Abstract
Objective
Weakness induced by critical illness (intensive care unit acquired weakness) is a major cause of disability in patients and is currently untreatable. We recently identified a defect in repetitive firing of lower motor neurons as a novel contributor to intensive care unit acquired weakness. In order to develop therapy for intensive care unit acquired weakness, it was necessary to determine the mechanism underlying the defect in repetitive firing.
Methods
Both computer simulation and in vivo dynamic voltage clamp of spinal motor neurons in septic rats were employed to explore potential mechanisms underlying defective repetitive firing.
Results
Our results suggested alteration in subthreshold voltage-activated currents might be the mechanism underlying defective repetitive firing. It has been shown previously that pharmacologic activation of serotonin receptors on motor neurons increases motor neuron excitability, in part by enhancing subthreshold voltage-activated inward currents. Administration of a food and drug administration approved serotonin agonist (lorcaserin) to septic rats greatly improved repetitive firing and motor unit force generation.
Interpretation
Our findings suggest activation of serotonin receptors with lorcaserin may provide the first ever therapy for intensive care unit acquired weakness in patients.
Introduction
The syndrome of profound weakness following critical illness is termed ICU acquired weakness. ICU acquired weakness is a common problem and greatly complicates patient recovery 1–3. Lack of understanding of mechanisms underlying weakness following critical illness has meant the only reliable therapy available for patients is supportive care. Non-specific treatments are applied in hopes of promoting long-term recovery of neuromuscular function, including tight glycemic control 4 and physical therapy during critical illness 5, but these therapies have little effect in patients with severe weakness. In order to develop targeted therapy for ICU acquired weakness it is necessary to develop an understanding of the cause(s) of weakness.
Until recently ICU acquired weakness was thought to be due entirely to myopathy and neuropathy 1–3. However, we identified patients in the early stages of recovery from critical illness in which neither myopathy nor neuropathy appeared sufficient to account for their severe weakness. These patients had poor recruitment of motor units, despite being alert and cooperative such that it appeared that a defect within the central nervous system was an important contributor to their ICU acquired weakness 6. To explore potential mechanisms underlying reduced motor unit recruitment in patients, we recorded from rat spinal cord in vivo in septic rats and identified a defect in motor neuron excitability 6. The defect in motor neuron excitability was the primary contributor to weakness in rats as there was little evidence of either myopathy or neuropathy 7. Taken together, our studies in rats and patients raise the possibility that reduced excitability of motor neurons is a significant contributor to ICU acquired weakness.
In order to develop therapy for ICU acquired weakness it is necessary to understand the mechanism underlying poor repetitive firing of motor neurons. Passive membrane properties and properties of single action potentials of rat motor neurons were normal, suggesting that the defect in excitability was specific to the currents that generate repetitive firing 6, 7. Persistent inward currents (PICs) have been identified as playing a central role in generation of repetitive firing of neurons 8–11. PIC in motor neurons is composed of both Na and Ca components, and the NaPIC in particular functions to bring motor neurons to threshold to promote repetitive firing 9, 12–15. Opposing PICs are subthreshold K currents (Ksthr) that reduce repetitive firing 15, 16.
We hypothesized that the defect in motor neuron firing induced by sepsis was due to a defect in the subthreshold currents that govern the approach to action potential threshold. Computer modeling and manipulations of currents in rat motor neurons in vivo supported our hypothesis that a defect in subthreshold currents was an important contributor to weakness. Using identification of this mechanism to guide development of therapy we identified a drug, FDA approved for weight loss, that greatly improved repetitive firing of motor neurons in septic rats. Our findings suggest a surprising approach to development of therapy for ICU acquired weakness.
Methods
Induction of sepsis and terminal recordings
We used the cecal ligation and puncture procedure to induce sepsis in rats and performed terminal recordings as previously described 6, 7. Additional detail on treatment of rats and the methods for making these measurements is provided in an online data supplement
Computer simulations
We used a cable model of a rat motor neuron implemented in NEURON 7.3 similar to that previously described 17 to simulate the behavior of septic motor neurons. We systematically varied the maximal conductances of the persistent sodium and Kv1 channels on the initial segment along with the level of injected current to reveal regions in parameter space associated with irregular firing (coefficients of variation of interspike intervals > 0.5; values for motor neurons are typically < 0.2 10). For a given combination of conductance values there were generally 3 different current levels (1 nA apart) that led to irregular firing; decreasing the level of injected current below this range eliminated discharge and increasing current above this range led to regular firing.
Dynamic clamp
In experiments on both septic and control motor neurons, we simulated the addition or subtraction of currents mediated by persistent sodium and low-threshold potassium channels using the dynamic clamp software in Signal (Cambridge Electronic Design). The activation of the simulated channels was described by a sigmoidal steady-state voltage dependence: 1/(1+exp((vm−vhalf)/vslp)), where vm is the measured membrane potential, vhalf is the half-activation voltage and vslp reflects the steepness of the voltage dependence. For Na channels vhalf=−42.5 mV and vslp=−5.5 mV, and for K channels vhalf=−45 mV and vslp=−6.5 mV. The time constants of activation exhibited a reverse sigmoidal voltage dependence: tmax − t1/(1+exp((vm−vhalf)/vslp)), where tmax-t1 and tmax are the minimum and maximum time constants and vhalf and vslp have the same values as the corresponding steady-state activation curve. For Na channels the time constants ranged from 0.3 to 0.5 ms and for K channels 1 to 2 ms.
Statistical Analysis
The effect under different conditions (i.e. control vs dynamic clamp, septic vs dynamic clamp, and septic vs lorcaserin) on motor neuron firing was identified via statistical comparison of multiple trials of stimulation within individual motor neurons with and without treatment. Statistical significance was tested using ANOVA, the level of significance was set at p < 0.05. Variability about the mean is expressed as means ± SEM.
Study Approval
All animal procedures were performed in accordance with the policies of the Animal Care and Use Committee of Wright State University and the United States Public Health Service’s Policy on Humane Care and Use of Laboratory Animals
Experiments were approved by Wright State University’s IACUC (#940).
Results
Identification of a candidate mechanism underlying reduced motor neuron excitability
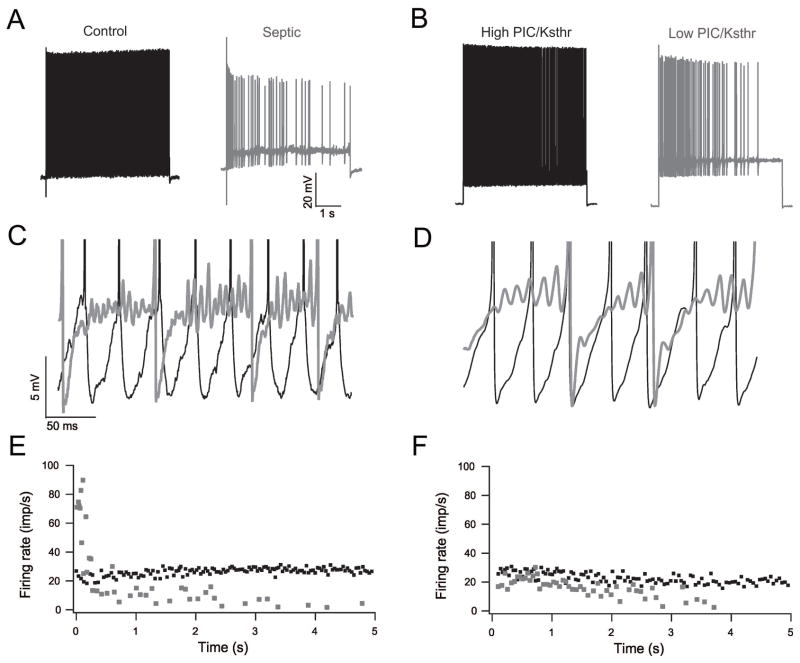
In order to identify potential mechanisms impairing repetitive firing of rat motor neurons in vivo following induction of sepsis we recorded from motor neurons from control rats and rats that had been septic for 1–3 days. In agreement with our previous study, we found no changes in passive membrane properties or properties of action potentials 6(Table 1). Given the lack of changes in passive properties and action potential properties we considered the possibility that subthreshold currents that are responsible for the approach to action potential threshold might be altered. We examined the approach of the membrane potential to action potential threshold in detail. Irregular motor neuron firing induced by sepsis was closely associated with high frequency (>100 Hz) membrane potential oscillations between spikes (Fig 1). Irregular firing associated with high frequency membrane potential oscillations has been previously described in a variety of neuron types 18, 19, including mouse motor neurons 14, 20. In sodium pentobarbitone anesthetized mice, irregular firing and membrane potential oscillations are seen in motor neurons during slow triangular ramps of current injection when current intensity is just suprathreshold 14, 20. Similarly, we found that control rat motor neurons occasionally exhibited this behavior at the lowest injected current intensities, but the oscillations always resolved with increased current injection such that firing rate became regular. In contrast, in septic motor neurons oscillations often could not be eliminated by increasing current injection.
Table 1.
| Control | Septic | |
|---|---|---|
| Resting potential (mV) | −72.0 ± 4.9 | −71.8 ± 5.8 |
| Input resistance (MΩ) | 2.2 ± 0.9 | 2.1 ± 0.7 |
| Rheobase (nA) | 10.1 ± 5.6 | 11.2 ± 6.5 |
| Action potential amplitude (mV) | 75.9 ± 8.1 | 75.6 ± 8.3 |
| Action potential overshoot (mV) | 3.9 ± 8.1 | 3.8 ± 8.4 |
| Maximum rate of rise dv/dt (mV/ms) | 208.6 ± 60.2 | 210.7 ± 52.3 |
| Maximum rate of decay dv/dt (mV/ms) | −191.3 ± 42.6 | −197.6 ± 39.7 |
| Action potential 1/2 width (ms) | 0.4 ± 0.1 | 0.4 ± 0.1 |
| After hyperpolarization amplitude (mV) | 1.7 ± 0.9 | 2.2 ± 1.5 |
| After hyperpolarization 1/2 width (ms) | 12.4 ± 3.1 | 12.6 ± 4.4 |
All septic motor neurons were recorded from 1–3 days after induction of sepsis.
N = 22 motor neurons for control and 29 for septic. Errors given are the standard deviation.
None of the differences were statistically significant.
Figure 1.
Reducing the PIC/Ksthr ratio in a motor neuron model reproduces sepsis-induced oscillations, reduced firing rate and increased variability of firing rate. A) Shown are comparison of action potential traces of control (black) and septic (gray) motor neurons. Firing of the septic motor neuron is slow and irregular. B) Action potential traces from modeled motor neuron with high PIC/Ksthr ratio (black) and low PIC/Ksthr ratio (gray). When PIC/Ksthr ratio is low, firing of the motor neuron is slow and irregular. C) Superimposed blow-ups focusing on the voltage range near action potential threshold for the real control (black) and septic (gray) traces from the trials shown in A. In the trace of the motor neuron from the control rat there are no subthreshold oscillations in membrane potential such that firing is rapid and regular. In the trace from the septic rat there are subthreshold oscillations in membrane potential such that firing is irregular. D) Superimposed blow-ups focusing on the voltage range near action potential threshold for the modeled motor neurons. In the trace of the modeled motor neuron with high PIC/Ksthr ratio (black) firing is rapid and regular. In the trace from the modeled motor neuron with low PIC/Ksthr ratio (gray) subthreshold oscillations are present. E) Plot of the instantaneous firing rate during the trials of the real motor neurons shown in A. The control motor neuron fires more rapidly and consistently at a similar level of current injection above rheobase current. F) Plot of the instantaneous firing rate during the trials of the modeled motor neurons shown in B. When the PIC/Ksthr ratio is reduced the mean firing rate is reduced and firing rate is more variable at the same level of current injection above rheobase current.
Oscillations in membrane potential during firing are thought to reflect the competing influence of inward and outward currents mediated by fast, voltage-dependent currents that are activated in the subthreshold range 14, 18–20. As subthreshold currents serve to drive the motor neuron to action potential threshold, the finding of oscillations suggested that dysregulation of subthreshold currents might be the mechanism underlying the defect in motor neuron repetitive firing induced by sepsis. Both Na+ and CaV1.3-type calcium channels contribute to a low-threshold persistent inward current (PIC) in motor neurons 9; however, the in situ kinetics of the Ca++ component appear to be too slow to contribute to fast membrane potential oscillations 21. The most likely candidates to account for subthreshold oscillations in motor neurons are the persistent sodium current (NaPIC) and low threshold Kv1-type potassium currents as channels mediating these currents are present in high concentration of the axon initial segments of rodent motor neurons 22. NaPIC is thought to help depolarize the cell to threshold and thus encourages repetitive firing 12, whereas low threshold Kv1 current opposes depolarization and thus opposes repetitive firing 15, 16.
We hypothesized that when the ratio of NaPIC to Kv1 currents (PIC/Ksthr ratio) is too low, achieving threshold becomes inconsistent such that oscillations in membrane potential are introduced. To test this hypothesis we used a computer cable model of rat motor neurons to determine whether reducing the PIC/Ksthr ratio was sufficient to reproduce the oscillations and the problems with motor neuron repetitive firing present in septic rats. The model had spike currents (transient and persistent sodium, delayed rectifier potassium), low threshold Kv1-type potassium conductance (Ksthr) on the initial segment compartments, after hyperpolarization currents on the soma compartment and included synaptic noise. Reducing the PIC/Ksthr ratio either by reducing PIC or by increasing Ksthr reproduced the sepsis-induced oscillations in membrane potential, the reduction in number of spikes fired and the increased variability of firing rate, while leaving properties of single spike unchanged (Fig 1). The results of computer simulations thus suggested reduction in the PIC/Ksthr ratio is sufficient to reproduce the defects in motor neuron repetitive firing present following sepsis. This encouraged us to pursue further our hypothesis that alteration of subthreshold currents is the mechanism underlying the defect in motor neuron excitability induced by sepsis.
Manipulating subthreshold currents can both reproduce and reverse the septic phenotype
Our ability to simulate defects in motor neuron repetitive firing with reduction in the PIC/Ksthr ratio encouraged us to test whether reducing this ratio could also trigger defects in repetitive firing in control motor neurons. We reduced the functional ratio of PIC/Ksthr in vivo using the dynamic clamp technique (Signal 6, Cambridge Electronic Design)14, 20. Dynamic clamp is a technique that allows for selective introduction and subtraction of currents mediated by voltage-dependent conductances. It has been validated in both cardiac myofibers and neurons 23–25. Although applying this technique via a somatic electrode cannot replicate voltage changes in dendrites due to the issue of space clamp, it does simulate changes in the balance of inward and outward currents near the soma and axon initial segment that are mediated by voltage-dependent currents activated in the subthreshold range. For introduction and subtraction of subthreshold currents to be effective in altering firing it was necessary that the interspike membrane potential covered a range where these simulated currents showed a steep change in their relative activation (~ −60 to −30 mV), and maximum conductance values were sufficiently large.
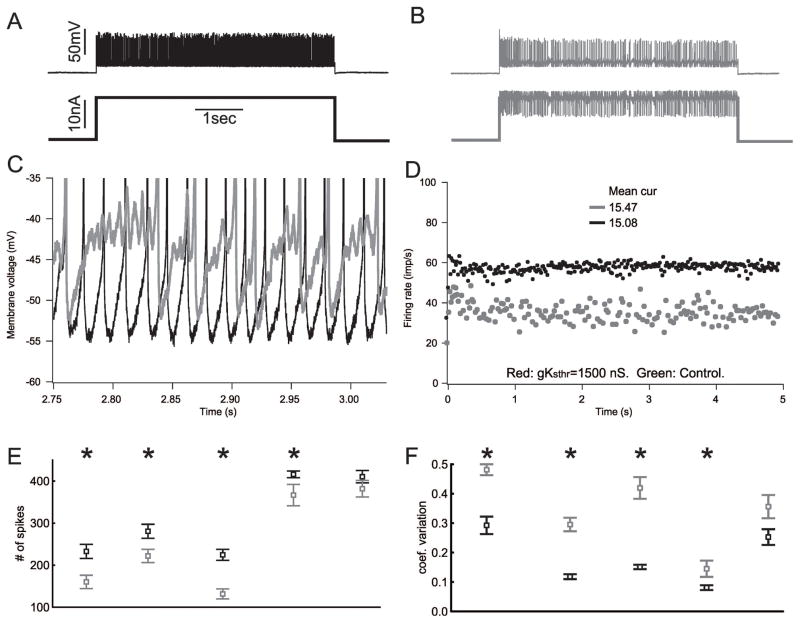
In each control motor neuron comparison of firing characteristics was made between alternating 5s current injections where dynamic clamp was employed and 5s current injections where standard square current pulses were used to depolarize the motor neuron to trigger firing. Care was taken to match the mean current injection between stimulations using a square pulse and stimulations using dynamic clamp. Decreasing the PIC/Ksthr ratio in control motor neurons lowered the mean firing rate, increased the variability of firing rate and introduced subthreshold oscillations in membrane potential (Fig 2). These data suggested that altering subthreshold currents in motor neurons from untreated rats in vivo is sufficient to induce defects in repetitive firing similar to those triggered by sepsis.
Figure 2.
Decreasing the PIC/Ksthr ratio in motor neurons from untreated rats causes defects in firing similar to those triggered by sepsis. A) The action potential (top) and current injection (bottom) traces recorded intracellularly from a motor neuron in an untreated rat. B) Traces from the same motor neuron using dynamic clamp to reduce the PIC/Ksthr ratio. Instead of firing continuously at a high rate, as shown in A, the motor neuron firing rate is irregular with brief pauses. C) Superimposed blow-ups focusing on the voltage range near action potential threshold for the traces from the trials shown in A (black) and B (gray). When the PIC/Ksthr ratio is normal (no dynamic clamp) there are no subthreshold oscillations in membrane potential such that firing is rapid and regular. When the ratio is reduced, there are subthreshold oscillations in membrane potential (gray) such that firing is irregular. D) Plot of the instantaneous firing rate during the trials shown in A and B. When the PIC/Ksthr ratio is reduced the mean firing rate is reduced from near 60 to below 40 and the instantaneous firing rate is more variable despite the mean current injection being higher than when PIC/Ksthr ratio is normal. E) Plot of the number of spikes fired during the 5s current injection for 5 motor neurons before (black) and after (gray) reduction of PIC/Ksthr ratio by dynamic clamp. Multiple trials were run with and without dynamic clamp for each motor neuron and the mean data plotted. In all cases the mean current injection was matched for the comparison of dynamic clamp to standard current injection. F) Plot of the coefficient of variation of firing rate before and after reduction of PIC/Ksthr ratio by dynamic clamp. In E and F, * indicates a statistically significant difference (p < .05) for the motor neuron studied.
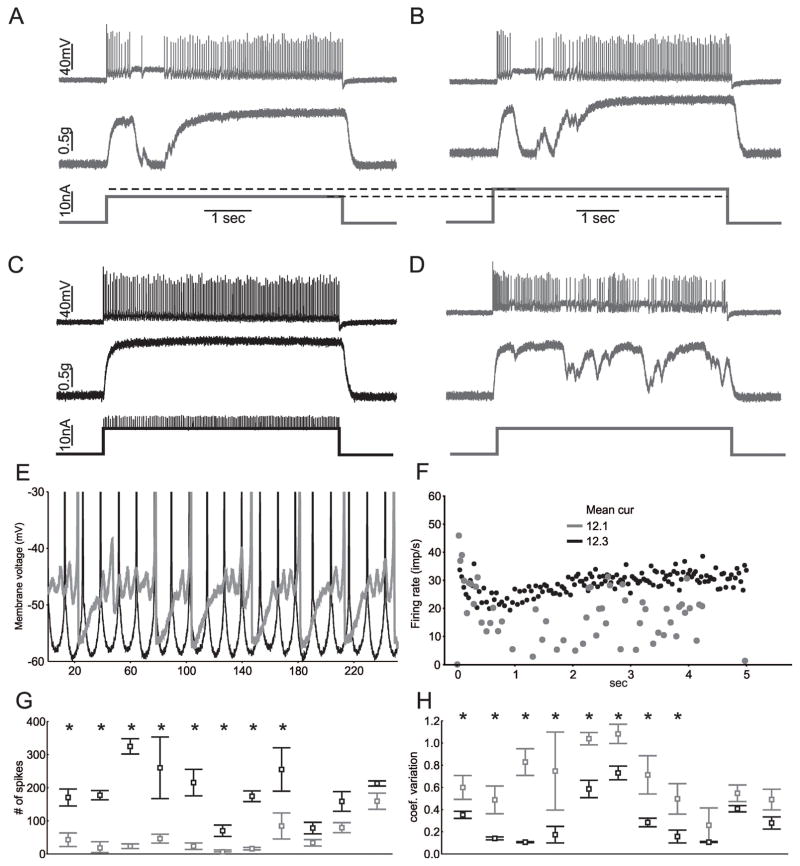
We next examined whether altering subthreshold currents was sufficient to improve firing of motor neurons following induction of sepsis. Prior to studies manipulating subthreshold currents in motor neurons from septic rats we confirmed that the deficit in firing was stable following impalement. Impalements (> 15 minutes) were performed in 8 motor neurons from rats 1–3 days following induction of sepsis and no improvements in repetitive firing was induced by holding the impalement (data not shown). We next manipulated subthreshold currents in 11 motor neurons from rats 1–3 days following induction of sepsis. In 4 of the recordings we were also able to record motor unit force. In all cases in which force was measured, transient loss of fusion of force was eliminated by increasing the PIC/Ksthr ratio by dynamic clamp (Fig 3). Increasing the PIC/Ksthr ratio in septic motor neurons caused a statistically significant increase in the number of spikes and significantly reduced the variability of firing rate in 8/11 motor neurons. In all cases subthreshold oscillations were reduced. These data suggest that shifting the balance of subthreshold currents towards excitatory inward currents in motor neurons in vivo reversed the sepsis-induced defect in excitability.
Figure 3.
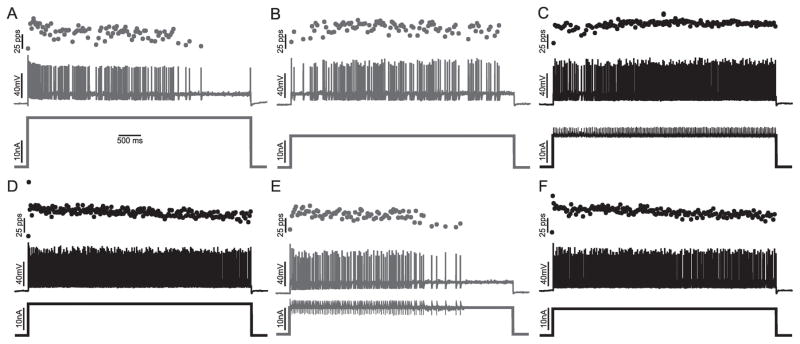
Increasing the PIC/Ksthr ratio is sufficient to improve firing and force production of motor units in septic rats. A) The action potentials, motor unit force and current injection traces for a single motor neuron during a 5s injection of current in vivo in a rat that had been septic for two days. There is an abnormal pause in firing of the motor neuron that causes motor unit force to fall to zero. B) Increasing current injection into the motor neuron does not improve firing. C) Dynamic clamp was used to add PIC and this normalizes firing and force production. Mean current injection was similar in B and C (12.1 vs 12.3 nA). D) Following the trial in C, a square current pulse was injected and again there are pauses in firing that cause muscle force to drop. E) Superimposed blow-ups focusing on the voltage range near action potential threshold for the trials shown in B (gray) and C (black). When the PIC/Ksthr ratio is increased with dynamic clamp, oscillations are eliminated and firing becomes fast and regular. F) Plot of the instantaneous firing rate during the trials shown in C and D. When the PIC/Ksthr ratio is increased the mean firing rate is increased and less variable. G) Plot of the number of spikes fired during the 5s current injection for 11 motor neurons before (gray) and after (black) increase of PIC/Ksthr ratio. Multiple trials were run for each motor neuron with the same current injection and the mean data plotted. In all cases the mean current injection was matched for the comparison of dynamic clamp to standard current injection. H) Plot of the coefficient of variation of firing rate before and after increasing the PIC/Ksthr ratio by dynamic clamp. In G and H, * indicates a statistically significant difference for the motor neuron studied.
Treatment with serotonin agonists improves repetitive firing of motor neurons in septic rats
To treat the defect in motor neuron firing we wished to pharmacologically increase motor neuron excitability in vivo. Activation of 5HT receptors has been shown to increase excitatory subthreshold PIC in motor neurons 26, 27. Individual motor neurons were impaled and the impalement was held during administration of drug to activate 5-HT receptors (15 minutes up to 2 hours). While this allowed for study of only one motor neuron per rat, it made possible comparison of individual motor neurons to themselves before and after activation of 5HT receptors.
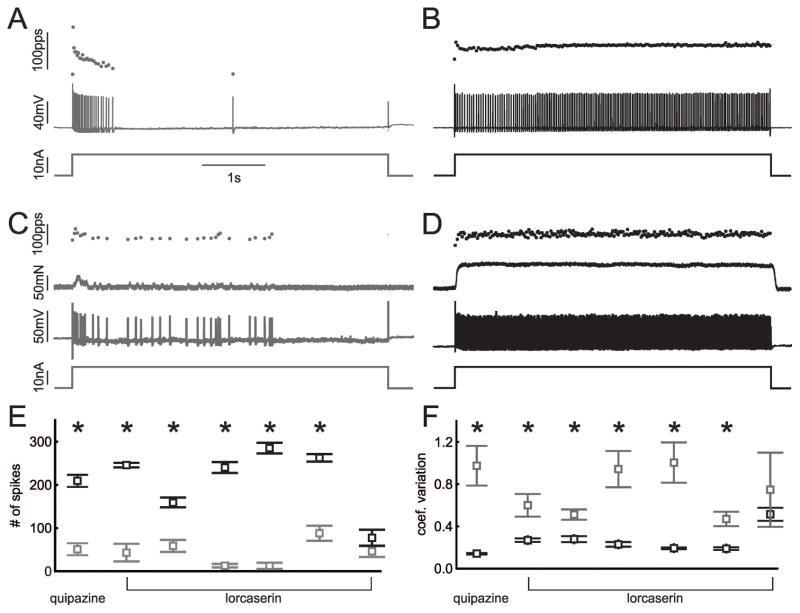
The first drug administered was relatively non-selective the 5 HT agonist quipazine, which has been shown to increase motor neurons excitability and motor output of the spinal cord 28, 29. In the motor neuron from the septic rat studied before and after intraperitoneal injection of 10 mg/kg quipazine i.p., there was a statistically significant improvement in repetitive firing (Fig 4). The findings with quipazine suggested increasing motor neuron excitability by activation of 5HT receptors might provide therapy for patients with ICU acquired weakness. However, as quipazine is not FDA approved for use in patients we did not test it further. PIC in rat motor neurons is increased by 5HT2B and 2C agonists 30. A search for FDA approved 5-HT2B and 2C agonists identified lorcaserin, which is FDA approved for weight loss and is a selective for 5-HT2C receptors 31. Treatment of rats with injection of 3 mg/kg of lorcaserin i.p. 32 triggered statistically significant improvements in repetitive firing in 5/6 motor neurons studied in rats 1–3 days following induction of sepsis (Fig 4). The increase in number of spikes fired and the reduction in coefficient of variation were greater than 2-fold in most cases.
Figure 4.
Activation of 5HT receptors improve firing of motor neurons in septic rats in vivo. A) Motor neuron firing in a rat two days after induction of sepsis. B) Firing of the same motor neuron to the same current injection 15 minutes after injection of 10 mg/kg quipazine. C and D are records from a motor neuron from a septic rat before and 15 minutes after injection of 3 mg/kg of lorcaserin. E) Plot of the number of spikes fired during the 5s current injection for 1 motor neuron before (gray) and after (black) 10 mg/kg quipazine and 6 motor neurons before and after 3 mg/kg lorcaserin. Multiple trials were run for each motor neuron with the same current injection before and at least 15 minutes after drug injection and the mean data plotted. In all cases the mean current injection was matched for the comparison of firing before and after administration of drug. F) Plot of the coefficient of variation of firing rate before and after quipazine and lorcaserin. In E and F, * indicates a statistically significant difference for the motor neuron studied.
We hypothesized that the improvement in firing following administration of lorcaserin is due to partial normalization of the sepsis-induced alteration in subthreshold currents. As a first test of this hypothesis we held the impalement of 2 motor neurons from septic rats throughout treatment with lorcaserin and used dynamic clamp to reduce the PIC/Ksthr ratio following the lorcaserin-induced improvement in repetitive firing (Fig 5). Decreasing the PIC/Ksthr ratio with dynamic clamp reversed the lorcaserin-induced improvement in firing in both neurons studied. These data demonstrate that lowering the PIC/Ksthr ratio in only the motor neuron being studied is sufficient to reverse the effect of lorcaserin.
Figure 5.
Lowering the PIC/Ksthr ratio in the motor neuron impaled is sufficient to reverse the improvement in motor neuron firing induced by treatment with lorcaserin. A and B) Shown are the current injection, action potentials, and instantaneous firing rates for a motor neuron during two 5s injections of high (A) and low (B) current in vivo in a rat that had been septic for two days. At both high and low levels of current injection the motor neuron fires erratically and cannot sustain firing throughout the 5s current injection. C) Dynamic clamp was used to increase the PIC/Ksthr ratio and this increases firing rate and lessens variability of firing rate throughout the 5s current injection. D) A further record from the same motor neuron 20 minutes after injection of 3 mg/kg lorcaserin. Motor neuron firing is rapid, and steady throughout the 5s injection of a square current pulse. E) Lowering the PIC/Ksthr ratio with dynamic clamp reverses the effect of lorcaserin such that firing becomes slow and the rate more variable. F) When dynamic clamp is turned off, the effect of lorcaserin treatment is again evident as firing is rapid and steady throughout the current injection.
Discussion
In earlier work we demonstrated that impaired motor neuron firing is the main cause of the early phase of ICU acquired weakness. Data presented in the present preclinical study suggest disruption in the normal balance of subthreshold currents that trigger action potentials contributes to defects in repetitive firing of motor neurons. Identification of this mechanism suggests an unexpected approach to therapy of ICU acquired weakness.
Mechanism and treatment of ICU acquired weakness in a rodent model of sepsis
The mechanisms contributing to ICU acquired weakness determine what therapies have the potential to improve strength. Both neuropathy and myopathy have been thought to be the primary contributors to ICU acquired weakness 1, 3, 33, 34. If these are the primary causes of weakness, development of effective therapy would not be possible because there is currently no treatment for either condition. However, we recently identified poor recruitment of motor units in patients with ICU acquired weakness, and this was the most important contributor to weakness in a rat model of sepsis 6, 7. Poor motor unit recruitment was due to a defect in excitability of lower motor neurons that was specific to repetitive firing 6, 7. In the current study we identified subthreshold oscillations in membrane potential in motor neurons with inability to repetitively fire. The occurrence of erratic firing together with oscillations constrained potential mechanisms. Oscillations in membrane potential and inability of motor neurons to repetitively fire have been shown in other systems to result from a reduction in the ratio of inward to outward subthreshold currents 14, 15 (often due to selective reduction of NaPIC 12). Study of the defect via both computer modeling and use of dynamic clamp in vivo were consistent in suggesting reduction in the persistent inward current/subthreshold K current (PIC/Ksthr) ratio could be the mechanism underlying the defect.
The voltage-dependence and kinetics of the dynamic clamp NaPIC current that was added or subtracted in our studies were specified to mimic NaPICs that have been described in a variety of neurons, specifically, a fast inward current that is significantly activated in a voltage range below the threshold for action potential generation, and that shows only slow inactivation. To our knowledge, there is no other voltage-activated current in mammalian neurons that exhibits these properties. For that reason we believe our results to have a high degree of specificity and indicate that increasing NaPIC alone is sufficient to restore repetitive firing in septic neurons and decreasing NaPIC alone is sufficient to induced septic-like behavior in normal motor neurons. However, it remains possible that deficits in other subthreshold currents play important roles in the motor neuron hypoexcitability induced by sepsis.
Our goal was to identify drugs that enhanced motor neuron repetitive firing in vivo following induction of sepsis. In animal studies it has been shown that activation of serotonin (5HT) receptors and noradrenergic receptors increases PICs and motor neuron excitability 26, 27, 35–37. We selected 5HT receptor agonists for use in in vivo experiments due to concern that activation of noradrenergic receptors might have significant cardiovascular side effects. Motor neurons express a wide variety of 5HT receptor subtypes 37. Drugs that activate 5HT2 receptors have been shown to increase PIC 26. Consistent with this, quipazine, a broad spectrum activator of 5HT receptors that increases motor neuron excitability and motor output of the spinal cord 28, 29, improved motor neuron repetitive firing in a septic rat. However, quipazine is not FDA approved. We thus tested lorcaserin, a selective 5HT2C agonist 31 that is FDA approved for weight loss and has fewer side effects 38. There was reason to hope that activation of 5HT2C receptors would increase motor neuron excitability 27, 30. Administration of lorcaserin rapidly improved repetitive firing of motor neurons in septic rats. A key question is whether treatment of sepsis-induced weakness with lorcaserin will translate from rats to humans.
The potential of activation of 5HT receptors to treat ICU acquired weakness in patients
For lorcaserin to provide effective therapy for ICU acquired weakness, three key findings in the rat model of sepsis must translate to patients with ICU acquired weakness: 1) A defect in motor neuron repetitive firing must be an important contributor to weakness, 2) Subthreshold currents must play a central role in human motor neuron repetitive firing, and 3) 5HT agonists such as lorcaserin that increase motor neuron excitability in rodents must also increase motor neuron excitability in patients. Each of these points is discussed below.
While myopathy and neuropathy have been suggested to be the primary causes of ICU acquired weakness, we identified poor recruitment of motor units as the most important contributor to weakness in patients in the early phase of recovery from ICU acquired weakness 6. This conclusion is bolstered by the finding that healthy humans injected with a single dose of bacterial lipopolysacharide, to acutely simulate sepsis, developed acute weakness that was due to reduced recruitment of motor units 39. These studies in patients suggest that reduced recruitment of motor units is an important contributor to weakness in the early stages of sepsis and recovery from sepsis. Thus, if motor neuron excitability could be increased to improve recruitment of motor units, this should significantly improve strength.
While it is not possible to study subthreshold currents directly in humans it is possible to study properties of motor unit recruitment that have been shown in animals to be dependent on subthreshold PIC 9, 40–42. The pattern of recruitment, firing and de-recruitment of motor units in humans strongly suggests a central role of PIC in motor unit firing 43–48. Thus up or down regulation of PIC in human motor neurons should have a profound effect on the ability of patients to recruit motor units to generate and sustain force.
To enhance motor neuron repetitive firing it is necessary to manipulate the currents responsible for repetitive firing. Administration of amphetamine, which increases release of norepinephrine, increases PIC amplitude in rats 49 and enhances firing of motor units in humans 50. Increasing the efficacy of 5HT in humans by drug treatment has been shown to increase the amplitude of spinal reflexes, consistent with an increase in motor neuron excitability 51. Further evidence that application of 5HT to patients increases motor neuron excitability comes from findings in serotonin syndrome, which is caused by overdose of serotonin agonists. Central features of the syndrome are over activity of the neuromuscular system including hyperreflexia, clonus and muscular rigidity 52. These symptoms are consistent with hyperexcitability of lower motor neurons. Thus, drugs shown to increase PIC in animals appear capable of increasing motor neuron excitability in humans.
All of the above leads to our proposal that giving serotonergic agonists to patients will provide effective therapy for ICUAW. If lorcaserin is effective in treating ICU acquired weakness, it would be the first therapy for this devastating complication of critical illness. As a recent study identified problems with repetitive firing of motor neurons in spinal muscular atrophy 53, administration of lorcaserin might also be useful in treating weakness in that disease of motor neurons.
Acknowledgments
This work was supported by National Institutes of Health Grant R01NS082354 (MMR). We would like to thank Lori Goss for performing surgery to induce sepsis in rats.
Footnotes
Author Contributions
PN, RP, TCC and MMR contributed to the conception and design of the study, drafting of the text and preparing the figures; PN and RP performed the acquisition and analysis of data.
Potential Conflicts of Interest
The authors declare no potential conflicts of interest.
References
- 1.Friedrich O, Reid MB, Van den Berghe G, et al. The Sick and the Weak: Neuropathies/Myopathies in the Critically Ill. Physiol Rev. 2015;95:1025–1109. doi: 10.1152/physrev.00028.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stevens RD, Marshall SA, Cornblath DR, et al. A framework for diagnosing and classifying intensive care unit-acquired weakness. Critical Care Medicine. 2009;37:S299–308. doi: 10.1097/CCM.0b013e3181b6ef67. [DOI] [PubMed] [Google Scholar]
- 3.Latronico N, Bolton CF. Critical illness polyneuropathy and myopathy: a major cause of muscle weakness and paralysis. Lancet Neurol. 2011;10:931–941. doi: 10.1016/S1474-4422(11)70178-8. [DOI] [PubMed] [Google Scholar]
- 4.van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 5.Truong AD, Fan E, Brower RG, Needham DM. Bench-to-bedside review: mobilizing patients in the intensive care unit--from pathophysiology to clinical trials. Crit Care. 2009;13:216. doi: 10.1186/cc7885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nardelli P, Khan J, Powers R, et al. Reduced motoneuron excitability in a rat model of sepsis. J Neurophysiol. 2013;109:1775–1781. doi: 10.1152/jn.00936.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nardelli P, Vincent JA, Powers R, et al. Reduced motor neuron excitability is an important contributor to weakness in a rat model of sepsis. Exp Neurol. 2016;282:1–8. doi: 10.1016/j.expneurol.2016.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harvey PJ, Li Y, Li X, Bennett DJ. Persistent sodium currents and repetitive firing in motoneurons of the sacrocaudal spinal cord of adult rats. J Neurophysiol. 2006;96:1141–1157. doi: 10.1152/jn.00335.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heckman CJ, Johnson M, Mottram C, Schuster J. Persistent inward currents in spinal motoneurons and their influence on human motoneuron firing patterns. Neuroscientist. 2008;14:264–275. doi: 10.1177/1073858408314986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heckman CJ, Enoka RM. Motor unit. Compr Physiol. 2012;2:2629–2682. doi: 10.1002/cphy.c100087. [DOI] [PubMed] [Google Scholar]
- 11.Powers RK, Nardelli P, Cope TC. Estimation of the contribution of intrinsic currents to motoneuron firing based on paired motoneuron discharge records in the decerebrate cat. J Neurophysiol. 2008;100:292–303. doi: 10.1152/jn.90296.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuo JJ, Lee RH, Zhang L, Heckman CJ. Essential role of the persistent sodium current in spike initiation during slowly rising inputs in mouse spinal neurones. J Physiol. 2006;574:819–834. doi: 10.1113/jphysiol.2006.107094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Do MT, Bean BP. Subthreshold sodium currents and pacemaking of subthalamic neurons: modulation by slow inactivation. Neuron. 2003;39:109–120. doi: 10.1016/s0896-6273(03)00360-x. [DOI] [PubMed] [Google Scholar]
- 14.Iglesias C, Meunier C, Manuel M, et al. Mixed mode oscillations in mouse spinal motoneurons arise from a low excitability state. J Neurosci. 2011;31:5829–5840. doi: 10.1523/JNEUROSCI.6363-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sciamanna G, Wilson CJ. The ionic mechanism of gamma resonance in rat striatal fast-spiking neurons. J Neurophysiol. 2011;106:2936–2949. doi: 10.1152/jn.00280.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldberg EM, Clark BD, Zagha E, et al. K+ channels at the axon initial segment dampen near-threshold excitability of neocortical fast-spiking GABAergic interneurons. Neuron. 2008;58:387–400. doi: 10.1016/j.neuron.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hines M. A program for simulation of nerve equations with branching geometries. Int J Biomed Comput. 1989;24:55–68. doi: 10.1016/0020-7101(89)90007-x. [DOI] [PubMed] [Google Scholar]
- 18.Golomb D, Donner K, Shacham L, et al. Mechanisms of firing patterns in fast-spiking cortical interneurons. PLoS Comput Biol. 2007;3:e156. doi: 10.1371/journal.pcbi.0030156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rho YA, Prescott SA. Identification of molecular pathologies sufficient to cause neuropathic excitability in primary somatosensory afferents using dynamical systems theory. PLoS Comput Biol. 2012;8:e1002524. doi: 10.1371/journal.pcbi.1002524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manuel M, Iglesias C, Donnet M, et al. Fast kinetics, high-frequency oscillations, and subprimary firing range in adult mouse spinal motoneurons. J Neurosci. 2009;29:11246–11256. doi: 10.1523/JNEUROSCI.3260-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Bennett DJ. Persistent sodium and calcium currents cause plateau potentials in motoneurons of chronic spinal rats. J Neurophysiol. 2003;90:857–869. doi: 10.1152/jn.00236.2003. [DOI] [PubMed] [Google Scholar]
- 22.Duflocq A, Chareyre F, Giovannini M, et al. Characterization of the axon initial segment (AIS) of motor neurons and identification of a para-AIS and a juxtapara-AIS, organized by protein 4.1B. BMC Biol. 2011;9:66. doi: 10.1186/1741-7007-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bebarova M. Advances in patch clamp technique: towards higher quality and quantity. Gen Physiol Biophys. 2012;31:131–140. doi: 10.4149/gpb_2012_016. [DOI] [PubMed] [Google Scholar]
- 24.Berecki G, Verkerk AO, van Ginneken AC, Wilders R. Dynamic clamp as a tool to study the functional effects of individual membrane currents. Methods Mol Biol. 2014;1183:309–326. doi: 10.1007/978-1-4939-1096-0_20. [DOI] [PubMed] [Google Scholar]
- 25.Bauer JA, Lambert KM, White JA. The past, present, and future of real-time control in cellular electrophysiology. IEEE Trans Biomed Eng. 2014;61:1448–1456. doi: 10.1109/TBME.2014.2314619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harvey PJ, Li X, Li Y, Bennett DJ. 5-HT2 receptor activation facilitates a persistent sodium current and repetitive firing in spinal motoneurons of rats with and without chronic spinal cord injury. J Neurophysiol. 2006;96:1158–1170. doi: 10.1152/jn.01088.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murray KC, Nakae A, Stephens MJ, et al. Recovery of motoneuron and locomotor function after spinal cord injury depends on constitutive activity in 5-HT2C receptors. Nat Med. 2010;16:694–700. doi: 10.1038/nm.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brumley MR, Robinson SR. The serotonergic agonists quipazine, CGS-12066A, and alpha-methylserotonin alter motor activity and induce hindlimb stepping in the intact and spinal rat fetus. Behav Neurosci. 2005;119:821–833. doi: 10.1037/0735-7044.119.3.821. [DOI] [PubMed] [Google Scholar]
- 29.Chopek JW, MacDonell CW, Power KE, et al. Removal of supraspinal input reveals a difference in the flexor and extensor monosynaptic reflex response to quipazine independent of motoneuron excitation. J Neurophysiol. 2013;109:2056–2063. doi: 10.1152/jn.00405.2012. [DOI] [PubMed] [Google Scholar]
- 30.Murray KC, Stephens MJ, Ballou EW, et al. Motoneuron excitability and muscle spasms are regulated by 5-HT2B and 5-HT2C receptor activity. J Neurophysiol. 2011;105:731–748. doi: 10.1152/jn.00774.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomsen WJ, Grottick AJ, Menzaghi F, et al. Lorcaserin, a novel selective human 5-hydroxytryptamine2C agonist: in vitro and in vivo pharmacological characterization. J Pharmacol Exp Ther. 2008;325:577–587. doi: 10.1124/jpet.107.133348. [DOI] [PubMed] [Google Scholar]
- 32.Higgins GA, Silenieks LB, Rossmann A, et al. The 5-HT(2C) Receptor Agonist Lorcaserin Reduces Nicotine Self-Administration, Discrimination, and Reinstatement: Relationship to Feeding Behavior and Impulse Control. Neuropsychopharmacology. 2012;37:1177–1191. doi: 10.1038/npp.2011.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bednarik J, Lukas Z, Vondracek P. Critical illness polyneuromyopathy: the electrophysiological components of a complex entity. Intensive Care Med. 2003;29:1505–1514. doi: 10.1007/s00134-003-1858-0. [DOI] [PubMed] [Google Scholar]
- 34.Khan J, Harrison TB, Rich MM, Moss M. Early development of critical illness myopathy and neuropathy in patients with severe sepsis. Neurology. 2006;67:1421–1425. doi: 10.1212/01.wnl.0000239826.63523.8e. [DOI] [PubMed] [Google Scholar]
- 35.Bennett DJ, Li Y, Siu M. Plateau potentials in sacrocaudal motoneurons of chronic spinal rats, recorded in vitro. J Neurophysiol. 2001;86:1955–1971. doi: 10.1152/jn.2001.86.4.1955. [DOI] [PubMed] [Google Scholar]
- 36.Lee RH, Heckman CJ. Enhancement of bistability in spinal motoneurons in vivo by the noradrenergic alpha1 agonist methoxamine. J Neurophysiol. 1999;81:2164–2174. doi: 10.1152/jn.1999.81.5.2164. [DOI] [PubMed] [Google Scholar]
- 37.Perrier JF, Rasmussen HB, Christensen RK, Petersen AV. Modulation of the intrinsic properties of motoneurons by serotonin. Curr Pharm Des. 2013;19:4371–4384. doi: 10.2174/13816128113199990341. [DOI] [PubMed] [Google Scholar]
- 38.Hurren KM, Berlie HD. Lorcaserin: an investigational serotonin 2C agonist for weight loss. Am J Health Syst Pharm. 2011;68:2029–2037. doi: 10.2146/ajhp100638. [DOI] [PubMed] [Google Scholar]
- 39.McNicol FJ, Hoyland JA, Cooper RG, Carlson GL. Skeletal muscle contractile properties and proinflammatory cytokine gene expression in human endotoxaemia. Br J Surg. 2010;97:434–442. doi: 10.1002/bjs.6868. [DOI] [PubMed] [Google Scholar]
- 40.Thomas CK, Butler JE, Zijdewind I. Patterns of pathological firing in human motor units. Adv Exp Med Biol. 2002;508:237–244. doi: 10.1007/978-1-4615-0713-0_29. [DOI] [PubMed] [Google Scholar]
- 41.Schwindt PC, Crill WE. Properties of a persistent inward current in normal and TEA-injected motoneurons. J Neurophysiol. 1980;43:1700–1724. doi: 10.1152/jn.1980.43.6.1700. [DOI] [PubMed] [Google Scholar]
- 42.Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Bistability of alpha-motoneurones in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. J Physiol. 1988;405:345–367. doi: 10.1113/jphysiol.1988.sp017336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gorassini M, Yang JF, Siu M, Bennett DJ. Intrinsic activation of human motoneurons: reduction of motor unit recruitment thresholds by repeated contractions. J Neurophysiol. 2002;87:1859–1866. doi: 10.1152/jn.00025.2001. [DOI] [PubMed] [Google Scholar]
- 44.Kiehn O, Eken T. Prolonged firing in motor units: evidence of plateau potentials in human motoneurons? J Neurophysiol. 1997;78:3061–3068. doi: 10.1152/jn.1997.78.6.3061. [DOI] [PubMed] [Google Scholar]
- 45.Walton C, Kalmar JM, Cafarelli E. Effect of caffeine on self-sustained firing in human motor units. J Physiol. 2002;545:671–679. doi: 10.1113/jphysiol.2002.025064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gorassini M, Yang JF, Siu M, Bennett DJ. Intrinsic activation of human motoneurons: possible contribution to motor unit excitation. J Neurophysiol. 2002;87:1850–1858. doi: 10.1152/jn.00024.2001. [DOI] [PubMed] [Google Scholar]
- 47.Vandenberk MS, Kalmar JM. An evaluation of paired motor unit estimates of persistent inward current in human motoneurons. J Neurophysiol. 2014;111:1877–1884. doi: 10.1152/jn.00469.2013. [DOI] [PubMed] [Google Scholar]
- 48.Revill AL, Fuglevand AJ. Inhibition linearizes firing rate responses in human motor units: implications for the role of persistent inward currents. J Physiol. 2017;595:179–191. doi: 10.1113/JP272823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rank MM, Li X, Bennett DJ, Gorassini MA. Role of endogenous release of norepinephrine in muscle spasms after chronic spinal cord injury. J Neurophysiol. 2007;97:3166–3180. doi: 10.1152/jn.01168.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Udina E, D’Amico J, Bergquist AJ, Gorassini MA. Amphetamine increases persistent inward currents in human motoneurons estimated from paired motor-unit activity. J Neurophysiol. 2010;103:1295–1303. doi: 10.1152/jn.00734.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wei K, Glaser JI, Deng L, et al. Serotonin affects movement gain control in the spinal cord. J Neurosci. 2014;34:12690–12700. doi: 10.1523/JNEUROSCI.1855-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352:1112–1120. doi: 10.1056/NEJMra041867. [DOI] [PubMed] [Google Scholar]
- 53.Fletcher EV, Simon CM, Pagiazitis JG, et al. Reduced sensory synaptic excitation impairs motor neuron function via Kv2.1 in spinal muscular atrophy. Nat Neurosci. 2017;20:905–916. doi: 10.1038/nn.4561. [DOI] [PMC free article] [PubMed] [Google Scholar]