Abstract
Objective
To determine the 6-month follow-up effects after intentional 6-month weight loss alone (WL) and with aerobic exercise (AEX+WL) on body composition, glucose metabolism, and CVD risk factors in older, postmenopausal women and mechanisms for weight regain.
Methods
Women (n=65, BMI>25 kg/m2) underwent VO2max, DXA, CT scans, and OGTT before and after 6-months AEX+WL or WL and at 12-months ad libitum follow-up and insulin sensitivity (M) (hyperinsulinemic-euglycemic clamp) at baseline and 6-months. Thirty WL and 35 AEX+WL women completed 12-month follow-up.
Results
Similar weight loss was observed (−8%) in both groups from 0–6 months. Total fat mass, FFM, visceral fat area, subcutaneous abdominal and mid-thigh fat areas, fasting glucose, insulin, HOMA-IR, insulin AUC, and triglyceride levels decreased similarly after WL and AEX+WL and remained lower at 12-months than at baseline despite weight re-gain at 12-months. Initial M was associated with weight regain (r=−0.40, P<0.01). Weight regain was related to independent changes in leptin and HOMA-IR from 6-to-12 months in a multiple regression model (r=0.77, P<0.0001).
Conclusions
Reductions in body fat and improvements in insulin sensitivity after AEX+WL and WL are maintained at 12-months despite modest weight regain. Baseline insulin resistance partially predicted the magnitude of weight regain in postmenopausal women.
Keywords: Obesity, insulin sensitivity, body composition, postmenopausal women, exercise intervention
Introduction
Overweight and obesity, present in over half of all middle-aged and older women (1), are associated with the development of type 2 diabetes (2). Weight loss of as little as 3% reduces the risk for the development of type 2 diabetes and reductions in obesity-associated cardiovascular risk factors (3). Yet, in adults who participate in weight loss studies, most subjects regain almost half the weight lost within the following two years and return to baseline weight within the next 3–5 years (4, 5, 6). Thus, long-term success in maintaining the reduced weight is a challenge.
Successful weight maintenance is likely multifactorial, dependent on both environmental and psychological factors such as dietary strategies (length of time weight loss is maintained, self-monitoring weight, eating breakfast), physical activity (~one hour/day), and having low levels of depression and disinhibition (7). Other predictors associated with weight maintenance following weight loss have included meal provision, type of diet, genetic factors, resting energy expenditure, and certain hormones (8, 9, 10, 11, 12). Adipose tissue hormones, including leptin, as well as gut hormones and neuropeptides affect appetite and thus, influence energy balance (13, 14). Leptin has beneficial effects on glucose metabolism, by decreasing glycemia, insulinemia and insulin resistance. There is also some evidence that insulin sensitivity predicts weight gain (15, 16), although limited studies (16) have utilized the measurement of insulin sensitivity by the glucose clamp to examine this relationship and others suggest that changes in insulin sensitivity during weight loss do not predict weight regain (17).
Weight regain may be accompanied by deterioration in cardiovascular disease (CVD) risk factors back to baseline (18, 19). Increases in total cholesterol, triglycerides, glucose, insulin, and Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) in postmenopausal women are directly associated with weight regain (20). Given the importance of abdominal fat in cardiovascular risk, it is possible that alterations in central body fat are important in the worsening of metabolic risk associated with weight regain.
We hypothesized that postmenopausal women who initially participate in a 6-month intervention of weight loss combined with aerobic exercise will have less weight regain and better metabolic profiles over a 6-month follow-up than women who initially participate in a weight loss only intervention. The aim of this study was to determine the 6-month follow-up effects after intentional 6-month weight loss alone (WL) and with aerobic exercise (AEX+WL) on body composition, glucose metabolism, and CVD risk factors in older postmenopausal women. We also investigated potential mechanisms for weight regain by examining the role of leptin and insulin sensitivity by hyperinsulinemic-euglycemic clamps.
Methods
Subjects
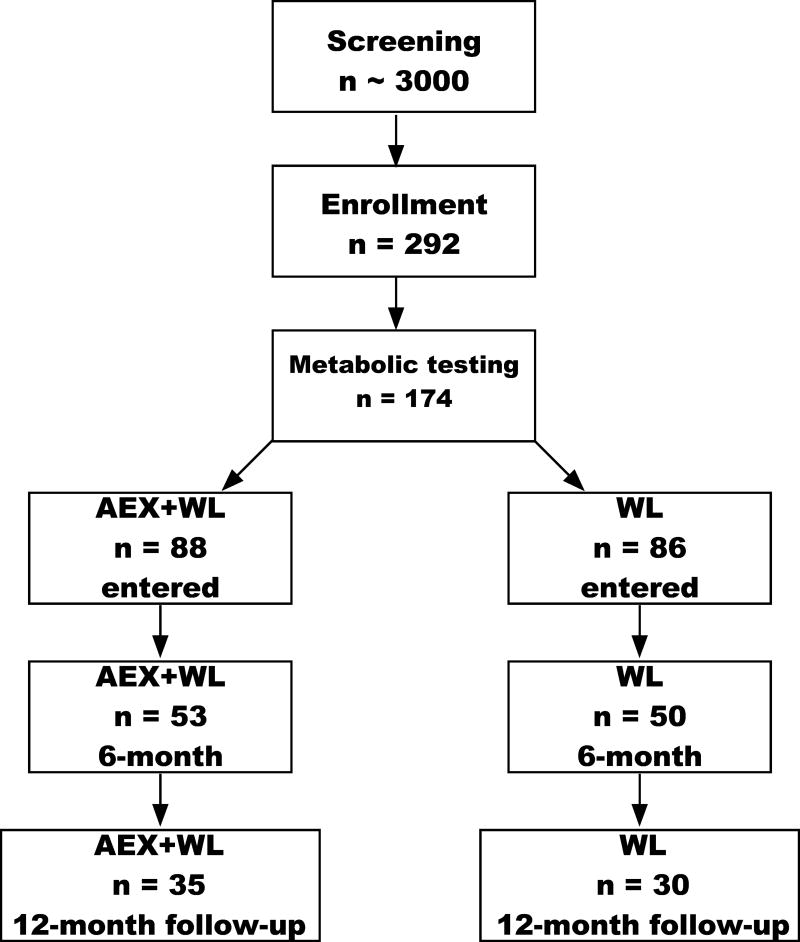
Subjects were African American and Caucasian women with overweight and obesity (BMI >25 kg/m2; range of 25–47 kg/m2) aged 45–76 years from Baltimore/Washington area. Women had not menstruated for at least one year and were weight stable (<2.0 kg weight change in past year) and sedentary (<20 min of aerobic exercise 2x/wk.). Individuals with untreated hypertension or hyperlipidemia were referred to their doctor for evaluation and entered the study if they were treated with an antihypertensive or lipid-lowering drug that did not affect glucose metabolism, were stable on the medications for at least three months, and agreed to remain on the medication throughout the study. Subjects were nonsmokers, showed no evidence of cancer, liver, renal or hematological disease, or other medical disorders and underwent a Bruce graded treadmill test to exclude those with asymptomatic coronary artery disease. A total of 103 women completed the initial study (WL, n=50 and AEX+WL, n=53) (21). Of these women, 65 (n=30 in WL and n=35 in AEX+WL) women (20 African American, 11 WL, 9 AEX+WL and 45 Caucasian, 24 WL, 21 AEX+WL) returned at the one-year mark for testing (Figure 1). Body composition and hyperinsulinemic-euglycemic clamp results were published before and after 6-months AEX+WL and WL (21); however, 12-month follow-up effects were not examined. Data are presented only for those who had some 12-month follow-up testing. Each participant provided written University of Maryland Baltimore IRB-approved informed consent.
Figure 1.
CONSORT diagram
Procedures
Subjects received instruction in maintaining a weight-stable, Therapeutic Lifestyle Changes (TLC) diet (22) (consuming <30% of total calories as total fat, 10% as saturated fat, 300 mg of cholesterol, and 2,400 mg of sodium per day), by a Registered Dietitian (RD) one day/week for 6–8 weeks, prior to initial testing. Subjects were weight stable ±2% on the TLC diet for at least two weeks prior to initial, 6-month, and 12-month testing. AEX+WL subjects had all metabolic tests performed 24–36 hours after the last exercise session.
VO2max and Body Composition
VO2max was measured using a continuous treadmill test protocol (21). Fat mass, lean tissue mass and bone mineral content (fat-free mass= lean+bone) were determined by dual-energy X-ray absorptiometry (Prodigy, LUNAR Radiation Corp., Madison, WI). A single computed tomography (Siemens Somatom Sensation 64 Scanner, Fairfield, CT) scan at L4–L5 region was used to determine visceral and subcutaneous abdominal adipose tissue area, and analyzed using Medical Image Processing, Analysis and Visualization, version 7.0.0 (NIH Center for Information Technology, Bethesda, MD). A second scan of the right mid-thigh was used to quantify intramuscular fat area (low-density lean tissue), subcutaneous fat, and muscle area (21).
Fasting Blood Draw and Oral Glucose Tolerance Test (OGTT)
Blood samples were drawn after a 12 hour fast and at 30-min intervals for 2 h after ingestion of 75g glucose. Plasma glucose concentrations were measured by the glucose oxidase method (2300 STAT Plus, YSI, Yellow Springs, OH) and plasma insulin and leptin by radioimmunoassay (RIA) (Millipore, St. Charles, MO). HOMA-IR was calculated as [(fasting insulin (µU/ml) × fasting glucose [mmol/l])/22.5] (23). All samples were measured in duplicate. Glucose tolerance status (24) was determined for each subject by ADA criteria. Plasma triglyceride and cholesterol levels were averaged from three fasting blood draws analyzed using enzymatic methods (UniCel DxC880i, Beckman Coulter, Inc., Brea, CA) (25, 26) and high-density lipoprotein cholesterol (HDL-C) measured in the supernatant after precipitation with dextran sulfate (low-density lipoprotein cholesterol (LDL-C)=total cholesterol– (TG/5 + HDL-C)) (27).
Indirect Calorimetry
Subjects reported to our lab first thing in the morning after a 12-hr fast. Resting metabolic rate was measured for 30 minutes canopy in a thermo-neutral environment by indirect calorimetry using a SensorMedics DeltaTrac (Yorba Linda, CA). Energy expenditure was calculated by the Weir equation (28).
Hyperinsulinemic-euglycemic Clamps
Subjects were provided meals as a eucaloric diet for two days before the clamp to control nutrient intake. Testing was performed in the morning after a 12-hr fast. Whole-body insulin sensitivity (M) was measured during a 180 min 80 mU˙m−2˙min−1 (HumulinR, Eli Lilly Co., Indianapolis, IN) hyperinsulinemic-euglycemic clamp (29), (30) in 45 women (n=20 WL, n=25 AEX+WL). Subject characteristics were not different between those who underwent the clamp and those who did not (data not shown). M was calculated from the amount of glucose infused after correction for glucose equivalent space (glucose space correction) and expressed per kg FFM. The glucose clamp was conducted before and after the 6-month AEX+WL and WL, but not at the 12-month follow-up.
Interventions
Women in AEX+WL and WL groups attended weekly weight loss classes led by a RD for instruction in the principles of hypocaloric diet (−500 kcal/d) according to TLC guidelines. Weight loss instructions included following the “heart healthy” dietary modification guidelines plus beginning a 250–350 kcal/d hypocaloric diet with a goal ~5–10% weight loss over the study duration. Diets were monitored by 7-day food records using the American Diabetes Association exchange list system. In addition to the weight loss classes, women in AEX+WL also exercised on a treadmill 3x/wk at our facility. Each exercise session included a 5-to-10-min warm-up phase and a 5-to-10-min cool-down phase. Exercise intensity was prescribed to a target heart rate and heart rate was monitored each session using monitors (Polar Electro Inc., Lake Success, NY). Duration of exercise began with 30-min sessions at ~50–60% of heart rate reserve and progressed to >85% for 45–50 min. for the 6-months (21). Average compliance to the exercise sessions was 87% and average compliance to the diet classes was 86% in months 0–6. During the 6-month follow-up period, women were given the option of attending a one-hour, face-to-face, 1x/month group nutrition class focused on the principles of the TLC diet and women were encouraged to continue monitoring intake; however, attendance and food recording were optional. Class topics refreshed participants knowledge, and included the following: Coping with Slips and Binges, Problem Solving, Healthy Habits, Self Talk, and Stress Management. The AEX+WL group could use the exercise facility 3x/wk, for 1-hour if desired where only exercise safety was monitored and not the exercise prescription, or exercise intensity. Women in the AEX+WL group attended ~27% of the exercise sessions and women attended ~29% of the RD sessions/month during the follow-up.
Data Analysis
Statistics were run only on subjects who completed 0, 6, and 12 months evaluations. Some women did not complete all testing at the 12-month evaluation; sample sizes were equal across each timepoint for each variable but not all variables have the same sample size. Statistical analyses were performed using a 2-way ANOVA with AEX+WL and WL as in-between variables, and time as within variable. The Bonferoni post-hoc test was used when the overall effects were significant. Pearson correlations and multiple regressions were used to assess relationships between key variables. Statistical significance was set at a two-tailed P<0.05. Data were analyzed using SPSS (IBM Analytics, Armonk, New York); results are expressed as mean±SEM.
Results
Effects on Body Weight and Composition
Weight and body fat did not differ between groups at baseline (Table 1). There were no group*time or group effect on body weight or body composition by DXA. There was a significant time effect for body weight, BMI, %fat, fat mass, and FFM. Both groups lost ~8% (P<0.0001) body weight at 6-months, regained ~1% and thus maintained a significant loss of 7% at 12 months (P<0.001). Mean weight gain from 6-to-12 months was 1.7±0.6 kg for WL and 1.6±0.4 kg for AEX+WL. The relationship between the change in weight between 0–6 months and the change 6–12 months was not significant (r=0.08, P=0.54), indicating that those that lost the most weight initially were not the same individuals that had the greatest weight regain. The reduction in BMI, % fat, fat mass and FFM with WL and AEX+WL at 6-months remained significant at 12-months (Table 1).
Table 1.
Physical characteristics at baseline, after the 6-month interventions, and at 12 months.
| Baseline | 6 Months | 12 Months | Group × Time |
Overall Group |
Overall Time |
6 vs. 0 | 12 vs. 6 | 12 vs. 0 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||||
| N | mean±SEM | mean±SEM | mean±SEM | P-value | P-value | P-value | P-value | P-value | P-value | ||||||||
| Weight (kg) | WL | 35 | 88 | ± | 3 | 80 | ± | 2 | 82 | ± | 3 | 0.98 | 0.17 | 0.000 | <0.001 | <0.001 | <0.001 |
| AEX+WL | 30 | 83 | ± | 3 | 75 | ± | 2 | 77 | ± | 3 | |||||||
|
| |||||||||||||||||
| BMI (kg/m2) | WL | 35 | 33.2 | ± | 0.9 | 30.3 | ± | 0.9 | 31.0 | ± | 0.9 | 0.96 | 0.23 | 0.000 | <0.001 | <0.001 | <0.001 |
| AEX+WL | 30 | 31.7 | ± | 1.0 | 28.8 | ± | 0.9 | 29.4 | ± | 1.0 | |||||||
|
| |||||||||||||||||
| Total body fat (%) | WL | 28 | 47.1 | ± | 0.8 | 43.8 | ± | 1.2 | 45.0 | ± | 1.1 | 0.39 | 0.61 | 0.000 | <0.001 | <0.001 | <0.001 |
| AEX+WL | 23 | 47.1 | ± | 1.0 | 42.7 | ± | 1.3 | 43.8 | ± | 1.3 | |||||||
|
| |||||||||||||||||
| Fat mass (kg) | WL | 28 | 41.1 | ± | 1.9 | 35.4 | ± | 2.0 | 36.4 | ± | 2.0 | 0.39 | 0.67 | 0.000 | <0.001 | <0.001 | <0.001 |
| AEX+WL | 23 | 40.3 | ± | 2.1 | 33.5 | ± | 2.2 | 35.2 | ± | 2.3 | |||||||
|
| |||||||||||||||||
| FFM (kg) | WL | 28 | 45.3 | ± | 1.2 | 43.8 | ± | 1.1 | 43.4 | ± | 1.2 | 0.13 | 0.78 | 0.000 | <0.001 | <0.001 | <0.001 |
| AEX+WL | 23 | 44.4 | ± | 1.4 | 43.2 | ± | 1.2 | 43.5 | ± | 1.3 | |||||||
|
| |||||||||||||||||
| Mid-thigh muscle area (cm2) | WL | 27 | 72.4 | ± | 2.8 | 69.9 | ± | 2.8 | 70.0 | ± | 3.0 | 0.001 | 0.38 | 0.43 | 0.08 | 0.93 | 0.20 |
| AEX+WL | 20 | 71.1 | ± | 2.8 | 76.2 | ± | 2.7* | 75.7 | ± | 3.3 | <0.01 | 0.99 | <0.05 | ||||
|
| |||||||||||||||||
| Mid-thigh subcutaneous fat area (cm2) | WL | 27 | 157.0 | ± | 8.9 | 131.5 | ± | 8.3 | 139.1 | ± | 8.1 | 0.54 | 0.78 | 0.000 | <0.001 | <0.001 | <0.001 |
| AEX+WL | 20 | 150.2 | ± | 11.0 | 131.0 | ± | 10.1 | 135.6 | ± | 11.0 | |||||||
|
| |||||||||||||||||
| Mid-thigh intramuscular fat area (cm2) | WL | 27 | 20.1 | ± | 1.5 | 19.4 | ± | 1.7 | 20.9 | ± | 1.9 | 0.12 | 0.76 | 0.56 | 0.81 | 1.0 | 1.0 |
| AEX+WL | 20 | 20.1 | ± | 1.4 | 19.6 | ± | 1.5 | 18.6 | ± | 1.5 | |||||||
|
| |||||||||||||||||
| RMR (kcal/d) | WL | 21 | 1472 | ± | 53 | 1388 | ± | 59 | 1446 | ± | 52 | 0.94 | 0.75 | 0.03 | <0.01 | <0.05 | 0.41 |
| AEX+WL | 17 | 1447 | ± | 59 | 1355 | ± | 66 | 1433 | ± | 57 | |||||||
|
| |||||||||||||||||
| VO2max (L/min) | WL | 12 | 1.52 | ± | 0.10 | 1.44 | ± | 0.06 | 1.38 | ± | 0.07 | 0.04 | 0.02 | 0.02 | 0.14 | 0.29 | 0.09 |
| AEX+WL | 17 | 1.80 | ± | 0.11* | 1.95 | ± | 0.15† | 1.65 | ± | 0.08* | <0.05 | <0.01 | <0.05 | ||||
P<0.05,
P<0.001,
AEX+WL vs WL
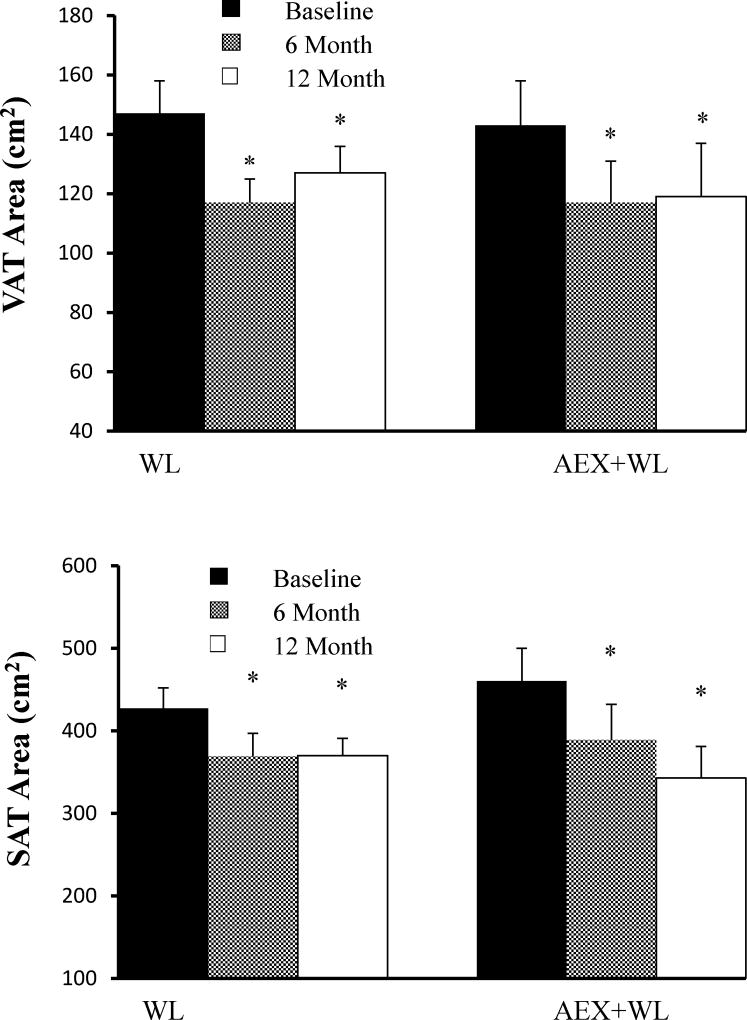
There were no baseline differences between groups. There was a group × time interaction in muscle area (P<0.001) which increased at six months after AEX+WL (P<0.01), did not significantly change from 6-to-12 months, and remained higher at 12-months (P<0.05) than at baseline. Mid-thigh muscle area did not change after 6 or 12-months in the WL group. There were no group*time or group effects for other CT outcomes. There were no significant changes in low density lean tissue area after WL and AEX+WL at 6 or 12-months. The reductions in visceral fat areas were comparable at 20% and 18% after WL and AEX+WL at 6-months (P<0.0001) and remained significantly lower at 12-months than initial despite an insignificant increase of 8.1% in the WL group and 1.3% in the AEX+WL group from 6-to-12 months (Figure 2). Subcutaneous abdominal fat area also decreased after WL and AEX+WL at 6-months (−14 and −15%, P<0.0001) and was still reduced at 12-months (P<0.0001, Figure 2). Overall, mid-thigh subcutaneous fat decreased at 6-months post-WL and AEX+WL (P<0.001) and was higher at 12-months than 6-months (P<0.001) but remained lower at 12-months than baseline (P<0.001).
Figure 2.
Visceral fat area and subcutaneous fat area at baseline, 6-months and 12-month follow-up in WL and AEX+WL groups.*P<0.0001 versus baseline
Effects on VO2max
There was a group × time interaction for VO2max (P<0.05). VO2max increased 8% with AEX+WL (P<0.05), which was significantly different (P<0.05) than the 4% decrease with WL. VO2max declined 13% from 6-to-12 months in the AEX+WL group (P<0.001), such that it was no longer different than the initial VO2max. In the WL group, VO2max decreased another 4% from 6-to-12 months, although this was not significant. Both groups had an overall −7% change in VO2max over the year.
Effects on Blood Parameters
There were no baseline differences between groups, no group × time interactions or group effects for triglycerides, total cholesterol, LDL-cholesterol, and HDL-cholesterol (Table 2). Overall, triglyceride levels decreased after 6-months WL and AEX+WL (P<0.01), did not change from 6-to-12 months and were lower at 12-months than baseline (P<0.01). Total cholesterol and LDL-cholesterol decreased at 6-months (both P<0.005). Overall, there was a significant increase from 6-to-12-months in total cholesterol (P<0.005) and LDL-cholesterol (P<0.01). Total cholesterol and LDL-cholesterol were not different between 12-months and baseline. Overall, HDL-cholesterol increased at 6-months (P<0.01), was higher at 12-months than 6-months (P<001), and remained higher at 12-months compared to baseline (P<0.001). There was not a group × time interaction or group effect for plasma leptin, which overall decreased from baseline to 6-months (P<0.001), increased from 6-to-12 months (P<0.005), and was not different at 12-months than baseline (P=0.09).
Table 2.
Metabolic characteristics at baseline, after the 6-month interventions, and at 12 months.
| Baseline | 6 Months | 12 Months | Group × Time |
Overall Group |
Overall Time |
6 vs. 0 | 12 vs. 6 | 12 vs. 0 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||||
| N | mean±SEM | mean±SEM | mean±SEM | P-value | P-value | P-value | P-value | P-value | P-value | ||||||||
| Triglycerides (mg/dl) | WL | 34 | 128 | ± | 11 | 113 | ± | 9 | 107 | ± | 8 | 0.49 | 0.24 | 0.000 | <0.01 | 1.0 | <0.01 |
| AEX+WL | 28 | 117 | ± | 7 | 94 | ± | 6 | 94 | ± | 6 | |||||||
|
| |||||||||||||||||
| Cholesterol (mg/dl) | WL | 34 | 197 | ± | 7 | 192 | ± | 6 | 204 | ± | 8 | 0.49 | 0.50 | 0.000 | <0.005 | <0.005 | 1.0 |
| AEX+WL | 28 | 198 | ± | 6 | 186 | ± | 5 | 194 | ± | 7 | |||||||
|
| |||||||||||||||||
| LDL-C (mg/dl) | WL | 34 | 121 | ± | 6 | 116 | ± | 5 | 128 | ± | 7 | 0.35 | 0.99 | 0.000 | <0.005 | <0.01 | 1.0 |
| AEX+WL | 28 | 123 | ± | 5 | 112 | ± | 4 | 118 | ± | 6 | |||||||
|
| |||||||||||||||||
| HDL-C (mg/dl) | WL | 34 | 55 | ± | 2 | 57 | ± | 2 | 61 | ± | 2 | 0.75 | 0.38 | 0.000 | <0.01 | <0.01 | <0.001 |
| AEX+WL | 28 | 52 | ± | 2 | 55 | ± | 2 | 57 | ± | 2 | |||||||
|
| |||||||||||||||||
| Fasting Glucose (mg/dl) | WL | 31 | 99 | ± | 2 | 92 | ± | 2 | 92 | ± | 2 | 0.30 | 0.41 | 0.000 | <0.001 | 1.0 | <0.001 |
| AEX+WL | 25 | 95 | ± | 2 | 90 | ± | 2 | 92 | ± | 2 | |||||||
|
| |||||||||||||||||
| Fasting Insulin (pmol/l) | WL | 26 | 93 | ± | 7 | 71 | ± | 5 | 72 | ± | 6 | 0.96 | 0.24 | 0.000 | <0.001 | 1.0 | <0.01 |
| AEX+WL | 18 | 80 | ± | 9 | 59 | ± | 5 | 58 | ± | 5 | |||||||
|
| |||||||||||||||||
| HOMA-IR | WL | 25 | 3.75 | ± | 0.31 | 2.75 | ± | 0.23 | 2.77 | ± | 0.26 | 0.99 | 0.28 | 0.000 | <0.001 | 1.0 | <0.005 |
| AEX+WL | 18 | 3.16 | ± | 0.37 | 2.24 | ± | 0.22 | 2.21 | ± | 0.20 | |||||||
|
| |||||||||||||||||
| Glucose AUC (mg˙dl−1˙120 min−1) | WL | 31 | 17578 | ± | 645 | 16492 | ± | 570 | 17080 | ± | 661 | 0.22 | 0.12 | 0.01 | <0.05 | 1.0 | <0.05 |
| AEX+WL | 25 | 16364 | ± | 718 | 15670 | ± | 634 | 15068 | ± | 736 | |||||||
|
| |||||||||||||||||
| Insulin AUC (pmol˙l−1˙120 min−1) | WL | 25 | 51258 | ± | 3947 | 43207 | ± | 3176 | 44883 | ± | 3457 | 0.09 | 0.63 | 0.000 | <0.001 | 1.0 | <0.001 |
| AEX+WL | 18 | 55466 | ± | 4651 | 41066 | ± | 3743 | 36120 | ± | 4075 | |||||||
|
| |||||||||||||||||
| Leptin (ng/ml) | WL | 15 | 29.2 | ± | 2.6 | 22.8 | ± | 2.4 | 28.9 | ± | 3.3 | 0.27 | 0.08 | 0.000 | <0.001 | <0.005 | 0.09 |
| AEX+WL | 21 | 25.4 | ± | 2.2 | 17.8 | ± | 2.0 | 20.1 | ± | 2.8 | |||||||
|
| |||||||||||||||||
| Systolic Blood Pressure (mmHg) | WL | 19 | 120 | ± | 3 | 114 | ± | 3 | 116 | ± | 3 | 0.93 | 0.90 | 0.003 | <0.01 | 0.49 | 0.62 |
| AEX+WL | 20 | 118 | ± | 3 | 114 | ± | 4 | 117 | ± | 4 | |||||||
|
| |||||||||||||||||
| Diastolic Blood Pressure (mmHg) | WL | 19 | 66 | ± | 2 | 64 | ± | 2 | 66 | ± | 2 | 0.57 | 0.88 | 0.07 | 0.06 | 1.0 | 0.50 |
| AEX+WL | 20 | 68 | ± | 2 | 65 | ± | 2 | 65 | ± | 2 | |||||||
Effects on Glucose Metabolism
Fasting and total areas under the curve (AUC) for glucose and insulin, leptin, and HOMA-IR were not different at baseline between groups (Table 2). There were not any group × time interactions or group effects. Fasting glucose, fasting insulin, and HOMA-IR decreased after 6-months (P<0.001) and did not change from 6-to-12-months. Overall, fasting glucose (P<0.001), fasting insulin (P<0.01), and HOMA-IR (P<0.005) remained lower at 12-months than baseline. Likewise, total insulin AUC decreased at 6-months (P<0.001), did not significantly change from 6-to-12-months, and remained lower at 12-months than baseline (P<0.001). Total glucose AUC also decreased from baseline to 6-months (P<0.05), did not change from 6-to-12-months, and remained lower at 12-months (P<0.05). There was no group × time interaction for M (WL: 61.6 ± 4.2 vs. 64.1 ± 4.0 µmol˙kgFFM−1˙min−1 and AEX+WL: 67.6 ± 3.4 vs. 75.4 ± 3.4 µmol˙kgFFM−1˙min−1), which increased from baseline to 6-months (64.9 ± 2.6 vs. 70.4 ± 2.7 µmol˙kgFFM−1˙min−1, P<0.05).
Effects on RMR
There was no group*time interaction or group effects for RMR. RMR declined after WL and AEX+WL (P<0.01), increased from 6-to-12-months (P<0.05), and was not different at 12-months than baseline (P=0.41).
Baseline Predictors of Weight Regain (6-to-12-months)
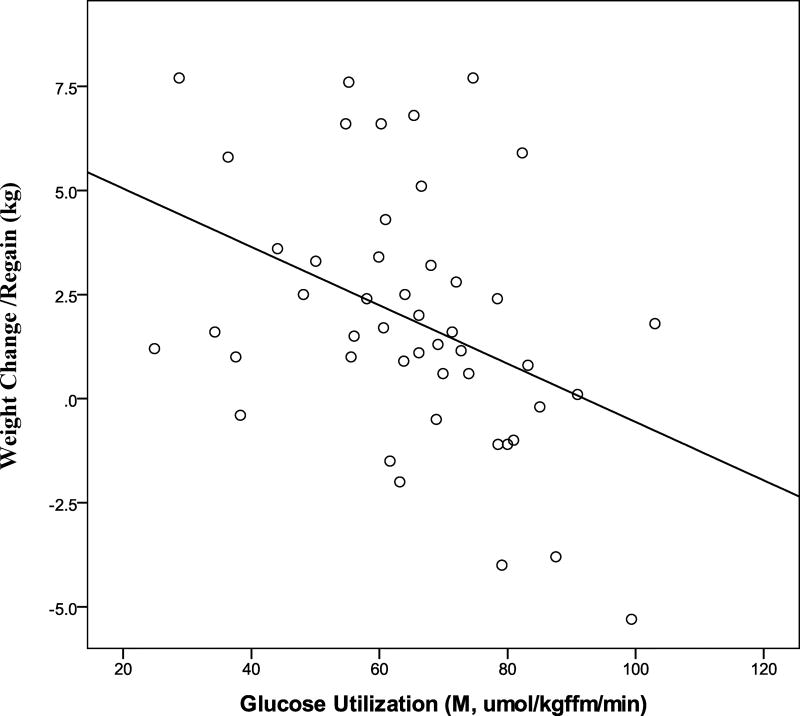
Initial M was associated with weight change/regain (r=−0.40, P<0.01, Figure 3). Initial RMRwas also associated with weight change/regain (r=0.27, P<0.05). However, baseline VO2max, fasting glucose, insulin, leptin, glucose120min, or glucose and insulin AUCs, were not associated with weight change/regain (r’s −0.01 to 0.10, P’s 0.97 to 0.12). In a multiple regression model with baseline leptin and M, only baseline M was independently correlated with weight change/regain (r2=0.18, P<0.05). Compliance to the exercise sessions from 6-to-12-months was associated with less weight change/regain from 6-to-12-months in the AEX+WL group (r=−0.44, P<0.05).
Figure 3.
Relationship of M to weight regain in postmenopausal women. (r = −0.40, P = 0.005).
Metabolic Changes Associated with Weight Regain (6-to-12-months)
Weight change/regain was associated with an increase in fasting glucose, insulin, leptin, insulin at 120 min, insulin AUC, and HOMA-IR from 6-to-12-months (Table 3). Weight change/regain was not associated with the change in VO2max. Weight change/regain was related to independent changes in leptin and HOMA-IR from 6-to-12-months in a multiple regression model (r=0.77, P<0.0001).
Table 3.
Weight Regain Predictors of Metabolism
|
ΔWeight r-value |
P-value | |
|---|---|---|
| ΔFasting glucose | 0.26 | 0.05 |
| ΔFasting insulin | 0.42 | 0.004 |
| ΔFasting leptin | 0.61 | 0.0001 |
| ΔGlucose @120min | 0.14 | 0.31 |
| ΔInsulin @120min | 0.39 | 0.008 |
| ΔGlucose AUC | 0.20 | 0.15 |
| ΔInsulin AUC | 0.39 | 0.01 |
| ΔRMR | 0.20 | 0.23 |
| ΔHOMA-IR | 0.49 | 0.001 |
| ΔVO2max | −0.31 | 0.098 |
Δ is from 6 to12 months.
AUC=area under curve, RMR=resting metabolic rate, HOMA-IR=homeostatic model assessment of insulin resistance, VO2max=maximal oxygen consumption
Discussion
This study compares the effects of WL alone and with AEX on body composition and metabolic changes during a 6-month ad libitum follow-up after intentional weight loss in postmenopausal women. Although the hypothesis was that the AEX+WL group would have less weight regain and better metabolic profiles, findings indicate that weight regain and metabolic changes at follow-up were not different between groups. Overall weight regain was associated with a worsening of glucose metabolism. Furthermore, greater baseline insulin sensitivity by the glucose clamp partially predicted less weight regain and changes in plasma leptin and HOMA-IR during the follow-up partially predicted weight regain. This suggests that initial insulin sensitivity is partially protective against weight regain.
Multiple factors are associated with the ability to sustain weight loss over a long period of time. A recent meta-analysis of 45 trials established that weight regain 12 months post weight loss was reduced by an average 1.5 kg when adults with obesity participate in lifestyle interventions focusing on both dietary intake and physical activity compared to a control/minimal care intervention (31). In the current study, weight regain was not different between groups independent of initial assignment. Groups were equally compliant to attending the weight maintenance classes. Yet, our results, contrary to our hypothesis, indicate that initial participation in an exercise program combined with weight loss is not better than WL alone as both groups had better CVD and diabetes risk factor profiles at 12 months and there was no difference between groups. This may be due to our findings that VO2max declined during the follow-up in the AEX+WL group as a whole and reverted to initial fitness values and that the women attended less than 30% of the available exercise sessions during follow-up. Although fitness declined in the AEX+WL group by 12 months, better compliance in continuing to exercise from 6 to 12 months was associated with less weight regain. It would have been useful to know exercise intensity adherence and to measure free-living physical activity at the 12 month visit to determine whether or not energy expenditure differed between the groups. Weekday total activity decreased 23% after 6 months WL but not AEX+WL indicating that both structured and non-structured activity is impacted if women undergo WL without participating in physical activity (32).
Despite an overall similar weight regain of ~2 kg in both groups at 12 months, total body fat, visceral fat, subcutaneous abdominal and mid-thigh fat remained lower than at baseline. The maintenance of loss of abdominal and mid-thigh subcutaneous fat despite minimal weight regain suggests that the weight regain occurred in other depots or is too small to contribute to the change in body weight. In the current study, both groups had improvements in triglyceride and HDL-cholesterol levels after the initial WL and AEX+WL, which were maintained at 12 months suggesting some residual effect of the initial treatment. Systolic blood pressure declined after initial weight loss and did not change during the follow-up. Perhaps, this is due to the fact that these postmenopausal women had, on average, normal blood pressure levels and many were medicated throughout the study. Thus, the benefits of initial WL or AEX+WL on body composition, triglycerides, HDL-cholesterol, and blood pressure were maintained despite weight regain. This should be considered in light of our findings that the moderate increase in VO2max might have contributed to the lack of difference in body composition and M value between the groups at 6-months and might have influenced the similar change between 6–12 months in AEX+WL and WL.
In the Study of the Effects of Diet on Metabolism and Nutrition (STEDMAN) project, fasting insulin and HOMA-IR declined after 6-months weight loss and showed a sustained reduction at 12 months despite weight regain of between 2–17.5 lbs, in the majority of subjects (n=19) with only two subjects remaining weight stable (<1 lb gain or loss) and 6 continuing to lose weight (18). Our results confirm these findings for WL alone and add that AEX+WL also resulted in a sustained improvement in glucose metabolism, including reductions in fasting glucose and insulin, and insulin AUC levels from the OGTT; each of which remained lower at 12 months than baseline values despite weight regain. Similarly, the decrease in HOMA-IR with WL and AEX+WL persisted at 12 months, suggesting a sustained reduction in insulin resistance. Thus, some metabolic improvements are maintained despite some regain of body weight.
Weight cycling (5 lb weight change) is associated with incident diabetes, higher fasting glucose, greater HOMA-IR and higher systolic blood pressure in adults followed for two years in the Diabetes Prevention Program (33). In the Look AHEAD (Action for Health in Diabetes) trial of adults aged 45–76 years, the initial weight loss and weight loss during the first year was predictive of greater improvements in glycosylated hemoglobin (HbA1c), HDL-cholesterol and systolic blood pressure at the four-year follow-up (34). In a study of postmenopausal women, total cholesterol, LDL-cholesterol, insulin, and HOMA-IR were worse at 12 months than baseline in women who regained greater than or equal to 2 kg of weight by 12 months than women who maintained or lost weight at 12 months (20). Our study supports this as the data indicate a relationship between weight regain and the change in metabolic profiles from 6-to 12 months. Although the amount of weight regain in our study was on average ~50% less than the average 4 kg regain reported by Beavers et al. (20) in which there was a worsening of metabolic profiles, their follow-up was twice as long (e.g. 12 months) and was associated with greater weight regain. This suggests that the magnitude of weight regain and duration of follow-up are important predictors of which metabolic improvements are maintained.
An examination of predictors of weight regain implicates the importance of insulin sensitivity in weight regain. In the Quebec Family Study, reduced OGTT G120 levels were associated with weight gain over a 6-year follow-up which was speculated to be due to an increase in hunger (15). In addition, subjects with obesity with the lowest glucose levels during the OGTT had the greatest weight regain after a WL program by drug therapy + energy restriction or WL by energy restriction alone (15). In a small sample (n=10) of women with moderate obesity who underwent a three-month weight loss program, weight regain at 12 and 18 months correlated with the change in insulin sensitivity by the glucose clamp from baseline to weight maintenance phase (16). In our study, neither initial fasting glucose, G120 levels or glucose AUC were associated with weight regain; however, women with the highest insulin sensitivity measured during the glucose clamp at baseline had the smallest weight regain at 12 months. A review of literature concludes that changes in leptin, taken alone, is not sufficient to predict weight regain following weight loss (11); whereas Rosenbaum et al. has shown that leptin administration in humans reverses the decline in energy expenditure of a weight-reduced state (35) and increases the work efficiency of skeletal muscle (36) suggesting a role of leptin in weight regain. In our model, both changes in leptin and HOMA-IR from 6-to-12 months were independently associated with weight regain suggesting that some combination of the two create a phenotype at risk for weight regain.
It should be noted that a greater exercise stimulus could have resulted in a difference in weight loss and greater change in insulin sensitivity between the two groups in the initial 6-months (21). However, our finding that AEX+WL and WL had similar changes in body weight allows, in fact, a better comparison for examining weight regain as both groups start the comparison after the same relative weight loss. Additionally, our results are in light of the 34% drop-out rate, which may reflect sample bias (i.e. different motivation/goals of those that completed 6 vs. 12 month), and limited the sample size available at the 12-month follow-up period.
In summary, these results show that weight loss, whether or not accompanied by exercise training, is associated with comparable weight loss and metabolic improvements that are sustained during ensuing modest weight regain over the subsequent six months. Future research could focus on cellular mechanisms for weight regain in postmenopausal women.
What is already known about this subject?
Weight loss of ~3% reduces the severity of obesity-associated cardiovascular risk factors.
Most adults return to baseline weight within the next 3–5 years after intentional weight loss.
Successful weight maintenance is multifactorial.
What does this study add?
Baseline insulin resistance partially predicts the magnitude of weight regain during 6 months of weight regain following intentional weight loss.
The amount of weight regained is associated with an increase in leptin and insulin resistance during follow-up.
The addition of aerobic exercise to intentional weight loss does not protect from weight regain and worsening metabolic profiles at follow-up.
Acknowledgments
Our appreciation is extended to the women who participated, clinical team, and research assistants.
Funding: This research was supported funds from a Senior Research Career Scientist Award (ASR) and Career Development Award IK2RX-000944 (MCS) from the United States Department of Veterans Affairs (VA) Rehabilitation R&D (Rehab RD) Service, VA Medical Center Baltimore Geriatric Research, Education and Clinical Center (GRECC), National Institutes of Health Grant RO1-AG19310, R01 AG20116, P30-AG028747, and P30 DK072488.
Footnotes
Clinical Trials: NCT00882141
Conflict of Interest: None
References
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Mokdad AH, Bales VS, Greenlund KJ, Mensah GA. Public health surveillance for disease prevention: lessons from the behavioral risk factor surveillance system. Ethn Dis. 2003;13:S19–S23. [PubMed] [Google Scholar]
- 3.Jensen MDRD, Donato KA, Apovian CM, Ard JD, Comuzzie AG, Hu FB, Hubbard VS, Jakicic JM, Kushner RF, Loria CM, Millen BE, Nonas CA, Pi-Sunyer FX, Stevens J, Stevens VJ, Wadden TA, Wolfe BM, Yanovski SZ. Guidelines (2013) for managing overweight and obesity in adults. Obesity. 2014;22:S1–S140. [Google Scholar]
- 4.Byrne S, Cooper Z, Fairburn C. Weight maintenance and relapse in obesity: a qualitative study. Int J Obes Relat Metab Disord. 2003;27:955–962. doi: 10.1038/sj.ijo.0802305. [DOI] [PubMed] [Google Scholar]
- 5.Visram S, Crosland A, Cording H. Triggers for weight gain and loss among participants in a primary care-based intervention. British journal of community nursing. 2009;14:495–501. doi: 10.12968/bjcn.2009.14.11.45008. [DOI] [PubMed] [Google Scholar]
- 6.Phelan S, Hill JO, Lang W, Dibello JR, Wing RR. Recovery from relapse among successful weight maintainers. Am J Clin Nutr. 2003;78:1079–1084. doi: 10.1093/ajcn/78.6.1079. [DOI] [PubMed] [Google Scholar]
- 7.Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr. 2005;82:222S–225S. doi: 10.1093/ajcn/82.1.222S. [DOI] [PubMed] [Google Scholar]
- 8.Pekkarinen T, Kaukua J, Mustajoki P. Long-term weight maintenance after a 17-week weight loss intervention with or without a one-year maintenance program: a randomized controlled trial. J Obes. 2015;2015:651460. doi: 10.1155/2015/651460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitham C, Mellor DD, Goodwin S, Reid M, Atkin SL. Weight maintenance over 12 months after weight loss resulting from participation in a 12-week randomised controlled trial comparing all meal provision to self-directed diet in overweight adults. Journal of human nutrition and dietetics : the official journal of the British Dietetic Association. 2014;27:384–390. doi: 10.1111/jhn.12178. [DOI] [PubMed] [Google Scholar]
- 10.Ebbeling CB, Swain JF, Feldman HA, Wong WW, Hachey DL, Garcia-Lago E, et al. Effects of dietary composition on energy expenditure during weight-loss maintenance. JAMA. 2012;307:2627–2634. doi: 10.1001/jama.2012.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strohacker K, McCaffery JM, MacLean PS, Wing RR. Adaptations of leptin, ghrelin or insulin during weight loss as predictors of weight regain: a review of current literature. Int J Obes (Lond) 2014;38:388–396. doi: 10.1038/ijo.2013.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roumans NJ, Vink RG, Gielen M, Zeegers MP, Holst C, Wang P, et al. Variation in extracellular matrix genes is associated with weight regain after weight loss in a sex-specific manner. Genes & nutrition. 2015;10:56. doi: 10.1007/s12263-015-0506-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lean ME, Malkova D. Altered gut and adipose tissue hormones in overweight and obese individuals: cause or consequence? Int J Obes (Lond) 2016;40:622–632. doi: 10.1038/ijo.2015.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 15.Boule NG, Chaput JP, Doucet E, Richard D, Despres JP, Bouchard C, et al. Glucose homeostasis predicts weight gain: prospective and clinical evidence. Diabetes Metab Res Rev. 2008;24:123–129. doi: 10.1002/dmrr.768. [DOI] [PubMed] [Google Scholar]
- 16.Yost TJ, Jensen DR, Eckel RH. Weight regain following sustained weight reduction is predicted by relative insulin sensitivity. Obes Res. 1995;3:583–587. doi: 10.1002/j.1550-8528.1995.tb00193.x. [DOI] [PubMed] [Google Scholar]
- 17.Wing RR. Insulin sensitivity as a predictor of weight regain. Obes Res. 1997;5:24–29. doi: 10.1002/j.1550-8528.1997.tb00279.x. [DOI] [PubMed] [Google Scholar]
- 18.Lien LF, Haqq AM, Arlotto M, Slentz CA, Muehlbauer MJ, McMahon RL, et al. The STEDMAN project: biophysical, biochemical and metabolic effects of a behavioral weight loss intervention during weight loss, maintenance, and regain. Omics : a journal of integrative biology. 2009;13:21–35. doi: 10.1089/omi.2008.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang P, Holst C, Wodzig WK, Andersen MR, Astrup A, van Baak MA, et al. Circulating ACE is a predictor of weight loss maintenance not only in overweight and obese women, but also in men. Int J Obes (Lond) 2012;36:1545–1551. doi: 10.1038/ijo.2011.278. [DOI] [PubMed] [Google Scholar]
- 20.Beavers DP, Beavers KM, Lyles MF, Nicklas BJ. Cardiometabolic risk after weight loss and subsequent weight regain in overweight and obese postmenopausal women. J Gerontol A Biol Sci Med Sci. 2013;68:691–698. doi: 10.1093/gerona/gls236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryan AS, Ortmeyer HK, Sorkin JD. Exercise with calorie restriction improves insulin sensitivity and glycogen synthase activity in obese postmenopausal women with impaired glucose tolerance. Am J Physiol Endocrinol Metab. 2012;302:E145–152. doi: 10.1152/ajpendo.00618.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, et al. Summary of American Heart Association Diet and Lifestyle Recommendations revision 2006. ArteriosclerThrombVascBiol. 2006;26:2186–2191. doi: 10.1161/01.ATV.0000238352.25222.5e. [DOI] [PubMed] [Google Scholar]
- 23.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 24.American Diabetes A. Diagnosis and Classification of Diabetes Mellitus. Diabetes. 2009;32:S62–67. doi: 10.2337/dc09-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–475. [PubMed] [Google Scholar]
- 26.Sampson EJ, Demers LM, Krieg AF. Faster enzymatic procedure for serum triglycerides. Clin Chem. 1975;21:1983–1985. [PubMed] [Google Scholar]
- 27.Warnick GR, Benderson J, Albers JJ. Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin Chem. 1982;28:1379–1388. [PubMed] [Google Scholar]
- 28.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 30.McGuire EA, Helderman JH, Tobin JD, Andres R, Berman M. Effects of arterial versus venous sampling on analysis of glucose kinetics in man. J Appl Physiol. 1976;41:565–573. doi: 10.1152/jappl.1976.41.4.565. [DOI] [PubMed] [Google Scholar]
- 31.Dombrowski SU, Knittle K, Avenell A, Araujo-Soares V, Sniehotta FF. Long term maintenance of weight loss with non-surgical interventions in obese adults: systematic review and meta-analyses of randomised controlled trials. BMJ. 2014;348:g2646. doi: 10.1136/bmj.g2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Serra MC, Treuth MS, Ryan AS. Dietary prescription adherence and non-structured physical activity following weight loss with and without aerobic exercise. The journal of nutrition, health & aging. 2014;18:888–893. doi: 10.1007/s12603-014-0481-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delahanty LM, Pan Q, Jablonski KA, Aroda VR, Watson KE, Bray GA, et al. Effects of weight loss, weight cycling, and weight loss maintenance on diabetes incidence and change in cardiometabolic traits in the Diabetes Prevention Program. Diabetes Care. 2014;37:2738–2745. doi: 10.2337/dc14-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neiberg RH, Wing RR, Bray GA, Reboussin DM, Rickman AD, Johnson KC, et al. Patterns of weight change associated with long-term weight change and cardiovascular disease risk factors in the Look AHEAD Study. Obesity (Silver Spring) 2012;20:2048–2056. doi: 10.1038/oby.2012.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenbaum M, Murphy EM, Heymsfield SB, Matthews DE, Leibel RL. Low dose leptin administration reverses effects of sustained weight-reduction on energy expenditure and circulating concentrations of thyroid hormones. J Clin Endocrinol Metab. 2002;87:2391–2394. doi: 10.1210/jcem.87.5.8628. [DOI] [PubMed] [Google Scholar]
- 36.Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S, et al. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest. 2005;115:3579–3586. doi: 10.1172/JCI25977. [DOI] [PMC free article] [PubMed] [Google Scholar]