Abstract
The most prevalent pathological features of many neurodegenerative diseases are the aggregation of misfolded proteins and the loss of certain neuronal populations. Autophagy, as major intracellular machinery for degrading aggregated proteins and damaged organelles, has been reported to be involved in the occurrence of pathological changes in many neurodegenerative disorders, including Alzheimer's disease, Parkinson's disease, Huntington's disease and amyotrophic lateral sclerosis. In this review, we summarize most recent research progress in this topic and provide a new perspective regarding autophagy regulation on the pathogenesis of neurodegenerative diseases. Finally, we discuss the signaling molecules in autophagy‐related pathways as therapeutic targets for the treatment of these diseases.
Keywords: autophagy, neurodegenerative diseases, protein aggregation
Introduction
Cellular aggregations of misfolded proteins are the most common pathological hallmark of many neurodegenerative diseases, including Alzheimer's disease (AD), Parkinson's disease (PD), Huntington's disease (HD) and amyotrophic lateral sclerosis (ALS) 3. The pathological abnormalities of various neurodegenerative diseases are often associated with corresponding protein aggregations that reside in different cellular environment and subcellular compartments. Some of them are resulted from specific genetic mutations that cause autosomal recessive or dominant familial type of neurodegenerative diseases, while diverse mechanisms leading to impaired proteostasis contribute to the protein aggregations in neurodegenerative diseases.
Autophagy is one of the major intracellular machinery to eliminate misfolded proteins and maintain proteostasis. Dysregulated autophagy is increasingly considered to play key roles in most neurodegenerative diseases, and the regulation of autophagy is therefore proposed as a potential therapeutic avenue for these diseases 73, 74, 108. Macroautophagy (referred as autophagy) literally means “self‐eating” in Greek that is responsible for removal of long‐lived proteins and damaged organelles which are too large for proteasome to degrade. Autophagy not only plays a vital role in development, cell differentiation, apoptosis, pathogen infection and starvation, but also contributes to cancer, immune diseases and neurodegenerative diseases 17, 47, 78. Many studies have shown that autophagy is closely linked with neurodegenerative diseases. For example, the amount of autophagic vacuole, an intermediate vesicular compartment in the process of autophagy, is much more in the brains of neurodegenerative diseases than in health controls, suggesting impaired maturation of autophagosome to autolysosome 35. Without any other causing factors, the depletion of key autophagy‐related genes (such as Atg5, Atg7) can lead to neurodegeneration in mouse central nervous system 54, 76.
Autophagy exerts a key role in degrading aggregate‐prone proteins, which have been implicated in the pathogenesis of various neurodegenerative diseases, such as mutant α‐synuclein in PD, mutant huntingtin in HD and mutant TAR DNA‐binding protein 43 (TDP‐43) in ALS. Once autophagy is inhibited, the clearance of these substrates is impeded. On the contrary, activation of autophagy may lead to enhanced clearance of those toxic proteins.
Lysosomal dysfunction in neurons is closely tied to neurodegeneration and cell death mechanisms 88. Growing genetic and biochemical evidence implicates the dysfunction of endosomal–lysosomal and autophagic lysosomal pathways during the pathogenesis of many neurodegenerative diseases, including AD, PD and ALS 31, 128. The therapeutic efficacy of autophagy/lysosome modulators in animal models of these diseases 88, 128 further underscores the significance of lysosomal impairments to the pathogenesis of neurodegenerative diseases.
In this review, we summarize recent research findings showing that the dysregulated autophagy contributes to protein aggregation, organelle impairment and neuronal loss, eventually leads to neurodegenerative diseases. Autophagy modulation can prevent the occurrence and progression of these diseases 132. Even though various factors underlie the pathology of these diseases, we aim at providing an interaction between autophagy and the cause/progression of neurodegenerative diseases. We also review autophagy‐inducing agents, both mTOR‐dependent and ‐independent, and evaluate their effectiveness in disease models in vitro and in vivo.
Autophagy Mechanism
Autophagy includes three subtypes: macroautophagy, microautophagy and chaperon mediated autophagy. Although subtypes of autophagy differ from cargo recognition, mechanism of molecular chaperon, they share lysosome as the unique place for cargo digestion and products recycling. An intact autophagy process is depicted as autophagic flux including autophagosome formation, fusion of autophagosome and lysosome, and cargo degradation in lysosome 15, 77. Firstly, misfolded proteins and damaged organelles are enwrapped by newly formed membrane termed as phagophore that is potentially derived from plasma membrane, Golgi, mitochondria or endoplasmic reticulum (ER) 99, 106, 134. Phagophore gradually sequesters cargoes through elongation till forming a closed autophagosome. By means of cytoskeletal microtubule systems, autophagosome traffics to lysosome and fuses with lysosome to form autolysosome. In autolysosome, cargoes are digested by lysosomal enzymes and recycled for reuse 2, 53, 55, 64.
Autophagy is a multi‐stage process containing numerous proteins, including several autophagy‐related proteins identified in mammals 79, 118. Autophagy is initiated by two major complexes UN51‐like Ser/Thr kinases (ULK) complex and the class III phosphatidylinositol‐3‐kinase (PI3K), which are recruited to the phagophore assembly site (PAS) 2, 91. The ULK complex contains ULK1/2 family, FAK family kinase interacting protein of 200 kDa (FIP200) and ATG13 121. The other complex PI3K, also named Beclin1 complex, consists of vacuolar protein sorting 34 (Vps34), p15 (VPS15), Beclin1 (ATG6) and Barkor (ATG14) 27. Notably, Beclin1 which localizes on ER membrane is regulated by anti‐apoptotic dimer BCL‐2 and BCL‐XL. When autophagy is activated, Beclin1 will be dissociated from BCL‐2 complex to coordinate with Vps34 36, 69, 95. Subsequently, bulk phosphatidylinositol 3‐phosphate [PI (3) P] will concentrate on the surface of phagophore 89, 99.
The extension and closure of autophagosome are exerted by two ubiquitin‐like complexes. At the first, with the interaction of Atg7, Atg5 links with Atg12 covalently 114. Then the covalent complex links with Atg16 to form Atg5–Atg12–Atg16 complex, responsible for elongating phagophore. Atg9 which binds Atg2 and Atg18 is essential for trafficking between the Trans‐Golgi‐network, endosomes and newly formed autophagosomes. In another ubiquitin‐like complex, microtubule‐associated protein 1 light chain 3 (LC3) is cleaved by Atg4B to generate LC3‐I 122. The Atg5–Atg12–Atg16 complex assists the transformation of LC3‐I to phosphatidylethanolamine (PE)‐conjugated LC3‐II. Since LC3‐II mainly resides on autophagosome, it is viewed as the significant marker for autophagosome 44.
Subsequently, maturated autophagosome needs kinesin and motor proteins to move along microtubules 101. Meanwhile, autophagosome fuses with lysosome in which multiple membrane proteins complexes such as the soluble NSF attachment protein receptor (SNAREs) are recruited 40. After the formation of autolysosome is completed, cargoes carried by autophagosomes are degraded by proteolysis. A detailed illustration of autophagy process is shown in Figure 1.
Figure 1.
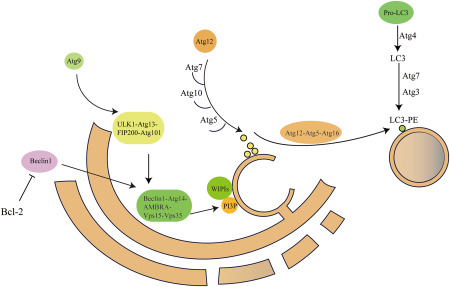
Autophagy induction and autophagosome formation. This diagram shows the process of autophagy induction and roles of Atg related proteins in autophagosome formation.
Autophagy plays an essential role in protein degradation and recycling. Though both of ubiquitin proteasome system and autophagy could clear ubiquitinated substrates, compared with proteasome, autophagy is the only one way to degrade large protein aggregates or impaired organelles which are too large to go into the narrow entrance of proteasome chamber.
Brains are the most vulnerable organ in most lysosome disorders, indicating that neurons might rely on autophagy even more heavily than other cells to maintain protein homeostasis. Unusual structures such as large dendritic and axonal cytoplasm in neurons cause difficulty for them to remove impaired organelles and other waste in time 5. Two key components of autophagy locate on distinct places that autophagic vacuoles generated in axons should travel long distances to lysosomes mainly locating near the cell body. In addition, unlike other mitotic cells, neurons are not able to divide to disperse harmful substances 62. Aging will worsen the situation that neurons are gradually losing the ability to efficiently clear wastes, eventually resulting in abnormal accumulated autophagic substrates. Altogether, neurons are prone to suffer from autophagic proteolytic damage.
Notably, numerous stress responses promote misfolded protein aggregation at ER. ER stress is associated with unfolded protein response (UPR) 38, which is initiated by Inositol‐requiring enzyme 1 α (IRE1α) 19. IRE1 is an ER transmembrane sensor that activates the UPR to maintain the ER and cellular function and downstream target c‐Jun N‐terminal kinase (JNK) 50, 90, 93, 130. ER stress can regulate Beclin1 in autophagosome formation 14. Protein misfolded diseases are usually accompanied with ER stress. UPR stands for a cell survival pathway to modulate autophagy to reduce protein aggregation and remain proteostasis 38, 93. In brief, various regulation mechanisms emerge to maintain proteostasis within cells making the modulation of autophagy as a promising strategy for therapeutic purposes.
Autophagy and Alzheimer's Disease
AD is the most common neurodegenerative disease that is characterized by extracellular amyloid‐β (Aβ) plaques which are cleaved products of amyloid precursor proteins (APPs) and intracellular neurofibrillary tangles which are composed of aggregated hyperphosphorylated tau protein 135.
Under normal circumstance, autophagosome vesicles are rare in brains. Strikingly, detailed ultrastructural analyses have shown that dystrophic neurites in AD brains contain autophagosome vesicles 35, 87. Further study demonstrates that increased autophagy vacuoles are found in Presenilin 1 (PS1)‐rich locations 35. Accumulation of autophagy vacuoles is likely arising from impaired clearance rather than the induction of autophagy itself, suggesting the modulation of late steps of autophagy as a possible therapeutic strategy for AD. Accordingly, treatment with autophagy enhancer rapamycin significantly increases autophagosome fusion with lysosome in vitro 35.
PS1 is a ubiquitous transmembrane protein, whose cleaved form is the catalytic subunit of γ‐secretase complex, which induces the intra‐membranous cleavage of APP 16. Generally, APP is firstly cleaved by β‐secretase to produce β‐C‐terminal fragment (βCTF), which is cleaved by Presenilin 1 (PS1) to produce Aβ. Mutant PS1 is considered to contribute to AD pathogenesis by interfering cleavage of APP. Recent investigations have shown that PS1 can also decrease Aβ levels by directing βCTF degradation through autophagy 8. Moreover, PS1 is involved in the fusion of autophagsome and lysosome. Lack of phosphorylation on PS1 1 Ser367 impedes the fusion of autophagosome and lysosome in mouse brain. And then, this inhibition of autophagy reduced βCTF degradation leading to the accumulation of Aβ in the brain 7. These observations imply that PS1 could be a promising target for the treatment of AD through autophagy.
However, PS1 is a vital mediator in lysosomal turnover of autophagic substrates. PS1 is an ER chaperone to facilitate maturation and targeting of the v‐ATPase V0a1 subunit to lysosomes, which is a key component in acidification and substrate degradation 60. Further investigation demonstrates that PS1 also maintains Ca2+ homeostasis by regulating acidification of lysosome 59. Loss of acidification leads to dysfunction of lysosome that impedes fusion of autophagosome to lysosome, thereby accumulation of autophagosomes. In addition, lysosome dysfunction causes cargo‐specific deficits of axonal transport leading to AD‐like neuritic dystrophy 61. Based on these observations, it is reasonable to suspect that restoring the proteolytic function of lysosome may enhance the removal of protein aggregations. In line with this notion, deletion of cystatin B, an inhibitor of lysosome cysteine protease in AD mouse models promotes the clearance of abnormal protein aggregations in lysosomal compartments 133.
Genome‐wide association studies (GWAS) have identified additional proteins involved in autophagy that are also closely linked with AD, such as the phosphatidylinositol binding clathrin assembly protein (PICALM/CALM). CALM is involved in endocytic trafficking to regulate endocytosis of SNAREs that enhance autophagy to clear tau aggregations 81.
Beclin1, a key factor in autophagosome formation has been shown to be transcriptionally suppressed in AD brains 96. Under pathogenic conditions, Caspase 3, a key component in apoptosis pathway, may cleave Beclin1 protein and lead to autophagy disruption. The cleaved form of Beclin1 is therefore regarded as a common in vitro marker for apoptosis in AD pathogenesis 104. Another potential marker for the pathology of AD is nuclear factor erythroid derived 2 like 2 (Nrf2). In response to oxidative stress, Nrf2 could induce autophagy receptor NDP52 51 to stimulate autophagy and remove aggregated tau proteins 43. Meanwhile, Nrf2 as a vital transcription factor can also regulate the transcription of autophagy related proteins 92.
Autophagy and Parkinson's Disease
PD is the second most common neurodegenerative disease that is characterized by selective loss of dopamine neurons in substantia nigra pars compacta, and intracellular inclusions of Lewy body and Lewy neurites composed of α‐synuclein and polyubiquitinated proteins 20. In the post‐mortem brain samples of PD patients, dysfunctional lysosomes and accumulation of autophagosomes were observed in neurons 22, indicating a pathogenic role of autophagy in PD. The main component of Lewy bodies is misfolded and aggregated α‐synuclein 20, 45, 123. When lysosome is inhibited, the level of α‐synuclein is increased, suggesting a close link between α‐synuclein degradation and autophagy. Previous studies have shown that basically all forms of α‐synuclein can be degraded by autophagy 22, 37, 58, while monomeric α‐synuclein is also degraded by the proteasome 126. Transcription factor EB (TFEB), a key modulator for autophagy 113, has been widely demonstrated to relieve pathology of neurodegenerative diseases. Over‐expression of TFEB could decrease the damage of lysosome by inducing its biogenesis, thus ameliorating the α‐synuclein pathology 21, 49. Taken together, these results suggest an essential role of autophagy in the prevention and treatment of synucleinopathy in PD.
Mutations in leucine rich repeat kinase 2 (LRRK2) represent the most common cause of autosomal dominant form of PD 123. Over‐expression of LRRK2 G2019S mutation in differentiated SH‐SY5Y cells results in shortening dentric and autophagosomes aggregation 98. In vivo experiments have demonstrated that the up‐regulation of LRRK2 G2019S impairs autophagic flux with aging 107. The VPS35 D620N mutation that causes autosomal‐dominant PD destabilizes WASH complex leading to defect of autophagosome formation and compromises trafficking of autophagy protein ATG9 137.
Besides, mutations in parkin RBR E3 ubiquitin protein ligase (PARKIN) and PTEN induced putative kinase 1 (PINK1) are the main causing factors for autosomal recessive forms of PD, accounting for 50% of familial cases in Europe 48. These two proteins coordinate mitophagy to selectively degrade mitochondria by autophagy. Damaged mitochondria are delivered and sequestered within double membrane autophagosome, ultimately cleared by autolysosome. In this process, the proteasome‐mediated degradation of PINK1 is stalled in depolarized mitochondria leading to accumulated PINK1 on the mitochondrial outer membrane where it phosphorylates ubiquitin and recruits parkin. In turn, the activated parkin can ubiquitinate outer membrane proteins, which are subsequently phosphorylated by PINK1. The outcome of these linkages greatly actives parkin and elicits a positive feedback involving more ubiquitinated proteins of mitochondria 46, 56, 70, 85, 86.
GWAS has identified a few lysosome related genes associated with PD. The protein ATP13A2 involved with lysosomal ATPase, is found mutated in autosomal recessive forms of early‐onset Parkinsonism 24, 100. Down‐regulation of ATP13A2 results in decreased lysosomal degradation in dopaminergic neurons and accumulation of α‐synuclein protein 120. Subsequently, depletion of ATP13A2 leads to ubiquitination and degradation of SYT11 that induces lysosome dysfunction and increases accumulation of mutant α‐synuclein 4.
Autosomal recessive mutations in the gene GBA which encodes lysosomal hydrolase cause defects in autophagosome‐lysosome pathway and aggregation of α‐synuclein 1. Depletion of ATP6AP2 which is essential for lysosomal acidification and function, has been associated with Parkinsonism 1. Moreover, loss of VPS13C function causes mitochondrial dysfunction and lysosome dysfunction and is associated with autosomal recessive Parkinsonism 1, 63.
Autophagy and Huntington's Disease
HD, the most common polyglutamine disease, is a devastating autosomal dominant neurodegenerative disease. HD is characterized by CAG repeat tri nucleotide in the first exon of the huntingtin (HTT) gene which leads to polyglutamine (polyQ) expansions and pathogenic aggregation 39, 42.
Aggregated autophagosomes could be observed in HD models 68, although autophagosome formation is not affected by HD pathology. Huntingtin plays a key role in autophagosome transport. In HD models, depletion of huntingtin results in abnormal accumulation of autophagosomes with engulfed mitochondria which is indicative of impaired cargo degradation 140.
In addition, there are aberrant interactions between autophagy and onset of HD. One polymorphism in the Atg7 is associated with an earlier onset form of HD 75. Beclin1 could reduce HTT mRNA level with aging 115. Dysfunction of loading into autophagosomes has been observed in cellular and animal models of HD, causing an impaired autophagic protein degradation despite of increased autophagic vesicles contents. The autophagy selective substrate p62/SQSTM1 is commonly treated as a crucial marker for autophagic flux especially in the cargo‐recognition machinery which transports substrates to autophagosomes. Deficiency in such machinery is prevalent in HD models 68. Moreover, up‐regulation of casein kinase 2 (CK2) which phosphorylates p62/SQSRM1 reduces large inclusion formation of mutant huntingtin 71.
Compared with mutant HTT, non‐mutant HTT seems to coordinate with autophagy in a different manner. For example, wild‐type HTT can bind p62 to enhance its role in autophagy and interact with ULK1 to evoke autophagy. In addition, Atg11 shares resemble structure with HTT to play a role in autophagosome formation. On the contrary, knock‐out of dynein reveals increased levels of autophagosomes and impaired autolysosomes, accompanied with increased aggregation of mutant huntingtin 101.
Autophagy and ALS
Amyotrophic lateral sclerosis (ALS) is a paralytic and fatal disease characterized by selective loss of motor neurons in brain and spinal cord giving rise to muscle weakness and atrophy. Most cases in ALS are sporadic, while the familial form accounts for approximately 10%. Mutations in chromosome 9 open reading frame 72 (C9ORF72), superoxide dismutase 1 (SOD1), TDP‐43 and fused in sarcoma/translated in lip sarcoma (FUS/TLS) are common causes for familial type of ALS 94.
Several reports have demonstrated that autophagy is associated with ALS. Immunostaining experiments in transgenic mice with mutant SOD1 G93A have shown that autophagy is activated 82. The aggregated autophagosomes in cytoplasm indicate that autophagy is activated in degenerated motor neurons in ALS cases 82. Notably, excess autophagosomes and autolysosomes are closely associated with p62/SQSTM1 positive inclusions, suggesting an impaired cargo digestion in lysosome 112. Other studies have shown that increased autophagosomes are tightly related with the decreased phosphorylation of mTOR in numerous genetic ALS models 82.
Growing evidence has shown that mutations in autophagy‐related proteins are closely associated with the onset of ALS. Earlier studies have indicated that depletion in subunits of endosomal sorting complexes required for transport (ESCRT) causes abnormal multivesicular bodies (MVBs) with autophagosomes and is considered to be associated with ALS 29. In addition, mutations in ESCRT subunit charged multi vesicular body protein‐2B (CHMP2B) are found in patients with ALS which impair ESCRT function leading to accumulation of ubiquitinated proteins and p62 29. Autophagy receptor p62/SQSTM1 which binds both LC3 and ubiquitin to target ubiquitinated substrates to autophagosomes has been involved in ALS cases. Clearance of mutant SOD1 via ubiquitin proteasome system or autophagy is coordinated by p62/SQSTM1. Similarly, over‐expression of p62/SQSTM1 could reduce TDP‐43 aggregation via autophagy or proteasome in vitro 6.
Moreover, multi groups have identified a link between serine/threonine kinase TANK‐binding kinase 1(TBK1) and ALS 52. One recent study has shown that TBK1 is the upstream regulator of autophagy receptor optineurin (OPTN) 80. Both TBK1 and OPTN play key roles in mitophagy 129. Since mitochondria are the place for not only generating energy but also executing cellular apoptosis, clearance of damaged mitochondria is essential for cellular homeostasis. These investigations suggest mitophagy as a new etiology of ALS. Ubiquilin2 (UBQLN2), a proteasome shuttle factor, plays a key role in formation of autophagosome. Mutations in UBQLN2 lead to cognitive deficits, shortened lifespan and neuron loss in mouse models 23, 57. A detailed illustration of alternations in neurodegenerative diseases in autophagic flux is shown in Figure 2.
Figure 2.
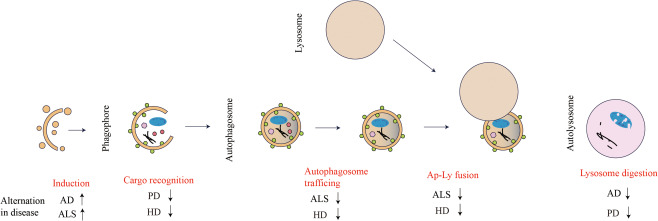
Overview of autophagic flux and impaired states in neurodegenerative diseases. This schematic diagram shows the procedures through the autophagic flux from formation of the autophagosome to fusion with the lysosome. Red text highlights refer to the dysfunctional steps in autophagy, along with related neurodegenerative diseases. Arrows' directions stand for activation or inhibition.
Autophagy as a Therapeutic Target for Neurodegenerative Diseases
Links between autophagy and neurodegenerative diseases promote an intriguing question: whether the modulation of autophagy could slow down disease progression. Emerging evidence has shown that autophagy enhancement could efficiently ameliorate neuropathology and neurodegeneration via either an mTOR‐dependent or ‐independent pathway. Thus various reagents targeting for autophagy have been investigated 102, 116, 127.
Autophagy is activated by diverse signaling classified as mammalian target of rapamycin (mTOR) dependent and mTOR independent pathway (Figure 3).
Figure 3.
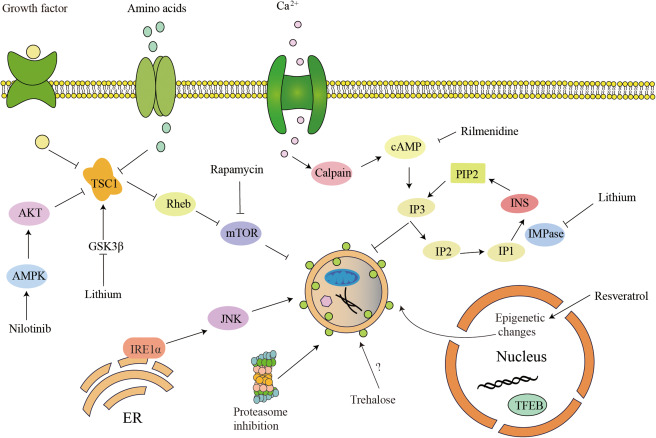
Molecular targets of autophagy up‐regulating agents. This schematic diagram shows representative molecular agents involved in autophagy activation through regulating autophagy‐related pathways. Either the mTOR‐dependent or mTOR‐independent pathway could play a negative role in autophagy. In addition, suppression of these pathways will result in activation of autophagy.
mTOR dependent pathway
The serine/threonine protein kinase mTOR is a core component of two distinct complexes, mTOR complex 1 (mTORC1) and mTORC2. mTORC1 negatively regulates autophagy, while mTORC2 does it in an opposite way 32. Under normal conditions, autophagy is suppressed by mTOR. Since mTORC1 phosphorylates and inhibits core autophagy complex composed of ULK1, Atg13 and FIP200. Rapamycin interacts with immunophilin FK506‐binding protein (FKBP12) to form a complex which inhibits the kinase activity of mTORC1, thus inducing autophagy 11, 66. Additionally, some drugs indirectly target mTOR such as Nilotinib, which could stimulate AMPK pathway in mTOR dependent manner to induce autophagy 136.
mTOR independent pathway
Besides autophagy, mTOR‐dependent pathway plays a wide role among several other biology reactions. To avoid potential adverse effects induced by mTOR multiple function, studies on mTOR‐independent pathway are increasingly emphasized. For example, more and more pharmacological drugs have been screened for regulating autophagy and have been shown to influence diverse signaling pathways including calcium flux, inositol phosphates and epigenetics.
Lithium decreases the level of Inositol 3‐phosphates (IP3), which is a second messenger binding to its receptor on the ER leading to Ca2+ release into cytoplasm. Rilmenidine reduces level of cAMP 110 which could bind IP3 to regulate intracytosolic Ca2+ levels 10. Resveratrol could active autophagy via epigenetic mechanisms 124. Nilotinib can upregulate AMPK pathway to active autophagy 37, 65. Although great achievements have been made in the discovering of novel mTOR‐independent autophagy modulators, till now, the detailed mechanisms underlying autophagy regulating effects of these molecules remains exclusive and efforts are still needed for their clinical application.
Alzheimer's disease
Previous studies have reported that the application of rapamycin can reduce fibrillary tangles and amyloid plaques in brains and rescue cognitive deficits 5, 9, 67. Rapamycin analogue temsirolimus also shows similar effects in AD mouse models 41. Arctigenin, a natural product from Arctium lappa, can inhibit Aβ production and promote Aβ clearance by activating autophagy through inhibiting AKT/mTOR signaling 141. Latrepirdine is a pro‐neurogenic compound that reduces accumulation of Aβ42 by stimulating autophagy 117. GTM‐1, a novel small molecule, can attenuate Aβ oligomer‐induced neurotoxicity via inducing autophagy in an mTOR‐independent manner 18. Nilotinib, a tyrosine kinase inhibitor, can enhance interaction of parkin and Beclin1 that lead to clearance of Aβ 65. Notably, it also plays a clearance role in PD‐related parkin mutant models 37. Trehalose, a natural disaccharide, is beneficial for removing abnormal proteins. It has been demonstrated to reduce accumulation of Aβ 103. Trehalose rescues the learning impairment by reducing Aβ deposits in APP/PS1 mice 25. Since trehalose is free of toxic effects at high concentrations suggesting a promising prospect for clinical applications in human tauopathies.
Parkinson's disease
Resveratrol induces autophagy via AMPK/SIRT1 pathway to protect neurons form rotenone induced toxicity in vitro 83. Administration of Nilotinib contributes to clearing α‐synuclein aggregation via autophagy, and rescues dopaminergic neuron loss 37. Notably, in proteasome inhibition‐induced mouse models, proteasome dysfunction leads to activation of autophagy that serves a compensatory mechanism to clear protein aggregation and decrease cell death 34. Further enhancement of autophagy by pharmacological drugs or molecular inhibitors can attain similar effects. Trehalose contributes to reducing α‐synuclein mutants in vitro 109. In addition, trehalose increases the number of dopamine neurons and the dopaminergic activity in the midbrain in PD mouse models 103. Lithium facilitates clearance of mutant α‐synuclein in vitro 28.
Huntington's disease
Rapamycin reduces huntingtin accumulation and cell death in cell models of HD 102, 111. Lithium could partially rescue cell death 12, 110. Trehalose could bind expanded polyglutamine to delay pathology in HD mouse models 109, 119. Rilmenidine could enhance autophagy to remove mutant huntingtin fragments in cell models via mTOR independent pathway 105. Lithium could reduce mutant huntingtin protein aggregates and cell death 30.
Amyotrophic lateral sclerosis
Interestingly, rapamycin plays two opposite roles in ALS animal models. For example, rapamycin treatment in SOD1G93A mouse models further augments motor neuronal degeneration and lead to more death of ALS mice 139. However, in mutant TDP‐43 models, rapamycin treatment decreases pathology of ALS 125. These contradictory findings may be because of different pathogenic proteins overexpressed and their different impact on autophagy in the two animal models of ALS. Further studies have demonstrated that rapamycin administration impairs autophagic flux, although it significantly increases the number of autophagosomes in mutant SOD1 models. Trehalose could induce autophagy via mTOR independent pathway and significantly decrease SOD1 aggregation, reduce ubiquitinated protein accumulation in the motor neurons of SOD1 mice 13, 138.
In addition, developing novel chemicals to modulate autophagy reveals a promising prospect. For example, single‐walled carbon nanotubes (SWNT) restore normal autophagy by reversing abnormal activation of mTOR signaling and deficits in lysosomal proteolysis, thereby facilitates elimination of autophagic substrates. These findings suggest that SWNT could serve as a novel neuroprotective approach to AD therapy 131.
Autophagy in Clinical Diagnosis of Neurodegenerative Diseases
Recently, emerging evidence in clinics implies that autophagy is in close association with neurodegenerative diseases. Biochemical analyses show the dramatic increase of the autophagosome marker LC3 in postmortem brains of AD patients and confirm its co‐localization with hyperphosphorylated tau 97. Besides, some proteins known to regulate autophagy have been newly implicated with the pathogenesis of AD. In postmortem brains of AD patients, increase of tetraspanin impedes the fusion of autophagosome with lysosome, which leads to the abnormal accumulation of APP 33. Another study shows that decrease of immunophilin FKBP52 (FK506‐Binding Protein of MW ∼ 52 kDa), a protein co‐localized with lysosome, is accompanied with accumulation of neurofibrillary tangles 72. In postmortem brains of PD patients, expression of toll‐like receptor 2 (TLR2) is elevated in neurons and spatially correlated with the pathological α‐synuclein aggregation and increase of autophagy receptor SQSTM1 26. Aberrant alternation of LAMP2, which is a significant marker in lysosome, is closely related with the early pathology of PD 84. These findings suggest that autophagy is commonly aberrant in neurodegenerative diseases. However, further studies are still required to explore specific autophagic pathways or signaling involved in different types or sub‐types of neurodegenerative diseases.
Concluding Remarks
Overall, increasing evidence indicates dysregulated autophagy plays a key role in the pathogenesis of neurodegenerative diseases, and implies potential therapeutic strategies to ameliorate neurodegenerative diseases through regulating autophagy. However, mechanisms of autophagy regulation on proteostasis and general metabolism remain to be further investigated, especially through linking the interplay between specific proteins involved in autophagy and progression of diseases. Furthermore, other resident cells in the brain such as microglia might also be involved in the process of autophagy. It remains largely unknown whether and how microglia cooperates with neurons and non‐neuronal cells to regulate autophagy. As to autophagy‐inducing agents, treatment dose and duration should be carefully chosen and examined, as over‐activation of autophagy could result in detrimental effects in accelerating the progression of neurodegenerative diseases.
Acknowledgment
This review is supported by the National Natural Science Foundation of China (81430021, 81370470), and is also support in part by the intramural program of National Institute on Aging, National Institutes of Health (HC: AG000928, 000959).
References
- 1. Abeliovich A, Gitler AD (2016) Defects in trafficking bridge Parkinson's disease pathology and genetics. Nature 539:207–216. [DOI] [PubMed] [Google Scholar]
- 2. Agarwal S, Tiwari SK, Seth B et al (2015) Activation of autophagic flux against xenoestrogen bisphenol‐A‐induced hippocampal neurodegeneration via AMP kinase (AMPK)/mammalian target of rapamycin (mTOR) pathways. J Biol Chem 290:21163–21184. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3. Aguzzi A, O'Connor T (2010) Protein aggregation diseases: pathogenicity and therapeutic perspectives. Nat Rev Drug Discov 9:237–248. [DOI] [PubMed] [Google Scholar]
- 4. Bento CF, Ashkenazi A, Jimenez‐Sanchez M, Rubinsztein DC (2016) The Parkinson/'s disease‐associated genes ATP13A2 and SYT11 regulate autophagy via a common pathway. Nat Commun 7:11803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boland B, Kumar A, Lee S, Platt FM, Wegiel J, Yu WH et al (2008) Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer's disease. J Neurosci 28:6926–6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brady OA, Meng P, Zheng Y, Mao Y, Hu F (2011) Regulation of TDP‐43 aggregation by phosphorylation andp62/SQSTM1. J Neurochem 116:248–259. [DOI] [PubMed] [Google Scholar]
- 7. Bustos V, Pulina MV, Bispo A, Lam A, Flajolet M, Gorelick FS et al (2017) Phosphorylated Presenilin 1 decreases β‐amyloid by facilitating autophagosome–lysosome fusion. Proc Natl Acad Sci USA 114:7142–7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bustos V, Pulina MV, Kelahmetoglu Y, Sinha SC, Gorelick FS, Flajolet M et al (2017) Bidirectional regulation of Aβ levels by Presenilin 1. Proc Natl Acad Sci USA pii:201705235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Caccamo A, Majumder S, Richardson A, Strong R, Oddo S (2010) Molecular interplay between mammalian target of rapamycin (mTOR), amyloid‐β, and Tau: effects on cognitive impairments. J Biol Chem 285:13107–13120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cárdenas C, Miller RA, Smith I, Bui T, Molgó J, Müller M et al (2010) Essential regulation of cell bioenergetics by constitutive insp3 receptor ca2+ transfer to mitochondria. Cell 142:270–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cardenas ME, Heitman J (1995) FKBP12‐rapamycin target TOR2 is a vacuolar protein with an associated phosphatidylinositol‐4 kinase activity. EMBO J 14:5892–5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carmichael J, Sugars KL, Bao YP, Rubinsztein DC (2002) Glycogen synthase kinase‐3β inhibitors prevent cellular polyglutamine toxicity caused by the Huntington's disease mutation. J Biol Chem 277:33791–33798. [DOI] [PubMed] [Google Scholar]
- 13. Castillo K, Nassif M, Valenzuela V, Rojas F, Matus S, Mercado G et al (2013) Trehalose delays the progression of amyotrophic lateral sclerosis by enhancing autophagy in motoneurons. Autophagy 9:1308–1320. [DOI] [PubMed] [Google Scholar]
- 14. Castillo K, Rojas‐Rivera D, Lisbona F, Caballero B, Nassif M, Court FA et al (2011) BAX inhibitor‐1 regulates autophagy by controlling the IRE1α branch of the unfolded protein response. EMBO J 30:4465–4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cecconi F, Levine B (2008) The role of autophagy in mammalian development: cell makeover rather than cell death. Dev Cell 15:344–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chávez‐Gutiérrez L, Bammens L, Benilova I, Vandersteen A, Benurwar M, Borgers M et al (2012) The mechanism of c ‐Secretase dysfunction in familial Alzheimer disease. EMBO J 31:2261–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Choi AMK, Ryter SW (2013) Autophagy in human health and disease. N Engl J Med 368:651–662. [DOI] [PubMed] [Google Scholar]
- 18. Chu C, Zhang X, Ma W, Li L, Wang W, Shang L et al (2013) Induction of autophagy by a novel small molecule improves aβ pathology and ameliorates cognitive deficits. PLoS One 8:e65367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Criollo A, Maiuri MC, Tasdemir E, Vitale I, Fiebig AA, Andrews D et al (2007) Regulation of autophagy by the inositol trisphosphate receptor. Cell Death Differ 14:1029–1039. [DOI] [PubMed] [Google Scholar]
- 20. Dauer W, Przedborski S (2003) Parkinson's disease: mechanisms and models. Neuron 39:889–909. [DOI] [PubMed] [Google Scholar]
- 21. Decressac M, Mattsson B, Weikop P, Lundblad M, Jakobsson J, Björklund A (2013) TFEB‐mediated autophagy rescues midbrain dopamine neurons from α‐synuclein toxicity. Proc Natl Acad Sci USA 110:E1817–E1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dehay B, Bové J, Rodríguez‐Muela N, Perier C, Recasens A, Boya P, Vila M (2010) Pathogenic lysosomal depletion in Parkinson's disease. J Neurosci 30:12535–12544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Deng HX, Chen W, Hong ST, Boycott KM, Gorrie GH, Siddique N et al (2012) Mutations in UBQLN2 cause dominant X‐linked juvenile and adult onset ALS and ALS/dementia. Nature 477:211–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Djarmati A, Hagenah J, Reetz K, Winkler S, Behrens MI, Pawlack H et al (2009) ATP13A2 variants in early‐onset Parkinson's disease patients and controls. Mov Disord 24:2104–2111. [DOI] [PubMed] [Google Scholar]
- 25. Du J, Liang Y, Xu F, Sun B, Wang Z. (2013) Trehalose rescues Alzheimer's disease phenotypes in APP/PS1 transgenic mice. J Pharm Pharmacol 65:1753–1756. [DOI] [PubMed] [Google Scholar]
- 26. Dzamko N, Gysbers A, Perera G, Bahar A, Shankar A, Gao J et al (2017) Toll‐like receptor 2 is increased in neurons in Parkinson's disease brain and may contribute to alpha‐synuclein pathology. Acta Neuropathol 133:303–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fan W, Nassiri A, Zhong Q (2011) Autophagosome targeting and membrane curvature sensing by Barkor/Atg14(L). Proc Natl Acad Sci USA 108:7769–7774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Feng HL, Leng Y, Ma CH, Zhang J, Ren M, Chuang DM (2008) Combined lithium and valproate treatment delays disease onset, reduces neurological deficits and prolongs survival in an amyotrophic lateral sclerosis mouse model. Neuroscience 155:567–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Filimonenko M, Stuffers S, Raiborg C, Yamamoto A, Malerød L, Fisher EM et al (2007) Functional multivesicular bodies are required for autophagic clearance of protein aggregates associated with neurodegenerative disease. J Cell Biol 179:485–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fornai F, Longone P, Cafaro L, Kastsiuchenka O, Ferrucci M, Manca ML et al (2008) Lithium delays progression of amyotrophic lateral sclerosis. Proc Natl Acad Sci USA 105:2052–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Frakes AE, Ferraiuolo L, Haidet‐Phillips AM, Schmelzer L, Braun L, Miranda CJ et al (2015) Microglia induce motor neuron death via the classical NF‐κB pathway in amyotrophic lateral sclerosis. Neuron 81:1009–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Granowitz EV, Vannier E, Poutsiaka DD, Dinarello CA (1992) Effect of interleukin‐1 (IL‐1) Blockade on cytokine synthesis: ii. il‐1 receptor antagonist inhibits lipopolysaccharide‐induced cytokine synthesis by human monocytes. Blood 79:2364–2369. [PubMed] [Google Scholar]
- 33. Guix FX, Sannerud R, Berditchevski F, Arranz AM, Horré K, Snellinx A et al (2017) Tetraspanin 6: a pivotal protein of the multiple vesicular body determining exosome release and lysosomal degradation of amyloid precursor protein fragments. Mol Neurodegener 12:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guo F, He XB, Li S, Le W (2016) A central role for phosphorylated p38a in linking proteasome inhibition‐induced apoptosis and autophagy. Mol Neurobiol 3:1–13. [DOI] [PubMed] [Google Scholar]
- 35. Yu WH, Cuervo AM, Kumar A, Peterhoff CM, Schmidt SD, Lee JH et al (2005) Macroautophagy ‐ A novel β‐amyloid peptide‐generating pathway activated in Alzheimer's disease. J Cell Biol 171:87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. He C, Levine B (2010) The beclin‐1 interactome. Curr Opin Cell Biol 22:140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hebron ML, Lonskaya I, Moussa CEH (2013) Nilotinib reverses loss of dopamine neurons and improves motor behavior via autophagic degradation of α‐synuclein in parkinson's disease models. Hum Mol Genet 22:3315–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hetz C, Mollereau B (2014) Disturbance of endoplasmic reticulum proteostasis in neurodegenerative diseases. Nat Rev Neurosci 15:233–249. [DOI] [PubMed] [Google Scholar]
- 39. Imarisio S, Carmichael J, Korolchuk V, Chen CW, Saiki S, Rose C et al (2008) Huntington's disease: from pathology and genetics to potential therapies. Biochem J 412:191–209. [DOI] [PubMed] [Google Scholar]
- 40. Itakura E, Kishi‐itakura C, Mizushima N (2012) The hairpin‐type tail‐anchored snare syntaxin 17 targets to autophagosomes for fusion with endosomes/lysosomes. Cell 151:1256–1269. [DOI] [PubMed] [Google Scholar]
- 41. Jiang T, Yu JT, Zhu XC, Tan MS, Wang HF, Cao L et al (2014) Temsirolimus promotes autophagic clearance of amyloid‐β and provides protective effects in cellular and animal models of Alzheimer's disease. Pharmacol Res 81:54–63. [DOI] [PubMed] [Google Scholar]
- 42. Jimenez‐Sanchez M, Licitra F, Underwood BR, Rubinsztein DC. (2016) Huntington's disease: mechanisms of pathogenesis and therapeutic strategies. Cold Spring Harb Perspect Med 7:a024240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jo C, Gundemir S, Pritchard S, Jin YN, Rahman I, Johnson GV (2014) Nrf2 reduces levels of phosphorylated tau protein by inducing autophagy adaptor protein NDP52. Nat Commun 5:3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T et al (2000) LC3, a mammalian homolog of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 19:5720–5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kalia LV, Kalia SK, McLean PJ, Lozano AM, Lang AE. (2013) α‐synuclein oligomers and clinical implications for Parkinson disease. Ann Neurol 73:155–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kane LA, Lazarou M, Fogel AI, Li Y, Yamano K, Sarraf SA et al (2014) PINK1 phosphorylates ubiquitin to activate parkin E3 ubiquitin ligase activity. J Cell Biol 205:143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kaushik S, Cuervo AM (2015) Proteostasis and aging. Nat Med 21:1406–1415. [DOI] [PubMed] [Google Scholar]
- 48. Kazlauskaite A, Muqit MMK (2015) PINK1 and Parkin ‐ Mitochondrial interplay between phosphorylation and ubiquitylation in Parkinson's disease. FEBS J 282:215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kilpatrick K, Zeng Y, Hancock T, Segatori L (2015) Genetic and chemical activation of TFEB mediates clearance of aggregated α‐synuclein. PLoS One 10:e0120819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kim I, Xu W, Reed JC (2008) Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov 7:1013–1030. [DOI] [PubMed] [Google Scholar]
- 51. Kim S, Lee D, Song JC, Cho SJ, Yun SM, Koh YH et al (2014) NDP52 associates with phosphorylated tau in brains of an Alzheimer disease mouse model. Biochem Biophys Res Commun 454:196–201. [DOI] [PubMed] [Google Scholar]
- 52. Kim YE, Oh KW, Noh MY, Nahm M, Park J, Lim SM et al (2017) Genetic and functional analysis of TBK1 variants in Korean patients with sporadic amyotrophic lateral sclerosis. Neurobiol Aging 50:170.e1–170.e6. [DOI] [PubMed] [Google Scholar]
- 53. Klionsky DJ, Abdalla FC, Abeliovich H, Abraham RT, Acevedo‐Arozena A, Adeli K et al (2012) Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 8:445–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I et al (2006) Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 441:880–884. [DOI] [PubMed] [Google Scholar]
- 55. Korolchuk VI, Menzies FM, Rubinsztein DC (2010) Mechanisms of cross‐talk between the ubiquitin‐proteasome and autophagy‐lysosome systems. FEBS Lett 584:1393–1398. [DOI] [PubMed] [Google Scholar]
- 56. Koyano F, Okatsu K, Kosako H, Tamura Y, Go E, Kimura M et al (2014) Ubiquitin is phosphorylated by PINK1 to activate parkin. Nature 510:162–166. [DOI] [PubMed] [Google Scholar]
- 57. Le NT, Chang L, Kovlyagina I, Georgiou P, Safren N, Braunstein KE et al (2016) Motor neuron disease, TDP‐43 pathology, and memory deficits in mice expressing ALS‐FTD‐linked UBQLN2 mutations. Proc Natl Acad Sci USA 113:E7580–E7589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lee HJ, Khoshaghideh F, Patel S (2004) Clearance of α‐synuclein oligomeric intermediates via the lysosomal degradation pathway. J Neurosci 24:1888–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lee JH, McBrayer MK, Wolfe DM, Haslett LJ, Kumar A, Sato Y et al (2015) Presenilin 1 maintains lysosomal ca2+ homeostasis via trpml1 by regulating vatpase‐mediated lysosome acidification. Cell Rep 12:1430–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lee JH, Yu WH, Kumar A, Lee S, Mohan PS, Peterhoff CM et al (2010) Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer‐related PS1 mutations. Cell 141:1146–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lee S, Sato Y, Nixon RA (2011) Primary lysosomal dysfunction causes cargo‐specific deficits of axonal transport leading to Alzheimer‐like neuritic dystrophy. Autophagy 7:1562–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lee S, Sato Y, Nixon RA (2011) Lysosomal proteolysis inhibition selectively disrupts axonal transport of degradative organelles and causes an alzheimer's‐like axonal dystrophy. J Neurosci 31:7817–7830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lesage S, Drouet V, Majounie E, Deramecourt V, Jacoupy M, Nicolas A et al (2016) Loss of VPS13C function in autosomal‐recessive parkinsonism causes mitochondrial dysfunction and increases PINK1/parkin‐dependent mitophagy. Am J Hum Genet 98:500–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Loos B, du Toit A, Hofmeyr J‐HS (2014) Defining and measuring autophagosome flux‐concept and reality. Autophagy 8627:37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lonskaya I, Hebron ML, Desforges NM, Schachter JB, Moussa CE (2014) Nilotinib‐induced autophagic changes increase endogenous parkin level and ubiquitination, leading to amyloid clearance. J Mol Med 92:373–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lorenz MC, Heitman J (1995) TOR mutations confer rapamycin resistance by preventing interaction with FKBP12‐rapamycin. J Biol Chem 270:27531–27537. [DOI] [PubMed] [Google Scholar]
- 67. Majumder S, Richardson A, Strong R, Oddo S (2011) Inducing autophagy by rapamycin before, but not after, the formation of plaques and tangles ameliorates cognitive deficits. PLoS One 6:e25416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Martinez‐Vicente M, Talloczy Z, Wong E, Tang G, Koga H, Kaushik S et al (2010) Cargo recognition failure is responsible for inefficient autophagy in Huntington's disease. Nat Neurosci 13:567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Martyniszyn L, Szulc L, Boratyńska A, Niemiałtowski MG (2011) Beclin 1 is involved in regulation of apoptosis and autophagy during replication of ectromelia virus in permissive L929 cells. Arch Immunol Ther Exp (Warsz) 59:463–471. [DOI] [PubMed] [Google Scholar]
- 70. Matsuda N, Sato S, Shiba K, Okatsu K, Saisho K, Gautier CA et al (2010) PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol 189:211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Matsumoto G1, Wada K, Okuno M, Kurosawa M, Nukina N (2011) Serine 403 phosphorylation of p62/SQSTM1 regulates selective autophagic clearance of ubiquitinated proteins. Mol Cell 44:279–289. [DOI] [PubMed] [Google Scholar]
- 72. Meduri G, Guillemeau K, Dounane O, Sazdovitch V, Duyckaerts C, Chambraud B et al (2016) Caspase‐cleaved Tau‐D421 is colocalized with the immunophilin FKBP52 in the autophagy‐endolysosomal system of Alzheimer's disease neurons. Neurobiol Aging 46:124–137. [DOI] [PubMed] [Google Scholar]
- 73. Menzies FM, Fleming A, Rubinsztein DC (2015) Compromised autophagy and neurodegenerative diseases. Nat Rev Neurosci 16:345–357. [DOI] [PubMed] [Google Scholar]
- 74. Menzies FM, Moreau K, Rubinsztein DC (2011) Protein misfolding disorders and macroautophagy. Curr Opin Cell Biol 23:190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Metzger S, Saukko M, Van Che H, Tong L, Puder Y, Riess O, Nguyen HP (2010) Age at onset in Huntington's disease is modified by the autophagy pathway: implication of the V471A polymorphism in Atg7. Hum Genet 128:453–459. [DOI] [PubMed] [Google Scholar]
- 76. Mizushima N, Hara T (2006) Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441:302–304. [DOI] [PubMed] [Google Scholar]
- 77. Mizushima N, Komatsu M (2011) Autophagy: renovation of cells and tissues. Cell 147:728–741. [DOI] [PubMed] [Google Scholar]
- 78. Mizushima N, Levine B, Cuervo AM, Klionsky DJ (2008) Autophagy fights disease through cellular self‐digestion. Nature 451:1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mizushima N, Yoshimori T, Ohsumi Y (2011) The role of Atg proteins in autophagosome formation. Annu Rev Cell Dev Biol 27:107–132. [DOI] [PubMed] [Google Scholar]
- 80. Moore AS, Holzbaur ELF (2016) Dynamic recruitment and activation of ALS‐associated TBK1 with its target optineurin are required for efficient mitophagy. Proc Natl Acad Sci U S A 113:E3349–E3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Moreau K, Fleming A, Imarisio S, Lopez Ramirez A, Mercer JL, Jimenez‐Sanchez M et al (2014) PICALM modulates autophagy activity and tau accumulation. Nat Commun 5:4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Morimoto N, Nagai M, Ohta Y, Miyazaki K, Kurata T, Morimoto M et al (2007) Increased autophagy in transgenic mice with a G93A mutant SOD1 gene. Brain Res 1167:112–117. [DOI] [PubMed] [Google Scholar]
- 83. Morselli E, Mariño G, Bennetzen MV, Eisenberg T, Megalou E, Schroeder S et al (2011) Spermidine and resveratrol induce autophagy by distinct pathways converging on the acetylproteome. J Cell Biol 192:615–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Murphy KE, Gysbers AM, Abbott SK, Spiro AS, Furuta A, Cooper A et al (2015) Lysosomal‐associated membrane protein 2 isoforms are differentially affected in early Parkinson's disease: early loss of LAMP2A protein in PD. Mov Disord 30:1639–1647. [DOI] [PubMed] [Google Scholar]
- 85. Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J et al (2010) PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol 8:e1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Narendra D, Tanaka A, Suen DF, Youle RJ (2008) Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol 183:795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Nixon RA, Wegiel J, Kumar A, Yu WH, Peterhoff C, Cataldo A, Cuervo AM (2005) Extensive involvement of autophagy in Alzheimer disease: an immuno‐electron microscopy study. J Neuropathol Exp Neurol 64:113–122. [DOI] [PubMed] [Google Scholar]
- 88. Nixon RA, Yang D‐SS (2012) Autophagy and neuronal cell death in neurological disorders. Cold Spring Harb Perspect Biol 4:a008839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Obara K, Ohsumi Y (2008) Dynamics and function of PtdIns (3) P in autophagy. Autophagy 8627:952–954. [DOI] [PubMed] [Google Scholar]
- 90. Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S et al (2006) Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol 26:9220–9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ohsumi Y, Mizushima N (2004) Two ubiquitin‐like conjugation systems essential for autophagy. Semin Cell Dev Biol 15:231–236. [DOI] [PubMed] [Google Scholar]
- 92. Pajares M, Jiménez‐Moreno N, García‐Yagüe ÁJ, Escoll M, de Ceballos ML, Van Leuven F et al (2016) Transcription factor NFE2L2/NRF2 is a regulator of macroautophagy genes. Autophagy 12:1902–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Park HS, Jun do Y, Han CR, Woo HJ, Kim YH (2011) Proteasome inhibitor MG132‐induced apoptosis via ER stress‐mediated apoptotic pathway and its potentiation by protein tyrosine kinase p56 lck in human Jurkat T cells. Biochem Pharmacol 82:1110–1125. [DOI] [PubMed] [Google Scholar]
- 94. Pasinelli P, Brown RH (2016) Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nat Rev Neurosci 7:710–723. [DOI] [PubMed] [Google Scholar]
- 95. Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N et al (2005) Bcl‐2 antiapoptotic proteins inhibit Beclin 1‐dependent autophagy. Cell 122:927–939. [DOI] [PubMed] [Google Scholar]
- 96. Pickford F, Masliah E, Britschgi M, Lucin K, Narasimhan R, Jaeger PA et al (2008) The autophagy‐related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid‐β accumulation in mice. J Clin Invest 118:2190–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Piras A, Collin L, Grüninger F, Graff C, Rönnbäck A (2016) Autophagic and lysosomal defects in human tauopathies: analysis of post‐mortem brain from patients with familial Alzheimer disease, corticobasal degeneration and progressive supranuclear palsy. Acta Neuropathol Commun 4:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Plowey ED, Cherra SJ 3rd, Liu YJ, Chu CT (2008) Role of autophagy in G2019S‐LRRK2‐associated neurite shortening in differentiated SH‐SY5Y cells. J Neurochem 105:1048–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Puri C, Renna M, Bento CF, Moreau K, Rubinsztein DC (2013) Diverse autophagosome membrane sources coalesce in recycling endosomes. Cell 154:1285–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ramirez A, Heimbach A, Gründemann J, Stiller B, Hampshire D, Cid LP et al (2006) Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P‐type ATPase. Nat Genet 38:1184–1191. [DOI] [PubMed] [Google Scholar]
- 101. Ravikumar B, Acevedo‐Arozena A, Imarisio S, Berger Z, Vacher C, O'Kane CJ et al (2005) Dynein mutations impair autophagic clearance of aggregate‐prone proteins. Nat Genet 37:771–776. [DOI] [PubMed] [Google Scholar]
- 102. Ravikumar B, Vacher C, Berger Z, Davies JE, Luo S, Oroz LG et al (2004) Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet 36:585–595. [DOI] [PubMed] [Google Scholar]
- 103. Rodríguez‐Navarro JA, Rodríguez L, Casarejos MJ, Solano RM, Gómez A, Perucho J et al (2010) Trehalose ameliorates dopaminergic and tau pathology in parkin deleted/tau overexpressing mice through autophagy activation. Neurobiol Dis 39:423–438. [DOI] [PubMed] [Google Scholar]
- 104. Rohn TT, Wirawan E, Brown RJ, Harris JR, Masliah E, Vandenabeele P (2011) Depletion of Beclin‐1 due to proteolytic cleavage by caspases in the Alzheimer's disease brain. Neurobiol Dis 43:68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Rohn TT, Wirawan E, Brown RJ, Harris JR, Masliah E, Vandenabeele P (2010) Rilmenidine attenuates toxicity of polyglutamine expansions in a mouse model of Huntington's disease. Hum Mol Genet 19:2144–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Rubinsztein DC, Shpilka T, Elazar Z (2012) Mechanisms of autophagosome biogenesis. Curr Biol 22:R29–R34. [DOI] [PubMed] [Google Scholar]
- 107. Saha S, Ash PE, Gowda V, Liu L, Shirihai O, Wolozin B (2015) Mutations in LRRK2 potentiate age‐related impairment of autophagic flux. Mol Neurodegener 10:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Sarkar S (2013) Regulation of autophagy by mTOR‐dependent and mTOR‐independent pathways: autophagy dysfunction in neurodegenerative diseases and therapeutic application of autophagy enhancers. Biochem Soc Trans 41:1103–1130. [DOI] [PubMed] [Google Scholar]
- 109. Sarkar S, Davies JE, Huang Z, Tunnacliffe A, Rubinsztein DC (2007) Trehalose, a novel mTOR‐independent autophagy enhancer, accelerates the clearance of mutant huntingtin and α‐synuclein. J Biol Chem 282:5641–5652. [DOI] [PubMed] [Google Scholar]
- 110. Sarkar S, Floto RA, Berger Z, Imarisio S, Cordenier A, Pasco M et al (2005) Lithium induces autophagy by inhibiting inositol monophosphatase. J Cell Biol 170:1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Sarkar S, Ravikumar B, Floto RA, Rubinsztein DC (2009) Rapamycin and mTOR‐independent autophagy inducers ameliorate toxicity of polyglutamine‐expanded huntingtin and related proteinopathies. Cell Death Differ 16:46–56. [DOI] [PubMed] [Google Scholar]
- 112. Sasaki S (2011) Autophagy in spinal cord motor neurons in sporadic amyotrophic lateral sclerosis. J Neuropathol Exp Neurol 70:349–359. [DOI] [PubMed] [Google Scholar]
- 113. Settembre C, Di Malta C, Polito VA, Garcia Arencibia M, Vetrini F, Erdin S et al (2013) TFEB links autophagy to lysosomal biogenesis. Science 332:1429–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Shao Y, Gao Z, Feldman T, Jiang X (2007) Stimulation of ATG12‐ATG5 conjugation by ribonucleic acid. Autophagy 3:10–16. [DOI] [PubMed] [Google Scholar]
- 115. Shibata M, Lu T, Furuya T, Degterev A, Mizushima N, Yoshimori T et al (2006) Regulation of intracellular accumulation of mutant huntingtin by beclin 1. J Biol Chem 281:14474–14485. [DOI] [PubMed] [Google Scholar]
- 116. Son JH, Shim JH, Kim KH, Ha JY, Han JY (2012) Neuronal autophagy and neurodegenerative diseases. Exp Mol Med 44:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Steele JW, Lachenmayer ML, Ju S, Stock A, Liken J, Kim SH et al (2013) Latrepirdine improves cognition and arrests progression of neuropathology in an Alzheimer's mouse model. Mol Psychiatry 18:889–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Suzuki K, Kubota Y, Sekito T, Ohsumi Y (2007) Hierarchy of Atg proteins in pre‐autophagosomal structure organization. Genes Cells 12:209–218. [DOI] [PubMed] [Google Scholar]
- 119. Tanaka M, Machida Y, Niu S, Ikeda T, Jana NR, Doi H et al (2004) Trehalose alleviates polyglutamine‐mediated pathology in a mouse model of Huntington disease. Nat Med 1:148–154. [DOI] [PubMed] [Google Scholar]
- 120. Usenovic M, Tresse E, Mazzulli JR, Taylor JP, Krainc D (2012) Deficiency of ATP13A2 leads to lysosomal dysfunction, α‐ synuclein accumulation and neurotoxicity. J Neurosci 257:4240–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Jung CH, Jun CB, Ro SH, Kim YM, Otto NM, Cao J et al (2009) ULK‐Atg13‐FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell 20:1992–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Fujita N, Hayashi‐Nishino M, Fukumoto H, Omori H, Yamamoto A, Noda T, Yoshimori T (2008) An Atg4B mutant hampers the lipidation of LC3 paralogues and causes defects in autophagosome closure. Mol Biol Cell 19:4651–4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Vekrellis K, Xilouri M, Emmanouilidou E, Rideout HJ, Stefanis L (2011) Pathological roles of α‐synuclein in neurological disorders. Lancet Neurol 10:1015–1025. [DOI] [PubMed] [Google Scholar]
- 124. Vingtdeux V, Giliberto L, Zhao H, Chandakkar P, Wu Q, Simon JE et al (2010) AMP‐activated protein kinase signaling activation by resveratrol modulates amyloid‐β peptide metabolism. J Biol Chem 285:9100–9113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Wang IF, Guo BS, Liu YC, Wu CC, Yang CH, Tsai KJ, Shen CK (2012) Autophagy activators rescue and alleviate pathogenesis of a mouse model with proteinopathies of the TAR DNA‐binding protein 43. Proc Natl Acad Sci USA 109:15024–15029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Webb JL, Ravikumar B, Atkins J, Skepper JN, Rubinsztein DC (2003) α‐synuclein is degraded by both autophagy and the proteasome. J Biol Chem 278:25009–25013. [DOI] [PubMed] [Google Scholar]
- 127. Williams AJ, Paulson HL (2008) Polyglutamine neurodegeneration: protein misfolding revisited. Trends Neurosci 31:521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Wolfe DM, Lee JH, Kumar A, Lee S, Orenstein SJ, Nixon RA (2013) Autophagy failure in Alzheimer's disease and the role of defective lysosomal acidification. Eur J Neurosci 37:1949–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Wong YC, Holzbaur ELF (2015) Temporal dynamics of PARK2/parkin and OPTN/optineurin recruitment during the mitophagy of damaged mitochondria. Autophagy 11:422–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Xu C, Bailly‐Maitre B, Reed J (2005) Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest 115:2656–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Xue X, Wang LR, Sato Y, Jiang Y, Berg M, Yang DS et al (2014) Single‐walled carbon nanotubes alleviate autophagic/lysosomal defects in primary glia from a mouse model of Alzheimer's disease. Nano Lett 14:5110–5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Yamamoto A, Simonsen A (2011) The elimination of accumulated and aggregated proteins: a role for aggrephagy in neurodegeneration. Neurobiol Dis 43:17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Yang DS, Stavrides P, Saito M, Kumar A, Rodriguez‐Navarro JA, Pawlik M et al (2014) Defective macroautophagic turnover of brain lipids in the TgCRND8 Alzheimer mouse model: prevention by correcting lysosomal proteolytic deficits. Brain 137:3300–3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Yen WL, Shintani T, Nair U, Cao Y, Richardson BC, Li Z et al (2010) The conserved oligomeric Golgi complex is involved in double‐membrane vesicle formation during autophagy. J Cell Biol 188:101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Yoon SY, Kim DH (2016) Alzheimer's disease genes and autophagy. Brain Res 1649:201–209. [DOI] [PubMed] [Google Scholar]
- 136. Yu HC, Lin CS, Tai WT, Liu CY, Shiau CW, Chen KF (2013) Nilotinib induces autophagy in hepatocellular carcinoma through AMPK activation. J Biol Chem 288:18249–18259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Zavodszky E, Seaman MN, Moreau K, Jimenez‐Sanchez M, Breusegem SY, Harbour ME, Rubinsztein DC (2014) Mutation in VPS35 associated with Parkinson's disease impairs WASH complex association and inhibits autophagy. Nat Commun 5:3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Zhang X, Chen S, Song L, Tang Y, Shen Y, Jia L, Le W (2014) MTOR‐independent, autophagic enhancer trehalose prolongs motor neuron survival and ameliorates the autophagic flux defect in a mouse model of amyotrophic lateral sclerosis. Autophagy 10:588–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Zhang X, Li L, Chen S, Yang D, Wang Y, Zhang X et al (2011) Rapamycin treatment augments motor neuron degeneration in SOD1 G93A mouse model of amyotrophic lateral sclerosis. Autophagy 7:412–425. [DOI] [PubMed] [Google Scholar]
- 140. Zheng S, Clabough EB, Sarkar S, Futter M, Rubinsztein DC, Zeitlin SO (2010) Deletion of the huntingtin polyglutamine stretch enhances neuronal autophagy and longevity in mice. PLoS Genet 6:e1000838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Zhu Z, Yan J, Jiang W, Yao XG, Chen J, Chen L et al (2013) Arctigenin effectively ameliorates memory impairment in Alzheimer's disease model mice targeting both beta‐amyloid production and clearance. J Neurosci 33:13138–13149. [DOI] [PMC free article] [PubMed] [Google Scholar]


