Abstract
Blood pressure responses to dietary sodium vary widely person-to-person. Salt sensitive rodent models display altered autonomic function, a trait thought to contribute to poor cardiovascular health. Thus, we hypothesized that increased salt sensitivity (SS) in normotensive humans would be associated with increased muscle sympathetic nerve activity (MSNA), decreased high frequency heart rate variability (HF-HRV), and decreased baroreflex sensitivity. Healthy normotensive men and women completed 1 week of high (300 mmol•day−1) and 1 week of low (20 mmol•day−1) dietary sodium (random order) with 24hr mean arterial pressure (MAP) assessed on the last day of each diet to assess SS. Participants returned to the lab under habitual sodium conditions for testing. Forty-two participants are presented in this analysis, 19 of which successful MSNA recordings were obtained (n=42: age 39±2 yrs, BMI 24.3±0.5 kg•(m2)−1, MAP 83±1 mmHg, habitual urine sodium 93±7 mmol•24 h−1; n=19: MSNA burst frequency 20±2 bursts•min−1). The variables of interest were linearly regressed over the magnitude of SS. Higher SS was associated with increased MSNA (burst frequency: r=0.469, p=0.041), decreased HF-HRV (r=−0.349, p=0.046), and increased LF/HF-HRV (r=0.363, p=0.034). SS was not associated with sympathetic or cardiac baroreflex sensitivity (p>0.05). Multiple regression analysis accounting for age found that age, not SS, independently predicted HF-HRV (age adjusted no longer significant; p=0.369) and LF/HF-HRV (age adjusted p=0.273). These data suggest that age-related salt sensitivity of blood pressure in response to dietary sodium is associated with altered resting autonomic cardiovascular function.
Keywords: Age, Sympathetic, MSNA, Sodium, Salt, Sensitive
INTRODUCTION
The responses of blood pressure to increased dietary sodium consumption vary widely person-to-person. What makes the blood pressure of some adults more salt sensitive than others is complex and not well understood, but evidence in rodent models suggests that numerous alterations in autonomic cardiovascular regulation may be involved (Brown et al., 1989; Ferrari et al., 1984; Miyajima and Buñag, 1986; Murphy and McCarty, 1995; Nedvídek and Zicha, 1993; Reddy et al., 1991). Proper functioning of both the parasympathetic and sympathetic branches of the autonomic nervous system are crucial to maintaining cardiovascular homeostasis, which may play a role in the poor cardiovascular outcomes of individuals with elevated salt sensitivity (SS) (Barba et al., 2007; Weinberger, 2002). A dearth of research exists examining resting autonomic function in relation to SS during habitual sodium consumption in normotensive adult humans.
Dysfunction in either branch of the autonomic nervous system could result in abnormal cardiovascular regulation that would impact blood pressure control. This is commonly found in adults with established pathologies such as hypertension (Mancia and Grassi, 2014), chronic heart failure (Silber et al., 1998), and chronic kidney disease (Park et al., 2015, 2013). Each of these conditions is associated with decreased parasympathetic activity, indexed in vivo by heart rate variability (HRV) analysis, and/or increased sympathetic activity, measured in vivo by microneurography. Vagal and sympathetic outflow are continuously modulated by the baroreflex to control blood pressure. Baroreflex sensitivity is low in multiple chronic disease states including the ones mentioned above (La Rovere et al., 2008). Studies in rodents suggest baroreflex function is also abnormal in normotensive highly salt sensitive animals fed a normal NaCl diet (Gordon and Mark, 1984). Thus investigating both autonomic nervous system branches and baroreflex function in relation to the degree of SS in normotensive adults would provide significant insight into SS.
Despite animal evidence suggestive of a link between SS and resting autonomic cardiovascular activity (Brown et al., 1989; Ferrari et al., 1984; Miyajima and Buñag, 1986; Murphy and McCarty, 1995; Nedvídek and Zicha, 1993; Reddy et al., 1991), little research has been done to examine this in healthy normotensive adults (Buchholz et al., 2003). Therefore, the purpose of this study was to test whether there was an association between degree of SS and autonomic cardiovascular function in healthy normotensive adults consuming habitual sodium intake. To accomplish this, we performed autonomic testing in a cohort of subjects that were previously assessed for their degree of SS (Brian et al., 2017). Based on the available literature (Brown et al., 1989; Ferrari et al., 1984; Miyajima and Buñag, 1986; Murphy and McCarty, 1995; Nedvídek and Zicha, 1993; Reddy et al., 1991), we hypothesized that higher SS would be associated with lower parasympathetic activity (HRV), lower vagal and sympathetic baroreflex function, and higher sympathetic activity (muscle sympathetic nerve activity, MSNA). While degree of SS was indexed as the change in 24 hour blood pressure during controlled low and high sodium diets, autonomic cardiovascular function was separately tested during habitual sodium intake conditions to reflect the participants’ normal diet state.
METHODS
Participants
All procedures and protocols in this investigation conform to the Declaration of Helsinki and were approved by the University of Delaware Institutional Review Board. All participants signed a written informed consent prior to participation. Eighty-seven healthy normotensive men and women underwent SS phenotyping. Of these 87 individuals, 50 agreed to return to the lab for testing under a habitual sodium intake condition. Eight were excluded from this analysis due to the presence of ectopic heart beats. Thus, 42 participants are presented in this analysis. An adequate MSNA signal was obtained in 19 participants.
Salt Sensitivity Phenotyping
Participants were originally recruited for participation in a separate study in which SS was assessed. The SS assessment protocol has been explained elsewhere (Brian et al., 2017; Matthews et al., 2015). In brief, participants completed a run-in week of recommended dietary sodium intake (100 mmol•day−1). The run-in phase was followed by one week of high (300 mmol•day−1) and one week of low (20 mmol•day−1) dietary sodium in random order. Twenty-four hour mean arterial pressure (MAP) (model 90207; Spacelabs Medical, Issaquah, WA, USA) was assessed on the last day of each diet. The change in 24hr MAP from low to high sodium was used as a continuous measurement variable for SS (de Leeuw and Kroon, 2013). Twenty-four hour urinary excretion of sodium was used to confirm participant compliance to the SS test diet and has been published elsewhere (Brian et al., 2017).
Baseline/Screening Visit
The 50 participants who agreed to return underwent a baseline/screening visit specific to the current study. The screening visits were performed at the University of Delaware Cardiovascular Research Laboratory and included the following: resting blood pressure, resting electrocardiogram, height, weight, and medical history questionnaire. Participants were excluded if they were not between 22–60 years of age, had known chronic health conditions affecting the cardiovascular system, had a BMI>30 kg•(m2)−1, used hormone replacement therapy, or used nicotine products.
Data Collection Visit
Prior to the data collection visit participants avoided alcohol, caffeine, and exercise for 24hrs, and fasted for 4hrs. Twenty-four hour urine collections were performed to assess habitual dietary sodium intake. All pre-menopausal women were studied during the first five days of their menstrual cycle. Participants laid supine during the data collection. The non-dominant hand was kept level with the heart and used to measure beat-by-beat arterial blood pressure with a photoplethysmograph finger cuff on the middle finger. The finger cuff was servo-controlled and calibrated to the manufacturer’s recommendations (Finometer; Finapres Medical Systems, Amsterdam, The Netherlands). Post hoc level correction was applied to finger pressures to match the baseline finger pressure to baseline brachial artery pressure (Dinamap Dash 2000; GE Medical Systems, Milwaukee, WI, USA) as done previously (Muller et al., 2011). Electrocardiograph lead II tracing (Dinamap Dash 2000; GE Medical Systems, Milwaukee, WI, USA) was collected. Respiratory movements were monitored using a piezo-electric respiration transducer (PneumoTrace; UFI, Morro Bay, CA, USA) to insure normal breathing patterns free of Valsalva maneuvers.
MSNA was recorded using microneurography (Sundlöf and Wallin, 1978). A tungsten microelectrode was inserted in the peroneal nerve posterior to the fibular head. A reference microelectrode was inserted 2–3 cm from the primary microelectrode. The nerve signal was amplified (factor = 70 000), bandpass filtered (700–2 000 Hz), rectified, and integrated (time constant 0.1 s) using a nerve traffic analyzer (Nerve Traffic Analyzer, model 662c-3; University of Iowa Bioengineering, Iowa City, IA, USA). The MSNA signal was confirmed to be free of skin sympathetic nervous activity using the following criteria: absence of afferent activity during light stroking of the skin, increased efferent activity during voluntary end-expiratory apnea, and spontaneous efferent signal gaiting by the cardiac cycle.
All measurements collected during the data collection visit were recorded at 1000 Hz using the PowerLab data acquisition system. Data were analyzed with LabChart 7 software (ADInstruments, Colorado Springs, Colorado, USA), or exported from LabChart and analyzed with a custom designed LabVIEW program (Matthews et al., 2016). This custom LabVIEW program was used to extract synchronized beat-by-beat data of MSNA, blood pressure, and ECG R-wave time. After instrumentation, participants rested quietly in a dimly lit room for at least 10 minutes prior to recording ≈5 minutes of resting data. Participants were allowed to breathe naturally, without paced breathing, during the data collection.
Heart Rate Variability Analysis
R-to-R intervals were calculated from ECG R-waves and analyzed with Kubios HRV software (Tarvainen et al., 2014) (University of Eastern Finland, Joensuu, Finland). The time domain and fast Fourier transformation frequency domain methods were used to analyze HRV. Frequency power was categorized as low frequency bands (0.04–0.15 Hz) and high frequency bands (0.15–0.4 Hz). The very low frequency band (<0.04 Hz) was not analyzed due to short recording time.
Muscle Sympathetic Nerve Activity Analysis
The MSNA signal was analyzed offline using a custom LabVIEW program as done previously (Matthews et al., 2016). This program identifies MSNA bursts by an R-wave gating approach. The mean value of the three largest bursts was assigned a value of 100 arbitrary units (AU) and all other bursts were scaled accordingly. Resting MSNA values are reported as burst frequency (burst per unit of time normalized to 1 minute, bursts•min−1) and burst incidence (bursts•100 heart beats−1).
Cardiac Baroreflex Sensitivity Analysis
Cardiac baroreflex sensitivity was calculated by means of the sequence method using HemoLab software (Harald Stauss Scientific, Iowa City, IA, USA). The R-to-R interval was regressed over systolic blood pressure for each sequence of four or more consecutive cardiac cycles where both variables increased (up sequences) or decreased (down sequences) in unison. Only sequences with a minimum acceptable r value of 0.8 were analyzed. The average of all regression line slopes for up and down sequences were calculated and used as indices of cardiac baroreflex sensitivity.
Sympathetic Baroreflex Sensitivity Analysis
Sympathetic baroreflex sensitivity was assessed using the spontaneous oscillations method as previously described (Greaney et al., 2013; Sundlöf and Wallin, 1978). This method quantifies baroreflex sensitivity around the operating point, and is well correlated to the modified Oxford technique (Hart et al., 2010). In this method each cardiac cycle is assigned to a 2 mmHg wide bin based on the diastolic blood pressure of the corresponding cardiac cycle. Burst incidence per bin (bursts•100 heart beats−1) and total MSNA per bin (burst height [AU]•heart beat−1) were regressed over diastolic blood pressure bins. All data was weighted to account for the number of cardiac cycles within each bin (Greaney et al., 2013; Ogoh et al., 2007). Bins without MSNA activity were included in the analysis. A minimum of r=0.5 was used as an inclusion criterion (Greaney et al., 2013; Ogoh et al., 2009). The slope of the linear regression line is used as an index value for sympathetic baroreflex sensitivity.
Statistical Analysis
Variables were regressed over the degree of SS (non-dichotomized). Secondary analysis used SS and age to create a multiple regression models to determine the effects of SS on key variables while adjusting for age (model 1 predictors: age; model 2 predictors age and SS). Variables of interest were also regressed over 24hr urinary sodium excretion under the habitual sodium condition. Alpha level of significance was set at p<0.05 for all statistical tests. Values expressed as mean ± SEM.
RESULTS
Table 1 shows participant characteristics. The blood and urine values contained in table 1 were collected during the data collection visit (habitual sodium intake), while all other information was collected during the baseline/screening visit (also under habitual sodium intake). Participant characteristic data including anthropometric measures, blood pressure, heart rate, hemoglobin, hematocrit, serum electrolytes, plasma osmolality, and urine electrolytes were unrelated to the degree of SS (range −12 to +10 ΔmmHg). Participants were primarily Caucasian (Caucasian=36, African American=3, African=1, Indian=1, biracial=1). Participant age (39±2 yrs, range 22–60 yrs) was significantly related to the degree of SS (Table 1), confirming previous findings (Weinberger and Fineberg, 1991). The 19 participants with successful MSNA recordings were representative of the total sample (36±3 yrs, range 22–59 yrs; BMI 24.7±0.7 kg•(m2)−1; MAP 84±2 mmHg; habitual urine sodium 77±6 mmol•24 h−1). In these 19 participants, age was similarly related to SS (r=0.475, p=0.038).
Table 1.
Participant Characteristics
| Baseline Demographic Data | Association with SS (r) |
p value | |||
|---|---|---|---|---|---|
| N (M,F) | 42 (24, 18) | - | - | ||
| Age (Years) | 39 | ± | 2 | 0.377 | 0.014 |
| Height (cm) | 174 | ± | 1 | −0.164 | 0.298 |
| Mass (kg) | 74 | ± | 2 | −0.018 | 0.912 |
| BMI (kg•(m2)−1) | 24 | ± | 0.5 | 0.099 | 0.531 |
| SBP (mmHg) | 114 | ± | 2 | −0.128 | 0.419 |
| DBP (mmHg) | 68 | ± | 1 | −0.292 | 0.060 |
| MAP (mmHg) | 83 | ± | 1 | −0.253 | 0.106 |
| Heart rate (Beats•min−1) | 59 | ± | 1 | 0.136 | 0.390 |
| Hemoglobin (g•dL−1) | 13.4 | ± | 0.2 | −0.114 | 0.540 |
| Hematocrit (%) | 41 | ± | 1 | −0.021 | 0.910 |
| Serum Na+ (mmol•L−1) | 138.2 | ± | 0.5 | −0.117 | 0.522 |
| Serum Cl− (mmol•L−1) | 104.3 | ± | 0.5 | −0.133 | 0.467 |
| Serum K+ (mmol•L−1) | 4.1 | ± | 0.1 | 0.232 | 0.200 |
| Plasma OsM (mOsm•kg H2O−1) | 287 | ± | 1 | −0.260 | 0.156 |
| Urine Na+ (mmol•24 h−1) | 163.2 | ± | 10 | −0.235 | 0.138 |
| Urine Cl− (mmol•24 h−1) | 195.2 | ± | 8 | −0.101 | 0.529 |
| Urine K+ (mmol•24 h−1) | 72.5 | ± | 4 | 0.144 | 0.374 |
Blood based values were collected at the data collection visit. All other values were collected from the baseline/screening visit. Values are means ± SE. BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial blood pressure; OsM, osmolality.
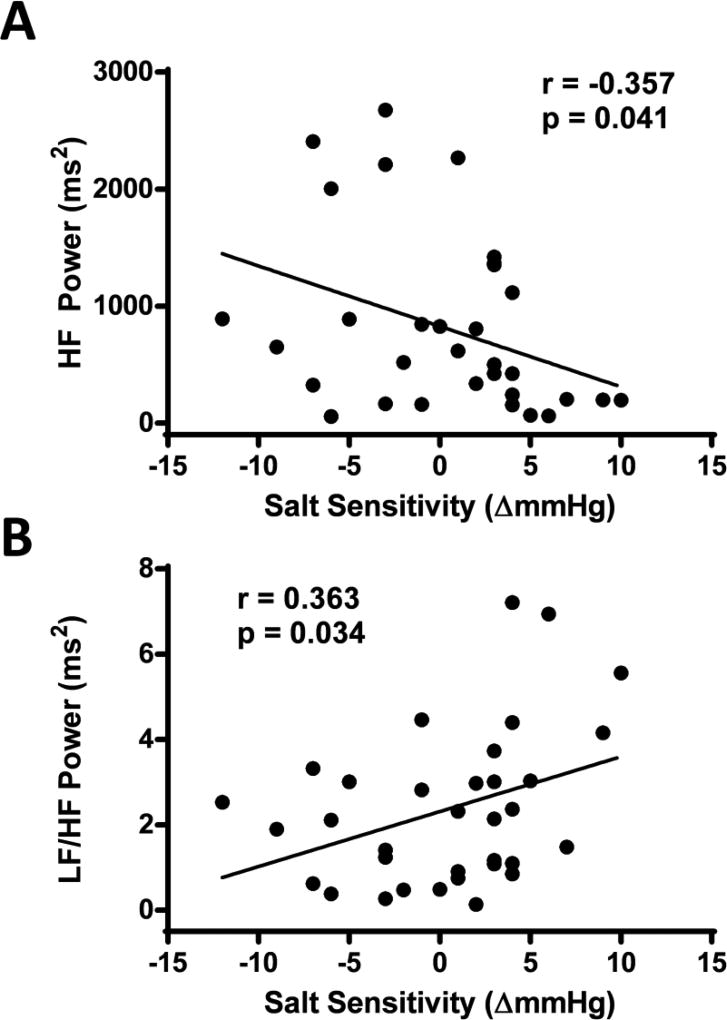
HRV high frequency power was assessed as an index of resting parasympathetic activity and was inversely related to the degree of SS (see figure 1A). Similarly, the ratio of low frequency to high frequency HRV displayed a significant and direct relation to SS (see figure 1B). There was no relation between the degree of SS and any other HRV values assessed (cardiac cycles analyzed 249±10 beats, R-to-R interval 1008±23 ms, heart rate 60±1 beats•min−1, RMSSD 463.3±4.3 ms, pNN50 22.5±3.4 %, SDNN 57.5±4.3 ms, low frequency power 1219±208 ms2, total power 3672±535 ms2; all p>0.05 for the degree of SS). Twenty-four hour urinary sodium excretion was not statistically related to any of these HRV variables (p>0.05).
Figure 1.
The association between the degree of salt sensitivity and heart rate variability high frequency power (panel A), and heart rate variability low frequency to high frequency power ratio (panel B).
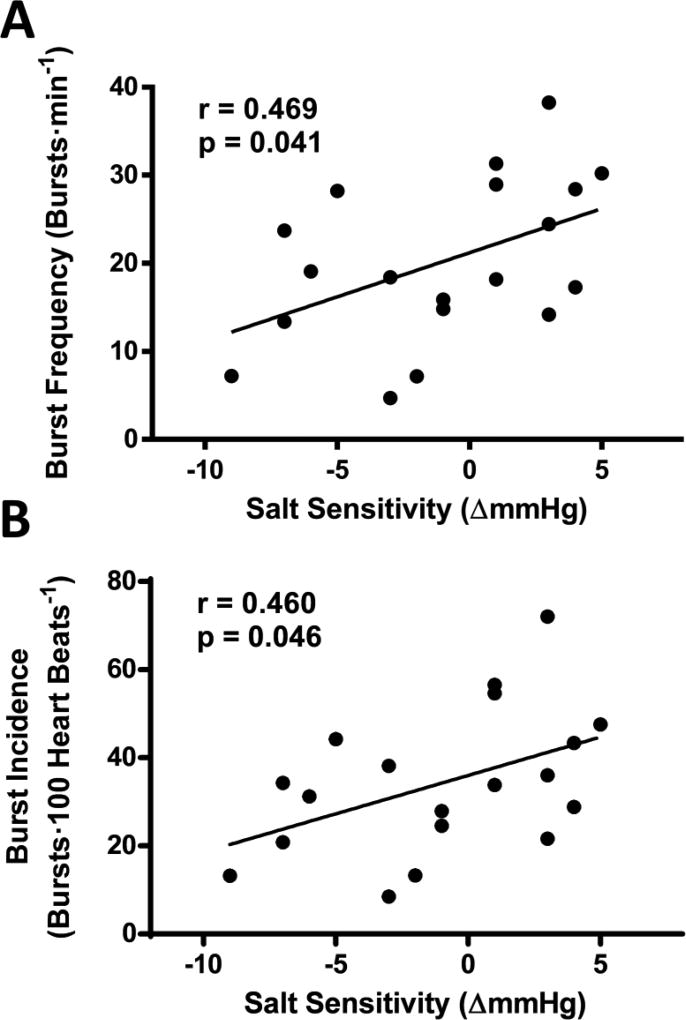
MSNA, a direct measurement of sympathetic outflow, was measured to assess sympathetic activity. Both MSNA burst frequency and burst incidence were significantly related to SS; MSNA values increased as the degree of SS increased. Twenty-four hour urinary sodium excretion was not statistically related to any of these MSNA variables (p>0.05).
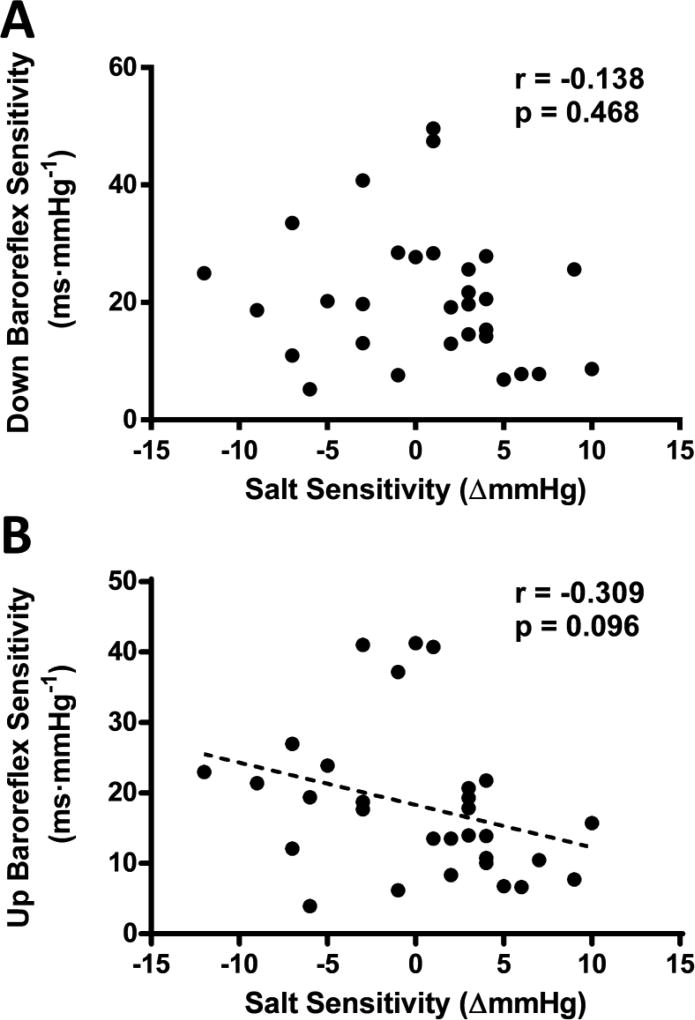
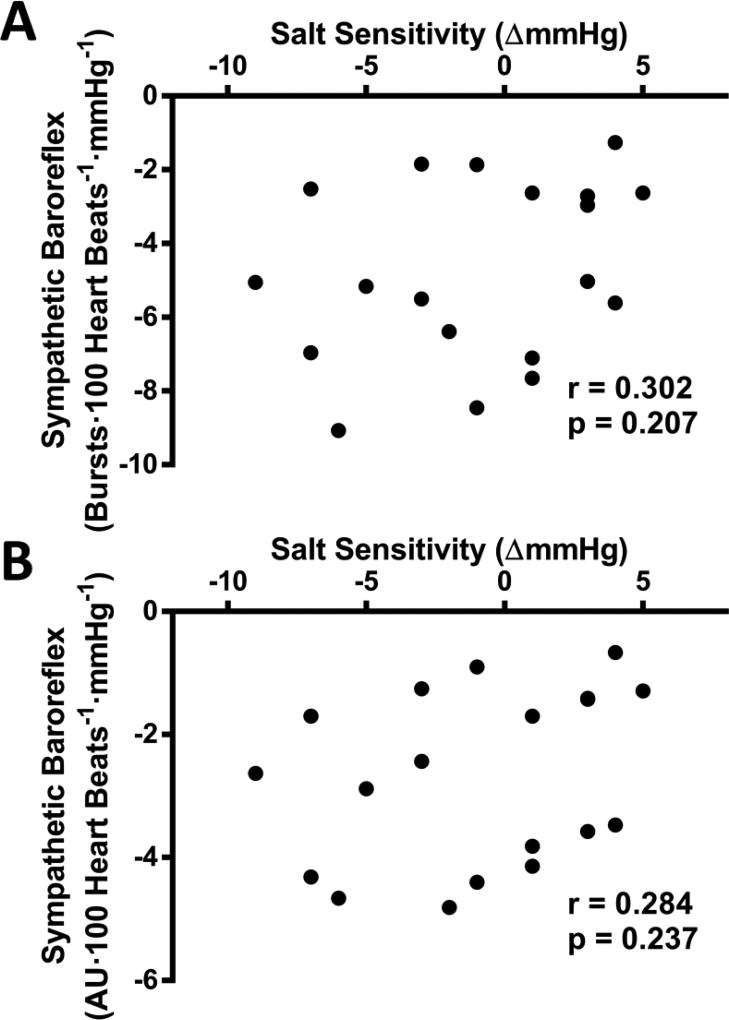
Both cardiac and sympathetic baroreflex sensitivity were assessed to determine their relation to SS. Down sequence cardiac baroreflex sensitivity was unrelated to the degree of SS (see figure 3A). Although up sequence cardiac baroreflex sensitivity was also not statistically significant, a slight trend (p=0.096) existed for decreased up sequence sensitivity with greater SS (see figure 4B). Twenty-four hour urinary sodium excretion was correlated with cardiac baroreflex up sequence sensitivity (r=0.445 p=0.013), but not down sequence sensitivity (r=0.111 p=0.559). Sympathetic baroreflex sensitivity displayed no relation with the degree of SS (see figure 4). Twenty-four hour urinary sodium excretion was not statistically related to any of these sympathetic baroreflex sensitivity measurements (p>0.05).
Figure 3.
The association between the degree of salt sensitivity and cardiac baroreflex sensitivity measured during down sequences (panel A), and up sequences (panel B). Dashed best-fit lines represent relations with p=0.05–0.10.
Figure 4.
The association between the degree of salt sensitivity and sympathetic baroreflex sensitivity measured by muscle sympathetic nerve activity (MSNA) burst incidence (panel A), and total MSNA (panel B).
As participant age was significantly related to the degree of SS (Table 1), secondary analysis was performed to re-evaluate significant associations with the SS by assessing the effects of age via multiple regression modeling. Individual multiple regression models were tested which included age (model 1) and age and the degree of SS (model 2) as predictors of the variables of interest. The multiple regression prediction models for high frequency power (model 1, r2=0.259 p=0.002; model 2, r2=0.279 p=0.007; model 1 - model 2, Δr2=0.020 p=0.369) and the low frequency to high frequency ratio (model 1, r2=0.235 p=0.004; model 2, r2=0.265 p=0.009; model 1 - model 2, Δr2=0.029 p=0.273) suggest the bivariate correlations between the degree of SS and HRV were driven by age as evidence by model 1 significance and lack of Δr2 significance in each comparison. The multiple regression prediction models for MSNA burst frequency (model 1, r2=0.096 p=0.197; model 2, r2=0.230 p=0.124; model 1 - model 2, Δr2=0.133 p=0.115) and MSNA burst incidence (model 1, r2=0.110 p=0.166; model 2, r2=0.228 p=0.126; model 1 - model 2, Δr2=0.118 p=0.137) display a lack of statistical significance within model 1 which suggests that the bivariate correlations between the degree of SS and MSNA cannot be driven solely by age. However, age does appear to partially confound the prediction of MSNA as the model 1 to model 2 Δr2 lacks statistical significance.
DISCUSSION
This investigation was the first to comprehensively explore the relation between the degree of SS and resting autonomic cardiovascular function under habitual conditions. The major findings of this analysis were that increases in SS were associated with decreased parasympathetic activity via high frequency HRV, and increased resting sympathetic activity via MSNA, during habitual sodium intake. Further analysis showed that the differences in high frequency HRV was due to the association between age and the degree of SS. The association between SS and sympathetic activity was not explained by age, but could not be shown as statistically independent either. This is likely due to the collinearity between SS and age in this dataset. Regardless, these results suggest age-related elevated sympathetic activity and age-related suppressed parasympathetic activity with increased SS.
A limited number of studies have examined tonic sympathetic or parasympathetic activity among normotensive adults characterized by SS. Three previous studies have examined parasympathetic activity, indexed by HRV analysis, while controlling for age. The results of these studies were mixed showing decreased (Buchholz et al., 2003; Deter et al., 2001) or no association (Weber et al., 2008) between HRV and the degree of SS. Miyajima and Yamada (Miyajima and Yamada, 1999) studied normotensive Japanese adult males (19–25 yrs) grouped according to their degree of SS and examined resting MSNA during a high (≈271 mmol•day−1) and low (≈68 mmol•day−1) sodium diet. The lower SS group exhibited a decrease in resting MSNA on the high sodium diet, while the group with higher SS did not. Although Miyajima and Yamada (Miyajima and Yamada, 1999) did not study their participants during habitual sodium intake, the results of our current analysis are consistent with their results as habitual sodium intake was high (163.2±10 mmol•24 h−1) in the current study. However, these studies (Buchholz et al., 2003; Deter et al., 2001; Miyajima and Yamada, 1999; Weber et al., 2008) were all performed in young (~25 yrs) men making a direct comparison difficult.
Rodent models bred to display phenotypic elevations in SS (i.e. Dahl Salt Sensitive rats) exhibit impaired cardiac (Brown et al., 1989; Ferrari et al., 1984; Murphy and McCarty, 1995; Nedvídek and Zicha, 1993; Reddy et al., 1991) and sympathetic (Gordon and Mark, 1984; Huang and Leenen, 1998; Miyajima and Buñag, 1986) baroreflex sensitivity. In turn, these altered autonomic reflexes may elevate resting sympathetic activity. These studies were performed using a wide range of sodium intakes. Interestingly, these Dahl-SS rodents displayed autonomic dysfunction while fed a normal salt intake prior to the development of hypertension. Collectively, this suggests that baroreflex function in this inbred strain is impaired regardless of dietary sodium consumption or resting blood pressure. Indeed, sinoaortic denervation will convert Sprague Dawley rats into a highly salt sensitive rat strain (Osborn and Hornfeldt, 1998). Our results do not show a significant relation between baroreflex sensitivity and the degree of SS in normotensive and healthy humans.
Autonomic dysfunction is a common characteristic among several pathologies that impact the cardiovascular system including hypertension (DiBona, 2013), heart failure (Leimbach et al., 1986), chronic kidney disease (Neumann et al., 2004), and others (Bruno et al., 2012). Elevated SS is predictive of future hypertension (Barba et al., 2007) and cardiovascular related death (Weinberger, 2002) in otherwise healthy individuals. For this reason, investigations like the current are important for establishing potential mechanisms for future experimental study. Determining which comes first, pathology or autonomic dysfunction, is difficult, as potential causal mechanisms have been established for both. In the current investigation we found an association between resting sympathetic activity and the degree of SS in currently normotensive adults, but these results were partially confounded with age. Future investigations into the role of sympathetic activity in the development of hypertension in adults with high SS are warranted.
Sympathoexcitatory stressors have the potential to exacerbate underlying conditions and result in cardiovascular events (Franklin et al., 1996), as well as predict future morbidity (Menkes et al., 1989; Stewart and France, 2001; Treiber et al., n.d.). Normotensive humans with high degrees of SS consuming habitual sodium intake have exaggerated blood pressure and HRV responses to mental stress (Buchholz et al., 2003; Deter et al., 2001, 1997; Weber et al., 2008), and enhanced startle modulation. These data suggest the central nervous system circuits that regulate blood pressure may be sensitized in adults with high SS (Buchholz et al., 2001). However, little is known about how the degree of SS affects responses to physical stressors; future investigations should seek to exam this.
We recognize there are limitations with the current study. We chose to utilize habitual sodium intake as it more closely reflects the factors influencing lifelong cardiovascular function. Not controlling for sodium intake may have increased the variability of some measurements, decreasing the likelihood of statistically significant findings. Additionally, the age range (22–60 yrs) of the participants in the current study was relatively large making age-independent associations difficult. However, even in a cohort of healthy normotensive adults, these data highlight the impact that increasing age has on autonomic cardiovascular function.
In conclusion, normotensive adults with greater blood pressure responses to dietary sodium exhibit lower parasympathetic and higher sympathetic tone, which is not due to alterations in baroreflex sensitivity. These data suggest that age-related salt sensitivity of blood pressure is associated with worsened resting autonomic cardiovascular function.
Figure 2.
The association between the degree of salt sensitivity and muscle sympathetic nerve activity (MSNA) burst frequency (panel A) and burst incidence (panel B)
Highlights.
Normotensive adult humans with greater salt sensitivity have lower parasympathetic and higher sympathetic tone.
This altered autonomic function is not due to alterations in baroreflex sensitivity.
This altered autonomic function does appear to be influenced by age.
Acknowledgments
The authors would like to thank Allen Prettyman PhD FNP-BC, & the University of Delaware Nurse Managed Primary Care Center for their assistance with this study.
GRANTS
This research was funded by an ACSM Foundation Pre-doctoral Grant, 2013 and NIH R01HL104106, 2011 and R01HL128388, 2016. The funding agencies had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Abbreviations
- SS
salt sensitivity
- HRV
heart rate variability
- MSNA
muscle sympathetic nerve activity
- MAP
mean arterial pressure
- AU
arbitrary units
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All data were collected at the University of Delaware.
CONFLICT OF INTEREST
No conflicts of interest are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: E.L.M. and W.B.F. conception and design of research; E.L.M., M.S.B., M.M.W., W.B.F. performed experiments; E.L.M. analyzed data; E.L.M. and W.B.F. interpreted results of experiments; E.L.M. prepared figures; E.L.M. drafted manuscript; E.L.M., M.S.B., D.G.E., S.D.S., M.M.W. and W.B.F edited and revised manuscript; E.L.M., M.S.B., D.G.E., S.D.S., M.M.W., and W.B.F approved final version of manuscript.
References
- Barba G, Galletti F, Cappuccio FP, Siani A, Venezia A, Versiero M, Della Valle E, Sorrentino P, Tarantino G, Farinaro E, Strazzullo P. Incidence of hypertension in individuals with different blood pressure salt-sensitivity: results of a 15-year follow-up study. J. Hypertens. 2007;25:1465–71. doi: 10.1097/HJH.0b013e3281139ebd. [DOI] [PubMed] [Google Scholar]
- Brian MS, Dalpiaz A, Matthews EL, Lennon-Edwards S, Edwards DG, Farquhar WB. Dietary sodium and nocturnal blood pressure dipping in normotensive men and women. J. Hum. Hypertens. 2017;31:145–150. doi: 10.1038/jhh.2016.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DR, Morgan DA, Peuler JD, Thoren P. 24-hour blood pressure recordings in Dahl rats on high- and low-salt diets. Am. J. Physiol. 1989;257:R1225–R1231. doi: 10.1152/ajpregu.1989.257.5.R1225. [DOI] [PubMed] [Google Scholar]
- Bruno RM, Ghiadoni L, Seravalle G, Dell’oro R, Taddei S, Grassi G. Sympathetic regulation of vascular function in health and disease. Front. Physiol. 2012;3:284. doi: 10.3389/fphys.2012.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz K, Schachinger H, Wagner M, Schorr U, Sharma AM, Deter HC. Enhanced Affective Startle Modulation in Salt-Sensitive Subjects. Hypertension. 2001;38:1325–1329. doi: 10.1161/hy1101.096055. [DOI] [PubMed] [Google Scholar]
- Buchholz K, Schächinger H, Wagner M, Sharma AM, Deter HC. Reduced vagal activity in salt-sensitive subjects during mental challenge. Am. J. Hypertens. 2003;16:531–6. doi: 10.1016/S0895-7061(03)00905-1. [DOI] [PubMed] [Google Scholar]
- de Leeuw PW, Kroon Aa. Salt and sensitivity. Hypertension. 2013;62:461–2. doi: 10.1161/HYPERTENSIONAHA.113.01831. [DOI] [PubMed] [Google Scholar]
- Deter HC, Buchholz K, Schorr U, Mathiak K, Sharma AM. Salt-sensitivity and other predictors of stress-related cardiovascular reactivity in healthy young males. Clin. Exp. Hypertens. 2001;23:213–25. doi: 10.1081/ceh-100102661. [DOI] [PubMed] [Google Scholar]
- Deter HC, Buchholz K, Schorr U, Schächinger H, Turan S, Sharma AM. Psychophysiological reactivity of salt-sensitive normotensive subjects. J. Hypertens. 1997;15:839–44. doi: 10.1097/00004872-199715080-00006. [DOI] [PubMed] [Google Scholar]
- DiBona GF. Sympathetic Nervous System and Hypertension. Hypertension. 2013;61:556–60. doi: 10.1161/HYPERTENSIONAHA.111.00633. [DOI] [PubMed] [Google Scholar]
- Ferrari AU, Gordon FJ, Mark AL. Primary impairment of cardiopulmonary baroreflexes in Dahl salt-sensitive rats. J. Hypertens. Suppl. 1984;2:S401–S403. [PubMed] [Google Scholar]
- Franklin BA, Bonzheim K, Gordon S, Timmis GC. Snow shoveling: a trigger for acute myocardial infarction and sudden coronary death. Am. J. Cardiol. 1996;77:855–8. doi: 10.1016/S0002-9149(97)89181-3. [DOI] [PubMed] [Google Scholar]
- Gordon FJ, Mark AL. Mechanism of impaired baroreflex control in prehypertensive Dahl salt- sensitive rats. Circ. Res. 1984;54:378–387. doi: 10.1161/01.RES.54.4.378. [DOI] [PubMed] [Google Scholar]
- Greaney JL, Schwartz CE, Edwards DG, Fadel PJ, Farquhar WB. The neural interaction between the arterial baroreflex and muscle metaboreflex is preserved in older men. Exp. Physiol. 2013;98:1422–31. doi: 10.1113/expphysiol.2013.073189. [DOI] [PubMed] [Google Scholar]
- Hart EC, Joyner MJ, Wallin BG, Karlsson T, Curry TB, Charkoudian N. Baroreflex control of muscle sympathetic nerve activity: a nonpharmacological measure of baroreflex sensitivity. Am. J. Physiol. Heart Circ. Physiol. 2010;298:H816–H822. doi: 10.1152/ajpheart.00924.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang BS, Leenen FHH. Both brain angiotensin II and “ouabain” contribute to sympathoexcitation and hypertension in Dahl S rats on high salt intake. Hypertension. 1998;32:1028–33. doi: 10.1161/01.HYP.32.6.1028. [DOI] [PubMed] [Google Scholar]
- La Rovere MT, Pinna GD, Raczak G. Baroreflex sensitivity: Measurement and clinical implications. Ann. Noninvasive Electrocardiol. 2008 doi: 10.1111/j.1542-474X.2008.00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leimbach WN, Wallin BG, Victor RG, Aylward PE, Sundlöf G, Mark AL. Direct evidence from intraneural recordings for increased central sympathetic outflow in patients with heart failure. Circulation. 1986;73:913–9. doi: 10.1161/01.cir.73.5.913. [DOI] [PubMed] [Google Scholar]
- Mancia G, Grassi G. The Autonomic Nervous System and Hypertension. Circ. Res. 2014;114 doi: 10.1161/CIRCRESAHA.114.302524. [DOI] [PubMed] [Google Scholar]
- Matthews EL, Brian MS, Coyle DE, Edwards DG, Stocker SD, Wenner MM, Farquhar WB. Peripheral venous distension elicits a blood pressure raising reflex in young and middle-aged adults. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016;310:R1128–33. doi: 10.1152/ajpregu.00438.2015. [DOI] [PubMed] [Google Scholar]
- Matthews EL, Brian MS, Ramick MG, Lennon-Edwards S, Edwards DG, Farquhar WB. High dietary sodium reduces brachial artery flow-mediated dilation in humans with salt-sensitive and salt-resistant blood pressure. J. Appl. Physiol. 2015;118:1510–5. doi: 10.1152/japplphysiol.00023.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menkes MS, Matthews KA, Krantz DS, Lundberg U, Mead LA, Qaqish B, Liang KY, Thomas CB, Pearson TA. Cardiovascular reactivity to the cold pressor test as a predictor of hypertension. Hypertens. (Dallas, Tex. 1979) 1989;14:524–30. doi: 10.1161/01.hyp.14.5.524. [DOI] [PubMed] [Google Scholar]
- Miyajima E, Buñag RD. Impaired sympathetic baroreflexes in prehypertensive Dahl hypertension-sensitive rats. Clin. Exp. Hypertens. A. 1986;8:1049–1061. doi: 10.3109/10641968609044085. [DOI] [PubMed] [Google Scholar]
- Miyajima E, Yamada Y. Reduced sympathetic inhibition in salt-sensitive Japanese young adults. Am. J. Hypertens. 1999;12:1195–1200. doi: 10.1016/S0895-7061(99)00122-3. [DOI] [PubMed] [Google Scholar]
- Muller MD, Gao Z, Drew RC, Herr MD, Leuenberger UA, Sinoway LI. Effect of cold air inhalation and isometric exercise on coronary blood flow and myocardial function in humans. J. Appl. Physiol. 2011;111:1694–702. doi: 10.1152/japplphysiol.00909.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CA, McCarty R. Baroreflex control of heart rate in Dahl hypertensive (SS/Jr) and normotensive (SR/Jr) rats. J. Hypertens. 1995;13:1145–1151. doi: 10.1097/00004872-199510000-00009. [DOI] [PubMed] [Google Scholar]
- Nedvídek J, Zicha J. Age-dependent changes of baroreflex efficiency in Dahl rats: effects of high salt intake. Physiol. Res. 1993;42:209–212. [PubMed] [Google Scholar]
- Neumann J, Ligtenberg G, Klein II, Koomans HA, Blankestijn PJ. Sympathetic hyperactivity in chronic kidney disease: pathogenesis, clinical relevance, and treatment. Kidney Int. 2004;65:1568–76. doi: 10.1111/j.1523-1755.2004.00552.x. [DOI] [PubMed] [Google Scholar]
- Ogoh S, Fisher JP, Raven PB, Fadel PJ. Arterial baroreflex control of muscle sympathetic nerve activity in the transition from rest to steady-state dynamic exercise in humans. Am J Physiol Hear. Circ Physiol. 2007;76107:2202–2209. doi: 10.1152/ajpheart.00708.2007. [DOI] [PubMed] [Google Scholar]
- Ogoh S, Fisher JP, Young CN, Raven PB, Fadel PJ. Transfer function characteristics of the neural and peripheral arterial baroreflex arcs at rest and during postexercise muscle ischemia in humans. Am. J. Physiol. Heart Circ. Physiol. 2009;296:H1416–H1424. doi: 10.1152/ajpheart.01223.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn JW, Hornfeldt BJ. Arterial baroreceptor denervation impairs long-term regulation of arterial pressure during dietary salt loading. Am. J. Physiol. 1998;275:H1558–H1566. doi: 10.1152/ajpheart.1998.275.5.H1558. [DOI] [PubMed] [Google Scholar]
- Park J, Liao P, Sher S, Lyles RH, Deveaux DD, Quyyumi AA. Tetrahydrobiopterin lowers muscle sympathetic nerve activity and improves augmentation index in patients with chronic kidney disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015;308:R208–18. doi: 10.1152/ajpregu.00409.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Quyyumi Aa, Middlekauff HR. Exercise pressor response and arterial baroreflex unloading during exercise in chronic kidney disease. J. Appl. Physiol. 2013;114:538–49. doi: 10.1152/japplphysiol.01037.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy RS, Baylis C, Kotchen TA. Hemodynamic responses to acute volume expansion in Dahl salt-sensitive rats. Am. J. Physiol. 1991;260:R32–R38. doi: 10.1152/ajpregu.1991.260.1.R32. [DOI] [PubMed] [Google Scholar]
- Silber DH, Sutliff G, Yang QX, Smith MB, Sinoway LI, Leuenberger UA. Altered mechanisms of sympathetic activation during rhythmic forearm exercise in heart failure. J. Appl. Physiol. 1998;84:1551–9. doi: 10.1152/jappl.1998.84.5.1551. [DOI] [PubMed] [Google Scholar]
- Stewart JC, France CR. Cardiovascular recovery from stress predicts longitudinal changes in blood pressure. Biol. Psychol. 2001;58:105–20. doi: 10.1016/s0301-0511(01)00105-3. [DOI] [PubMed] [Google Scholar]
- Sundlöf G, Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J. Physiol. 1978;274:621–37. doi: 10.1113/jphysiol.1978.sp012170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarvainen MP, Niskanen J-P, Lipponen JA, Ranta-Aho PO, Karjalainen PA. Kubios HRV--heart rate variability analysis software. Comput. Methods Programs Biomed. 2014;113:210–20. doi: 10.1016/j.cmpb.2013.07.024. [DOI] [PubMed] [Google Scholar]
- Treiber FA, Kamarck T, Schneiderman N, Sheffield D, Kapuku G, Taylor T. Cardiovascular reactivity and development of preclinical and clinical disease states. Psychosom. Med. 65:46–62. doi: 10.1097/00006842-200301000-00007. n.d. [DOI] [PubMed] [Google Scholar]
- Weber CS, Thayer JF, Rudat M, Sharma AM, Perschel FH, Buchholz K, Deter HC. Salt-sensitive men show reduced heart rate variability, lower norepinephrine and enhanced cortisol during mental stress. J. Hum. Hypertens. 2008;22:423–31. doi: 10.1038/jhh.2008.11. [DOI] [PubMed] [Google Scholar]
- Weinberger MH. Salt sensitivity is associated with an increased mortality in both normal and hypertensive humans. J. Clin. Hypertens. (Greenwich) 2002;4:274–6. doi: 10.1111/j.1524-6175.2002.00924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger MH, Fineberg NS. Sodium and volume sensitivity of blood pressure. Age and pressure change over time. Hypertension. 1991;18:67–71. doi: 10.1161/01.HYP.18.1.67. [DOI] [PubMed] [Google Scholar]