assay. Two-fold dilutions of titrated control (pink) and Gardasil-treated (purple) mouse sera (starting dilution 1:25) were incubated with diluted HPV16 Luciferase PsV for two hours. Luciferase activity was measured after incubation with 293TT cells for 72 hours. Gardasil-treated mice display increased viral neutralization capacity up to 1:800 dilution compared to control sera from non-vaccinated mice.


