Abstract
Organ donors are sources of physiologically healthy organs and tissues for life-saving transplantation, and have been recently used for human immunology studies which are typically confined to the sampling of peripheral blood. Donors comprise a diverse population with different causes of death and clinical outcomes during hospitalization, and the effects of such variations on immune parameters in blood and tissues are not known. We present here coordinate analyses of innate and adaptive immune components in blood, lymphoid (bone marrow, spleen, lymph nodes), and mucosal (lungs, intestines) sites from a population of brain-dead organ donors (3-months-93-years; n=291) across eight clinical parameters. Overall, the blood of donors exhibited similar monocyte and lymphocyte content and low serum levels of pro-inflammatory cytokines as healthy controls; however, donor blood had increased neutrophils and serum levels of IL-8, IL-6 and MCP-1 which varied with cause of death. In tissues, the frequency and composition of monocytes, neutrophils, B lymphocytes and T cell subsets in lymphoid or mucosal sites did not vary with clinical state, and was similar in donors independent of the extent of clinical complications. Our results reveal that organ donor maintain tissue homeostasis, and are a valuable resource for fundamental studies in human immunology.
INTRODUCTION
Our understanding of the healthy human immune system and its related immunopathology—infection, autoimmunity, inflammation, allergy, transplant rejection, cancer—has advanced tremendously largely due to advances in animal models (1–5). However, many aspects of the human experience, including a long lifespan, prolonged exposure to diverse microbial and environmental antigens, and extensive genetic heterogeneity cannot be recapitulated in rodent models. In-depth studies of the human immune system are therefore necessary to address unanswered questions in fundamental immunology and develop more effective therapies for transplantation, autoimmunity, and infectious diseases.
The most readily accessible site for studying human immune responses is peripheral blood, containing neutrophils and monocytes as the predominant innate immune cells, and T and B lymphocytes as the cellular components of adaptive immunity. However, the majority of innate and adaptive immune cells reside in tissues, including subsets of immune cells that do not enter circulation (e.g. dendritic cells, macrophages, and tissue-resident memory T cells). The anatomical localization and tissue compartmentalization of immune cells is emerging as a key factor in their function, regulation, and maintenance (6–8). Because of the logistical and ethical difficulties involved in acquiring human tissues, immunology studies have used surgical explants or biopsies of single sites derived from diseased tissues of small cohorts (9–16). Results from such studies, while providing views into tissue immune cell populations, cannot be extrapolated to understanding baseline immune health in the diverse population.
Compared to the multiple caveats involved in use of tissue biopsies and explants, tissues from recently deceased organ donors provide multiple advantages for the study of human immunity including: 1) multiple tissues from a single individual can be obtained at the time of acquisition for life-saving transplantation enabling isolation of live, functional immune cell populations (unlike post-mortem autopsy samples); 2) donors in our cohort were free of cancer, Hepatitis B, Hepatitis C, and HIV, and have similar baseline medical comorbidities as compared to the US adult population; and 3) key information on donors including age, gender, BMI, viral serology, HLA typing, and clinical information (comorbidities, cause of death (COD), blood cultures, cell counts, etc.) is available.
We, along with our collaborators, have embarked on a multi-dimensional analysis of innate and adaptive immune cells in diverse tissue types (blood-rich, mucosal, lymphoid) from research-consented organ donors through a protocol and collaboration with our local organ procurement organization in the New York Metropolitan area, LiveOnNY (17–24). Using this resource, we have analyzed immune cell subset distribution and heterogeneity throughout the body and across the lifespan in tissues from over 100 individuals (19, 21, 23), revealing that T cell and dendritic cell subset composition and differentiation are specific to the tissue site, with some age-associated changes in specific compartments (17, 21, 23, 24). While these findings suggest that organ donor tissues provide a reliable assessment of baseline immune responses, the extent to which clinical variations of the donors including circumstances of death or hospitalization, are affecting our results is not clear.
Here, we conducted a novel analysis of the impact of clinical changes on innate and adaptive immune parameters in blood and multiple lymphoid and mucosal tissues in a large cohort of organ donors. Compared to healthy controls, the blood of organ donors contains similar myeloid and lymphocyte cellular compositions and low serum levels of multiple pro-inflammatory cytokines; however, organ donor blood had elevated neutrophils and serum levels of MCP-1, IL-6, and IL-8. Despite these circulatory changes, there were few significant variations in the monocyte, neutrophil, or T cell subset composition in lymphoid, mucosal, or peripheral tissues over a range of eight clinical parameters, including COD, length of stay (LOS), and the presence of infection. Our results indicate that organ donor tissues contain stably maintained immune cell populations, and can provide a unique view into human immunological health and homeostasis.
Materials and Methods
Experimental design
Blood, mucosal and lymphoid tissues were obtained from research-consented organ donors aged 2 months-93yrs at the time of procurement for clinical transplantation through a protocol and material transfer agreement (MTA) with LiveOnNY from February 2011 through October 2016 (n=291, Table S1). Tissue sites (ten total) included blood, bone marrow (BM), spleen, lung tissue, lymph nodes (LN) basins (lung hilum, mesenteric, and iliac), and intestines (jejunum, ileum, colon). Exclusion criteria for donors included active or recent cancer, and seropositivity for Hepatitis B, C, or HIV. The acquisition of tissue from deceased (brain dead) individuals does not qualify as human subjects research as confirmed by the Columbia University IRB. Peripheral blood samples were also drawn via venipuncture from consented volunteers, through an approved IRB protocol (Columbia University).
Isolation of cells from tissue sites
Tissue collection occurred after the donor organs were flushed with cold preservation solution and clinical procurement was completed. Tissues were processed within 2–4hrs of organ procurement resulting in high yields of live immune cells (17, 19, 23) (see online methods). For blood samples, lymphocytes were isolated by centrifugation through lymphocyte separation medium (Corning); monocytes and neutrophils were isolated using whole blood lysis techniques with ACK lysing buffer (Lonza, Walkersville, Md.).
Immune cell analysis by flow cytometry
Single-cell suspensions were stained with fluorochrome-conjugated antibodies (Table S2) in staining buffer (PBS/1% fetal bovine serum/0.1% sodium azide). Stained cells were acquired on a 6-laser LSRII analytical flow cytometer (BD Biosciences, San Jose, CA) or a 3-laser BD Fortessa flow cytometer. Controls included unstained samples, single fluorochrome-stained compensation beads (OneComp ebeads, eBioscience), and fluorescence minus one (FMO) controls. Flow cytometry data were analyzed using FlowJo software (Treestar, Ashland, OR).
Quantitation of Serum Cytokines
Blood was stored in EDTA coated collection tubes and then centrifuged to isolate the serum fraction. Serum cytokine content was quantitated using cytometric bead arrays (LEGENDplex™ Human Inflammation 13-plex Panel; Biolegend, San Diego, CA), and assessed by flow cytometry compared to control standards as indicated by the manufacturer.
Clinical Data
All clinical data were obtained from DonorNet®, the Organ Procurement and Transplantation Network’s computer system for transplant data collection and organ allocation. Eight clinical factors were chosen based on their known potential effects on organ physiology and immune responses: 1. Cause of death (COD; cerebrovascular stroke (CVA) vs. non-CVA used in risk indices in liver and kidney transplantation); 2. requirement for cardiopulmonary resuscitation (CPR) as cardiovascular instability is correlated with increased immune cell infiltration into tissues; 3. brain death duration based on reported cellular changes in animal models (25); 4. hospitalization length of stay (LOS) which correlates with overall worse outcomes and infections; 5. steroid administration known to inhibit immune responses; 6. acute lung injury (ALI) defined by the Berlin criteria also associated with tissue damage and infection (26); 7. Transfusions, including packed RBC or platelet transfusions, associated with changes in cytokines and lymphocytes (27); and 8. Positive blood or urine cultures indicating infections/ongoing immune responses. Donors with multiple factors were defined as having two or more of the above clinical characteristics. Donors were stratified into age categories: pediatric (0–15-years), young adult (16–35years), middle years (36–65yrs), and senior (> 65yrs). Clinical factors were compiled from all donors; immunological analysis was compiled from donors ≥16 years.
Statistical analysis
Donor demographics and clinical characteristics were analyzed with Stata MP (Stata Corp version 14.1, College Station, Texas). Categorical variables were analyzed using Pearson’s chi-squared test or test for trend. Continuous variables were presented as means (with standard deviation) or medians (with inter-quartile range), analyzed with either Student’s t-test, ANOVA, or their non-parametric equivalent (Mann-Whitney U or Kruskal-Wallis tests, respectively). Frequency comparison two-tailed p values for each cell type/tissue by each clinical factor was then correlated with eight different clinical factors (see above) by two-way ANOVA analyses in GraphPad PRISM, using Holm-Sidak post hoc correction for multiple comparisons (Graphpad software, La Jolla, CA). Family-wise level of significance was set at p < 0.05.
RESULTS
Study population and donor characteristics
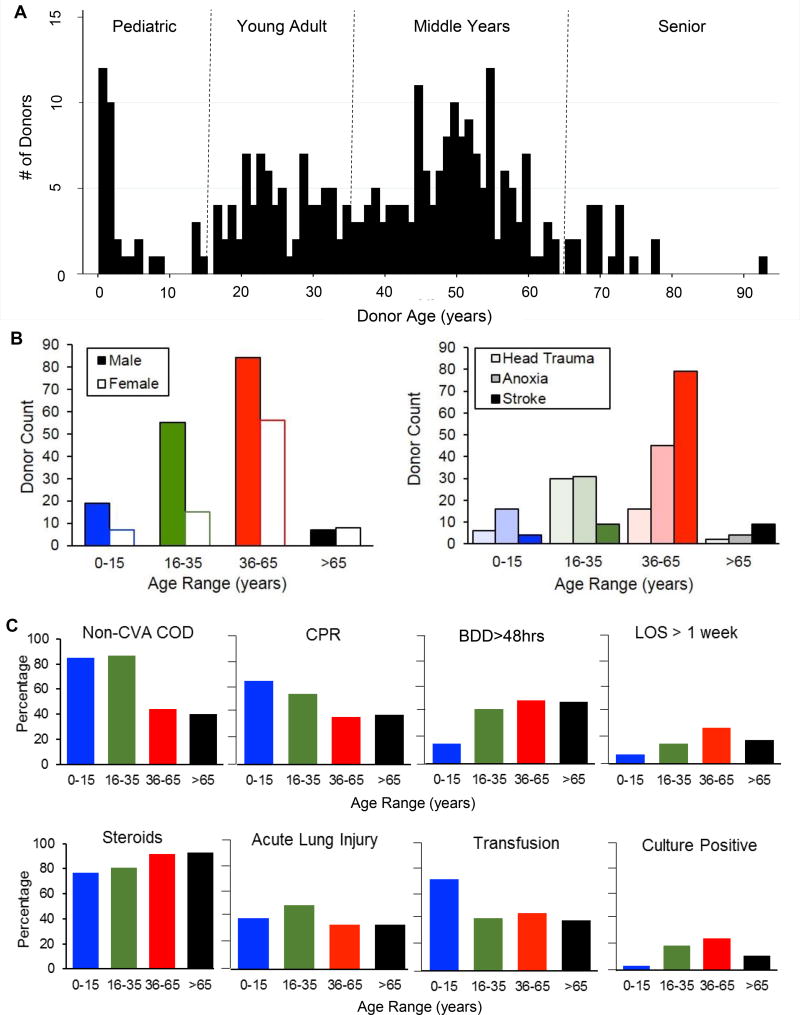
Between February 2011 and October 2016 we obtained tissues from 291 research-consented organ donors (Table 1). Donors were aged two-months to 93-years of age (mean=39.7 years, 66% male, Figure 1A). All donors met brain death criteria: 22% from head trauma (HT), 38% from anoxia, and 40% from stroke. COD varied significantly by age; younger donors were more likely to die from HT (p<0.001) while older donors of stroke (p<0.001) (Table 1 and Figure 1B). CPR was required prior to organ procurement in 47% of donors, with the highest rate of CPR (88%) in donors who died of anoxic compared to other causes (p<0.001, Table 1). In terms of medical comorbidities, 14% of donors had Type II diabetes mellitus (DM), 31% were obese, 37% had hypertension, 6% had coronary artery disease, 6% were intravenous drug abusers, and 18% were smokers (Table 1)—frequencies similar to the general US adult population (Table 2). Serological data indicated that 94% and 61% of adult donors were seropositive for persistent viruses Epstein-Barr virus (EBV+) and cytomegalovirus (CMV+), respectively, consistent with known seropositivities in the general US population (28). This analysis shows that by multiple clinical and health criteria, the donor population approximated the general state of health of the adult US population overall.
TABLE 1.
Descriptive Statistics - Population Characteristics of our cohort of deceased, brain dead organ donors from February 2011 through March 2016 (n=251)
| Cause of Death | Comparisons (p-value)χ | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Head Trauma (n=54) | Anoxia (n=96) | CVA/Stroke (n=101) | Total (n=251) | HT vs. Anoxia | HT vs. CVA | Anoxia vs. CVA | ||
|
|
||||||||
| Population Characteristics | ||||||||
| Demographics | ||||||||
| Age (mean years [SD]) | 30.29 (15.29) | 35.98 (20.74) | 48.16 (14.12) | 39.69 (18.58) | 0.0801A | <0.001A | <0.001A | |
| 0–15 (column %) | 6 (11.11) | 16 (16.67) | 4 (3.96) | 26 (10.36) | ||||
| 16–35 (column %) | 30 (55.56) | 31 (32.29) | 9 (8.91) | 70 (27.89) | 0.049✓ | <0.001✓ | <0.001✓ | |
| 36–65 (column %) | 16 (29.63) | 45 (46.88) | 79 (78.22) | 140 (55.78) | ||||
| >65 (column %) | 2 (3.70) | 4 (4.17) | 9 (8.91) | 15 (5.98) | ||||
| Female (%) | 8 (14.81) | 37 (38.54) | 41 (40.59) | 86 (34.26) | 0.002 | 0.001 | 0.768 | |
| Race (%) | ||||||||
| White | 18 (33.33) | 62 (64.58) | 41 (40.59) | 121 (48.21) | <0.001 | 0.375 | 0.001 | |
| Black | 14 (25.93) | 16 (16.67) | 27 (26.73) | 57 (22.71) | 0.174 | 0.914 | 0.087 | |
| Hispanic | 21 (38.89) | 17 (17.71) | 28 (27.72) | 66 (26.29) | 0.004 | 0.154 | 0.094 | |
| Asian | 1 (1.85) | 1 (1.04) | 5 (4.9) | 7 (2.79) | 0.592 | 0.318 | 0.118 | |
| BMI (median [IQR]) | 23.7 (20.8, 29.2) | 25.9 (21.9, 31.8) | 27.6 (23.3, 32.7) | 26.0 (22.1, 31.1) | 0.1376╤ | 0.0035╤ | 0.1262╤ | |
| Obese (%) | 10 (18.52) | 32 (33.68) | 35 (34.65) | 77 (30.80) | 0.048 | 0.035 | 0.886 | |
| Comorbidities (%) | ||||||||
| HTN | 3 (5.56) | 34 (35.42) | 55 (55.50) | 92 (36.80) | <0.001 | <0.001 | 0.006 | |
| DM | 2 (3.70) | 16 (16.67) | 18 (18.00) | 36 (14.40) | 0.019 | 0.012 | 0.805 | |
| CAD | 2 (3.70 ) | 9 (9.38) | 5 (5.00) | 6 (6.40) | 0.201 | 0.712 | 0.234 | |
| COPD or Asthma | 4 (7.41) | 17 (17.89) | 10 (9.90) | 31 (12.40) | 0.077 | 0.606 | 0.105 | |
| Cigarette smoker (at least 20 pack-year history) | 3 (5.66) | 17 (17.89) | 24 (24.24) | 44 (17.74) | 0.039 | 0.004 | 0.263 | |
| IVDA | 1 (1.85) | 12 (12.50) | 1 (1.00) | 14 (5.60) | 0.032 | 0.99 | 0.001 | |
| Serology (% ) | ||||||||
| EBV | 46 (86.79) | 78 (83.87) | 93 (93.94) | 217 (88.57) | 0.635 | 0.133 | 0.025 | |
| CMV | 30 (56.60) | 46 (49.46) | 64 (64.65) | 140 (57.14) | 0.406 | 0.331 | 0.034 | |
| EBV/CMV double negative | 6 (11.32) | 10 (10.75) | 6 (6.06) | 22 (8.98) | 0.916 | 0.252 | 0.24 | |
| Donor Clinical Characteristics | ||||||||
| ECD (%) | 3 (5.56) | 14 (14.58) | 28 (28.0) | 45 (18.0) | 0.094 | 0.001 | 0.022 | |
| Brain Death duration (median hours [IQR]) | 44.5 (37, 62) | 43.5 (30, 58.5) | 45 (36, 60) | 44 (34.5, 62) | 0.3690╤ | 0.7412╤ | 0.4345╤ | |
| KDPI (median [IQR]) | 31 (13, 52) | 57.5 (38, 83) | 74 (54, 89) | 59.5 (38, 82.5) | <0.001╤ | <0.001╤ | 0.0061╤ | |
| CPR (%) | 13 (24.07) | 84 (88.42) | 20 (19.80) | 117 (46.80) | <0.001 | 0.536 | <0.001 | |
| CPR duration (median minutes [IQR]) if cardiac arrest | 15 (14, 20) | 24 (15, 45) | 15 (10, 29) | 20 (14.5, 40) | 0.0777╤ | 0.6452╤ | 0.0635╤ | |
| LOS (median days [IQR]) | 4.5 (3, 7) | 6 (4, 8) | 5 (3, 7) | 5 (4, 7) | 0.0084╤ | 0.7796╤ | 0.0061╤ | |
| Acute Lung Injury (% during hospital stay)⌿ | 27 (51.92) | 47 (54.02) | 34 (34.69) | 108 (45.47) | 0.81 | 0.041 | 0.008 | |
| Positive Cultures (% with one or more positive during hospital stay) | ||||||||
| Sputum | 7 (13.46) | 13 (14.94) | 24 (24.24) | 44 (18.49) | 0.81 | 0.119 | 0.113 | |
| Blood | 2 (3.85) | 2 (2.33) | 5 (5.05) | 9 (3.80) | 0.632 | 0.99 | 0.452 | |
| Urine | 3 (5.77) | 8 (9.20) | 6 (6.06) | 17 (7.14) | 0.469 | 0.943 | 0.419 | |
| Clinical Interventions (% ) | ||||||||
| Steroids (1g Solumedrol every 24 hours) | 46 (86.79) | 73 (81.11) | 93 (93.00) | 212 (87.94) | 0.38 | 0.205 | 0.014 | |
| Thyroid Hormone | 43 (91.49) | 74 (85.06) | 88 (89.80) | 205 (88.36) | 0.286 | 0.747 | 0.33 | |
| DDAVP | 8 (17.78) | 11 (13.10) | 20 (21.74) | 39 (17.65) | 0.474 | 0.589 | 0.133 | |
| Blood Products (either packed red blood cells or platelets transfused) | 42 (79.25) | 42 (46.15) | 50 (50.51) | 134 (55.14) | <0.001 | 0.001 | 0.549 | |
| Vasopressors (on at least one vasopressor at time of procurement) | 49 (92.45) | 75 (83.33) | 84 (84.85) | 208 (85.95) | 0.121 | 0.177 | 0.776 | |
Our donor population - descriptive statistics by cause of death analysis. Abbreviations: BMI = body mass index; ECD = extended criteria donor; KDPI = kidney donor profile index (range 0–100: lower = better quality kidney for transplant); CPR = cardio-pulmonary resuscitation; LOS = length of hospital stay in days; HTN = hypertension; DM = diabetes mellitus; CAD = coronary artery disease; COPD = chronic obstructive pulmonary disease; IVDA = intravenous drug abuse; EBV = Ebstein Barr virus; CMV = cytomegalovirus; DDAVP = desmopressin.
Acute Lung Injury/Mild ARDS = defined by Berlin criteria (bilateral opacities on Chest X-ray or CT involving at least 3 quadrants that are not fully explained by pleural effusions, atelectasis, or nodules; developed in < 1 week; non-cardiac in origin; and a PaO2/FiO2 ratio < 300)
statistical significance defined as p value < 0.05
chi-squared analysis (%) unless otherwise indicated
chi-squared for trend
ANOVA (mean [SD])
Kruskal Wallis
Figure 1. Characteristics of deceased organ donors analyzed in this study.
Tissues from research-consented organ donors were obtained for analysis of immune cells from 291 donors between February, 2011 and October 2016. (A) Graph shows number of donors of each age spanning 3 months to 93 years of age (n=291), stratified into the following age categories: Pediatric, 0–15 years of age; Young adult, 16–35 years of age; Middle years, 36–65 years of age; Senior, > 65-years of age. (B) Graph shows stratification of gender (left) and cause of death (right) by age range. (C) Bar graphs showing eight clinical characteristics stratified by donor age category. Clinical characteristics include: non-cerebrovascular accident (CVA) as cause of death (COD); need for cardiopulmonary resuscitation (CPR); brain death duration greater than 48 hours (BDD>48hrs); length of stay (LOS) greater than one week; administration of steroids (1g Solumedrol every 24 hours) after the diagnosis of brain death; presence of acute lung injury (ALI) as diagnosed by Berlin criteria (see supplemental methods); whether or not the donor received a packed red blood cell or platelet transfusion during their hospitalization (“Transfusion”); and, whether or not the donor had a positive blood or urine culture at any point during their hospitalization (Culture positive).
TABLE 2.
Medical comorbidities - Donors compared to the US adult population
| Our Adult Donors (%) |
US Population (%) |
|
|---|---|---|
| HTN | 41.6 | 32† |
| DM | 16.3 | 9.3† |
| CAD | 7.2 | 6† |
| COPD/Asthma | 13.6 | 11✓ |
| Obese | 34.4 | 35.9† |
| IV Drug abuse | 6.33 | < 1╤ |
| Smoker (defined as > 20 pack years in our donors) | 20.1 | 19.3† |
Medical comorbidities in adult deceased donors vs. US adult population (adult = 18 years of age or older).
CDC estimates
American Lung Association estimates (current smokers)
Health and Human Services estimate (Substance Abuse and Mental Health Services Administration (SAMHSA)
We analyzed how key clinical parameters related to the COD, length of brain death, LOS, and treatments and drugs administered prior to organ acquisition varied among donors (Table 1; Figure 1B,C). The median LOS for the organ donors was five days, and the median duration of brain death prior to organ procurement was under two days (44 hours). Consistent with most current donor management strategies, ~85–90% of donors received steroids, vasopressors, or thyroid hormone after brain death declaration; 55% of donors received a transfusion (either packed red blood cells or platelets) during their hospitalization; and 46% of donors sustained ALI during their hospitalization (29–31). A small fraction of donors (10.5%) also developed infections, as measured by positive cultures in blood or urine.
Certain clinical factors varied by age (Figure 1B,C; Table 1). COD varied significantly, in which median age was highest for donors who died of stroke and lowest for donors who died of HT (p<0.001). Pediatric and young adult donors were most likely to receive CPR and transfusions prior to organ donation, and to have shorter lengths of stay and brain death durations. Young adult donors were more likely to sustain ALI prior to organ donation. Finally, young adults and middle-aged donors had the highest rates of infections, also associated with anoxia (Table 1). Thus, the conditions and circumstances of death and subsequent hospitalization varied significantly by age.
Variation in blood immune cells and cytokines among donors
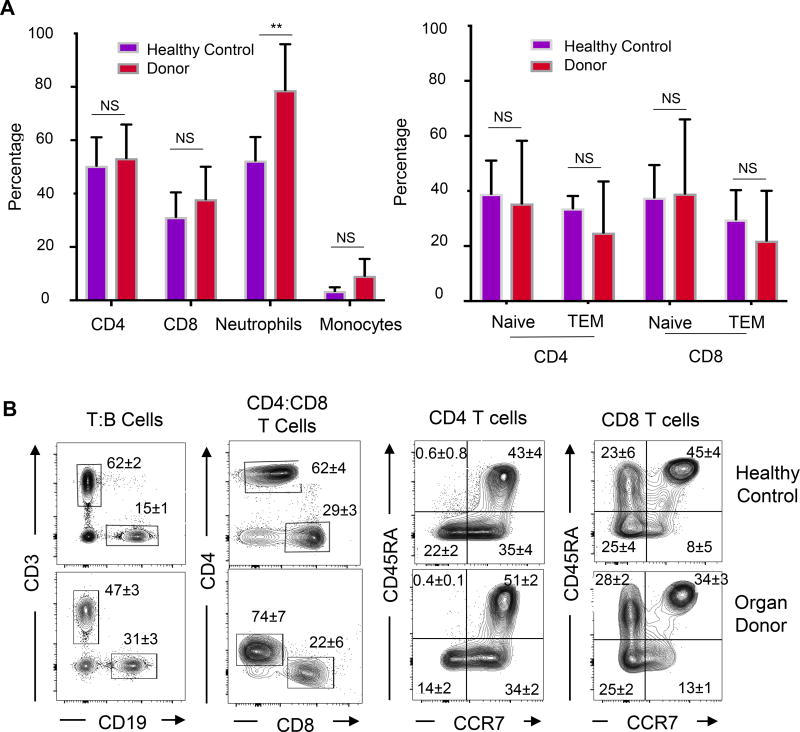
We initially examined the frequency of innate (myeloid lineage) and adaptive (T and B cells) immune cells in the blood of organ donors compared to healthy controls. While T cell subset frequencies and CD4+:CD8+ T cell ratios did not vary significantly between donors and healthy controls, donor blood contained a significantly higher percentage of neutrophils compared to that of healthy controls (Figure 2A; mean of 85% vs 64%, p<0.001). Monocyte frequencies for donors and healthy subjects were within normal limits (32) and not significantly different. Frequencies of naïve, effector, and memory T cell subsets did not vary significantly between organ donors and healthy controls (Figures 2A,B), indicating no overt immune cell activation in either population. These results indicate an increase in circulating neutrophils in donor compared to control blood, but similar lymphocyte frequencies.
Figure 2. Immune cell composition in donor blood compared to healthy controls.
Immune cell content in the blood of organ donors and healthy living controls was analyzed by flow cytometry. (A) Left: Frequency of CD4+ and CD8+ T cells, neutrophils and monocytes in peripheral blood of organ donors (n=82, T cells; n=40, monocyte/neutrophil) compared to healthy controls (n=7) expressed as %CD45+ cells (** indicates p<0.001). Right: Graph shows percentage of naïve (CD45RA+CCR7+) and effector-memory (TEM, CD45RA−CCR7−) subsets for CD4+ and CD8+ T cells. N.S.=not significant. (B) Representative flow cytometry plots of lymphocytes in healthy control (top) and deceased organ donors (bottom) showing gating for T, B, CD4+, CD8+ T cells and T cell subset delineation by CD45RA and CCR7 expression. All plots are shown as a function of live (DAPI−), singlet, CD45+ cells. Numbers in quadrants indicate mean ± SEM for entire cohort.
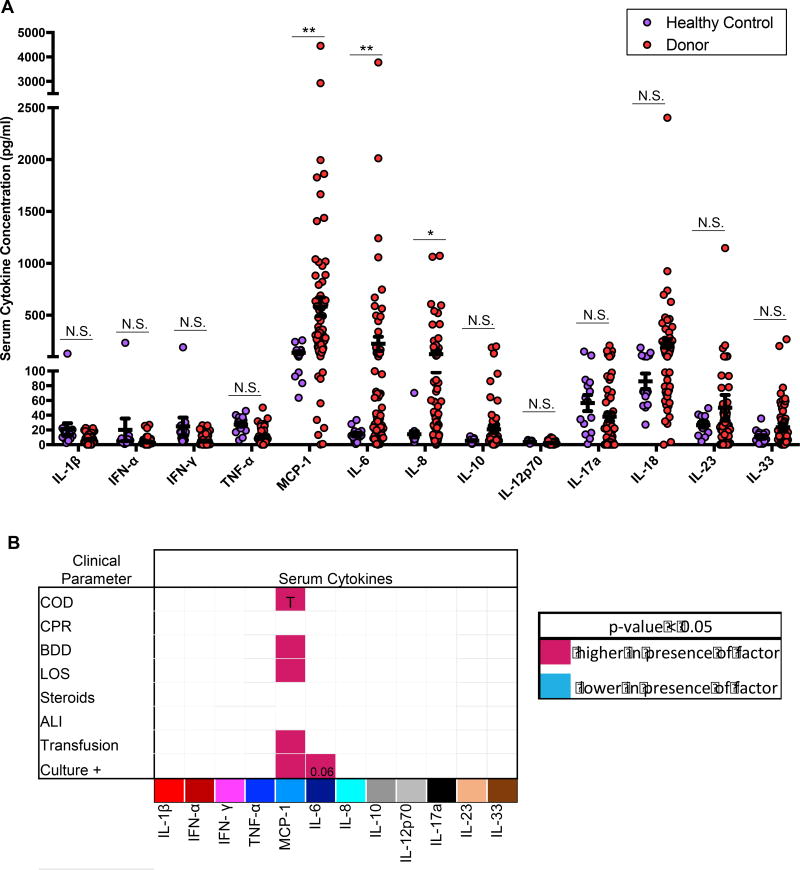
We measured the concentration of multiple cytokines in the serum of donors and healthy controls (Figures 3A). Notably, donor and healthy control serum both contained low or negligible levels of multiple inflammatory cytokines, including Type I IFN, TNF-α, IFN-γ, IL-17, IL-12, IL-23, IL-1β and IL-18, and the Th2-promoting cytokine IL-33 (Figure 3A). (Elevated levels of IL1-β, IFN-γ, and IFN-α were consistently observed in samples obtained from a healthy male (28yrs), suggesting natural variation in serum cytokines among the healthy population.) There were, however, statistically significant elevations in three pro-inflammatory cytokines in donor compared to control serum, including monocyte chemotactic factor (MCP-1; also known as CCL2); IL-6 a multi-functional pro-inflammatory cytokine; and IL-8 a neutrophil chemotactic factor. Levels of the anti-inflammatory cytokine IL-10 trended higher in organ donors but did not reach significance (p=0.129). These results indicate that donor serum exhibited specific elevations in certain cytokines, rather an overall global inflammatory signature.
Figure 3. Comparison of Serum cytokine levels in organ donors and healthy controls.
(A) Levels of multiple cytokines in the serum of organ donors (n=47) and healthy controls (n=13) expressed as pg/ml (mean ± SEM). N.S.=not significant. Significant differences between levels in donors and healthy controls were identified for MCP-1 (p<0.001), IL-6 (p=0.0041), and IL-8 (p=0.0129) (* = p<0.05, ** = p< 0.01). (B) Matrix shows serum cytokine concentrations as a function of eight measured clinical characteristics as defined in Figure 1C (COD, cause of death; CPR, cardiopulmonary resuscitation; LOS, length of stay > 1 week with the mean LOS being 5.5 days; BDD, brain death duration greater than 48 hours which was the mean length of brain death; Transfusion, whether or not a donor received any packed red blood cell or platelet transfusion; Cx Data, whether or not donors had a positive blood or urine culture during their hospitalization.) The matrix grid shows an ANOVA analysis analyzing the presence/absence of each factor in each tissue. Shaded pink boxes indicating significant (p<0.05) positive correlation with the factor and shaded blue boxes indicating negative correlation with the factor. For MCP-1, the significance for each factor was: p=0.0280 for higher levels of MCP-1 in HT vs. stroke; p=0.006 for higher MCP-1 in donors with BDD> 48 hours; p=0.0034 for higher MCP-1 in donors with LOS >1 week; p<0.001 for higher MCP-1 levels in donors who had transfusions; and p<0.001 for higher MCP-1 levels in culture+ donors. IL-6 was higher in donors with infections (p=0.06).
We analyzed whether changes in levels of MCP-1, IL-6, IL-8, and other cytokines were associated with specific variations in donor clinical state, based on eight clinical parameters identified in Figure 1A (Figure 3B). Serum MCP-1 concentration was higher in donors receiving transfusions, in donors with infections, and in donors with multiple clinical factors present (Figure 3B). Serum IL-6 levels were higher in donors with infections compared to those without, while elevated levels of IL-8 and IL-10 did not correlate with any clinical change (Figure 3B). Table 3 shows a correlation matrix revealing that donors with one elevated cytokine are more likely to have elevations in other cytokines. Together, these results show that elevated levels of these three cytokines are specific features of organ donors and correlate with worsened clinical state (e.g. MCP-1) or infections (MCP-1, IL-6).
TABLE 3.
Correlation of serum cytokine levels in organ donors
| MCP-1 | IL-6 | IL-8 | IL-10 | IL-18 | |
|---|---|---|---|---|---|
|
|
|||||
| MCP-1 | 1 | ||||
|
| |||||
| IL-6 | 0.4783 | 1 | |||
| 0.0001* | |||||
|
| |||||
| IL-8 | 0.3833 | 0.1545 | 1 | ||
| 0.0014* | 0.2119 | ||||
|
| |||||
| IL-10 | 0.5606 | 0.1594 | 0.312 | 1 | |
| 0.0001* | 0.1976 | 0.0102* | |||
|
| |||||
| IL-18 | 0.0232 | −0.0297 | 0.1075 | 0.3327 | 1 |
| 0.8522 | 0.8117 | 0.3866 | 0.0059* | ||
Serum cytokine correlation among the five highest serum cytokine levels seen in the deceased organ donor population (n=47).
Top row = correlation coefficient (>0 means positive correlation; <0 means negative correlation).
Bottom row/shaded row = p-value (< 0.05)
Myeloid and lymphoid lineage analysis and variation by clinical parameter
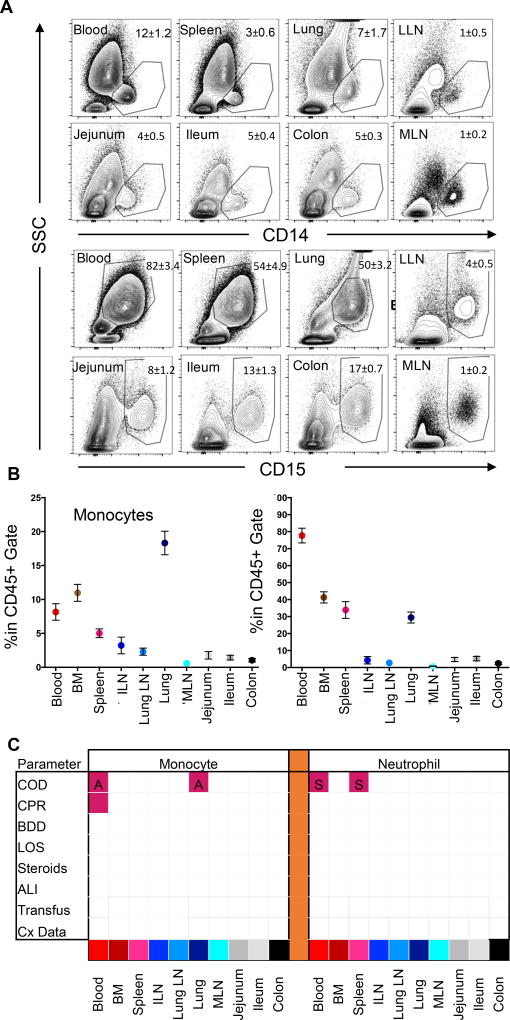
We performed a systematic analysis of myeloid (monocyte, neutrophil) and lymphocyte (T, B) lineage cells in lymphoid and mucosal tissues compiled from many donors (Figure 4). Among leukocytes (CD45+), the frequency of CD14+ monocytes and CD15+ neutrophils differs for each site as observed in flow cytometry plots from representative individual donors and compiled data from 40 donors (Figure 4A,B). Notably, blood and blood-rich tissues including BM, spleen, and lungs have the highest frequencies of monocytes (5–20%) and neutrophils (30–80%) compared to lymph nodes (LN), with intestinal sites having the lowest frequency (<5%,) (Figure 4B). These compiled results indicate that the composition of innate immune cells differs between tissues, and this tissue-specific composition does not vary significantly between individuals.
Figure 4. Low variability of Monocyte and neutrophil content as a function of donor clinical changes.
(A) Representative flow cytometry plots of CD14+ monocyte (first two rows) and CD15+ neutrophil (third and fourth rows) staining in relation to their side scatter properties in blood and seven tissue sites of a representative individual organ donor including blood, spleen, lung, lung-drining lymph nodes (LLN), jejunum, ileum, colon and mesenteric lymph node (MLN). Plots show profiles gated on live (DAPI−), singlet, CD45+ cells. Numbers in quadrants indicate mean ± SEM for entire cohort. (B) Compiled flow cytometry data showing percentage of monocytes (mean ± SEM) (left) and neutrophils (right) in blood and nine tissue sites (n=40). Tissue abbreviations as in (A) also showing bone marrow (BM) and iliac lymph node (ILN). (C) Matrix shows significant changes (p<0.05) in monocyte (left) or neutrophil (right) levels as a function of eight clinical factors as defined in Figure 1C (COD, cause of death; CPR, cardiopulmonary resuscitation; LOS, length of stay > 1 week; BDD, brain death duration greater than 48hrs; Transfusion; Cx Data) The matrix grid shows an ANOVA analysis analyzing the presence/absence of each factor in each tissue. Shaded pink boxes indicating significant (p<0.05) positive correlation with the factor and shaded blue boxes indicating negative correlation with the factor. Significance level for each factor was as follows: for monocytes, a higher frequency in the blood (p=0.0043) and lung (p<0.001) was associated with anoxia (labeled ‘A’) compared to donors who died of stroke, while higher monocyte frequency in blood was also associated with CPR (p=0.0293). For neutrophils, donors who died of stroke (labeled ‘S’) had significantly higher frequencies in the blood (p=0.0417) and spleen (p=0.0326) than donors who died of HT.
In order to examine how cell frequencies changed in each tissue site in relation to donor clinical variations, we performed a cross-table analysis comparing monocyte and neutrophil variation across eight clinical parameters between each tissue site (Figure 4B). Monocyte frequency was significantly higher in the blood and lungs of donors who died of anoxia compared to stroke or HT, while neutrophil frequency was higher in the blood and spleen of donors who died of stroke compared to HT, with COD and CPR as the only factors associated with significant differences in the blood (Figure 4C). However, there were no significant differences in any of the LN basins or intestinal sites when analyzed in relation to the eight clinical factors (Figure 4C). These results indicate that despite some variation in myeloid lineage cells in blood-rich sites based on COD, the tissue composition of monocytes and neutrophils was a feature of the tissue sites and remained stable between clinically-diverse donors.
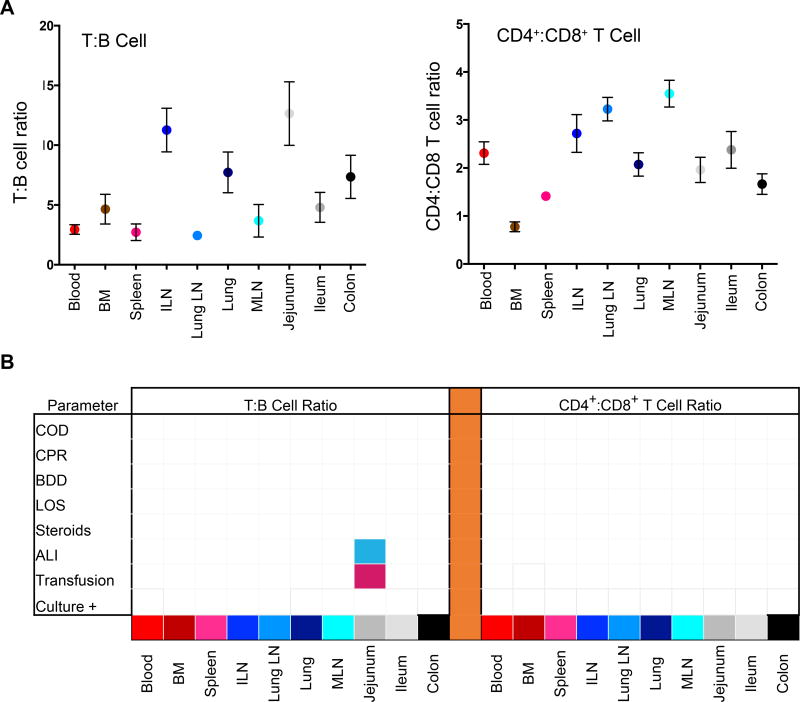
For lymphocyte composition, the T:B and CD4+:CD8+ T cell ratio was a feature of the tissue site as determined in a large donor cohort (Figure 5A, n=82), with lymphoid sites having higher T cell content and predominant CD4+ T cells compared to mucosal sites consistent with our results in smaller cohorts (21, 23). When lymphocyte composition (T, B, CD4+, CD8+ T cells) was analyzed for variations across eight clinical parameters as above, there was no significant variation in any of the 10 sites examined except for some jejunal variation in T:B ratio only (Figure 5B). Specifically, blood transfusion was associated with a higher T:B ratio and ALI correlated with a lower T:B ratio (Figure 5B). Together these findings show minimal detectable variation in lymphocyte composition in tissues of diverse organ donors based on multiple clinical parameters, revealing stability of tissue lymphocyte populations in organ donors.
Figure 5. Stability of lymphocyte frequencies in donor blood and tissues as a function of donor clinical changes.
(A) Compiled Lymphocyte data showing mean ± SEM for T:B cell (left) and CD4+:CD8+ T cell (right) ratios in each tissue site (n=82). Tissue abbreviations: BM, bone marrow; ILN, iliac lymph node; MLN, mesenteric lymph node. (B) Matrices show significant changes (p<0.05) in T:B cell (left) or CD4+:CD8+ T cell (right) ratios as a function of eight measured clinical characteristics as defined in Figure 1C. The matrix grid shows an ANOVA analysis analyzing the presence/absence of each factor in each tissue. Shaded pink boxes indicating significant (p<0.05) positive correlation with the factor and shaded blue boxes indicating negative correlation with the factor. A lower T:B ratio in jejunum was associated with ALI (p<0.001) while a higher T:B cell ratio was associated with transfusions (p=0.0091).
T cell subset distribution is stably maintained
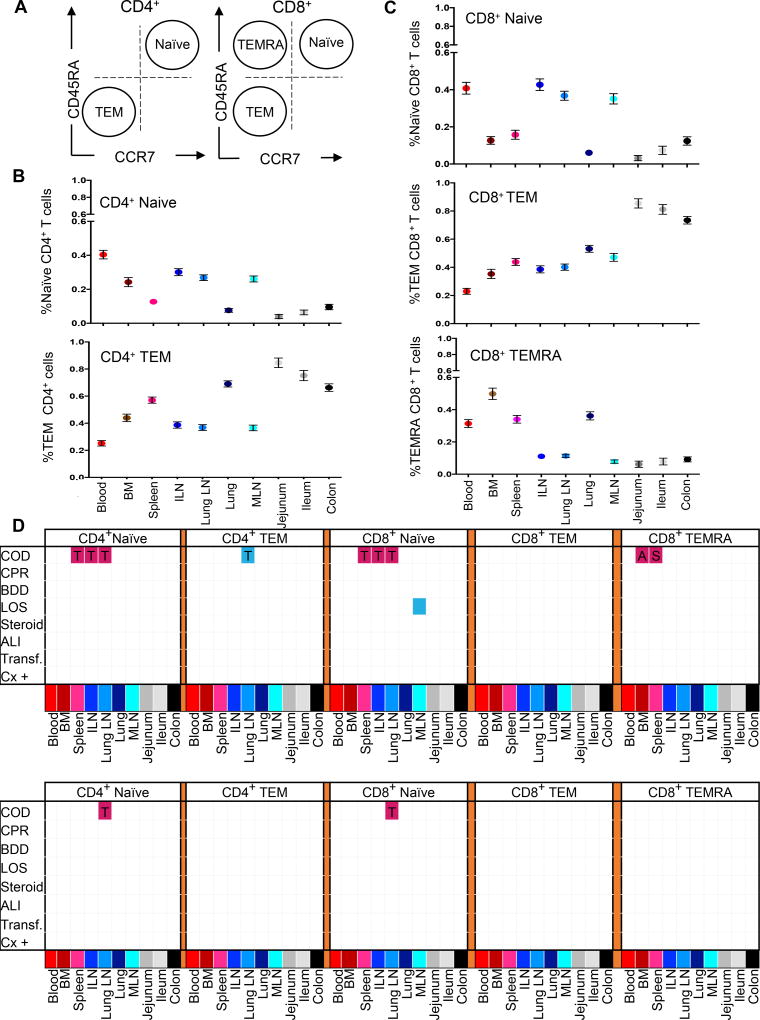
T cells exist in distinct subsets that reflect their activation history and functional capacity and can be delineated by CD45RA and CCR7 expression into naïve (CD45RA+CCR7+), effector-memory (TEM: CD45RA−CCR7−), and terminal effector (TEMRA: CD45RA+CCR7−) subsets (Figure 6A) with the relative proportions of these subset a function of the tissue site, age and lineage (21, 23). We analyzed the proportion of these major subsets in each site compiled from 82 different donors across eight clinical factors (Figure 6B–D). For both CD4+ and CD8+T cells, naïve T cells were prevalent in lymphoid sites, TEM cells were the predominant subset in mucosal sites and present in significant frequencies (30–50%) in lymphoid sites, while CD8+ TEMRA cells were found significantly only in blood and blood-rich sites (BM, spleen, lung; Figure 6B,C). The frequency of these T cell subsets did not exhibit significant variations across 8 clinical factors in most sites, with the exception of COD which was associated with variations in naïve T cells in spleen, ILN, and LLN and TEMRA in BM and spleen (Figure 6D, top). When adjusting for effects of age, however, these variations in T cell subset composition were no longer significant. These results show stability of T cell subset composition in donors despite clinical variation and when controlling for age.
Figure 6. T cell subset composition in tissues varies more by age and less by clinical factors.
(A) CD4+ and CD8+ T cell subsets are defined as a function of CD45RA and CCR7: Terminal Effector (TEMRA: CD45RA+CCR7−), Naïve (CD45RA+CCR7+), and Effector Memory (TEM: CD45RA−CCR7−). (B) Compiled subset results for CD4+ T cells showing naive (upper) and TEM (lower) percentages (mean ± SEM) in blood and 9 tissue sites (n=82). (C) Compiled subset results for CD8+ T cells showing naive (upper), TEM (middle) and TEMRA (lower) percentages (mean ± SEM) in blood and 9 tissue sites (n=82). (D) Matrix of significant association (p<0.05) of T cell subset frequencies with eight clinical parameters without adjustments for age (top), and adjusted for age as a covariate (bottom), as defined in Figure 1C. The matrix grid shows an ANOVA analysis analyzing the presence/absence of each factor in each tissue. Shaded pink boxes indicating significant (p<0.05) positive correlation with the factor and shaded blue boxes indicating negative correlation with the factor. In the age-adjusted analysis, HT as a COD was associated with significantly higher CD4+ naïve cells (p<0.001) and CD8+ naïve cells (p=0.002) in the LLN compared to donors who died of a stroke.
Immune cell variations by quantitative association with clinical risk factors
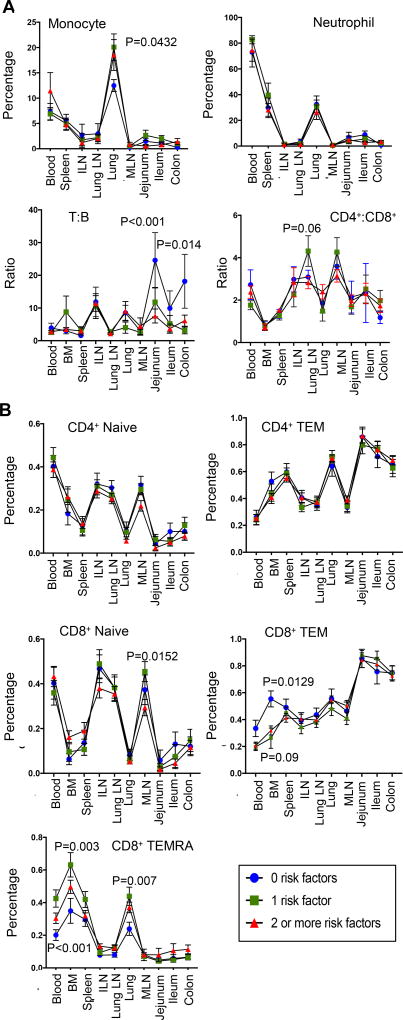
To further investigate potential associations between immune cell content in blood and tissues with donor clinical status, we stratified immune cell results based on the aggregate number of clinical factors (Figure 7, see methods). Interestingly, the monocyte, neutrophil and lymphocyte (T:B, CD4+:CD8+ T cell) content in blood and tissues was similar in donors independent of clinical factors, (Figure 7A). However, significant variations were observed for CD8+ TEM and TEMRA cells in blood, BM, and lung. The frequency of CD8+ TEMRA cells was higher in the blood and BM of individuals with >1 factor, compared to donors with none (Figure 7B). Conversely, the proportion of CD8+ TEM cells was lower in the blood and BM of donors with one or more clinical factors compared to donors with none (Figure 7B). Correlating serum cytokines with immune cell frequencies in the blood (Table 4), indicates that MCP-1 is associated with increased frequencies of monocytes and neutrophils while IL-8 is associated with higher frequencies of CD8+ TEMRAs. These data show measurable changes in the frequency of circulating TEMRA cells and serum IL-8 with increased clinical factors, while myeloid cells and tissue populations remain stable.
Figure 7. Immune cell variations for lymphoid and myeloid subsets across tissue site by number of donor clinical risk factors.
Cellular frequencies were compared between donors across three categories in all ten tissue sites: donor categories included, donors with 0 clinical risk factors; donors with one risk factor; and donors with two or more risk factors as defined in Figure 1C. (A) Monocyte/Neutrophil (n=40; top) as well as T:B and CD4+:CD8+ (n=82; bottom) ratios (mean value ± SEM) compared across tissue sites as a function of number of donor clinical risk factors. (B) CD4+ and CD8+ T cell subset frequencies compared across tissue sites as a function of number of donor clinical risk factors.
TABLE 4.
Correlation between serum cytokine levels and immune cell frequencies in peripheral blood of organ donors
| MCP-1 | IL-6 | IL-8 | ||||
|---|---|---|---|---|---|---|
| Correlation Coeff. |
P-value | Correlation Coeff. |
P-value | Correlation Coeff. |
P-value | |
|
|
||||||
| Blood | ||||||
| Monocyte | 0.6779 | 0.0312 | −0.1968 | 0.5859 | 0.1267 | 0.7273 |
| Neutrophil | 0.6338 | 0.0915 | 0.2118 | 0.6145 | −0.3813 | 0.3514 |
| T:B cell ratio | 0.1346 | 0.6465 | 0.2387 | 0.4111 | 0.273 | 0.3449 |
| CD4:CD8 ratio | −0.3565 | 0.3463 | −0.3358 | 0.377 | −0.3214 | 0.3989 |
| CD4 naïve | −0.2333 | 0.4028 | −0.2781 | 0.3156 | −0.1464 | 0.6026 |
| CD4 TEM | 0.3338 | 0.224 | 0.336 | 0.2208 | 0.1957 | 0.4845 |
| CD8 naïve | −0.2187 | 0.4336 | −0.2174 | 0.4365 | −0.275 | 0.3212 |
| CD8 TEM | −0.2367 | 0.3956 | −0.3012 | 0.2754 | −0.3908 | 0.1498 |
| CD8 TEMRA | 0.3777 | 0.1651 | 0.4133 | 0.1258 | 0.5682 | 0.0271 |
Correlation data between serum cytokine concentrations and peripheral blood immune cell frequencies (n=47).
Light grey (p <0.10); dark grey (p < 0.05).
DISCUSSION
We have obtained tissues from organ donors at the time of procurement for life-saving transplantation for fundamental studies on human immunology, through a research protocol and collaboration with LiveOnNY. This resource has enabled the study of immune cells to a depth and scope not previously possible—in 10–15 tissue sites from a single individual and from diverse individuals of all ages. Our studies have yielded consistent results on tissue-specific immune cell populations between disparate individuals (17, 19, 21, 23, 24); however, donors experience diverse COD and clinical states, raising the important question of whether clinical variations impact immune parameters.
To address this question, we analyzed lymphocyte data from a large donor cohort together with new results on myeloid cells and cytokines in tissue and/or circulation across eight clinical factors known to impact organ physiology and immune responses, including COD, duration of brain death or hospitalization, CPR, infections, ALI, and/or transfusions. Overall, we found negligible variation in the composition of innate and adaptive immune cells in tissues from analysis of over 100 donors across these clinical parameters. However, there were measurable, but limited, changes in circulation in certain serum cytokines and immune subtypes when stratified by clinical factors. Together, these findings indicate the remarkable stability of tissue immunity in the context of variations in circulation, and indicate that organ donor tissues can provide a unique view of human tissue immunity.
The cohort of research-consented organ donors analyzed here exhibited a profile of health consistent with the overall US adult population. The key variables that differed among these donors were age-related with COD being a major variable associated with clinical complications. Younger donors exhibited highest rates of HT and anoxia (related to accidents, suicides, and drug overdoses) compared to older donors where CVS was the predominant cause. As a result, younger donors were more likely to experience ALI and infections which can occur as a result of aspiration following traumatic injury or overdose. Younger donors had shorter LOS likely due to superior organ quality and shorter search for an accepting transplant center.
The myeloid and lymphocyte composition in blood and tissues of donors did not exhibit significant variations over eight clinical parameters. Overall, we did not find significant variations in the tissue-intrinsic composition of immune cells in tissue sites based on COD, CPR, brain death duration, LOS, transfusions, steroids, ALI, or when combing multiple clinical factors. Notably, lymph nodes, intestines, and lungs maintained stable frequencies between donors. Variations in monocyte, neutrophil and CD8+ TEMRA composition was observed in blood and/or blood-rich sites such as spleen and BM with COD and CPR, and associated with multiple clinical factors. These findings indicate that variations in donor immune cell parameters due to clinical state are nearly exclusively observed in circulation, with tissues maintaining stable immune populations.
Compared to healthy controls, donor blood contained an increase in neutrophils and serum concentrations of MCP-1, IL-6 and IL-8, while levels of multiple inflammatory cytokines and monocytes/lymphocytes numbers were similar. The blood neutrophilia in organ donors could be due to increased IL-6 and IL-8 which function in neutrophil chemotaxis (33) and/or steroids, which are administered to donors to replace adrenal function (34–36), but can trigger elevations in neutrophils by demargination (37). MCP-1 and IL-6 levels were increased in donors with infections, consistent with findings in transplant patients (38, 39). The immune cell stability in tissues despite these variations in serum cytokines suggests that immune cells in tissues may be segregated into protective “niches” where with specific cytokine and cellular environments. Our discrepant findings in the circulation and tissues further suggest a level of separation exists between tissue resident cells and their circulating counterparts.
Limitations to our study include the possibility that relatively few changes related to immune cell subset and phenotype in tissues, more sensitive measures such as transcriptome profiling may uncover variations. It is also possible that additional clinical factors from the ones chosen may impact tissue immune status. Several studies utilizing organ biopsies in deceased vs. living donors have attempted this type of analysis with some differences identified (40–44), although there has been some debate regarding the timing of biopsies as related to cold ischemia time (40). All of our donors met brain death criteria, not allowing us to discern living vs. deceased changes.
Our understanding of human immunology is largely dependent on knowledge obtained from sampling of blood and extrapolated from animal models. Therefore, a major goal in modern immunology is to develop a method to study human immunity in situ. Our findings show stable maintenance of tissue immune cell populations in diverse sites indicate that organ donation cannot only provide life-saving therapies in the short-term, but can serve as a unique and valuable research resource for fundamental studies on human immunology for future life-saving therapies.
Supplementary Material
Supplemental Materials and Methods
Table S1: Donor Information
Table S2: Antibodies used for flow cytometry
Table S3: Representative CD4/CD8 Ratio Analysis by Clinical Factor
Acknowledgments
D.L.F. is supported by NIH grants AI100119, AI106697, and HL116136. Studies were performed in the CCTI Flow Cytometry Core funded in part through an S10 Shared Instrumentation Grant, 1S10RR027050 and 5P30DK063608. D.J.C is supported by NIH T32 Training Grant, T32HL007854. We wish to gratefully acknowledge the generosity of the donor families and the outstanding efforts of LiveOnNY transplant coordinators and staff for making this study possible.
Abbreviations
- ALI
Acute lung injury
- BM
Bone marrow
- CPR
Cardiopulmonary resuscitation
- COD
Cause of death
- CVA
Cerebrovascular accident
- HT
Head Trauma
- LOS
Length of stay
- BM
Bone Marrow
- LN
Lymph Node
- MCP-1
Monocyte chemoattractant protein-1
- TEM
Effector memory T cells
- TEMRA
Terminal Differentiated Effector T cells
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Hein WR, Griebel PJ. A road less travelled: large animal models in immunological research. Nat Rev Immunol. 2003;3(1):79–84. doi: 10.1038/nri977. [DOI] [PubMed] [Google Scholar]
- 2.Ngiow SF, Loi S, Thomas D, Smyth MJ. Mouse Models of Tumor Immunotherapy. Adv Immunol. 2016;130:1–24. doi: 10.1016/bs.ai.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Zhou X, Ramachandran S, Mann M, Popkin DL. Role of lymphocytic choriomeningitis virus (LCMV) in understanding viral immunology: past, present and future. Viruses. 2012;4(11):2650–69. doi: 10.3390/v4112650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pearson JA, Wong FS, Wen L. The importance of the Non Obese Diabetic (NOD) mouse model in autoimmune diabetes. J Autoimmun. 2016;66:76–88. doi: 10.1016/j.jaut.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sachs DH, Kawai T, Sykes M. Induction of tolerance through mixed chimerism. Cold Spring Harb Perspect Med. 2014;4(1):a015529. doi: 10.1101/cshperspect.a015529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schenkel JM, Masopust D. Tissue-Resident Memory T Cells. Immunity. 2014;41(6):886–97. doi: 10.1016/j.immuni.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thome JJ, Farber DL. Emerging concepts in tissue-resident T cells: lessons from humans. Trends Immunol. 2015;36(7):428–35. doi: 10.1016/j.it.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hashimoto D, Miller J, Merad M. Dendritic cell and macrophage heterogeneity in vivo. Immunity. 2011;35(3):323–35. doi: 10.1016/j.immuni.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kleinschek MA, Boniface K, Sadekova S, Grein J, Murphy EE, Turner SP, et al. Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. J Exp Med. 2009;206(3):525–34. doi: 10.1084/jem.20081712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong MT, Ong DE, Lim FS, Teng KW, McGovern N, Narayanan S, et al. A High-Dimensional Atlas of Human T Cell Diversity Reveals Tissue-Specific Trafficking and Cytokine Signatures. Immunity. 2016;45(2):442–56. doi: 10.1016/j.immuni.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Purwar R, Campbell J, Murphy G, Richards WG, Clark RA, Kupper TS. Resident memory T cells (T(RM)) are abundant in human lung: diversity, function, and antigen specificity. PLoS One. 2011;6(1):e16245. doi: 10.1371/journal.pone.0016245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hombrink P, Helbig C, Backer RA, Piet B, Oja AE, Stark R, et al. Programs for the persistence, vigilance and control of human CD8+ lung-resident memory T cells. Nat Immunol. 2016;17(12):1467–78. doi: 10.1038/ni.3589. [DOI] [PubMed] [Google Scholar]
- 13.Dedeoglu B, de Weerd AE, Huang L, Langerak AW, Dor FJ, Klepper M, et al. Lymph node and circulating T cell characteristics are strongly correlated in end-stage renal disease patients, but highly differentiated T cells reside within the circulation. Clin Exp Immunol. 2017;188(2):299–310. doi: 10.1111/cei.12934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Havenith SH, Remmerswaal EB, Idu MM, van Donselaar-van der Pant KA, van der Bom N, Bemelman FJ, et al. CXCR5+CD4+ follicular helper T cells accumulate in resting human lymph nodes and have superior B cell helper activity. Int Immunol. 2014;26(3):183–92. doi: 10.1093/intimm/dxt058. [DOI] [PubMed] [Google Scholar]
- 15.Lazuardi L, Jenewein B, Wolf AM, Pfister G, Tzankov A, Grubeck-Loebenstein B. Age-related loss of naive T cells and dysregulation of T-cell/B-cell interactions in human lymph nodes. Immunology. 2005;114(1):37–43. doi: 10.1111/j.1365-2567.2004.02006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinet KZ, Bloquet S, Bourgeois C. Ageing combines CD4 T cell lymphopenia in secondary lymphoid organs and T cell accumulation in gut associated lymphoid tissue. Immun Ageing. 2014;11:8. doi: 10.1186/1742-4933-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Granot T, Senda T, Carpenter DJ, Matsuoka N, Weiner J, Gordon CL, et al. Dendritic Cells Display Subset and Tissue-Specific Maturation Dynamics over Human Life. Immunity. 2017;46(3):504–15. doi: 10.1016/j.immuni.2017.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12(11):1045–54. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sathaliyawala T, Kubota M, Yudanin N, Turner D, Camp P, Thome JJ, et al. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity. 2013;38(1):187–97. doi: 10.1016/j.immuni.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sonnenberg GF, Monticelli LA, Alenghat T, Fung TC, Hutnick NA, Kunisawa J, et al. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science. 2012;336(6086):1321–5. doi: 10.1126/science.1222551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thome JJ, Bickham KL, Ohmura Y, Kubota M, Matsuoka N, Gordon C, et al. Early-life compartmentalization of human T cell differentiation and regulatory function in mucosal and lymphoid tissues. Nat Med. 2016;22(1):72–7. doi: 10.1038/nm.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thome JJC, Grinshpun B, Kumar BV, Kubota M, Ohmura Y, Lerner H, et al. Longterm maintenance of human naive T cells through in situ homeostasis in lymphoid tissue sites. Science Immunol. 2016 doi: 10.1126/sciimmunol.aah6506. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thome JJC, Yudanin NA, Ohmura Y, Kubota M, Grinshpun B, Sathaliyawala T, et al. Spatial Map of Human T Cell Compartmentalization and Maintenance over Decades of Life. Cell. 2014;159:814–28. doi: 10.1016/j.cell.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gordon CL, Miron M, Thome JJ, Matsuoka N, Weiner J, Rak MA, et al. Tissue reservoirs of antiviral T cell immunity in persistent human CMV infection. J Exp Med. 2017;214(3):651–67. doi: 10.1084/jem.20160758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Hoeven JA, Molema G, Ter Horst GJ, Freund RL, Wiersema J, van Schilfgaarde R, et al. Relationship between duration of brain death and hemodynamic (in)stability on progressive dysfunction and increased immunologic activation of donor kidneys. Kidney international. 2003;64(5):1874–82. doi: 10.1046/j.1523-1755.2003.00272.x. [DOI] [PubMed] [Google Scholar]
- 26.Force ADT, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–33. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 27.Pahwa S, Sia C, Harper R, Pahwa R. T lymphocyte subpopulations in high-risk infants: influence of age and blood transfusions. Pediatrics. 1985;76(6):914–7. [PubMed] [Google Scholar]
- 28.Pembrey L, Raynor P, Griffiths P, Chaytor S, Wright J, Hall AJ. Seroprevalence of cytomegalovirus, Epstein Barr virus and varicella zoster virus among pregnant women in Bradford: a cohort study. PLoS One. 2013;8(11):e81881. doi: 10.1371/journal.pone.0081881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mascia L, Mastromauro I, Viberti S, Vincenzi M, Zanello M. Management to optimize organ procurement in brain dead donors. Minerva Anestesiol. 2009;75(3):125–33. [PubMed] [Google Scholar]
- 30.Naik PM, Angel LF. Special issues in the management and selection of the donor for lung transplantation. Semin Immunopathol. 2011;33(2):201–10. doi: 10.1007/s00281-011-0256-x. [DOI] [PubMed] [Google Scholar]
- 31.Wilkes DS, Egan TM, Reynolds HY. Lung transplantation: opportunities for research and clinical advancement. Am J Respir Crit Care Med. 2005;172(8):944–55. doi: 10.1164/rccm.200501-098WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prevention CfDCa, editor. Statistics NCfH. Reference Manuals and Reports: Manual for Medical Technicians and Laboratory Procedures Used for NHANES III (CD-ROM) Hyattsville, MD: 1996. [Google Scholar]
- 33.Suwa T, Hogg JC, English D, Van Eeden SF. Interleukin-6 induces demargination of intravascular neutrophils and shortens their transit in marrow. Am J Physiol Heart Circ Physiol. 2000;279(6):H2954–60. doi: 10.1152/ajpheart.2000.279.6.H2954. [DOI] [PubMed] [Google Scholar]
- 34.Salim A, Velmahos GC, Brown C, Belzberg H, Demetriades D. Aggressive organ donor management significantly increases the number of organs available for transplantation. J Trauma. 2005;58(5):991–4. doi: 10.1097/01.ta.0000168708.78049.32. [DOI] [PubMed] [Google Scholar]
- 35.Shah VR. Aggressive management of multiorgan donor. Transplantation proceedings. 2008;40(4):1087–90. doi: 10.1016/j.transproceed.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 36.Straznicka M, Follette DM, Eisner MD, Roberts PF, Menza RL, Babcock WD. Aggressive management of lung donors classified as unacceptable: excellent recipient survival one year after transplantation. J Thorac Cardiovasc Surg. 2002;124(2):250–8. doi: 10.1067/mtc.2002.123813. [DOI] [PubMed] [Google Scholar]
- 37.Oppong E, Cato ACB. Effects of Glucocorticoids in the Immune System. In: Wang J-C, Harris C, editors. Glucocorticoid Signaling: From Molecules to Mice to Man. New York, NY: Springer New York; 2015. pp. 217–33. [DOI] [PubMed] [Google Scholar]
- 38.Nakagiri T, Inoue M, Minami M, Shintani Y, Okumura M. Immunology mini-review: the basics of T(H)17 and interleukin-6 in transplantation. Transplantation proceedings. 2012;44(4):1035–40. doi: 10.1016/j.transproceed.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 39.Raza A, Firasat S, Khaliq S, Khan AR, Mahmood S, Aziz T, et al. Monocyte Chemoattractant Protein-1 (MCP-1/CCL2) Levels and Its Association with Renal Allograft Rejection. Immunol Invest. 2017;46(3):251–62. doi: 10.1080/08820139.2016.1248559. [DOI] [PubMed] [Google Scholar]
- 40.Barklin A. Systemic inflammation in the brain-dead organ donor. Acta anaesthesiologica Scandinavica. 2009;53(4):425–35. doi: 10.1111/j.1399-6576.2008.01879.x. [DOI] [PubMed] [Google Scholar]
- 41.Novitzky D, Horak A, Cooper DK, Rose AG. Electrocardiographic and histopathologic changes developing during experimental brain death in the baboon. Transplantation proceedings. 1989;21(1 Pt 3):2567–9. [PubMed] [Google Scholar]
- 42.Stangl M, Zerkaulen T, Theodorakis J, Illner W, Schneeberger H, Land W, et al. Influence of brain death on cytokine release in organ donors and renal transplants. Transplantation proceedings. 2001;33(1–2):1284–5. doi: 10.1016/s0041-1345(00)02479-9. [DOI] [PubMed] [Google Scholar]
- 43.Weiss S, Kotsch K, Francuski M, Reutzel-Selke A, Mantouvalou L, Klemz R, et al. Brain death activates donor organs and is associated with a worse I/R injury after liver transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2007;7(6):1584–93. doi: 10.1111/j.1600-6143.2007.01799.x. [DOI] [PubMed] [Google Scholar]
- 44.Nijboer WN, Schuurs TA, van der Hoeven JA, Leuvenink HG, van der Heide JJ, van Goor H, et al. Effects of brain death on stress and inflammatory response in the human donor kidney. Transplantation proceedings. 2005;37(1):367–9. doi: 10.1016/j.transproceed.2004.12.262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Materials and Methods
Table S1: Donor Information
Table S2: Antibodies used for flow cytometry
Table S3: Representative CD4/CD8 Ratio Analysis by Clinical Factor