Summary
Asymmetry in the outer membrane has long defined the cell envelope of Gram-negative bacteria. This asymmetry, with lipopolysaccharide (LPS) or lipooligosaccharide (LOS) exclusively in the outer leaflet of the membrane, establishes an impermeable barrier that protects the cell from a number of stressors in the environment. Work done over the past 5 years has shown that Acinetobacter baumannii has the remarkable capability to survive with inactivated production of lipid A biosynthesis and the absence of LOS in its outer membrane. The implications of LOS-deficient A. baumannii are far-reaching – from impacts on cell envelope biogenesis and maintenance, bacterial physiology, antibiotic resistance, and virulence. This review examines recent work that has contributed to our understanding of LOS-deficiency and compares it to studies done on Neisseria meningitidis and Moraxella catarrhalis; the two other organisms with this capability.
Introduction
A defining characteristic of Gram-negative bacteria is the asymmetric outer membrane, with lipopolysaccharide (LPS) localized exclusively to the outer leaflet (Kamio and Nikaido, 1976; Funahara and Nikaido, 1980). This structure provides an intrinsic barrier against a number of noxious compounds and potential antibiotics in the environment – a barrier that is typically essential for survival and has been reviewed extensively (Nikaido, 2003; Needham and Trent, 2013; Whitfield and Trent, 2014; Henderson et al., 2016). The core sugars and O-antigen of LPS are anchored to the membrane by the glycolipid lipid A, which is essential for cell survival and is a prerequisite for localization of the molecule to the cell surface (Okuda et al., 2016). Until 2012, there were limited exceptions to this rule. Two species, Neisseria meningitidis and Moraxella catarrhalis, had been shown to survive in the absence of LOS (an analogous molecule lacking the O-antigen polymer) via a directed mutation in a lipid A biosynthetic gene (Steeghs et al., 1998; Peng et al., 2005). The ability for other Neisseria and Moraxella species to survive in the absence of LOS is most likely not conserved, as attempts to make similar mutations in N. gonorrhoeae failed to yield viable colonies (Bos and Tommassen, 2005).
In 2012, it was first reported that Acinetobacter baumannii could survive in the absence of lipid A as well (Moffatt et al., 2010). This came at a time when A. baumannii was an emerging nosocomial epidemic and public health concern across the world; recently being placed as a critical priority pathogen for new antimicrobials by the World Health Organization (WHO | Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics, 2017). While the benefit of an asymmetric outer membrane relative to a standard phospholipid bilayer is apparent due to the impermeable barrier it provides, its lack of essentiality in multiple organisms provides an opportunity to understand mechanisms that are unique to maintenance of asymmetry versus a standard lipid bilayer. A. baumannii is the first organism to select for inactivated lipid A biosynthesis in the presence of polymyxins, a class of lipopeptide antibiotics that target LPS. With its clinical relevance combined with its tractability, non-fastidious growth, an expanding repertoire of genetic tools, and animal models, A. baumannii represents an important system for studying the outer membrane in the context of antimicrobial resistance and Gram-negative physiology.
The Outer Membrane Barrier
The impermeability of the outer membrane aids in resistance to various antimicrobials including antibiotics, toxic metabolites, antimicrobial peptides, and components of the innate immune response (Delcour, 2009). This impermeability is due in large part to the asymmetry of the outer membrane, with LPS molecules occupying the outer leaflet and glycerophospholipids in the inner leaflet. Intact LPS is a molecule comprised of three distinct moieties – the lipid A anchor, core oligosaccharide, and O-antigen (Raetz, 1990).
Lipid A is a hydrophobic glycolipid, for which the biosynthetic pathway is highly conserved and considered essential for Gram-negative bacteria (Figure 1) (Whitfield and Trent, 2014). Despite this conservation, variations occur downstream of lipid A biosynthesis by way of modifying enzymes that allow for the modification and adaptation of the lipid A species to particular niches increasing bacterial fitness. These modifying enzymes provide a valuable tool for establishing resistance to certain types of antibiotics and altering the permeability of the outer membrane (Raetz et al., 2007).
Fig. 1. Proposed biosynthesis and transport of A. baumannii lipooligosaccharide (LOS).
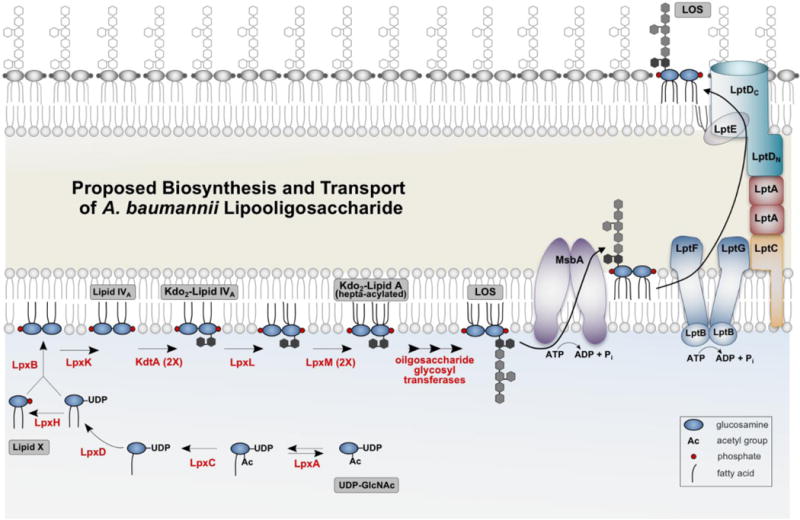
Typically, the Kdo2-lipid A domain of LPS or LOS is required for growth of gram-negative bacteria. The A. baumannii genome contains homologs of the necessary Lpx enzymes to synthesize the Kdo2-lipid A substructure similar to that of E. coli, although the Acinetobacter enzymes have not been fully characterized. Each step of Kdo2-lipid synthesis requires a single enzyme (red) with the early steps of the pathway catalyzed by soluble enzymes and the latter steps catalyzed by membrane associated proteins. Each acyl transferase has a preferred acyl chain specificity that is dictated by an active site hydrocarbon ruler and acyl-ACP (acyl carrier protein) serves as the acyl donor. Although the Kdo sugars are part of the inner core-oligosaccharide, in some organisms Kdo addition is required for lipid A biosynthesis as the final two steps, catalyzed by LpxL and LpxM, require the presence of covalently attached Kdo. In E. coli, both LpxL and LpxM catalyze the addition of a single acyl chain producing the final hexa-acylated lipid A species. However, A. baumannii LpxM catalyzes the addition of two acyl chains resulting in an hepta-acylated lipid A structure (Boll et al., 2015). The remaining sugars of the oligosaccharide of LOS are extended on the cytoplasmic leaflet of the inner membrane prior to transport by the ABC transporter MsbA that flips A. baumannii LOS to the periplasmic face of the inner membrane. Although absent in Acinetobacter, for organisms with a complete LPS structure, the O-antigen domain is added on the periplasmic face of the inner membrane. Finally, the intermembrane transport of LOS is performed by the Lpt (LPS transport) proteins that comprise an envelope-spanning translocation machine.
The highly conserved lipid A moiety is a glucosamine-based phospholipid that is phosphorylated at the 1 and 4ˈ positions. While this structure is conserved, the acylation patterns vary in different species. A. baumannii produces hepta-acylated lipid A as their major lipid A species, in contrast to hexa-acylated lipid A in Escherichia coli (Figure 2) (Boll et al., 2015a). The increased acylation of lipid A provides a higher degree of hydrophobicity relative to standard phospholipids in biological membranes. Lipid A serves as the anchor to two 3-deoxy-D-manno-oct-2-ulsonic acid (Kdo) residues, which along with an oligomer of sugars comprises the core region of LPS (Brabetz et al., 1997). Lastly, a repeating O-antigen can be attached to the core oligosaccharide, generating an intact LPS structure. Certain genera, including Acinetobacter, lack the lengthy O-antigen polysaccharide, but display an extended core-oligosaccharide. These endotoxin molecules are termed lipooligosaccharides (LOS) (Preston et al., 1996) (Figure 1). The lipid A (Figure 2) and core moieties in Acinetobacter are phosphorylated to varying extents, generating an overall negative charge for the endotoxin molecule (Vinogradov et al., 2002). Furthermore, divalent cation bridging between LPS molecules serves to fortify the membrane through balancing the electrostatic net (Nikaido and Vaara, 1985). This intact outer membrane provides an intrinsic barrier to many potentially toxic compounds, but the unique composition of the outer membrane also makes the cell susceptible to a particular class of antimicrobials – cationic antimicrobial peptides (CAMPS) (Olaitan et al., 2014).
Fig. 2. Comparison of lipid A structures of E. coli and A. baumannii.
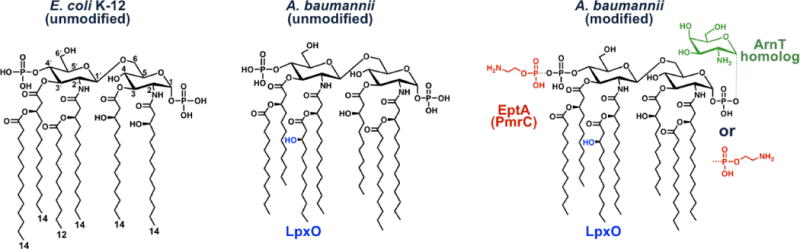
The predominant lipid A species of E. coli K-12 is hexa-acylated and phosphorylated at the 1- and 4′-positions. A. baumannii is similar to that of E. coli except an additional acyl chain is found in an acyloxyacyl-linkage at the 2-position arising from a second acylation event catalyzed by LpxM (Figure 1). Wildtype A. baumannii lipid A also contains an additional hydroxyl group on the 2´-linked secondary acyl chain that likely arises from the action of an LpxO homolog found in the A. baumannii genome (Boll et al., 2015). In colistin resistant A. baumannii that maintain LOS, the lipid A phosphate groups can be modified with galactosamine and with phosphoethanolamine residues. Phosphoethanolamine modification can occur at both phosphates (Arroyo et al., 2011), whereas only single modified species of galactosamine have been reported (Pelletier et al., 2013) thus far. EptA (PmrC) catalyzes the addition of phosphoethanolamine in A. baumannii (Arroyo et al., 2011) and galactosamine addition is likely catalyzed by an A. baumannii ArnT homolog. In Salmonella and E. coli, ArnT transfers the sugar L-4-aminoarabinose to lipid A phosphate groups (Needham and Trent, 2013).
CAMPs, such as polymyxins, are highly effective against Gram-negative bacteria. This class of antibiotics includes two FDA-approved clinical antibiotics; polymyxin B and polymyxin E (colistin). These antibiotics, despite being discovered in 1949, remain the “last-resort” treatment for multi-drug resistant infections due to their high nephrotoxicity in humans (Falagas and Kasiakou, 2006). The amphipathic nature of these compounds with a positively charged cyclic group, makes them highly attracted to the anionic lipid A molecule on the bacterial surface (Velkov et al., 2013). High concentrations of polymyxins displace the divalent cation bridging between LPS/LOS molecules and the acyl chain allows for pore formation and permeation of the outer membrane. Once in the periplasm, this pore formation occurs in the cytoplasmic membrane, causing membrane leakage and disruption of the proton motive force – effectively lethal to the cell (Hancock and Chapple, 1999).
Many bacterial species have modifying enzymes that can reduce the negative charge of LPS/LOS through modification of the lipid A phosphate groups with various amine-containing residues, such as phosphoethanolamine or cationic sugars (e.g. aminoarabinose and galactosamine) (Figure 2) (Trent et al., 2001; Arroyo et al., 2011; Pelletier et al., 2013; Chin et al., 2015). Additional modifications have been demonstrated on the inner core oligosaccharide, which would further reduce the negative charge of LPS/LOS (Kanipes et al., 2001; Tamayo et al., 2005). Remodeling of the bacteria cell surface results in resistance to polymyxins; however, the extent of resistance varies based on the organism and type of LPS/LOS modification.
Despite having modifying enzymes for decreasing polymyxin susceptibility, A. baumannii_is the only species to date that employs the response of inactivating lipid A biosynthesis as an alternative mechanism of resistance.
Mechanisms of LOS-deficiency in A. baumannii
The lipid A biosynthetic pathway, except for certain late acyl-transferases (Fig. 1), is considered essential in Gram-negative bacteria. Mutants that are defective in key processes of lipid A biosynthesis or its transport are inviable with gross morphological changes and disrupted membrane biogenesis (Doerrler et al., 2001; Reynolds and Raetz, 2009; Tomaras et al., 2014; García-Quintanilla et al., 2016; Zhang et al., 2017).
The lipid A biosynthetic pathway consists of nine enzymes, the first seven of which are essential for lipid A biosynthesis and cell survival under standard laboratory conditions. (Figure 1) (Reynolds and Raetz, 2009). The absence of these genes typically results in lethality. This was recently confirmed to be the case in A. baumannii through the inducible expression of two key lipid A biosynthesis genes. It was shown that in the absence of inducer, lpxH and lpxK mutants were inviable as expected. The use of LpxC inhibitor CHIR-090 (Barb et al., 2007) restored growth, indicating that intermediates from the lipid A biosynthetic pathway, and not the loss of lipid A itself, were responsible for toxicity. This is consistent with the fact that these intermediates are amphipathic, detergent like compounds (Figure 1) (Richie et al., 2016; Wei et al., 2017). This work aligns itself with precedents established in E. coli (Garrett et al., 1998; Babinski et al., 2002).
Despite this, multiple groups have now reported that when grown on high concentrations of colistin (10 μg/mL which is ~20X higher than the average MIC for wild-type A. baumannii) colonies appear that are viable (Moffatt et al., 2010; Boll et al., 2016). The colonies lacked lipid A and LOS which is consistent with mutations in early genes of the lipid A biosynthetic pathway as determined by whole-genome sequencing (Boll et al., 2016). These mutations vary by strain and laboratory; however, they all occur in one of the first three genes (lpxA, lpxC, or lpxD) of the lipid A biosynthetic pathway (Figure 1) (Moffatt et al., 2010; Beceiro et al., 2014; Boll et al., 2016). Acinetobacter joins N. meningitidis and M. catarrhalis as the three Gram-negative species viable in the absence of lipid A, excluding the genus Spirochete which have distinctive outer membranes in a class of its own (Haake and Zückert, 2015). N. meningitidis was the first organism shown to survive without lipid A and LOS, which was demonstrated in 1998 when a viable lpxA mutant was generated in vitro (Steeghs et al., 1998). Later, Moraxella catarrhalis, a close relative of the genus Acinetobacter, was also shown to be viable in the absence of LOS (Peng et al., 2005). An interesting distinction is that both Neisseria and Moraxella became LOS-deficient through in vitro directed mutations. To date, A. baumannii is the only organism shown to accumulate lipid A biosynthesis mutants readily under significant outer membrane stress caused by polymyxin antibiotics. That these lpx mutations occur in the presence of high colistin suggests the process is determined stochastically rather than through a strictly regulated process. While it is possible these three genes are hot spots for mutations to occur, it is more likely that inactivation of downstream lipid A biosynthetic genes are inviable and drop out under selection.
Concurrent with this work is a study that explores the physiology of A. baumannii using a lipooligosaccharide transport mutant (lptD) in A. baumannii ATCC19606. Remarkably, the lptD mutant is viable despite its essentiality in other Gram-negative organisms. LptD is the terminal step in LOS translocation to the outer membrane, and in its absence the entirety of the lpt complex is nonfunctional if it forms at all (Okuda et al., 2016). As such, it is interesting that an lptD mutant is viable in wild-type A. baumannii, as this would lead to an accumulation of intact LOS in the cytoplasm or potentially the periplasm depending on the functionality of the partial Lpt pathway. This work builds on studies that determined the lpt pathway is not essential in Neisseria meningitidis and the cell can tolerate internal accumulation of LOS (Bos et al., 2004). The accumulation of LOS or lipid A should be lethal regardless of A. baumannii’s (or any other bacterial species) ability to survive in its absence. Despite this, electron microscopy showed minimal disturbances to the cell. The authors report the lptD mutant having intact LOS, suggesting either the cells have a bypass mechanism to get LOS to the outer membrane or A. baumannii is remarkably suited to tolerating internal accumulations of intact LOS. Either outcome would have a significant impact on the field, and warrants further study and examination of these mutants (Bojkovic et al., 2015; Richie et al., 2016; Wei et al., 2017).
Impacts of LOS-deficiency on A. baumannii physiology
LOS-deficiency alters the physical and chemical characteristics of the Gram-negative cell envelope leading to effects on membrane biogenesis and physiology. The use of various -omics approaches has worked to decipher these changes en masse.
Gene Regulation
A. baumannii has a high degree of strain variation making broad generalizations difficult in the absence of multi-strain studies (Maifiah et al., 2016; Boll et al., 2016). A large-scale transcriptomics study on multiple, commonly used lab strains allowed for the first comparative analysis of transcriptional regulation in the absence of lipid A/LOS. In the four strains studied, 38 distinct gene clusters were up- or down-regulated in A. baumannii relative to their LOS-positive counterparts. Strikingly, only five of these were conserved across the strains tested (Boll et al., 2016). These five groups are (1) the lol pathway for lipoprotein transport, (2) six lipoproteins, (3) multidrug efflux pumps, (4) the baeRS two component-system, and (5) the mla pathway for phospholipid transport. All of these are involved in cell envelope maintenance (Raffa and Raivio, 2002; Malinverni and Silhavy, 2009; Lin et al., 2015; Yoon et al., 2016; Grabowicz and Silhavy, 2017; Ekiert et al., 2017).
Outer Membrane Maintenance: the lol and mla pathways
The lol and mla pathways are proposed mechanisms for anterograde lipoprotein and retrograde phospholipid transport, respectively (Malinverni and Silhavy, 2009; Konovalova and Silhavy, 2015). LOS-deficient Acinetobacter upregulate both of these pathways, although the effects on the outer membrane are unclear. In ATCC strain19606, a common type strain, there are anywhere from 5–25 upregulated lipoproteins upon loss of LOS (Henry et al., 2012; Boll et al., 2016). Acinetobacter sp. and Escherichia coli have between 80–100 putative lipoproteins in their genomes to provide context (Babu et al., 2006). Some of these lipoproteins localize to the outer leaflet, but their function is unknown (Boll et al., 2016). Increased abundance of lipoproteins in the outer leaflet could simply help occupy space in the outer membrane in addition to glycerophospholipids in the absence of LOS (Figure 3) and this is still under investigation in our laboratory.
Fig. 3. Potential changes in cell-surface architecture during LOS/lipid A deficiency.
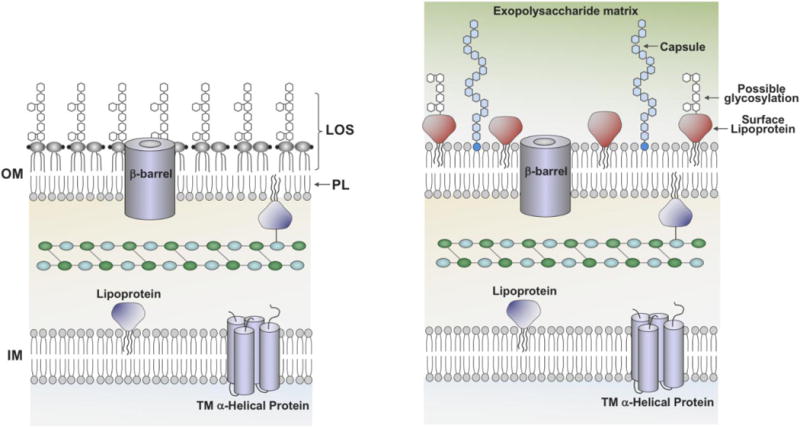
Left: A wild-type Gram-negative cell envelope with typical asymmetry in the outer membrane. Right: A depiction of putative mechanisms for altering the cell envelope and fortifying the membrane in the absence of asymmetry. Lipoproteins exposed to the surface (Boll et al., 2016) could play a critical role in filling space in a more porous outer leaflet. The capacity for A. baumannii to glycosylate these lipoproteins hints at a potential mechanism for mimicking LOS. The generation of capsular polysaccharide and exopolysaccharide provide an additional level of protection that could impede the influx of noxious compounds and buffer the LOS-deficient cell from the environment.
The mla ABC-transport system is thought to remove phospholipids from the outer membrane to the inner membrane, actively maintaining asymmetry (Malinverni and Silhavy, 2009). In the absence of LOS, canonical asymmetry is no longer possible. The replacement of lipid A with glycerophospholipids (as no unique lipid species have been detected in LOS-deficient A. baumannii to date (Maifiah et al., 2016; Boll et al., 2016) in the outer leaflet most likely provides a constant stress signal to the cell leading to mla upregulation. Mislocalization of glycerophospholipids to the outer leaflet of the outer membrane also results in activation of the outer membrane phospholipase PldA and glycerophosphospholipid degradation (Brok et al., 1996; Langen et al., 2001; Bishop, 2008). While these mechanisms are in place to protect asymmetry, in the absence of lipid A/LOS it is likely their functions are detrimental. Subsequent retrograde removal of phospholipids from the outer membrane would require equivalent anterograde transport of phospholipids to ensure a well-packed membrane. It important to note that the mla transport system lacks concrete biochemical evidence for its directionality, a topic under investigation by several laboratories.
Activation of cellular stress response mechanisms
The upregulation of efflux pumps and the baeRS two-component system are indicative of a cell under duress (Grabowicz and Silhavy, 2017). The BaeRS system is a conserved response system for cell envelope stress. In E. coli, it has a small regulon that includes key efflux pumps. While there is limited data as to the entire regulon of BaeRS in A. baumannii, it is reasonable to assume that the subsequent upregulation of efflux pumps suggests a similar function (Lin et al., 2015). Efflux pump activity would be a logical first step at ameliorating the influx of toxic metabolites in the absence of asymmetry (Henry et al., 2012; Boll et al., 2016). Curiously, none of the other major cell envelope stress response systems were upregulated in A. baumannii – perhaps due to an expanded role of BaeRS in this species.
Constituting a Fortified Membrane in the Absence of Lipid A
Phospholipid alterations
It remains unclear what makes the outer membrane a functional barrier in the absence of LOS for A. baumannii. The overall composition of phospholipids does not appear to drastically change, although the phospholipid phenotype is strain-dependent. While several studies have analyzed multiple strains, the changes seen between LOS-positive and LOS-deficient cells are minor (Maifiah et al., 2016; Boll et al., 2016). In one case, the LOS-deficient strain synthesized phospholipids with shorter acyl-chain lengths, decreasing the overall hydrophobicity of the membrane (Maifiah et al., 2016).
Mimicking LOS through display of surface carbohydrates
Carbohydrate modifications to outer membrane proteins have been shown to be integral to their function (Tytgat and de Vos, 2016). Many Gram-negative bacteria have the capability to glycosylate proteins; however, the mechanisms vary. The two forms of glycosylation, N-linked and O-linked, utilize completely different methods for generating and attaching glycans to target proteins. Generally, if a bacterial species contains a mechanism for protein glycosylation, it contains either the N-linked or O-linked system (Nothaft and Szymanski, 2010). A genomics study of A. baumannii reveals its capability for O-linked glycosylation (Iwashkiw et al., 2012). This hints at the possibility for it to use protein glycosylation as an LOS-mimic in the absence of canonical endotoxin (Figure 3). Furthermore, while strains tend to produce only a single glycan, the glycan is promiscuous in its utility and can be used for glycosylation of proteins and capsule formation (Lees-Miller et al., 2013).
A. baumannii secretes an abundance of the exopolysaccharide poly-N acetylglucosamine (PNAG) (Choi et al., 2009). In certain strains, expression of key PNAG genes is upregulated in the absence of LOS (Henry et al., 2012). The upregulation of exopolysaccharide in stressed cells has been previously documented (Ren et al., 2016), and increased exopolysaccharide could provide a buffer to a compromised outer membrane. Additionally, the generation of outer membrane attached capsule could achieve a similar purpose (Figure 3). This case is best made in N. meningitidis, although there are conflicting data. The initial studies of LOS-deficient N. meningitidis suggested capsule was essential for survival in this condition (Steeghs et al., 2001). However, the isolation of suppressor mutants in a capsule-deficient, LOS-deficient N. meningitidis suggests that capsular polysaccharide is not truly essential (Bos and Tommassen, 2005). The suppressor mutations and subsequent mechanism responsible for this viability remain undetermined.
LOS-deficiency; an in vitro phenomenon?
In light of evolution, an outer membrane complete with lipid A and LOS/LPS must be the most fit version of the Gram-negative cell envelope. With the existence of both Gram-negative and Gram-positive bacteria, there is the underlying question of which came first. A majority of bacterial phyla are diderms, containing an inner and outer membrane (Hug et al., 2016). The monoderm bacteria are restricted largely to the Firmicutes and Actinobacteria, although key exceptions of diderm Firmicutes allow for an interesting comparative study on the evolution of the cell envelope. Antunes and co-workers (Antunes et al., 2016) examined the diderms in the Firmicutes and found intact genes for lipid A biosynthesis, LPS transport, lipoprotein transport, and unique diderm flagellar genes. That these genes exist in the Firmicutes suggests that monoderm bacteria arose multiple times in evolutionary history through several independent events. It is curious to look at the loss of LOS in the context of a step towards transitioning from being a diderm to a monoderm. While mimicking millions of years of evolution in a test tube is entirely infeasible, the ability to inactivate lipid A biosynthesis could be a critical first step in eventually shedding an outer membrane. Remarkably, the fact that LOS-deficient A. baumannii maintains an outer membrane is a testament to the mechanisms in place to safeguard asymmetry and an outer membrane as a barrier.
The ability to survive in the absence of lipid A and LOS is not necessarily conserved among a given genus or even species. When a number of A. baumannii isolates were tested for LOS-deficiency, only about 50% were capable of surviving in the absence of LOS: a fact that highlights the immense lack of knowledge in terms of signals and physiological prerequisites for inactivating lipid A biosynthesis (Boll et al., 2016). In one particular case, this inability was linked to the expression of PBP1a, which is a penicillin-binding protein involved in peptidoglycan synthesis and maintenance (Boll et al., 2016). There is no clear evidence based on sequence similarity of A. baumannii genomes for why particular strains have high or low levels of PBP1A (unpublished results, Trent Lab).
A. baumannii contains two bifunctional penicillin-binding proteins, PBP1a and PBP1b, that are capable of both transglycosylase and transpeptidase activity (Cayô et al., 2011). In E. coli, these proteins function in different capacities despite performing identical chemistry and loss of both is synthetically lethal (Typas et al., 2010). The expression and localization has not been explored in A. baumannii. The current dogma in E. coli suggests that outer membrane lipoproteins regulate the activity and localization of PBP1a and PBP1b, yet a lack of any obvious homologs for regulatory lipoproteins suggests their mechanisms of maintenance and activity are unique from E. coli (Lupoli et al., 2014). Potentially, PBP1a-mediated cell elongation is fatal in LOS-deficient cells due to a compromised membrane and an increase in overall osmotic and turgor stress that results from it. This is supported in part by a small group of studies that examined cell morphology of LOS-deficient cells; however, more in-depth analysis of cell shape morphology with high-resolution techniques will bolster these claims (Soon et al., 2011; Soon et al., 2012). This link between cell shape and LOS-deficiency warrants further exploration and study.
LOS-deficiency in the Clinic
That A. baumannii can survive in the absence of LOS in vitro is an interesting phenotype in and of itself; however, its impact on physiology suggests it is largely deleterious to its virulence. LOS-deficient A. baumannii fail to activate toll-like receptor 4 (TLR4), the main TLR that detects endotoxin in eukaryotic hosts (Kim et al., 2013; Moffatt et al., 2013; Boll et al., 2016). LOS-deficient N. meningitidis and A. baumannii both have significant in vitro growth defects (Bos and Tommassen, 2005; Beceiro et al., 2014). Although a direct comparison is inappropriate due to difficulty in monitoring growth rate in vivo, LOS-deficient A. baumannii exhibit reduced fitness in both C. elegans and a mouse infection model (Beceiro et al., 2014). Furthermore, inhibition of lipid A biosynthesis through a large concentration of LpxC inhibitors in vivo inhibited lethal infection from A. baumannii due to its inability to shed LPS molecules and activate TLR4. The inhibitor used, LpxC-1, had no in vitro activity against A. baumannii, although it is undetermined whether LpxC-1 treated A. baumannii were truly LOS-deficient in this study or if the compound is simply ineffective against A. baumannii LpxC. (Lin et al., 2012; Tomaras et al., 2014). Additionally, inhibition of canonical virulence factors including type-VI secretion factors and surface-based pili likely contribute to reduced virulence in vivo (Henry et al., 2012).
While LOS-deficient A. baumannii can be readily isolated in vitro, its isolation in the clinic is overwhelmingly rare. The lack of published clinical cases of LOS-deficient A. baumannii is presumably due to lipid A modification mechanisms (Fig. 2) that allow for survival in the presence of clinical levels of colistin and polymyxin B without a significant growth defect (Lesho et al., 2013). A transcriptomic study of eight patients infected with A. baumannii yielded a number of clinical isolates with single nucleotide polymorphisms increasing transcription of pmrAB that encodes for a two-component regulatory system controlling lipid A modifications (Kröger et al., 2016). The same study identified nonsynonymous mutations in a lipopolysaccharide transport gene (lptF) and lipid A transport gene (msbA). The in vitro resistance to colistin of these clinical isolates was only 0.5 μg/mL. Thus, these strains would be considered highly susceptible to the antibiotic and would lack lipid A modifications consistent with colistin resistance (> 2 μg/mL). This makes it likely that host-induced factors lead to active remodeling of the outer membrane and this phenotype was not stable in vitro (Wright et al., 2017). That the expected phenotype was not detected in vitro suggests these mutations might be an artifact of next-generation sequencing, and is consistent with similar findings in the Trent Lab (unpublished results, Trent Lab). In contrast, virulent LOS-deficient N. meningitidis have been isolated, albeit infrequently, in clinical settings. Interestingly, this isolate contained a mutation in lpxH, which is further downstream than any viable isolate generated in vitro (Piet et al., 2014).
The clinical threshold for colistin and polymyxin B concentrations in vivo is low due to its nephrotoxicity in humans. The delivery of colistin via a convertible pro-drug means that its accumulation to target concentrations in the body is slow in contrast to polymyxin B which is delivered as the active drug (Nation et al., 2014). While the dosing regimen in the clinic appears to have no effect on the efficacy of the antibiotic, A. baumannii was capable of developing resistance in a stable (no reversion of resistance) and unstable (reversion of resistance) fashion (Cheah et al., 2016). Whether A. baumannii inactivates LOS/lipid A in the host remains to be determined. The low isolation frequency of LOS-deficient cells from the host environment suggests that this phenomenon has an unknown impact on virulence that needs to be further explored. However, with limited knowledge of the natural reservoirs of A. baumannii, it is possible this endotoxin-free, non-immunogenic state could be important for bacterial persistence.
Conclusion
Over the past five years, significant progress has elucidated a number of physiological changes that occur in Gram-negative cells that lack lipid A and LOS in their outer membrane. This organism represents a new frontier for understanding membrane biology, and represents a continued shift in the paradigm of LPS-essentiality for bacterial membranes. Despite this progress, there are serious gaps in our knowledge of LOS-deficient membranes, its regulation, and mechanisms for membrane fortification in A. baumannii.
Acknowledgments
Funding from the National Institutes of Health (NIH) (grants AI076322, AI129940, AI064184, AI119879 to M.S.T.), the Army Research Office (grant W911NF-12-1-0390 to M.S.T). and the National Science Foundation (Graduate Research Fellowship to M.J. P.) is gratefully acknowledged.
Contributor Information
Matthew Joseph Powers, University of Georgia Infectious disease department, Athens, Georgia, United States.
Dr M. Stephen Trent, University of Georgia, Infectious Diseases Department, Athens Georgia United States, strent@uga.edu.
References
- Antunes LC, Poppleton D, Klingl A, Criscuolo A, Dupuy B, Brochier-Armanet C, et al. Phylogenomic analysis supports the ancestral presence of LPS-outer membranes in the Firmicutes. eLife. 2016;5 doi: 10.7554/eLife.14589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo LA, Herrera CM, Fernandez L, Hankins JV, Trent MS, Hancock REW. The pmrCAB operon mediates polymyxin resistance in Acinetobacter baumannii ATCC 17978 and clinical isolates through phosphoethanolamine modification of lipid A. Antimicrob Agents Chemother. 2011;55:3743–3751. doi: 10.1128/AAC.00256-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babinski KJ, Kanjilal SJ, Raetz CRH. Accumulation of the lipid A precursor UDP-2,3-diacylglucosamine in an Escherichia coli mutant lacking the lpxH gene. J Biol Chem. 2002;277:25947–25956. doi: 10.1074/jbc.M204068200. [DOI] [PubMed] [Google Scholar]
- Babu MM, Priya ML, Selvan AT, Madera M, Gough J, Aravind L, Sankaran K. A database of bacterial lipoproteins (DOLOP) with functional assignments to predicted lipoproteins. J Bacteriol. 2006;188:2761–2773. doi: 10.1128/JB.188.8.2761-2773.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barb AW, McClerren AL, Snehelatha K, Reynolds CM, Zhou P, Raetz CRH. Inhibition of lipid A biosynthesis as the primary mechanism of CHIR-090 antibiotic activity in Escherichia coli. Biochemistry (Mosc) 2007;46:3793–3802. doi: 10.1021/bi6025165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beceiro A, Moreno A, Fernández N, Vallejo JA, Aranda J, Adler B, et al. Biological cost of different mechanisms of colistin resistance and their impact on virulence in Acinetobacter baumannii. Antimicrob Agents Chemother. 2014;58:518–526. doi: 10.1128/AAC.01597-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop RE. Structural biology of membrane-intrinsic beta-barrel enzymes: sentinels of the bacterial outer membrane. Biochim Biophys Acta. 2008;1778:1881–1896. doi: 10.1016/j.bbamem.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojkovic J, Richie DL, Six DA, Rath CM, Sawyer WS, Hu Q, Dean CR. Characterization of an Acinetobacter baumannii lptD Deletion Strain: Permeability Defects and Response to Inhibition of Lipopolysaccharide and Fatty Acid Biosynthesis. J Bacteriol. 2015;198:731–741. doi: 10.1128/JB.00639-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boll JM, Crofts AA, Peters K, Cattoir V, Vollmer W, Davies BW, Trent MS. A penicillin-binding protein inhibits selection of colistin-resistant, lipooligosaccharide-deficient Acinetobacter baumannii. Proc Natl Acad Sci U A. 2016;113:E6228–E6237. doi: 10.1073/pnas.1611594113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boll JM, Tucker AT, Klein DR, Beltran AM, Brodbelt JS, Davies BW, Trent MS. Reinforcing Lipid A Acylation on the Cell Surface of Acinetobacter baumannii Promotes Cationic Antimicrobial Peptide Resistance and Desiccation Survival. MBio. 2015a;6:e00478–15. doi: 10.1128/mBio.00478-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boll JM, Tucker AT, Klein DR, Beltran AM, Brodbelt JS, Davies BW, Trent MS. Reinforcing Lipid A Acylation on the Cell Surface of Acinetobacter baumannii Promotes Cationic Antimicrobial Peptide Resistance and Desiccation Survival. MBio. 2015b;6:e00478–15. doi: 10.1128/mBio.00478-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos MP, Tefsen B, Geurtsen J, Tommassen J. Identification of an outer membrane protein required for the transport of lipopolysaccharide to the bacterial cell surface. Proc Natl Acad Sci U S A. 2004;101:9417–9422. doi: 10.1073/pnas.0402340101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos MP, Tommassen J. Viability of a capsule- and lipopolysaccharide-deficient mutant of Neisseria meningitidis. Infect Immun. 2005;73:6194–6197. doi: 10.1128/IAI.73.9.6194-6197.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabetz W, Müller-Loennies S, Holst O, Brade H. Deletion of the heptosyltransferase genes rfaC and rfaF in Escherichia coli K-12 results in an Re-type lipopolysaccharide with a high degree of 2-aminoethanol phosphate substitution. Eur J Biochem. 1997;247:716–724. doi: 10.1111/j.1432-1033.1997.00716.x. [DOI] [PubMed] [Google Scholar]
- Brok RG, Belandia IU, Dekker N, Tommassen J, Verheij HM. Escherichia coli outer membrane phospholipase A: role of two serines in enzymatic activity. Biochemistry (Mosc) 1996;35:7787–7793. doi: 10.1021/bi952970i. [DOI] [PubMed] [Google Scholar]
- Cayô R, Rodríguez MC, Espinal P, Fernández-Cuenca F, Ocampo-Sosa AA, Pascual A, et al. Analysis of genes encoding penicillin-binding proteins in clinical isolates of Acinetobacter baumannii. Antimicrob Agents Chemother. 2011;55:5907–5913. doi: 10.1128/AAC.00459-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheah SE, Johnson MD, Zhu Y, Tsuji BT, Forrest A, Bulitta JB, et al. Polymyxin Resistance in Acinetobacter baumannii: Genetic Mutations and Transcriptomic Changes in Response to Clinically Relevant Dosage Regimens. Sci Rep. 2016;6:26233. doi: 10.1038/srep26233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin CY, Gregg KA, Napier BA, Ernst RK, Weiss DS. A PmrB-Regulated Deacetylase Required for Lipid A Modification and Polymyxin Resistance in Acinetobacter baumannii. Antimicrob Agents Chemother. 2015;59:7911–7914. doi: 10.1128/AAC.00515-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi AHK, Slamti L, Avci FY, Pier GB, Maira-Litrán T. The pgaABCD locus of Acinetobacter baumannii encodes the production of poly-beta-1-6-N-acetylglucosamine, which is critical for biofilm formation. J Bacteriol. 2009;191:5953–5963. doi: 10.1128/JB.00647-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcour AH. Outer membrane permeability and antibiotic resistance. Biochim Biophys Acta. 2009;1794:808–816. doi: 10.1016/j.bbapap.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerrler WT, Reedy MC, Raetz CR. An Escherichia coli mutant defective in lipid export. J Biol Chem. 2001;276:11461–11464. doi: 10.1074/jbc.C100091200. [DOI] [PubMed] [Google Scholar]
- Ekiert DC, Bhabha G, Isom GL, Greenan G, Ovchinnikov S, Henderson IR, et al. Architectures of Lipid Transport Systems for the Bacterial Outer Membrane. Cell. 2017;169:273–285.e17. doi: 10.1016/j.cell.2017.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falagas ME, Kasiakou SK. Toxicity of polymyxins: a systematic review of the evidence from old and recent studies. Crit Care. 2006;10:R27. doi: 10.1186/cc3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahara Y, Nikaido H. Asymmetric localization of lipopolysaccharides on the outer membrane of Salmonella typhimurium. J Bacteriol. 1980;141:1463–1465. doi: 10.1128/jb.141.3.1463-1465.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Quintanilla M, Caro-Vega JM, Pulido MR, Moreno-Martínez P, Pachón J, McConnell MJ. Inhibition of LpxC Increases Antibiotic Susceptibility in Acinetobacter baumannii. Antimicrob Agents Chemother. 2016;60:5076–5079. doi: 10.1128/AAC.00407-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett TA, Que NL, Raetz CR. Accumulation of a lipid A precursor lacking the 4’-phosphate following inactivation of the Escherichia coli lpxK gene. J Biol Chem. 1998;273:12457–12465. doi: 10.1074/jbc.273.20.12457. [DOI] [PubMed] [Google Scholar]
- Grabowicz M, Silhavy TJ. Envelope Stress Responses: An Interconnected Safety Net. Trends Biochem Sci. 2017;42:232–242. doi: 10.1016/j.tibs.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haake DA, Zückert WR. The leptospiral outer membrane. Curr Top Microbiol Immunol. 2015;387:187–221. doi: 10.1007/978-3-662-45059-8_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock RE, Chapple DS. Peptide antibiotics. Antimicrob Agents Chemother. 1999;43:1317–1323. doi: 10.1128/aac.43.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson JC, Zimmerman SM, Crofts AA, Boll JM, Kuhns LG, Herrera CM, Trent MS. The Power of Asymmetry: Architecture and Assembly of the Gram-Negative Outer Membrane Lipid Bilayer. Annu Rev Microbiol. 2016 doi: 10.1146/annurev-micro-102215-095308. http://dx.doi.org/10.1146/annurev-micro-102215-095308. [DOI] [PubMed]
- Henry R, Vithanage N, Harrison P, Seemann T, Coutts S, Moffatt JH, et al. Colistin-resistant, lipopolysaccharide-deficient Acinetobacter baumannii responds to lipopolysaccharide loss through increased expression of genes involved in the synthesis and transport of lipoproteins, phospholipids, and poly-β-1,6-N-acetylglucosamine. Antimicrob Agents Chemother. 2012;56:59–69. doi: 10.1128/AAC.05191-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hug LA, Baker BJ, Anantharaman K, Brown CT, Probst AJ, Castelle CJ, et al. A new view of the tree of life. Nat Microbiol. 2016;1 doi: 10.1038/nmicrobiol.2016.48. nmicrobiol201648. [DOI] [PubMed] [Google Scholar]
- Iwashkiw JA, Seper A, Weber BS, Scott NE, Vinogradov E, Stratilo C, et al. Identification of a general O-linked protein glycosylation system in Acinetobacter baumannii and its role in virulence and biofilm formation. PLoS Pathog. 2012;8:e1002758. doi: 10.1371/journal.ppat.1002758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamio Y, Nikaido H. Outer membrane of Salmonella typhimurium: accessibility of phospholipid head groups to phospholipase c and cyanogen bromide activated dextran in the external medium. Biochemistry (Mosc) 1976;15:2561–2570. doi: 10.1021/bi00657a012. [DOI] [PubMed] [Google Scholar]
- Kanipes MI, Lin S, Cotter RJ, Raetz CR. Ca2+-induced phosphoethanolamine transfer to the outer 3-deoxy-D-manno-octulosonic acid moiety of Escherichia coli lipopolysaccharide. A novel membrane enzyme dependent upon phosphatidylethanolamine. J Biol Chem. 2001;276:1156–1163. doi: 10.1074/jbc.M009019200. [DOI] [PubMed] [Google Scholar]
- Kim CH, Jeong YJ, Lee J, Jeon SJ, Park SR, Kang MJ, et al. Essential role of toll-like receptor 4 in Acinetobacter baumannii-induced immune responses in immune cells. Microb Pathog. 2013;54:20–25. doi: 10.1016/j.micpath.2012.08.008. [DOI] [PubMed] [Google Scholar]
- Konovalova A, Silhavy TJ. Outer membrane lipoprotein biogenesis: Lol is not the end. Philos Trans R Soc Lond B Biol Sci. 2015;370 doi: 10.1098/rstb.2015.0030. http://dx.doi.org/10.1098/rstb.2015.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kröger C, Kary SC, Schauer K, Cameron ADS. Genetic Regulation of Virulence and Antibiotic Resistance in Acinetobacter baumannii. Genes. 2016;8 doi: 10.3390/genes8010012. http://dx.doi.org/10.3390/genes8010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langen GR, Harper JR, Silhavy TJ, Howard SP. Absence of the outer membrane phospholipase A suppresses the temperature-sensitive phenotype of Escherichia coli degP mutants and induces the Cpx and sigma(E) extracytoplasmic stress responses. J Bacteriol. 2001;183:5230–5238. doi: 10.1128/JB.183.18.5230-5238.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees-Miller RG, Iwashkiw JA, Scott NE, Seper A, Vinogradov E, Schild S, Feldman MF. A common pathway for O-linked protein-glycosylation and synthesis of capsule in Acinetobacter baumannii. Mol Microbiol. 2013;89:816–830. doi: 10.1111/mmi.12300. [DOI] [PubMed] [Google Scholar]
- Lesho E, Yoon EJ, McGann P, Snesrud E, Kwak Y, Milillo M, et al. Emergence of colistin-resistance in extremely drug-resistant Acinetobacter baumannii containing a novel pmrCAB operon during colistin therapy of wound infections. J Infect Dis. 2013;208:1142–1151. doi: 10.1093/infdis/jit293. [DOI] [PubMed] [Google Scholar]
- Lin L, Tan B, Pantapalangkoor P, Ho T, Baquir B, Tomaras A, et al. Inhibition of LpxC protects mice from resistant Acinetobacter baumannii by modulating inflammation and enhancing phagocytosis. MBio. 2012;3 doi: 10.1128/mBio.00312-12. http://dx.doi.org/10.1128/mBio.00312-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MF, Lin YY, Lan CY. The Role of the Two-Component System BaeSR in Disposing Chemicals through Regulating Transporter Systems in Acinetobacter baumannii. PLoS One. 2015;10:e0132843. doi: 10.1371/journal.pone.0132843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupoli TJ, Lebar MD, Markovski M, Bernhardt T, Kahne D, Walker S. Lipoprotein activators stimulate Escherichia coli penicillin-binding proteins by different mechanisms. J Am Chem Soc. 2014;136:52–55. doi: 10.1021/ja410813j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maifiah MHM, Cheah SE, Johnson MD, Han ML, Boyce JD, Thamlikitkul V, et al. Global metabolic analyses identify key differences in metabolite levels between polymyxin-susceptible and polymyxin-resistant Acinetobacter baumannii. Sci Rep. 2016;6:22287. doi: 10.1038/srep22287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinverni JC, Silhavy TJ. An ABC transport system that maintains lipid asymmetry in the gram-negative outer membrane. Proc Natl Acad Sci U A. 2009;106:8009–8014. doi: 10.1073/pnas.0903229106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffatt JH, Harper M, Harrison P, Hale JDF, Vinogradov E, Seemann T, et al. Colistin resistance in Acinetobacter baumannii is mediated by complete loss of lipopolysaccharide production. Antimicrob Agents Chemother. 2010;54:4971–4977. doi: 10.1128/AAC.00834-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffatt JH, Harper M, Mansell A, Crane B, Fitzsimons TC, Nation RL, et al. Lipopolysaccharide-deficient Acinetobacter baumannii shows altered signaling through host Toll-like receptors and increased susceptibility to the host antimicrobial peptide LL-37. Infect Immun. 2013;81:684–689. doi: 10.1128/IAI.01362-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nation RL, Velkov T, Li J. Colistin and polymyxin B: peas in a pod, or chalk and cheese? Clin Infect Dis. 2014;59:88–94. doi: 10.1093/cid/ciu213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham BD, Trent MS. Fortifying the barrier: the impact of lipid A remodelling on bacterial pathogenesis. Nat Rev Microbiol. 2013;11:467–481. doi: 10.1038/nrmicro3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol Mol Biol Rev. 2003;67:593–656. doi: 10.1128/MMBR.67.4.593-656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H, Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985;49:1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothaft H, Szymanski CM. Protein glycosylation in bacteria: sweeter than ever. Nat Rev Microbiol. 2010;8:765–778. doi: 10.1038/nrmicro2383. [DOI] [PubMed] [Google Scholar]
- Okuda S, Sherman DJ, Silhavy TJ, Ruiz N, Kahne D. Lipopolysaccharide transport and assembly at the outer membrane: the PEZ model. Nat Rev Microbiol. 2016;14:337–345. doi: 10.1038/nrmicro.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaitan AO, Morand S, Rolain JM. Mechanisms of polymyxin resistance: acquired and intrinsic resistance in bacteria. Front Microbiol. 2014;5:643. doi: 10.3389/fmicb.2014.00643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier MR, Casella LG, Jones JW, Adams MD, Zurawski DV, Hazlett KRO, et al. Unique structural modifications are present in the lipopolysaccharide from colistin-resistant strains of Acinetobacter baumannii. Antimicrob Agents Chemother. 2013;57:4831–4840. doi: 10.1128/AAC.00865-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng D, Hong W, Choudhury BP, Carlson RW, Gu XX. Moraxella catarrhalis bacterium without endotoxin, a potential vaccine candidate. Infect Immun. 2005;73:7569–7577. doi: 10.1128/IAI.73.11.7569-7577.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piet JR, Zariri A, Fransen F, Schipper K, van der Ley P, van de Beek D, van der Ende A. Meningitis caused by a lipopolysaccharide deficient Neisseria meningitidis. J Infect. 2014;69:352–357. doi: 10.1016/j.jinf.2014.06.005. [DOI] [PubMed] [Google Scholar]
- Preston A, Mandrell RE, Gibson BW, Apicella MA. The lipooligosaccharides of pathogenic gram-negative bacteria. Crit Rev Microbiol. 1996;22:139–180. doi: 10.3109/10408419609106458. [DOI] [PubMed] [Google Scholar]
- Raetz CR. Biochemistry of endotoxins. Annu Rev Biochem. 1990;59:129–170. doi: 10.1146/annurev.bi.59.070190.001021. [DOI] [PubMed] [Google Scholar]
- Raetz CRH, Reynolds CM, Trent MS, Bishop RE. Lipid A modification systems in gram-negative bacteria. Annu Rev Biochem. 2007;76:295–329. doi: 10.1146/annurev.biochem.76.010307.145803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffa RG, Raivio TL. A third envelope stress signal transduction pathway in Escherichia coli. Mol Microbiol. 2002;45:1599–1611. doi: 10.1046/j.1365-2958.2002.03112.x. [DOI] [PubMed] [Google Scholar]
- Ren G, Wang Z, Li Y, Hu X, Wang X. Effects of Lipopolysaccharide Core Sugar Deficiency on Colanic Acid Biosynthesis in Escherichia coli. J Bacteriol. 2016;198:1576–1584. doi: 10.1128/JB.00094-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CM, Raetz CRH. Replacement of lipopolysaccharide with free lipid A molecules in Escherichia coli mutants lacking all core sugars. Biochemistry (Mosc) 2009;48:9627–9640. doi: 10.1021/bi901391g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richie DL, Takeoka KT, Bojkovic J, Metzger LE, 4th, Rath CM, Sawyer WS, et al. Toxic Accumulation of LPS Pathway Intermediates Underlies the Requirement of LpxH for Growth of Acinetobacter baumannii ATCC 19606. PLoS One. 2016;11:e0160918. doi: 10.1371/journal.pone.0160918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soon RL, Li J, Boyce JD, Harper M, Adler B, Larson I, Nation RL. Cell surface hydrophobicity of colistin-susceptible vs resistant Acinetobacter baumannii determined by contact angles: methodological considerations and implications. J Appl Microbiol. 2012;113:940–951. doi: 10.1111/j.1365-2672.2012.05337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soon RL, Nation RL, Harper M, Adler B, Boyce JD, Tan CH, et al. Effect of colistin exposure and growth phase on the surface properties of live Acinetobacter baumannii cells examined by atomic force microscopy. Int J Antimicrob Agents. 2011;38:493–501. doi: 10.1016/j.ijantimicag.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeghs L, de Cock H, Evers E, Zomer B, Tommassen J, van der Ley P. Outer membrane composition of a lipopolysaccharide-deficient Neisseria meningitidis mutant. EMBO J. 2001;20:6937–6945. doi: 10.1093/emboj/20.24.6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeghs L, den Hartog R, den Boer A, Zomer B, Roholl P, van der Ley P. Meningitis bacterium is viable without endotoxin. Nature. 1998;392:449–450. doi: 10.1038/33046. [DOI] [PubMed] [Google Scholar]
- Tamayo R, Choudhury B, Septer A, Merighi M, Carlson R, Gunn JS. Identification of cptA, a PmrA-regulated locus required for phosphoethanolamine modification of the Salmonella enterica serovar typhimurium lipopolysaccharide core. J Bacteriol. 2005;187:3391–3399. doi: 10.1128/JB.187.10.3391-3399.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaras AP, McPherson CJ, Kuhn M, Carifa A, Mullins L, George D, et al. LpxC inhibitors as new antibacterial agents and tools for studying regulation of lipid A biosynthesis in Gram-negative pathogens. MBio. 2014;5:e01551–14. doi: 10.1128/mBio.01551-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trent MS, Ribeiro AA, Lin S, Cotter RJ, Raetz CR. An inner membrane enzyme in Salmonella and Escherichia coli that transfers 4-amino-4-deoxy-L-arabinose to lipid A: induction on polymyxin-resistant mutants and role of a novel lipid-linked donor. J Biol Chem. 2001;276:43122–43131. doi: 10.1074/jbc.M106961200. [DOI] [PubMed] [Google Scholar]
- Typas A, Banzhaf M, Saparoea B, van den B, Verheul J, Biboy J, Nichols RJ, et al. Regulation of Peptidoglycan Synthesis by Outer-Membrane Proteins. Cell. 2010;143:1097–1109. doi: 10.1016/j.cell.2010.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tytgat HLP, de Vos WM. Sugar Coating the Envelope: Glycoconjugates for Microbe-Host Crosstalk. Trends Microbiol. 2016;24:853–861. doi: 10.1016/j.tim.2016.06.004. [DOI] [PubMed] [Google Scholar]
- Velkov T, Roberts KD, Nation RL, Thompson PE, Li J. Pharmacology of polymyxins: new insights into an “old” class of antibiotics. Future Microbiol. 2013;8:711–724. doi: 10.2217/fmb.13.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradov EV, Duus JØ, Brade H, Holst O. The structure of the carbohydrate backbone of the lipopolysaccharide from Acinetobacter baumannii strain ATCC 19606. Eur J Biochem. 2002;269:422–430. doi: 10.1046/j.0014-2956.2001.02647.x. [DOI] [PubMed] [Google Scholar]
- Wei JR, Richie DL, Mostafavi M, Metzger LE, Rath CM, Sawyer WS, et al. LpxK Is Essential for Growth of Acinetobacter baumannii ATCC 19606: Relationship to Toxic Accumulation of Lipid A Pathway Intermediates. mSphere. 2017;2:e00199–17. doi: 10.1128/mSphere.00199-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield C, Trent MS. Biosynthesis and export of bacterial lipopolysaccharides. Annu Rev Biochem. 2014;83:99–128. doi: 10.1146/annurev-biochem-060713-035600. [DOI] [PubMed] [Google Scholar]
- WHO. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. WHO. http://www.who.int/medicines/publications/global-priority-list-antibiotic-resistant-bacteria/en/. Accessed July 27, 2017.
- Wright MS, Jacobs MR, Bonomo RA, Adams MD. Transcriptome Remodeling of Acinetobacter baumannii during Infection and Treatment. MBio. 2017;8 doi: 10.1128/mBio.02193-16. http://dx.doi.org/10.1128/mBio.02193-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon EJ, Balloy V, Fiette L, Chignard M, Courvalin P, Grillot-Courvalin C. Contribution of the Ade Resistance-Nodulation-Cell Division-Type Efflux Pumps to Fitness and Pathogenesis of Acinetobacter baumannii. MBio. 2016;7 doi: 10.1128/mBio.00697-16. http://dx.doi.org/10.1128/mBio.00697-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Chan A, Lippa B, Cross JB, Liu C, Yin N, et al. Structure-based discovery of LpxC inhibitors. Bioorg Med Chem Lett. 2017;27:1670–1680. doi: 10.1016/j.bmcl.2017.03.006. [DOI] [PubMed] [Google Scholar]


