Summary
Emerging lipidomic technologies have enabled researchers to dissect the complex roles of phospholipases in lipid metabolism, cellular signaling, and immune regulation. Host phospholipase products are involved in stimulating and resolving the inflammatory response to pathogens. While many pathogen-derived phospholipases also manipulate the immune response, they have recently been shown to be involved in lipid remodeling and scavenging during replication. Animal and plant hosts as well as many pathogens contain a family of patatin-like phospholipases, which have been shown to have phospholipase A2 activity. Proteins containing patatin-like phospholipase domains have been identified in protozoan parasites within the Apicomplexa phylum. These parasites are the causative agents of some of the most widespread human disease. Malaria, caused by Plasmodium spp., kills nearly half a million people worldwide each year. Toxoplasma and Cryptosporidium infect millions of people each year with lethal consequences in immunocompromised populations. Parasite-derived patatins are likely effective drug targets and progress in the tools available to the Apicomplexan field will allow for a closer look at the interplay of lipid metabolism and immune regulation during host infection.
Keywords: Apicomplexa, Toxoplasma, Plasmodium, Cryptosporidium, Patatin-like phospholipases
Abbreviated Summary
Animal and plant hosts as well as many pathogens contain a family of patatin-like phospholipases, which have been shown to have phospholipase A2 activity. Proteins containing patatin-like phospholipase domains have been identified in protozoan parasites within the Apicomplexa phylum. Parasite-derived patatins are likely effective drug targets and progress in the tools available for Apicomplexa will allow for a closer look at the interplay of lipid metabolism and immune regulation during host infection.
Introduction
The phospholipase A2 (PLA2) superfamily contains a wide range of enzymes that cleave the sn-2 ester bond of phospholipids to release free fatty acids and lysophospholipids (Burke & Dennis, 2009). However, there is great diversity among this family of enzymes (Six & Dennis, 2000). The most common groups of human PLA2s are secreted PLA2 (sPLA2), calcium-independent PLA2 (iPLA2), cytosolic PLA2 (cPLA2), and patatin-like PLA2 (PLP). The sPLA2s are the most extensive group of PLA2s and play a variety of roles in the inflammatory properties of venoms, digestion of dietary phospholipids, production of eicosanoids, and signal transduction in the inflammatory response (Lambeau & Gelb, 2008). iPLA2 enzymes are important in phospholipid remodeling and homeostasis as well as signal transduction in many cell types (Winstead et al., 2000). cPLA2 enzymes have a high specificity for arachidonic acid (AA) at the sn-2 position of its substrate phospholipid (Kramer & Sharp, 1997). PLP enzymes were first described as lipid acyl hydrolases involved in potato tuber storage (Senda et al., 1996; Shewry, 2003). In many plants, PLPs are induced by infection to help control the spread of disease, whereas in mammals, PLPs are mostly used in lipid metabolism and turnover.
Lipoxygenases (LOX) are a family of iron-binding enzymes that catalyze the dioxygenation of polyunsaturated fatty acids (PUFA), primarily AA, released from lipids by PLA2s. Cyclooxygenases (COX) catalyze the bis-dioxygenation and subsequent reduction of PUFAs (Rouzer & Marnett, 2008). In mammals, the products of LOX and COX are eicosanoids, which can help regulate immune responses via both pro-inflammatory and anti-inflammatory activities (Tam, 2013). Eicosanoids are transported from the cell via the multidrug resistance-associated protein family of ATP-dependent efflux transporters, which export compounds conjugated to anionic molecules (Gao et al., 1998). Once these eicosanoids are transported out of the cell, they can bind their specific lipoxin or leukotriene receptors to activate various signaling cascades resulting in a range of downstream effects on both the inflammatory response and cell proliferation (Wang & DuBois, 2010).
The enzyme cascades that produce eicosanoids have been increasingly studied in the context of infection. Both host and pathogens produce the enzymes involved in these pathways to regulate metabolism and the inflammatory response. Most studies of PLA2s focus on either their role in lipid metabolism or on the downstream production of eicosanoids. In this review, we will show the connection between the two pathways (Fig. 1), with a focus on pathogen-derived enzymes during host infection. We will also discuss a family of apicomplexan-derived PLPs, which have been largely uncharacterized at the time of this review.
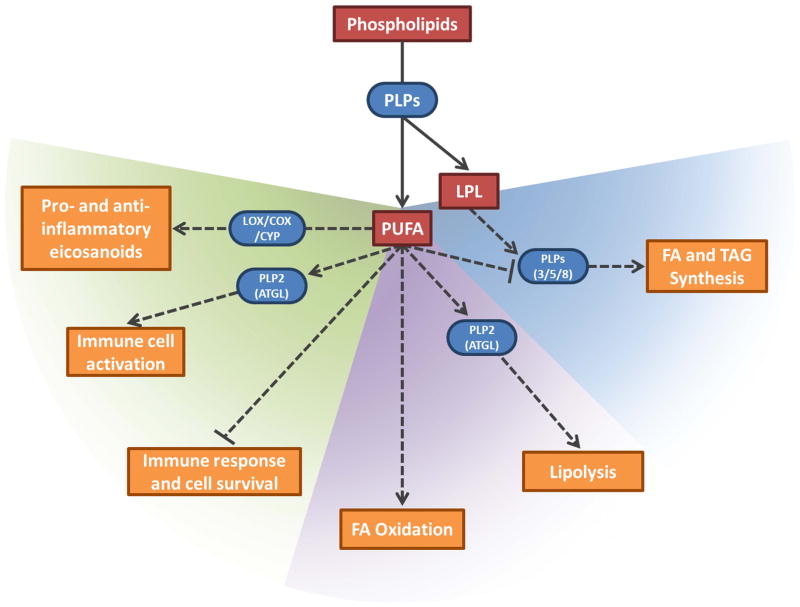
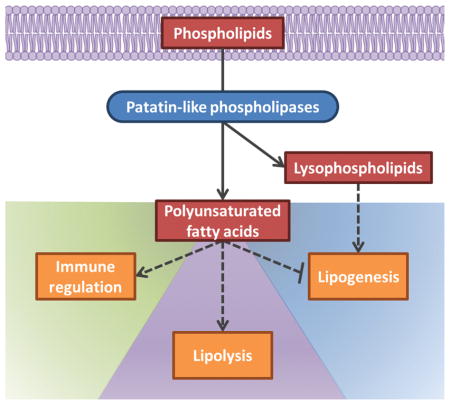
Fig. 1.
Diverse roles of patatin-like phospholipases (PLPs) and their products in immune regulation (left/green region), lipolysis (middle/purple region), and lipogenesis (right/blue region). PLPs release polyunsaturated fatty acids (PUFA) and lysophospholipids (LPL) from phospholipids. These products can directly alter membrane fluidity and can also act as signaling molecules to regulate lipid metabolism and cellular signaling pathways. PUFAs can be oxidized by LOX, COX, and CYP to form both pro- and anti-inflammatory eicosanoids (figure 3 for details). Some PLPs (e.g. PNPL2/ATGL, PNPLA3, PNPLA5, and PNPLA8 in mammals) can be regulated by PUFAs and LPLs to maintain stringent control over immune responses and lipid homeostasis (figure 2 for details). Substrates and signaling molecules are red, enzymes are blue and rounded, and altered downstream processes are orange. Activation and inhibition are indicated with arrows and bar-headed lines, respectively. Dashed lines indicate indirect pathways.
Patatin-like phospholipases in lipid hydrolysis
Humans have 9 PLPs, annotated in mammals as PNPLA, within the broader lipid hydrolase family. All of the PNPLAs have a catalytic serine lipase motif G-X-S-X-G, a serine-aspartate dyad, and a glycine-rich oxyanion hole. While all studied members have shown acyl-hydrolase activity, several have lacked significant phospholipase activity under the conditions tested in vitro. The mammalian PNPLAs have diverse and often specialized functions, but most are involved in lipid metabolism and turnover (diagram in Fig. 2). PNPLA2, or adipose triglyceride lipase (ATGL), is the rate-determining enzyme in triglyceride lipolysis (Kienesberger et al., 2008; Qiao et al., 2011). ATGL expression is upregulated by fasting glucocorticoids and downregulated by feeding and insulin (Villena et al., 2004; Lake et al., 2005; Kershaw et al., 2006). However, PNPLA3 and PNPLA5 show the opposite pattern, reminiscent of genes involved in lipogenesis, such as FAS (Baulande et al., 2001; Liu et al., 2017). The insulin-dependent activation of PNPLA3 is mediated by the sterol regulatory element-binding protein (SREBP) transcription factors to promote triglyceride synthesis (Kim et al., 2016). ATGL, PNPLA3, and PNPLA5 contain two distinct C-terminal lipid droplet targeting motifs responsible for their recruitment to lipid droplets (Murugesan et al., 2013). PNPLA8 is also regulated by the SREBP family, but PNPLA8 associates with mitochondria and peroxisomes and has both PLA1 and PLA2 activity. PNPLA8 activation induces lipid droplet mobilization through autophagy to provide the cell with new lipids faster and with less energy consumption than uptake or de novo synthesis (Kim et al., 2016). The fatty acids released by lipid hydrolysis can provide twice as much ATP as carbohydrates relative to their mass and are important in energy storage, especially in adipose tissue. Fatty acids are catabolized by the fatty acid oxidation, or β-oxidation, pathway (Carracedo et al., 2013). The diverse cellular localizations and tissue expression patterns of the PNPLA family suggest non-redundant roles in lipid metabolism.
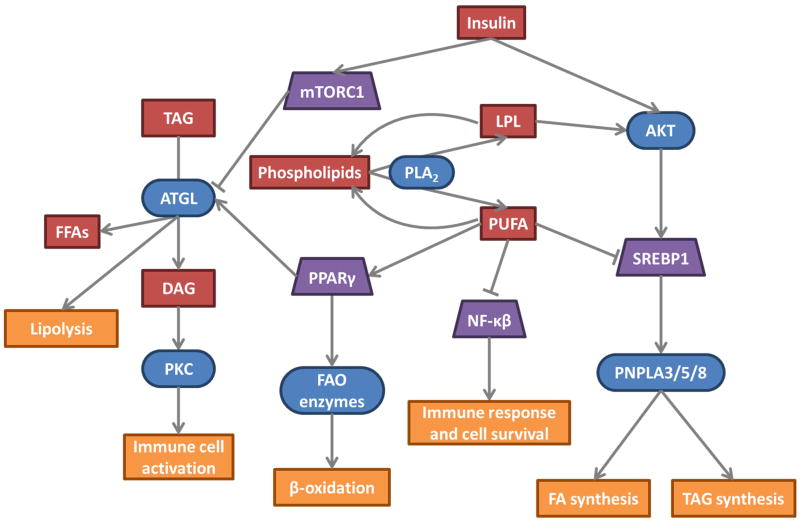
Fig. 2.
PLA2 enzymes in lipid metabolism and cellular signaling. PUFAs and lysophospholipids (LPLs) are cleaved from phospholipids by PLA2. These products can be recycled back into the lipid membrane or serve as signaling molecules to regulate lipolysis, lipogenesis, and the immune responses. Downstream patatin-like PLA2s (ATGL, PNPLA3, PNPLA5, and PNPLA8) respond to insulin levels, adding another layer of regulation on these lipid metabolism and cellular signaling pathways. Substrates and signaling molecules are red, enzymes are blue and rounded, transcription factors are purple trapezoids, and altered downstream processes are orange. Activation and inhibition are indicated with arrows and bar-headed lines, respectively. mTORC1 (mammalian target of rapamycin complex 1), Akt (Protein kinase B), PKC (Protein kinase C), DAG (diacylglycerol), PPARγ (Peroxisome proliferator-activated receptor gamma)
Patatin-like phospholipases in host cellular signaling pathways
The polyunsaturated fatty acids (PUFA) and lysophospholipids released by PLA2 activity can act as secondary messengers for downstream cellular responses; however, in mammalian hosts these secondary messengers come from sPLA2, iPLA2 and cPLA2 but not the PLPs. In contrast, plant PLPs play an active role in defense signaling. Arabidopsis encodes 10 PLPs and the PUFAs produced can inhibit the protein phosphatase MP2C, which is involved in the wound-induced MAP kinase pathway (Scherer et al., 2010). Arabidopsis PLP2 and PLP7 are pathogen-induced patatin-like phospholipases. PLP2 promotes a hypersensitive reaction, or programmed cell death, in infected cells that are critical in the resistance of Arabidopsis to cucumber mosaic virus but detrimental during infection with the fungus Botrytis cinerea and the bacteria Pseudomonas syringae. Higher induction of the downstream oxylipins in response to B. cinerea and P. syringae compared to the virus led to increased host damage from the induced hypersensitive reaction (Camera et al., 2005; Camera et al., 2009). Similarly, infection of tobacco leaves with tobacco mosaic virus strongly induced transcription and activity of three tobacco PLPs. Fatty acid conversion to 12-oxophytodienoic and jasmonic acid defense-signaling eicosanoids induced the onset of necrotic lesions to inhibit viral spread (Dhondt et al., 2000, Ryu, 2004). There are also complex feedback loops involving other phospholipases. For example, phospholipase D (PLD)-derived PA can stimulate PLA2 activity in animals, whereas PLD is inhibited by the LPE generated by PLA2 in both plants and animals. PLD can also be activated by the PUFAs generated by PLA2 (Ryu, 2004).
Patatin-like phospholipases in microbes
In addition to their mammalian and plant hosts, PLPs are also found in many microorganisms. In a screen of 123 bacterial genomes, 55 strains contained at least one gene with a PLP domain including Bacillus sp., Brucella sp., Rickettsia sp., Staphylococcus aureus, and Yersinia pestis. Genomes of pathogens and symbionts had significantly higher numbers of PLP-containing genes than the genomes of free-living bacteria, suggesting a role in host-pathogen interaction for these PLPs (Banerji & Flieger, 2004). One of the best characterized PLP is ExoU in Pseudomonas aeruginosa (Phillips et al., 2003; Sato & Frank, 2004). ExoU is a cytotoxic effector protein secreted through the type III secretion system upon cellular contact (Sawa et al., 2016). Host ubiquitination is required to activate the PLA2 activity of ExoU leading to destruction of infected cell membranes (Anderson et al., 2011; Anderson et al., 2015). Rickettsia typhi encodes two PLPs, one of which has similar cytotoxic activity to ExoU and also requires a eukaryotic host cofactor for activation (Rahman et al., 2010). The intracellular pathogen Legionella pneumophila encodes four PLPs, three of which are secreted into the host cell through the type IVB secretion system (Banerji et al., 2008). The best characterized of these PLPs, VipD, hydrolyzes phosphatidylcholine (PC) and phosphatidylethanolamine (PE) specifically on the mitochondrial membrane and not the plasma membrane. The release of fatty acids and lysophospholipids destabilizes the membrane and allows for the release of cytochrome c from the mitochondria. Cytochrome C activates caspase 3, a major regulator of apoptotic cell death (Zhu et al., 2013). VipD also interferes with endosomal trafficking and subsequent lysosomal degradation that contributes to bacterial survival within the host cell (Banerji et al., 2008; Ku et al., 2012).
PLPs released by fungi during infection in a mammalian host have been implicated in nutrient acquisition, tissue invasion, and modulation of the host immune response (Köhler et al., 2006). The triacylglycerol (TAG) lipases in Saccharomyces cerevisiae contain patatin-like domains and release TAG from lipid storage particles (Athenstaedt & Daum, 2003; Athenstaedt & Daum, 2005). S. cerevisiae also encodes a human PNPLA6 homolog involved in phosphatidylcholine turnover. Pathogenic fungi like Candida albicans produce PLPs, but their role in pathogenesis and virulence is still unclear.
Eicosanoid production and regulation
Eicosanoids are lipid mediators made by the oxidation of PUFAs and are involved in the regulation of inflammation (diagram in Fig. 3). Eicosanoids were first discovered in fungi and are involved in sexual maturation and life cycle regulation as well as pathogenesis in virulent strains (Kock et al., 1991). Eicosanoids are largely produced by cells of the innate immune system, using arachidonic acid (AA as the PUFA substrate (Harizi et al., 2008). The three major enzymes involved in eicosanoid production are cytochrome P450, LOX, and COX. AA metabolized by cytochrome P450 forms epoxides and hydroxyeicosatetreanoic acids (HETE), which likely contributes to tumor initiation and progression (Nebert & Dalton, 2006). COX-mediated AA oxidation forms prostaglandins (PG) and thromboxanes (TX). COX-1 and COX-2 are the two isoforms that have been identified in humans. COX-1 is constitutively expressed in most tissues, while COX-2 is induced in response to inflammation (Botting, 2007). PGs are produced by most human cells, are associated with both pro- and anti-inflammatory activity, and are responsible for many physiological symptoms of an inflammatory response. Aspirin and other nonsteroidal anti-inflammatory drugs function by blocking the active site of COX to reduce the general malaise caused by PG and TX activity (Harizi et al., 2008).
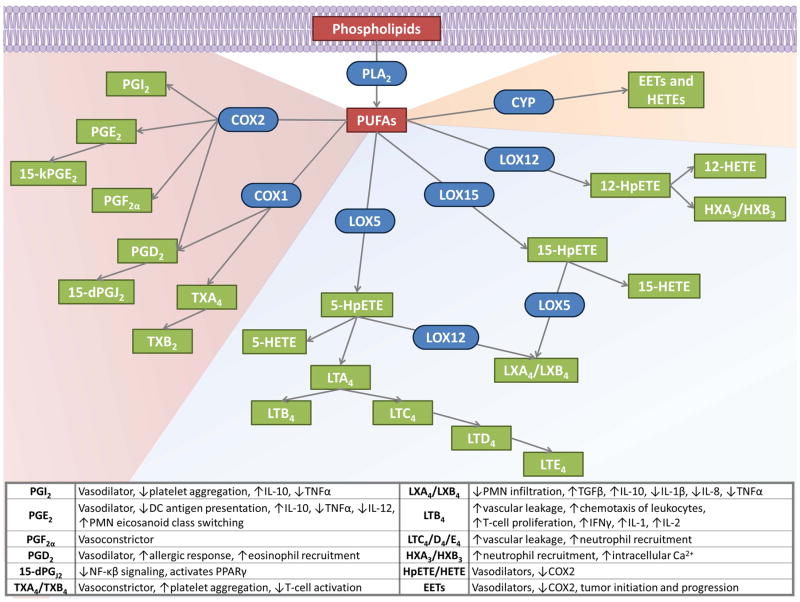
Fig. 3.
Eicosanoid production and inflammatory roles. PUFAs, released from phospholipids by PLA2, are oxidized by three main pathways to form both pro- and anti-inflammatory eicosanoids. COX pathways to produce PGs and TXs are shown in the red-shaded region. LOX production of HETEs, HXs, LTs, and LXs are shown in the blue-shaded region. Cytochrome P450 (CYP) products are shown in the orange-shaded region. Substrates are red, products are green, and enzymes are blue and rounded. The table summarizes some of the discussed downstream pro- and anti-inflammatory effects of the eicosanoids.
The LOX superfamily is found widely in plants, animals, fungi and bacteria. The main human LOXs are 5-, 12- and 15-LOX and the names reflect the specific carbon target for oxidation on the PUFA substrate. Soybean LOX was one of the first characterized and while it displays 15-LOX activity, it only shares 25% identity with any mammalian 15-LOX. The human 15-LOXs themselves only share 35% identity with each other, highlighting the sequence diversity of this enzymatic family (Lagocki et al., 1976; Steczko et al., 1992; Shureiqi & Lippman, 2001). LOX-mediated AA dioxygenation forms hydroperoxyeicosatetraenoic acids (HpETE), which are rapidly reduced to HETEs. In plants, these intermediates are converted to jasmonic acid and aldehydes, which are both involved in plant defense responses (Chehab et al., 2008). In other organisms, LOX-derived HETEs can be further converted to hepoxilins (HX), leukotrienes (LT), and lipoxins (LX). HXs are associated with stimulated intracellular calcium release, including stimulation of AA, diacylglycerol and insulin secretion (Nigam et al., 1990; Mrsny et al., 2004). LTs are mainly produced by inflammatory cells and exhibit strong pro-inflammatory properties including T cell proliferation, chemotaxis induction and secretion of cytokines involved in the Th1 response (Samuelsson et al., 1987; Wang & DuBois, 2010; Norris et al., 2014). Conversely, LXs are involved in inflammation resolution through downregulation of pro-inflammatory chemokines and cytokines, reduced PMN infiltration, and increased IL-10 production (Serhan, 2014; Petri et al., 2015).
Mediators like PGs and LTs are produced in response to infection, but failure to resolve inflammation can be as damaging as the initial infection, so pro-resolving eicosanoids like LXs, resolvins, protectins, and maresins are produced to protect the host tissues (Tam, 2013). Signaling networks between the eicosanoids that help mediate this balance between pro- and anti-inflammatory responses. In fungi, inhibition of PGs leads to an increase in LOX products to compensate for the loss of eicosanoids (Noverr et al., 2003). PGE2 enhances IL-10 production in dendritic cells, which inhibits activation of the 5-LOX activating protein and results in lower LTB4 production (Harizi et al., 2003). Both PGE2 and PGD2 can upregulate 15-LOX, leading to increased synthesis of pro-resolving LXs (Tam, 2013). Differences in eicosanoid production by immune cells contribute to their downstream effects on the immune response. PMNs are the first leukocyte responders to an inflammatory insult. At the onset of inflammation, LTB4, PGE2 and PGD2 are the most abundant eicosanoids produced by PMNs and these eicosanoids enhance further leukocyte recruitment and vascular permeability. The PGs then stimulate lipid mediator class switching and upregulation of resolving eicosanoids at the sites of inflammation (Dalli & Serhan, 2012). Secreted products produced in one cell type can also be metabolized in a subsequent cell to generate the final lipid mediator. 15-HpETE or 15-HETE produced by 15-LOX in epithelial cells or monocytes can serve as substrates for neutrophil 5-LOX to produce LXA4 and LXB4. Myeloid 5-LOX produces pro-inflammatory LTA4, which is then converted by platelet 12-LOX to anti-inflammatory lipoxins (Fierro & Serhan, 2001). Although there has not yet been a direct link between microbial PLPs and eicosanoid production, a mammalian PLP has been implicated in human mast cell eicosanoid production (described below under lipid scavenging). The balance of cytokines and lipid mediators is critical for an effective host response to infection, but pathogens can take advantage of host eicosanoid production to modulate the immune response. P. aeruginosa secretes a 15-LOX homolog which regulates the host response through the production of anti-inflammatory 15-HETE molecules (Vance et al., 2004). Listeria monocytogenes infection of macrophages activates host cPLA2 and COX-2 activity leading to increased production of PGs. The resulting downregulation of TNFα by the PGs helps L. monocytogenes survive (Noor et al., 2008). As these mechanisms for immune regulation are studied further, the roles of microbial PLPs in eicosanoid production will likely be elucidated.
Interplay of host and parasitic PLA2 activity during infection
Eicosanoids produced by pathogens often play a dual role in metabolism or maturation of the organism and cross-talk with the host inflammatory response (Noverr et al., 2003). In protozoan parasites specifically, the PLA2 family of enzymes have been implicated in invasion, membrane remodeling, virulence, and disease progression. PGE2 inhibition of IL-12 plays a critical role in disease progression of Leishmania major (Passero et al., 2008). These intracellular parasites are cholesterol auxotrophs and salvage lipids from the host during infection. An L. major-derived PLA2 is responsible for hydrolyzing platelet-activating factor and contributes to virulence in vivo (Pawlowic & Zhang, 2012). Ca2+-dependent PLA2 produced by Trypanosoma brucei releases AA and stimulates Ca2+ influx into the parasite, which has been associated with control of infection (Eintracht et al., 1998). Similar AA-regulated Ca2+ influx was seen in T. cruzi and L. donovani (Catisti et al., 2000). T. brucei also has a lysoPLA that can release AA by sequentially hydrolyzing the sn1 and sn2 acyl chains in phospholipids (Ridgley & Ruben, 2001). Activation of T. cruzi-infection macrophages by LTB4 induces the production of TNFα. This pro-inflammatory cytokine drives the release of NO, which mediates parasite killing (Talvani et al., 2002). A lytic factor important for the virulence of Trichomonas vaginalis was determined to be a PLA2 and contributes to tissue damage and inflammation during infection through the downstream production of PGs and LTs (Lubick & Burgess, 2004).
PLA2s have been emerging as critical enzymes in the pathogenesis of apicomplexans in recent years as well. T. gondii secretes both a Ca2+-dependent and -independent PLA2 that are involved in host cell penetration by facilitating membrane fusion during invasion and synthesis of LOX and COX eicosanoid products (Saffer et al., 1989; Saffer & Schwartzman, 1991; Thardin et al., 1993). The Ca2+-independent PLA2 is resistant to most PLA2 inhibitors tested unlike the host enzymes, which could be exploited for drug development (Cassaing et al., 2000). The released PUFAs themselves could also aid host cell invasion by increasing the fluidity and permeability of the host cell membrane (Li et al., 2008). IFNγ blocks the activities of the T. gondii PLA2s, suggesting another mechanism of protection for this Th1-type cytokine against active invasion by T. gondii (Gomez-Marín et al., 2002). While the Th1 immune response is used by the host to clear parasitic infection, the cytokines from this immune response signals T. gondii to shift into the chronic bradyzoite stage suggesting that both stimulation and resolution of the inflammatory response aid survival of T. gondii to its chronic stage. Fatty acids from T. gondii reduce the secretion of TNFα, which is normally induced by protozoan glycolipids, through the inhibition of NF-κβ activation in the host (Debierre-Grockiego et al., 2007).
Phospholipases in lipid remodeling and scavenging in Apicomplexa
Apicomplexans are auxotrophs for many lipid species such as cholesterol and phospholipids (Gupta et al., 2005; Milovanović et al., 2017). Phospholipids are important components of membranes and are also involved in signaling, protein turnover and attachment to membranes, and membrane fluidity (Ramakrishnan et al., 2013). Apicomplexans exploit the host lipidome through a variety of mechanisms (Rub et al., 2013). T. gondii can acquire lipid precursors from its environment and use them to synthesize all of its major glycerophospholipids (Charron & Sibley, 2002). Previous studies suggest that the parasite is capable of synthesizing only 50% of the phosphatidylserine (PS) and less than 10% of the phosphatidylcholine (PC) necessary to maintain the rate of replication (Gupta et al., 2005). Accumulating enough phospholipids for replication of the parasitic membranes requires both internal synthesis and scavenging from the host and is likely a rate-limiting step in replication. The high abundance of PC in the T. gondii membrane, about 75%, increases membrane fluidity, which may help the parasite accommodate a wide range of hosts and environments (Gupta et al., 2005; Seeber et al., 2008). All of Apicomplexa have much higher levels of PC than other eukaryotic cells, which typically have 30–40% of their phospholipids comprised of PC (Mitschler et al., 1994).
A mechanism for hijacking host resources involves accumulation of lipid droplets in host cells during infection and the concomitant downregulation of host genes involved in lipid metabolism, such as the mammalian patatin-like phospholipase ATGL (Hu et al., 2017). In human mast cells, silencing ATGL caused an increase in neutral lipids in lipid droplets as well as a decrease in eicosanoid production (Dichlberger et al., 2014). LOX and COX enzymes have been shown to localize to lipid droplets and are considered to represent a site of prostaglandin and leukotriene synthesis, so ATGL release of arachidonic acid from lipid droplet-associated triacylglycerol could be a crucial step for eicosanoid production in mast cells (Schreiber & Zechner, 2014). During Apicomplexa infection, host lipid droplets localize near and within the parasitophorous vacuole but are too large for direct endocytosis, so host- and parasite-derived enzymes, such as PLPs, are likely involved in releasing lipids from the droplets (Nolan et al., 2017). In mammalian cells, phospholipids can move freely between membranes without vesicular trafficking using mitochondrial-associated membranes. The PVM of T. gondii parasites can associate with the ER and mitochondria of the host, which could allow for easier lipid exchange between the host and parasite (Coppens, 2006).
The most abundant phospholipid in Plasmodium membranes is also PC at 40–50% of the total phospholipids (Dechamps et al., 2010) Plasmodium cannot synthesize fatty acids or cholesterol de novo (Labaied, 2011; Fish, 1995). They have multiple pathways to overcome this by either scavenging lipid precursors such as choline and ethanolamine to be metabolized further by the parasite or actively taking up FAs and phospholipids from the erythrocyte membranes and human serum (Mitamura & Palacpac, 2003; Krishnegowda & Gowda, 2003) Plasmodium parasites induce a 6-fold increase in the overall phospholipid content in the membranes of their host cells, particularly infected erythrocytes (Dechamps et al., 2010). The membranes become highly susceptible to phospholipase activity due to a rearrangement of those phospholipids to the outer leaflet of the host cell membrane bilayer (Joshi & Gupta, 1988). Of note, lipid metabolism is effectively absent in uninfected mature erythrocytes, so the parasite must rely on its own phospholipid synthetic machinery to meet its requirements for replication (Hsiao et al., 1991) P. berghei PLA2 contributes to several life cycle transitions. PLA2 activity is involved in oocyst rupture and release of sporozoites, epithelial cell membrane penetration for migration to the liver, and finally PVM rupture in the infected hepatocyte (Burda et al., 2015) and egress of merozoites into the blood stream (Bhanot et al., 2005). Regulation of the immune response is also critical in the pathogenesis of cerebral malaria (CM). Patients who died or developed long-term side effects from CM had higher plasma PLA2 levels than those who recovered. This increase in PLA2 was also associated with excessive production of pro-inflammatory cytokines, likely leading to the severe pathogenesis (Gupta et al., 2017). The PUFAs docosahexaenoic acid, eicosapentaenoic acid, AA, and linoleic acid were able to inhibit P. falciparum growth, but oleic acid and docosanoic acid did not inhibit growth. Addition of the oxidized derivatives of the PUFAs led to direct killing of the parasites (Kumaratilake et al., 1992). PUFAs, but not their hydroperoxy derivatives, markedly increased neutrophil-mediated killing of parasites (Kumaratilake et al., 1997).
A PLA2 secreted by Cryptosporidium parvum is critical for host cell invasion, but the specific gene has not yet been identified (Pollok et al., 2003). In C. parvum, PC was 65% of the total phospholipids (Mitschler et al., 1994). These high levels of PC in the parasitophorous vacuole may help avoid fusion with the lysosome, as PC is more stable as a bilayer than other phospholipids. While T. gondii has similarly high levels of PC, Plasmodium may not require such high levels during the intraerythrocytic stages as red blood cells lack most fusogenic organelles. Eimeria neischulzi has 75–85% PC in both sporulated and unsporulated oocysts and the aqueous insolubility and relative inertness may contribute to their resistant nature (Mitschler, 1994).
Apicomplexa-derived patatin-like phospholipases
A T. gondii PLP, named TgPL1, was discovered during a mutagenesis screen to identify genes important for the inhibition of NO production from activated macrophages (Mordue et al., 2007). This mutant had a defect in its ability to survive in active macrophages and increased cytokines with fewer inflammatory lesions in late chronic infection compared to wild type. TgPL1 localizes within the parasite during the tachyzoite stage but moves out to the PV and cyst wall during the bradyzoite stage, further supporting its role in late chronic infection (Tobin et al., 2014). TgPL1 lacks the predicted catalytic serine essential for PLA2 activity, so its effects are likely not due to PLA2 activity (Tobin & Knoll, 2012). This led to a screen of the T. gondii genome where six potential PLP enzymes were found, four of which had retained the catalytic serine (Fig. 4). Recently TgPL2 was shown to be critical in maintaining membrane integrity of the apicoplast (Lévêque et al., 2017). Lipid analysis revealed that TgPL2 is important for maintaining levels of apicoplast-generated fatty acids and regulating PC and LPC levels in the parasite. A third PLP, TgPL3, was previously identified in a screen for T. gondii virulence in vivo and is currently under investigation (Frankel et al., 2007). In P. falciparum, the localization of a putative PLP, Pf3D7_0209100, was shown to be cytosolic during all asexual and gametocyte stages with additional nuclear localization during the trophozoite and gametocyte stages (Pappa et al., 2017). Another predicted Plasmodium PLP, Pf3D7_092400, was downregulated in response to choline kinase inhibition. Choline kinase is a key enzyme in the synthesis of PC, a phospholipid comprising a large portion of the parasitophorous vacuole membrane, and is critical for Plasmodium replication during the asexual stages. Pf3D7_092400 inhibition in response to reduced PC synthesis could indicate a role for the PLP in maintaining membrane lipid homeostasis (Ridzuan et al., 2014).
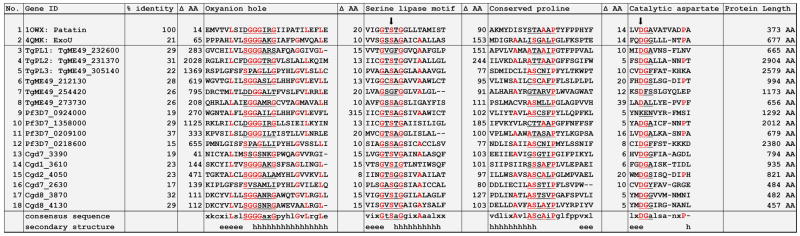
Fig. 4.
Alignment of conserved patatin domains in Apicomplexa PLPs. 16 Toxoplasma, Plasmodium, and Cryptosporidium PLP genes were aligned to PDB structures of Patatin and P. aeruginosa ExoU using the CLUSTAL Omega and MAFFT methods in MegAlign Pro (DNASTAR). PROMALS3D and SWISS-MODEL were used to confirm the predicted secondary and tertiary structures aligned with the known PLP structures. The known conserved patatin motifs are underlined and residues conserved in more than 60% of the sequences are colored red. Arrows indicate the catalytic S/A dyad. % identity = 100*(1 – distance) where distance is the uncorrected pairwise distance between the full sequence of each gene compared to patatin. ΔAA is the number of residues before and after each motif.
A search of Apicomplexa genomes reveals that these PLP domains are present broadly and in high numbers across the phylum. In addition to the 6 putative PLPs found in the T. gondii genome, there are 6 in Cryptosporidium and Hammondia, 5 in Eimeria and Neospora, and 4 in Plasmodium and Sarcocystis. Most PLPs contain a conserved proline between the lipase domain and the catalytic aspartate. The proline motif in bacterial PLPs was typically ASxxxP, whereas in mammalian PLPs, the motif was AAP (Banerji & Flieger, 2004). An alignment of the Toxoplasma, Plasmodium and Cryptosporidium PLPs revealed a mixture of both motifs but the majority aligned with ASxxxP, suggesting these PLPs have a bacterial origin (Fig. 4). TgPL2 and its Plasmodium ortholog, Pf3D7_1358000, notably contained the AAP motif. As discussed earlier, mammalian PLPs contribute mainly to lipid metabolism and turnover, similar to the role TgPL2 plays in the apicoplast. It will be intriguing to see if this motif distinction predicts the role of the apicomplexan PLPs in either lipid metabolism or inflammation. The Apicomplexa PLPs fall roughly into 6 ortholog groups with an additional 5 ortholog groups specific for Cryptosporidium or Eimeria species and a handful of PLPs that did not fall into any of the ortholog groups (Fig. 5). Several ortholog groups contain signal peptides and/or transmembrane domains that will direct the localization and specialized activities of the PLPs. For example, TgPL1 has a signal peptide that may be responsible for the localization shift of the protein out to the cyst wall during chronic infection. PLPs in the ortholog group named OG5_129101 have signal peptides, multiple transmembrane domains, and a domain of unknown function (DUF3336). While functionally uncharacterized, this domain has been described adjacent to the GxSxG lipase domain in Tgl3 Tgl4 and Tgl5 in Saccharomyces cerevisiae. All three have triacyglycerol lipase activity and localize to lipid particles (Athenstaedt & Daum, 2003; Athenstaedt & Duam, 2005). Tgl4 is a functional ortholog of mammalian ATGL and has additional steryl ester hydrolase and phospholipase activities (Rajakumari & Duam 2010). These data give insight into the potential role of this Apicomplexa PLP ortholog group and will help guide the enzymatic assays and conditions used during further experiments. The conservation of multiple PLPs across the phylum suggests these enzymes play a critical role for the parasites. Based on the diversity, these enzymes are likely to fill unique roles to address the changing needs across the life cycle of the parasites in both lipid metabolism and host immune regulation.
Future perspectives
As seen in the PLP sequence alignments, Apicomplexa PLPs more closely resemble bacterial PLPs than eukaryotic PLPs. Additionally, compounds identified as potent inhibitors of ExoU PLA2 activity did not inhibit the activity of mammalian-derived PLA2s (Lee et al., 2007). These parasite PLPs will likely be excellent drug targets as TgPL2 has already been characterized as a critical gene to parasite growth and replication (Lévêque et al., 2017). Treatment of T. gondii with atglistatin, an inhibitor of ATGL, led to a decrease in parasite replication proportional to drug concentration and disorganization of the parasites within the PV. There was also accumulation of lipids in the lumen of the PV and between the parasites, indicative of dysfunctional lipid metabolism (Nolan et al., 2017). This paper suggested the effects of the drug were based solely on the mammalian ATGL, however the drug could also be acting on one or more of the six parasite-derived PLPs. Anti-bacterial drugs gentamicin and amikacin have modest effects on Plasmodium development from the ring stage to mature trophozoites and schizonts. While the ring stage matures in erythrocytes, it ingests host cell cytoplasm through endocytosis. The endocytic vesicle fuses with the parasite food vacuole and phospholipases contribute to the digestion of its contents. Drug treatment resulted in reduced host cytosol digestion concurrent with reduced PLA2 activity in the parasite cytosol, suggesting these drugs may function through the inhibition of a parasite-derived PLA2 (Krugliak et al., 1987). Anti-malarials chloroquine, quinine and arteether also produce minor inhibition of PLA2 activity associated with P. falciparum infection of erythrocytes with IC50s of 1.3, 1.0 and 1.8 mM, respectively (Zidovetzki et al., 1993). Arteether and quinine have been shown to disrupt the structure of lipid membranes, which could alter the ability of PLA2s to access their substrates leading to reduced activity. In chloroquine-sensitive parasites, the drug enters the parasite in a neutral form that can move freely across the food vacuole membrane, but in this acidic environment, it becomes charged and can no longer diffuse out of the vacuole (Saliba et al., 1998). The accumulation of chloroquine to higher concentrations within the food vacuole compared to the rest of the parasite may give some therapeutic potential for the current IC50 of this drug on PLA2 activity. However, these drugs are likely more useful as a starting point in designing more specific and potent drugs against the parasitic PLA2 enzymes. A mammalian PLA2 inhibitor, AACOCF3, had an inhibitory anti-malarial effect at low μM concentrations, but whether this AA analogue is targeting host or parasite-derived PLA2 is not yet known (Pappa et al., 2017). An alternative route to fight the spread of malaria is to target the mosquito vector where oocyst formation occurs. When active or inactive PLA2 from snake and bee venoms was fed to mosquitoes, the protein associated with the midgut surface and prevented ookinete attachment and invasion of the midgut epithelium, critical for oocyst formation (Zieler et al., 2001). Further, mosquitoes were genetically manipulated to express an inactive form of PLA2 under the control of a midgut-specific promoter and showed similar inhibition of oocyst development (Rodrigues et al., 2008).
There is crosstalk among the many pathways discussed here and inhibition of one enzyme can stimulate activity in another pathway to maintain a balance (Buczynski et al., 2009; Quehenberger & Dennis, 2011; Norris & Dennis, 2012). A systemic approach is needed to fully understand the entire lipidomic response to host and pathogen interactions. The emerging field of lipidomics is largely driven by the improving mass spectrometry methods integrated with better detection and characterization of the enzymes involved in lipid anabolism and catabolism. In addition to the lipidomics advancements, Apicomplexa have become more genetically manipulatable through new methods such as CRISPR/Cas9 (Sidik et al., 2014; Brown et al., 2014). Combining drug development with lipidomics and targeted parasite genetic manipulation will allow for a better understanding of the interwoven roles these PLPs play in parasite development and survival in the host.
Supplementary Material
References
- Anderson DM, Schmalzer KM, Sato H, Casey M, Terhune SS, Haas AL, Feix JB, Frank DW. Ubiquitin and ubiquitin-modified proteins activate the Pseudomonas aeruginosa T3SS cytotoxin, ExoU. Mol Microbiol. 2011;82:1454–1467. doi: 10.1111/j.1365-2958.2011.07904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DM, Sato H, Dirck AT, Feix JB, Frank DW. Ubiquitin activates patatin-like phospholipases from multiple bacterial species. J Bacteriol. 2015;197:529–541. doi: 10.1128/JB.02402-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athenstaedt K, Daum G. YMR313c/TGL3 encodes a novel triacylglycerol lipase located in lipid particles of Saccharomyces cerevisiae. J Biol Chem. 2003;278:23317–23323. doi: 10.1074/jbc.M302577200. [DOI] [PubMed] [Google Scholar]
- Athenstaedt K, Daum G. Tgl4p and Tgl5p, two triacylglycerol lipases of the yeast Saccharomyces cerevisiae are localized to lipid particles. J Biol Chem. 2005;280:37301–37309. doi: 10.1074/jbc.M507261200. [DOI] [PubMed] [Google Scholar]
- Banerji S, Flieger A. Patatin-like proteins: A new family of lipolytic enzymes present in bacteria? Microbiology. 2004;150:522–525. doi: 10.1099/mic.0.26957-0. [DOI] [PubMed] [Google Scholar]
- Banerji S, Aurass P, Flieger A. The manifold phospholipases A of Legionella pneumophila - Identification, export, regulation, and their link to bacterial virulence. Int J Med Microbiol. 2008;298:169–181. doi: 10.1016/j.ijmm.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Baulande S, Lasnier F, Lucas M, Pairault J. Adiponutrin, a transmembrane protein corresponding to a novel dietary- and obesity-linked mRNA specifically expressed in the adipose lineage. J Biol Chem. 2001;276:33336–33344. doi: 10.1074/jbc.M105193200. [DOI] [PubMed] [Google Scholar]
- Bhanot P, Schauer K, Coppens I, Nussenzweig V. A surface phospholipase is involved in the migration of Plasmodium sporozoites through cells. J Biol Chem. 2005;280:6752–6760. doi: 10.1074/jbc.M411465200. [DOI] [PubMed] [Google Scholar]
- Botting RM. Cyclooxygenases in Biology and Disease. Encycl Pain. 2007;12:511–515. [Google Scholar]
- Brown KM, Lee TD, Sibley LD. Efficient gene disruption in diverse strains of Toxoplasma gondii using CRISPR / CAS9 Efficient Gene Disruption in Diverse. MBio. 2014;5:1–11. doi: 10.1128/mBio.01114-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buczynski M, Dumlao D, Dennis EA. An integrated omics analysis of eicosanoid biology. J Lipid Res. 2009;50:1015–1038. doi: 10.1194/jlr.R900004-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burda PC, et al. A Plasmodium phospholipase is involved in disruption of the liver stage parasitophorous vacuole membrane. PLoS Pathog. 2015;11:e1004760. doi: 10.1371/journal.ppat.1004760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JE, Dennis EA. Phospholipase A2 structure/function, mechanism, and signaling. Journal of lipid research. 2009;50:S237–S242. doi: 10.1194/jlr.R800033-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camera SL, et al. A pathogen-inducible patatin-like lipid acyl hydrolase facilitates fungal and bacterial host colonization in Arabidopsis. Plant J. 2005;44:810–825. doi: 10.1111/j.1365-313X.2005.02578.x. [DOI] [PubMed] [Google Scholar]
- Camera SL, et al. The Arabidopsis Patatin-Like Protein 2 (PLP2) Plays an Essential Role in Cell Death Execution and Differentially Affects Biosynthesis of Oxylipins and Resistance to Pathogens. Mol Plant-Microbe Interact. 2009;22:469–481. doi: 10.1094/MPMI-22-4-0469. [DOI] [PubMed] [Google Scholar]
- Carracedo A, Cantley LC, Pandolfi PP. Cancer metabolism: fatty acid oxidation in the limelight. Nat Rev Cancer. 2013;13:227–32. doi: 10.1038/nrc3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassaing S, et al. Toxoplasma gondii secretes a calcium-independent phospholipase A2. Int J Parasitol. 2000;30:1137–1142. doi: 10.1016/s0020-7519(00)00101-6. [DOI] [PubMed] [Google Scholar]
- Catisti R, Uyemura SA, Docampo R, Vercesi AE. Calcium mobilization by arachidonic acid in trypanosomatids. Mol Biochem Parasitol. 2000;105:261–271. doi: 10.1016/s0166-6851(99)00186-3. [DOI] [PubMed] [Google Scholar]
- Charron AJ, Sibley D. Host cells: mobilizable lipid resources for the intracellular parasite Toxoplasma gondii. J cell Sci. 2002;115:3049–3059. doi: 10.1242/jcs.115.15.3049. [DOI] [PubMed] [Google Scholar]
- Chehab EW, et al. Distinct roles of jasmonates and aldehydes in plant-defense responses. PLoS One. 2008;3:1–10. doi: 10.1371/journal.pone.0001904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppens I. Contribution of host lipids to Toxoplasma pathogenesis. Cell Microbiol. 2006;8:1–9. doi: 10.1111/j.1462-5822.2005.00647.x. [DOI] [PubMed] [Google Scholar]
- Dalli J, Serhan CN. Specific lipid mediator signatures of human phagocytes: Microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood. 2012;120:60–72. doi: 10.1182/blood-2012-04-423525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debierre-Grockiego F, et al. Fatty acids isolated from Toxoplasma gondii reduce glycosylphosphatidylinositol-induced tumor necrosis factor alpha production through inhibition of the NF-kappaB signaling pathway. Infect Immun. 2007;75:2886–93. doi: 10.1128/IAI.01431-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechamps S, Shastri S, Wengelnik K, Vial HJ. Glycerophospholipid acquisition in Plasmodium – A puzzling assembly of biosynthetic pathways. Int J Parasitol. 2010;40:1347–1365. doi: 10.1016/j.ijpara.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Dhondt S, Geoffroy P, Stelmach BA, Legrand M, Heitz T. Soluble phospholipase A2 activity is induced before oxylipin accumulation in tobacco mosaic virus-infected tobacco leaves and is contributed by patatin-like enzymes. Plant J. 2000;23:431–440. doi: 10.1046/j.1365-313x.2000.00802.x. [DOI] [PubMed] [Google Scholar]
- Dichlberger A, Schlager S, Maaninka K, Schneider WJ, Kovanen PT. Adipose triglyceride lipase regulates eicosanoid production in activated human mast cells. J Lip Res. 2014;55:2471–2478. doi: 10.1194/jlr.M048553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eintracht J, Maatha R, Mellors A, Ruben L. Calcium entry in Trypanosoma brucei is regulated by phospholipase A2 and arachidonic acid. Biochem J. 1998;336:659–666. doi: 10.1042/bj3360659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierro IM, Serhan CN. Mechanisms in anti-inflammation and resolution: The role of lipoxins and aspirin-triggered lipoxins. Brazilian J Med Biol Res. 2001;34:555–566. doi: 10.1590/s0100-879x2001000500002. [DOI] [PubMed] [Google Scholar]
- Fish WR. Lipid and Membrane Metabolism of the Malaria Parasite and the African Trypanosome. Mol Biochem Parasitol. 1995;8:133–145. [Google Scholar]
- Frankel MB, Mordue DG, Knoll LJ. Discovery of parasite virulence genes reveals a unique regulator of chromosome condensation 1 ortholog critical for efficient nuclear trafficking. Proc Natl Acad Sci USA. 2007;104:10181–10186. doi: 10.1073/pnas.0701893104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M, Yamazaki M, Loe DW, Westlake CJ, Grant CE, Cole SP, Deeley RG. Multidrug Resistance Protein Identification of Regions Required for Active Transport of Leukotriene C4. Journal of Biological Chemistry. 1998;273:10733–10740. doi: 10.1074/jbc.273.17.10733. [DOI] [PubMed] [Google Scholar]
- Gomez-Marín JE, et al. Involvement of secretory and cytosolic phospholipases a2 during infection of THP1 human monocytic cells with Toxoplasma gondii. Effect of interferon γ. Parasitol Res. 2002;88:208–216. doi: 10.1007/s00436-001-0525-z. [DOI] [PubMed] [Google Scholar]
- Gupta N, Zahn MM, Coppens I, Joiner KA, Voelker DR. Selective disruption of phosphatidylcholine metabolism of the intracellular parasite Toxoplasma gondii arrests its growth. J Biol Chem. 2005;280:16345–16353. doi: 10.1074/jbc.M501523200. [DOI] [PubMed] [Google Scholar]
- Gupta S, et al. Extensive alterations of blood metabolites in pediatric cerebral malaria. PLoS One. 2017;12:1–13. doi: 10.1371/journal.pone.0175686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harizi H, Corcuff JB, Gualde N. Arachidonic-acid-derived eicosanoids: roles in biology and immunopathology. Trends Mol Med. 2008;14:461–469. doi: 10.1016/j.molmed.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Harizi H, Juzan M, Moreau JF, Gualde N. Prostaglandins inhibit 5-lipoxygenase-activating protein expression and leukotriene B4 production from dendritic cells via an IL-10-dependent mechanism. J Immunol. 2003;170:139–146. doi: 10.4049/jimmunol.170.1.139. [DOI] [PubMed] [Google Scholar]
- Hsiao LL, Howard RJ, Aikawa M, Taraschi TF. Modification of host cell membrane lipid composition by the intra-erythrocytic human malaria parasite Plasmodium falciparum. Biochem J. 1991;274:121–132. doi: 10.1042/bj2740121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Binns D, Reese ML. The coccidian parasites Toxoplasma and Neospora dysregulate mammalian lipid droplet biogenesis. J Biol Chem. 2017;292:11009–11020. doi: 10.1074/jbc.M116.768176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley BP, Siccardi D, Mrsny RJ, McCormick BA. Polymorphonuclear Cell Transmigration Induced by Pseudomonas aeruginosa Requires the Eicosanoid Hepoxilin A3. J Immunol. 2004;173:5712–5720. doi: 10.4049/jimmunol.173.9.5712. [DOI] [PubMed] [Google Scholar]
- Joshi P, Gupta CM. Abnormal membrane phospholipid organization in Plasmodium falciparum-infected human erythrocytes. Br J Haematol. 1988;68:255–259. doi: 10.1111/j.1365-2141.1988.tb06198.x. [DOI] [PubMed] [Google Scholar]
- Kershaw EE, Hamm JK, Verhagen LA, Peroni O, Katic M, Flier JS. Adipose triglyceride lipase: function, regulation by insulin, and comparison with adiponutrin. Diabetes. 2006;55:148–157. [PMC free article] [PubMed] [Google Scholar]
- Kienesberger PC, Oberer M, Lass A, Zechner R. Mammalian patatin domain containing proteins: a family with diverse lipolytic activities involved in multiple biological functions. J Lipid Res. 2008;50:S63–S68. doi: 10.1194/jlr.R800082-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KY, et al. SREBP-2/PNPLA8 axis improves non-alcoholic fatty liver disease through activation of autophagy. Sci Rep. 2016;6:35732. doi: 10.1038/srep35732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kock J, Coetzee D, Van Dyk M, Truscott M, Cloete P, Van Wyk V, Augustyn O. Evidence for pharmacologically active prostaglandins in yeasts. S Afr J Sci. 1991;87:73–76. [Google Scholar]
- Köhler GA, et al. Phospholipase A2 and Phospholipase B activities in fungi. Biochim Biophys Acta - Mol Cell Biol Lipids. 2006;1761:1391–1399. doi: 10.1016/j.bbalip.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer RM, Sharp JD. Structure, function and regulation of Ca2+-sensitive cytosolic phospholipase A2 (cPLA2) FEBS letters. 1997;410:49–53. doi: 10.1016/s0014-5793(97)00322-0. [DOI] [PubMed] [Google Scholar]
- Krishnegowda G, Gowda DC. Intraerythrocytic Plasmodium falciparum incorporates extraneous fatty acids to its lipids without any structural modification. Mol Biochem Parasitol. 2003;132:55–58. doi: 10.1016/j.molbiopara.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Krugliak M, Waldman Z, Ginsburg H. Gentamicin and amikacin repress the growth of Plasmodium falciparum in culture, probably by inhibiting a parasite acid phospolipase. Life Sci. 1987;40:1253–1257. doi: 10.1016/0024-3205(87)90581-9. [DOI] [PubMed] [Google Scholar]
- Ku B, et al. VipD of Legionella pneumophila targets activated Rab5 and Rab22 to interfere with endosomal trafficking in macrophages. PLoS Pathog. 2012;8:e1003082. doi: 10.1371/journal.ppat.1003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaratilake LM, Ferrante A, Robinson BS, Jaeger T, Poulos A. Enhancement of neutrophil-mediated killing of Plasmodium falciparum asexual blood forms by fatty acids: Importance of fatty acid structure. Infect Immun. 1997;65:4152–4157. doi: 10.1128/iai.65.10.4152-4157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaratilake LM, Robinson BS, Ferrante A, Poulos A. Antimalarial Properties of n-3 and n-6 polyunsaturated fatty acids: In vitro effects on Plasmodium falciparum and in vivo effects on P. berghei. J Clin Invest. 1992;89:961–967. doi: 10.1172/JCI115678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labaied M, Jayabalasingham B, Bano N, Cha SJ, Sandoval J, Guan G, Coppens I. Plasmodium salvages cholesterol internalized by LDL and synthesized de novo in the liver. Cell Microbiol. 2011;13:569–586. doi: 10.1111/j.1462-5822.2010.01555.x. [DOI] [PubMed] [Google Scholar]
- Lagocki JW, Emken EA, Law JH, Kezdy FJ. Kinetic Analysis of the Action of Soybean Lipoxygenase Linoleic Acid. J Biol Chem. 1976;251:6001–6006. [PubMed] [Google Scholar]
- Lake AC, Sun Y, Li JL, Kim JE, Johnson JW, Li D, et al. Expression, regulation, and triglyceride hydrolase activity of Adiponutrin family members. J Lipid Res. 2005;46:2477–2487. doi: 10.1194/jlr.M500290-JLR200. [DOI] [PubMed] [Google Scholar]
- Lambeau G, Gelb MH. Biochemistry and physiology of mammalian secreted phospholipases A2. Annu Rev Biochem. 2008;77:495–520. doi: 10.1146/annurev.biochem.76.062405.154007. [DOI] [PubMed] [Google Scholar]
- Lee VT, et al. Pseudolipasin A is a specific inhibitor for phospholipase A2 activity of Pseudomonas aeruginosa cytotoxin ExoU. Infect Immun. 2007;75:1089–1098. doi: 10.1128/IAI.01184-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévêque MF, et al. TgPL2, a patatin-like phospholipase domain-containing protein, is involved in the maintenance of apicoplast lipids homeostasis in Toxoplasma. Mol Microbiol. 2017;0:1–17. doi: 10.1111/mmi.13694. [DOI] [PubMed] [Google Scholar]
- Li L, Li X, Yan J. Alterations of concentrations of calcium and arachidonic acid and agglutinations of microfilaments in host cells during Toxoplasma gondii invasion. Vet Parasitol. 2008;157:21–33. doi: 10.1016/j.vetpar.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Liu Y, et al. PNPLA5-knockout rats induced by CRISPR/Cas9 exhibit abnormal bleeding and lipid level. J Integr Agric. 2017;16:169–180. [Google Scholar]
- Lubick KJ, Burgess DE. Purification and Analysis of a Phospholipase A2-Like Lytic Factor of Trichomonas vaginalis. Infect Immun. 2004;72:1284–1290. doi: 10.1128/IAI.72.3.1284-1290.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milovanović I, Busarčević M, Trbovich A, Ivović V, Uzelac A, Djurković-Djaković O. Evidence for host genetic regulation of altered lipid metabolism in experimental toxoplasmosis supported with gene data mining results. PLoS One. 2017;12:e0176700. doi: 10.1371/journal.pone.0176700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitamura T, Palacpac NM. Lipid metabolism in Plasmodium falciparum-infected erythrocytes: possible new targets for malaria chemotherapy. Microbes Infect. 2003;5:545–552. doi: 10.1016/s1286-4579(03)00070-4. [DOI] [PubMed] [Google Scholar]
- Mitschler RR. Doctoral thesis. Kansas State University; Kansas, USA: 1994. Lipid biology of Cryptosporidium parvum and Eimeria nieschulzi (Apicomplexa) [Google Scholar]
- Mitschler RR, Welti R, Upton SJ. A Comparative Study of Lipid Compositions of Crypfosporidium parvum (Apicomplexa) and Madin-Darby Bovine Kidney Cells. J Euk Microbiol. 1994;41:8–12. doi: 10.1111/j.1550-7408.1994.tb05927.x. [DOI] [PubMed] [Google Scholar]
- Moltke J, Trinidad NJ, Moayeri M, Kintzer AF, Wang SB, Rooijen N, Brown CR, Krantz BA, Leppla SH, Gronert K, Vance RE. Rapid induction of inflammatory lipid mediators by the inflammasome in vivo. Nature. 2012;490:107–111. doi: 10.1038/nature11351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordue DG, Scott-Weathers CF, Tobin CM, Knoll LJ. A patatin-like protein protects Toxoplasma gondii from degradation in activated macrophages. Mol Microbiol. 2007;63:482–496. doi: 10.1111/j.1365-2958.2006.05538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrsny RJ, Gewirtz AT, Siccardi D, Savidge T, Hurley BP, Madara JL, McCormick BA. Identification of Hepoxilin A3 in inflammatory events: a required role in neutrophil migration across intestinal epithelia. Proc Natl Acad Sci USA. 2004;101:7421–7426. doi: 10.1073/pnas.0400832101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugesan S, Goldberg EB, Dou E, Brown WJ. Identification of Diverse Lipid Droplet Targeting Motifs in the PNPLA Family of Triglyceride Lipases. PLoS One. 2013;8:e64950. doi: 10.1371/journal.pone.0064950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert DW, Dalton TP. The role of cytochrome P450 enzymes in endogenous signaling pathways and environmental carcinogenesis. Nat Rev Cancer. 2006;6:947–960. doi: 10.1038/nrc2015. [DOI] [PubMed] [Google Scholar]
- Nigam S, Nodes J, Cichon G, Corey EJ, Pace-Asciak CR. Receptor-mediated action of hepoxilin A3 releases diacylglycerol and arachidonic acid from human neutrophils. Biochem Biophys Res Commun. 1990;171:944–948. doi: 10.1016/0006-291x(90)90775-i. [DOI] [PubMed] [Google Scholar]
- Nolan SJ, Romano JD, Coppens I. Host lipid droplets: An important source of lipids salvaged by the intracellular parasite Toxoplasma gondii. PLoS Pathog. 2017;13:e1006362. doi: 10.1371/journal.ppat.1006362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noor S, et al. Activation of cytosolic phospholipase A2α in resident peritoneal macrophages by Listeria monocytogenes involves listeriolysin O and TLR2. J Biol Chem. 2008;283:4744–4755. doi: 10.1074/jbc.M709956200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris PC, Gosselin D, Reichart D, Glass CK, Dennis EA. Phospholipase A2 regulates eicosanoid class switching during inflammasome activation. Proc Natl Acad Sci. 2014;111:12746–12751. doi: 10.1073/pnas.1404372111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris PC, Dennis EA. Omega-3 fatty acids cause dramatic changes in TLR4 and purinergic eicosanoid signaling. Proc Natl Acad Sci USA. 2012;109:8517–8522. doi: 10.1073/pnas.1200189109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noverr MC, Erb-Downward JR, Huffnagle GB. Production of eicosanoids and other oxylipins by pathogenic eukaryotic microbes. Clin Microbiol Rev. 2003;16:517–533. doi: 10.1128/CMR.16.3.517-533.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappa V, Mony V, Fajardo JE, Guo P, Kieken F, Ankarklev J, Noy T, Almo SC, Fiser A, Daily JP. Plasmodium falciparum PLA2: a potential novel anti-malarial target. Mol & Biochem Parasitology. 2017 In press. [Google Scholar]
- Passero LFD, Laurenti MD, Tomokane TY, Corbett CEP, Toyama MH. The effect of phospholipase A2 from Crotalus durissus collilineatus on Leishmania (Leishmania) amazonensis infection. Parasitol Res. 2008;102:1025–1033. doi: 10.1007/s00436-007-0871-6. [DOI] [PubMed] [Google Scholar]
- Pawlowic MC, Zhang K. Leishmania parasites possess a platelet-activating factor acetylhydrolase important for virulence. Mol Biochem Parasitol. 2012;186:11–20. doi: 10.1016/j.molbiopara.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri MH, Thul S, Ovchinnikova O, Back M. Differential regulation of monocytic expression of leukotriene and lipoxin receptors. Prostaglandins Other Lipid Mediat. 2015;121:138–143. doi: 10.1016/j.prostaglandins.2015.07.005. [DOI] [PubMed] [Google Scholar]
- Phillips RM, Six DA, Dennis EA, Ghosh P. In Vivo Phospholipase Activity of the Pseudomonas aeruginosa Cytotoxin ExoU and Protection of Mammalian Cells with Phospholipase A2 Inhibitors. J Biol Chem. 2003;278:41326–41332. doi: 10.1074/jbc.M302472200. [DOI] [PubMed] [Google Scholar]
- Pollok RCG, McDonald V, Kelly P, Farthing MJG. The role of Cryptosporidium parvum-derived phospholipase in intestinal epithelial cell invasion. Parasitol Res. 2003;90:181–6. doi: 10.1007/s00436-003-0831-8. [DOI] [PubMed] [Google Scholar]
- Qiao A, et al. Mouse patatin-like phospholipase domain-containing 3 influences systemic lipid and glucose homeostasis. Hepatology. 2011;54:509–521. doi: 10.1002/hep.24402. [DOI] [PubMed] [Google Scholar]
- Quehenberger O, Dennis EA. The human plasma lipidome. N Engl J Med. 2011;365:1812–1823. doi: 10.1056/NEJMra1104901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MS, Ammerman NC, Sears KT, Ceraul SM, Azad AF. Functional characterization of a phospholipase A2 homolog from Rickettsia typhi. J Bacteriol. 2010;192:3294–3303. doi: 10.1128/JB.00155-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajakumari S, Duam G. Multiple Functions as Lipase, Steryl Ester Hydrolase, Phospholipase, and Acyltransferase of Tgl4p from the Yeast Saccharomyces cerevisiae. J Biol Chem. 2010;285:5769–15776. doi: 10.1074/jbc.M109.076331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnana S, Serricchio M, Striepen B, Bütikofer P. Lipid Synthesis in Protozoan Parasites: a Comparison Between Kinetoplastids and Apicomplexans. Prog Lipid Res. 2013;52:488–512. doi: 10.1016/j.plipres.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgley EL, Ruben L. Phospholipase from Trypanosoma brucei releases arachidonic acid by sequential sn-1, sn-2 deacylation of phospholipids. Mol Biochem Parasitol. 2001;114:29–40. doi: 10.1016/s0166-6851(01)00234-1. [DOI] [PubMed] [Google Scholar]
- Ridzuan M, et al. Effect of choloine kinase inhibitor hexadecyltrimethylammonium bromide on Plasmodium falciparum gene expression. Southeast Asian J Trop Med Public Health. 2014;45:259–266. [PubMed] [Google Scholar]
- Rodrigues FG, et al. Expression of a mutated phospholipase A 2 in transgenic Aedes fluviatilis mosquitoes impacts Plasmodium gallinaceum development. Insect Mol Biol. 2008;17:175–183. doi: 10.1111/j.1365-2583.2008.00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouzer CA, Marnett LJ. Cyclooxygenases: structural and functional insights. J Lipid Res. 2008;50:S29–S34. doi: 10.1194/jlr.R800042-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rub A, Arish M, Husain SA, Ahmed N, Akhter Y. Host-lipidome as a potential target of protozoan parasites. Microbes and Infect. 2013;15:649–660. doi: 10.1016/j.micinf.2013.06.006. [DOI] [PubMed] [Google Scholar]
- Ryu SB. Phospholipid-derived signaling mediated by phospholipase A in plants. Trends Plant Sci. 2004;9:229–235. doi: 10.1016/j.tplants.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Saffer LD, Schwartzman JD. A soluble phospholipase of Toxoplasma gondii associated with host cell penetration. J Protozool. 1991;38:454–460. doi: 10.1111/j.1550-7408.1991.tb04816.x. [DOI] [PubMed] [Google Scholar]
- Saffer LD, Long Krug SA, Schwartzman JD. The role of phospholipase in host cell penetration by Toxoplasma gondii. Am J Trop Med Hyg. 1989;40:145–149. doi: 10.4269/ajtmh.1989.40.145. [DOI] [PubMed] [Google Scholar]
- Saliba KJ, Folb PI, Smith PJ. Role for the Plasmodium falciparum digestive vacuole in chloroquine resistance. Biochem Pharmacol. 1998;56:313–320. doi: 10.1016/s0006-2952(98)00140-3. [DOI] [PubMed] [Google Scholar]
- Samuelsson B, Dahlen SE, Lindgren JA, Rouzer CA, Serhan CN. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987;237:1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- Sato H, Frank DW. ExoU is a potent intracellular phospholipase. Mol Microbiol. 2004;53:1279–1290. doi: 10.1111/j.1365-2958.2004.04194.x. [DOI] [PubMed] [Google Scholar]
- Sawa T, et al. Pseudomonas aeruginosa Type III secretory toxin ExoU and its predicted homologs. Toxins. 2016;8:1–15. doi: 10.3390/toxins8110307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer GFE, Ryu SB, Wang X, Matos AR, Heitz T. Patatin-related phospholipase A: Nomenclature, subfamilies and functions in plants. Trends Plant Sci. 2010;15:693–700. doi: 10.1016/j.tplants.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Schreiber R, Zechner R. Lipolysis meets inflammation–arachidonic acid mobilization from fat. J Lip Res. 2014;55:C055673. doi: 10.1194/jlr.C055673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeber F, Limenitakis J, Soldati-Favre D. Apicomplexan mitochondrial metabolism: a story of gains, losses and retentions. Trends Parasitol. 2008;24:468–478. doi: 10.1016/j.pt.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Senda K, Yoshioka H, Doke N, Kawakita K. A cytosolic phospholipase A2 from potato tissues appears to be patatin. Plant Cell Physiol. 1996;37:347–353. doi: 10.1093/oxfordjournals.pcp.a028952. [DOI] [PubMed] [Google Scholar]
- Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewry PR. Tuber storage proteins. Annals of botany. 2003;91:755–769. doi: 10.1093/aob/mcg084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shureiqi I, Lippman SM. Lipoxygenase modulation to reverse carcinogenesis. Cancer Res. 2001;61:6307–6312. [PubMed] [Google Scholar]
- Sidik SM, Hackett CG, Tran F, Westwood NJ, Lourido S. Efficient genome engineering of Toxoplasma gondii using CRISPR/Cas9. PLoS One. 2014;9:e100450. doi: 10.1371/journal.pone.0100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Six DA, Dennis EA. The expanding superfamily of phospholipase A 2 enzymes: classification and characterization. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids. 2000;1488:1–19. doi: 10.1016/s1388-1981(00)00105-0. [DOI] [PubMed] [Google Scholar]
- Steczko J, Donoho GP, Clemens JC, Dixon JE, Axelrod B. Conserved histidine residues in soybean lipoxygenase: functional consequences of their replacement. Biochemistry. 1992;31:4053–4057. doi: 10.1021/bi00131a022. [DOI] [PubMed] [Google Scholar]
- Talvani A, et al. Leukotriene B4 Induces Nitric Oxide Synthesis in Trypanosoma cruzi -Infected Murine Macrophages and Mediates Resistance to Infection. Infect Immun. 2002;70:4247–4253. doi: 10.1128/IAI.70.8.4247-4253.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam VC. Lipidomic profiling of bioactive lipids by mass spectrometry during microbial infections. Semin Immunol. 2013;25:240–248. doi: 10.1016/j.smim.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thardin JF, et al. Eicosanoid production by mouse peritoneal macrophages during Toxoplasma gondii penetration: Role of parasite and host cell phospholipases. Infect Immun. 1993;61:1432–1441. doi: 10.1128/iai.61.4.1432-1441.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin CM, Knoll LJ. A patatin-like protein protects Toxoplasma gondii from degradation in a nitric oxide-dependent manner. Infect Immun. 2012;80:55–61. doi: 10.1128/IAI.05543-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin CM, Pittman KJ, Moser LA, Boldon KM, Knoll LJ. A Toxoplasma patatin-like protein changes localization and alters the cytokine response during Toxoplasmic encephalitis. Infect Immun. 2014;82:618–625. doi: 10.1128/IAI.00444-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance RE, Hong S, Gronert K, Serhan CN, Mekalanos JJ. The opportunistic pathogen Pseudomonas aeruginosa carries a secretable arachidonate 15-lipoxygenase. Proc Natl Acad Sci USA. 2004;101:2135–2139. doi: 10.1073/pnas.0307308101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villena JA, Roy S, Sarkadi-Nagy E, Kim KH, Sul HS. Desnutrin, an adipocyte gene encoding a novel patatin domain-containing protein, is induced by fasting and glucocorticoids: ectopic expression of desnutrin increases triglyceride hydrolysis. J Biol Chem. 2004;279:47066–47075. doi: 10.1074/jbc.M403855200. [DOI] [PubMed] [Google Scholar]
- Wang D, DuBois RN. Eicosanoids and cancer. Nature Reviews Cancer. 2010;10:181–193. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstead MV, Balsinde J, Dennis EA. Calcium-independent phospholipase A2: structure and function. Biochimica et Biophysica Acta. 2000;1488:28–39. doi: 10.1016/s1388-1981(00)00107-4. [DOI] [PubMed] [Google Scholar]
- Zhu W, Hammad LA, Hsu F, Mao Y, Luo ZQ. Induction of caspase 3 activation by multiple Legionella pneumophila Dot/Icm substrates. Cell Microbiol. 2013;15:1783–1795. doi: 10.1111/cmi.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zidovetzki R, Sherman IW, O’Brien L. Inhibition of Plasmodium falciparum Phospholipase A2 by Chloroquine, Quinine, and Arteether. J Parasitol. 1993;79:565–570. [PubMed] [Google Scholar]
- Zieler H, Keister DB, Dvorak JA, Ribeiro JM. A snake venom phospholipase A2 blocks malaria parasite development in the mosquito midgut by inhibiting ookinete association with the midgut surface. J Exp Biol. 2001;204:4157–4167. doi: 10.1242/jeb.204.23.4157. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.