Abstract
Previous studies have demonstrated an association between high body mass index (BMI) and acute myeloid leukemias (AML), particularly acute promyelocytic leukemia (APL). However, the effect of obesity and overweight on the incidence of AML is not supported by all studies, and the relationship between obesity and prognosis of AML and APL has not been established. Thus, we conducted a meta-analysis to determine the role of BMI on the risk and clinical outcome of AML, including APL. Twenty-six eligible studies enrolling 12,971 AML (including 866 APL) patients were retrieved and analyzed. Overweight and obesity was associated with an increased incidence of AML (relative risk [RR], 1.23; 95% confidence interval [CI], 1.12–1.35; P < 0.001). High BMI did not significantly affect overall survival (OS) (hazard ratio [HR], 0.97; 95% CI, 0.92–1.03; P = 0.323) or disease-free survival (HR, 0.98; 95% CI, 0.88–1.10; P = 0.755) in patients with non-APL AML. By contrast, APL patients with high BMI had shorter OS (HR, 1.77; 95% CI, 1.26–2.48; P = 0.001) and a higher risk of differentiation syndrome (HR, 1.53; 95% CI, 1.03–2.27, P = 0.04). Overall, our findings suggest that patients with overweight or obesity have a higher incidence of AML, and high BMI is a predictor of adverse clinical outcomes in APL.
Introduction
The prevalence of obesity has been increasing worldwide over the past decades. According to World Health Organization (WHO), overweight and obesity are defined by a body mass index (BMI) of 25–29.9 kg/m2 and ≥30 kg/m2, respectively. Currently, the proportion of overweight or obesity in adults has increased to approximately 40% worldwide1. It has been reported that overweight or obesity is associated with over 20 types of cancer, such as cancers of the breast, colon, uterus, gallbladder, and cervix, as well as leukemia2,3.
Leukemia is a clonal hematopoietic disorder characterized by the aggressive proliferation of immature hematopoietic progenitor cells arrested at an early stage of differentiation. Acute myeloid leukemia (AML) is one of the most common myeloid malignancies in adults4. Although much effort has been devoted to studying this disease, the etiology and pathophysiology of AML are still poorly understood and the outcome remains unsatisfactory. Epidemiologic observations have suggested that obesity is a risk factor for AML5–7. However, obesity did not affect risk for AML in several studies8–10, and Wong et al. even reported that obesity is protective against AML11. Overweight may have a similar effect on the incidence of AML, although this relationship is less certain6,8,12. The most recent meta-analysis on this topic was performed in 2012 and suggested that obesity, but not overweight, increases incidence of AML7. However, the number of patients with AML was limited because this meta-analysis included all types of leukemia and only included five studies on AML. Since 2012, five new studies, including two large multinational survey programs, have been published5,6,10,12,13. Thus, including these new studies in an updated analysis is needed to comprehensively evaluate the association between BMI and risk for AML.
In addition to the incidence of AML, the association between high BMI and the prognosis of AML is unclear due to the lack of a meta-analysis, although several studies have investigated this issue14–16. Reports on the prognostic value of obesity for AML are somewhat inconsistent. Several studies have found that obesity adversely affects the clinical outcome of AML17 whereas others have failed to confirm such an association15,18,19.
Notably, several studies have shown that the percentage of obese patients with acute promyelocytic leukemia (APL), a unique subtype of AML, is higher than that observed in patients with non-APL AML15,20,21. APL generally carries the PML/RARα fusion protein linking the retinoic acid receptor alpha gene (RARα) on chromosome 17 with the PML gene on chromosome 15. Historically, APL was one of the most fatal forms of acute leukemia but currently has a cure rate of approximately 80–85% with the introduction of all-trans retinoic acid (ATRA) and arsenic trioxide (ATO)15,22. Emerging studies have demonstrated that obesity has adverse effects on the treatment outcome of APL15,23. However, to our knowledge, a quantitative analysis that evaluates this association with APL is not available.
With the aim of evaluating the prognostic value of obesity in patients with AML (including APL), we conducted the first comprehensive meta-analysis to clarify the potential association between AML, particularly APL, with overweight and obesity as defined by BMI. Another objective of our study was to investigate the association between obesity and the incidence of AML.
Methods
BMI calculation and classification
BMI was calculated by dividing weight in kilograms by the square of height in meters (weight [kg]/height [m2]). According to the criteria from the World Health Organization, BMI was categorized as underweight (<18.5 kg/m2), normal (18.5–25 kg/m2), overweight (25–29.9 kg/m2), and obese (≥30 kg/m2). Most of the studies used 25 and 30 kg/m2 as the thresholds for overweight and obesity, respectively. Studies that did not meet these criteria11,13,24 were eligible for analysis of the association based on high BMI but were excluded from the overweight and obese subgroup analysis. Lin et al. used body weight (BW) ≥130% ideal body weight (IBW) as the criteria to define obesity25.
Literature search
A literature search (last search updated to Dec. 4, 2016) was conducted in PubMed, EMBASE, Web of Science and the Cochrane Library for articles assessing the effect of overweight and obesity on the incidence and clinical outcome of AML using the keywords as follows: (obesity OR overweight OR body mass OR BMI OR body weight OR anthropometric) AND leukemia. References lists from the retrieved studies were also examined. The results were limited to peer-reviewed English language studies.
Eligibility criteria
The studies were considered eligible if they reported the effect of overweight and obesity on the incidence or clinical outcome of patients with AML and provided sufficient data to determine an estimate of relative risk (RR) or hazard ratio (HR) and a 95% confidence interval (CI). Odd ratios (OR) were converted to RRs using methods reported by Zhang et al.26. When the patient populations overlapped between studies, only the most recent or most complete publication was included to avoid duplications.
Data extraction and quality assessment
The data extracted for our meta-analysis included the first author’s name, year of publication, country, number of patients analyzed, enrollment period, source of the cohort, adjustments, and other relevant data stratified by BMI. Quality assessment of eligible papers was conducted according to the Newcastle-Ottawa Scale (NOS)7. The NOS score includes an assessment of subject selection (four points), comparability of groups (two points), and exposure or clinical outcome (three points). The maximum score is 9 and the scores of eligible papers included in our meta-analysis is ranged from 5 to 9 (Supplementary Table S1).
Statistical analysis
RR or HR from each article was extracted directly from the original reports or calculated using the method reported by Tierney et al.27. The potential heterogeneity across studies was evaluated using the Cochran’s Q-test and expressed using the I2 index. The pooled results for RR and HR were calculated by the fixed-effects model (I2 ≤ 50%) or the random-effects model (I2 > 50%). Publication bias was evaluated by the funnel plot and Egger’s and Begg’s tests. The effect of publication bias on the pooled findings was evaluated by trim-and-fill analyses. The stability of the pooled findings was confirmed by one-way sensitivity and subgroup analyses. All statistical analyses were conducted using STATA version 11.0.
Results
Identification of relevant studies
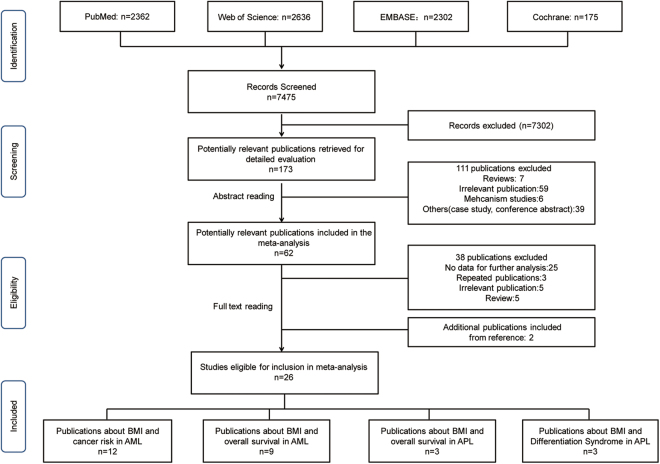
A total of 7,475 studies were retrieved from the preliminary literature search (PubMed:2,362, EMBASE:2,302, Web of Science:2,636, and the Cochrane library:175), and an additional two studies were identified from a review of citations. 173 potential relevant publications were retrieved for detailed evaluation. Of these publications, 111 articles were excluded after reading the title and abstract because they were irrelevant to our meta-analysis, or because they were mechanistic studies, case reports, conference abstracts, or reviews. An additional 38 publications were then excluded because they were irrelevant to AML, provided no data for further analysis, or were duplicate publications or reviews. Finally, 26 studies were included in our meta-analysis (Fig. 1, Table 1), including 12 papers about relative risk (RR) with 6,724 AML patients5,6,8–13,28–31, 11 papers regarding AML survival with 5,505 non-APL AML patients and 538 APL patients14–19,24,25,32–34, and three papers on the subject of differentiation syndrome in APL (after excluding duplicate publications32,35,36) with 243 patients23,37,38.
Figure 1.
Results of the search strategy. BMI, body mass index; AML, acute myeloid leukemia; APL, acute promyelocytic leukemia.
Table 1.
Characteristics of studies included in the meta-analysis.
| First author, year | Country | Subtype (patient #) | Enrollment period | Source of the cohort | Adjustments |
|---|---|---|---|---|---|
| Studies on relative risk | |||||
| Samanic 2004 | USA | AML (1894) | 1969–1996 | Veterans Affairs (VA) Hospitals | age, race and calendar-year |
| Ross 2004 | USA | AML (74) | 1986–2001 | The Iowa Women’s Health Study | age and regular physical activity |
| Kasim 2005 | Canada | AML (307) | 1994–1997 | Canadian National Enhanced Cancer Surveillance System | gender and pack-years of smoking |
| Samanic 2006 | Sweden | AML (267) | 1971–1992 | The Population- based Swedish Cancer Registry, The Nationwide Mortality Registry, the Migration Register | age and smoking status |
| Engeland 2007 | Norway | AML (1374) | 1963–2001 | Norwegian Nationwide Screening Program | birth cohort and age |
| Wong 2009 | China | non-APL AML (598)APL (124) | 2003–2007 | Twenty-nine Hospitals in Shanghai | gender, age |
| Soderberg 2009 | Sweden, Finland | AML (66) | 1961–2002 Sweden1975–2004 Finland | The Swedish Twin Registry and one Finnish Twin Cohort | age, sex, country, alcohol intake, education, smoking, diabetes and exercise |
| Strom 2012 | USA | AML (638) | 2003–2007 | The University of Texas M. D. Anderson Cancer Center | education and family history of hematopoietic cancer |
| Nagel 2012 | Norway, Sweden, Austria | AML (231) | 1988–2002 Norway1972–2003Sweden1974–2005 Austria | The Metabolic Syndrome and Cancer Project (Me-Can) | age, smoking status |
| Murphy 2013 | UK | AML (578) | 1996–2001 | The United Kingdom National Breast Cancer Screening Programme | height, alcohol consumption, smoking and socioeconomic status |
| Hosnijeh 2013 | Denmark, France, Greece, Germany, Italy, Netherlands, Norway, Spain, Sweden, UK | AML (153) | 1992–2000 | European Prospective Investigation into Cancer and Nutrition (EPIC) | physical activity, educational level, smoking status, alcohol intake, history of diabetes and family history of cancer |
| Poynter 2016 | USA | AML (420) | 2005–2009 | Minnesota Cancer Surveillance System | age, income, physical activity, and exposure to chemotherapy or benzene |
| Studies on overall survival | |||||
| Jeddi 2010 | Tunisia | APL (39) | 2004–2008 | Aziza Othmana University Hospital | age, sex, baseline WBC, serum creatinine, platelet count and immune phenotyping |
| Lee 2012 | USA | non-APL AML (329) | 1990–2008 | Roswell Park Cancer Institute | age, gender, AML presentation, WBC, smoking history, treatment decade and karyotype |
| Medeiros 2012 | USA | non-APL AML (1974) | 1980s–2000s | Southwest Oncology Group (Trial S8600, S9031, S9126, S9333, S9500, S9617, S9918, and S0106) | age, gender, performance status, karyotype, WBC, platelet and peripheral blast counts |
| Lin 2013 | USA | non-APL AML (63) | 2006–2010 | University of Washington Medical Center | prior malignancy, FLT3-ITD and NPM-1 status |
| Wenzell 2013 | USA | non-APL AML (247) | 2002–2009 | Cleveland Clinic | age, sex, WBC, cytogenetic risk, etiology and bacteremia |
| Brunner 2013 | USA | non-APL AML (97) | 1992–2011 | Massachusetts General Hospital | a history of CAD or diabetes, patient gender and race, patient cytogenetics |
| Kempf 2014a | France | non-APL AML (233) | 2003–2013 | Saint-Antoine hospital | age, gender and cytogenetic |
| Wang 2015 | China | APL (53) | 2004–2010 | Institute of Hematology and Blood Diseases Hospital | not available |
| Finn 2015 | USA | non-APL AML (295) | 1995–2012 | Mayo Clinic in Florida and Arizona | not available |
| Castillo 2016a | USA | APL (446) non-APL AML (1648) | APL (1999–2005)AML (1993–2010) | APL from CALGB9710, non-APL AML from CALGB9621, 10503 and 19808 | age, sex, performance status, race, ethnicity, treatment and WBC |
| Tavitian 2016a | France | non-APL AML (619) | 2004–2012 | Toulouse University Hospital | age, WBC, AML status and ECOG performance status |
| Studies on differentiation syndrome | |||||
| Jeddi 2010 | Tunisia | APL (39) | 2004–2008 | Aziza Othmana University Hospital | age, sex, WBC, serum creatinine, platelet count and immunophenotyping |
| Breccia 2012 | Rome | APL (144) | 1993–2010 | Sapienza University of Rome | age, sex, FAB classification, transcript type, WBC, platelet count and hemoglobin level |
| Leblebjian 2013 | USA | APL (60) | 2004–2010 | Dana Farber/Brigham and Women’s Hospital Cancer Center (DF/BWHCC) | age, sex, WBC, percent blast count, serum creatinine, platelet count, uric acid, lactate dehydrogenase, albumin and type of chemotherapy used with ATRA |
Abbreviations: AML, acute myeloid leukemia; APL, acute promyelocytic leukemia; DS, differentiation syndrome; OR, odds ratio; RR, relative ratio; OS, overall survival; WBC, white blood count.
aContaining disease-free survival (DFS) data.
Relationship between high BMI and incidence of AML
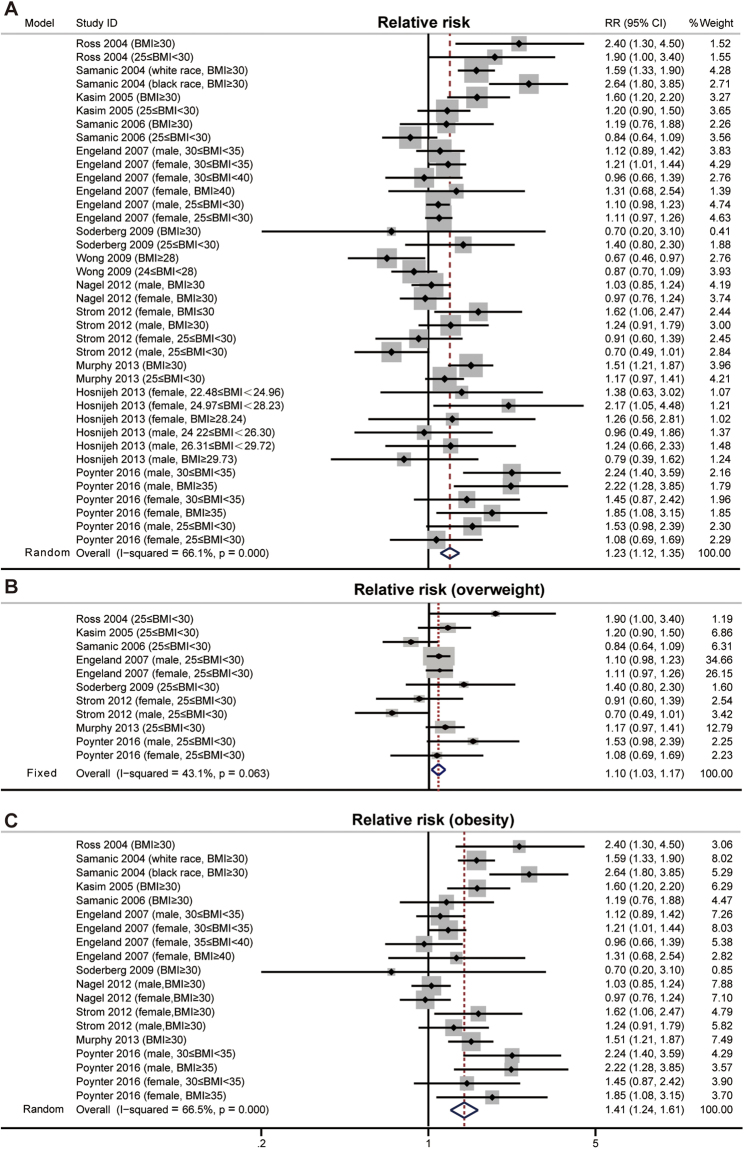
AML is one of the most common myeloid malignancies in adults. To explore the association between BMI and the incidence of AML, we analyzed 12 studies that reported on incidence estimates for AML according to BMI (Table 1). All ORs were transformed into RRs before pooled analysis. The pooled RR indicated that high BMI was associated with increased risk for AML (RR, 1.23; 95% CI, 1.12–1.35; P < 0.001; I2 = 66.0%; random effects; Fig. 2A). Although no significant publication bias was identified by Begg’s and Egger’s tests (PBegg = 0.227; PEgger = 0.156), the funnel plot suggested the possibility of missing studies (Supplementary Fig. S1A). After performing a trim-and-fill analysis (Supplementary Fig. S1B), we found that six studies might be missing. When these potentially missing studies were added to the analysis, the adjusted RR would be 1.14 (95% CI, 1.04–1.26, P = 0.008).
Figure 2.
The effects of high BMI on the incidence of AML. (A) Meta-analysis of the relative risk (RR) of AML according to BMI with random-effects model. (B) Estimates of RR in overweight AML individuals with the fixed-effects model. (C) Estimates of RR in obese AML individuals with the random-effects model.
We further divided the patients into overweight and obesity subgroups. As shown in Fig. 2B and C, both overweight and obesity were associated with higher incidence of AML (for overweight, RR, 1.10; 95% CI, 1.03–1.17; P = 0.007; I2 = 43.1%; fixed effects; and for obesity, RR, 1.41; 95% CI, 1.24–1.61; P < 0.001; I2 = 66.5%; random effects). One-way sensitivity analysis further confirmed the stability of our results (Supplementary Fig. S1C).
Relationship between high BMI and clinical outcome of AML
Next, we investigated the prognostic value of BMI for clinical outcomes of AML. Because treatment strategy and prognosis of APL and non-APL are significantly different, we analyzed their clinical outcomes separately.
No association between high BMI and clinical outcome of non-APL AML
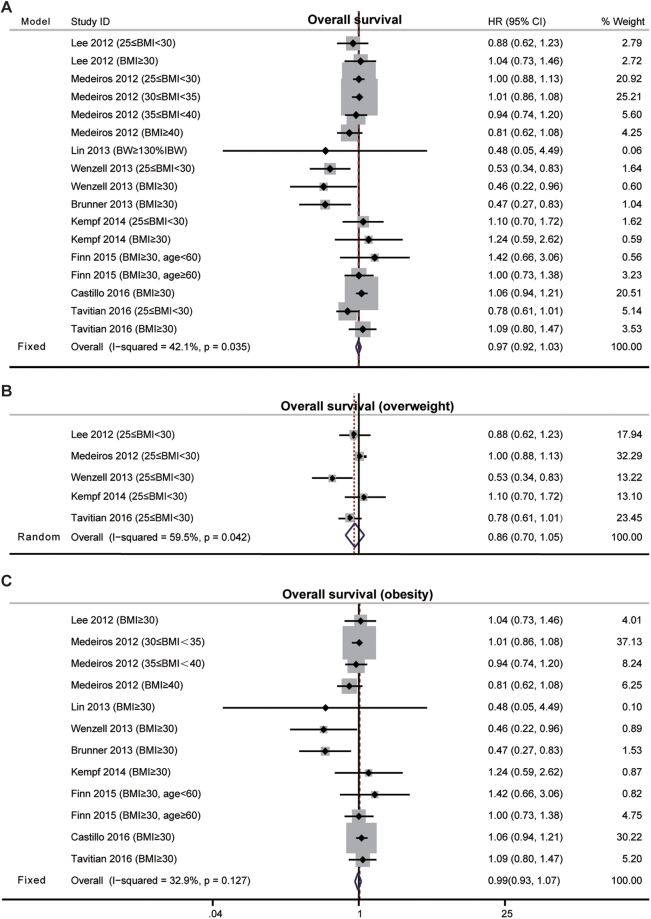
Nine studies were included in the survival estimates for BMI in non-APL AML (Table 1). Our data suggested that high BMI did not significantly affect the survival of non-APL AML patients (HR, 0.97; 95% CI, 0.92–1.03; P = 0.323; I2 = 42.1%, fixed effects, Fig. 3A). No significant publication bias was identified by Begg’s and Egger’s tests (PBegg = 0.434; PEgger = 0.25). Although the funnel plot showed asymmetry (Supplementary Fig. S2A), no missing study were identified by the trim-and-fill analysis (Supplementary Fig. S2B).
Figure 3.
The effects of high BMI on the overall survival of non-APL AML patients. (A) Meta-analysis of the overall survival (OS) of AML according to BMI with the fixed-effects model. (B) Estimates of OS in overweight AML individuals with the random-effects model. (C) Estimates of OS in obese AML individuals with fixed-effects model.
We divided these patients into overweight and obese subgroups. Similarly, neither overweight nor obesity affected OS of patients with non-APL AML relative to patients of normal weight (for overweight, HR, 0.86; 95% CI, 0.70–1.05; P = 0.14; I2 = 59.5%; random effects; and for obesity, HR, 0.99; 95% CI, 0.93–1.07; P = 0.281; I2 = 32.9%; fixed effects; Fig. 3B,C). One-way sensitivity analysis further confirmed the stability of our pooled findings (Supplementary Fig. S2C).
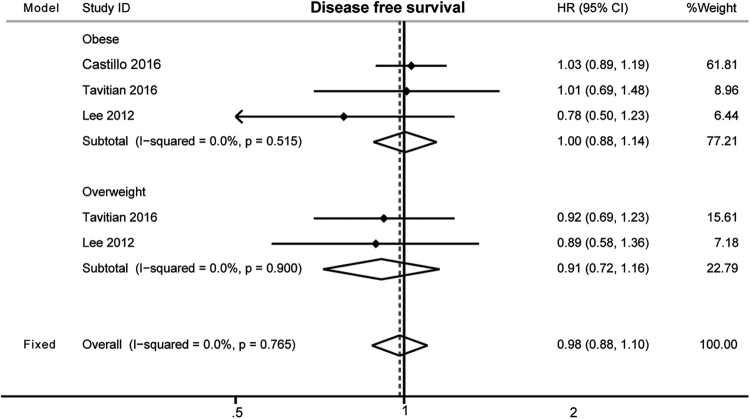
The HRs for disease free survival (DFS) were available in three studies about non-APL AML (Table 1). The results indicated that high BMI at diagnosis did not affect DFS of non-APL AML (HR, 0.98; 95% CI, 0.88–1.10; fixed effects, Fig. 4). The heterogeneity among studies was absent (P = 0.765; I2 = 0%). Specifically with respect to obesity, there was still no association between obesity and DFS (HR, 1.00; 95% CI, 0.88–1.14; P = 0.951; I2 = 0%; fixed effects, Fig. 4).
Figure 4.
The effects of high BMI on the disease free survival of non-APL AML patients.
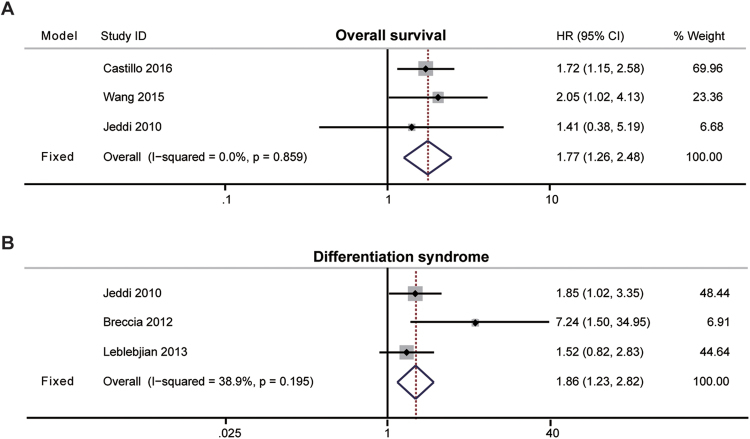
Significant association between high BMI and clinical outcome of APL
APL is a unique subtype of AML. Surprisingly, we found that among patients with APL, those with high BMI had a shorter duration of OS than those with low BMI after pooling three relevant studies (HR, 1.77; 95% CI, 1.26–2.48; P = 0.001; I2 = 0%; fixed effects; Fig. 5A)15,24,32. Moreover, high BMI in patients with APL was associated with an increased risk of developing differentiation syndrome, a severe complication of chemotherapy, (HR, 1.86; 95% CI, 1.23–2.82; P = 0.003; I2 = 38.9%; fixed effects; Fig. 5B), according to three related studies23,37,38. The DFS data of APL was available in only one publication, which demonstrated that obese patients have worse DFS (HR, 1.53; 95% CI, 1.03–2.27, p = 0.04)15.
Figure 5.
The effects of high BMI on the clinical outcome of APL patients. (A) Meta-analysis of the overall survival (OS) of APL according to BMI with the fixed- effects model. (B) Meta-analysis of the differentiation syndrome of APL according to BMI with the fixed-effects model.
Discussion
Increasing evidence has demonstrated that obesity is correlated to the incidence and clinical outcome of AML5–7,29,39. In our meta-analysis, we systematically collected related literature and analyzed the association between obesity and AML, including APL. First, we observed that both overweight and obesity increased the risk for AML. This is an important update since previous studies showed no correlation between overweight and incidence of AML7,39,40. Second, our study was the first to evaluate the relationship between obesity and the clinical outcomes (OS and DFS) of AML using a meta-analytical methodology. We found that obesity had no effect on the OS or DFS of non-APL AML, whereas overweight or obese patients with APL had a poorer OS and a higher risk of developing differentiation syndrome. Overall, obesity was closely related to AML, especially APL.
Exploring the association between obesity and AML is important for drug development and the battle against AMLs. However, mechanistic studies investigating the adverse association between obesity and AML are still in the early stages. Recently, epigenome-wide association studies identified that BMI is associated with widespread changes in DNA methylation41. In fact, fatty acid binding protein 4 (FABP4)/IL-6/STAT3/DNA methyltransferase 1 (DNMT1) have been shown to link obesity to the growth of AML cells42. Li et al. demonstrated that the obesity-associated gene FTO is highly expressed in AML with the MLL-fusion gene, thereby promoting leukemogenesis and inhibiting ATRA-induced cell differentiation43. Moreover, studies have shown that leukemia stem cells with a high expression of CD36 (fatty acid transporter) are prone to be chemoresistance44, and CD36+ patients with AML have a poorer leukemia-free survival45.
Apart from the mechanisms illustrated above, the function of obesity in contributing to leukemogenesis requires more research. Adipose tissue is a complex and active endocrine organ and can secrete various bioactive adipokines, hormones, inflammatory factors, angiogenesis factors, and free fatty acids. These secretory factors have been shown to participate in production and progression of tumors46,47, suggesting they may also contribute to the leukemogenesis of AML. Furthermore, as adipocytes are one of the most abundant cell types in blood-producing bone marrow48, direct cell-to-cell interaction cannot be disregarded, as Tabe et al. have demonstrated that the direct cell-to-cell interaction between adipocytes and APL cells is required for adipocyte-induced anti-apoptotic effect49. Also, it should be noted that obese individuals have unique pharmacokinetics compared to normal-weight controls. Reduced efficacy of chemotherapy drugs in overweight and obese patients, probably as a result of altered liver enzyme function, is a likely and often underestimated event related to cancer mortality46. It is also unclear whether the increased incidence and adverse outcome of obese patients with leukemia are associated to complications from obesity-related co-morbidities and a chronic inflammatory state or to obesity itself. In addition, microbiota can modulate the response to cancer therapy and susceptibility to toxic side effects, and obesity-induced gut microbial metabolites alterations may influence the clinical outcomes of AML patients47.
When evaluating the prognosis of patients with AML, the treatment strategies and patient’s characteristics should also be factors to consider, including gender, age, chromosome karyotype, genetic changes, and ECOG. In our meta-analysis, multivariate analysis was conducted to avoid the interference of confounding factors in most studies (Table 1 and Supplementary Table S2). Another important issue is that the dosing strategies may be different for the obese AML patients in different studies. For this, Percival et al. summarized several retrospective analyses and concluded that dosing according to body surface area based on ideal body weight (cap) or actual body weight (not cap) have similar complete remission rates, toxicity, and overall survival in obese patients with AML50.
Our finding that obesity has no effect on the OS of adult patients with AML was somewhat surprising, particularly because a high BMI is associated with poorer OS in pediatric patients with AML51. This discrepancy may be due to the physiological and metabolic differences between children and adults. However, we did find that obesity was associated with poorer OS in adult patients with APL. Actually, Tabe et al. showed that the leptin receptor is highly expressed on APL cells, and co-culturing of mesenchymal stem cell-derived adipocytes with APL cells significantly reduced ATRA and doxorubicin-induced apoptosis of APL cells49. More mechanistic studies are required to investigate the association between obesity and APL.
Our meta-analysis may carry inherent limitations within the published literature. First, there is risk for publication, ecological, and racial bias. Second, although the survival data included was primarily obtained through multivariable analyses considering the most influential confounder, some confounders may have still been ignored. Third, BMI is inappropriate for evaluating adiposity in individuals at extreme heights or older ages. Moreover, self-reported height and weight in some studies may be inaccurate and thus result in classification errors. Obese individuals vary in their distribution of visceral and subcutaneous fat. Since the function and metabolism between the two types are different, individuals who have the same BMI may respond differently to risk factors and cancer drugs due to variance in fat distributions. Finally, the research on APL is relatively limited because its incidence is lower than in other subtypes of AML. Further studies should be conducted to sufficiently address these issues.
In conclusion, both overweight and obesity were associated with increased risk of AML, with obesity producing more palpable effects. However, obesity does not significantly affect the OS and DFS of non-APL AML. APL patients are, more obese, on average, than those with other forms of AML, and obesity is an adverse prognostic factor for clinical outcome in APL (demonstrated by reduced OS and the increased incidence of differentiation syndrome). Thus, APL is more specifically associated with obesity.
Evaluating the underlying mechanisms responsible for their relationship is necessary to develop the novel therapeutic approaches for AML. Also, given that high BMI increases the incidence of AML, controlling body weight may be considered for obese individuals. In addition, for obese APL patients, more attention should be given to possible ATRA-associated complications during induction therapy.
Electronic supplementary material
Acknowledgements
This work was supported in part by National Natural Science Foundation Grants of China (81530003), the National Key Research and Development Program of China (2016YFC0902800), Shanghai Leading Talent Projects (2015008) and the Academic Leader Program of Shanghai Science and Technology Committee (2015137).
Author Contributions
S.F.L. and K.K.W. designed the study, S.F.L. and L.C. searched and screened the studies, S.F.L., Y.L.M. and X.F.M. extracted and analyzed the data, F.Y.D., W.J., H.M.Z. and J.M.L. performed statistical analysis, S.F.L. and K.K.W. wrote the manuscript, all the authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Shufen Li and Li Chen contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-18278-x.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. LANCET387, 1377 (2016). [DOI] [PMC free article] [PubMed]
- 2.Hefetz-Sela S, Scherer PE. Adipocytes: impact on tumor growth and potential sites for therapeutic intervention. Pharmacol Ther. 2013;138:197. doi: 10.1016/j.pharmthera.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhaskaran K, et al. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5.24 million UK adults. LANCET. 2014;384:755. doi: 10.1016/S0140-6736(14)60892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Estey E, Dohner H. Acute myeloid leukaemia. LANCET. 2006;368:1894. doi: 10.1016/S0140-6736(06)69780-8. [DOI] [PubMed] [Google Scholar]
- 5.Poynter JN, et al. Obesity over the life course and risk of acute myeloid leukemia and myelodysplastic syndromes. Cancer Epidemiol. 2016;40:134. doi: 10.1016/j.canep.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy F, et al. Body size in relation to incidence of subtypes of haematological malignancy in the prospective Million Women Study. Brit J Cancer. 2013;108:2390. doi: 10.1038/bjc.2013.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castillo JJ, et al. Obesity but not overweight increases the incidence and mortality of leukemia in adults: A meta-analysis of prospective cohort studies. Leukemia Res. 2012;36:868. doi: 10.1016/j.leukres.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 8.Söderberg KC, et al. Overweight, obesity and risk of haematological malignancies: A cohort study of Swedish and Finnish twins. Eur J Cancer. 2009;45:1232. doi: 10.1016/j.ejca.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Engeland A, Tretli S, Hansen S, Bjorge T. Height and Body Mass Index and Risk of Lymphohematopoietic Malignancies in Two Million Norwegian Men and Women. Am J Epidemiol. 2006;165:44. doi: 10.1093/aje/kwj353. [DOI] [PubMed] [Google Scholar]
- 10.Nagel G, et al. Metabolic factors and blood cancers among 578,000 adults in the metabolic syndrome and cancer project (Me-Can) Ann Hematol. 2012;91:1519. doi: 10.1007/s00277-012-1489-z. [DOI] [PubMed] [Google Scholar]
- 11.Wong O, Harris F, Yiying W, Hua F. A hospital-based case-control study of acute myeloid leukemia in Shanghai: Analysis of personal characteristics, lifestyle and environmental risk factors by subtypes of the WHO classification. Regul Toxicol Pharm. 2009;55:340. doi: 10.1016/j.yrtph.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Strom SS, Oum R, Elhor Gbito KY, Garcia-Manero G, Yamamura Y. De novo acute myeloid leukemia risk factors. Cancer-Am Cancer Soc. 2012;118:4589. doi: 10.1002/cncr.27442. [DOI] [PubMed] [Google Scholar]
- 13.Saberi Hosnijeh F, et al. Anthropometric characteristics and risk of lymphoid and myeloid leukemia in the European Prospective Investigation into Cancer and Nutrition (EPIC) Cancer Cause Control. 2013;24:427. doi: 10.1007/s10552-012-0128-2. [DOI] [PubMed] [Google Scholar]
- 14.Medeiros BC, Othus M, Estey EH, Fang M, Appelbaum FR. Impact of body-mass index on the outcome of adult patients with acute myeloid leukemia. Haematologica. 2012;97:1401. doi: 10.3324/haematol.2011.056390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castillo JJ, et al. Relationship between obesity and clinical outcome in adults with acute myeloid leukemia: A pooled analysis from four CALGB (alliance) clinical trials. Am J Hematol. 2016;91:199. doi: 10.1002/ajh.24230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tavitian S, et al. Impact of obesity in favorable-risk AML patients receiving intensive chemotherapy. Am J Hematol. 2016;91:193. doi: 10.1002/ajh.24228. [DOI] [PubMed] [Google Scholar]
- 17.Finn L, et al. Epidemiology of adult acute myeloid leukemia: Impact of exposures on clinical phenotypes and outcomes after therapy. Cancer Epidemiol. 2015;39:1084. doi: 10.1016/j.canep.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Brunner AM, et al. Association between baseline body mass index and overall survival among patients over age 60 with acute myeloid leukemia. Am J Hematol. 2013;88:642. doi: 10.1002/ajh.23462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wenzell CM, et al. Outcomes in obese and overweight acute myeloid leukemia patients receiving chemotherapy dosed according to actual body weight. Am J Hematol. 2013;88:906. doi: 10.1002/ajh.23530. [DOI] [PubMed] [Google Scholar]
- 20.Estey E, et al. Association between increased body mass index and a diagnosis of acute promyelocytic leukemia in patients with acute myeloid leukemia. Leukemia. 1997;11:1661. doi: 10.1038/sj.leu.2400783. [DOI] [PubMed] [Google Scholar]
- 21.Tedesco J, Qualtieri J, Head D, Savani BN, Reddy N. High Prevalence of Obesity in Acute Promyelocytic Leukemia (APL): Implications for Differentiating Agents in APL and Metabolic Syndrome. Ther Adv Hematol. 2011;2:141. doi: 10.1177/2040620711408490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coombs CC, Tavakkoli M, Tallman MS. Acute promyelocytic leukemia: where did we start, where are we now, and the future. Blood Cancer J. 2015;5:e304. doi: 10.1038/bcj.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Breccia M, et al. Increased BMI correlates with higher risk of disease relapse and differentiation syndrome in patients with acute promyelocytic leukemia treated with the AIDA protocols. Blood. 2012;119:49. doi: 10.1182/blood-2011-07-369595. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, et al. Long-term follow-up of homoharringtonine plus all-trans retinoic acid-based induction and consolidation therapy in newly diagnosed acute promyelocytic leukemia. Int J Hematol. 2015;101:279. doi: 10.1007/s12185-014-1730-8. [DOI] [PubMed] [Google Scholar]
- 25.Lin A, Othus M, McQuary A, Chi M, Estey E. Influence of obesity on efficacy and toxicity of induction chemotherapy in patients with newly diagnosed acute myeloid leukemia. Leukemia Lymphoma. 2012;54:541. doi: 10.3109/10428194.2012.717278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. Jama. 1998;280:1690. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 27.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kasim K, Levallois P, Abdous B, Auger P, Johnson KC. Lifestyle factors and the risk of adult leukemia in Canada. Cancer Cause Control. 2005;16:489. doi: 10.1007/s10552-004-7115-1. [DOI] [PubMed] [Google Scholar]
- 29.Ross JA, Parker E, Blair CK, Cerhan JR, Folsom AR. Body mass index and risk of leukemia in older women. Cancer Epidemiol Biomarkers Prev. 2004;13:1810. doi: 10.1158/1055-9965.EPI-03-2135. [DOI] [PubMed] [Google Scholar]
- 30.Samanic C, Chow W, Gridley G, Jarvholm B, Fraumeni JF. Relation of body mass index to cancer risk in 362,552 Swedish men. Cancer Cause Control. 2006;17:901. doi: 10.1007/s10552-006-0023-9. [DOI] [PubMed] [Google Scholar]
- 31.Samanic C, et al. Obesity and cancer risk among white and black United States veterans. Cancer Causes Control. 2004;15:35. doi: 10.1023/B:CACO.0000016573.79453.ba. [DOI] [PubMed] [Google Scholar]
- 32.Jeddi R, et al. Treatment of acute promyelocytic leukemia with PETHEMA LPA 99 protocol: a Tunisian single center experience. Hematology. 2010;15:204. doi: 10.1179/102453309X12583347114176. [DOI] [PubMed] [Google Scholar]
- 33.Kempf E, et al. Prognosis of body mass index and chemotherapy dose capping in acute myeloid leukaemia. Leukemia Res. 2014;38:1425. doi: 10.1016/j.leukres.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 34.Lee HJ, et al. Is obesity a prognostic factor for acute myeloid leukemia outcome? Ann Hematol. 2012;91:359. doi: 10.1007/s00277-011-1319-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeddi R, et al. Predictive factors of all-trans-retinoic acid related complications during induction therapy for acute promyelocytic leukemia. Hematology. 2008;13:142. doi: 10.1179/102453308X316112. [DOI] [PubMed] [Google Scholar]
- 36.Jeddi R, et al. Treatment of Acute Promyelocytic Leukemia with AIDA Based Regimen. Update of a Tunisian Single Center Study. Mediterr J Hematol Infect Dis. 2011;3:e2011033. doi: 10.4084/mjhid.2011.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jeddi R, et al. High body mass index is an independent predictor of differentiation syndrome in patients with acute promyelocytic leukemia. Leukemia Res. 2010;34:545. doi: 10.1016/j.leukres.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 38.Leblebjian H, et al. Predictive factors for all-trans retinoic acid-related differentiation syndrome in patients with acute promyelocytic leukemia. Leukemia Res. 2013;37:747. doi: 10.1016/j.leukres.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 39.Lichtman MA. Obesity and the Risk for a Hematological Malignancy: Leukemia, Lymphoma, or Myeloma. The Oncologist. 2010;15:1083. doi: 10.1634/theoncologist.2010-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Larsson SC, Wolk A. Overweight and obesity and incidence of leukemia: A meta-analysis of cohort studies. Int J Cancer. 2008;122:1418. doi: 10.1002/ijc.23176. [DOI] [PubMed] [Google Scholar]
- 41.Wahl, S. et al. Epigenome-wide association study of body mass index, and the adverse outcomes of adiposity. Nature (2016). [DOI] [PMC free article] [PubMed]
- 42.Yan, F. et al. Fatty acid-binding protein FABP4 mechanistically links obesity with aggressive AML by enhancing aberrant DNA methylation in AML cells. Leukemia (2016). [DOI] [PMC free article] [PubMed]
- 43.Li, Z. et al. FTO Plays an Oncogenic Role in Acute Myeloid Leukemia as a N6-Methyladenosine RNA Demethylase. Cancer Cell (2016). [DOI] [PMC free article] [PubMed]
- 44.Ye H, et al. Leukemic Stem Cells Evade Chemotherapy by Metabolic Adaptation to an Adipose Tissue Niche. Cell Stem Cell. 2016;19:23. doi: 10.1016/j.stem.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perea G, et al. Adverse prognostic impact of CD36 and CD2 expression in adult de novo acute myeloid leukemia patients. Leuk Res. 2005;29:1109. doi: 10.1016/j.leukres.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 46.Mazzarella L. Why does obesity promote cancer? Epidemiology, biology, and open questions. Ecancermedicalscience. 2015;9:554. doi: 10.3332/ecancer.2015.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tahergorabi Z, Khazaei M, Moodi M, Chamani E. From obesity to cancer: a review on proposed mechanisms. Cell Biochem Funct. 2016;34:533. doi: 10.1002/cbf.3229. [DOI] [PubMed] [Google Scholar]
- 48.Scheller EL, Cawthorn WP, Burr AA, Horowitz MC, MacDougald OA. Marrow Adipose Tissue: Trimming the Fat. Trends Endocrinol Metab. 2016;27:392. doi: 10.1016/j.tem.2016.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tabe Y. PML-RAR is associated with leptin-receptor induction: the role of mesenchymal stem cell-derived adipocytes in APL cell survival. Blood. 2004;103:1815. doi: 10.1182/blood-2003-03-0802. [DOI] [PubMed] [Google Scholar]
- 50.Percival MM, Medeiros BC. Chemotherapy dose in obese AML patients: To cap or not to cap? Leukemia Res. 2015;39:30. doi: 10.1016/j.leukres.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Orgel E, et al. Association of body mass index and survival in pediatric leukemia: a meta-analysis. Am J Clin Nutr. 2016;103:808. doi: 10.3945/ajcn.115.124586. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.