Abstract
Multiple studies have reported that individuals with low birth weights (LBW, <2500 g) have a lower intelligence quotient (IQ) than those with normal birth weights (NBW, ≥2500 g). Based on 57 eligible individual studies including 12,137 participants, we performed a meta-analysis to estimate the association between low birth weight and individuals’ IQ scores (IQs). The pooled weight mean difference (WMD) in IQs between NBW and LBW individuals was 10 (95% CI 9.26–11.68). The WMD was stable regardless of age. No publication bias was detected. The mean IQs of the extremely low birth weight (ELBW, <1000 g), very low birth weight (VLBW, 1000–1499 g), moderately low birth weight (MLBW, 1500–2499 g) and NBW individuals were 91, 94, 99 and 104, respectively. Additionally, the WMD in IQs with NBW were 14, 10 and 7 for ELBW, VLBW, and MLBW individuals, respectively. Two studies permitted estimates of the influence of social determinants of health to the discrepancy in IQs, which was 13%. Since IQ is inherited and influenced by environmental factors, parental IQs and other factors contribute to residual confounding of the results. As the conclusion was based on population studies, it may not be applicable to a single individual.
Introduction
Infants with low birth weight (LBW), very low birth weight (VLBW) and extremely low birth weight (ELBW) are considered to be at a high risk of cognitive dysfunction1–3, such as attention deficit4,5, executive function issues6–8 and low average to borderline intelligence quotient (IQ)1,4,6–8. With the development of perinatal care and neonatal medicine, the survival rates of LBW infants are greatly improved5, followed by an increasing number of LBW individuals with cognitive deficit2,9, which has become a serious public health burden5,10.
Numerous studies have focused on the cognitive outcomes of VLBW individuals in recent decades11. More than 50% of VLBW children required special education services, and approximately 20% of VLBW children repeated at least one grade12. ELBW individuals without major disabilities (mental retardation, cerebral palsy, deafness, or blindness)5 had subtle neurodevelopmental disabilities (language disorders, hyperactivity, behavioural problems, or motor dysfunction, etc.) in the school and teenage years13,14. Evidence from cohort studies in four western countries showed that more than 50% of adolescents with ELBW had learning difficulties (mathematics, writing, reading, or spelling)15,16. The effect of LBW accounted for a 0.4 standard deviation (SD) decrease in math and a 0.25 SD decrement in reading17. Those cognitive disadvantages would lead to low school achievements and persist into early adulthood18–21, thus resulting in low socio-economic status (SES) in the future3.
The IQ score (IQs) is often used to indicate individuals’ cognitive outcomes worldwide22. The IQ is relatively stable and can be easily measured23. Additionally, there are some internationally recognized assessment scales which make it possible to compare the IQs in different populations. The consistent finding was that LBW individuals had lower IQs than those with normal birth weights (NBW)8,9,24. The size of this discrepancy varied across studies, ranging from 3 to 23 points9,25, and the discrepancy was directly proportional to their birth weight20 (R2 = 0.51; P < 0.001)2. Some studies found that a gradient relationship existed, in which lower birth weight was associated with lower IQs1,26. In other words, the ELBW individuals’ IQs were the lowest, followed by those with VLBW and moderately low birth weight (MLBW)27,28. However, most of the previous individual studies were based on a small number of participants, so it was necessary to use meta-analysis to enlarge the sample size and assess the gradient relationship.
A recent meta-analysis containing 15 individual studies on the relationship between LBW and IQs in adolescent and early adulthood (age ≥ 13)29 found that LBW individuals scored an average of 8 IQ points lower than NBW individuals. As is already known, there have been more relevant studies focusing on preschool and school-aged children. We integrated those studies into our meta-analysis to identify the age-related change in IQs between LBW and NBW individuals.
Data from the US Centres for Disease Control showed that 45% of babies born preterm were < 2500 g29. Using 27 eligible individual studies published between 1980–2009, Kerr-Wilson et al.30 performed a meta-analysis on preterm delivery and intelligence, which showed that the preterm children had significantly lower IQs compared with term children. The weighted mean difference (WMD) was 12 [95% confidence interval (CI) 10.47–13.42]. The group’s analysis included duplicated populations (Caldú31, Narberhaus32), and some control groups were used more than once in the model, which may enlarge the weight of some individual studies. Despite the overlap of LBW and prematurity, they may have different relationships with IQs29. To more specifically reflect on the relationship between LBW and IQs, we performed this meta-analysis on LBW and IQs.
In this meta-analysis, we aimed to use 57 eligible individual studies to estimate the pooled discrepancy in IQs between LBW individuals and NBW individuals and the changes in discrepancy across age. We also used subgroup analysis to assess the gradient relationship with IQs for the different levels of LBW.
Results
Search results
The search strategy generated a total of 3,124 potentially relevant papers. After reviewing the title and abstract, 2,548 papers were excluded because of irrelevance. Another 281 articles were also excluded because they were reviews (n = 40) or intervention studies (n = 14). Furthermore, 225 studies focusing on relevant factors for LBW and 2 studies in other than English were also excluded. Thus, we reviewed 295 articles with full text. Among them, 238 were excluded because they did not meet the inclusion criteria. The flow chart for exclusion/inclusion of individual studies is presented in Fig. 1.
Figure 1.
Flow chart of meta-analysis for exclusion/inclusion of individual studies. ∗Deficiency of data cited references63–95.
Characteristics of included studies
There were 57 eligible studies published over 36 years based on our search strategies, four of which13,25,33,34 had two pairs of groups in the study population. Therefore, the meta-analysis included 61 study groups with 6,683 LBW individuals and 5,454 NBW comparisons. The participants included both children and adults, with ages ranging from 4 to 26. These studies were performed in 21 countries, including 18 developed countries, where most of the studies were conducted (n = 53). Forty-four studies used different versions of the Wechsler scale to measure IQ. Five studies used the K-ABC (Kaufman Assessment Battery for Children), whereas three used the Stanford-Binet intelligence scale. The MIQS (McCarthy IQ Scale) and BAS (British Ability Scales) were used in other studies. Most studies (n = 50) were cohort studies, and 7 were case-control studies. The descriptive information of the included studies is shown in Table 1.
Table 1.
Characteristics of included studies of LBW and IQ.
| No. | Study | Country | Years of Birth | LBW(n) | Birth Weight (g) | Gestational Age (week) | Measurement Tool | Age at evaluation | IQ Scores | Study Design | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NBW(n) | mean (SD/range) | Mean (SD)/range | mean (SD) | ||||||||
| 1 | Yi KH et al.96, 2016 | Korea | 2003-2009 | L | 46 | 2110(315) | ≥37 | WISC-III | 12 | 100.52(15.24) | case-control study |
| N | 46 | 3280(460) | ≥37 | 109.52(12.54) | |||||||
| 2 | Serenius et al.10, 2016 | Sweden | 2004–2007 | L | 371 | 779(170) | 25.4(1.07) | WISC-IV | 6.5 | 83.4(14.8) | cohort study |
| N | 367 | 3617(482) | 39.9(1.13) | 100.3(11.7) | |||||||
| 3 | Breeman et al.45, 2015 | German | 1985–1986 | L3 | 216 | 1311(320) | <32 | WISC-III | 26 | 86.2(20.25) | cohort study |
| N3 | 197 | 3371(452) | >37 | 102.6(12.89) | |||||||
| 4 | Molloy et al.97, 2014 | Australia | 1994–1995 | L | 221 | 883(161) | 26.6(2.0) | WASI | 18 | 95.18 (16.33) | cohort study |
| N | 159 | 3394(454) | 39.2(1.4) | 106.46(13.72) | |||||||
| 5 | Ritter et al.8, 2014 | Switzerland | 1998–2003 | L | 52 | <1500 | <32 | WISC (HAWIK-IV) | 101.17(10.34) | cohort study | |
| N | 36 | >2500 | >37 | 10 | 109.28(7.77) | ||||||
| 6 | Guarini et al.25, 2014 | Italy | 1998–2001 | L1 | 56 | 1155(331) | 29.8(2.3) | K-BIT | 6 | 93.4(10.5) | case-control study |
| N1 | 60 | >2800 | >37 | 96.7(11.4) | |||||||
| 1996–1999 | L2 | 84 | 1224(284) | 30.1(2.3) | 8 | 103.1(12.9) | |||||
| N2 | 26 | >2800 | >37 | 106.5(9.4) | |||||||
| 7 | McNicholas et al.98, 2013 | Ireland | 1995–1997 | L | 52 | 1172(219) | 29.9(2.8) | WISC-IV | 11 | 89.7(12.5) | cohort study |
| N | 48 | NA | NA | 101.3(11.7) | |||||||
| 8 | Cheong et al.39, 2013 | Australia | 1991–1992 | L | 148 | 897(177) | 25.8(1.1) | WASI | 18 | 95.7(15.9) | cohort study |
| N | 132 | 3441(457) | 39.3(1.3) | 107.6(12.8) | |||||||
| 9 | Hutchinson et al.99, 2013 | Australia | 1997 | L | 189 | 833(164) | 26.5(2.0) | WISC-IV | 8 | 93.1(16.1) | cohort study |
| N | 173 | 3506(1455) | 39.3(1.1) | 105.6(12.4) | |||||||
| 10 | Lundequist et al.100, 2013 | Sweden | 1988–1993 | L | 145 | 1050(266) | 28.1(2.8) | WPPSI-R | 5 | 95.7(16.1) | cohort study |
| N | 117 | 3493(453) | 39.8(1.2) | 102.3(11.0) | |||||||
| 11 | Aarnoudse-Moens et al.6, 2013 | Netherlands | 1996–2004 | L | 200 | 1013(287) | 28.1(1.4) | WISC-III | 8 | 93.3(15.8) | cohort study |
| N | 230 | 3578(482) | 39.9(1.2) | 105.0(13.4) | |||||||
| 12 | Munck et al.101, 2012 | Finland | 2001–2006 | L | 124 | 1061(260) | 28.7(2.8) | WPPSI-R | 5 | 99.3(17.7) | cohort study |
| N | 168 | 3659(454) | 40.1(1.2) | 111.7(14.5) | |||||||
| 13 | Pyhala et al.19, 2011 | Finland | 1978–1985 | L | 103 | 1140(217) | 29.3(2.3) | WAIS-III | 25 | 102.2(15.3) | cohort study |
| N | 105 | 3609(489) | 40.1(1.2) | 110.6(12.0) | |||||||
| 14 | Potharst et al.22, 2011 | Netherlands | 2002–2004 | L | 104 | 1045(254) | 28.7(1.6) | WPPSI | 5 | 92(17) | cohort study |
| N | 95 | 3436(512) | 39.8(1.7) | 103(11) | |||||||
| 15 | Ni et al.7, 2011 | China | 2002–2003 | L | 37 | 1158(266) | 29.5(2.8) | WISC-IV | 6 | 100.1(10.7) | cohort study |
| N | 22 | 3162(404) | 38.3(1.5) | 103.9(11.1) | |||||||
| 16 | Løhaugen et al.102, 2010 | Norway | 1986–1988 | L | 55 | 1217(233) | 29.1(2.5) | WAIS-III | 19 | 88(13) | cohort study |
| N | 81 | 3707(433) | 39.7(1.2) | 101(12) | |||||||
| 17 | Soria-Pastor et al.40, 2009 | Spain | 1996–1998 | L | 20 | 1794 | 30–34 | WISC-IV | 9 | 105.8(13.8) | case-control study |
| N | 22 | 3392 | 40 | 121.9(15.3) | |||||||
| 18 | Aarnoudse-Moens et al.103, 2009 | Netherlands | 1998–2000 | L | 50 | 1042(31) | 28.0(1.4) | WPPSI-R | 6 | 92.5(17.5) | case-control study |
| N | 50 | 3579(510) | 39.7(1.3) | 109.0(19.2) | |||||||
| 19 | Woodward et al.28, 2009 | New Zealand | 1998–2000 | L1 | 43 | 807(233) | <28 | WPPSI-R | 4 | 93.86(17.57) | cohort study |
| L2 | 62 | 1237(223) | 28–33 | 95.65(13.88) | |||||||
| N | 107 | 3574(409) | 38–41 | 104.70(13.45) | |||||||
| 20 | Mu et al.104, 2008 | China | 1995–1997 | L | 130 | 1165(238) | 29.5(2.7) | WISC-III | 8 | 93.14(16.33) | case-control study |
| N | 59 | 3312(379) | 39.3(1.1) | 111.05(14.81) | |||||||
| 21 | Gaddlin et al.34, 2008 | Sweden | 1987–1988 | L1 | 59 | 1214(212) | 30.7(2.4) | WISC-III | 15 | 84.9(17.5) | cohort study |
| N1 | 57 | 3637(524) | 40.2(1.3) | 97.1(13.3) | |||||||
| L2 | 31 | 1213(191) | 32.0(2.5) | 84.1(19.9) | |||||||
| N2 | 28 | 3477(440) | 39.9(1.0) | 85.7(14.7) | |||||||
| 22 | Allin et al.18, 2008 | UK | 1982–1984 | L | 94 | <2500 | <33 | WASI | 19 | 96.6(13.7) | cohort study |
| N | 44 | NA | 38–42 | 105.3(12.8) | |||||||
| 23 | Saavalainen et al.105, 2007 | Finland | 1984–1986 | L | 35 | 1440(440) | 30(2) | WISC-R | 9 | 96.3(11.3) | cohort study |
| N | 31 | 3427(410) | 40(1.3) | 100.3(10.6) | |||||||
| 24 | Nosarti et al.106, 2007 | UK | 1979–1982 | L | 61 | 1296(295) | 29.5(1.8) | WASI | 22 | 105.14(11.99) | cohort study |
| N | 64 | >2500 | 37–42 | 111.75(10.56) | |||||||
| 25 | Narberhaus et al.32, 2007 | Spain | 1983–1994 | L1 | 9 | 899 | 26.4 | WISC-R or WAIS-III | 14 | 91.4(14.4) | case-control study |
| L2 | 19 | 1140 | 29 | 100.5(16.2) | |||||||
| L3 | 25 | 1534 | 31.7 | 103.2(15.7) | |||||||
| L4 | 11 | 2445 | 34.6 | 112.7(13.8) | |||||||
| N | 53 | 3416 | 39.6 | 113.6(11.5) | |||||||
| 26 | Hoff et al.107, 2006 | Denmark | 1994–1995 | L | 191 | 922(167) | 27.5(1.8) | WPPSI-R | 5 | 96.4(14.1) | cohort study |
| N | 76 | 3530(518) | 40.1(1.2) | 107.3(11.4) | |||||||
| 27 | Martinez-Cruz et al.27, 2006 | Mexico | 1997 | L1 | 25 | 875(107) | 31.4(1.7) | Stanford-Binet | 6 | 95.3(11.3) | case-control study |
| L2 | 52 | 1297(130) | 32.5(1.2) | 103.1(14.4) | |||||||
| L3 | 66 | 1940(247) | 33.6(1.8) | 105.1(12.3) | |||||||
| N | 41 | 3239(410) | 39.6(1.8) | 106.8(11.7) | |||||||
| 28 | Hack et al.24, 2005 | USA | 1992–1995 | L | 219 | 810(124) | 26.4(2) | KABC | 8 | 87.8(18) | cohort study |
| N | 176 | 3300(513) | ≥37 | 99.8(15) | |||||||
| 29 | Lefebvre et al.21, 2005 | France | 1976–1981 | L | 69 | 912(79) | 28.5(2.4) | WAIS | 18 | 94(12) | cohort study |
| N | 44 | 3419(418) | 39.8(1.1) | 108(14) | |||||||
| 30 | Marlow et al.11, 2005 | UK | 1995 | L | 241 | <2500 | <26 | K-ABC | 6 | 82.1(19.2) | cohort study |
| N | 160 | NA | >37 | 105.7(11.8) | |||||||
| 31 | Kilbride et al.108, 2004 | USA | 1983–1990 | L | 25 | 702(76) | 26.0(1.6) | Stanford-Binet | 4 | 85(12) | cohort study |
| N | 25 | 3215(509) | 38.8(1.5) | 95(11) | |||||||
| 32 | Short et al.109, 2003 | USA | 1989–1991 | L | 75 | 1256(176) | 30(2) | WISC-III | 8 | 91.7(16) | cohort study |
| N | 99 | 3451(547) | 40(1) | 101.9(15) | |||||||
| 33 | Cooke et al.110, 2003 | UK | 1991–1992 | L | 268 | 1467 | 29.8 | WISC-III | 7 | 89.4(14.2) | cohort study |
| N | 198 | NA | NA | 100.5(13.7) | |||||||
| 34 | Grunau et al.111, 2002 | Canada | 1982–1987 | L | 74 | 718(480–800) | 26.0(23–33) | WISC-R | 9 | 99.3(10.9) | cohort study |
| N | 30 | 3540(2948–4706) | 40.0(28–40) | 117.3(13.0) | |||||||
| 35 | Magill-Evans et al.112, 2002 | Canada | NA | L | 20 | 2104 | <37 | WISC-III | 10 | 98(14.9) | cohort study |
| N | 23 | 3515 | NA | 101.5(11.9) | |||||||
| 36 | Breslau et al.17, 2001 | USA | 1983–1985 | L1 | 231 | <2500 | NA | WISC-R | 11 | 88.1(14.7) | cohort study |
| N1 | 143 | ≥2500 | 94.1(13.6) | ||||||||
| L2 | 180 | <2500 | 107.8(14.8) | ||||||||
| N2 | 163 | ≥2500 | 112.8(14.3) | ||||||||
| 37 | Rickards et al.113, 2001 | Australia | 1980–1982 | L | 120 | 1167(215) | 29.3(2.0) | WISC-III | 14 | 96.2(15.5) | cohort study |
| N | 41 | 3417(432) | 39.9(1.0) | 105.0(13.3) | |||||||
| 38 | Nadeau et al.114, 2001 | Canada | 1987–1990 | L | 61 | 1024(204) | 27.4(1.1) | MIQS | 5 | 100.3(19.1) | cohort study |
| N | 44 | 3453(498) | 39.8(1.6) | 112.8(16.2) | |||||||
| 39 | Taylor et al.115, 2000 | USA | 1982–1986 | L1 | 60 | 665(68) | 25.7(1.8) | KABC | 11 | 83.49(19.7) | cohort study |
| L2 | 55 | 1173(217) | 29.4(2.4) | 96.81(14.4) | |||||||
| N | 49 | 3300(660) | 40 | 106.24(14.3) | |||||||
| 40 | Tandon et al.33, 2000 | India | 1985–1995 | L1 | 27 | 1810(248) | 36.2(2.9) | Stanford-Binet | 8 | 105.6(13.4) | cohort study |
| N1 | 28 | 2850(363) | 39.2(1.2) | 116(11.6) | |||||||
| L2 | 32 | 1740(195) | 36(2.5) | 99.6(11.8) | |||||||
| N2 | 29 | 2850(331) | 39.8(1.3) | 11 | 110.6(7.3) | ||||||
| 41 | Saigal et al.116, 2000 | Canada | 1977–1982 | L | 150 | 833(126) | 27(2.4) | WISC-R | 14 | 89(19) | cohort study |
| N | 124 | 3395(483) | 40 | 102(13) | |||||||
| 42 | Hughes et al.117, 1999 | USA | 1979–1981 | L1 | 95 | 964(208) | 28.5(2.1) | WISC-R | 9 | 86.16(17.67) | cohort study |
| L2 | 311 | 1157(272) | 30.6(2.3) | 95.56(17.63) | |||||||
| N | 188 | 2776(707) | 39.4(1.7) | 99.79(16.51) | |||||||
| 43 | Stjernqvist et al.118, 1999 | Sweden | 1985–1986 | L | 61 | 1042(242) | 27.1(1.03) | WISC-III-R | 10 | 89.8(15.1) | cohort study |
| N | 61 | 3648(533) | 40.1(1.43) | 106.5(15.0) | |||||||
| 44 | Botting et al.119, 1998 | UK | 1980–1983 | L | 138 | <1500 | NA | WISC-III | 12 | 89.7(17.2) | cohort study |
| N | 163 | >2500 | >37 | 97.8(17.4) | |||||||
| 45 | Whitfield et al.120, 1997 | Canada | 1974–1985 | L | 90 | 731(520–800) | 26(23–28) | WISC or Stanford-Binet | 9 | 98.7(12.6) | cohort study |
| N | 50 | 3488(2614–4706) | 40(38–42) | 111.6(13.1) | |||||||
| 46 | Rose et al.121, 1996 | USA | 1979–1981 | L | 50 | 1154(233) | 31.2(1.8) | WISC-R | 11 | 89.6(11.3) | cohort study |
| N | 40 | NA | NA | 98.9(11.9) | |||||||
| 47 | Sommerfelt et al.46, 1995 | Norway | 1986–1988 | L | 144 | 1555(368) | 32(3) | WPPSI-R | 5 | 97(14) | cohort study |
| N | 163 | >3000 | 40 | 104(14) | |||||||
| 48 | Levy-Shiff et al.61, 1994 | Israel | NA | L | 90 | 1190(209) | 29(2.3) | WISC-R | 7 | 105.1(10.5) | cohort study |
| NA | N | 90 | 3225(334) | 39(1.2) | 114.4(9.8) | ||||||
| 49 | Sommerfelt et al.14, 1993 | Norway | 1981–1982 | L | 29 | 1251(166) | 31.1(2.6) | WISC-R | 8 | 93.2(16) | cohort study |
| N | 29 | 3650(490) | 40(0) | 104.2(14) | |||||||
| 50 | Hack et al.122, 1992 | USA | 1977–1979 | L | 249 | 1176 | 29.2 | WISC-R | 8 | 95.7(18) | cohort study |
| N | 363 | >2500 | >37 | 100.6(17.6) | |||||||
| 51 | Teplin et al.123, 1991 | USA | 1980 | L | 28 | 905(86) | 28(1.5) | KABC | 6 | 86.3(13.6) | cohort study |
| N | 26 | NA | >37 | 98.7(14.3) | |||||||
| 52 | Smith et al.124, 1990 | UK | 1981 | L | 43 | 1306(164) | > 28 | MIQS | 5 | 88.56(16.94) | cohort study |
| N | 43 | 3342(429) | >37 | 101(13.04) | |||||||
| 53 | McDonald et al.125, 1989 | USA | NA | L | 16 | 1776(510) | 31.4(3) | WPPSI | 5 | 113(21) | cohort study |
| N | 18 | 3359(481) | 40 | 124(13) | |||||||
| 54 | Klein et al.126, 1989 | USA | 1976 | L | 65 | 1190(197) | 30(2) | WISC-R | 9 | 92(14) | cohort study |
| N | 65 | >3000 | >37 | 98(16) | |||||||
| 55 | Portnoy et al.127, 1988 | UK | 1980–1981 | L | 15 | 909 | NA | MIQS | 5 | 93(20) | cohort study |
| N | 15 | >2500 | 38–42 | 103(13) | |||||||
| 56 | Lloyd BW et al.128, 1988 | UK | 1978–79 | L | 44 | 1302 | 26–37 | BAS | 7 | 93.1(15) | cohort study |
| N | 44 | 40 | 100.4(12.9) | ||||||||
| 57 | Kitchen WH et al.129, 1980 | Australia | 1966–1970 | L1 | 10 | <1000 | NA | WISC-R | 8 | 79.4(15.7) | cohort study |
| L2 | 143 | <1501 | NA | 89.4(15.7) | |||||||
| N | 43 | >2500 | NA | 98.8(15.7) | |||||||
LBW: low birth weight; NBW: normal birth weight; NA: not available; L: LBW; N: NBW; WISC-III: Wechsler Intelligence Scale for Children, Third Edition; WISC-R: Wechsler Intelligence Scale for Children, Revised; WISC-IV: Wechsler Intelligence Scale for Children, Fourth Edition; WPPSI: Wechsler Preschool and Primary Scales of Intelligence Test; WPPSI-R: Wechsler Preschool and Primary scale of intelligence, Revised; WASI: Wechsler Abbreviated Scales of Intelligence; WAIS-III: Wechsler Adult Intelligence Scale; KABC, Kaufman Assessment Battery for Children; K-Bit: Kauf-man Brief Intelligence Test, Italian version; MIQS: McCarthy IQ Scale; BAS: British Abilities Scale.
Overall analysis
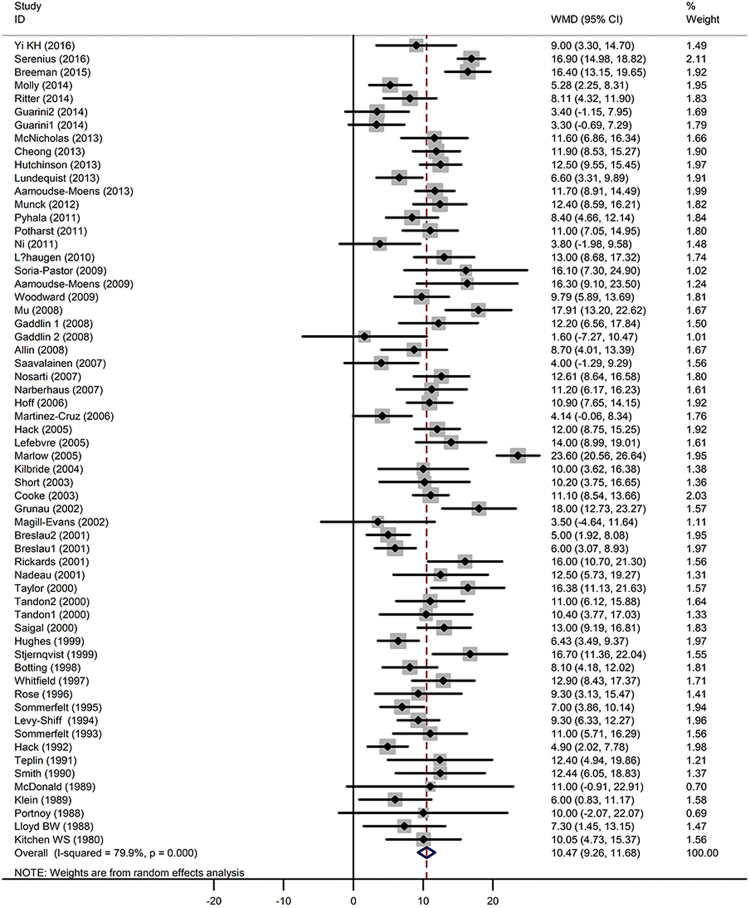
All studies revealed that the LBW individuals had lower IQs compared with the NBW group. The pooled WMD was 10 (Z = 17.12, P < 0.001), with a 95% CI of 9.26–11.68, which means that the LBW individuals’ IQs were significantly lower than those of the NBW controls (Fig. 2). Between-study heterogeneity was detected [Q = 298.79 (P < 0.001) and I2 = 79.9% (P < 0.001)]. The mean IQs of the ELBW, VLBW, LBW and NBW individuals were 91, 94, 99 and 104, respectively. A gradient relationship was observed between birth weight and IQ.
Figure 2.
Random-effect analysis of the association between low birth weight and IQs. WMD: weight mean difference; CI:confidence interval.
Sensitivity analysis and publication bias
After excluding one study at a time, the sensitivity analysis confirmed the significant association between LBW and IQs (with 95% CI ranging from 0.68 to 0.76) (Figure S1). No publication bias was detected (Begg’s test: P = 0.49 and Egger’s test: P = 0.50). Figure 3 shows a basic funnel plot depicting potential bias.
Figure 3.
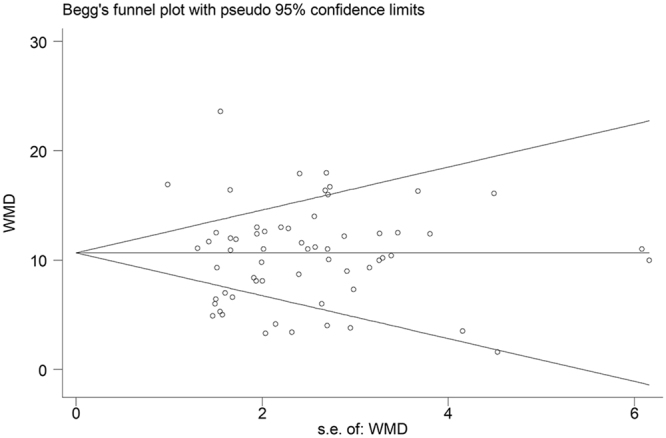
Begg’s funnel plot of individual studies included in the analysis according to random-effect WMD estimates.
Sources of heterogeneity
We used meta-regression models to probe the source of heterogeneity. The variables included sample size, birth year, age of assessment, and the birth weight of LBW individuals. The model showed that the birth weight of the LBW participants was associated with an IQ difference between NBW and LBW individuals (coefficient = −0.005, adjusted R2 = 13.22%, P =0.003). Other variables did not reach the significance level (Table S1). Low birth weight contributed to 30.5% of the heterogeneity after further analysis, with T2 reduced from 17.10 to 11.88. Figure 4 shows the meta-regression model of the effect of low birth weight on IQ. The results from the Galbraith plot (Figure S2) indicated that two populations (Serenius10, Marlow11) with the highest WMD may be the main cause of high heterogeneity. After excluding these two studies, the adjusted pooled WMD was 10 (95% CI 9.02–11.03, I2 = 67.4%, P < 0.01). Approximately 15.6% of the heterogeneity was attributable to these two studies.
Figure 4.
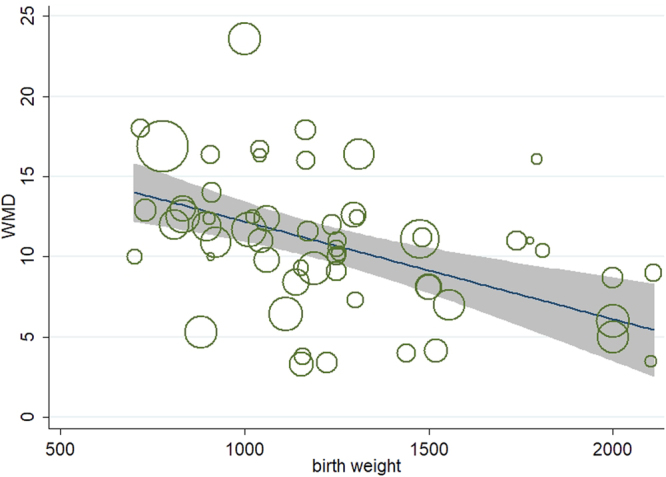
Meta-regression of birth weight on IQs difference between NBW and LBW individuals.
Subgroup analysis
We performed subgroup analysis to examine whether a gradient relationship existed between different LBW levels and IQs. As shown in Table 2, the WMD was 7 (95% CI 4.76–8.89), 10 (95% CI 8.43–11.28), and 14 (95% CI 11.71–16.20) for MLBW, VLBW and ELBW, respectively (Figures S3–S5). To identify age-related changes in IQs between LBW and NBW individuals, all studies were divided into three groups, i.e., under 10 years, 10–18 years, and over 18 years. The WMD was 11 (95% CI 8.87–12.30), 10 (95% CI 7.88–11.75), and 11 (95% CI 8.42–11.68), respectively. Thus, the discrepancy was stable regardless of age (Table 2; Figure S6).
Table 2.
Subgroups analysis based on birth weight and age at assessment.
| No. of study group | WMD | 95% CI | Z test P value | I 2 (%) | Q test P value | Egg’s test P value | |
|---|---|---|---|---|---|---|---|
| birth weight | |||||||
| <1000 | 20 | 13.95 | 11.71–16.20 | <0.001 | 81.7 | <0.001 | 0.739 |
| 1000–1499 | 35 | 9.85 | 8.43–11.28 | <0.001 | 70.6 | <0.001 | 0.764 |
| 1500–2499 | 11 | 6.83 | 4.76–8.89 | <0.001 | 43.7 | 0.06 | 0.382 |
| age | |||||||
| <10 | 36 | 10.58 | 8.87–12.30 | <0.001 | 83.5 | <0.001 | 0.278 |
| 10~18 | 17 | 9.82 | 7.88–11.75 | <0.001 | 66.4 | <0.001 | 0.151 |
| ≥18 | 8 | 11.28 | 8.42–11.68 | <0.001 | 76.8 | <0.001 | 0.588 |
| social determinants | |||||||
| matched | 39 | 9.90 | 8.42–11.39 | <0.001 | 76.8 | <0.001 | 0.172 |
| non-matched | 22 | 11.42 | 9.31–13.53 | <0.001 | 84.4 | <0.001 | 0.409 |
Another subgroup meta-analysis was based on social determinants of health. The LBW and NBW groups were matched by social determinants of health in 39 individual studies, whereas other studies had different social determinant distributions for the two groups. The results showed that the WMDs between NBW and LBW individuals were 10 (95% CI 8.42–11.39) and 11 (95% CI 9.31–13.53) for social determinants between matched groups and non-matched groups (Figure S7), respectively. Therefore, approximately 13% of the IQ discrepancy was due to social determinants of health.
Discussion
Our study supported the evidence that individuals’ low birth weight had a negative association with IQ4,29. The lower birth weight categories had lower IQs on average. The average IQs of ELBW individuals were the lowest, followed by VLBW individuals and those with MLBW. Specifically, low birth weight individuals had approximately 10–11 points lower IQs than NBW individuals from childhood to adulthood (4–26 in age). There was a gradient relationship between low birth weight and the discrepancy in IQs between LBW and NBW individuals, with the WMDs from large to small being 14 (ELBW), 10 (VLBW), and 7 (MLBW). In addition, social determinants of health were associated with individuals’ IQs, which explained approximately 13% of the IQ difference between LBW and NBW individuals.
The gradient relationship obviously depicted the IQ gap between individuals with different levels of LBW and those with NBW. The M LBW infants were closer to preterm (<37 weeks)29, while the VLBW and ELBW infants tended to be less than 32 weeks in gestational age3. Because of the high degree of immaturity of respiratory organs and the nervous system, they were susceptible to bronchopulmonary dysplasia35, neonatal brain injury (cerebral palsy, periventricular leukomalacia, hydrocephalus, hypoxic-ischaemic encephalopathy)9,35–37, and other medical complications, which may result in cognitive impairment. Additionally, children born with low birth weight had less connected and less complex brain networks38, smaller brain volumes39–41, and less cortical surface area42 compared with NBW children. The different degree of neonatal immaturity in LBW infants is considered to be associated with cognitive outcomes34,43.
We also found a stable difference in IQs between LBW individuals and NBW individuals. The discrepancy was approximately 10–11 points regardless of the age of assessment. This finding was inconsistent with previous reports that showed that the discrepancy would decrease over time29. Some LBW individuals may have cognitive catch-up growth44, but it is not a universal rule among those with LBW. A long-term follow-up study on a population sample aged from 5 months to 26 years showed that the IQs were more stable in very preterm (VP)/VLBW individuals than in term-born individuals across all time points45. However, this conclusion was based on the entire LBW group and may not be applicable to a single individual.
Social determinants of health, such as social class, parental/maternal education and occupation, marital status, etc., are known to contribute to suboptimal cognitive development of LBW children. Previous studies have indicated that LBW continues to be associated with cognitive disadvantage at each SES level21 and that the risk of impaired cognitive development increases with decreasing SES46. A study by Sommerfelt et al. reported that 23% of the variance in child’s IQ at age 5 could be attributed to parental and family variables in Norway. Our results showed that social determinants of health explained approximately 13% of the lower IQ values. Because of the diversity of social determinants in different societies and the variations in study design, the common practice of simply matching social determinants of health (social class, occupation, parental/maternal education) may result in an underestimation of cognitive impairment caused by social determinants of health or other similar risk factors.
Intelligence is a product of genetic and environmental variables47.Genetic variation is the main cause of individual differences in IQ48. Previous studies have reported that the “heritability” (h 2) for IQ ranges from 20% in infancy, to 40–50% by late adolescence and to 60–80% in adulthood49. Environmental factors, such as perinatal factors50, schooling, family environment, nutrition and so on49 also contribute to individuals’ IQs. The aetiology of LBW individuals’ lower IQs is complex and unclear. Various adversities occur among LBW infants, such as preterm birth, the stress of intensive care and more frequent morbidities, which may also affect individuals’ IQs. It may be that low birth weight is an event along this causal pathway. However, two cohort studies from Denmark51 and Estonia52 demonstrated the associations between birth weight and IQs, and the associations remained significant after controlling for a wide range of confounders. These correlations were modest, ranging from 0.05 to 0.1352,53.
As poor cognitive outcomes may be related to lower school achievements12,54, inferior SES5, an unhealthy lifestyle47,55,5, and even some chronic diseases56, improving the cognitive outcome of LBW infants is essential and urgent. Previous evidence showed that the LBW individuals can benefit from early interventions for cognitive outcomes3,9. Some randomized controlled trials, such as the Newborn Individualized Developmental Care and Assessment Program (NIDCAP)57 and a sensitizing parental intervention programme58, showed that breastfeeding59 and kangaroo care had beneficial effects on LBW infants’ cognitive outcomes. It is recommended to assess the cognitive ability of LBW individuals first in order to determine the need for interventions. Periodic cognitive assessment of LBW children can evaluate the intervention’s effectiveness, thus providing more accurate interventions for each individual60. The cognitive benefits from early intervention may persist into preschool age or adolescence58. Therefore, long-term interventions may play a role in the long run. Although there were few long-term intervention programmes reported, it is necessary for child care centres and parents to offer long-term neuropsychological rehabilitation to LBW individuals even if they do not suffer from severe cognitive disabilities.
Strengths and limitations
Strengths
Compared with previous meta-analyses of LBW/preterm individuals’ IQs, we included more eligible and recent individual studies, with a total of 12,137 participants and without a duplicated study population. We conducted subgroup analyses to show the gradient in the IQ gap between individuals with different levels of LBW and those with NBW, as well as the stability of the difference in IQs between LBW and NBW individuals. Although the selected studies used different cognitive tests to measure individuals’ IQs, each test/scale had similar normative data (mean = 100; SD = 15), which made the results from different studies comparable.
Limitations
We tried to include all relevant studies, but some studies may be missed in this meta-analysis due to our search strategies or incomplete databases. Additionally, grey literature publications were not included. However, the large sample size of this study made the results more stable and credible.
According to individual studies, parental/maternal education was either a variable of socio-economic status or an independent social determinant. Three individual studies only matched by parental/maternal education were also included in the social determinant-matched group. Since there is not a perfect fit between education and socio-economic status, residual confounding may exist in the subgroup analysis based on social determinants of health.
IQ is a complex trait that is influenced by genetic and environmental factors, such as parental IQs17,45, medical complications61, early home environment, schooling, and so on36,60,62. We didn’t take this residual confounding into account. These factors may also contribute to the heterogeneity. The association between these factors and LBW IQs will be explored in a further study.
Methods
Literature and search strategy
We searched the PubMed and the Embase databases for full-text articles in English published between January 1980 and November 2016. The following terms were used to perform the literature search: “low birth weight” or “preterm” or “premature”, and “intelligent quotient” or “IQ” or “cogni*” or “neuro*” or “mental” or “psycho*” or “outcome”.
Inclusion criteria
Each study should meet all of the inclusion criteria.
Participants with LBW (< 2500 g) were compared with those with NBW (≥ 2500 g).
The individuals’ ages were ≥ 4 years.
Full-scale IQ was measured by a standardized and global scale with the mean and standard deviation of the IQs listed.
Full-text articles were available from the two databases.
We excluded reviews, studies of the non-LBW group, and those without NBW individuals as a control group. If more than one study was based on the same cohort, only the study with the larger sample was included in the meta-analysis. When the study had two or more LBW groups, we calculated the weighted mean and deviation to represent the LBW individuals’ IQs in the meta-analysis (Figure S8). For the subgroup analysis, we used the raw data from each study.
Data extraction
The following information was extracted from each study:
(1) first author’s name; (2) year of publication; (3) country of origin; (4) birth year of the participants; (5) size of study population; (6) birth weight; (7) gestational age; (8) measurement tools; (9) age at assessment; and (10) mean and standard deviation of the IQs.
Statistical analysis
A random-effects meta-analysis was performed using the WMD in IQs between LBW and NBW individuals. The significance of the WMD was determined using a Z test (P < 0.05 was considered statistically significant). To assess the heterogeneity, we consulted the Cochrane Q test and I2 statistics. Publication bias was assessed by Begg’s test and Egger’s test. We also used a funnel plot to depict the potential publication bias. We constructed meta-regression models and a Galbraith radial plot to probe the source of heterogeneity.
The subgroup analysis was conducted based on birth weight, age at assessment and social determinants of health. In the first subgroup analysis, we divided the studies into three subgroups according to the LBW participants’ birth weight, i.e., moderately low birth weight (MLBW, 1500–2499 g), very low birth weight (VLBW, 1000–1499 g) and extremely low birth weight (ELBW, <1000 g). Then, we grouped individual studies into three groups by the subjects’ age at assessment (under 10 years, 10 to 18 years, 18 years or older) in the second subgroup analysis. Because social determinants of health are associated with individuals’ IQs, we compared the social determinant-matched group with the social determinant-unmatched group to evaluate how much of the lower IQ values were due to social determinants of health. All analyses were conducted using STATA version 11 (Stata Corp LP, College Station, Texas, USA).
Conclusion
Individuals with LBW had lower IQs compared to those with NBW, and the discrepancy was approximately 10–11 points from childhood to adulthood (4–26 in age). We also demonstrated a gradient relationship between different levels of LBW and IQs. The social determinants of health explained approximately 13% of the IQ difference. These findings contribute to our understanding of the association between LBW and IQs. Our results will help physicians and parents to pay more attention to regular cognitive assessment and early intervention, as well as to long-term neuropsychological rehabilitation for LBW infants.
Electronic supplementary material
Acknowledgements
This research was supported by National Natural Science Foundation of China (81673194) to S.R. and Project from Health and Family Planning Commission of Hubei Province (WJ2015MB019).
Author Contributions
G.H. and S.R. designed the research; G.H and W.L. wrote the paper; G.H., W.L., L.L., H.F., L.X., W.J., M.H., and D. P. reviewed and extracted data from eligible studies; Z.J. and W.L. performed the statistical analysis; L.J. and L.G. prepared the tables and figures. All authors have reviewed and approved the manuscript as submitted.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Huaiting Gu and Lixia Wang contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-18234-9.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dombrowski N, Martin Low birth weight and cognitive outcomes: Evidence for a gradient relationship in an urban, poor, African American birth cohort. Sch Psychol Q. 2007;22:26–43. doi: 10.1037/1045-3830.22.1.26. [DOI] [Google Scholar]
- 2.Doyle LW, Victorian Infant Collaborative Study G. Changing availability of neonatal intensive care for extremely low birthweight infants in Victoria over two decades. Med J Aust. 2004;181:136–139. doi: 10.5694/j.1326-5377.2004.tb06203.x. [DOI] [PubMed] [Google Scholar]
- 3.Spittle, A., Orton, J., Anderson, P. J., Boyd, R. & Doyle, L. W. Early developmental intervention programmes provided post hospital discharge to prevent motor and cognitive impairment in preterm infants. Cochrane Database Syst Rev., CD005495, 10.1002/14651858.CD005495.pub4 (2015). [DOI] [PMC free article] [PubMed]
- 4.Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJ. Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. Jama. 2002;288:728–737. doi: 10.1001/jama.288.6.728. [DOI] [PubMed] [Google Scholar]
- 5.Moster D, Lie RT, Markestad T. Long-term medical and social consequences of preterm birth. N Engl J Med. 2008;359:262–273. doi: 10.1056/NEJMoa0706475. [DOI] [PubMed] [Google Scholar]
- 6.Aarnoudse-Moens CS, Weisglas-Kuperus N, Duivenvoorden HJ, van Goudoever JB, Oosterlaan J. Executive function and IQ predict mathematical and attention problems in very preterm children. PloS one. 2013;8:e55994. doi: 10.1371/journal.pone.0055994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ni TL, Huang CC, Guo NW. Executive function deficit in preschool children born very low birth weight with normal early development. Early Hum Dev. 2011;87:137–141. doi: 10.1016/j.earlhumdev.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 8.Ritter BC, Perrig W, Steinlin M, Everts R. Cognitive and behavioral aspects of executive functions in children born very preterm. Child Neuropsychol. 2014;20:129–144. doi: 10.1080/09297049.2013.773968. [DOI] [PubMed] [Google Scholar]
- 9.Jarjour IT. Neurodevelopmental outcome after extreme prematurity: a review of the literature. Pediatr Neurol. 2015;52:143–152. doi: 10.1016/j.pediatrneurol.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 10.Serenius F, et al. Neurodevelopmental Outcomes Among Extremely Preterm Infants 6.5 Years After Active Perinatal Care in Sweden. JAMA pediatrics. 2016;170:954–963. doi: 10.1001/jamapediatrics.2016.1210. [DOI] [PubMed] [Google Scholar]
- 11.Marlow N, Wolke D, Bracewell MA, Samara M, Group EPS. Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med. 2005;352:9–19. doi: 10.1056/NEJMoa041367. [DOI] [PubMed] [Google Scholar]
- 12.Saigal S, et al. School-Age Outcomes in Children Who Were Extremely Low Birth Weight From Four International Population-Based Cohorts. Pediatrics. 2003;112:943–950. doi: 10.1542/peds.112.4.943. [DOI] [PubMed] [Google Scholar]
- 13.Lorenz JM. The Outcomeof Extreme Prematurity. Semin Perinatol. 2001;25:348–359. doi: 10.1053/sper.2001.27164. [DOI] [PubMed] [Google Scholar]
- 14.Sommerfelt K, Ellertsen B, Markestad T. Personality and behaviour in eight-year-old, non-handicapped children with birth weight under 1500 g. Acta Paediatr. 1993;82:723–728. doi: 10.1111/j.1651-2227.1993.tb12546.x. [DOI] [PubMed] [Google Scholar]
- 15.Aylward GP. Update on neurodevelopmental outcomes of infants born prematurely. J Dev Behav Pediatr. 2014;35:392–393. doi: 10.1097/DBP.0000000000000075. [DOI] [PubMed] [Google Scholar]
- 16.Aarnoudse-Moens CS, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. 2009;124:717–728. doi: 10.1542/peds.2008-2816. [DOI] [PubMed] [Google Scholar]
- 17.Breslau N, et al. Stability and change in children’s intelligence quotient scores: a comparison of two socioeconomically disparate communities. Am J Epidemiol. 2001;154:711–717. doi: 10.1093/aje/154.8.711. [DOI] [PubMed] [Google Scholar]
- 18.Allin M, et al. Cognitive maturation in preterm and term born adolescents. J Neurol Neurosurg Psychiatry. 2008;79:381–386. doi: 10.1136/jnnp.2006.110858. [DOI] [PubMed] [Google Scholar]
- 19.Pyhala R, et al. Neurocognitive abilities in young adults with very low birth weight. Neurology. 2011;77:2052–2060. doi: 10.1212/WNL.0b013e31823b473e. [DOI] [PubMed] [Google Scholar]
- 20.Hack M, et al. Outcomes in young adulthood for very-low-birth-weight infants. N Engl J Med. 2002;346:149–157. doi: 10.1056/NEJMoa010856. [DOI] [PubMed] [Google Scholar]
- 21.Lefebvre F, Mazurier E, Tessier R. Cognitive and educational outcomes in early adulthood for infants weighing 1000 grams or less at birth. Acta Paediatr. 2005;94:733–740. doi: 10.1080/08035250510025987. [DOI] [PubMed] [Google Scholar]
- 22.Potharst ES, et al. High incidence of multi-domain disabilities in very preterm children at five years of age. J Pediatr.. 2011;159:79–85. doi: 10.1016/j.jpeds.2010.12.055. [DOI] [PubMed] [Google Scholar]
- 23.Mortensen EL, Andresen J, Kruuse E, Sanders SA, Reinisch JM. IQ stability: the relation between child and young adult intelligence test scores in low-birthweight samples. Scand J Psychol. 2003;44:395–398. doi: 10.1111/1467-9450.00359. [DOI] [PubMed] [Google Scholar]
- 24.Hack M, et al. Chronic conditions, functional limitations, and special health care needs of school-aged children born with extremely low-birth-weight in the 1990s. Jama. 2005;294:318–325. doi: 10.1001/jama.294.3.318. [DOI] [PubMed] [Google Scholar]
- 25.Guarini A, et al. Basic numerical processes in very preterm children: a critical transition from preschool to school age. Early Hum Dev. 2014;90:103–111. doi: 10.1016/j.earlhumdev.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Breslau N, et al. A gradient relationship between low birth weight and IQ at age 6 years. Arch Pediatr Adolesc Med. 1994;148:377–383. doi: 10.1001/archpedi.1994.02170040043007. [DOI] [PubMed] [Google Scholar]
- 27.Martinez-Cruz CF, Poblano A, Fernandez-Carrocera LA, Jimenez-Quiroz R, Tuyu-Torres N. Association between intelligence quotient scores and extremely low birth weight in school-age children. Arch Med Res. 2006;37:639–645. doi: 10.1016/j.arcmed.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Woodward LJ, et al. Very preterm children show impairments across multiple neurodevelopmental domains by age 4 years. Arch Dis Child Fetal Neonatal Ed. 2009;94:F339–344. doi: 10.1136/adc.2008.146282. [DOI] [PubMed] [Google Scholar]
- 29.Kormos CE, Wilkinson AJ, Davey CJ, Cunningham AJ. Low birth weight and intelligence in adolescence and early adulthood: a meta-analysis. J Public Health (Oxf). 2014;36:213–224. doi: 10.1093/pubmed/fdt071. [DOI] [PubMed] [Google Scholar]
- 30.Kerr-Wilson CO, Mackay DF, Smith GC, Pell JP. Meta-analysis of the association between preterm delivery and intelligence. J Public Health (Oxf). 2012;34:209–216. doi: 10.1093/pubmed/fdr024. [DOI] [PubMed] [Google Scholar]
- 31.Caldú X, Junqué NA, Giménez C, Vendrell M, Bargalló P. N. Corpus Callosum Size and Neuropsychologic Impairment in Adolescents Who Were Born Preterm. J Child Neurol. 2006;21:406–410. doi: 10.1177/08830738060210050801. [DOI] [PubMed] [Google Scholar]
- 32.Narberhaus A, et al. Gestational age at preterm birth in relation to corpus callosum and general cognitive outcome in adolescents. J Child Neurol. 2007;22:761–765. doi: 10.1177/0883073807304006. [DOI] [PubMed] [Google Scholar]
- 33.Tandon A, et al. Intellectual psycho-educational and functional status of low birth weight survivors beyond 5 years of age. Indian J Pediatr. 2000;67:791–796. doi: 10.1007/BF02726220. [DOI] [PubMed] [Google Scholar]
- 34.Gaddlin PO, Finnstrom O, Wang C, Leijon I. A fifteen-year follow-up of neurological conditions in VLBW children without overt disability: Relation to gender, neonatal risk factors, and end stage MRI findings. Early Hum Dev. 2008;84:343–349. doi: 10.1016/j.earlhumdev.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 35.Patel RM. Short- and Long-Term Outcomes for Extremely Preterm Infants. Am J Perinatol. 2016;33:318–328. doi: 10.1055/s-0035-1571202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krageloh-Mann I, Cans C. Cerebral palsy update. Brain Dev. 2009;31:537–544. doi: 10.1016/j.braindev.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 37.Sansavini A, Guarini A, Caselli MC. Preterm birth: neuropsychological profiles and atypical developmental pathways. Dev Disabil Res Rev. 2011;17:102–113. doi: 10.1002/ddrr.1105. [DOI] [PubMed] [Google Scholar]
- 38.Thompson DK, et al. Structural connectivity relates to perinatal factors and functional impairment at 7years in children born very preterm. NeuroImage. 2016;134:328–337. doi: 10.1016/j.neuroimage.2016.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheong JL, et al. Contribution of brain size to IQ and educational underperformance in extremely preterm adolescents. PloS one. 2013;8:e77475. doi: 10.1371/journal.pone.0077475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soria-Pastor S, et al. Decreased regional brain volume and cognitive impairment in preterm children at low risk. Pediatrics. 2009;124:e1161–1170. doi: 10.1542/peds.2009-0244. [DOI] [PubMed] [Google Scholar]
- 41.Allin M, et al. Cognitive and motor function and the size of the cerebellum in adolescents born very pre-term. Brain. 2001;124:60–66. doi: 10.1093/brain/124.1.60. [DOI] [PubMed] [Google Scholar]
- 42.Rimol LM, et al. Cortical trajectories during adolescence in preterm born teenagers with very low birthweight. Cortex. 2016;75:120–131. doi: 10.1016/j.cortex.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 43.Larroque B, et al. Neurodevelopmental disabilities and special care of 5-year-old children born before 33 weeks of gestation (the EPIPAGE study): a longitudinal cohort study. The Lancet. 2008;371:813–820. doi: 10.1016/S0140-6736(08)60380-3. [DOI] [PubMed] [Google Scholar]
- 44.Luu TM, Vohr BR, Allan W, Schneider KC, Ment LR. Evidence for catch-up in cognition and receptive vocabulary among adolescents born very preterm. Pediatrics. 2011;128:313–322. doi: 10.1542/peds.2010-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Breeman LD, Jaekel J, Baumann N, Bartmann P, Wolke D. Preterm Cognitive Function Into Adulthood. Pediatrics. 2015;136:415–423. doi: 10.1542/peds.2015-0608. [DOI] [PubMed] [Google Scholar]
- 46.Sommerfelt K, Ellertsen B, Markestad T. Parental factors in cognitive outcome of non-handicapped low birthweight infants. Arch Dis Child Fetal Neonatal Ed. 1995;73:F135–142. doi: 10.1136/fn.73.3.F135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shenkin SD, Starr JM, Deary IJ. Birth weight and cognitive ability in childhood: a systematic review. Psychol Bull. 2004;130:989–1013. doi: 10.1037/0033-2909.130.6.989. [DOI] [PubMed] [Google Scholar]
- 48.Franic S, et al. Genetic and environmental stability of intelligence in childhood and adolescence. Twin Res Hum Genet. 2014;17:151–163. doi: 10.1017/thg.2014.26. [DOI] [PubMed] [Google Scholar]
- 49.Neisser U, et al. Intelligence: Knowns and Unknowns. Am Psychol. 1996;51:77–101. doi: 10.1037/0003-066X.51.2.77. [DOI] [Google Scholar]
- 50.Liu L, et al. Descriptive epidemiology of prenatal and perinatal risk factors in a Chinese population with reading disorder. Sci. Rep. 2016;6:36697. doi: 10.1038/srep36697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flensborg-Madsen T, Mortensen EL. Birth Weight and Intelligence in Young Adulthood and Midlife. Pediatrics. 2017;139:2016–3161. doi: 10.1542/peds.2016-3161. [DOI] [PubMed] [Google Scholar]
- 52.Rahu K, Rahu M, Pullmann H, Allik J. Effect of birth weight, maternal education and prenatal smoking on offspring intelligence at school age. Early Hum Dev. 2010;86:493–497. doi: 10.1016/j.earlhumdev.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 53.Broman SH, Nichols PL, Kennedy WA. Preschool IQ: Prenatal and early developmental correlates. Am J Psychol. 1975;89:343–344. [Google Scholar]
- 54.Johnson S, Wolke D, Hennessy E, Marlow N. Educational outcomes in extremely preterm children: neuropsychological correlates and predictors of attainment. Dev Neuropsychol. 2011;36:74–95. doi: 10.1080/87565641.2011.540541. [DOI] [PubMed] [Google Scholar]
- 55.Batty GD, Deary IJ, Schoon I, Gale CR. Childhood mental ability in relation to food intake and physical activity in adulthood: the 1970 British Cohort Study. Pediatrics. 2007;119:e38–45. doi: 10.1542/peds.2006-1831. [DOI] [PubMed] [Google Scholar]
- 56.Modig Wennerstad K, Silventoinen K, Tynelius P, Bergman L, Rasmussen F. Association between intelligence and type-specific stroke: a population-based cohort study of early fatal and non-fatal stroke in one million Swedish men. J Epidemiol Community Health. 2010;64:908–912. doi: 10.1136/jech.2008.084020. [DOI] [PubMed] [Google Scholar]
- 57.Peters KL, et al. Improvement of short- and long-term outcomes for very low birth weight infants: Edmonton NIDCAP trial. Pediatrics. 2009;124:1009–1020. doi: 10.1542/peds.2008-3808. [DOI] [PubMed] [Google Scholar]
- 58.Nordhov SM, et al. Early intervention improves cognitive outcomes for preterm infants: randomized controlled trial. Pediatrics. 2010;126:e1088–1094. doi: 10.1542/peds.2010-0778. [DOI] [PubMed] [Google Scholar]
- 59.Lucas A, Morley R, Cole TJ, Lister G, Leeson-Payne C. Breast milk and subsequent intelligence quotient in children born preterm. Lancet. 1992;339:261–264. doi: 10.1016/0140-6736(92)91329-7. [DOI] [PubMed] [Google Scholar]
- 60.Baron IS, Rey-Casserly C. Extremely preterm birth outcome: a review of four decades of cognitive research. Neuropsychol Rev. 2010;20:430–452. doi: 10.1007/s11065-010-9132-z. [DOI] [PubMed] [Google Scholar]
- 61.Levy-Shiff R, Einat G, Mogilner MB, Lerman M, Krikler R. Biological and environmental correlates of developmental outcome of prematurely born infants in early adolescence. J Pediatr Psychol. 1994;19:63–78. doi: 10.1093/jpepsy/19.1.63. [DOI] [PubMed] [Google Scholar]
- 62.Aylward GP. Neurodevelopmental outcomes of infants born prematurely. J Dev Behav Pediatr. 2005;26:427–440. doi: 10.1097/00004703-200512000-00008. [DOI] [PubMed] [Google Scholar]
- 63.Maggi EF, Magalhaes LC, Campos AF, Bouzada MC. Preterm children have unfavorable motor, cognitive, and functional performance when compared to term children of preschool age. J Pediatr (Rio J). 2014;90:377–383. doi: 10.1016/j.jped.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 64.Tanskanen P, et al. Is prematurity associated with adult cognitive outcome and brain structure? Pediatr Neurol. 2011;44:12–20. doi: 10.1016/j.pediatrneurol.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 65.Rautava L, et al. Development and behaviour of 5-year-old very low birthweight infants. Eur Child Adolesc Psychiatry. 2010;19:669–677. doi: 10.1007/s00787-010-0104-x. [DOI] [PubMed] [Google Scholar]
- 66.Schmidt CL, Lawson KR. Caregiver attention-focusing and children’s attention-sharing behaviours as predictors of later verbal IQ in very low birthweight children. J Child Lang. 2002;29:3–22. doi: 10.1017/S0305000901004913. [DOI] [PubMed] [Google Scholar]
- 67.Liu X, Sun Z, Neiderhiser JM, Uchiyama M, Okawa M. Low birth weight, developmental milestones, and behavioral problems in Chinese children and adolescents. Psychiatry Res. 2001;101:115–129. doi: 10.1016/S0165-1781(00)00244-4. [DOI] [PubMed] [Google Scholar]
- 68.Reuner, G., Hassenpflug, A., Pietz, J. & Philippi, H. Long-term development of low-risk low birth weight preterm born infants: neurodevelopmental aspects from childhood to late adolescence. Early Hum Dev. 85, 409–413 (2009).
- 69.Ozbek A, Miral S, Eminagaoglu N, Ozkan H. Development and behavior of non-handicapped preterm children from a developing country. Pediatr Int. 2005;47:532–540. doi: 10.1111/j.1442-200x.2005.02108.x. [DOI] [PubMed] [Google Scholar]
- 70.Vermeylen D, Franco P, Wermenbol V, Muller MF, Pardou A. Neurodevelopment of extremely low birthweight infants born in Erasmus Hospital between 1992 and 2001. Rev Med Brux. 2004;25:449–455. [PubMed] [Google Scholar]
- 71.Pietz J, et al. Physical growth and neurodevelopmental outcome of nonhandicapped low-risk children born preterm. Early Hum Dev. 2004;79:131–143. doi: 10.1016/j.earlhumdev.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 72.Elgen I, Johansson KA, Markestad T, Sommerfelt K. A non-handicapped cohort of low-birthweight children: growth and general health status at 11 years of age. Acta Paediatr. 2005;94:1203–1207. doi: 10.1111/j.1651-2227.2005.tb02075.x. [DOI] [PubMed] [Google Scholar]
- 73.Lubetzky O, Weitzman A, Gilat I, Tyano S. Premature birth and cognitive functioning in adolescence. Harefuah. 1999;137(380-383):430. [PubMed] [Google Scholar]
- 74.Vegni C, et al. Growth and neurodevelopmental outcome at medium term in very low birth weight (VLBW) infants. J Perinat Med. 1994;22(Suppl 1):156–163. doi: 10.1515/jpme.1994.22.s1.156. [DOI] [PubMed] [Google Scholar]
- 75.Lee H, Barratt MS. Cognitive development of preterm low birth weight children at 5 to 8 years old. J Dev Behav Pediatr. 1993;14:242–249. doi: 10.1097/00004703-199308010-00006. [DOI] [PubMed] [Google Scholar]
- 76.Smedler AC, Faxelius G, Bremme K, Lagerstrom M. Psychological development in children born with very low birth weight after severe intrauterine growth retardation: a 10-year follow-up study. Acta Paediatr. 1992;81:197–203. doi: 10.1111/j.1651-2227.1992.tb12203.x. [DOI] [PubMed] [Google Scholar]
- 77.Stalnacke J, Lundequist A, Bohm B, Forssberg H, Smedler AC. Individual cognitive patterns and developmental trajectories after preterm birth. Child Neuropsychol. 2015;21:648–667. doi: 10.1080/09297049.2014.958071. [DOI] [PubMed] [Google Scholar]
- 78.Ornstein M, Ohlsson A, Edmonds J, Asztalos E. Neonatal follow-up of very low birthweight/extremely low birthweight infants to school age: a critical overview. Acta Paediatr Scand. 1991;80:741–748. doi: 10.1111/j.1651-2227.1991.tb11943.x. [DOI] [PubMed] [Google Scholar]
- 79.Winstanley A, et al. Consistency of maternal cognitions and principles across the first five months following preterm and term deliveries. Infant Behav Dev. 2014;37:760–771. doi: 10.1016/j.infbeh.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dunn HG, et al. Neurological, psychological and educational sequelae of low birth weight. Brain Dev. 1980;2:57–67. doi: 10.1016/S0387-7604(80)80008-8. [DOI] [PubMed] [Google Scholar]
- 81.Lundgren EM, Cnattingius S, Jonsson B, Tuvemo T. Intellectual and psychological performance in males born small for gestational age. Horm Res. 2003;59(Suppl 1):139–141. doi: 10.1159/000067850. [DOI] [PubMed] [Google Scholar]
- 82.Klamer A, Lando A, Pinborg A, Greisen G. Ages and Stages Questionnaire used to measure cognitive deficit in children born extremely preterm. Acta Paediatr. 2005;94:1327–1329. doi: 10.1111/j.1651-2227.2005.tb02095.x. [DOI] [PubMed] [Google Scholar]
- 83.Roussounis SH, Hubley PA, Dear PR. Five-year-follow-up of very low birthweight infants: neurological and psychological outcome. Child: care, health and development. 1993;19:45–59. doi: 10.1111/j.1365-2214.1993.tb00712.x. [DOI] [PubMed] [Google Scholar]
- 84.Schothorst PF, Swaab-Barneveld H, van Engeland H. Psychiatric disorders and MND in non-handicapped preterm children. Prevalence and stability from school age into adolescence. Eur Child Adolesc Psychiatry. 2007;16:439–448. doi: 10.1007/s00787-007-0617-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smith L, Ulvund SE, Lindemann R. Prediction of IQ among children with birth weight under 1,501 gms. Tidsskr Nor Laegeforen. 2001;121:1886–1891. [PubMed] [Google Scholar]
- 86.Koc O, et al. School Performance and Neurodevelopment of Very Low Birth Weight Preterm Infants: First Report From Turkey. J Child Neurol. 2016;31:170–176. doi: 10.1177/0883073815587028. [DOI] [PubMed] [Google Scholar]
- 87.Imamura A, et al. Cognitive function and MRI findings in very low birth weight infants. No To Hattatsu. 1996;28:299–305. [PubMed] [Google Scholar]
- 88.Rickards AL, Kitchen WH, Doyle LW, Kelly EA. Correction of developmental and intelligence test scores for premature birth. Aust Paediatr J. 1989;25:127–129. doi: 10.1111/j.1440-1754.1989.tb01434.x. [DOI] [PubMed] [Google Scholar]
- 89.Ochiai M, et al. Longitudinal study of very low birth weight infants until 9 years of age; attention deficit hyperactivity and autistic features are correlated with their cognitive functions. Early Hum Dev. 2015;91:783–786. doi: 10.1016/j.earlhumdev.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 90.Olivieri I, et al. Outcome of extremely low birth weight infants: what’s new in the third millennium? Neuropsychological profiles at four years. Early Hum Dev. 2012;88:241–250. doi: 10.1016/j.earlhumdev.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 91.Kwinta P, et al. Intellectual and motor development of extremely low birth weight (</ = 1000 g) children in the 7th year of life; a multicenter, cross-sectional study of children born in the Malopolska voivodship between 2002 and 2004. Med Wieku Rozwoj. 2012;16:222–231. [PubMed] [Google Scholar]
- 92.van Baalen A, et al. Gaussian distribution of intelligence in VLBW preterm infants at age 5: very low correlation with very low birth weight. Z Geburtshilfe Neonatol. 2008;212:57–63. doi: 10.1055/s-2008-1004763. [DOI] [PubMed] [Google Scholar]
- 93.Ong LC, Boo NY, Chandran V. Predictors of neurodevelopmental outcome of Malaysian very low birthweight children at 4 years of age. J Paediatr Child Health. 2001;37:363–368. doi: 10.1046/j.1440-1754.2001.00694.x. [DOI] [PubMed] [Google Scholar]
- 94.Hawdon JM, Hey E, Kolvin I, Fundudis T. Born too small–is outcome still affected? Dev Med Child Neurol. 1990;32:943–953. doi: 10.1111/j.1469-8749.1990.tb08116.x. [DOI] [PubMed] [Google Scholar]
- 95.Largo RH, Molinari L, Kundu S, Hunziker U, Duc G. Neurological outcome in high risk weight appropriate for gestational age preterm children at early school age. Eur J Pediatr. 1990;149:835–844. doi: 10.1007/BF02072070. [DOI] [PubMed] [Google Scholar]
- 96.Yi KH, Yi YY, Hwang IT. Behavioral and intelligence outcome in 8- to 16-year-old born small for gestational age. Korean J Pediatr. 2016;59:414–420. doi: 10.3345/kjp.2016.59.10.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Molloy CS, et al. Visual memory and learning in extremely low-birth-weight/extremely preterm adolescents compared with controls: a geographic study. J Pediatr Psychol. 2014;39:316–331. doi: 10.1093/jpepsy/jst088. [DOI] [PubMed] [Google Scholar]
- 98.McNicholas F, et al. Medical, cognitive and academic outcomes of very low birth weight infants at age 10–14 years in Ireland. Ir J Med Sci. 2013;183:525–532. doi: 10.1007/s11845-013-1040-9. [DOI] [PubMed] [Google Scholar]
- 99.Hutchinson EA, et al. School-age outcomes of extremely preterm or extremely low birth weight children. Pediatrics. 2013;131:e1053–1061. doi: 10.1542/peds.2012-2311. [DOI] [PubMed] [Google Scholar]
- 100.Lundequist A, Bohm B, Smedler AC. Individual neuropsychological profiles at age 5(1/2) years in children born preterm in relation to medical risk factors. Child Neuropsychol. 2013;19:313–331. doi: 10.1080/09297049.2011.653331. [DOI] [PubMed] [Google Scholar]
- 101.Munck P, et al. Stability of cognitive outcome from 2 to 5 years of age in very low birth weight children. Pediatrics. 2012;129:503–508. doi: 10.1542/peds.2011-1566. [DOI] [PubMed] [Google Scholar]
- 102.Løhaugen GC, et al. Cognitive profile in young adults born preterm at very low birthweight. Dev Med Child Neurol. 2010;52:1133–1138. doi: 10.1111/j.1469-8749.2010.03743.x. [DOI] [PubMed] [Google Scholar]
- 103.Aarnoudse-Moens CS, Smidts DP, Oosterlaan J, Duivenvoorden HJ, Weisglas-Kuperus N. Executive function in very preterm children at early school age. J Abnorm Child Psychol. 2009;37:981–993. doi: 10.1007/s10802-009-9327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mu SC, et al. Cognitive Development at Age 8 Years in Very Low Birth Weight Children in Taiwan. J Formos Med Assoc. 2008;107:6. doi: 10.1016/S0929-6646(09)60014-0. [DOI] [PubMed] [Google Scholar]
- 105.Saavalainen P, et al. Spatial span in very prematurely born adolescents. Dev Neuropsychol. 2007;32:769–785. doi: 10.1080/87565640701539535. [DOI] [PubMed] [Google Scholar]
- 106.Nosarti C, et al. Impaired executive functioning in young adults born very preterm. J Int Neuropsychol Soc. 2007;13:571–581. doi: 10.1017/S1355617707070725. [DOI] [PubMed] [Google Scholar]
- 107.Hoff Esbjørn B, Hansen BM, Greisen G, Mortensen EL. Intellectual development in a Danish cohort of prematurely born preschool children: specific or general difficulties? J Dev Behav Pediatr. 2006;27:477–484. doi: 10.1097/00004703-200612000-00004. [DOI] [PubMed] [Google Scholar]
- 108.Kilbride HW, Thorstad K, Daily DK. Preschool Outcome of Less Than 801-Gram Preterm Infants ComparedWith Full-Term Siblings. Pediatrics. 2004;113:742–747. doi: 10.1542/peds.113.4.742. [DOI] [PubMed] [Google Scholar]
- 109.Short EJ, et al. Cognitive and academic consequences of bronchopulmonary dysplasia and very low birth weight: 8-year-old outcomes. Pediatrics. 2003;112:e359–366. doi: 10.1542/peds.112.5.e359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cooke RW, Foulder-Hughes L. Growth impairment in the very preterm and cognitive and motor performance at 7 years. Arch Dis Child. 2003;88:482–487. doi: 10.1136/adc.88.6.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Grunau RE, Whitfield MF, Davis C. Pattern of learning disabilities in children with extremely low birth weight and broadly average intelligence. Arch Pediatr Adolesc Med. 2002;156:615–620. doi: 10.1001/archpedi.156.6.615. [DOI] [PubMed] [Google Scholar]
- 112.Magill-Evans J, Harrison MJ, Van der Zalm J, Holdgrafer G. Cognitive and language development of healthy preterm infants at 10 years of age. Phys Occup Ther Pediatr. 2002;22:41–56. doi: 10.1080/J006v22n01_04. [DOI] [PubMed] [Google Scholar]
- 113.Rickards AL, Kelly EA, Doyle LW, Callanan C. Cognition, academic progress, behavior and self-concept at 14 years of very low birth weight children. J Dev Behav Pediatr. 2001;22:11–18. doi: 10.1097/00004703-200102000-00002. [DOI] [PubMed] [Google Scholar]
- 114.Nadeau L, Boivin M, Tessier R, Lefebvre F, Robaey P. Mediators of behavioral problems in 7-year-old children born after 24 to 28 weeks of gestation. J Dev Behav Pediatr. 2001;22:1–10. doi: 10.1097/00004703-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 115.Taylor HG, Klein N, Minich NM, Hack M. Middle-school-age outcomes in children with very low birthweight. Child Dev. 2000;71:1495–1511. doi: 10.1111/1467-8624.00242. [DOI] [PubMed] [Google Scholar]
- 116.Saigal S, Hoult LA, Streiner DL, Stoskopf BL, Rosenbaum PL. School difficulties at adolescence in a regional cohort of children who were extremely low birth weight. Pediatrics. 2000;105:325–331. doi: 10.1542/peds.105.2.325. [DOI] [PubMed] [Google Scholar]
- 117.Hughes C. et al. Cognitive pereformance at school age of very low birth weight infants with bronchpulmonary dysplasia. J Dev Behav Pediatr. 20 (1999). [DOI] [PubMed]
- 118.Stjernqvist K, Svenningsen NW. Ten-year follow-up of children born before 29 gestational weeks: health, cognitive development, behaviour and school achievement. Acta Paediatr. 1999;88:557–562. doi: 10.1111/j.1651-2227.1999.tb00175.x. [DOI] [PubMed] [Google Scholar]
- 119.Botting N, Powls A, Cooke RW, Marlow N. Cognitive and educational outcome of very-low-birthweight children in early adolescence. Dev Med Child Neurol. 1998;40:652–660. doi: 10.1111/j.1469-8749.1998.tb12324.x. [DOI] [PubMed] [Google Scholar]
- 120.Whitfield MF, Grunau RV, Holsti L. Extremely premature (< or = 800 g) schoolchildren: multiple areas of hidden disability. Arch Dis Child Fetal Neonatal Ed. 1997;77:F85–90. doi: 10.1136/fn.77.2.F85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rose SA, Feldman JF. Memory and processing speed in preterm children at eleven years: a comparison with full-terms. Child Dev. 1996;67:2005–2021. doi: 10.2307/1131606. [DOI] [PubMed] [Google Scholar]
- 122.Hack M, et al. The effect of very low birth weight and social risk on neurocognitive abilities at school age. J Dev Behav Pediatr. 1992;13:412–420. doi: 10.1097/00004703-199212000-00005. [DOI] [PubMed] [Google Scholar]
- 123.Teplin SW, Burchinal M, Johnson-Martin N, Humphry RA, Kraybill EN. Neurodevelopmental, health, and growth status at age 6 years of children with birth weights less than 1001 grams. J Pediatr. 1991;118:768–777. doi: 10.1016/S0022-3476(05)80045-9. [DOI] [PubMed] [Google Scholar]
- 124.Smith AE, Knight-Jones EB. The abilities of very low-birthweight children and their classroom controls. Dev Med Child Neurol. 1990;32:590–601. doi: 10.1111/j.1469-8749.1990.tb08543.x. [DOI] [PubMed] [Google Scholar]
- 125.McDonald MA, Sigman M, Ungerer JA. Intelligence and behavior problems in 5-year-olds in relation to representational abilities in the second year of life. J Dev Behav Pediatr. 1989;10:86–91. doi: 10.1097/00004703-198904000-00005. [DOI] [PubMed] [Google Scholar]
- 126.Klein NK, Hack M, Breslau N. Children who were very low birth weight: development and academic achievement at nine years of age. J Dev Behav Pediatr. 1989;10:32–37. doi: 10.1097/00004703-198902000-00007. [DOI] [PubMed] [Google Scholar]
- 127.Portnoy S, Callias M, Wolke D, Gamsu H. Five-year follow-up study of extremely low-birthweight infants. Dev Med Child Neurol. 1988;30:590–598. doi: 10.1111/j.1469-8749.1988.tb04796.x. [DOI] [PubMed] [Google Scholar]
- 128.Lloyd BW, Wheldall K, Perks D. Controlled study of intelligence and school performance of very low-birthweight children from a defined geographical area. Dev Med Child Neurol. 1988;30:36–42. doi: 10.1111/j.1469-8749.1988.tb04724.x. [DOI] [PubMed] [Google Scholar]
- 129.Kitchen WH, et al. A longitudinal study of very low-birthweight infants. IV: An overview of performance at eight years of age. Dev Med Child Neurol. 1980;22:172–188. doi: 10.1111/j.1469-8749.1980.tb04326.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.