Abstract
The authors examined the relationship of prepregnancy body mass index (BMI) and gestational weight gain (GWG) with child neurodevelopment. Mother–child dyads were a subgroup (n = 2,084) of the Child Health and Development Studies from the Oakland, California, area enrolled during pregnancy from 1959 to 1966 and followed at child age 9 years. Linear regression was used to examine associations between prepregnancy BMI, GWG, and standardized Peabody Picture Vocabulary Test and Raven Progressive Matrices scores and to evaluate effect modification of GWG by prepregnancy BMI. Before pregnancy, 77% of women were normal weight, 8% were underweight, 11% were overweight, and 3% were obese. Associations between GWG and child outcomes did not vary by prepregnancy BMI, suggesting no evidence for interaction. In multivariable models, compared to normal prepregnancy BMI, prepregnancy overweight and obesity were associated with lower Peabody scores (b: −1.29; 95% CI [−2.6, −0.04] and b: −2.7; 95% CI [−5.0, −0.32], respectively). GWG was not associated with child Peabody score [b: −0.03 (95% CI: −0.13, 0.07)]. Maternal BMI and GWG were not associated with child Raven score (all P >0.05). Maternal prepregnancy overweight and obesity were associated with lower scores for verbal recognition in mid‐childhood. These results contribute to evidence linking maternal BMI with child neurodevelopment. Future research should examine the role of higher prepregnancy BMI values and the pattern of pregnancy weight gain in child cognitive outcomes.
Keywords: child development, cognitive development, maternal obesity, pregnancy
1. INTRODUCTION
Overweight and obesity affect 60% of women of childbearing age in the United States (Flegal, Kruszon‐Moran, Carroll, Fryar, & Ogden, 2016; Ogden, Carroll, Kit, & Flegal, 2014) and are associated with several adverse health outcomes for mothers and children (Gilmore, Klempel‐Donchenko, & Redman, 2015; Marchi, Berg, Dencker, Olander, & Begley, 2015). Recently, maternal prepregnancy body mass index (BMI) and gestational weight gain (GWG) have emerged as potentially modifiable risk factors for adverse child cognitive development (Hinkle, Albert, Sjaarda, Grewal, & Grantz, 2016; Hinkle, Sharma, Kim, & Schieve, 2013; Hinkle et al., 2012; Huang et al., 2014; Jo et al., 2015; Keim & Pruitt, 2012; Pugh et al., 2015; Pugh et al., 2016a; Pugh et al., 2016b). Several non‐mutually exclusive biological mechanisms are postulated to be involved in these associations. During pregnancy, hormonal and inflammatory perturbations (Mehta, Kerver, Sokol, Keating, & Paneth, 2014; Sullivan, Nousen, & Chamlou, 2014; van der Burg et al., 2016), as well as excessive or suboptimal nutrient intake (e.g., high‐fat diet (Niculescu & Lupu, 2009, Sasaki, de Vega, Sivanathan, St‐Cyr, & McGowan, 2014, Sullivan et al., 2014)), could lead to deficits in fetal brain development, including inadequate synapse formation as well as perturbed neuronal proliferation and differentiation, resulting in abnormalities in neuronal structure and function (Bouret, 2010; Edlow et al., 2014; Neri & Edlow, 2016; Tozuka, Wada, & Wada, 2009). Evidence from animal models suggests that a high‐fat diet combined with excessive GWG can lead to high levels of inflammatory cytokines (Niculescu & Lupu, 2009; Tozuka et al., 2009) that can potentially permanently alter fetal brain development trajectories (Bolton & Bilbo, 2014). In contrast, other evidence suggests that appropriate GWG may mitigate adverse effects of higher BMI values on child neurodevelopment (Huang et al., 2014; Pugh et al., 2015; Pugh et al., 2016a; Rodriguez et al., 2008).
Some, but not all (Brion et al., 2011; Hinkle et al., 2016; Keim & Pruitt, 2012), studies find associations between maternal BMI and/or GWG and adverse cognitive outcomes in children, with a majority of studies reporting associations for specific BMI categories, such as obesity (Daraki et al., 2017; Hinkle et al., 2013; Huang et al., 2014; Jo et al., 2015; Krakowiak et al., 2012; Mann, McDermott, Hardin, Pan, & Zhang, 2013; Pugh et al., 2015; Rodriguez, 2010; Rodriguez et al., 2008; Tanda, Salsberry, Reagan, & Fang, 2013; Tavris & Read, 1982; Torres‐Espinola et al., 2015; Yeung, Sundaram, Ghassabian, Xie, & Buck, 2017) or low and/or high BMI values (Basatemur et al., 2013; Hinkle et al., 2012; Huang et al., 2014; Neggers, Goldenberg, Ramey, & Cliver, 2003; Pugh et al., 2016a). For GWG, several studies have reported adverse effects of low GWG (Gage, Lawlor, Tilling, & Fraser, 2013), high GWG (Huang et al., 2014; Pugh et al., 2016a; Tavris & Read, 1982) or excessive GWG (>2009 Institute of Medicine [IOM] guidelines; Gage et al., 2013; Pugh et al., 2015), while others report positive associations between GWG and cognitive outcomes (Gage et al., 2013). Altogether, the preponderance of evidence suggests that higher BMI values and low or high GWG may have adverse consequences for child neurodevelopment, but several gaps remain. Some previous studies were conducted with very small numbers of overweight or obese women, limiting their ability to evaluate differential effects of prepregnancy BMI. Other studies only reported results from early childhood (Bitsko et al., 2016; Casas et al., 2013; Hinkle et al., 2012; Hinkle et al., 2013; Hinkle et al., 2016; Jo et al., 2015; Rodriguez, 2010; Torres‐Espinola et al., 2015) or did not account for concurrent weight or height of the child in analyses (Gage et al., 2013; Hinkle et al., 2012; Hinkle et al., 2016; Huang et al., 2014; Keim & Pruitt, 2012; Pugh et al., 2015; Pugh et al., 2016a; Pugh et al., 2016b; Tavris & Read, 1982; Torres‐Espinola et al., 2015). Finally, several studies only evaluated effects of BMI or GWG alone, and did not evaluate the joint associations between these factors (Basatemur et al., 2013; Brion et al., 2011; Hinkle et al., 2013; Krakowiak et al., 2012; Mann et al., 2013; Neggers et al., 2003; Rodriguez, 2010; Rodriguez et al., 2008; Tanda et al., 2013; Tavris & Read, 1982; Torres‐Espinola et al., 2015). Our objective was to determine the relationship of maternal prepregnancy BMI and GWG with child neurodevelopment in mid‐childhood among mother–child dyads who participated in the Child Health and Development Studies (CHDS). We hypothesized that higher prepregnancy BMI and higher GWG would have negative associations with child cognitive outcomes. We also hypothesized that associations of GWG with child cognitive outcomes would vary by prepregnancy BMI, with stronger negative associations in women with higher BMI.
Key messages.
There is mixed evidence of the association between prepregnancy BMI, pregnancy weight gain, and child cognitive development with some, but not all, studies reporting associations between these factors and neurodevelopment in childhood.
In a large cohort, this study found that maternal overweight and obesity, but not pregnancy weight gain, were associated with lower verbal recognition scores in mid‐childhood.
Maternal prepregnancy BMI and pregnancy weight gain were not associated with perceptual reasoning scores in mid‐childhood.
2. METHODS
The CHDS is a prospective birth cohort that has been previously described (van den Berg, Christianson, & Oechsli, 1988). Briefly, the CHDS enrolled 98% of eligible pregnant women who were receiving obstetric care between 1959 and 1966 from the Kaiser Foundation Health Plan in the Oakland, California, area. Women were enrolled after confirmation of pregnancy and followed through delivery. Dyads were followed post‐partum to at least child age 5 years. Detailed information on maternal characteristics was obtained through maternal interview. After delivery, maternal and paediatric records were abstracted for information about prenatal care and pregnancy outcomes. From 1959 to 1967, the CHDS cohort included 14,909 live births with a maternal interview. Follow up relevant to this analysis was conducted on a subset of dyads with live births enrolled from 1960 to 1963 (n = 9,708). Of the dyads enrolled during this period that continued to reside in the Bay Area at child age 9 years (n = 6,614), 3,737 dyads were brought in for a follow‐up visit at this time.
For this analysis, women with implausible gestational ages (>44 weeks), last measured prenatal weights >4 weeks before delivery, twin gestations or missing BMI, GWG, or outcome (i.e., child cognition at age 9 years) data were excluded. Women (n = 44) with implausible weight change from their self‐reported prepregnancy weight to their first measured weight at ~14 weeks of pregnancy, defined using the 1st and 99th percentiles for weight change (weight loss >6.23 kg and weight gain >12.94 kg), were also excluded. This yielded an analytic sample of 2,084 singleton children and their mothers from the original CHDS population (Figure 1). This analysis was approved by the Institutional Review Board at Columbia University Medical Center.
Figure 1.
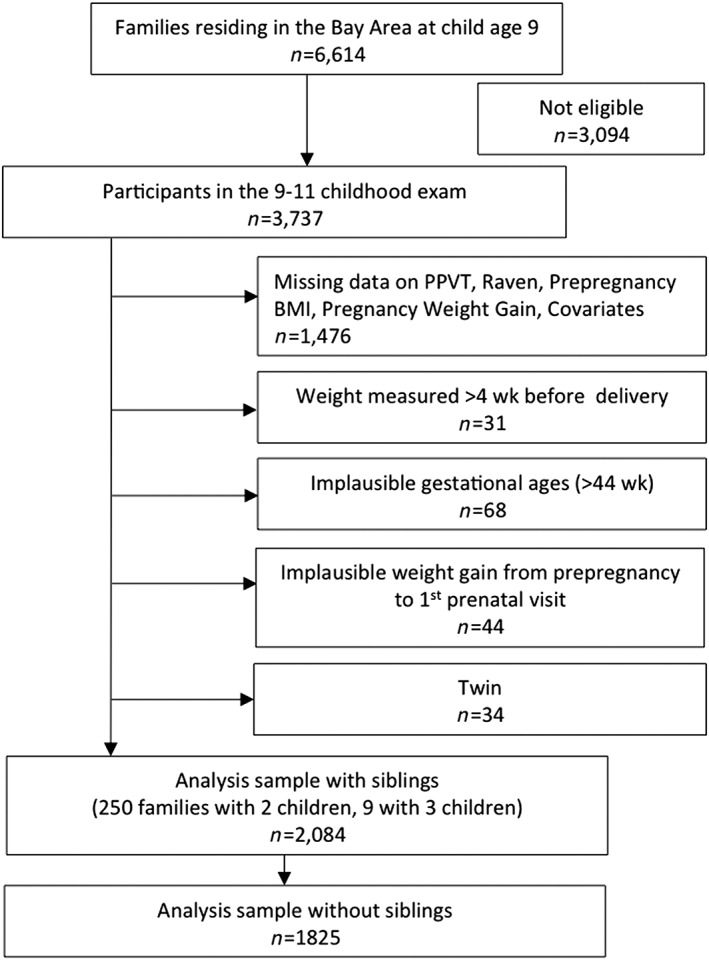
Participant flow chart for a study of prepregnancy body mass index (BMI), pregnancy weight gain, and child neurodevelopment (Child Health and Development Studies (1960 to 1963))
2.1. Exposures
Prepregnancy BMI (kg/m2) was calculated from self‐reported prepregnancy weight and maternal height measured at enrolment. Prepregnancy BMI categories were defined as underweight (BMI <18.5 kg/m2), normal weight (18.5–24.99 kg/m2), overweight (25.0–29.99 kg/m2), and obese (≥30 kg/m2). We calculated total GWG as the difference between prepregnancy weight and the last measured weight prior to delivery. Of women weighed <28 days before delivery, the last prenatal weight was obtained on average 2.5 days before delivery, with 95% of women measured within 9 days of delivery.
2.2. Outcomes
At ages 9–11 years, children were administered the Peabody Picture Vocabulary Test (Osicka, 1976), which tests receptive vocabulary, and the Raven Coloured Progressive Matrices Tests (Raven, 1965, 1962), which assess perceptual reasoning. The Peabody test remains widely used to assess receptive vocabulary in children and adults, and recent studies have explored changes in development assessed with the Peabody test from childhood to adulthood (Armstrong et al., 2016) and use of different versions of the test (Armstrong et al., 2016; Hoffman, Templin, & Rice, 2012). The Raven test has been widely applied to assess basic cognitive functioning (Mackintosh & Bennett, 2005), and the change and stability of scores have previously been reviewed (Raven, 2000). Trained research staff administered tests. Raw scores were standardized for age, race, and sex, with a mean of 50 and a standard deviation of 10.
2.3. Covariates
Potential covariates examined included family income (<40% of 1960 census median, 40–60% census median, >60% census median), maternal education (<high school, high school and/or vocational school, ≥college), marital status (married/not married), smoking status during pregnancy (smoker/not smoker), race (White, Black, Hispanic, Asian), age (years, continuous), parity (n, continuous), and gestational age at last measured prenatal weight (weeks, continuous), as well as child gestational age at delivery (weeks between last menstrual cycle and delivery, continuous), sex (dichotomous), weight at birth (g, continuous, abstracted from medical record), and measured weight (kg, continuous) and height (cm, continuous) at exam. In a subset (80%) of participants, maternal Peabody Score (continuous) was assessed at the 9‐ to 11‐year visit.
2.4. Statistical analyses
Analyses were conducted with Stata 14.0 (College Station, TX). An alpha of 0.05 was used for statistical tests of associations and interactions. Preliminary analyses compared the baseline characteristics of dyads in the analytic sample (n = 2,084) to those who were not in the analysis but who had child cognitive outcomes measured at ages 9–11 years (n = 1,653) using chi‐square tests for categorical variables and t‐tests for continuous normally distributed variables and Wilcoxon rank‐sum test for non‐normally distributed continuous variables.
As previously described (Widen et al., 2016) and to contextualize GWG in our cohort to the present day, we evaluated adherence to the 2009 IOM guidelines in descriptive analyses using GWG adequacy ratios, which accounts for the gestational age at the last measured weight (GWG adequacy = observed total GWG/expected GWG), where expected GWG = IOM recommended first trimester GWG + (gestational age at last weight—13 weeks) × the recommended rate of GWG for the second and third trimesters (Institute of Medicine, 2009). Ratios exceeding the recommendations were coded as excessive GWG, while ratios below the recommendations were coded as inadequate GWG.
Multivariable linear regression with robust standard errors and clustering by mother (to account for multiple children per family) was used to examine the relationship of prepregnancy BMI and GWG, as a continuous variable in kg, to standardized Peabody and Raven scores. Our first model set included prepregnancy BMI categories, GWG and an interaction term between pregnancy BMI category and GWG, to test for interaction on the additive scale. We decided a priori that if no effect modification was observed, the variables would be evaluated for independent main effects. Potential covariates based on prior literature were evaluated for inclusion in adjusted models and retained if they changed the primary exposure effect estimate by 10%. We hypothesized that childhood body size was likely on the causal pathway between maternal BMI/pregnancy weight gain, with which it is strongly associated, and child cognition. However, due to the complexity of these associations and the multifaceted biological drivers of cognition, we chose to explore this pathway by examining the primary models both with and without adjustment for child height, weight, and birthweight. Maternal age, income, race, education, and parity met the criteria for inclusion in the adjustment set.
Several sensitivity analyses were conducted: (1) Quartiles of GWG: Analyses were conducted with quartiles of GWG, derived from the continuous variable, to assess for linearity of associations. (2) Siblings: A sensitivity analysis was conducted restricting to the oldest CHDS child per family. (3) Timing of last prenatal weight: A sensitivity analysis was conducted excluding dyads with last measured prenatal weights obtained >7 days before delivery. (4) Excluding preterm deliveries: A sensitivity analysis was conducted excluding preterm deliveries (<37 weeks gestational age). (5) Maternal Peabody IQ score: A sensitivity analysis was conducted including maternal Peabody score as a covariate. (6) Selection bias: An analysis was conducted using inverse probability weighting to assess the effects of incomplete follow up at the 9‐ to 11‐year visit (Widen et al., 2016). This approach allows for estimation of bias if missing data were differential by exposure or key covariates. A logistic regression model was used to estimate the covariate‐adjusted probability of inclusion in this analysis. The model included the following variables: maternal BMI at first study visit, race, age, education, and parity. The inverse of the predicted probability of inclusion was used for sample weighting in linear regression analyses with survey commands in Stata.
3. RESULTS
There were no differences in GWG, parity, race, maternal age, marital status, child sex, or birthweight between those included in this analysis and those with missing data; however, included children were younger, had slightly higher standardized Peabody and Raven scores, and were shorter and lighter at the 9‐year measurement than excluded children, and included mothers were slightly heavier, more highly educated, and had higher income than excluded mothers (Table 1).
Table 1.
Characteristics of 2,084 mothers and children for a study of prepregnancy BMI, pregnancy weight gain, and child neurodevelopment (Child Health and Development Studies participants in the 9‐ to 11‐year follow‐up study)
| Maternal | |
|---|---|
| Prepregnancy BMI, kg/m2 (Mean ± SD) | 22.0 ± 3.4 |
| Prepregnancy BMI category, % | |
| Underweight (<18.5 kg/m2) | 8.7 |
| Normal weight (18.5–24.9 kg/m2) | 77.2 |
| Overweight (25–29.9 kg/m2) | 11.2 |
| Obese (≥30 kg/m2) | 2.8 |
| Pregnancy weight gaina, kg (Mean ± SD) | 12.0 ± 4.3 |
| Gestational age at delivery, weeks (Mean ± SD) | 39.4 ± 1.9 |
| Maternal race, % | |
| White | 71.6 |
| Black | 21.4 |
| Hispanic | 2.7 |
| Asian | 4.4 |
| Multiparous, % yes | 77.8 |
| Maternal age, years (Mean ± SD) | 28.4 ± 5.9 |
| Maternal education, % | |
| Less than high school | 12.7 |
| High school and/or trade school | 43.2 |
| Some college/college degree | 44.1 |
| Maternal raw Peabodyb, score | 125.5 ± 18.4 |
| Married (yes/no), % | 98.6 |
| Income, relative to 1960 census median, % | |
| Below median | 34.5 |
| At median | 16.3 |
| Above median | 49.2 |
| Child | |
| Male sex, % | 51.7 |
| Preterm deliveryc, % | 6.3 |
| Birthweight, g (Mean ± SD) | 3352 ± 508 |
| Age at 9‐year exam, years (Mean ± SD) | 9.8 ± 0.82 |
| Weight at age 9, kg (Mean ± SD) | 33.5 ± 7.2 |
| Height at age 9, cm (Mean ± SD) | 137.6 ± 7.6 |
| Standardized Peabody scored (Mean ± SD) | 50.6 ± 10.0 |
| Standardized Raven scored (Mean ± SD) | 50.4 ± 10.0 |
BMI = body mass index; SD = standard deviation.
Pregnancy weight gain was coded as a continuous variable defined as the last measured weight prior to delivery minus the self‐reported prepregnancy weight.
Maternal Peabody was administered in a subset of women in the CHDS, and in our analytic sample, 1,652 women had Peabody scores.
Defined as gestational age at delivery <37 weeks.
Standardized for race, age, and sex.
Table 1 shows the characteristics of the analytic sample (n = 2,084). Before pregnancy, a majority of mothers had BMIs in the normal range, while fewer were overweight or underweight, and a small proportion were obese. Overall, 41.2% of women had GWG below the 2009 IOM recommendations, 37.4% had GWG within the recommended ranges, and 21.4% had GWG above IOM recommendations (Institute of Medicine, 2009). Adherence to IOM recommendations varied by prepregnancy BMI (P < 0.001; Figure S1). Specifically, a larger proportion of overweight and obese women exceeded the IOM GWG guidelines, while a larger proportion of underweight and normal weight women gained less than the recommended amounts.
In our multivariable models, we were interested in evaluating whether child body size mediated associations of maternal prepregnancy BMI category and GWG with child outcomes. While inclusion of birthweight decreased estimated beta‐coefficients by >10% (data not shown), inclusion of child current body size in the model increased the estimated beta‐coefficients for prepregnancy BMI categories by >10% (data not shown). This was in contrast to what we expected: We hypothesized that if child body size mediated the relationship, inclusion in the model would decrease the beta‐coefficient. We therefore concluded that current child body size was acting as a confounder and birthweight was acting as a mediator.
Our first model set included prepregnancy BMI categories, GWG, and an interaction between prepregnancy BMI categories and GWG to evaluate if effects of GWG varied by BMI, adjusting for covariates. In this model, associations with GWG did not vary by prepregnancy BMI (P‐value for interaction >0.05), so the interaction term was not retained in subsequent models. The second model set included both prepregnancy BMI category and GWG as independent exposures and covariates, and is denoted the primary model set for comparisons. In the Peabody model, we observed that compared to women with normal prepregnancy BMI, women who were overweight and obese had children with lower Peabody scores at 9 years (Figure 2; Table S2, Model 2), controlling for GWG. In this model, GWG was not independently associated with child Peabody score (Table S2, Model 2). Neither prepregnancy BMI category nor GWG was associated with child Raven scores (Table S2, Model 2); however, the estimated beta coefficients relating prepregnancy BMI categories and GWG to the Raven score followed a similar inverse trend to the Peabody scores. Our third model set was the unadjusted model and included only prepregnancy BMI category and GWG; unadjusted results were similar to the adjusted primary model (Table S2, Model 3).
Figure 2.
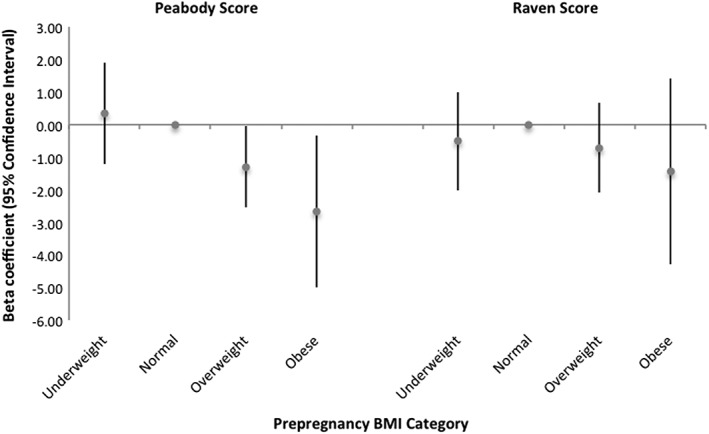
Association between maternal prepregnancy body mass index (BMI) category and child cognitive test scores at age 9 years (n = 2,084) with normal weight BMI as the referent group. Results shown are estimated beta‐coefficients for standardized continuous Peabody and Raven scores from multivariable linear regression models, controlling for covariates
In sensitivity analyses conducted using quartiles of GWG (n = 2,084), including only the oldest CHDS child per family (n = 1,825), and excluding preterm deliveries (n = 1,935), observed associations were similar to the full cohort adjusted model without interaction terms (Model 2; Table S2, Models 4, 5, and 7, respectively). In a sensitivity analysis that included only women with weights measured within a week of delivery (n = 1,882) and that adjusted for maternal Peabody IQ score (n = 1,652), estimated beta‐coefficients were similar in direction, but smaller in magnitude compared to the full cohort adjusted model without interaction terms (Model 2; Table S2, Models 6 and 8, respectively).
Calculation of inverse probability weights for those included in this analysis compared to those whose cognitive abilities were measured at 9 years but were not included in this analysis showed that maternal education was associated with inclusion, while higher maternal BMI at the first study visit was associated with a reduced likelihood of inclusion; race, parity, and age were not associated with inclusion. Weighting the data for successful follow up did not appreciably alter associations between maternal BMI categories and child outcomes (Table S1, Model 9).
4. DISCUSSION
We found evidence of associations between maternal BMI and cognitive scores in mid‐childhood. Maternal overweight and obesity were associated with lower scores for the Peabody Picture Vocabulary Test, an indicator of verbal recognition. Maternal BMI categories were not associated with the Raven Progressive Matrices Tests, a non‐verbal indicator of perceptual reasoning, although results were in the expected direction. Because these tests reflect different cognitive domains, our findings suggest that verbal abilities could perhaps be more susceptible to the adverse effects of maternal obesity during pregnancy. However, the biological rationale for these differences is not clear, and these differential findings may also be explained by the challenges in measuring these different components of cognition. Our results also indicate that GWG is not associated with cognitive scores in mid‐childhood.
We conducted several sensitivity analyses. We observed similar results for prepregnancy BMI with use of GWG quartiles, rather than continuous GWG. As data on maternal IQ was not available on all participants, we conducted a sensitivity analysis with the additional adjustment for IQ in a subset of participants. The beta‐coefficients for maternal overweight and obesity were modestly attenuated compared to the primary model, but due to a smaller sample size (1652 vs. 2084), the results were no longer significant. The other sensitivity analyses, including inverse probability weighting for successful follow‐up, confirmed the results of the primary analysis.
Our results for maternal prepregnancy overweight and obesity are consistent with two other studies that reported inverse associations between maternal BMI and child cognition. Specifically, higher BMI was associated with lower kindergarten standardized readings scores in a nationally representative U.S. cohort (Hinkle et al., 2013) and poorer performance on the British Ability Scales at ages 5 and 7 years in a United Kingdom‐based cohort (Basatemur et al., 2013). Although these BMI results are consistent with our findings, GWG was not examined in these reports.
Several studies have examined both BMI and GWG and are consistent with our findings. In U.S. dyads from Pittsburgh, prepregnancy overweight and obesity were associated with lower child IQ at age 10 years, while there was no overall association between GWG and child IQ (Pugh et al., 2015). In a low‐income cohort of African American women, prepregnancy obesity was associated with lower general IQ and nonverbal abilities scores at child age 5 years, while GWG was not associated with child outcomes (Neggers et al., 2003). In a nationally representative U.S. cohort, prepregnancy obesity was associated with a reduction in child reading and mathematics scores at ages 5–7 years on the Peabody Individual Achievement Test, whereas high GWG (>2009 IOM recommendations) was not associated with child outcomes (Tanda et al., 2013).
Two studies reported results consistent with ours for prepregnancy body size, but in contrast to our report, observed associations between GWG and child outcomes. In a Pittsburgh cohort, maternal prepregnancy BMI values above 22 kg/m2 and high GWG were each associated with lower reading, math, and spelling scores ages 6, 10, and 14 years (Pugh et al., 2016a). In a United Kingdom cohort, prepregnancy weight was inversely associated with IQ at age 8 years, and GWG from early to mid‐pregnancy (0–28 weeks) was positively associated with child IQ (Gage et al., 2013). Because GWG was examined by phase of pregnancy in this study, the results cannot be directly compared to ours. In the U.S. Collaborative Perinatal Project cohort, prepregnancy obesity was associated with lower IQ at age 7 years, with stronger associations among mothers with high GWG (Huang et al., 2014).
This study has several limitations. First, our results may not be fully generalizable to the current population of women of childbearing age, as the prevalence of overweight and obesity in our cohort is much lower than the current prevalence in the United States (Flegal et al., 2016; Ogden et al., 2014); however, there is no evidence that the biological association between maternal body size, GWG, and neurodevelopment has changed over time (Pugh et al., 2015). Although our study population was all receiving health insurance during pregnancy and we accounted for several measures of socioeconomic status in our analyses, there may still be residual confounding due to socioeconomic status or other unmeasured factors. We had limited numbers of participants with both adequate GWG and maternal obesity (n = 10), and thus were underpowered to evaluate for interactions between prepregnancy BMI and GWG according to IOM guideline adherence in this cohort (Institute of Medicine, 2009). Furthermore, there was potential for selection bias due to restrictions on who was included in our analysis. We conducted several sensitivity analyses, including inverse probability weighting, to evaluate whether our results were biassed based on our inclusion criteria, and the results were not meaningfully changed. We had a smaller sample of women with data on IQ, and the effect sizes for prepregnancy BMI category were somewhat attenuated after adjustment for maternal IQ in this sub‐sample; however, the beta‐coefficients were not appreciably different. We were not able to account for paternal obesity or gestational diabetes in our analyses (Daraki et al., 2017; Yeung et al., 2017), which may be important to consider in future work. In addition, gestational age was assessed from the last menstrual period, as ultrasound was not common at the time of the study, which could result in bias of our last maternal weight measurement timing due to measurement error, which would likely be nondifferential and bias results towards the null (Savitz et al., 2002). Finally, the cognitive tests reported are domain‐specific measures and are not direct measures of academic skills; however, our results for prepregnancy BMI are similar to another report with academic outcomes in childhood (Pugh et al., 2016a). Strengths include our ability to examine two cognitive outcomes, reflecting different cognitive domains, with a relatively large sample size and with adjustment for a range of confounders, including maternal education and, in a subset, maternal IQ.
5. CONCLUSION
In summary, we found that maternal prepregnancy overweight and obesity were associated with a reduction in verbal recognition cognitive test scores in mid‐childhood. Furthermore, we observed that GWG was not associated with child cognitive test scores. These findings suggest that strategies to ensure a healthy BMI before pregnancy may have long‐term effects on child cognitive outcomes. Although the observed associations are small, these findings may be of public health importance for population education, employment, and earning potential, especially because of the present‐day high prevalence of overweight and obesity in women of childbearing age (Flegal et al., 2016; Ogden et al., 2014). Future studies in contemporary populations should consider the role of higher prepregnancy BMI values, such as class II and class III obesity (BMI >35 kg/m2), and the pattern of weight gain across the three trimesters of pregnancy in child neurodevelopment.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
CONTRIBUTIONS
EMW conceptualized and designed the study, conducted data analyses, and drafted the initial manuscript. LGK, PFL and KLK provided input on the data analyses, critically reviewed, and revised the manuscript. BC and PC provided the data, provided input on the data analyses, and critically reviewed the manuscript. All authors were involved in writing the paper and had final approval of the submitted and published versions.
Supporting information
Table S1. Comparison of characteristics for participants included versus excluded from the analytic sample
Table S2. Adjusted Associations between Maternal Prepregnancy BMI, Pregnancy Weight Gain and Child Cognitive Scores at age 9, Child Health and Development Studies Enrolled from 1960 to 1963
Figure S1. Adherence to 2009 Institute of Medicine pregnancy weight gain recommendations for each prepregnancy BMI category (n = 2,084), Child Health and Development Studies (1960 to 1963). 8.7% of women were underweight before pregnancy, 77.2% were normal weight, 11.2% were overweight, and 2.8% were obese.
Widen EM, Kahn LG, Cirillo P, Cohn B, Kezios KL, Factor‐Litvak P. Prepregnancy overweight and obesity are associated with impaired child neurodevelopment. Matern Child Nutr. 2018;14:e12481 10.1111/mcn.12481
REFERENCES
- Armstrong, R. , Scott, J. , Copland, D. , McMahon, K. , Khan, A. , Najman, J. M. , … Arnott, W. (2016). Predicting receptive vocabulary change from childhood to adulthood: A birth cohort study. Journal of Communication Disorders, 64, 78–90. [DOI] [PubMed] [Google Scholar]
- Basatemur, E. , Gardiner, J. , Williams, C. , Melhuish, E. , Barnes, J. , & Sutcliffe, A. (2013). Maternal prepregnancy BMI and child cognition: A longitudinal cohort study. Pediatrics, 131, 56–63. [DOI] [PubMed] [Google Scholar]
- Bitsko, R. H. , Holbrook, J. R. , Robinson, L. R. , Kaminski, J. W. , Ghandour, R. , Smith, C. , & Peacock, G. (2016). Health care, family, and community factors associated with mental, behavioral, and developmental disorders in early childhood—United States, 2011–2012. MMWR. Morbidity and Mortality Weekly Report, 65, 221–226. [DOI] [PubMed] [Google Scholar]
- Bolton, J. L. , & Bilbo, S. D. (2014). Developmental programming of brain and behavior by perinatal diet: Focus on inflammatory mechanisms. Dialogues in Clinical Neuroscience, 16, 307–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret, S. G. (2010). Neurodevelopmental actions of leptin. Brain Research, 1350, 2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brion, M. J. , Zeegers, M. , Jaddoe, V. , Verhulst, F. , Tiemeier, H. , Lawlor, D. A. , & Smith, G. D. (2011). Intrauterine effects of maternal prepregnancy overweight on child cognition and behavior in 2 cohorts. Pediatrics, 127, e202–e211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas, M. , Chatzi, L. , Carsin, A. E. , Amiano, P. , Guxens, M. , Kogevinas, M. , … Vrijheid, M. (2013). Maternal pre‐pregnancy overweight and obesity, and child neuropsychological development: Two Southern European birth cohort studies. International Journal of Epidemiology, 42, 506–517. [DOI] [PubMed] [Google Scholar]
- Daraki V., Roumeliotaki T., Koutra K., Georgiou V., Kampouri M., Kyriklaki A., … Chatzi L. (2017) Effect of parental obesity and gestational diabetes on child neuropsychological and behavioral development at 4 years of age: The Rhea mother–child cohort, Crete, Greece. Eur Child Adolesc Psychiatry. [DOI] [PubMed]
- Edlow, A. G. , Vora, N. L. , Hui, L. , Wick, H. C. , Cowan, J. M. , & Bianchi, D. W. (2014). Maternal obesity affects fetal neurodevelopmental and metabolic gene expression: A pilot study. PloS One, 9, e88661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal, K. M. , Kruszon‐Moran, D. , Carroll, M. D. , Fryar, C. D. , & Ogden, C. L. (2016). Trends in obesity among adults in the United States, 2005 to 2014. JAMA, 315, 2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage, S. H. , Lawlor, D. A. , Tilling, K. , & Fraser, A. (2013). Associations of maternal weight gain in pregnancy with offspring cognition in childhood and adolescence: Findings from the Avon Longitudinal Study of Parents and Children. American Journal of Epidemiology, 177, 402–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore, L. A. , Klempel‐Donchenko, M. , & Redman, L. M. (2015). Pregnancy as a window to future health: Excessive gestational weight gain and obesity. Seminars in Perinatology, 39, 296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkle, S. N. , Albert, P. S. , Sjaarda, L. A. , Grewal, J. , & Grantz, K. L. (2016). Trajectories of maternal gestational weight gain and child cognition assessed at 5 years of age in a prospective cohort study. Journal of Epidemiology and Community Health, 70, 696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkle, S. N. , Schieve, L. A. , Stein, A. D. , Swan, D. W. , Ramakrishnan, U. , & Sharma, A. J. (2012). Associations between maternal prepregnancy body mass index and child neurodevelopment at 2 years of age. International Journal of Obesity, 36, 1312–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkle, S. N. , Sharma, A. J. , Kim, S. Y. , & Schieve, L. A. (2013). Maternal prepregnancy weight status and associations with children's development and disabilities at kindergarten. International Journal of Obesity, 37, 1344–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman, L. , Templin, J. , & Rice, M. L. (2012). Linking outcomes from peabody picture vocabulary test forms using item response models. Journal of Speech, Language, and Hearing Research, 55, 754–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, L. , Yu, X. , Keim, S. , Li, L. , Zhang, L. , & Zhang, J. (2014). Maternal prepregnancy obesity and child neurodevelopment in the Collaborative Perinatal Project. International Journal of Epidemiology, 43, 783–792. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine . (2009). In Rasmussen K. M., & Yaktine A. L. (Eds.), Weight gain during pregnancy: Reexamining the guidelines. Washington (DC): National Academies Press (US) National Academy of Sciences. [PubMed] [Google Scholar]
- Jo, H. , Schieve, L. A. , Sharma, A. J. , Hinkle, S. N. , Li, R. , & Lind, J. N. (2015). Maternal prepregnancy body mass index and child psychosocial development at 6 years of age. Pediatrics, 135, e1198–e1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keim, S. A. , & Pruitt, N. T. (2012). Gestational weight gain and child cognitive development. International Journal of Epidemiology, 41, 414–422. [DOI] [PubMed] [Google Scholar]
- Krakowiak, P. , Walker, C. K. , Bremer, A. A. , Baker, A. S. , Ozonoff, S. , Hansen, R. L. , et al. (2012). Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics, 129, e1121–e1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackintosh, N. J. , & Bennett, E. (2005). What do Raven's matrices measure? An analysis in terms of sex differences. Intelligence, 33, 663–674. [Google Scholar]
- Mann, J. R. , McDermott, S. W. , Hardin, J. , Pan, C. , & Zhang, Z. (2013). Pre‐pregnancy body mass index, weight change during pregnancy, and risk of intellectual disability in children. BJOG, 120, 309–319. [DOI] [PubMed] [Google Scholar]
- Marchi, J. , Berg, M. , Dencker, A. , Olander, E. K. , & Begley, C. (2015). Risks associated with obesity in pregnancy, for the mother and baby: A systematic review of reviews. Obesity Reviews, 16, 621–638. [DOI] [PubMed] [Google Scholar]
- Mehta, S. H. , Kerver, J. M. , Sokol, R. J. , Keating, D. P. , & Paneth, N. (2014). The association between maternal obesity and neurodevelopmental outcomes of offspring. The Journal of Pediatrics, 165, 891–896. [DOI] [PubMed] [Google Scholar]
- Neggers, Y. H. , Goldenberg, R. L. , Ramey, S. L. , & Cliver, S. P. (2003). Maternal prepregnancy body mass index and psychomotor development in children. Acta Obstetricia et Gynecologica Scandinavica, 82, 235–240. [DOI] [PubMed] [Google Scholar]
- Neri, C. , & Edlow, A. G. (2016). Effects of maternal obesity on fetal programming: Molecular approaches. Cold Spring Harbor Perspectives in Medicine, 6, a026591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niculescu, M. D. , & Lupu, D. S. (2009). High fat diet‐induced maternal obesity alters fetal hippocampal development. International Journal of Developmental Neuroscience, 27, 627–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden, C. L. , Carroll, M. D. , Kit, B. K. , & Flegal, K. M. (2014). Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA, 311, 806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osicka, C. J. (1976). Peabody Picture Vocabulary Test: Comments on administration and two methods of scoring. Psychological Reports, 38, 1135–1144. [Google Scholar]
- Pugh, S. J. , Hutcheon, J. A. , Richardson, G. A. , Brooks, M. M. , Himes, K. P. , Day, N. L. , & Bodnar, L. M. (2016a). Child academic achievement in association with pre‐pregnancy obesity and gestational weight gain. Journal of Epidemiology and Community Health, 70, 534–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh S. J., Hutcheon J. A., Richardson G. A., Brooks M. M., Himes K. P., Day N. L., & Bodnar L. M. (2016b). Gestational weight gain, prepregnancy body mass index and offspring attention‐deficit hyperactivity disorder symptoms and behaviour at age 10. Bjog, 123, 2094–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh, S. J. , Richardson, G. A. , Hutcheon, J. A. , Himes, K. P. , Brooks, M. M. , Day, N. L. , & Bodnar, L. M. (2015). Maternal obesity and excessive gestational weight gain are associated with components of child cognition. The Journal of Nutrition, 145, 2562–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven, J. (1965, 1962). Coloured progressive matricies, sets A, Ab, B. London: Lewis. [Google Scholar]
- Raven, J. (2000). The Raven's progressive matrices: Change and stability over culture and time. Cognitive Psychology, 41, 1–48. [DOI] [PubMed] [Google Scholar]
- Rodriguez, A. (2010). Maternal pre‐pregnancy obesity and risk for inattention and negative emotionality in children. Journal of Child Psychology and Psychiatry, 51, 134–143. [DOI] [PubMed] [Google Scholar]
- Rodriguez, A. , Miettunen, J. , Henriksen, T. B. , Olsen, J. , Obel, C. , Taanila, A. , … Jarvelin, M. R. (2008). Maternal adiposity prior to pregnancy is associated with ADHD symptoms in offspring: Evidence from three prospective pregnancy cohorts. International Journal of Obesity, 32, 550–557. [DOI] [PubMed] [Google Scholar]
- Sasaki, A. , de Vega, W. , Sivanathan, S. , St‐Cyr, S. , & McGowan, P. O. (2014). Maternal high‐fat diet alters anxiety behavior and glucocorticoid signaling in adolescent offspring. Neuroscience, 272, 92–101. [DOI] [PubMed] [Google Scholar]
- Savitz, D. A. , Terry, J. W. Jr. , Dole, N. , Thorp, J. M. Jr. , Siega‐Riz, A. M. , & Herring, A. H. (2002). Comparison of pregnancy dating by last menstrual period, ultrasound scanning, and their combination. American Journal of Obstetrics and Gynecology, 187, 1660–1666. [DOI] [PubMed] [Google Scholar]
- Sullivan, E. L. , Nousen, E. K. , & Chamlou, K. A. (2014). Maternal high fat diet consumption during the perinatal period programs offspring behavior. Physiology & Behavior, 123, 236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda, R. , Salsberry, P. J. , Reagan, P. B. , & Fang, M. Z. (2013). The impact of prepregnancy obesity on children's cognitive test scores. Maternal and Child Health Journal, 17, 222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavris, D. R. , & Read, J. A. (1982). Effect of maternal weight gain on fetal, infant, and childhood death and on cognitive development. Obstetrics and Gynecology, 60, 689–694. [PubMed] [Google Scholar]
- Torres‐Espinola, F. J. , Berglund, S. K. , Garcia‐Valdes, L. M. , Segura, M. T. , Jerez, A. , Campos, D. , … Campoy, C. (2015). Maternal obesity, overweight and gestational diabetes affect the offspring neurodevelopment at 6 and 18 months of age—A follow up from the PREOBE cohort. PloS One, 10, e0133010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozuka, Y. , Wada, E. , & Wada, K. (2009). Diet‐induced obesity in female mice leads to peroxidized lipid accumulations and impairment of hippocampal neurogenesis during the early life of their offspring. The FASEB Journal, 23, 1920–1934. [DOI] [PubMed] [Google Scholar]
- van den Berg, B. J. , Christianson, R. E. , & Oechsli, F. W. (1988). The California Child Health and Development Studies of the School of Public Health, University of California at Berkeley. Paediatric and Perinatal Epidemiology, 2, 265–282. [DOI] [PubMed] [Google Scholar]
- van der Burg, J. W. , Sen, S. , Chomitz, V. R. , Seidell, J. C. , Leviton, A. , & Dammann, O. (2016). The role of systemic inflammation linking maternal BMI to neurodevelopment in children. Pediatric Research, 79, 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widen E. M., Whyatt R. M., Hoepner L. A., Mueller N. T., Ramirez‐Carvey J., Oberfield S. E., … Rundle A. G. (2016). Gestational weight gain and obesity, adiposity and body size in African‐American and Dominican children in the Bronx and Northern Manhattan. Matern Child Nutr, 12, 918–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung, E. H. , Sundaram, R. , Ghassabian, A. , Xie, Y. , & Buck, L. G. (2017). Parental obesity and early childhood development. Pediatrics, 139, e20161459. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Comparison of characteristics for participants included versus excluded from the analytic sample
Table S2. Adjusted Associations between Maternal Prepregnancy BMI, Pregnancy Weight Gain and Child Cognitive Scores at age 9, Child Health and Development Studies Enrolled from 1960 to 1963
Figure S1. Adherence to 2009 Institute of Medicine pregnancy weight gain recommendations for each prepregnancy BMI category (n = 2,084), Child Health and Development Studies (1960 to 1963). 8.7% of women were underweight before pregnancy, 77.2% were normal weight, 11.2% were overweight, and 2.8% were obese.


