Abstract
Objectives
Bipolar disorder (BD) is associated with elevated reward sensitivity and persistent positive affect, yet the neural mechanisms underlying these patterns are not well understood. In the present study, we examined putative disruptions in communication within a well-known corticolimbic reward circuit during reward processing as a potential contributing mechanism to these symptoms.
Methods
The present investigation employed a within- and between-subjects design utilizing a monetary and social incentive delay task among adults with bipolar disorder type I (BD; N=24) and a healthy non-psychiatric control group (HC; N=25) during fMRI. Participants in the BD group were remitted at the time of testing.
Results
Functional connectivity analyses revealed increased connectivity between the ventral striatum (VS) seed region and orbitofrontal cortex (OFC) as well as the amygdala during processing of reward receipt in the BD group. After omission of expected rewards, the BD group showed decreased functional connectivity between the ventral striatum and a medial frontopolar cortex (mFPC) region associated with consideration of behavioral alternatives. Follow-up analyses within the BD group showed that increased VS-OFC connectivity after reward receipt, and decreased VS-mFPC connected after reward omission, were associated with higher levels of subthreshold mania symptoms.
Conclusions
Results point toward potential mechanisms implicated in elevated reward sensitivity in BD. Enhanced VS-OFC connectivity after reward receipt may be involved in elevated valuation of rewards whereas blunted VS-mFPC connectivity after reward omission may reflect a failure to consider behavioral alternatives to reward pursuit.
Keywords: Reward, Bipolar Disorder, Social, Monetary, Ventral Striatum
Introduction
Bipolar disorder (BD) is a chronic and severe psychiatric condition, characterized by extreme shifts in mood and emotion over time (1–3). Disturbances in positive emotion and reward are central to many symptoms of the disorder; these include persistently elevated positive emotion and sustained reward pursuit across contexts (4–8). Elevated reward sensitivity, in turn, has been found to predict worsening of mania symptoms and the onset of new manic episodes over time (9–10). Efforts to better understand these behavioral patterns at the neural level have primarily focused on regional neural activity during reward tasks. For example, studies have reported increased activation in the ventral striatum (VS; 11–15) and orbitofrontal cortex (OFC; 15–17) of patients with BD compared to healthy controls during reward processing. While these data provide an important foundation, they cannot fully explain the pathophysiology of reward processing dysfunctions in BD, as these regions do not operate independently but interact as components of complex neural networks (18–23). Thus, an important next step is to begin to explore the functional connectivity between implicated regions or structures. Understanding the extent to which elevated reward sensitivity may be related to patterns of functional connectivity between neural regions may provide more precise targets for therapeutic intervention.
Bipolar Disorder Symptoms and Reward Processing
Reward processing disturbances have been proposed as a putative endophenotype for BD (24). Specifically, BD is associated with heightened sensitivity to reward, as well as behavioral patterns including persistent reward pursuit and excessive engagement in pleasurable activities (6, 24–26). Euthymic individuals with BD (i.e., neither currently hypo/manic, depressed, or mixed) self-report heightened positive affect at the prospect of future rewards in their daily lives and in response to emotional stimuli (6), compared to healthy controls (10). Similarly, in a college student sample, high BD risk (based on Hypomanic Personality Scale scores) was found to predict higher expectancies for future success and more ambitious goal-setting after an initial monetary reward (26). Individuals at risk for BD have also been found to report heightened positive affect after receiving false success feedback (27). Individuals with BD and those at risk for developing BD also report elevated reward responsiveness (4, 10, 28), which may be a key contributor to the development and maintenance of BD (4, 5, 25). Moreover, rewarding life events (i.e., goal attainment events such as graduations) have been found to predict increases in manic symptoms over time (29–30). Taken together, elevated sensitivity to rewards is central to the development and symptom course of BD.
Brain Networks and Reward Processing Disturbances in BD
Reward processing is supported by an interconnected, dopamine-rich brain network (21, 31, 32). Within this network, the striatum can be conceptualized as a central hub for the transmission of reward-relevant signals within multiple circuits mediating motivation as well as reward-based decision-making and behavior (33, 34). Two regions of the PFC have been shown to interact with the striatum in particularly relevant ways for the study of individual differences in reward-related responding. First, a ventral striatal-orbitofrontal circuit has been implicated in motivation and reward learning (35–41). In one study, Jung and colleagues (42) examined functional connectivity during a task in which participants could risk monetary gain or loss by playing a trial, or pass to the next trial without gain or loss. They reported that OFC-VS connectivity positively correlated with the number of persistent responses made during the task, suggesting that individual differences in frontostriatal functional connectivity may be implicated in behavioral persistence toward reward in uncertain conditions.
A more anterior region of the PFC, the frontopolar cortex (FPC) has shown functional connectivity with the striatum during set shifting (43), suggesting that connectivity between the FPC and striatum may be important for facilitating change in behavioural strategies for reward-pursuit. Consistent with this interpretation, FPC activation has been found to track with the range of behavioral options being considered (44), and with the value of alternative choices that were not selected (45). On the basis of these findings, this region has been conceptualized as maintaining a representation of possible courses of action in the near future (46, 47). This is consistent with work showing disruption to FPC in BD, resulting in impairments of information maintenance (48), and response selection (49). Taken together, previous work appears to suggest that striatal functional connectivity with the OFC is implicated in persistence in reward pursuit strategy, while functional connectivity between the striatum and FPC is implicated in facilitating consideration and selection of alternative strategies. Given the well-documented persistent reward pursuit behavior, including failures to downregulate reward pursuit after an initial reward receipt observed clinically in BD (6, 50), we hypothesized that this group would be characterized by alterations in functional connectivity between the striatum and one or both of these prefrontal regions.
Finally, connectivity between the amygdala and ventral striatum has been strongly implicated in reward-motivated behavior and reward learning. The amygdala has been found to encode the motivational or affective significance of events (51). Afferent projections from the amygdala to the VS have been found to facilitate reward seeking (52, 53), and play an important role in reward-based learning (51, 54). In a study examining connectivity-based parcellation of the human striatum in relation to personality characteristics, Cohen and colleagues (55) found that individuals who self-reported higher levels of novelty-seeking had relatively stronger fiber tracts between the amygdala and ventral and mesial regions of the striatum. Based on behavioral findings of heightened reward seeking and affective responding to rewards in BD (6, 7), as well as work directly implicating altered amgydala activity in BD (56, 57), we predicted enhanced functional connectivity between the striatum and amygdala in response to reward receipt in the BD group.
The Present Investigation
In an earlier analysis of these data, we found that the VS exhibited elevated reactivity to reward receipt in euthymic BD, compared to a healthy control group (13). Notably, no task-related differences in VS reactivity to reward receipt emerged. The goal of the present investigation is to build on these findings, characterizing group differences in functional connectivity with this region during reward processing. As such, the present analysis collapses across reward types. To this end, we seeded the VS and examined the temporal coupling between this seed region with other neural regions during processing three types of trial outcomes, including 1) reward receipt, 2) neutral outcomes, and 3) the omission of expected rewards, across both monetary and social reward types. Second, we examined relationships between functional connectivity and individual difference variables related to mania and reward sensitivity in the BD group. We predicted that ventral striatum functional connectivity with the amygdala and OFC would be enhanced after win outcomes. This pattern would align with persistent reward pursuit, particularly after experiences involving reward receipt in BD (8, 50, 58), and the roles for these regions in reward valuation and appetitive motivation (39, 42, 51). In addition, we predicted that functional connectivity between the ventral striatum and FPC would be blunted for the BD, compared to the HC group after failing to obtain an available reward (no-win outcome). This pattern would fit with the role of the FPC in facilitating change in reward-pursuit strategy (43–47), and clinical findings demonstrating a deficit in this ability in BD (6, 7, 50).
Importantly, participants were in remission at the time of testing. This allowed us to examine the differences in functional connectivity between individuals with BD and a HC group without the inherent confound of mood symptoms at the time of testing (16, 59). If group differences emerge in the context of remission, they are less likely attributable to transient mood symptoms. In addition, we employed the well-validated monetary incentive delay (MID) task (60) alongside a novel social incentive delay (SID) task recently developed by our group (13). In doing so, we were able to examine connectivity during processing of monetary and social rewards, consistent with the variability in reward types that individuals typically experience in their daily lives.
Materials and Methods
Participants
As described in a previous analysis of this data (13) participants were 28 individuals diagnosed with BD type I, currently remitted, and 27 healthy controls (HC) who did not meet current or past criteria for any DSM-IV-TR Axis I disorder. Additional exclusion criteria for both groups were history of severe head trauma, stroke, neurological disease, severe medical illness (e.g., autoimmune disorder, HIV/AIDS), left-handedness, medications affecting cerebral blood flow (e.g., blood pressure medications), MRI safety incompatibility, pregnancy, suicidal ideation, or alcohol or substance abuse or dependence in the past six months. Four BD and two HC participants were excluded from the final data analysis due to excessive motion during fMRI (>5mm movements during at least 4 of 8 runs), leaving a final sample of 24 BD and 25 HC participants. Participants were recruited using online advertisements and flyers posted in New Haven, CT and surrounding communities.
Diagnostic Evaluation
All diagnoses were confirmed using the Structured Clinical Interview for DSM-IV (SCID-IV; 61) administered by trained researchers. Trained researchers (i.e., licensed clinical psychologist, clinical psychology Ph.D. students, post-baccalaureate fellow, or advanced research assistant) administered the diagnostic and symptom assessments after completing training that included role-playing interviews, co-interviews with trained staff, and live observation of trainee clinical interviews. Approximately one-fourth (n=12; 24.49%) of videotaped interviews were selected and independently rated by an additional clinical interviewer and major discrepancies (i.e., errors) were corrected during informal consensus meetings. Final (i.e., post-meeting) inter-rater reliability matched 100% for BD diagnosis (κ= 1.00) and non-psychiatric control diagnosis (κ = 1.00) (i.e., the absence of current or lifetime psychiatric diagnoses, according to DSM-IV-TR criteria). This indicates strong interrater reliability, though the “skip out” following the standard SCID format could have reduced opportunities for disagreement among independent raters.
Mood Symptoms
Current symptoms of mania were measured using the Young Mania Rating Scale (YMRS; 62) and current symptoms of depression were measured using the Inventory of Depressive Symptomatology (IDS-C; 63). Remitted mood status (i.e., neither manic, depressed, nor mixed mood state) for the BD group was verified according to SCID-IV mood module criteria for the past month and cutoff scores on the YMRS (≤ 7), and IDS-C (≤ 11) for the past week. The IDS-C and YMRS were administered on the day of the diagnostic interview, and re-administered again on the day of the scan to ensure that participants scored below cutoffs on both days (see Table 1). Intra-class correlation coefficients (ICC; 64) for absolute agreement between the original interviewer and an independent rater for approximately one fifth of study participants were strong for both the IDS-C (n=11; ICC=1.00) and YMRS (n=10; ICC=0.96).
Table 1.
Participant Characteristics
| BD (n=24) |
HC (n=25) |
Statistic | Effect Size | |
|---|---|---|---|---|
| Demographic | ||||
| Age (Yrs) | 31.38 (11.86) | 29.44 (8.84) | F=0.42 | ηp2=0.01 |
| Female (%) | 62.5% | 60.0% | χ2=0.03 | V=0.03 |
| Caucasian (%) | 95.8% | 84.0% | χ2=1.87 | V=0.20 |
| Education (Yrs) | 15.20 (2.00) | 16.16 (1.57) | F=3.47 | ηp2=0.07 |
| Employed (%) | 50.0% | 72.0% | χ2=2.50 | V=0.23 |
| Married (%) | 12.5% | 4.0% | χ2=1.18 | V=0.16 |
| Cognitive | ||||
| MMSE | 27.96 (1.76) | 28.68 (1.57) | F=0.15 | ηp2=0.05 |
| WAIS-IV Letter Number Task | 11.92 (2.56) | 12.08 (3.37) | F=0.15 | ηp2=0.003 |
| Reward Sensitivity | ||||
| BAS Reward Responsiveness | 16.71 (2.07) | 16.68 (1.55) | F=0.003 | ηp2=0.000 |
| Clinical | ||||
| YMRS | 1.50 (1.72) | 1.04 (1.46) | F=1.02 | ηp2=0.17 |
| IDS-C | 3.58 (2.08) | 1.40 (1.47) | F=18.07* | ηp2=0.99 |
| GAF | 70.63 (10.75) | 88.96 (3.93) | F=63.84* | ηp2=0.58 |
| Age at Onset (Yrs) | 16.33 (7.14) | – | – | – |
| Illness Duration (Yrs) | 14.78 (11.45) | – | – | – |
| # Comorbid Disorders | 0.42 (0.65) | – | – | – |
| # Depressive Episodes | 13.00 (19.13) | – | – | – |
| # Manic Episodes | 16.69 (37.40) | – | – | – |
| # Antidepressants | 0.33 (0.56) | – | – | – |
| # Lithium | 0.20 (0.41) | – | – | – |
| # Benzodiazepines | 0.13 (0.34) | – | – | – |
| # Typical Neuroleptics | 0.00 (0.00) | – | – | – |
| # Atypical Neuroleptics | 0.21 (0.41) | – | – | – |
Note: BD=Bipolar disorder group; HC=Healthy control group; MMSE=Mini Mental State Exam; WAIS-IV Letter Number Task =Letter-Number Sequencing subtest of the Wechsler Adult Intelligence Scale-IV; YMRS=Young Mania Rating Scale; IDS-C=Inventory of Depressive Symptoms – Clinician Rated; Age at Onset=Age of BD Onset; # Manic Episodes=Number of Lifetime Manic Episodes; # Depressive Episodes=Number of Lifetime Major Depressive Episodes; # Comorbid Disorders=Number of Comorbid DSM-IV-TR Axis I Diagnoses; Mean values are displayed with standard deviations in parentheses where applicable. Clinical information collected at initial laboratory visit.
p<0.05 comparison of BD and HC groups.
Medication Assessment
At the baseline laboratory visit, participants reported use and dosage of psychiatric medications over the past month recorded using the Somatotherapy Index (65). Medication classes coded included antidepressants, anticonvulsants, lithium, valproate, benzodiazepines, typical and atypical neuroleptics, buspirone, zolpidem, lamictal, and alternative therapies (see Table 1).
Cognitive Functioning
Baseline cognitive functioning was assessed using the Mini Mental Status Examination, a brief objective measure of cognitive status and impairment (MMSE; 66). Raw scores (range: 0–30) were calculated as the total number of trials correct and all participants exceeded the eligibility cutoff score (≥24; 66) (see Table 1).
Executive Functioning
Executive functioning was measured using the letter-number sequencing subtest of the Wechsler Adult Intelligence Scale-IV (WAIS-IV; 67). Raw scores were calculated as the total number of trials correct (range: 5–20), from which WAIS-IV age-normed scaled scores were used in final analyses (range: 5–19) (see Table 1).
Reward Sensitivity
Participants completed the self-report questionnaire the Behavioral Inhibition System/Behavioral Approach System (BIS/BAS) Scales (68). Subscale scores were calculated according to instructions provided by the authors, and the Reward Responsiveness subscale was operationalized as a measure of trait-level reward responsivity (see Table 1).
Procedures
Participants completed two study sessions, including an initial baseline diagnostic visit and a second fMRI scanning session approximately 2.5 months apart (M=78.51 days, SD=65.79). Between the two visits there was a second unrelated fMRI scanning session during which symptoms were also reassessed to ensure continuity of remitted mood status in the BD group.
Baseline diagnostic visit
At baseline, participants completed a diagnostic evaluation in the laboratory that included the SCID-IV, YMRS, BRMS, IDS-C, Positive Qualities Questionnaire (see below), medication information, and demographics (along with additional questionnaires not part of the current investigation).
Positive Qualities Questionnaire (PQQ)
The PQQ is a 10-item questionnaire designed for the present study to elicit self-reported information about perceived positive qualities. It was used to derive personalized social feedback for use in the Social Incentive Delay (SID) task, described below. Specifically, participants were asked to “describe some positive events in your life, as well as some positive personal qualities and beliefs”, and to respond to each question in a few sentences. PQQ items span several domains including personal values (e.g., “Name some values that you believe are very important, and describe why they are important to you”), personal qualities (e.g., “Describe a quality that makes you unique”), social relationships (e.g., “Describe a time when you felt love for someone else”), and achievement (e.g., “Describe one of your greatest accomplishments”; see Appendix 1). The PQQ was used to generate the positive adjectives presented in the SID task. Specifically, content analysis was performed to match adjectives from a validated database of 555 positive adjectives (69) with individual responses to items on the PQQ. In order to assess the relative intensity of each positive adjective, 134 community participants rated 100 of these adjectives for their positive value on a scale of 1 (not at all positive) to 5 (extremely positive) in an anonymous online survey. Following this, all adjectives were standardized and assigned percentile rankings based on their average positivity rating. These rankings were used to categorize positive adjectives into one of two categories: (1) Level 2 adjectives were defined as ‘highly positive’ and consisted of adjectives with the highest 50th percentile; (2) Level 1 adjectives were defined as ‘moderately positive’ and consisted of adjectives with percentile rankings in the lowest 50th percentile. See (13) for details of adjective selection for individual participants.
fMRI scanning visit
The fMRI visit included four parts; namely, a pre-scan assessment, pre-scan task training, fMRI task, and post-scan phase. During the pre-scan assessment, the YMRS and IDS-C were re-administered to ensure that participants were below symptom thresholds and met remitted symptom status (70). Next, the presence of current substance use was assessed using the Medimpex Multi-Drug Urine Test (United Inc.) and participants who tested positive for cocaine, amphetamines, methamphetamines, opiates, or benzodiazepines were excluded unless prescribed by a physician (see Table 1).
Next, participants were trained on the MID and SID tasks for approximately 30 minutes. First, the experimenter explained the task as instructions were presented on a laptop. Next, participants completed 45 practice trials for each task. During the practice, average reaction times were calculated, and these were used to titrate the duration of the target presentation during the fMRI session to ensure even distribution (~50%) of win trials on the MID and SID tasks, following prior research (e.g., 71). Specifically, half the targets were presented for one standard deviation longer than the participant’s average reaction time (predicted ‘win’ trials), and the other half were presented for one standard deviation shorter than the participant’s average reaction time (predicted ‘no-win’ trials). During the fMRI scan, participants completed four runs of each task lasting approximately seven minutes each, presented in random order. Each run consisted of 22–23 trials, totaling 90 MID and 90 SID trials, broken down into 18 neutral/no-win trials (20%), 36 low reward trials (40%), and 36 high reward trials (40%). After the scan, participants completed post-task questionnaires in a testing room, received compensation, and were debriefed.
Monetary and social incentive delay tasks
Participants completed the previously-validated Monetary Incentive Delay (MID) task (60) and a Social Incentive Delay (SID) task developed by our group (13) (see Figure 1 for task schematics). Each task consisted of 90 trials, yielding a total of 180 trials. During pre-scan task training, participants were given a cover story for the SID task. The cover story suggested that the object of the task was to view trained experimenters’ feedback on the best aspects of their personalities, based on their interactions with experimenters during the laboratory session and their questionnaire responses. Each task varied across three levels of reward (e.g., neutral, low reward, and high reward). For further details of the SID task cover story and levels of reward across tasks, see (13).
Figure 1.
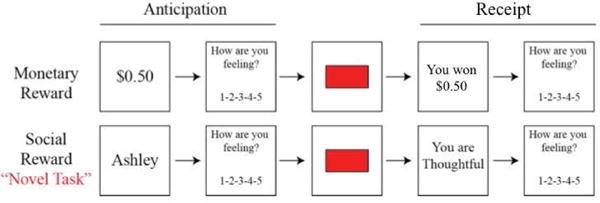
Monetary Incentive Delay (MID) and Social Incentive Delay (SID) task schematics. First, participants saw a cue indicating either how much money was available (MID: e.g., $0.50), or whose feedback they were playing for (SID: e.g., Ashley) on that trial. Next, participants rated how they felt about the potential for winning the amount of money displayed in the cue, on a 1 (negative) to 5 (positive) scale. Next, a red box flashed briefly, and participants responded as quickly as possible. If they responded quickly enough, they would see a win outcome consisting either of monetary rewards (MID: e.g., You won $0.50) or praise (SID: e.g., “You are thoughtful). If they did not, a no-win outcome would appear. Finally, participants rated their affective responses to the outcome on a 1 (negative) to 5 (positive) scale.
Participants completed four runs of the MID task and four runs of the SID task, for a total of eight runs. Each run consisted of approximately 22–23 trials. The order in which the four MID and four SID task runs were presented was randomly selected for each participant at the time of testing using E-Prime 2.0. The temporal sequence of a single trial can be seen in Figure 1 and is described in more detail in (13). Trials were separated by an inter-trial interval of variable duration (ITI; jittered ~3s; range: 2.0–7.0s). To assess emotion during reward anticipation and receipt, participants reported their current feelings in response to the prompt, “How are you feeling?” on a Likert scale from 1 (negative) to 5 (positive) before and following reward outcome.
Data Acquisition
Behavioral and self-report data
E-Prime 2.0 software (Psychology Software Tools, Inc.) was used for stimulus presentation and self-report data collection. During the fMRI scan, stimuli were projected onto a screen behind the scanner, and participants viewed the stimuli via an angled mirror affixed to the head coil. Responses were made using a five-button response box with the right hand.
fMRI data acquisition
Data were collected on a Siemens TIM Trio 3T scanner (Siemens Medical Solutions, Erlangen, Germany). Functional images were acquired with a T2-weighted EPI BOLD sequence (TR=2000ms; TE=35ms; FOV=220mm; voxel dimensions 3.4×3.4×4.0mm; 28 slices). Structural images were obtained using a T1-weighted MPRAGE acquisition (TR=2530ms; TE=2.77 msec; FOV=256mm; voxel dimensions=1.0×1.0×1.0mm; 176 slices).
fMRI data analysis
Preprocessing was carried out using the fMRI Expert Analysis Tool (FEAT) Version 5.0.8, part of FSL (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl), For the EPI images, we performed brain extraction, motion estimation using MCFLIRT, and spatial smoothing with a Gaussian kernel (FWHM=5 mm). The data were then high pass filtered at 0.01 Hz. After pre-processing, EPI images were linearly co-registered to each participant’s anatomical T1-weighted image with FLIRT, and then non-linearly normalized to MNI152 (2mm) template space with FNIRT. Finally, we extracted motion parameters along six dimensions (3 rotations and 3 translations). T1-weighted anatomical images were individually segmented into gray matter, white matter and CSF for each participant.
To ensure that group differences were not driven by motion confounds, especially given the impact of motion confounds on functional connectivity (see 72, 73), we ran a separate analysis to test for group differences along the six motion parameters. To do so, we ran separate multi-level models regressing diagnostic group onto each of the six motion parameters, nesting each motion parameter estimates within run, and nesting runs within participants.
For each participant, we computed the values for each individual trial by regressing the gamma-convolved model of a given trial against the activation of each voxel in the brain using the general linear model, and the individual modulation option in AFNI (3dDeconvolve, -stim_times_IM; see 74 for a similar strategy). The six motion parameters were entered as nuisance covariates to further control for motion confounds. The served as the first stage GLM in our beta-series correlation approach to functional connectivity, which has been shown to be a high-powered alternative to psychophysiological interaction methods (75) for event-related task designs (73, 76). Specifically, this approach involves deriving beta estimates for each trial, which serves as a data reduction step. This step allows for the data to be modeled in a single mixed-effects model, parsing trial-level (e.g. task condition) and individual-level (e.g. diagnostic label) variance and thereby maximizing statistical power.
To compute seed-based beta-series correlation estimates (i.e. functional connectivity), we delineated anatomically-based masks of the left and right VS from the Harvard-Oxford Subcortical Structural Atlas included in FSL (See Figure 2). The mean beta values across trials for voxels within the right and left VS regions were calculated for each seed, and were then entered in a whole-brain voxel-wise two-level multi-level model which predicted the beta series for every voxel in the brain from the interaction term between the mean beta series of each seed, the task outcome (i.e. win, no-win, neutral), and the diagnostic group of the individual (see 77 for a similar beta-series correlation approach). These fixed effects were nested within each participant by estimating a random intercept for each participant, using an unstructured covariance matrix and the between-within method of estimating degrees of freedom. The mean beta-values corresponding to white matter and CSF were extracted from the participant-specific white matter and CSF masks, and entered as nuisance covariates in this multi-level model.
Figure 2.
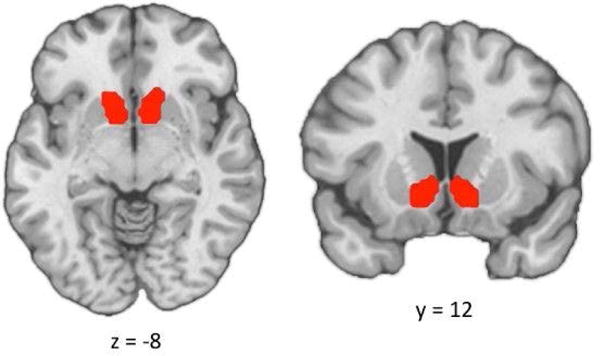
Ventral striatum region-of-interest (ROI). ROI was defined using the Nucleus Accumbens mask from the Harvard-Oxford Subcortical Structural Atlas.
Multiple Comparisons Correction
F-maps of the three-way interactions were then submitted to cluster-wise multiple comparisons correction to keep FWE > 0.05. For each of the two seeds, the residual standard deviation map from the multilevel connectivity analysis was used to estimate the spatial auto-correlation function in AFNI (3dFWHMx –acf; 74); these parameters were then used to estimate minimum cluster sizes (Supplementary Table 1). The F-maps were thresholded with a CDT of p<0.001 (see 78), and resulting clusters smaller than the minimum cluster sizes were removed.
Results
Participant Characteristics
As shown in Table 1, groups did not differ in age, gender, ethnicity, employment status, or years of education. Groups also did not differ on subthreshold mania symptoms. The BD group did score significantly higher on subthreshold depression symptoms, though both groups scored well below clinical thresholds. As expected, the BD group scored significantly lower on global functioning than the HC group. Groups did not differ on MMSE or WAIS-IV Letter Number Sequencing Task scores. Groups did not differ along any of the six motion parameters, which included the x-rotation [F(1,47)=0.006, p=0.940], y-rotation [F(1,47)=0.723, p=0.400], z-rotation [F(1,47)=1.855, p=0.180], x-translation [F(1,47)=2.798, p=0.101], y-translation [F(1,47)=0.205, p=0.653], and z-translation [F(1,47)=0.132, p= 0.719] (See Supplementary Figure 1).
Whole Brain Seed-Based Functional Connectivity Analysis
Results revealed significant three-way interactions between VS beta estimates, task outcome, and group for both the left and right VS seeds, though the patterns differed. Because the spatial boundaries of these resulting clusters extended across multiple anatomical boundaries, we masked the significant voxels within this cluster that overlapped with anatomical defined by the H-O probabilistic atlas, with thresholds set at 0.25. For the left VS seed, significant three-way interactions were found in voxels within the bilateral striatum, anterior fusiform gyrus, posterior inferior temporal, anterior parahippocampal gyri, and OFC, the left amygdala, and the right lingual gyrus and anterior insula. For the right VS seed, significant three-way interactions were found in voxels within the right striatum, bilateral posterior fusiform gyri and posterior inferior temporal gyrus, left amygdala, right lingual gyrus, and medial frontopolar cortex (mFPC) (See Figure 3 and Table 2). Furthermore, the whole-brain functional connectivity clusters were nearly identical for both the left and right VS-seeded analyses when including subthreshold depression symptoms (IDS-C scores), presence vs. absence of antipsychotic medication, and presence vs. absence of comorbid anxiety as covariates.1 Presence vs. absence of antipsychotic medication was included as a covariate given existing evidence that this type of medication may influence neural reward processing (79).
Figure 3.
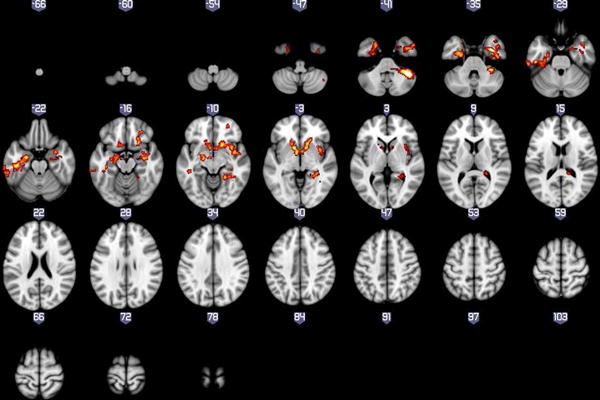
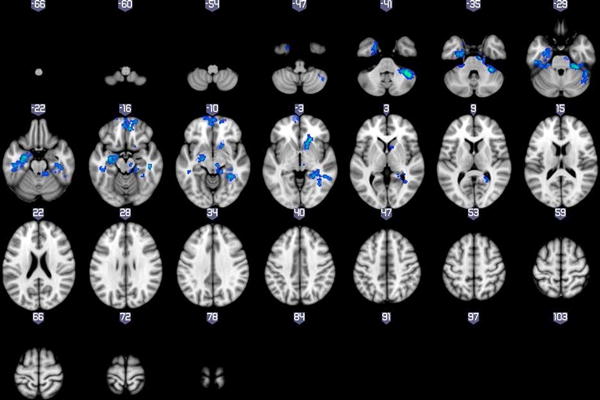
Whole brain results showing significant three-way interactions between diagnosis, task condition, and the Left(A) and Right (B) VS seeds.
Table 2.
Significant Three-Way Interaction Clusters
| Peak Coordinates | ||||||
|---|---|---|---|---|---|---|
| Region | Laterality | x | y | z | Brodmann Area | Cluster Size |
| Right VS Seed | ||||||
| Post. Temporal Fusiform Gyrus | L | −44 | −24 | −24 | 20 | 150 |
| Post. Temporal Fusiform Gyrus | R | 28 | −34 | −26 | N/A | 130 |
| Post. Inferior Temporal Gyrus | L | −46 | −26 | −26 | 20 | 103 |
| Post. Inferior Temporal Gyrus | R | 42 | −30 | −26 | 37 | 37 |
| Amygdala | L | −20 | −12 | −12 | N/A | 214 |
| Lingual Gyrus | R | 32 | −42 | −6 | 36 | 73 |
| Med. Frontopolar Cortex | – | 8 | 46 | −16 | 11 | 379 |
| Ventral Striatum | R | 12 | 12 | −6 | 48 | 30 |
| Left VS Seed | ||||||
| Ant. Temporal Fusiform Gyrus1 | L | −26 | −10 | −40 | 36 | 81 |
| Ant. Temporal Fusiform Gyrus | R | 36 | −4 | −34 | 20 | 100 |
| Post. Inferior Temporal gyrus | L | −46 | −26 | −26 | 20 | 263 |
| Post. Inferior Temporal gyrus | R | 44 | −16 | −36 | 20 | 24 |
| Ant. Insula | R | 42 | 2 | −8 | 13 | 215 |
| Orbitofrontal Cortex | L | −12 | 16 | −16 | 11 | 19 |
| Orbitofrontal Cortex | R | 24 | 20 | −16 | N/A | 76 |
| Ant. Parahippocampal Gyrus | L | −26 | −12 | −32 | 36 | 192 |
| Ant. Parahippocampal Gyrus | R | 30 | 0 | −34 | 36 | 38 |
| Amygdala | L | −18 | −12 | −12 | N/A | 131 |
| Lingual Gyrus | R | 30 | −40 | −6 | 36 | 97 |
| Ventral Striatum | L | −12 | 10 | −6 | N/A | 50 |
| Ventral Striatum1 | R | 12 | 12 | −6 | 48 | 58 |
Note: Peak coordinates for main clusters (≥100 voxels) are reported in Montreal Neurological Institute (MNI) space (x, y, z). Results are cluster corrected at p<0.05. Brodmann Areas indicated correspond to the peak coordinates for each cluster.
These regions were no longer significant after clinical covariates (subthreshold depression symptoms, presence vs. absence of antipsychotic medication, and presence vs. absence of comorbid anxiety) were entered into the regression model.
Given our a priori interest and the reward-based paradigm employed, we sought to explore the nature of the three-way interactions between the VS and regions that comprise a well-known corticolimbic reward circuit: the left amygdala (for both left and right VS seeds), left and right OFC (for the left VS seed only), and mFPC (for the right VS seed only). To this end, we examined group differences in VS connectivity within each condition by probing the simple effects of the three-way interaction (between VS, diagnosis group, and task condition) from the omnibus multi-level model described above, separately for the three target regions.
Left VS Seed
Results indicated that for left VS, significant three-way interactions were found for both the left OFC [F(2,8689)=12.883, p<0.0001] and right OFC [F(2,8689)=19.4802, p<0.0001]. For the left OFC analysis, simple effect tests showed differences between the bipolar and control groups in left VS – left OFC connectivity in the win and no-win conditions, with stronger connectivity in the bipolar group, but not in the neutral condition (Supplementary Figure 2A). For the right OFC analysis, groups differed in left VS – right OFC connectivity in the neutral condition (with HC showing greater connectivity) and the no-win condition (with the BD group showing greater connectivity), but not in the win condition (Supplementary Figure 2B). We also found significant three-way interactions between the left VS and the left amygdala [F(2,8689)=18.378, p<0.0001]. Group differences in left VS – left amygdala connectivity were found only in the win condition, with the BD group demonstrating greater positive connectivity (Supplementary Figure 2C).
In order to examine the extent to which the findings may be driven primarily by outliers in the data, we repeated the analyses excluding trials in which beta coefficients estimated from the single-trial deconvolution were greater or less than three standard deviations from the mean. We continued to find significant three-way interactions in the omnibus ANOVA for the left OFC [F(2,8183)=8.820, p=0.0001], right OFC [F(2,8183)=4.063, p=0.017], and left amygdala [F(2,8183)=7.158, p=0.0008]. However, when we probed the simple effects, the only group differences that remained were in the left VS – left OFC connectivity in the win condition (Figure 4a) and in left VS – left amygdala connectivity in the win condition (Figure 4c), with the BD group demonstrating greater connectivity strength in both.
Figure 4.
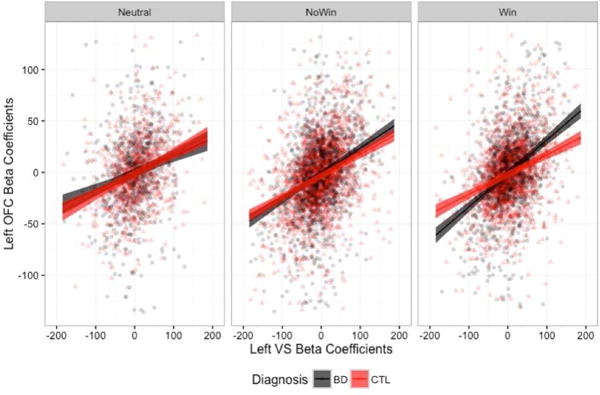
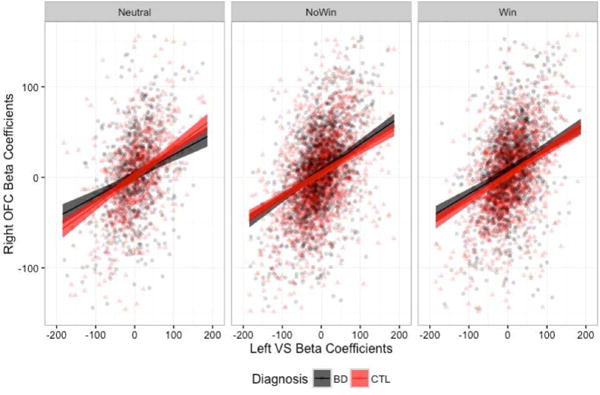
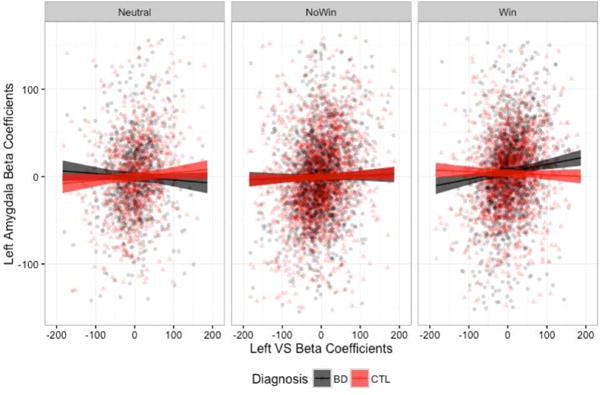
Left VS connectivity slopes to the left OFC (A), right OFC (B), and left amygdala (C), when beta estimates +/− 3 standard deviations from the mean were removed.
Right VS Seed
For the right VS seed, we found significant three-way interactions in the mFPC [F(2,8689)=15.185, p<0.001] and the left amygdala [F(2, 8689)=24.561, p<0.0001]. Probing the simple effects revealed that differences in right VS– mFPC connectivity between groups in the neutral and no-win, but not win, conditions, with the HC group demonstrating greater positive slopes for both (Supplementary Figure 3a). We also found differences in right VS – left amygdala connectivity in the win condition only, with stronger connectivity slopes in the BD group, similar to the results for the left VS – left amygdala described above (Supplementary Figure 3b).
Again, we examined these results after excluding beta estimate outliers (+/− 3 SD from the mean). We continued to find significant three-way interactions in the omnibus ANOVA for both the mFPC [F(2,8334)=9.011, p=0.0001] and left amygdala [F(2, 8334)=13.221, p<0.0001]. When we probed the simple effects, the only group differences that remained for the right VS – mFPC connectivity were in the no-win, with the stronger connectivity slope in the HC group (Figure 5a). Results for the right VS – left amygdala connectivity, with the BD group showing a stronger connectivity slope only in the win condition, remained robust (Figure 5b). All proceeding analyses were conducted using the data with outliers removed.2
Figure 5.
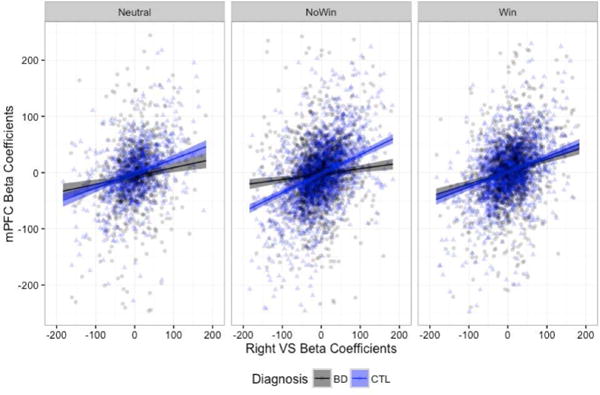
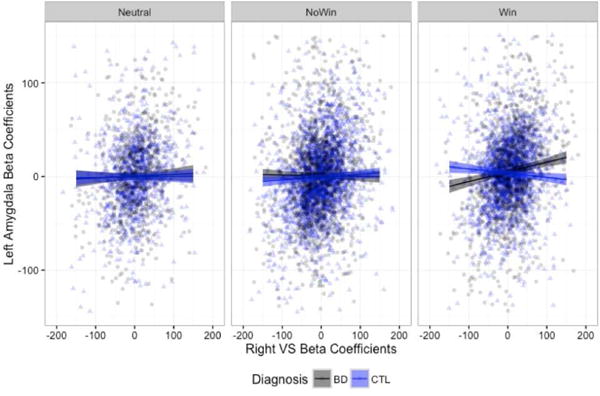
Right VS connectivity slopes to the mPFC (A) and left amygdala (B), when beta estimates +/− 3 standard deviations from the mean were removed.
Frontostriatal Connectivity and Clinical Status
In the next stage of the analysis, we sought to build on our initial findings to better understand their clinical implications within the BD group. First, we predicted that OFC-VS connectivity during win outcomes would be associated with clinical measures of mania recency (i.e., time since last manic episode) as well as mania risk. This hypothesis was based on existing research documenting associations between OFC-VS connectivity and persistence (44), and clinical observations of persistent reward pursuit in individuals with BD (50). Conversely, we predicted that mFPC-VS connectivity during no-win outcomes would be negatively associated with mania recency and risk. This hypothesis was based on evidence for the role of mFPC-striatum connectivity in facilitating change in reward-pursuit strategy (46–49), and the clinical deficits in the ability to shift goal-pursuit strategies observed in individuals with and at risk for mania (50). This mechanism could help to explain failure to ‘put on the brakes’ or re-evaluate reward pursuit strategies even in the context of environmental cues to do so. Finally, we hypothesized that amygdala-VS connectivity during win outcomes would be positively associated with mania recency and risk. This hypothesis was based on the role of the afferent projections from the amygdala to the VS in facilitating reward seeking (52, 53), and the pathologically elevated reward-seeking behavior observed clinically among individuals at risk for BD (58).
We tested these hypotheses by employing a moderation approach that probed how VS activity interacted with each of 4 individual difference variables to predict the mFPC (right VS seed), left OFC (left VS seed), and left amygdala (right and left VS seed). We examined four key individual difference variables related to mania and reward sensitivity, including (1) the Young Mania Rating Scale (YMRS; 62) which assessed subthreshold mania symptoms on the day of the scan; (2) number of months since the most recent manic episode; (3) quality of the most recent mood episode (mania vs. depression), and (4) scores on the Reward Responsiveness subscale of the Behavioral Activation Scale (BAS-RR; 68).
First, we probed whether the four clinical variables interacted with the left VS to predict left OFC beta values. We found significant moderation effects with YMRS ratings [F(1,1554)=9.375, p=0.0022], and positive coefficients (β = 0.05) indicated that greater subthreshold mania symptoms were associated with increased connectivity during the reward outcomes. Moderation effects for the remaining individual difference measures were not significant.
For the right VS – mFPC connectivity, we found significant interactions with YMRS [F(1, 1832)= 5.64, p=0.018] and months since the most recent manic episode [F(1, 1611)= 9.535, p=0.002] only. Negative coefficients (β = −0.095) indicated that greater subthreshold mania symptoms were associated with more attenuated right VS – mFPC connectivity during reward omission. Similarly, as the temporal proximity of the most recent manic episode decreased, this attenuation of the right VS – mFPC connectivity in reward omission decreased as well (β = 0.003), with the right VS – mFPC connectivity slopes more closely resembling that of the HC group.2
For the left VS – left amygdala connectivity, we found significant moderation effects with the number of months since the most recent manic episode [F(1,1350)=12.392, p=0.0004], and the nature of the most recent mood episode [F(1, 1350)=7.748, p=0.0055]. Examining the coefficients showed that months since mania weakly negatively predicted left VS – left Amygdala connectivity (β = −0.003), but this connectivity was stronger if the most recent episode was mania, rather than depression (β = −0.172). We found the same pattern for the right VS – left amygdala connectivity: months since most recent manic episode [β =−0.002, F(1,1350)= 9.627, p=0.0020]; most recent mood episode type [β=−0.135, F(1,1350)= 4.852, p=0.028].
Discussion
The goal of the current study was to identify and characterize patterns of functional connectivity during reward processing in BD, and explore relationships between these patterns and reward-related symptoms. By elucidating the patterns of communication between neural regions that may contribute to persistent reward seeking in BD, we sought to contribute to a broader understanding of the pathophysiology of BD. Three main findings emerged from the present study. First, we identified elevated left VS – left OFC functional connectivity to reward outcomes in the BD group, and found that within the BD group elevated VS-OFC functional connectivity was associated with greater subthreshold mania on the day of the scan. Second, we found reduced right VS- mFPC functional connectivity in response to omission of expected rewards in BD, and found that within the BD group, this reduction was associated with more recent mania and higher levels of subthreshold mania on the day of the scan. Finally, we found enhanced bilateral VS – left amygdala functional connectivity in response to reward outcomes in BD, and within this group enhanced VS – left amygdala connectivity was associated with less recent mania. Below, we discuss each of these findings in turn, and their implications for understanding the pathophysiology of BD.
First, as predicted, we found elevated left VS – left OFC functional connectivity in the BD group in response to reward outcomes. We interpreted this finding as a possible mechanism by which incentive motivation and reward sensitivity are elevated in BD. This interpretation is consistent with previous work documenting the role of a ventral striatal-orbitofrontal circuit in motivation and reward learning, including tracking the reward value of reinforcers and regulating appetitive motivation (37–41). Also consistent with this interpretation, we found that elevated VS-OFC connectivity to rewards in BD was associated with greater subthreshold mania on the day of the scan. Together, these findings suggest that enhanced VS-OFC connectivity in response to rewards may contribute to the pathologically persistent reward pursuit observed clinically in BD (6–9).
Second, also consistent with our hypotheses, we found that right VS – mFPC connectivity was blunted in the BD group when an expected reward was omitted (no-win outcomes). This finding emerged in the context of previous work demonstrating FPC – striatum functional connectivity during set shifting (43), and the role of the FPC in tracking alternative courses of future action (46, 47). As such, decreased connectivity between the mFPC and VS when an expected reward is omitted may be one mechanism by which individuals with BD fail to re-evaluate reward pursuit or change strategies in response to environmental cues to do so. If alternative courses of action are not considered in response to environmental cues during reward pursuit, this could contribute to the persistent reward seeking behavior that has been observed in BD at the trait level (5, 50, 58), and particularly during manic episodes (8, 80). This pattern of reduced functional connectivity is also consistent with previous work showing that decreased activation in frontopolar regions of the PFC are related to deficits in inhibiting behavioral responses (49). Consistent with this interpretation, blunted mFPC -VS connectivity after no-win outcomes was associated with more recent mania and higher levels of subthreshold mania on the day of the scan in the BD group. Together, these findings suggest that disrupted mFPC -VS connectivity to omitted rewards may be a mechanism by which individuals with BD fail to consider behavioral alternatives when reward pursuit is unsuccessful. In turn, this may contribute to the persistent reward pursuit characteristic of BD and mania risk.
Finally, we found elevated bilateral VS-amygdala functional connectivity to reward outcomes in the BD group. This finding was interpreted in light of previous work documenting the role of afferent projections from the amygdala to the VS in facilitating reward seeking and reward-based learning (51–54). In this context, we initially interpreted this finding as a possible mechanism underlying elevated reward seeking in BD. However, our follow-up analyses indicated that VS-amygdala functional connectivity was negatively associated with mania recently. While this association was relatively weak, it does not support our initial interpretation of this finding. Although this finding was surprising, one alternative interpretation is that the elevated VS-amygdala functional connectivity observed in this group was related to a more general deficit in modulation of amygdala responding to affective stimuli observed previously in BD (81). If this pattern is representative of a more trait-like feature of BD, rather than a characteristic of BD or mania risk, this could explain the prominence of this pattern particularly when mania is less recent.
Taken together, the results of the present study have highlighted three possible mechanisms within a corticolimbic reward circuit that may contribute to the clinical patterns of elevated reward sensitivity, persistent reward pursuit, and difficulty downregulating approach motivation in BD. Future studies could build on these findings by exploring trial-by-trial changes in reward predictions and incentive motivation during a reward processing task. If, for example, elevated OFC-VS functional connectivity after one reward receipt predicted faster reaction times to reward cues on a subsequent trial, this would be consistent with a role for this mechanism in facilitating pathologically persistent reward pursuit in BD. In addition, it will be important to explore associations between striatal connectivity and dysfunctions in reward-based learning, as well as predictions about the likelihood of obtaining short- and long-term future rewards. Finally, future studies should examine prospectively whether disrupted striatal functional connectivity predicts changes in symptoms or behavior for individuals with BD.
These results should be interpreted within the confines of several caveats. First, our sample size was relatively small in our final data analysis. While this sample size is consistent with, or larger than, the majority of neuroimaging studies of individuals with severe psychopathology including adults diagnosed with BD (e.g., 15, 82), direct replication in future studies with larger sample sizes will help to ensure the generalizability of these results. Second, our BD sample was taking a variety of medications at the time of testing as is common among this population thus assuring the ecological validity of the sample (83). Although our main results remained consistent when taking into account use of antipsychotic medications, future studies should aim to recruit samples of individuals with BD on specific subclasses of medication. Third, in the present study, the 5mm motion-artifact threshold allowed us to retain the maximal number of participants with usable data (after discarding four BD and two HC participants for excessive motion beyond our 5mm threshold). Future studies could use more restrictive motion thresholds in order to reduce noise and improve spatial specificity of findings. Finally, without a clinical comparison group of individuals with abnormal reward processing (e.g., remitted major depressive disorder or pathological gambling), we cannot state with absolute certainty that these results are specific to BD versus transdiagnostic across other disorders of aberrant reward processing. To build on these findings, future studies should employ this or similar paradigms across mood states among individuals with BD and in comparison with other psychiatric disorders characterized by dysfunctional reward processing.
Supplementary Material
Acknowledgments
This study was supported by a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation (JG), CTSA Grant Number UL1 RR024139 from the National Center for Research Resources (NCRR) and the National Center for Advancing Translational Science (NCATS), NIH roadmap for Medical Research (JG and HK), grant K12-DA00167 from the National Institute of Drug Abuse (HK), and by grant CIHR-111257 from the Canadian Institutes of Health Research (WC). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH or CIHR. This study was also supported by a grant from the American Psychological Foundation (SD) and Sigma Xi Grants-in-Aid of Research (SD). We thank John Purcell, Franziska Goer, Cameron DeLeone, Maggie Mae Mell, Amanda Purcell, and Elizabeth Reeves for their assistance with data collection and scoring.
Appendix 1. Positive Qualities Questionnaire
| Instructions: This questionnaire will ask you to describe some positive events in your life, as well as some positive personal qualities and beliefs. Please respond to each question thoughtfully and honestly, with roughly 2–3 sentences in the space provided. | |
| 1. | Describe something you are very good at. |
| 2. | Describe a quality that makes you unique. |
| 3. | Name someone you admire, and describe the qualities you admire most about them. |
| 4. | Describe a time when you felt love for someone else. |
| 5. | Name some values that you believe are very important, and describe why they are important to you. |
| 6. | Describe something you hope to achieve in the future. |
| 7. | Describe a time when you felt hopeful about the future. |
| 8. | Describe one of your greatest accomplishments. |
| 9. | Describe a time when you felt content or at ease. |
| 10. | If we asked your friends and family about your best qualities, what might they say? |
Note. Each question was presented with an open-ended response box below so the participant could provide a response.
Footnotes
DR. SUNNY J DUTRA (Orcid ID: 0000-0003-2956-5913)
With the exception of the right ventral striatum cluster and left anterior temporal fusiform gyrus cluster results for the right ventral striatum seed. These clusters were no longer significant after clinical covariates were entered into the regression model.
To evaluate the extent to which outlier trials may or may not be clinically meaningful, we examined demographic and clinical variables in relation to participants’ proportion of outlier trials. Each participant’s percentage of trials +/− 3 SDs from the mean was calculated separately for right and left VS seeds. One-way ANOVAs revealed that groups did not differ in the percentage of outlier trials for right (p=0.69) or left (p=0.75) VS seeds. Percentage of outlier trials did not correlate with age within the BD (Right VS r=0.21, p=0.32, Left VS r=−0.24, p=0.27) or HC (Right VS r=−0.24, p=0.25, Left VS r=−0.35, p=0.09) groups. In addition, one-way ANOVAs revealed that males and females did not differ in the percentage of outlier trials within the BD (Right VS p=0.36; Left VS p=0.24) or HC (Right VS p=0.32; Left VS p=0.32) groups. Within the BD group, percentage of outlier trials was not correlated with subthreshold mania (YMRS; Right VS r=−0.04, p=0.85, Left VS r=−0.04, p=0.87) or subthreshold depression symptoms (IDS-C; Right VS r=−0.10, p=0.64; Left VS r=−0.08, p=0.71) on the day of testing.
In order to examine whether outlier trials may be related to motion in the scanner, we calculated each participant’s maximum motion in mm across the 8 task runs, and examined the correlation between participants’ average maximum motion and percentage of outlier trials. Correlations were highly significant across both hemispheres within the BD group (Right VS r=0.59, p=0.003; Left VS r=0.64, p=0.001) and the HC group (Right VS r=0.46, p=0.02; Left VS r=0.56, p=0.004), suggesting that outlier FC beta values are likely attributable to artifact related to excess motion in the scanner.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 2.Tondo L, Isacsson G, Baldessarini R. Suicidal behavior in bipolar disorder: risk and prevention. CNS Drugs. 2003;17:491–511. doi: 10.2165/00023210-200317070-00003. [DOI] [PubMed] [Google Scholar]
- 3.Woods SW. The economic burden of bipolar disease. J Clin Psychiatry. 2000;61(Supp 13):31–41. [PubMed] [Google Scholar]
- 4.Alloy LB, Abramson LY, Walshaw PD, Cogswell A, Smith JM, Neeren AM, et al. Behavioral Approach System (BAS) sensitivity and bipolar spectrum disorders: A retrospective and concurrent behavioral high-risk design. Motiv Emotion. 2006;30:143–155. [Google Scholar]
- 5.Alloy LB, Abramson LY. The role of the Behavioral Approach System (BAS) in bipolar spectrum disorders. Curr Dir Psychol. 2010;19:189–194. doi: 10.1177/0963721410370292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gruber J. A review and synthesis of positive emotion and reward disturbance in bipolar disorder. Clin Psychol Psychother. 2011;18:356–365. doi: 10.1002/cpp.776. [DOI] [PubMed] [Google Scholar]
- 7.Gruber J. Can feeling too good be bad? Positive emotion persistence (PEP) in bipolar disorder. Curr Dir Psychol. 2011;20:217–221. [Google Scholar]
- 8.Johnson SL. Mania and dysregulation in goal pursuit: a review. Clin Psychol Rev. 2005;25:241–262. doi: 10.1016/j.cpr.2004.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alloy LB, Abramson LY, Walshaw PD, Cogswell A, Grandin LD, Hughes ME, et al. Behavioral approach system and behavioral inhibition system sensitivities and bipolar spectrum disorders: Prospective prediction of bipolar mood episodes. Bipolar Disord. 2008;10:310–322. doi: 10.1111/j.1399-5618.2007.00547.x. [DOI] [PubMed] [Google Scholar]
- 10.Meyer B, Johnson SL, Winters R. Responsiveness to threat and incentive in bipolar disorder: Relations of the BIS/BAS scales with symptoms. J Psychopathol Behav. 2001;23:133–143. doi: 10.1023/A:1010929402770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abler B, Erk S, Walter H. Human reward system activation is modulated by a single dose of olanzapine in healthy subjects in an event-related, double-blind, placebo-controlled fMRI study. Psychopharmacology. 2007;191:823–833. doi: 10.1007/s00213-006-0690-y. [DOI] [PubMed] [Google Scholar]
- 12.Caseras X, Lawrence NS, Murphy K, Wise RG, Phillips ML. Ventral striatum activity in response to reward: Differences between bipolar I and II disorders. Am J Psychiatry. 2013;170:533–541. doi: 10.1176/appi.ajp.2012.12020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dutra S, Cunningham WA, Kober H, Gruber J. Elevated striatal reactivity across monetary and social rewards in bipolar I disorder. J Abnorm Psychol. 2015;124:890–904. doi: 10.1037/abn0000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hassel S, Almeida JR, Kerr N, Nau S, Ladouceur CD, Fissell K, et al. Elevated striatal and decreased dorsolateral prefrontal cortical activity in response to emotional stimuli in euthymic bipolar disorder: no associations with psychotropic medication load. Bipolar Disord. 2008;10:916–927. doi: 10.1111/j.1399-5618.2008.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nusslock R, Almeida JR, Forbes EE, Versace A, Frank E, Labarbara EJ, et al. Waiting to win: Elevated striatal and orbitofrontal cortical activity during reward anticipation in euthymic bipolar disorder adults. Bipolar Disord. 2012;14:249–260. doi: 10.1111/j.1399-5618.2012.01012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bermpohl F, Kahnt T, Dalanay U, Hagele C, Sajonz B, Wegner T, et al. Altered representation of expected value in the orbitofrontal cortex in mania. Hum Brain Mapp. 2010;31:958–969. doi: 10.1002/hbm.20909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Linke J, King AV, Rietschel M, Strohmaier J, Hennerici M, Gass A, et al. Increased medial orbitofrontal and amygdala activation: Evidence for a systems-level endophenotype of bipolar disorder. Am J Psychiatry. 2012;169:316–325. doi: 10.1176/appi.ajp.2011.11050711. [DOI] [PubMed] [Google Scholar]
- 18.Bressler SL. Large-scale cortical networks and cognition. Brain Res Rev. 1995;20:288–304. doi: 10.1016/0165-0173(94)00016-i. [DOI] [PubMed] [Google Scholar]
- 19.Fuster JM. The module: Crisis of a paradigm. Neuron. 2000;26:51–53. [Google Scholar]
- 20.Haber SN, Kim K-S, Mailly P, Calzavara R. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. J Neurosci. 2006;26:8368–8376. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haber SN, Knutson B. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McIntosh AR. Towards a network theory of cognition. Neural Net. 2000;13:861–870. doi: 10.1016/s0893-6080(00)00059-9. [DOI] [PubMed] [Google Scholar]
- 23.Sporns O. Structure and function of complex brain networks. Dialogues Clin Neurosci. 2013;15:247–262. doi: 10.31887/DCNS.2013.15.3/osporns. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hasler G, Drevets WC, Gould TD, Gottesman II, Manji HK. Toward constructing an endophenotype strategy for bipolar disorders. Biol Psychiatry. 2006;60:93–105. doi: 10.1016/j.biopsych.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Alloy LB, Abramson LY, Urosevic S, Bender RE, Wagner CA. Longitudinal predictors of bipolar spectrum disorders: A behavioral approach system (BAS) perspective. Clin Psychol. 2009;16:206–226. doi: 10.1111/j.1468-2850.2009.01160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson SL, Ruggero CJ, Carver CS. Cognitive, behavioral, and affective responses to reward: Links with hypomanic symptoms. J Soc Clin Psychol. 2005;24:894–906. [Google Scholar]
- 27.Meyer TD, Baur M. Positive and negative affect in individuals at high and low risk for bipolar disorders. J Ind Diff. 2009;30:169–175. [Google Scholar]
- 28.Meyer B, Johnson SL, Carver CS. Exploring behavioral activation and inhibition sensitivities among college students at risk for bipolar spectrum symptomatology. J Psychopathol Behav. 1999;21:275–292. doi: 10.1023/A:1022119414440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edge MD, Miller CJ, Muhtadie L, Johnson SL, Carver CS, Marquinez N, et al. People with bipolar I disorder report avoiding rewarding activities and dampening positive emotion. J Affect Disord. 2013;146:407–413. doi: 10.1016/j.jad.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson SL, Sandrow D, Meyer B, Winters R, Miller I, Solomon D, et al. Increases in manic symptoms after life events involving goal attainment. J Abnorm Psychol. 2000;109:721–727. doi: 10.1037//0021-843x.109.4.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 32.Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- 33.Frank MJ. Computational models of motivated action selection in corticostriatal circuits. Curr Opin Neurobiol. 2011;21:381–386. doi: 10.1016/j.conb.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 34.Groenewegen HJ, Trimble M. The ventral striatum as an interface between the limbic and motor systems. CNS Spectrums. 2007;12:887–892. doi: 10.1017/s1092852900015650. [DOI] [PubMed] [Google Scholar]
- 35.Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: A unifying interpretation with special reference to reward-seeking. Brain Res Rev. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- 36.Knutson B, Bhanji JP, Cooney RE, Atlas LY, Gotlib IH. Neural responses to monetary incentives in major depression. Biol Psychiatry. 2008;63:686–692. doi: 10.1016/j.biopsych.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: Evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72:341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 38.Richard JM, Berridge KC. Prefrontal cortex modulates desire and dread generated by nucleus accumbens glutamate disruption. Biol Psychiatry. 2012;73:360–370. doi: 10.1016/j.biopsych.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gottfried JA, O’Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;5636:1104–1107. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- 40.Arnsten AF, Rubia K. Neurobiological circuits regulating attention, cognitive control, motivation, and emotion: disruptions in neurodevelopmental psychiatric disorders. J Am Acad Child Adolesc Psychiatry. 2012;51:356–367. doi: 10.1016/j.jaac.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 41.Frank MJ, Claus ED. Anatomy of a decision: striato-orbitofrontal interactions in reinforcement learning, decision making, and reversal. Psychol Rev. 2006;113:300–326. doi: 10.1037/0033-295X.113.2.300. [DOI] [PubMed] [Google Scholar]
- 42.Jung Y-C, Ku J, Namkoong K, Lee W, Kim SI, Kim J-J. Human orbitofrontal-striatum functional connectivity modulates behavioral persistence. Neuroreport. 2010;21:502–506. doi: 10.1097/WNR.0b013e3283383482. [DOI] [PubMed] [Google Scholar]
- 43.Nagano-Saito A, Leyton M, Monchi O, Goldberg YK, He Y, Dagher A. Dopamine depletion impairs frontostriatal functional connectivity during a set-shifting task. J Neurosci. 2008;28:3697–3706. doi: 10.1523/JNEUROSCI.3921-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshida W, Ishii S. Resolution of uncertainty in prefrontal cortex. Neuron. 2006;50:781–789. doi: 10.1016/j.neuron.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 45.Boorman ED, Behrens TEJ, Woolrich MW, Rushworth MFS. How green is the grass on the other side? Frontopolar cortex and the evidence in favor of alternative courses of action. Neuron. 2009;62:733–743. doi: 10.1016/j.neuron.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 46.Koechlin E, Hyafil A. Anterior prefrontal function and the limits of human decision manking. Science. 2007;318:594–598. doi: 10.1126/science.1142995. [DOI] [PubMed] [Google Scholar]
- 47.Rushworth MF, Noonan MP, Boorman ED, Walton ME, Behrens TE. Frontal cortex and reward-guided learning and decision-making. Neuron. 2011;70:1054–1069. doi: 10.1016/j.neuron.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 48.Jogia J, Dima D, Kumari V, Frangou S. Frontopolar cortical inefficiency may underpin reward and working memory dysfunction in bipolar disorder. World J Biol Psychiatry. 2012;13:605–615. doi: 10.3109/15622975.2011.585662. [DOI] [PubMed] [Google Scholar]
- 49.Kaladjian A, Jeanningros R, Azorin JM, Nazarian B, Roth M, Anton JL, Mazzola-Pomietto P. Remission from mania is associated with a decrease in amygdala activation during motor response inhibition. Bipolar Disord. 2009;11:530–538. doi: 10.1111/j.1399-5618.2009.00722.x. [DOI] [PubMed] [Google Scholar]
- 50.Fulford D, Johnson SL, Llabre MM, Carver CS. Pushing and coasting in dynamic goal pursuit: coasting is attenuated in bipolar disorder. Psychol Sci. 2010;21:1021–1027. doi: 10.1177/0956797610373372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balleine BW, Killcross S. Parallel incentive processing: an integrated view of amygdala function. Trends Neurosci. 2006;29:272–279. doi: 10.1016/j.tins.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 52.Ambroggi F, Ishikawa A, Fields HL, Nicola SM. Basolateral amygdala neurons facilitate reward-seeking behavior by exciting nucleus accumbens neurons. Neuron. 2008;59:648–661. doi: 10.1016/j.neuron.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stubner GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baxter MG, Murray EA. The amygdala and reward. Nature Rev Neurosci. 2002;3:563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- 55.Cohen MX, Schoene-Bake JC, Elger CE, Weber B. Connectivity-based segregation of the human striatum predicts personality characteristics. Nat Neurosci. 2009;12:32–35. doi: 10.1038/nn.2228. [DOI] [PubMed] [Google Scholar]
- 56.Almeida JRC, Versace A, Hassel S, Kupfer DJ, Phillips ML. Elevated amygdala activity to sad facial expressions: a state marker of bipolar but not unipolar depression. Biol Psychiatry. 2010;67:414–421. doi: 10.1016/j.biopsych.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn Sci. 2012;16:61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 58.Alloy LB, Bender RE, Whitehouse WG, Wagner CA, Liu RT, Grant DA, et al. High Behavioral Approach System (BAS) sensitivity, reward responsiveness, and goal-striving predict first onset of bipolar spectrum disorders: A prospective behavioral high-risk design. J Abnorm Psychol. 2012;121:339–351. doi: 10.1037/a0025877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abler B, Greenhouse I, Ongur D, Walter H, Heckers S. Abnormal reward system activation in mania. Neuropsychopharmacology. 2008;33:2217–2227. doi: 10.1038/sj.npp.1301620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Knutson B, Westdorp A, Kaiser E, Hommer D. fMRI visualization of brain activity during a monetary incentive delay task. NeuroImage. 2000;12:20–27. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- 61.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV axis I disorders-clinician version (SCID-CV) Washington, DC: American Psychiatric Association Press; 1996. [Google Scholar]
- 62.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Brit J Psychiat. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 63.Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): Psychometric properties. Psychol Med. 1996;26:477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- 64.Shrout PE, Fleiss JL. Intraclass correlations: Uses in assessing rater reliability. Psychol Bull. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 65.Bauer MS, McBride L, Shea N, Gavin C, Holden F, Kendall S. Impact of an easy-access VA clinic-based program for patients with bipolar disorder. Psychiatr Serv. 1997;48:491–496. doi: 10.1176/ps.48.4.491. [DOI] [PubMed] [Google Scholar]
- 66.Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 67.Wechsler D. Adult Intelligence Scale. Fourth. San Antonio, TX: Pearson; 2008. [Google Scholar]
- 68.Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. J Pers Soc Psychol. 1994;67:319–333. [Google Scholar]
- 69.Anderson NH. Likableness ratings of 555 personality-trait words. J Pers Soc Psychol. 1968;9:272–279. doi: 10.1037/h0025907. [DOI] [PubMed] [Google Scholar]
- 70.Tohen M, Frank E, Bowden CL, Colom F, Ghaemi SN, Yatham LN, et al. The International Society for Bipolar Disorders (ISBD) Task Force report on the nomenclature of course and outcome in bipolar disorders. Bipolar Disorders. 2009;11:453–473. doi: 10.1111/j.1399-5618.2009.00726.x. [DOI] [PubMed] [Google Scholar]
- 71.Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, et al. Reduced caudate and nucleus accumbens response to rewards in unmedicated subjects with major depressive disorder. Am J Psychiat. 2009;166:702–710. doi: 10.1176/appi.ajp.2008.08081201. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mumford JA, Turner BO, Ashby FG, Poldrack RA. Deconvolving BOLD activation in event-related designs for multivoxel pattern classification analyses. Neuroimage. 2012;59:2636–2643. doi: 10.1016/j.neuroimage.2011.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 76.Cisler JM, Bush K, Steele JS. A comparison of statistical methods for detecting context-modulated functional connectivity in fMRI. Neuroimage. 2014;84:1042–1052. doi: 10.1016/j.neuroimage.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rissman J, Gazzaley A, D’Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage. 2004;23:752–763. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 78.Cox RW, Reynolds RC, Taylor PA. AFNI and clustering: false positive rates redux. BioRxiv. 2016:065862. doi: 10.1089/brain.2016.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abler B, Erk S, Walter H. Human reward system activation is modulated by a single dose of olanzepine in healthy subjects in an event-related, double-blind, placebo-controlled fMRI study. Psychopharmacology. 2007;191:823–833. doi: 10.1007/s00213-006-0690-y. [DOI] [PubMed] [Google Scholar]
- 80.Johnson SL, Edge MD, Holmes MK, Carver CS. The behavioral activation system and mania. Annu Rev Clin Psychol. 2012;8:243–267. doi: 10.1146/annurev-clinpsy-032511-143148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Foland LC, Altshuler LL, Bookheimer SY, Eisenberger N, Townsend J, Thompson PM. Evidence for deficient modulation of amygdala response by prefrontal cortex in bipolar mania. Psychiat Res – Neuroim. 2008;162:27–37. doi: 10.1016/j.pscychresns.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Delvecchio G, Fossati P, Boyer P, Brambilla P, Falkai P, Gruber O, et al. Common and distinct neural correlates of emotional processing in bipolar disorder and major depressive disorder: A voxel-based meta-analysis of functional magnetic resonance imaging studies. Eur Neuropsychopharmacol. 2012;22:100–113. doi: 10.1016/j.euroneuro.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 83.Ghaemi SN, Hsu DJ, Thase ME, Wisniewski SR, Nierenberg AA, Miyahara S, Sachs G. Pharmacological treatment patterns at study entry for the first 500 STEP-BD participants. Psych Serv. 2006;57:660–665. doi: 10.1176/ps.2006.57.5.660. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


