Abstract
Underactive bladder (UAB) is a symptom syndrome reflecting the urodynamic observation of detrusor underactivity (DU), a voiding contraction of reduced strength and/or duration, leading to prolonged or incomplete bladder emptying. An International Continence Society Working Group has described UAB as characterised by a slow urinary stream, hesitancy and straining to void, with or without a feeling of incomplete bladder emptying and dribbling, often with storage symptoms. Since DU often coexists with bladder outlet obstruction, or storage dysfunction (detrusor overactivity or incontinence), the exact contribution of the DU to the presenting complaints can be difficult to establish. The presence of voiding and post voiding lower urinary tract symptoms (LUTS) is implicitly expected in UAB, but a reduced sensation of fullness is reported by some patients, and storage LUTS are also an important factor in many affected patients. These may result from a postvoid residual, but often they do not. The storage LUTS are often the key driver in leading the patient to seek healthcare input. Nocturia is particularly common and bothersome, but what the role of DU is in all the range of influences on nocturia has not been established. Qualitative research has established a broad impact on everyday life as a result of these symptoms. In general, people appear to manage the voiding LUTS relatively well, but the storage LUTS may be problematic.
Keywords: Detrusor underactivity, Lower urinary tract symptoms, Overactive urinary bladder, Underactive bladder
INTRODUCTION
The International Continence Society (ICS) derived the terminology of lower urinary tract function [1], and this is the main basis for the wordings and definitions used most widely in clinical practice. Although overactive bladder syndrome is comparatively well understood, its analogous counterpart, underactive bladder (UAB) syndrome is relatively underresearched. There has been much interest generated in this topic in recent years and efforts made to reach professional consensus on symptomatic definitions.
There are common linkages of lower urinary tract symptoms (LUTS) which can be used to derive symptom syndromes. The best recognised of this is overactive bladder (OAB), which is the existence of urgency with or without urgency urinary incontinence usually with increased daytime frequency and nocturia [2]. The importance of this symptom syndrome is the ability to identify individuals who potentially incurred benefit from specific intervention to reduce the severity of their LUTS. The usual approach is to give fluid advice, recommend bladder training and to consider pharmaceutical interventions. This approach enables wider recognition of problems and institution of initial therapy in primary care settings. “Overactive bladder” is generally considered a reasonably clear term by most patients, and intuitively fits with how they perceive their own symptoms. When investigated further by healthcare professionals, patients with OAB are sometimes identified as having detrusor overactivity (DO). This is a urodynamic observation of bladder contractions during filling which may be spontaneous or provoked [1]. Alternatively, there may be findings of inflammation in the lower urinary tract or some other factor which might sensitise the sensory nerves from the lower urinary tract. Thus the storage LUTS are largely encompassed by a symptom syndrome (OAB) and urodynamic observation (DO). These 2 terms are not interchangeable, since OAB patients may not have DO on filling cystometry; likewise DO may occur with no associated urgency. A similar approach to voiding LUTS, based on a symptom syndrome and a urodynamic observation, seems to be a legitimate aspiration in further developing the field. Detrusor underactivity (DU), an increasingly recognised cause of troublesome LUTS, is proposed as the analogous urodynamic observation to the UAB symptom complex [3,4].
Normal voiding is a complex process reliant on several potentially vulnerable contributors throughout the lower urinary tract and the neuraxis [5]. The bladder is a muscular organ with the detrusor constituting the muscle responsible for pressure generation, and hence voiding. The muscle is driven by its efferent innervation, which ramifies throughout the detrusor to trigger contraction. The efferent innervation derives from the autonomic parasympathetic nucleus in the sacral part of the spinal cord. In the storage phase, this nucleus is inhibited; during voiding, the descending drive to trigger detrusor contraction is received from the pontine micturition center [6]. The permissive signal that sets off the process of voiding is determined by the cerebral cortex, predominantly the prefrontal cortex, a particularly important area for decision-making and planning. This rather complex arrangement is responsible for ensuring voiding proceeds as a normal behavioural activity in the social context of the organism. Reflexes in the neuraxis also allows the ability to overcome perturbations which could otherwise impair or prevent voiding. For example, some adaptation of bladder contraction strength may well be needed to overcome alterations in the dynamics of voiding [7], notably with change in position, or increasing bladder outlet obstruction (BOO) (a common feature in the aging male, as a result of intrusive enlargement of the prostate gland). Thus DU may often occur alongside other urodynamic problems such as DO, BOO, or urodynamic stress incontinence [8,9,10]. The overlap of LUTS associated with these conditions, such as a slow flow, increased urinary frequency and incontinence [3,4,11], presents a further challenge for the isolation and definition of a UAB symptom complex which may be attributed to DU.
TERMINOLOGY OF UNDERACTIVE BLADDER
Urodynamically diagnosed DU is defined by the ICS as “a contraction of reduced strength and/or duration, resulting in prolonged bladder emptying and/or a failure to achieve complete bladder emptying in a normal time span” [1]. However, the ICS definition of DU does not include any specific thresholds to identify reduced strength, reduced duration, prolonged voiding, completeness of bladder emptying, or “normal” time span. Complex aetiologies that lead to DU [12,13,14] and lack of consensus of what constitutes a contraction of reduced strength and prolonged urination time [4], are some of the hindrances to having standardized parameters which define urodynamic DU. Nevertheless, studies which attempt to provide epidemiological data or elucidate symptoms that may be attributed to DU give indications of reasonable thresholds that can be considered. Urodynamic inclusion criteria for DU in some recent studies are given in Table 1. Uren et al. [15] and Gammie et al. [16] use a bladder contractility index (BCI) of <100 and a bladder outlet obstruction index (BOOI) of <20 in men. The acceptable degree of BOO is therefore restricted, with the result that patients with likely coexisting DU and BOO are excluded (those with a low BCI yet relatively high BOOI). Women were required to have a detrusor pressure at maximum flow (PdetQmax) of <20 cmH2O and a maximum flow rate (Qmax) of <15 mL/s. These thresholds were later endorsed by Fode and Sønksen [17]. However, these parameters are not likely to be definitive, given the ongoing research efforts of many, and further complications introduced through the proposed classification by aetiology [18].
Table 1. Urodynamic inclusion criteria for detrusor underactivity in recent studies.
| Study | Sample size with DU (n) | Age (y) | DU urodynamic diagnostic criteria |
|---|---|---|---|
| Abarbanel and Marcus (2007) [29] | Male, 82; female, 99 | ≥70 | Qmax<10 mL/s, PdetQmax <30 cmH2O |
| Jeong et al. (2012) [9] | Male, 632; female, 547 | >65 | Male: BCI<100 |
| Female: PdetQmax ≤10 cmH2O and Qmax ≤12 mL/s | |||
| Hoag and Gani (2015) [8] | Male, 25; female, 54 | Mean: 59.2 (range, 19–90) | BCI<100 and absence of identifiable BOO |
| Gammie et al. (2016) [16] | Male, 129; female, 308 | Median: male 63, female 55 | Male: BCI<100, BOOI<20, BVE<90% |
| Female: PdetQmax <20 cmH2O and Qmax <15ml/s | |||
| Uren et al. (2017) [15] | Male, 29; female, 15 | Mean: 64 (range, 27–88) | Male: BCI<100 and BOOI <20 |
| Female: PdetQmax of <20 cmH2O and Qmax of <15 mL/s |
PdetQmax, detrusor pressure at maximum flow; Qmax, maximum flow rate; BCI, bladder contractility index; BOOI, bladder outlet obstruction index; BVE, bladder voiding efficiency.
BCI=PdetQmax+5Qmax. BOOI=PdetQmax–2Qmax. BVE=(voided volume/total bladder capacity)×100.
Working back from this nonspecific definition of DU, a working symptomatic definition for UAB was derived by a consensus group at the 2014 International Consultation on Incontinence – Research Society as follows; “The underactive bladder is a symptom complex suggestive of detrusor underactivity and is usually characterised by prolonged urination time with or without sensation of incomplete bladder emptying, usually with hesitancy, reduced sensation on filling, and a slow stream” [3]. However, the need for further qualitative and quantitative research on which a symptomatic UAB definition may be based was increasingly recognised [3,4,19]. The latest symptomatic definition proposed by a Working Group set up by the ICS in 2016 is: “Underactive bladder is characterised by a slow urinary stream, hesitancy and straining to void, with or without a feeling of incomplete bladder emptying and dribbling, often with storage symptoms” [20]. The ICS Working Group gives some explanatory notes. Firstly, that UAB “occurs in association with diverse pathologies and based on current knowledge there is no single distinguishing symptom.” This is in contrast to OAB, for which urgency is the central defining symptom. Secondly, that “storage symptoms in UAB are varied and may be highly prevalent, including nocturia, increased daytime frequency, reduced sensation of filling, and incontinence”. Finally, “underlying mechanisms of storage symptoms are diverse and are often related to a significant post voiding residual urine volume.” Recent contributions by Dewulf et al. [18] and Fode and Sønksen (2017) [17] also recognise the potential overlap of symptoms with BOO within their definitions. Fode and Sønksen [17] give theirs as: “Underactive bladder is the subjective feeling of prolonged urination time, slow stream, and hesitancy, which may or may not be associated with poor bladder emptying and subsequent storage symptoms in men and women without evidence of any outlet obstruction.”
Crucially, the terminology used must meet the needs of both patients and healthcare professionals. The days when obscure terms were used by professionals regardless of whether the patient was able to identify their own position with the descriptive terms are receding. It is a specific expectation that communication between professionals and patients should be transparent and understandable. Underactive bladder is a comparatively simple term, and one that is reasonably straightforward for patients to take on board. There are some distinct weaknesses, nonetheless. The word “bladder” identifies a specific organ, yet it is not definite that that particular organ is necessarily the basis of the problem in all individuals. Furthermore, the bladder has definite fundamental tasks for which it has very contrasting behaviours. Specifically, storage requires minimal activity of the bladder while voiding requires active contraction. Because the terminology does not make the contrasting storage and voiding functions clear, patients can often be confused by the possibility that they could have both an OAB and an UAB, and the healthcare professional then has to explain the relationship between these terms and the micturition cycle. No consensus has yet been achieved on how to overcome this limitation. Healthcare professionals do not generally have any problem with technical terms like detrusor underactivity. However, the limitation here is the vague description that has to be employed in defining it. Since there is no normative data or clear cut thresholds, the term is vague in its description, and rather discursive. Healthcare professionals tend to be rather dismissive of the term as a result, even though it is absolutely certain that some patients do genuinely manifest weakness of their bladder contractility when attempting to void.
SYMPTOMS OF UNDERACTIVE BLADDER
The symptoms important to patients may reflect underlying DU, but this may be compounded by additional lower urinary tract dysfunctions. In a large scale analysis of a UK database, Gammie et al. [16] have recently made attempts to identify differences in relative occurrence of the signs and symptoms of patients with urodynamic DU, in comparison with patients with BOO and those with ‘normal’ pressure flow studies (PFS). Many symptoms and medical history factors showed a statistically significant difference in relative occurrence. In male patients, the symptoms of decreased urinary stream (56% in patients with DU, 82% of patients with BOO, and 30% in those with normal PFS) and hesitancy (51% in DU, 69% BOO, 26% with normal PFS 26%) were in high frequency. In women, a decreased urinary stream was present in 29% of DU patients, 20% of BOO patients and 4% of those with normal PFS. However, as symptoms that were specific to a particular group were low in frequency, and the relative differences in symptom occurrence generally indistinct; information that could be to symptomatically differentiate DU from BOO still remains unclear [20].
In a qualitative study by Uren et al. [15] storage LUTS (nocturia, increased daytime frequency, urgency, and incontinence), and voiding symptoms (slow stream, hesitancy, and straining) were reported by over half the patients with DU. Table 2 lists the commonly reported symptoms in this study. The symptom of straining (which patients may employ to initiate, maintain, or finish urination) is of particular note as it common in the group of patients with DU in isolation. Straining is not included in some important symptomatic definitions of UAB [3]. However, it was included in the more recent definition of the ICS Working Group [20]. Storage symptoms were key to the patient reported UAB experience with a high prevalence of spontaneous reporting and often severe associated bother. However, the notable observation of incontinence was mostly attributed to the inclusion of a group with DU and coexisting DO or SUI. Nocturia was the most commonly reported symptom but the underlying aetiology of nocturia is complex and age-related [21,22], so ascertaining the underlying mechanism and to what extent nocturia can be attributed to DU is uncertain. Post voiding symptoms, including a sensation of incomplete emptying, the need to revoid within a short period of time, and dribbling, were also frequently reported in the sample. A few patients described a perceived reduction in sensation of the fullness of the bladder, similar to the phrasing in the symptomatic definition (‘reduced sensation on filling’) [3]. As might be expected from other studies [8], many had a postvoid residual (PVR) (77% had a PVR of >30 mL) and perhaps correspondingly, there was a high proportion of patients who were historically or currently self-catheterising and experienced UTIs.
Table 2. Commonly reported signs and symptoms of underactive bladder (based on Uren et al. [15]).
| Symptoms |
| Slow (and or interrupted) stream of long duration and of small volume |
| Increased daytime urinary frequency |
| Nocturia |
| Straining |
| Hesitancy |
| Sensation of incomplete emptying |
| Urgency |
| Urinary incontinence |
| Postmicturition dribble |
| Reduced bladder sensation |
| Bladder discomfort or pain |
| Signs |
| High postvoid residual |
| History of urinary tract infections |
| History of acute retention episodes |
IMPACT OF UNDERACTIVE BLADDER
Qualitative research into the impact of LUTS show that there can be a broad impact on patients' lives associated with these symptoms [23,24,25]. Disruption to sleep due to waking several times in the night and the lifestyle inconveniences caused by increased daytime urinary frequency can be particularly bothersome. The necessity to plan ahead for awareness of the location of toilets, impairment of social life, embarrassment in particular situations and reduced self-esteem are a feature of qualitative studies in male patients with LUTS [25,26]. However, overall, UAB does appear to be a condition that can often be tolerated and well-managed by patients. Patients in Uren et al. [15] described how they adapt their lives around their condition so that impact was minimised, asserting that they had become ‘used to it,’ perhaps due to the chronic nature of their condition. This also appears to be the case in other studies investigating the impact on patients with LUTS [25,27].
PRESENTATION AND DIAGNOSIS OF UNDERACTIVE BLADDER
The ongoing scientific debate surrounding the definitions of DU/UAB and that DU is fundamentally a urodynamic observation, means the extent of the underlying contribution of DU to the prevalence of LUTS in the general population is difficult to establish [28]. However, the current epidemiological research for DU suggests it is a commonly a factor in men and women presenting with LUTS. In referred populations who have undergone urodynamic studies, the prevalence of DU was 40% of men and 13% of women [9], and as much as 48% in male patients >70 years of age [29].
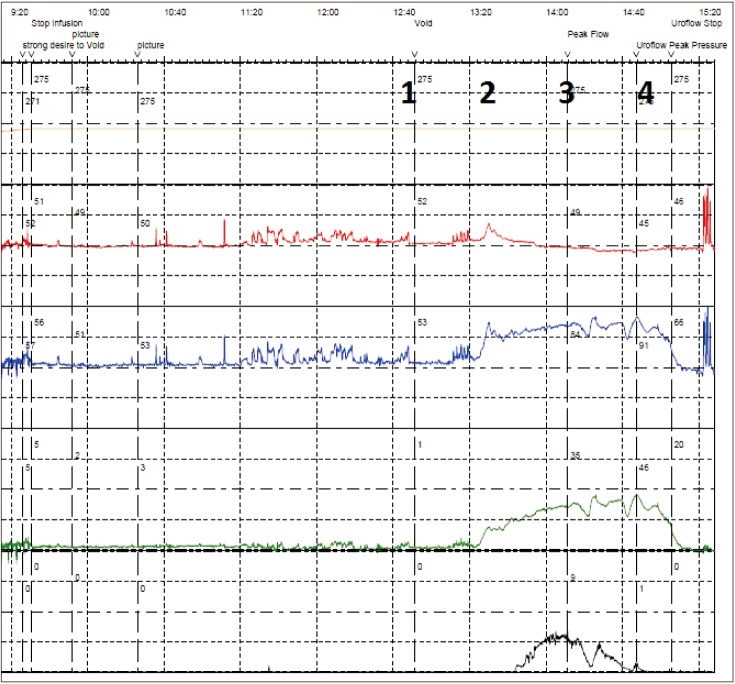
As a primary condition, individuals affected by UAB may constitute a group of people which is not widely recognised in the healthcare professions, as they may not present if their only symptoms are voiding LUTS. From research into OAB, it is clear that voiding LUTS tend to be rather less problematic to patients in their day-to-day lives than storage LUTS [27]. Many patients with a comparatively poor stream or mild hesitancy may not give this sufficient priority to present to healthcare professionals for assessment. Presentation probably becomes more likely if storage LUTS emerge. Patients are substantially more bothered by urgency, nocturia and increased daytime frequency [27] and this may well be the factor that prompts a medical presentation. Such a situation can emerge if patients develop incomplete bladder emptying, resulting in a PVR. This probably reduces the functional bladder capacity, resulting in an increased daytime frequency and perhaps urgency and nocturia. However, it is important to recognise that the PVR is not necessarily the sole driver of storage LUTS in people with an UAB. Some patients may present with voiding LUTS in combination with storage LUTS and only a modest PVR (Fig. 1). This paradoxical situation, if investigated urodynamically, tends to show a stable detrusor during filling, as illustrated, even though a symptom score and bladder diary suggest urgency and small typical voided volumes. Recently, some theoretical physiology has been advanced to try to explain this [30].
Fig. 1. A young male patient (aged 36 years) presenting with storage, voiding and postmicturition LUTS. Urodynamic trace of the end of the filling cystometry and the pressure flow study (PFS), illustrating rectal pressure (red), bladder pressure (blue), subtracted detrusor pressure (green), and flow (black). Permission to void was given at 1, and a slowly-building detrusor contraction results, which the patient augments with abdominal straining at 2. Flow took a minute to start, and reached peak flow (Qmax) at point 3. Detrusor pressure was 35 cmH2O and Qmax was 9 mL/s (bladder contractility index 80, which is below the threshold for normal of 100). The detrusor contraction concludes at 4, giving a total duration for the PFS of two and a half minutes.
UAB may become a feature in a secondary setting, for example, if medications, neurological disease or autonomic disease leads to impairment of the detrusor contraction. This is more likely to become an issue for those patients with additional challenges for lower urinary tract function, for example, the presence of benign prostate enlargement in men, giving rise to the need for raised detrusor pressure to achieve urination (i.e., BOO), since DU impairs this compensatory increased contraction. Invasive urodynamics is currently used to differentiate suspected DU from BOO as there may be little benefit to corrective surgery for LUTS in patients with DU [31]. Indeed, there would be considerable advantage for patients and clinical practice if it was possible to differentiate these conditions by noninvasive means. Further work such as the ongoing development of a validated, condition-specific patient reported outcome instrument [15,32], along with employing techniques such as penile cuff-urodynamics [33] and ultrasonographic measurement of detrusor wall thickness [34] may add additional diagnostic information. However these methods have not yet been evaluated by clinical trials.
CONCLUSIONS
People experience voiding as a rather transient and minor aspect of their day-to-day lives. They are unaware of the complexities underlying voiding and simply expect it to be a prompt, efficient and complete process, conveniently achieved at the timing that suits them best. Whenever this is their actual experience, it is unlikely that a normal individual would give it much further thought. People referred for urodynamic tests may be found to have a weak and poorly sustained detrusor contraction, termed detrusor underactivity. When the presenting symptoms of these people are reviewed, they encompass storage, voiding and postvoiding LUTS. Bringing them together, the ICS Working Group describes UAB as being characterised by a slow urinary stream, hesitancy, and straining to void, with or without a feeling of incomplete bladder emptying and dribbling, often with storage symptoms. Presentations are driven by the most bothersome symptoms, which rather paradoxically in UAB appear to be storage LUTS. The exact contribution of UAB in the context of the coexisting urological conditions which commonly present is a conundrum which needs to be addressed by healthcare professionals for each individual patient.
Footnotes
CONFLICTS OF INTEREST: Alan D. Uren was sponsored by Astellas. Marcus J. Drake is Advisory Boards, Research, Speaker panel for Allergan, Astellas, Ferring and speaker panel for Hikma, Pfizer.
References
- 1.Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61:37–49. doi: 10.1016/s0090-4295(02)02243-4. [DOI] [PubMed] [Google Scholar]
- 2.Wein AJ, Rovner ES. Definition and epidemiology of overactive bladder. Urology. 2002;60(5 Suppl 1):7–12. doi: 10.1016/s0090-4295(02)01784-3. discussion 12. [DOI] [PubMed] [Google Scholar]
- 3.Chapple CR, Osman NI, Birder L, van Koeveringe GA, Oelke M, Nitti VW, et al. The underactive bladder: a new clinical concept? Eur Urol. 2015;68:351–353. doi: 10.1016/j.eururo.2015.02.030. [DOI] [PubMed] [Google Scholar]
- 4.Osman NI, Chapple CR, Abrams P, Dmochowski R, Haab F, Nitti V, et al. Detrusor underactivity and the underactive bladder: a new clinical entity? A review of current terminology, definitions, epidemiology, aetiology, and diagnosis. Eur Urol. 2014;65:389–398. doi: 10.1016/j.eururo.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 5.Birder L, de Groat W, Mills I, Morrison J, Thor K, Drake M. Neural control of the lower urinary tract: peripheral and spinal mechanisms. Neurourol Urodyn. 2010;29:128–139. doi: 10.1002/nau.20837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drake MJ, Fowler CJ, Griffiths D, Mayer E, Paton JFR, Birder L. Neural control of the lower urinary and gastrointestinal tracts: supraspinal CNS mechanisms. Neurourol Urodyn. 2010;29:119–127. doi: 10.1002/nau.20841. [DOI] [PubMed] [Google Scholar]
- 7.Drake MJ. The integrative physiology of the bladder. Ann R Coll Surg Engl. 2007;89:580–585. doi: 10.1308/003588407X205585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoag N, Gani J. Underactive bladder: clinical features, urodynamic parameters, and treatment. Int Neurourol J. 2015;19:185–189. doi: 10.5213/inj.2015.19.3.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeong SJ, Kim HJ, Lee YJ, Lee JK, Lee BK, Choo YM, et al. Prevalence and clinical features of detrusor underactivity among elderly with lower urinary tract symptoms: a comparison between men and women. Korean J Urol. 2012;53:342–348. doi: 10.4111/kju.2012.53.5.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Resnick NM, Yalla SV. Detrusor hyperactivity with impaired contractile function. An unrecognized but common cause of incontinence in elderly patients. JAMA. 1987;257:3076–3081. doi: 10.1001/jama.257.22.3076. [DOI] [PubMed] [Google Scholar]
- 11.Miyazato M, Yoshimura N, Chancellor MB. The other bladder syndrome: underactive bladder. Rev Urol. 2013;15:11–22. [PMC free article] [PubMed] [Google Scholar]
- 12.van Koeveringe GA, Vahabi B, Andersson KE, Kirschner-Herrmans R, Oelke M. Detrusor underactivity: a plea for new approaches to a common bladder dysfunction. Neurourol Urodyn. 2011;30:723–728. doi: 10.1002/nau.21097. [DOI] [PubMed] [Google Scholar]
- 13.van Koeveringe GA, Rademakers KL, Birder LA, Korstanje C, Daneshgari F, Ruggieri MR, et al. Detrusor underactivity: pathophysiological considerations, models and proposals for future research. ICI-RS 2013. Neurourol Urodyn. 2014;33:591–596. doi: 10.1002/nau.22590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith PP. Aging and the underactive detrusor: a failure of activity or activation? Neurourol Urodyn. 2010;29:408–412. doi: 10.1002/nau.20765. [DOI] [PubMed] [Google Scholar]
- 15.Uren AD, Cotterill N, Harding C, Hillary C, Chapple C, Klaver M, et al. Qualitative exploration of the patient experience of underactive bladder. Eur Urol. 2017;72:402–407. doi: 10.1016/j.eururo.2017.03.045. [DOI] [PubMed] [Google Scholar]
- 16.Gammie A, Kaper M, Dorrepaal C, Kos T, Abrams P. Signs and symptoms of detrusor underactivity: an analysis of clinical presentation and urodynamic tests from a large group of patients undergoing pressure flow studies. Eur Urol. 2016;69:361–369. doi: 10.1016/j.eururo.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 17.Fode M, Sønksen J. Towards a greater understanding of underactive bladder. Eur Urol. 2017;72:408–409. doi: 10.1016/j.eururo.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 18.Dewulf K, Abraham N, Lamb LE, Griebling TL, Yoshimura N, Tyagi P, et al. Addressing challenges in underactive bladder: recommendations and insights from the Congress on Underactive Bladder (CURE-UAB) Int Urol Nephrol. 2017;49:777–785. doi: 10.1007/s11255-017-1549-3. [DOI] [PubMed] [Google Scholar]
- 19.Chapple CR, Osman NI. Crystallizing the definition of underactive bladder syndrome, a common but under-recognized clinical entity. Low Urin Tract Symptoms. 2015;7:71–76. doi: 10.1111/luts.12101. [DOI] [PubMed] [Google Scholar]
- 20.Wein A, Chapple C. Introduction and terminology. In: Chapple C, Wein A, Osman N, editors. Underactive bladder. Switzerland: Springer; 2017. pp. ix–xiii. [Google Scholar]
- 21.Drake MJ. Should nocturia not be called a lower urinary tract symptom? Eur Urol. 2015;67:289–290. doi: 10.1016/j.eururo.2014.09.024. [DOI] [PubMed] [Google Scholar]
- 22.Gulur DM, Mevcha AM, Drake MJ. Nocturia as a manifestation of systemic disease. BJU Int. 2011;107:702–713. doi: 10.1111/j.1464-410X.2010.09763.x. [DOI] [PubMed] [Google Scholar]
- 23.Coyne KS, Sexton CC, Kopp Z, Chapple CR, Kaplan SA, Aiyer LP, et al. Assessing patients' descriptions of lower urinary tract symptoms (LUTS) and perspectives on treatment outcomes: results of qualitative research. Int J Clin Pract. 2010;64:1260–1278. doi: 10.1111/j.1742-1241.2010.02450.x. [DOI] [PubMed] [Google Scholar]
- 24.Gannon K, Glover L, O'Neill M, Emberton M. Men and chronic illness: a qualitative study of LUTS. J Health Psychol. 2004;9:411–420. doi: 10.1177/1359105304042350. [DOI] [PubMed] [Google Scholar]
- 25.Glover L, Gannon K, McLoughlin J, Emberton M. Men's experiences of having lower urinary tract symptoms: factors relating to bother. BJU Int. 2004;94:563–567. doi: 10.1111/j.1464-410X.2004.05001.x. [DOI] [PubMed] [Google Scholar]
- 26.Wareing M. Lower urinary tract symptoms: a hermeneutic phenomenological study into men’s lived experience. J Clin Nurs. 2005;14:239–246. doi: 10.1111/j.1365-2702.2004.01003.x. [DOI] [PubMed] [Google Scholar]
- 27.Coyne KS, Wein AJ, Tubaro A, Sexton CC, Thompson CL, Kopp ZS, et al. The burden of lower urinary tract symptoms: evaluating the effect of LUTS on health-related quality of life, anxiety and depression: EpiLUTS. BJU Int. 2009;103(Suppl 3):4–11. doi: 10.1111/j.1464-410X.2009.08371.x. [DOI] [PubMed] [Google Scholar]
- 28.Osman N, Hillary C, Chapple C. Epidemiology of underactive bladder. In: Chapple C, Wein A, Osman N, editors. Underactive bladder. Switzerland: Springer; 2017. pp. 25–31. [Google Scholar]
- 29.Abarbanel J, Marcus EL. Impaired detrusor contractility in community-dwelling elderly presenting with lower urinary tract symptoms. Urology. 2007;69:436–440. doi: 10.1016/j.urology.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 30.Drake MJ, Kanai A, Bijos DA, Ikeda Y, Zabbarova I, Vahabi B, et al. The potential role of unregulated autonomous bladder micromotions in urinary storage and voiding dysfunction; overactive bladder and detrusor underactivity. BJU Int. 2017;119:22–29. doi: 10.1111/bju.13598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho MC, Ha SB, Park J, Son H, Oh SJ, Kim SW, et al. Impact of detrusor underactivity on surgical outcomes of laser prostatectomy: comparison in serial 12-month follow-up outcomes between potassium-titanyl-phosphate photoselective vaporization of the prostate (PVP) and holmium laser enucleation of the prostate (HoLEP) Urology. 2016;91:158–166. doi: 10.1016/j.urology.2015.11.052. [DOI] [PubMed] [Google Scholar]
- 32.Uren AD, Cotterill N, Harding C, Hillary C, Chapple C, Klaver M, et al. Qualitative development of a new patient reported outcome measure for underactive bladder; International Continence Society 2016 Tokyo; 2016 Sep 13-16; Tokyo, Japan. Available from: https://www.ics.org/Abstracts/Publish/326/000075.pdf. [Google Scholar]
- 33.McIntosh SL, Drinnan MJ, Griffiths CJ, Robson WA, Ramsden PD, Pickard RS. Noninvasive assessment of bladder contractility in men. J Urol. 2004;172(4 Pt 1):1394–1398. doi: 10.1097/01.ju.0000139470.58006.dd. [DOI] [PubMed] [Google Scholar]
- 34.FORCE Research Group, Maastricht and Hannover. Rademakers KL, van Koeveringe GA, Oelke M. Ultrasound detrusor wall thickness measurement in combination with bladder capacity can safely detect detrusor underactivity in adult men. World J Urol. 2017;35:153–159. doi: 10.1007/s00345-016-1902-7. [DOI] [PubMed] [Google Scholar]