Abstract
Fabry disease (FD) is a pan-ethnic, X-linked, progressive lysosomal storage disorder caused by pathogenic mutations in the GLA gene. Published case reports and abstracts suggest that decreased reproductive fitness may occur in males with FD. In order to understand the impact of FD on reproductive fitness and increase the accuracy of reproductive genetic counseling, this study examines a large, multi-centered population of individuals with FD to determine if males have reduced reproductive fitness. Study data were collected on 376 patients through two, gender-specific surveys distributed across the United States and Canada. The number of biological live-born children among individuals with FD was compared to statistics from the general population. Information was also collected on reduced sperm count, depression, pain, use of assisted reproductive technology, and reproductive choice. On average, females affected by FD had more biological live-born children (1.8) than males affected by FD (1.1). However, males affected by FD had an increased mean number of biological children (1.1) compared to the mean number of biological children fathered by men in the United States (0.9). Sixteen of the 134 males with FD reported oligospermia, which suggests that an infertility work up may be indicated for males having difficulty impregnating their partners. In our large multicenter sample, males and females with FD do not exhibit reduced reproductive fitness; on average they have more biological children than the general population in the United States. This information should assist clinicians in providing accurate reproductive genetic counseling and treatment for individuals with FD.
Introduction
Fabry disease (FD) (OMIM 301500) is a pan-ethnic, X-linked, multi-systemic, progressive lysosomal storage disorder caused by mutations in the GLA gene. These mutations result in decreased or deficient levels of the enzyme α-galactosidase A (α-gal A) (EC 3.2.1.22). This deficiency of α-gal A causes the accumulation of globotriaosylceramide (GL3 or GB3) and related glycosphingolipids in the vascular endothelium (Desnick et al. 2001; Eng et al. 2006). The glycosphingolipid storage initiates a cascade of events beginning with the dysfunction of basic metabolic processes on the cellular level and moving into cell death, inflammatory events, and progressive major organ dysfunction (Eng et al. 2006).
The incidence of FD ranges from 1 in 1,250 to 1 in 117,000 live male births worldwide, with an increased incidence of mutations expected to result in non-classical or later onset FD (Hopkins et al. 2015; Inoue et al. 2013; Meikle et al. 1999; Mechtler et al. 2012; Lin et al. 2009; Zarate and Hopkin 2008; Hwu et al. 2009; Spada et al. 2006). The symptoms and progression of FD are highly variable, from classical FD with early and severe onset of symptoms to non-classical or later onset FD with symptoms that may have a more severe impact on a particular organ system (cardiac, neurological, or renal) or may present in adolescence or later rather than childhood (Eng et al. 2006; Smid et al. 2015; Biegstraaten et al. 2010; Desnick et al. 2001; Laney et al. 2015). These symptoms affect both males and females and vary among individuals (Eng et al. 2006; Wang et al. 2007; Desnick et al. 2001; Hopkin et al. 2008).
Unlike the characteristic features and symptoms of FD, reproductive health issues have only been reported in individual case reports and not examined systematically in a large FD population. In particular, there have been several pathology-focused case reports of males with azoospermia and FD, males with storage of GL3 in testicular biopsies, and diminished fertility and FD (de Groot 1964; Guin et al. 1976; Lacombe et al. 2010; Papaxanthos-Roche et al. 2007; Török et al. 1980). The most informative case report focused on biopsies from a deceased 32-year-old male with FD. As reported, biopsies revealed multiple areas of significant GL3 storage (Leydig cells, epithelial of ductuli efferentes, ductus of the epididymis, testicular interstitium, and seminiferous tubules) but undisturbed fertility in the patient despite the reduced diameter of the seminiferous tubules (Nistal et al. 1983). In a separate report on two testicular biopsies which found storage of GL3 in the Leydig cells by optic and electronic microscopic analysis in two individuals with azoospermia and FD, authors suggest male patients desiring children may need to use assisted reproductive technologies such as testicular sperm extraction (TESE) and intracytoplasmic sperm injection (ICSI) (Papaxanthos-Roche et al. 2007; Lacombe et al. 2010; Török et al. 1980).
Another reproductive health issue that could have a negative impact on reproductive fitness in FD, priapism, has also been reported via case reports in males with FD (Labarthe et al. 2010; Foda et al. 1996). However, in a study of 48 male patients with FD, Biegstraaten et al. found nearly normal male sexual function and autonomic control of the cardiovascular system using the Autonomic Symptom Profile in their sampling of patient with FD as compared to a control population (Biegstraaten et al. 2010).
Similarly, a small scale study from Hauser et al. assessed ovarian, testicular, and adrenal function in a cohort of 13 patients with FD (6 females and 7 males, including 3 males on hemodialysis) and found undisturbed hormonal function and a normal fertility rate in both male and female patients when compared with the corresponding Austrian populations. The three males on maintenance hemodialysis had abnormal plasma levels of LH, prolactin, and testosterone consistent with their uremic state. All 13 patients had α-gal A enzyme levels below normal at a mean of 17.70 nMol/mg protein/h ± 19.51 (1–48) (Hauser et al. 2005).
A single-site pilot study in 2007 investigated the differing rate of live-born children between males and females with FD and found that males affected by FD who have completed their families have a reduced rate of live-born children (1.7 children per male on average) as compared to females affected by FD (3 children per female on average) (Laney et al. 2008). This data was counter to the existence of several very large families with males with many offspring that had been published in the clinical setting (Veronik et al. 2004; Spence et al. 1978).
Finally, males with FD may choose to reduce the number of or not have biological children, as previous studies have shown that individuals with genetic conditions make different reproductive decisions than individuals without known genetic risks (Beeson and Golbus 1985; Hershberger et al. 2012; Read 2002). If males with FD are choosing to have fewer children, this raises the possibility that they have reduced reproductive fitness when compared to females with FD and/or the national rates of reproduction.
The scientific literature offers several theoretical factors that could contribute to decreased fitness in males with FD including: mechanical blockage of the vas deferens due to glycolipid storage, autonomic dysfunction, reduced life expectancy, depression and decreased social adaptive functioning, or personal choice (Laney et al. 2010; Wagner et al. 2014; Arends et al. 2015; Germain et al. 2015; Waldek et al. 2009). In order to provide accurate genetic counseling about their reproductive fitness to individuals affected by FD, we conducted a multi-center, large scale study to determine if reproductive fitness is decreased in males with FD and, if so, delineate specific risk factors or issues related to decreased fitness. We anticipate this information will increase the accuracy of comprehensive monitoring and treatment plans, and help determine if there is skewing in the incidence calculation of FD.
Methods
Institutional Review Board Approval
This study has been reviewed and approved by the Emory University Institutional Review Board. All participants completed a consent process prior to participating in the study. Documents approved for this study included recruitment flyers, recruitment emails, consent information forms, and surveys.
Participants and Recruitment
Recruitment for the study was a multifaceted effort. Recruitment flyers were mailed to Emory Lysosomal Storage Disease Center patients who met inclusion criteria. In addition, notice of this study was included on the Fabry Support and Information Group (FSIG) and the National Fabry Disease foundation (NFDF) website and Facebook pages. Study investigators attended and recruited at local and regional FD meetings sponsored by NFDF and FSIG. Recruitment flyers were also distributed to genetic counselors around the United States within Lysosomal Storage Disease Centers of Excellence to provide to qualified patients who may have an interest in participating. Participating institutions included the University of Iowa, Children’s Hospital of Wisconsin, the University of Washington, and Massachusetts General Hospital.
Survey Population
Participants who completed the informed consent process were screened to determine if they met study entry criteria. Inclusion criteria for participating in the study required all participants to be affected by FD, be over age 18 years, and be able to provide informed consent. Subjects not meeting these criteria were excluded. This includes five subjects who completed the surveys, but did not record their sex on the questionnaires. Demographic and reproductive data was used to ensure that subjects’ survey data was not counted more than once. The target participant population was 450 participants from all regions of the United States and Canada.
Survey Design
Following completion of the consent process, patients were asked to complete either a paper or online self-response survey entitled “Reproductive Fitness in Individuals Affected by Fabry Disease (FIT).” Separate questionnaires were completed if the subject was male or female. The survey for males consisted of 30 questions and was divided into 4 parts: demographics, reproductive history, medical history, and questions about their family and children. The survey for females consisted of 36 questions and was divided into 5 parts: demographics, reproductive history, medical history, gynecological history, and questions about their family and children. Key questions from the surveys are provided in Appendix 1.
Statistical Analysis
Raw data was obtained from self-response queries and analyzed using SAS 9.4 (SAS Institute, Cary, North Carolina, USA). Demographic variables were summarized using frequencies and proportions. Demographic variables of interest were compared by sex, using chi-square tests and Fisher’s exact tests, where appropriate. The number of biological live-born children was summarized with medians and interquartile ranges. Any children born to a parent affected by FD who used a donor egg or sperm in place of their biological contribution were not reported as biological children. The proportion of male participants who reported various characteristics related to infertility was compared to reported statistics for the general population. Cohen’s h was calculated to assess the standardized difference between the general population proportions and those in our sample (Cohen 1988). As Cohen suggests, h values of 0.2, 0.5, and 0.8 were interpreted as “small,” “medium,” and “large” effect sizes, respectively. Nonparametric Kruskal-Wallis tests were used to compare the number of biological live-born children by sex and all other demographic and survey variables of interest. These included key health and behavior issues such as azoospermia, oligospermia, depression, erectile dysfunction, environmental exposures, having burning pain in hand/feet at less than 10 years of age, renal failure, and knowing FD status prior to having children. The mean number of biological live-born children was compared with national population estimates from the United States National Health statistics report, the World Health Organization fertility data, and World Bank fertility data by using paired comparison tests (Martinez et al. 2012; The World Bank 2015; Cooper et al. 2010; Sharlip et al. 2002). Chi-square tests were used to compare the distribution of the number of live-born children among participants with FD to the distribution of live-born children among the United States. All statistical tests were assessed using an alpha = 0.05.
Although we did not collect data on genotypes, studies have shown that the majority of men with classical FD experience neuropathic extremity pain (acroparesthesias) in childhood (Hopkin et al. 2015; Laney et al. 2015; Smid et al. 2015). To focus on the males most likely to have classical FD, we separately analyzed the number of live-born children, infertility issues (including azoospermia and oligospermia), and use of assisted reproductive technology (ART) in males with FD who reported onset of neuropathic pain at age 10 or under.
Results
A total of 376 subjects (134 males, 242 females) consented to participate in the study and completed the survey. The mean age of participants who completed the survey was 46.5 years with a range of 19–78 years and a median of 46 years. The majority of individual respondents were Caucasian (332/376, 90%) with additional representation from the Hispanic (18/376, 4.8%), Arab American (1/376, 0.27%), Native American (2/376, 0.50%), African-American (4/376, 1.1%), and Asian (5/376, 1.3%) populations (Table 1).
Table 1.
Demographic characteristics of study subjects with Fabry disease, United States, 2013–2015
| Characteristic | Total N = 376 |
Females n = 242 |
Males n = 134 |
P valuea | |
|---|---|---|---|---|---|
| N | % | n (%) | n (%) | ||
| Recruitment source | 0.330 | ||||
| Paper survey | 131 | (34.8) | 80 (33.1) | 51 (38.1) | |
| Survey monkey | 245 | (65.2) | 162 (66.9) | 83 (615) | |
| Age in 2014 (in years) | 0.920 | ||||
| 18–21 | 10 | (2.7) | 6 (2.5) | 4 (3.0) | |
| 22–29 | 30 | (8.1) | 21 (8.8) | 9 (6.7) | |
| 30–35 | 55 | (14.8) | 34 (14.3) | 21 (15.7) | |
| 36–40 | 43 | (11.6) | 29 (12.2) | 14 (10.5) | |
| 41+ | 234 | (62.9) | 148 (62.2) | 86 (642) | |
| Race/ethnicity | 0.318b | ||||
| Caucasian, single race | 332 | (90.0) | 211 (88.7) | 121 (92.4) | |
| Hispanic | 18 | (4.9) | 11 (4.6) | 7 (53) | |
| African-American, single race | 4 | (1.1) | 4 (17) | 0 (0.0) | |
| Other or mixed race | 15 | (4.1) | 12 (5.0) | 3 (23) | |
| Highest level of education | 0.327 | ||||
| No school | 2 | (0.5) | 1 (0.4) | 1 (0.8) | |
| High school or less | 75 | (20.1) | 51 (21.3) | 24 (17.9) | |
| Some college or technical degree | 139 | (37.2) | 96 (40.0) | 43 (32.1) | |
| Four-year degree | 60 | (16.0) | 34 (14.2) | 26 (19.4) | |
| Graduate education | 98 | (26.2) | 58 (24.2) | 40 (29.9) | |
| Average annual household income | 0.267 | ||||
| $0–$24,999 | 58 | (17.8) | 42 (20.4) | 16 (13.3) | |
| $25,000–$39,999 | 38 | (11.7) | 20 (9.7} | 18 (15.0) | |
| $40,000–$49,999 | 32 | (9.8) | 21 (10.2) | 11 (9.2) | |
| $50,000–$74,999 | 76 | (23.3) | 53 (25.7) | 23 (19.2) | |
| $75,000–$99,999 | 47 | (14.4) | 27 (13.1) | 20 (16.7) | |
| $100,000–$124,999 | 36 | (11.0) | 23 (11.2) | 13 (103) | |
| $125,000–$149,999 | 14 | (4.3) | 8 (3.9) | 6 (5.0) | |
| Over $150,000 | 25 | (7.7) | 12 (5.8) | 13 (10.8) | |
| Any biological children | 253 | (69.7) | 178 (77.4) | 75 (56.4) | <0.001 |
| med | (IQR) | med (IQR) | med (IQR) | ||
| Number of liveborn children | 2.0 | (0–2) | 2.0 (1–3) | 1.0 (0–2) | <0.001c |
aCalculated by chi-square test with an alpha = 0.05, unless otherwise noted
bCalculated by Fisher’s exact text with an alpha = 0.05
cCalculated by Kruskal Wallis test with an alpha = 0.05
Fabry Related Syndrome and Health History
The majority of male respondents with FD reported experiencing the characteristic burning pain in the hands/feet seen frequently in FD (115/134, 85.8%). A subset of those males (75/134, 56%) reported onset of burning pain at age 10 or younger. Additional health issues experienced in more than 25% of the male respondents included depression (55/134, 41.0%), anxiety (50/134, 37.3%), and renal failure (45/134, 33.6%) (Table 2). In the majority of cases, the onset of renal failure in the males occurred after the reported age range at which they had their first biological child. In males, erectile dysfunction (ED) was reported in 19 males (19/134, 14.2%), with the majority reporting onset after the birth of their first biological child (onset at an average age of 50 with a range of 30–64 years of age). Priapism was also reported in 2 out of the 134 males with FD (1.5%) and did occur during the subjects’ main time of reproduction. Data on Fabry related symptoms and health history in females can be found in Table 3.
Table 2.
Reproductive history of 134 males with Fabry disease, United States, 2013–2015
| Characteristic | Level | N | Number of live-born biological children | ||
|---|---|---|---|---|---|
| Mean | Median | Kruskal-Wallis P-value | |||
| Age at birth of first child, in years | 12–20 | 1 | 2.0 | 2 | 0.76 |
| 21–29 | 32 | 2.0 | 2 | ||
| 30–35 | 23 | 1.7 | 2 | ||
| 36–40 | 7 | 1.7 | 2 | ||
| 41–45 | 3 | 1.7 | 2 | ||
| Age at birth of last child, in years | 21–29 | 24 | 1.8 | 2 | 0.51 |
| 30–35 | 28 | 1.9 | 2 | ||
| 36–40 | 14 | 2.0 | 2 | ||
| 41–45 | 4 | 2.5 | 2 | ||
| Ever had depression | No | 78 | 1.1 | 1 | 0.53 |
| Yes | 55 | 1.0 | 1 | ||
| Ever had renal failure | No | 88 | 1.0 | 1 | 0.40 |
| Yes | 45 | 1.2 | l | ||
| Ever smoked | No | 52 | 1.0 | 1 | 0.06 |
| Yes | 23 | 0.5 | 0 | ||
| Reported any pain | No | 18 | 0.8 | 0 | 0.16 |
| Yes | 115 | 1.1 | l | ||
| Burning pain before age 10 | No | 40 | 1.3 | 1 | 0.28 |
| Yes | 75 | 1.0 | 1 | ||
| Anxiety | No | 83 | 1.1 | l | 0.73 |
| Yes | 50 | 1.0 | 1 | ||
| Erectile dysfunction | No | 114 | 1.0 | 1 | 0.51 |
| Yes | 19 | 1.3 | 1 | ||
| Reported any infertility problems | No | 57 | 1.6 | 2 | 0.01 |
| Yes | 32 | 0.9 | 0 | ||
| Ever had an infertility evaluation | No | 99 | 1.1 | 1 | 0.96 |
| Yes | 29 | 1.0 | 1 | ||
| Low sperm count (oligospermia) | No | 117 | 1.1 | 1 | 0.39 |
| Yes | 16 | 0.8 | 1 | ||
| Low sperm motility | No | 127 | 1.1 | 1 | 0.70 |
| Yes | 6 | 1.2 | 2 | ||
| Used assisted reproductive technology | No | 121 | 1.1 | l | 0.46 |
| Yes | 12 | 0.8 | 0 | ||
Table 3.
Reproductive history of 242 females with Fabry disease, United States, 2013–2015
| Characteristic | Number of live-born biological children | ||||
|---|---|---|---|---|---|
| Level | N | Mean | Median | Kruskal–Wallis P-value | |
| Ever had depression | No | 133 | 1.9 | 2 | 0.98 |
| Yes | 97 | 1.7 | 2 | ||
| Ever had renal failure | No | 140 | 1.8 | 2 | 0.16 |
| Yes | 10 | 1.1 | 1 | ||
| Reported any pain | No | 33 | 1.5 | 1 | 0.20 |
| Yes | 197 | 1.9 | 2 | ||
| Burning pain before age 10 | No | 112 | 2.0 | 2 | 0.20 |
| Yes | 85 | 1.8 | 2 | ||
| Anxiety | No | 131 | 1.9 | 2 | 0.77 |
| Yes | 99 | 1.8 | 2 | ||
| Panic | No | 196 | 1.8 | 2 | 0.90 |
| Yes | 34 | 1.8 | 2 | ||
| Reported any infertility problems | No | 157 | 2.2 | 2 | 0.04 |
| Yes | 40 | 1.6 | 1 | ||
| Ever had an infertility evaluation | No | 194 | 1.8 | 2 | 0.63 |
| Yes | 31 | 1.8 | 2 | ||
| Used assisted reproductive technology | No | 200 | 1.9 | 2 | 0.91 |
| Yes | 30 | 1.7 | 2 | ||
Rate of Biological Children
The majority (253/376, 69.7%) of individuals with FD in the study reported having at least one biological child. Stratified by gender, 56.4% of males (75/134) and 77.4% of females (178/242) reported having at least one biological child (Table 4), with males having an average of 1.1 biological children and females having an average of 1.8 biological children (Table 4). Females affected by FD have significantly more live-born children than males affected by FD (p < 0.001, Figs. 1 and 2). However, the mean number of biological children among females with FD in our study (1.8 children) is higher than the general United States population average of 1.3 children (Table 4). This is also true when compared to the Caucasian, non-Hispanic United States population average of 1.1 children. Males affected by FD also had an increased mean number of children (1.1 in all males with FD) as compared to the mean number of biological children fathered by all men in the United States (0.9) and among Caucasian, non-Hispanic men in the United States (0.8) (Table 4).
Table 4.
Mean number of children by sex and data source
| Data source | N | Mean (S.E.) | Percent distribution (S.E.) for number of live-born childrenb | ||||
|---|---|---|---|---|---|---|---|
| None | 1 | 2 | 3 | 4+ | |||
| Males (all) | |||||||
| National dataa, 2006–2010 | − | 0.9 (0.0) | 55.2 (1.1) | 15.8 (0.6) | 17.0 (0.7) | 7.9 (0.5) | 4.1 (0.3) |
| Fabry data, 2015 | 133 | 1.1 (0.1) | 43.6 (4.3) | 17.3 (3.3) | 30.8 (4.0) | 6.0 (2.1) | 2.3 (1.3) |
| Females (all) | |||||||
| National dataa, 2006–2010 | − | 1.3 (0.0) | 44.4 (1.1) | 16.2 (0.5) | 21.0 (0.8) | 11.5 (0.5) | 6.9 (0.5) |
| Fabry data, 2015 | 230 | 1.8 (0.1) | 22.6 (2.8) | 20.4 (2.7) | 30.9 (3.1) | 13.9 (2.3) | 12.2 (2.2) |
| White, non-Hispanic (single race) | |||||||
| Women | |||||||
| National dataa, 2006–2010 | − | 1.1 (0.0) | 47.7 (1.4) | 15.7 (0.7) | 21.8 (1.0) | 10.6 (0.6) | 4.2 (0.4) |
| Fabry data, 2015 | 201 | 1.8 (0.1) | 23.4 (3.0) | 19.4 (2.8) | 31.8 (3.3) | 13.4 (2.4) | 11.9 (2.3) |
| Men | |||||||
| National dataa, 2006–2010 | − | 0.8 (0.0) | 58.9 (1.5) | 14.9 (0.9) | 17.0 (1.0) | 6.6 (0.6) | 2.6 (0.3) |
| Fabry data, 2015 | 120 | 1.1 (0.1) | 44.2 (4.6) | 18.3 (3.6) | 28.3 (4.1) | 6.7 (2.3) | 2.5 (1.0) |
Abbreviations: SE standard error
aNational data is based on a survey of 10,403 men and 12,279 women which were weighted to reflect approximately 62 million men and 62 million women aged 15–44
bChi-square tests were used to compare the percent distribution of number of live-born children among patients with Fabry’s disease to corresponding national data. All statistical comparisons were significant as a result of the large sample size in the national data
Fig. 1.
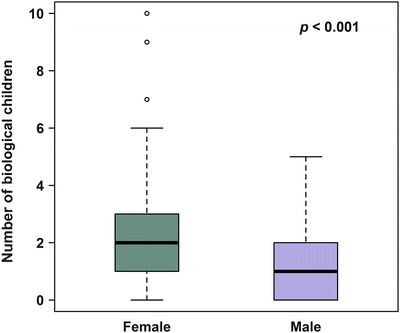
Distribution of biological children counts by gender
Fig. 2.
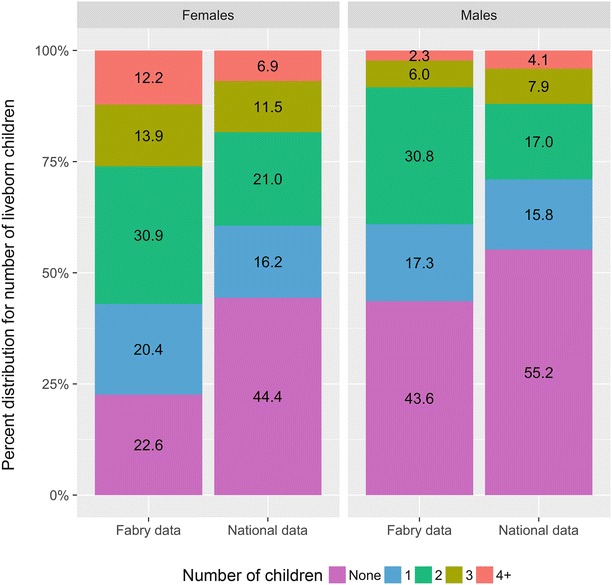
Percent distribution of the number of liveborn children born to individuals in the general population as compared to individuals with Fabry disease
Infertility in Males
Approximately 32 males with FD reported issues with infertility (32/89, 36.0%). This is higher than the reported general population rate of 7% of males of reproductive age who reported having infertility issues (Krausz 2011). Similarly, 29 males (29/128, 22.7%) reported seeking an infertility evaluation to address specific male infertility issues (Table 5). Although our data did not identify the age at which the males with FD sought evaluation, the percentage of males with FD seeking an infertility evaluation is twice the general population rate in which 9.4% of all sexually experienced men younger than age 45 report undergoing an infertility evaluation (Chandra et al. 2014). There were 16 males (15/132, 11.4%) who reported being diagnosed with low sperm count (oligospermia), 1 with no sperm detected (azoospermia) (1/133, 0.8%), 4 with low testosterone (4/134, 2.99%), 1 with a varicocele (1/134, 0.75%), and 6 with low sperm motility (6/134, 4.49%). Looking at a selected population of males with FD who reported onset of neuropathic pain at age 10 or younger, 9 out of 75 (11.4%) males reported having either azoospermia or oligospermia. The rate of oligospermia in our population of males with FD (11.4%) was comparable to general population data which found that 15% of males in the general population are defined to have oligospermia (sperm concentration was below 15 million/ml) (Jørgensen et al. 2012). (Table 5) This is slightly lower than the rate of fertile men (male partners of pregnant women) in a previous Jørgensen study in which 8% had oligospermia (Jørgensen et al. 2001). The percent of males with FD and azoospermia in our study (0.75%) was lower than the general population rate in which 2% of all men are reported to have azoospermia (Willott 1982).
Table 5.
Reproductive characteristics of participants with Fabry disease compared to the general population, by sex
| Characteristic | Total respondents | n | (%) | P in general population (%) | Cohen’s h a |
|---|---|---|---|---|---|
| Males | |||||
| Reported having infertility issues | 89 | 32 | (36.0) | 7% (Krausz 2011) | 0.75 |
| Reported undergoing an infertility evaluation | 128 | 29 | (22.7) | 9.4% (Chandra et al. 2014) | 0.37 |
| Low sperm count (oligospermia) | 132 | 15 | (11.4) | 15% (Jørgensen et al. 2012) | 0.11 |
| Azoospermia | 133 | 1 | (0.8) | 2% (Willott 1982) | 0.11 |
| Used ART | 133 | 12 | (9.0) | 0.7% (Chandra et al. 2014) | 0.44 |
| Females | |||||
| Reported having infertility issues | 197 | 40 | (20.3) | 12% (Chandra and Copen 2013) | 0.23 |
| Reported undergoing an infertility evaluation | 225 | 31 | (13.8) | 13.7% (Krausz 2011) | 0.00 |
| Used ART | 230 | 30 | (13.0) | 0.7% (Chandra et al. 2014) | 0.57 |
aCohen suggests that h values 0.20, 0.50, and 0.80 can be interpreted as “small,” “medium,” and “large” effect sizes, respectively (Cohen 1988)
Out of the 134 males with FD, 12 (9.0%) used infertility services to attempt to impregnate their partners including: intrauterine insemination, use of donor sperm, in vitro fertilization, in vitro fertilization and use of donor sperm, and partner taking hormones to increase ovulation. Of the males who used donor sperm, three reported having oligo or azoospermia and one chose to use a donor sperm in order to avoid the risk of transmitting FD to his children.
Infertility in Females
Approximately 40 females with FD reported issues with infertility (40/197, 20.3%) with 31 females (31/225, 13.8%) seeking an infertility evaluation to address specific female infertility issues. The number of females with FD reporting issues with infertility is higher than the general population rate of 12% of women aged 25–44 who report ever having infertility issues, but equivalent to the number of females in the general population seeking an infertility evaluation (Chandra and Copen 2013; Chandra et al. 2014; Kessler et al. 2013). Out of the 242 females with FD, 30 (12.4%) used infertility services to attempt a pregnancy including: use of donor egg, in vitro fertilization, and taking hormones to increase ovulation (Tables 3 and 5). It is important to note that at least three females used Pre-implantation Genetic Screening (PGS) for FD and one used a donor egg to avoid passing on FD rather than for reasons of infertility.
Adoption
In our survey, 13 males and 15 females with FD reported adopting a least one child (28/376; 7.4%). Eleven of the 13 males with FD had adopted children and did not have any biological children. Two of the 13 males with FD had at least one biological child in addition to their adopted children. Several males reported that their adopted children were their stepchildren, their wife’s biological children. Three of the females with FD reported adoptive children and no biological children, while 12 had both adopted and at least one biological study. Approximately 4% of US families on average have at least one adopted child. Individuals with Fabry disease in this study report a higher than average incidence of having an adopted child in their family at 7.4% (Kreider and Lofquist 2014).
Impact of Known Diagnosis of Fabry Disease
Elective Terminations
The psychosocial impact of having FD and making the decision to have children could impact the rate of reproductive fitness via personal choice. There were 21 out of the 273 females affected by FD that reported having an elective pregnancy termination. Of the 21 reported terminations, 3 were of male fetuses prenatally diagnosed with FD, 3 were over the fear that the baby could be affected by FD, and one was due to fetal exposure to the teratogenic medication Dilantin for treatment of FD related symptoms. In males who completed the question, 18 reported having a partner who had electively terminated at least one pregnancy. No further information about the reasons for the elective terminations was given in males. These rates are comparable to the general population data which found that 21% of all pregnancies (excluding miscarriages) end in elective terminations with non-Hispanic white women accounting for 36% of abortions (Jones and Jerman 2014; Jones et al. 2010).
Use of Preimplantation Genetic Diagnosis or Other Assisted Reproductive Technology
Preimplantation genetic diagnosis (PGD) is available in the United States for individuals with a known family GLA mutation or for those who wish to implant only male or female embryos. PGD is expensive and often not covered by insurance; however, it offers an alternative to prenatal testing. Using a donor sperm or egg to replace the biological contribution of an affected individual is also an option for parent who chooses not to pass on FD. Of these males with FD, only four used donor sperm and one used preimplantation genetic diagnosis for FD. Of female respondents, three used PGD for FD in the past, one used a donor egg, and four plan to use PGD to avoid passing on FD in future pregnancies (one of these already has frozen eggs to this end). Although this option may cause a larger impact on reproductive fitness in the future, at this time the option appears to be used minimally.
Reproductive Decision-Making and Fabry Disease
Survey questions specifically focused on personal reproductive choice in individuals with FD and the possibility that study subjects chose to have fewer children based on their risk of passing on FD. These data were limited in that individuals needed to know that they were affected by FD prior to attempting pregnancies in order to have a choice. In summary, 41/134 males and 71/242 females knew that they were affected by FD prior to having children and completed the survey questions about the impact of FD risk and number of children. In females, 39 (39/242) respondents said their knowledge impacted their decision-making process and 19 (19/242) said it did not. In males, 14 respondents (14/134) said having FD did affect their reproductive decision-making process and 12 respondents (12/134) said it did not. The impact that knowledge of FD had on reproductive decision making was very diverse with an interesting range of free response and comment sections about how it affected their personal choices. Some felt it made little difference in their choices and they proceeded to have the number of children they wished. Examples of free response answers in this category are available in Appendix 2. The knowledge that FD could be passed on to their children did negatively impact the choice to have children or have more children in approximately 50% of males and 67% of female respondents who knew they had FD, but many of them still had at least one live-born biological child and the rate of live-born children does not seem to be greatly reduced based on the numbers reported.
Discussion
Analysis of self-reported reproductive data in a large cross section of males and females with FD did not find reduced reproductive fitness as compared to general population data; instead, it indicated increased reproductive fitness in males and females with FD. On average, participants with FD had more biological, live-born children than the general population in the United States. There was an increased number of biological children seen in females with FD compared with males with FD, which mirrors the general population rates, but may also be affected by the expected incidence of affected individuals with FD. However, there is a subset of males with FD who did self-report infertility, oligospermia, and the existence of at least one related sperm or semen problems. Given the number of males with oligospermia, the standard genetic counseling for FD should include discussion of the option of semen analysis for sperm count and motility where appropriate as well as referral to reproductive centers and in vitro fertilization with testicular sperm extraction and intracytoplasmic sperm injection as appropriate and desired by the patient. Furthermore, the data on the impact of a FD diagnosis on reproductive decision making emphasizes the need to explore the topic with patients. These discussions would be added to the current genetic counseling recommendations including review of the inheritance pattern of FD, identification of at-risk family members, addressing the psychological impact of FD, and options for preimplantation and prenatal genetic testing (Laney et al. 2013).
The specific rationale for increased reproductive fitness in males and females with FD as compared to the general population rate was not uncovered in this study, but the authors have a few hypotheses to explore in future studies. The first is related to the impact of FD on daily life, career, and motivation. Other studies have found that individuals with FD have reduced social adaptive function, increased fatigue, and more school absences than their peers due to medical issues and appointments (Hopkin et al. 2008; Laney et al. 2010). These factors could reduce the number of individuals who continue their education after high school. When women pursue higher education, they have a delayed fertility pattern (Dye 2010). The opposite may be true for women who do not pursue high education. Another hypothesis, albeit with more anecdotal than firm evidence, could be that given relationship stress caused by having a chronic disease that includes fatigue, depression, anxiety, and decreased social adaptive functioning may lead individuals with FD to more divorces or dissolutions of long-term relationships than the general population, leading to an increased rate of serial long-term partners at younger ages increasing the chances for additional children.
The incidence of oligospermia in males diagnosed with FD still seems more frequent than in the general population and may be related to the significant glycolipid storage in the male reproductive tract seen on pathology; however, it doesn’t appear to significantly affect fertility in the majority of males. This is consistent with a study that found that, despite significant GL3 storage and narrowed vas deferens, one subject still produced three children (Nistal et al. 1983). It is also consistent with other studies that found largely normal endocrine hormone profiles and normal male sexual function, and with the observed after that the fertility rate in both female and male patients was equal to or higher than the local fertility rate (Nistal et al. 1983; Hauser et al. 2005; Biegstraaten et al. 2010). However, as the National Survey of Family Growth found “sperm or semen problems” in 14% of general population couples seeking infertility evaluation, attention to the matter is still indicated (Martinez et al. 2006).
There were no significant associations between the number of biological children and other factors that could theoretically impact reproduction in males with FD including erectile dysfunction, pain, or depression. It appears that though these factors may decrease quality of life, they do not have a direct impact on reproductive fitness.
Limitations of the study include: the majority of respondents were Caucasian, there may be a skewing toward individuals with internet access and those who are active in the FD community through attending in person meetings, being on support group mailing lists, or participating in online support groups. In addition, data on reproductive history, Fabry disease related symptoms, infertility, and health history symptom data was self-reported and not verified by medical record review. The study design also did not provide specific definitions for “infertility” which was interpreted differently by different subjects based on free response answers used for clarification. The study design introduces another bias in that only those interested in this area may have participated, and we did not directly collect genotypes to distinguish between classical and nonclassical/later onset FD. In order to mimic classical FD in analysis, we attempted to focus on “classically affected males” by analyzing separately those who reported pain onset in childhood at 10 years of age or younger; however, we did not find a statistically significant difference between males with early onset of pain and those without. Assumptions about classical vs. nonclassical genotype based on symptoms reported was not possible, given the variable presentation of symptoms in females with FD.
Future studies may choose to prospectively examine changes in sperm count over time as compared to serum glycolipid storage levels such as lysoGL3 in classically affected males, as it remains possible that fertility decreases due to glycolipid storage over time leading to lower fertility in males with classical FD later in life. Another important direction to explore would be the impact of treatment with enzyme replacement therapy (ERT) on sperm count and fertility in men with classical FD. Knowledge about the reproductive impact of early treatment with enzyme replacement therapy following newborn or family screening will help patients understand the broader effects of therapy and impact on their reproductive choices.
Acknowledgments
Our thanks to Genzyme, a Sanofi company, for support of this project. Additional thanks to the individuals who took the time to complete our survey and candidly provide their thoughts on a sensitive issue. We also thank all the collaborating institutions who helped spread the word of this survey to their patients. Our acknowledgment and gratitude also go to the National Fabry Disease Foundation and Fabry Support and Information Group for allowing us to distribute information about our study to their members.
Summary
Males and females with Fabry disease do not exhibit reduced reproductive fitness; on average they have more biological children than the general population in the United States.
Compliance with Ethics Guidelines
Allison Foley, Myrl Holida, Scott Gillespie, Eric Hall, Morgan Simmons, Alexandrea Wadley, and Virginia Clark declare they have no conflicts of interest.
Dawn Laney consults for Genzyme and Shire, and is a study coordinator in clinical trials sponsored by Genzyme, Amicus, and Protalix. She has also received research funding from Alexion, Amicus, Genzyme, Pfizer, Retrophin, Shire, and Synageva. Grant funding to conduct this investigator initiated studies was provided through an educational grant from Genzyme, A Sanofi company. These activities are monitored and are in compliance with the conflict of interest policies at Emory University School of Medicine.
Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentations (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study. This chapter does not contain any studies with animal subjects performed by any of the authors.
Contributions
Planning of this study, oversight, data collection, analysis, and reporting of work were coordinated by Dawn Laney. Statistical analysis and manuscript authorship by Scott Gillespie and Eric Hall. Subject recruitment, data collection, and manuscript creation review by Alexandrea Wadley. Data analysis and manuscript review and editing by Morgan Simmons and Allison Foley. Subject recruitment and manuscript authorship/review by Myrl Holida and Virginia Clarke.
Appendix 1: FIT Survey Sample Questions. Questions Were Presented to Be Answered as Self-Response Multiple Choice, Yes or No, and Free Response Answers
| FIT survey | Sample question asked |
|---|---|
| Males | |
| Part 2. General Questions | “Do you have any biological children?” Yes: _____ Number of boys ____ Number of girls _____ No, but I have adopted children No, I do not have any children biological or adopted |
| “Have you ever gotten one of your partners pregnant?” | |
| “Have you ever had difficulties getting your partner pregnant?” Yes (please describe) __________________ No I have never tried to make my partner pregnant | |
| “Have you ever experienced issues with infertility?” | |
| “Have you ever been used assisted reproductive technology?” Yes, my partner took hormones to increase ovulation Yes, we used a donor egg Yes, we used a donor sperm Yes we used in vitro fertilization Yes, we used in vitro fertilization and preimplantation genetic diagnosis No, we have never used assisted reproductive technology | |
| Part 3. Medical History | “Have you ever experienced any of the following health issues?” Hypertension/High Blood pressure, beginning at age ___ Burning pain in your hands/feet, beginning at age ___ Fabry pain crises, beginning at age ___ Depression, beginning at age ___ Anxiety, beginning at age ___ Panic Attacks, beginning at age ___ Kidney failure, beginning at age ___ Liver disease, beginning at age ___ Erectile dysfunction, beginning at age ___ Low sperm count, beginning at age ____ No, none of the above. |
| “Do you take any medications for high blood pressure?” Yes, ______________ (medication) beginning at age ___________ | |
| “Have you ever been treated for cancer or a tumor?” | |
| “Have you ever worked in a job that included frequent exposure to industrial chemical, pesticides, lead, heavy metals, or radiation/Xrays?” | |
| Part 4. Family History | “Did you know that you had Fabry disease before you considered having children?” |
| “If you did know that you had Fabry disease prior to having children, did having Fabry disease affect your decision to have children?” | |
| “Anything else about your reproductive history or having babies while living with Fabry disease that you think we should know?” | |
| Females | |
| Part 2. Medical History | “Have you ever experienced any of the following health issues?” Burning pain in your hands/feet, beginning at age ____ Depression, beginning at age ____ Kidney failure, beginning at age ____ Polycystic ovaries, beginning at age ___ Abnormal uterine shape Endometriosis, beginning at age ___ An STD such as herpes, which one? ___ Other chronic health issue(s) (other than Fabry disease) not mentioned above |
| Part 3. Reproductive History | “Have you ever been pregnant” If yes, number of times and how old were you during the first trimester of each? If no, have you ever tried to become pregnant? |
| “At what age did you begin having your periods?” | |
| “Have you ever had difficulties getting pregnant?” Yes (please describe _________) No I have never tried to become pregnant. | |
| “Have you ever had any concerns about your ability to have children?” | |
| “Have you ever experienced any issues with infertility?” | |
| “Have you ever been evaluated for infertility concerns?” | |
| “Have you ever been used assisted reproductive technology?” Yes, I took hormones to increase ovulation Yes, I used a donor egg Yes, I used a donor sperm Yes, I used in vitro fertilization Yes, I used in vitro fertilization and preimplantation genetic diagnosis No, I have never used assisted reproductive technology | |
| “Have you ever experienced menopause?” | |
| Part 4. Pregnancy History | “How many times have you been pregnant?” |
| “Have you ever had a miscarriage?” | |
| “Have you ever had an elective termination of a pregnancy?” | |
| “Have you ever had a termination of a pregnancy for medication reasons?” | |
| “How many children have you given birth to ___boys ___girls?” | |
| “Were any of your children stillborn or died soon after birth?” | |
| “Do you have any adopted children?” | |
| Part 5. Family history | “Did you know that you had Fabry disease before having children?” |
| If you did know that you had Fabry disease prior to having children, did having Fabry disease affect your decision to have children? | |
Appendix 2: Reproductive Decision-Making and Fabry Disease (Selection of Free Response Answers)
| Impact reported on the reproductive decision-making process of the knowledge that they had Fabry disease | Patient quotes |
|---|---|
| No negative impact | “No, it <having Fabry disease> didn’t matter … My wife and I discussed Fabrys, and decided we wanted at least 2 children.” |
| “No, not at all. My dad always told me he had enjoyed his life and was glad he was born and hated when his dad told him if he knew his boys would have Fabrys he wouldn’t have had them.” | |
| “No, but it has changed that decision for some of my family members. I figure my life, even though not easy, is worth it. So my kids if they got it would feel the same way. Some of my family have a harder time with that feeling.” | |
| Mixed impact | “Yes, from age 18 I was certain I did not want children because I did not want the disease to continue. I was the youngest person in my family that had Fabry and the disease would end with me. My brother had two kids, but male, so I would be the last of it. But then I got married and I changed my mind and wanted children.” |
| Negative impact | “The treat<ment> is great for many reasons, but the pain from neuropathy is not controlled enough for me to be comfortable in having a child who could possibly go through the type of pain I did growing up.” |
| “YES … I had a tubal ligation at age 24” | |
| “Once I knew I had Fabry it was sort of the final straw in deciding if we should have kids or not. I felt selfish possibly bringing a child into the world with Fabry. I know this doesn’t take into consideration all of the other facets of a child, and that if my mom had chosen that, then I wouldn’t be here. But for me I would feel so guilty watching a child suffer the symptoms of Fabry, knowing I chose that for them, in sense.” |
Contributor Information
Dawn A. Laney, Email: dawn.laney@emory.edu
Collaborators: Matthias Baumgartner, Marc Patterson, Shamima Rahman, Verena Peters, Eva Morava, and Johannes Zschocke
References
- Arends M, Hollak CE, Biegstraaten M. Quality of life in patients with Fabry disease: a systematic review of the literature. Orphanet J Rare Dis. 2015;10:77. doi: 10.1186/s13023-015-0296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeson D, Golbus MS. Decision making: whether or not to have prenatal diagnosis and abortion for X-linked conditions. Am J Med Genet. 1985;20:107–114. doi: 10.1002/ajmg.1320200113. [DOI] [PubMed] [Google Scholar]
- Biegstraaten M, van Schaik IN, Wieling W, Wijburg FA, Hollak CE. Autonomic neuropathy in Fabry disease: a prospective study using the autonomic symptom profile and cardiovascular autonomic function tests. BMC Neurol. 2010;10:38. doi: 10.1186/1471-2377-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra A, Copen CE. Infertility and impaired fecundity in the United States, 1981-2010: data from the National Survey of Family Growth. Natl Health Stat Rep. 2013;67:1–19. [PubMed] [Google Scholar]
- Chandra A, Copen CE, Stephen EH. Infertility service use in the United States: data from the National Survey of Family Growth, 1982-2010. Natl Health Stat Rep. 2014;73:1982–2010. [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavior science. Lawrence Erlbaum Association: Hillsdale; 1988. [Google Scholar]
- Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, Haugen TB, Kruger T, Wang C, Mbizvo MT, Vogelsong KM. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16(3):231–245. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- de Groot WP. Angiokeratoma corporis diffusum Fabry (thesaurismosis hereditaria Ruiter-Pompen-Wyers) Dermafologica. 1964;128:321. doi: 10.1159/000254765. [DOI] [PubMed] [Google Scholar]
- Desnick RJ, Ioannou YA et al (2001) Alpha-galactosidase A 364 deficiency: Fabry disease. In: CRBA S, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited disease, 8th edn. McGraw Hill, New York, pp 3733–3774
- Dye JL (2010) Fertility of American women: 2008. Current Population Reports, P20–563. www.census.gov/prod/2010pubs/p20-563.pdf. Accessed 17 Jan 2017
- Eng CM, Germain DP, et al. Fabry disease: guidelines for the evaluation and management of multi-organ system involvement. Genet Med. 2006;8(9):539–548. doi: 10.1097/01.gim.0000237866.70357.c6. [DOI] [PubMed] [Google Scholar]
- Foda MM, Mahmood K, Rasuli P, Dunlap H, Kiruluta G, Schillinger JF. High-flow priapism associated with Fabry’s disease in a child: a case report and review of the literature. Urology. 1996;48(6):949–952. doi: 10.1016/S0090-4295(96)00320-2. [DOI] [PubMed] [Google Scholar]
- Germain DP, Charrow J, Desnick RJ, Guffon N, Kempf J, Lachmann RH, Lemay R, Linthorst GE, Packman S, Scott CR, Waldek S, Warnock DG, Weinreb NJ, Wilcox WR. Ten-year outcome of enzyme replacement therapy with agalsidase beta in patients with Fabry disease. J Med Genet. 2015;52(5):353–358. doi: 10.1136/jmedgenet-2014-102797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guin GH, Burns WA, Saini N, Jones WP. Diffuse angiokeratoma (Fabry’s disease) Mil Med. 1976;141:259–263. [PubMed] [Google Scholar]
- Hauser AC, Gessl A, Harm F, Wiesholzer M, Kleinert J, Wallner M, Voigtländer T, Bieglmayer C, Sunder-Plassmann G. Hormonal profile and fertility in patients with Anderson-Fabry disease. Int J Clin Pract. 2005;59(9):1025–1028. doi: 10.1111/j.1742-1241.2005.00620.x. [DOI] [PubMed] [Google Scholar]
- Hershberger PE, Gallo AM, Kavanaugh K, Olshansky E, Schwartz A, Tur-Kaspa I. The decision-making process of genetically at-risk couples considering preimplantation genetic diagnosis: initial findings from a grounded theory study. Soc Sci Med. 2012;74(10):1536–1543. doi: 10.1016/j.socscimed.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkin RJ, Bissler J, Banikazemi M, et al. Characterization of Fabry disease in 352 pediatric patients in the Fabry Registry. Pediatr Res. 2008;64:550–555. doi: 10.1203/PDR.0b013e318183f132. [DOI] [PubMed] [Google Scholar]
- Hopkin RJ, Jefferies JL, Laney DA, Lawson VH, Mauer M, Taylor MR, Wilcox WR, Fabry Pediatric Expert Panel The management and treatment of children with Fabry disease: a United States-based perspective. Mol Genet Metab. 2015;117:104–113. doi: 10.1016/j.ymgme.2015.10.007. [DOI] [PubMed] [Google Scholar]
- Hopkins PV, Campbell C, Klug T, Rogers S, Raburn-Miller J, Kiesling J. Lysosomal storage disorder screening implementation: findings from the first six months of full population pilot testing in Missouri. J Pediatr. 2015;166(1):172–177. doi: 10.1016/j.jpeds.2014.09.023. [DOI] [PubMed] [Google Scholar]
- Hwu WL, Chien YH, Lee NC, Chiang SC, Dobrovolny R, Huang AC, et al. Newborn screening for Fabry disease in Taiwan reveals a high incidence of the later onset GLA mutation c.936+919G4A (IVS4+919G4A) Hum Mutat. 2009;30:1397–1405. doi: 10.1002/humu.21074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Hattori K, Ihara K, Ishii A, Nakamura K, Hirose S. Newborn screening for Fabry disease in Japan: prevalence and genotypes of Fabry disease in a pilot study. J Hum Genet. 2013;58:548–552. doi: 10.1038/jhg.2013.48. [DOI] [PubMed] [Google Scholar]
- Jones RK, Jerman J. Abortion incidence and service availability in the United States, 2011. Perspect Sex Reprod Health. 2014;46(1):3–14. doi: 10.1363/46e0414. [DOI] [PubMed] [Google Scholar]
- Jones RK, Finer LB, Singh S. Characteristics of U.S. abortion patients, 2008. New York: Guttmacher Institute; 2010. [Google Scholar]
- Jørgensen N, Andersen AG, Eustache F, et al. Regional differences in semen quality in Europe. Hum Reprod. 2001;16:1012e19. doi: 10.1093/humrep/16.5.1012. [DOI] [PubMed] [Google Scholar]
- Jørgensen N, Joensen UN, Jensen TK, Jensen MB, Almstrup K, Olesen IA, Juul A, Andersson AM, Carlsen E, Petersen JH, et al. Human semen quality in the new millennium: a prospective cross-sectional population-based study of 4867 men. BMJ Open. 2012;2:1–14. doi: 10.1136/bmjopen-2012-000990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler LM, Craig BM, Plosker SM, Reed DR, Quinn GP. Infertility evaluation and treatment among women in the United States. Fertil Steril. 2013;100(4):1025–1031. doi: 10.1016/j.fertnstert.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krausz C. Male infertility: pathogenesis and clinical diagnosis. Best Pract Res Clin Endocrinol Metab. 2011;25(2):271–285. doi: 10.1016/j.beem.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Kreider RM, Lofquist DM (2014) Adopted children and stepchildren: 2010. Current Population Reports, P20-572, US Census Bureau, Washington, DC
- Labarthe F, de Bodman C, Maruani A, Szwarc C, Froissart R, Lorette G, Lardy H. Priapism: a severe paediatric complication of Fabry disease. Rev Med Interne. 2010;31(Suppl 2):S217–S219. doi: 10.1016/S0248-8663(10)70015-1. [DOI] [PubMed] [Google Scholar]
- Lacombe D, Germain DP, Papaxanthos-Roche A. Azoospermia as a new feature of Fabry disease. Rev Med Interne. 2010;31(Suppl 2):S214–S216. doi: 10.1016/S0248-8663(10)70014-X. [DOI] [PubMed] [Google Scholar]
- Laney DA, Gruskin DJ, Metha A. Reproductive fitness in individuals affected by Fabry disease. Mol Genet Metab. 2008;93(2):28. doi: 10.1016/j.ymgme.2007.10.070. [DOI] [Google Scholar]
- Laney DA, Gruskin DJ, Fernhoff PM, Cubells JF, Ousley OY, Hipp H, Mehta AJ. Social-adaptive and psychological functioning of patients affected by Fabry disease. J Inherit Metab Dis. 2010;33(Suppl 3):S73–S81. doi: 10.1007/s10545-009-9025-6. [DOI] [PubMed] [Google Scholar]
- Laney DA, Bennett RL, Clarke V, Fox A, Hopkin RJ, Johnson J, O'Rourke E, Sims K, Walter G. Fabry disease practice guidelines: recommendations of the National Society of Genetic Counselors. J Genet Couns. 2013;22(5):555–564. doi: 10.1007/s10897-013-9613-3. [DOI] [PubMed] [Google Scholar]
- Laney DA, Peck DS, Atherton AM, Manwaring LP, Christensen KM, Shankar SP, Grange DK, Wilcox WR, Hopkin RJ. Fabry disease in infancy and early childhood: a systematic literature review. Genet Med. 2015;17(5):323–330. doi: 10.1038/gim.2014.120. [DOI] [PubMed] [Google Scholar]
- Lin HY, Chong KW, Hsu JH, et al. High incidence of the cardiac variant of Fabry disease revealed by newborn screening in the Taiwan Chinese population. Circ Cardiovasc Genet. 2009;2:450–456. doi: 10.1161/CIRCGENETICS.109.862920. [DOI] [PubMed] [Google Scholar]
- Martinez G, Daniels K, Chandra A. Division of vital statistics, fertility of men and women aged 15–44 years in the United States: National Survey of Family Growth, 2006–2010. Natl Health Stat Rep. 2012;51:1–28. [PubMed] [Google Scholar]
- Martinez GM, Chandra A, Abma JC, Jones J, Mosher WD. Fertility, contraception, and fatherhood: data on men and women from cycle 6 (2002) of the 2002 national survey of family growth. Vital Health Stat. 2006;23(26):1–142. [PubMed] [Google Scholar]
- Mechtler TP, Stary S, Metz TF, De Jesus VR, et al. Neonatal screening for lysosomal storage disorders: feasibility and incidence from a nationwide study in Austria. Lancet. 2012;379:335–341. doi: 10.1016/S0140-6736(11)61266-X. [DOI] [PubMed] [Google Scholar]
- Meikle PJ, Hopwood JJ, Clague AE, Carey WF. Prevalence of lysosomal storage disorders. JAMA. 1999;281:249–254. doi: 10.1001/jama.281.3.249. [DOI] [PubMed] [Google Scholar]
- Nistal M, Paniagua R, Picazo ML. Testicular and epididymal involvement in Fabry’s disease. J Pathol. 1983;141:113–124. doi: 10.1002/path.1711410203. [DOI] [PubMed] [Google Scholar]
- Papaxanthos-Roche A, Deminière C, Bauduer F, Hocké C, Mayer G, Lacombe D. Azoospermia as a new feature of Fabry disease. Fertil Steril. 2007;88(1):212.e15–212.e18. doi: 10.1016/j.fertnstert.2006.11.036. [DOI] [PubMed] [Google Scholar]
- Read C. Reproductive decisions of parents of children with metabolic disorders. Clin Genet. 2002;61:268–276. doi: 10.1034/j.1399-0004.2002.610405.x. [DOI] [PubMed] [Google Scholar]
- Sharlip ID, Jarow JP, Belker AM, Lipshultz LI, Sigman M, Thomas AJ, Schlegel PN, Howards SS, Nehra A, Damewood MD, Overstreet JW, Sadovsky R. Best practice policies for male infertility. Fertil Steril. 2002;77(5):873–882. doi: 10.1016/S0015-0282(02)03105-9. [DOI] [PubMed] [Google Scholar]
- Smid BE, van der Tol L, Biegstraaten M, Linthorst GE, Hollak CE, Poorthuis BJ. Plasma globotriaosylsphingosine in relation to phenotypes of Fabry disease. J Med Genet. 2015;52(4):262–268. doi: 10.1136/jmedgenet-2014-102872. [DOI] [PubMed] [Google Scholar]
- Spada M, Pagliardini S, Yasuda M, et al. High incidence of later-onset Fabry disease revealed by newborn screening. Am J Hum Genet. 2006;79:31–40. doi: 10.1086/504601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence MW, Clarke JT, D’Entremont DM, Sapp GA, Smith ER, Goldbloom AL, Davar G. Angiokeratoma corporis diffusum (Anderson-Fabry disease) in a single large family in Nova Scotia. J Med Genet. 1978;15(6):428–434. doi: 10.1136/jmg.15.6.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Török L, Szekeres L, Reszler M. Angiokeratoma corporis diffusum in 2 brother. Hautarzt. 1980;31(7):376–380. [PubMed] [Google Scholar]
- Veronik F, Benko D, Vujkovac B, Linthorst GE. Remarkable variability in renal disease in a large Slovenian family with Fabry disease. Eur J Hum Genet. 2004;12(8):678–681. doi: 10.1038/sj.ejhg.5201184. [DOI] [PubMed] [Google Scholar]
- Wagner M, Krämer J, Blohm E, Vergho D, Weidemann F, Breunig F, Wanner C. Kidney function as an underestimated factor for reduced health related quality of life in patients with Fabry disease. BMC Nephrol. 2014;15:188. doi: 10.1186/1471-2369-15-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldek S, Patel MR, Banikazemi M, Lemay R, Lee P. Life expectancy and cause of death in males and females with Fabry disease: findings from the Fabry Registry. Genet Med. 2009;11(11):790–796. doi: 10.1097/GIM.0b013e3181bb05bb. [DOI] [PubMed] [Google Scholar]
- Wang RY, Lelis A, et al. Heterozygous Fabry women are not 400 just carriers, but have a significant burden of disease and impaired quality of life. Genet Med. 2007;9(1):34–45. doi: 10.1097/GIM.0b013e31802d8321. [DOI] [PubMed] [Google Scholar]
- Willott GM. Frequency of azoospermia. Forensic Sci Int. 1982;20:9. doi: 10.1016/0379-0738(82)90099-8. [DOI] [PubMed] [Google Scholar]
- The World Bank (2015) Fertility rate, total (births per woman), United States, 2011–2015. http://data.worldbank.org/indicator/SP.DYN.TFRT.IN. Accessed 17 Dec 2015
- Zarate YA, Hopkin RJ. Fabry’s disease. Lancet. 2008;372:1427–1435. doi: 10.1016/S0140-6736(08)61589-5. [DOI] [PubMed] [Google Scholar]


