Abstract
Background
Randomized controlled trials (RCTs) inform clinical practice and have provided the evidence base for introducing minimally invasive surgery (MIS) in surgical oncology. Crossover (unplanned intraoperative conversion of MIS to open surgery) may affect clinical outcomes and the effect size generated from RCTs with homogenization of randomized groups.
Objectives
Our aims were to identify modifiable factors associated with crossover and assess the impact of crossover on clinical endpoints.
Methods
A systematic review was performed to identify all RCTs comparing MIS with open surgery for gastrointestinal cancer (1990–2017). Meta-regression analysis was performed to analyze factors associated with crossover and the influence of crossover on endpoints, including 30-day mortality, anastomotic leak rate, and early complications.
Results
Forty RCTs were included, reporting on 11,625 patients from 320 centers. Crossover was shown to affect one in eight patients (mean 12.6%, range 0–45%) and increased with American Society of Anesthesiologists score (β = + 0.895; p = 0.050). Pretrial surgeon volume (β = − 2.344; p = 0.037), composite RCT quality score (β = − 7.594; p = 0.014), and site of tumor (β = − 12.031; p = 0.021, favoring lower over upper gastrointestinal tumors) showed an inverse relationship with crossover. Importantly, multivariate weighted linear regression revealed a statistically significant positive correlation between crossover and 30-day mortality (β = + 0.125; p = 0.033), anastomotic leak rate (β = + 0.550; p = 0.004), and early complications (β = + 1.255; p = 0.001), based on intention-to-treat analysis.
Conclusions
Crossover in trials was associated with an increase in 30-day mortality, anastomotic leak rate, and early complications within the MIS group based on intention-to-treat analysis, although our analysis did not assess causation. Credentialing surgeons by procedural volume and excluding high comorbidity patients from initial trials are important in minimizing crossover and optimizing RCT validity.
Electronic supplementary material
The online version of this article (10.1245/s10434-017-6210-y) contains supplementary material, which is available to authorized users.
Randomized controlled trials (RCTs) provide the highest level of evidence informing clinical practice, and provide the basis for national and international guidance of disease-treatment algorithms and protocols.1 Several RCTs have suggested benefits in short-term endpoints from minimally invasive surgery (MIS) for gastrointestinal cancer,2–5 which has led to substantial growth in the adoption of these techniques and incorporation into national and international guidelines.6
Adherence to the tested intervention is a fundamental principle of scientific investigation. Crossover (unplanned intraoperative conversion of MIS to open surgery) can have important consequences on trial validity, with large-scale, uncontrolled crossover potentially invalidating trial results.7 This is frequently the result of a combination of mechanisms, which include disruption of randomization, bias, systematic error, and loss of statistical power. Crossover can thus convert the trial from randomized to a hybrid of randomized and observational study with survival, the primary endpoint in surgical oncology trials, being compromized.8
The primary objective of this systematic review was to evaluate the influence of crossover within RCTs regarding MIS for gastrointestinal cancer on short-term endpoints and overall survival. The secondary objective was to identify potentially modifiable factors that significantly affect the incidence of crossover within these RCTs.
Methods
Search Strategy and Selection Criteria
The study was executed and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.9 All studies published between 1990 and 2017 without language restriction and reporting on MIS versus open treatment modality in patients undergoing surgical treatment for gastrointestinal cancer were identified. The MEDLINE, EMBASE, Google Scholar, Cochrane, PsycINFO, and ERIC electronic databases were searched (latest search 3 September 2017) using the following medical subject heading (MeSH) terms: ((“randomized controlled trial”[Publication Type] OR “randomized controlled trials as topic”[MeSH Terms] OR “randomised controlled trial”[All Fields] OR “randomized controlled trial”[All Fields]) AND (“Surg Oncol”[Journal] OR (“surgical”[All Fields] AND “oncology”[All Fields]) OR “surgical oncology”[All Fields] OR “J Surg Oncol”[Journal] OR (“surgical”[All Fields] AND “oncology”[All Fields]) OR “surgical oncology”[All Fields])) AND Clinical Trial[All Fields]). The ‘related articles’ function and reference list of each of the identified publications was used to widen the literature search. Any relevant review articles were also screened.
For inclusion in the analysis, studies had to be RCTs that compared MIS versus open surgery for the treatment of gastrointestinal cancer. Furthermore, studies had to report the endpoints of interest (described below) and comprise an adult patient group (> 18 years). All trials had to be registered. Studies were excluded if they reported a previously published dataset (in which case the most recent publication was included) and/or if they primarily investigated adjuvant (or neoadjuvant) treatments (e.g. chemotherapy and/or radiotherapy) as opposed to the type of surgical approach (i.e. MIS vs. the open approach).
Data Extraction
Two reviewers (GG and SRM) independently assessed the articles and relevant data were extracted without cross-referencing. Any conflicts in data extraction or quality assessment were resolved by the senior authors (AD and TA) prior to analysis. The parameters and endpoints captured are described below. The quality of the included studies was assessed using a recently introduced tool for evaluating the rigor of surgical RCTs, and a composite quality score was computed for each study, taking into account the Jadad and risk-of-bias scores.10 A composite quality score > 3 was defined as a threshold of rigor (electronic supplementary Table S1).11
Variables
The following demographic data were extracted: study type, patient numbers, year and country of publication, tumor site, disease stage, American Society of Anesthesiologists (ASA) score, treatments compared, number of centers participating in the trial, pretrial surgeon volume/experience to be allowed to enter each trial, Jadad score, risk-of-bias score, composite quality score, and presence of crossover (including percentage, time it occurred [i.e. pre- vs. intraoperative], and reasons given for it).
Endpoints
Endpoints studied were 30-day mortality, 30-day (early) complications (specifically pulmonary complications and anastomotic leak rate), length of stay, and overall survival.
Factors Affecting the Incidence of Crossover
The measure of crossover for each study was defined as the percentage of patients who crossed over from one study arm to the other. The effect of number of centers, pretrial surgeon volume, sample size, site of cancer [upper (esophageal and gastric) versus lower (colonic and rectal) gastrointestinal tumors, and further subdivision of lower gastrointestinal tumors into colonic and rectal], ASA score (3 + 4 vs. 1 + 2), disease stage (III + IV vs. I + II), and composite quality score of the RCT were studied. Subsequently, univariate and multivariate linear regression models were utilized to evaluate the association between crossover and the above parameters. The level of significance permitting multivariate analysis inclusion was set at p < 0.05.
Crossover and Study Endpoints
Meta-regression analysis was performed to quantitatively assess the impact of (1) composite quality score, (2) number of participating centers, (3) pretrial surgeon volume, (4) sample size, (5) ASA score, and (6) crossover on the overall effect for each endpoint (30-day mortality, anastomotic leak rate, pulmonary complications, length of stay, and early complications). All variables were checked for interaction and multicollinearity. The significance level was set at p < 0.05.
The study was performed in line with Cochrane recommendations, following the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group guidelines (electronic supplementary Table S2)12 using the statistical software STATA 14 (StataCorp LP, College Station, TX, USA).
Results
Selected Studies
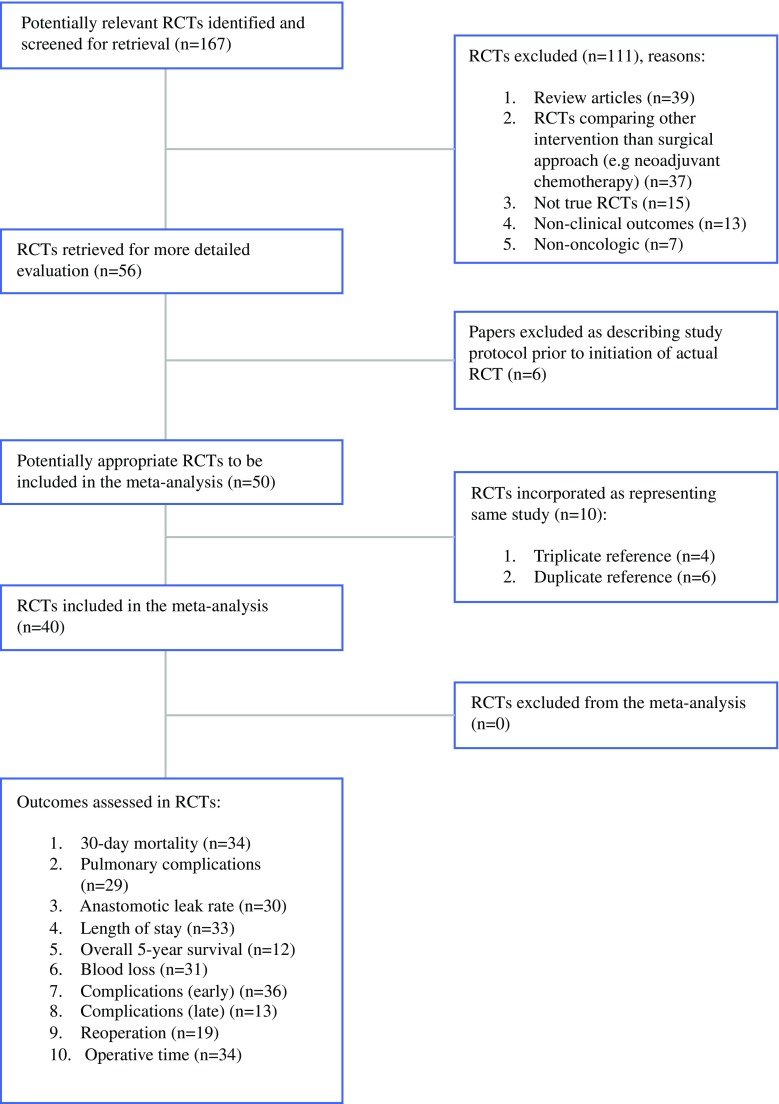
The literature search yielded 56 articles4,5,13–62 reporting results from 40 RCTs that met the inclusion criteria and were included in this study. The PRISMA statement flow diagram illustrating the search strategy is shown in Fig. 1. Of these RCTs, one compared MIS against open surgery for esophageal cancer,14 11 for gastric cancer,25–27,35,37,42,43,47,50,57,60 25 for colorectal cancer,5,13,16 22,28–30,34,36,38,40,44–46,48,49,51,52,58,61 and 15 for rectal cancer.4,15,23,24,31–33,39,41,53–56,59,62 Overall, 11,625 patients were included from 320 centers. In total, 6210 patients were randomized to MIS; however, on completion of these RCTs, the actual number of patients who underwent MIS was 5423 as 787 patients had crossed over to the open surgery arm, resulting in a mean crossover rate of 12.6% (range 0–45%). In other words, one in eight patients randomized to MIS underwent open surgery, although they were analyzed as part of the MIS group as intention-to-treat analysis was employed.
Fig. 1.
PRISMA statement flow diagram illustrating the search strategy used. PRISMA preferred reporting items for systematic reviews and meta-analyses, RCTs randomized controlled trials
Trial Characteristics and Crossover Rates
The characteristics of each RCT, including composite quality score, description of crossover (MIS to open surgery) in terms of percentage, time (i.e. pre- vs. intraoperative), and reasons given for it are presented in electronic supplementary Table S3. The percentage crossover ranged from 0 to 45% (mean 12.6%, standard deviation 10.6%) and primarily related to unplanned intraoperative open conversion. Electronic supplementary Table S4 illustrates further information, including patient demographics, cancer site and stage, morbidity, 30-day mortality, and overall survival (1–5 years, including median survival for each approach) for each RCT.
The Effect of Pretrial Surgeon Volume, Composite Randomized Controlled Trial Quality Score, Site of Tumor, and American Society of Anesthesiologists Score on Crossover
The pretrial surgeon volume (β = − 2.344; p = 0.037), composite RCT quality score (β = − 7.594; p = 0.014), and site of tumor (β = − 12.031; p = 0.021, favoring lower over upper gastrointestinal tumors) showed an inverse relationship with crossover, while ASA score showed a positive relationship with crossover (β = + 0.895; p = 0.050) [Table 1]. When performing a subanalysis of lower gastrointestinal tumors into colonic and rectal sites, mean crossover rate was found to be higher in the former (11.3 vs. 10.7%), although the difference did not reach statistical significance (p > 0.05). This finding is likely to be a result of differences in comorbidity between patients included in these studies, with the rectal cancer studies consisting of patients with lower mean ASA score (i.e. less comorbid population compared with the colonic cancer studies).
TABLE 1.
Results of univariate and multivariate linear regression illustrating the relationship between different factors and crossover (MIS to open surgery)
| Univariate linear regression model | Coefficients | p-Valuea | 95% CI for B | Collinearity statistics | ||
|---|---|---|---|---|---|---|
| B | SE | Lower bound | Upper bound | Variance inflation factor | ||
| No. of centers | − 0.279 | 0.138 | 0.136 | − 0.717 | 0.159 | 6.436 |
| Pretrial surgeon volume | 3.844 | 1.133 | 0.047 | 0.546 | 12.142 | 19.073 |
| Sample size | 0.010 | 0.004 | 0.070 | − 0.002 | 0.023 | 4.075 |
| Composite quality score | − 9.992 | 1.733 | 0.010 | − 15.505 | − 4.478 | 3.235 |
| Year of publication | 0.219 | 0.121 | 0.367 | − 0.165 | 0.603 | 28.403 |
| Site of tumor (upper vs. lower GI cancer) | − 23.031 | 6.224 | 0.034 | − 42.837 | − 3.224 | 9.073 |
| ASA score (3 + 4 versus 1 + 2) | 0.795 | 0.270 | 0.049 | 0.013 | 1.653 | 12.534 |
| Disease stage (III + IV versus I + II) | 0.239 | 0.070 | 0.042 | 0.015 | 0.462 | 11.279 |
| Dependent variable: MIS to open crossover (%) | ||||||
| Multivariate linear regression model | Coefficients | p-Valuea | 95% CI for B | ||
|---|---|---|---|---|---|
| B | SE | Lower bound | Upper bound | ||
| Pretrial surgeon volume | − 2.344 | 0.323 | 0.037 | 0.546 | 1.142 |
| Composite quality score | − 7.594 | 1.984 | 0.014 | − 15.505 | − 4.478 |
| Site of tumor (upper vs. lower GI cancer) | − 12.031 | 3.387 | 0.021 | − 22.376 | − 2.340 |
| ASA score (3 + 4 vs. 1 + 2) | 0.895 | 0.279 | 0.050 | 0.001 | 1.253 |
| Disease stage (III + IV vs. I + II) | 0.239 | 1.070 | 0.122 | − 0.789 | 1.462 |
| Dependent variable: MIS to open crossover (%) | |||||
a Boldface denotes statistical significance
SE standard error, CI confidence interval, MIS minimally invasive surgery, GI gastrointestinal, ASA American Society of Anesthesiologists
The Effect of Crossover on 30-Day Mortality and 30-Day Complications
Meta-regression analysis revealed a statistically significant positive correlation between crossover and 30-day mortality (β = + 0.125; p = 0.033), anastomotic leak rate (β = + 0.550; p = 0.004), and 30-day complications (β = + 1.255; p = 0.001), based on intention-to-treat analysis (Table 2). No statistically significant correlations were found between crossover and pulmonary complications (β = + 0.223; p = 0.728) or length of hospital stay (β = − 1.718; p = 0.939). It was not possible to perform the regression for the effect of crossover on overall 5-year survival due to insufficient data reported.
Table 2.
Results of univariate and multivariate weighted linear regression illustrating the relationship between crossover (MIS to open surgery) and surgical endpoints
| Univariate weighted linear regression | Multivariate weighted linear regression | ||||
|---|---|---|---|---|---|
| Factor | β coefficient | p-Valuea | β coefficient | 95% CI | p-Valuea |
| 30-Day mortality | |||||
| MIS to open crossover | 0.028 | 0.001 | 0.125 | 0.012–0.238 | 0.033 |
| Composite quality score | 0.317 | 0.452 | 0.317 | − 0.494 to 1.128 | 0.404 |
| Number of centers | − 0.035 | 0.763 | − 0.035 | − 0.130 to 0.061 | 0.439 |
| Pretrial surgeon volume | 0.005 | 0.356 | 0.005 | − 0.018 to 0.028 | 0.640 |
| Sample size | 0.001 | 0.645 | 0.001 | − 0.004 to 0.003 | 0.907 |
| ASA score | 0.021 | 0.049 | 0.019 | 0.001–0.321 | 0.049 |
| Anastomotic leak | |||||
| MIS to open crossover | 0.467 | 0.003 | 0.550 | 0.259–0.841 | 0.004 |
| Composite quality score | 0.748 | 0.847 | 0.605 | − 1.037 to 2.248 | 0.402 |
| Number of centers | − 0.076 | 0.456 | − 0.097 | − 0.298 to 0.108 | 0.283 |
| Pretrial surgeon volume | − 0.287 | 0.245 | − 0.009 | − 0.053 to 0.034 | 0.613 |
| Sample size | − 0.038 | 0.837 | − 0.005 | − 0.013 to 0.003 | 0.181 |
| ASA score | 0.056 | 0.050 | 0.089 | − 0.023 to 0.009 | 0.161 |
| Pulmonary complications | |||||
| MIS to open crossover | 0.223 | 0.874 | 0.223 | − 1.754 to 2.201 | 0.728 |
| Composite quality score | − 2.033 | 0.710 | − 2.033 | − 20.16 to 16.09 | 0.745 |
| Number of centers | 0.247 | 0.653 | 0.247 | − 1.251 to 1.745 | 0.636 |
| Pretrial surgeon volume | − 0.010 | 0.873 | − 0.010 | − 0.201 to 0.180 | 0.873 |
| Sample size | − 0.012 | 0.541 | − 0.012 | − 0.049 to 0.025 | 0.368 |
| ASA score | 0.142 | 0.123 | − 0.098 | − 0.187 to 0.289 | 0.256 |
| Length of stay | |||||
| MIS to open crossover | − 2.234 | 0.939 | − 1.718 | − 47.92 to 44.49 | 0.939 |
| Composite quality score | − 7.276 | 0.977 | − 4.899 | − 350.2 to 340.3 | 0.977 |
| Number of centers | − 0.962 | 0.965 | − 0.661 | − 32.02 to 30.69 | 0.965 |
| Pretrial surgeon volume | − 2.882 | 0.685 | − 1.800 | − 10.985 to 7.386 | 0.685 |
| Sample size | − 0.198 | 0.952 | − 0.038 | − 1.324 to 1.249 | 0.952 |
| ASA score | − 0.763 | 0.456 | − 0.305 | − 0.013 to 0.403 | 0.381 |
| Early (30-day) complications | |||||
| MIS to open crossover | 1.255 | 0.001 | 1.255 | 0.929–4.412 | 0.001 |
| Composite quality score | 0.372 | 0.085 | 0.372 | − 0.685 to 8.711 | 0.085 |
| Number of centers | − 0.158 | 0.586 | − 0.158 | − 0.031 to 0.019 | 0.586 |
| Pretrial surgeon volume | 0.119 | 0.613 | 0.119 | − 0.106 to 0.170 | 0.613 |
| Sample size | − 0.174 | 0.586 | − 0.174 | − 0.031 to 0.019 | 0.586 |
| ASA score | 0.987 | 0.009 | 1.008 | 0.328–3.910 | 0.039 |
aBoldface denotes statistical significance
CI confidence interval, MIS minimally invasive surgery, ASA American Society of Anesthesiologists
Discussion
The findings of this study demonstrate that crossover in surgical oncology RCTs is common, affecting one in eight patients. Moreover, its incidence was shown to reduce with increasing surgeon experience and decreasing patient comorbidity (as indicated by pretrial volume and ASA score, respectively). Importantly, the clinical consequences of this crossover within RCTs included increases in 30-day mortality, anastomotic leak rate, and 30-day complications demonstrated by meta-regression.
It thus becomes apparent that in the presence of crossover, an intention-to-treat analysis may underestimate the underlying mortality benefit associated with MIS, i.e. the benefit that would have been observed had crossover not occurred due to partial homogenization of the study groups. Similarly, the anastomotic leak rate may be overestimated in the MIS group. In other words, in the presence of crossover, a simple intention-to-treat analysis may result in bias that will be equal to the difference between the underlying mortality difference and the observed one (in the presence of crossover); however, the extent of this bias remains unknown (as the underlying mortality difference is not directly observed). Based on our analytical methodology, the interpretation of results offers an association between outcomes with counterfactual treatment effects, although this is not a measure of causation.63 What is clear though is that as long as there is a difference between MIS and open surgery, some bias will inevitably exist as a direct result of crossover.64
Hence, in the presence of crossover, any clinical, cost effectiveness, and economic evaluation relying on intention-to-treat analysis is prone to generate inaccurate results, which may in turn lead to inappropriate resource allocation.64 This is of special importance in the current healthcare climate characterized by severe financial restraints and competing national priorities for investment.1,65
Crossover represents a particularly challenging problem for bodies such as Health Technology Assessment (HTA) and the National Institute for Health and Care Excellence (NICE) in the UK, and the Agency for Healthcare Research and Quality (AHRQ) in the US, which rely on the findings of RCTs to form the basis of health policy.66 Surgical oncology is particularly prone to crossover due to adverse events or technical challenges experienced with the MIS techniques. Moreover, surgical RCTs in general have additional limitations.1 For example, when evaluating a novel medical device or surgical technique, assessment of endpoints is not commonly blind and may be affected by assessor bias added to crossover.67 – 70
Thus, it becomes apparent that there is a need to pre-emptively address factors that influence crossover. To optimize surgical oncology RCT validity, prevention strategies need to be employed to minimize and reduce the effects of crossover. Of course, crossover cannot be completely abolished for several reasons, including clinical (e.g. need for open conversion due to technical reasons or adverse event), logistic (e.g. equipment malfunction or no available surgeon competent in MIS technique for oncologic purposes), and ethical (e.g. post-randomization patient choice).1
In terms of prevention strategies, the current study shows that credentialing surgeons and having a minimum procedural volume threshold to enter the study reduces the degree of crossover from MIS to open surgery. Prior studies have highlighted the presence of a proficiency-gain curve with the introduction of MIS, which can substantially influence rates of conversion (crossover) and clinical endpoints, including mortality at a national level.71 The length of this proficiency-gain curve must be accurately defined in order to provide appropriately validated procedural volume thresholds for surgeon inclusion within RCTs. This will ensure that surgeons within their MIS proficiency-gain curve are excluded from the study.
Another prevention strategy may involve alternative processes of surgeon credentialing with video assessment and data monitoring in a pretrial phase prior to inclusion within the RCT, in a similar manner to a driving test before embarking on driving independently. COLOR-III is a good example, given the relative novelty of the procedure (transanal total mesorectal excision) and the presumption that many surgeons will currently be within their proficiency-gain curve, a pretrial phase with careful surgeon credentialing has been designed to ensure surgeons entering the RCT are beyond their period of gaining proficiency.72
Moreover, given its independence as a predictor of crossover, as well as its potential effect on complications, it may be advisable to include low comorbidity (ASA score) patients in RCTs only when initially comparing novel MIS techniques with open surgery. This strategy aims to further minimize crossover, promote patient safety (by reducing complications), and optimize trial validity. Depending on initial RCT findings, inclusion criteria can then be expanded in subsequent RCTs to include higher comorbidity patients.
As crossover is not random, simple methods to address it, such as excluding or censoring patients, will only lead to further bias. This is especially true when crossover is associated with prognosis, the primary endpoint in the majority of surgical oncology RCTs. Although more complex statistical methods have been developed to account for the crossover effect, it is important to appreciate that no method is superior, each suffering its own limitations.64
A more pragmatic approach could involve reporting endpoints in three rather than two groups, i.e. the (completed) MIS, open, and converted (crossover) trial arms in addition to the traditional intention-to-treat analysis. Only three RCTs reported endpoints in this way.34,38,46 This type of analysis will also allow the evaluation of factors associated with crossover, and thus try to predict which patients would not constitute good candidates for MIS due to the high risk of conversion and thus complications. Interestingly, one RCT reported endpoints using both intention-to-treat analysis and actual treatment groups separately for the same patient cohort.19 This is the only RCT that used both statistical methods, and illustrates the significantly worse outcomes associated with the crossover group over both the open and MIS groups.
It is important to acknowledge the limitations of this study. First, this was a study-based (as opposed to individual patient data) meta-regression analysis, and hence individual clinicopathological parameters were not included. However, in the context of RCTs, these parameters are carefully controlled for in the study design. Second, assessing the role of crossover itself is not directly quantifiable (i.e. how outcomes would differ had there been no crossover) based on the current dataset.19
Conclusions
There are two key findings from this study. First, pretrial surgeon volume and patient ASA score are the two modifiable factors associated with crossover in surgical oncology RCTs. Second, the presence of crossover is associated with an increase in 30-day mortality, anastomotic leak rate, and early complications within the MIS group based on intention-to-treat-analysis. However, the association reported here is not a measure of causation. Credentialing surgeons by procedural volume is an important method of ensuring surgeons have completed their proficiency-gain curve and thus reduce the incidence of crossover, as is including low comorbidity patients only when initially comparing novel MIS techniques with open surgery. Future RCTs must develop and implement strategies, including pretrial phases and surgeon credentialing by volume or video assessment, in addition to excluding high-comorbidity patients during initial trials to reduce the incidence of crossover and thus maintain randomized homogenous groups to adequately test the study hypotheses.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
Dr. George Garas, MD, FRCS, holds a Royal College of Surgeons of England Doctoral Research Fellowship (Grant Number GG 1037600/2017-2018) and is also supported by Imperial College London (Grant Number CID 337755/2015-2018) and the Alexander S. Onassis Public Benefit Foundation (Grant Number F ZM 014-1/2016-2017). Dr. Sheraz R. Markar, MD, PhD, MRCS, is supported by the National Institute for Health Research (Grant Number NIHR-CTF-2015-04-09). Professor Athanassios Argiris, MD, PhD, FACP, is funded by the National Cancer Institute (NCI R21 Grant) and serves as the principal investigator for several trials (ECOG E1302 and E1305, SWOG S1206) with the NCI-funded national cooperative groups of the US. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Funding
No funding or sponsoring was received in support of this work.
Disclosure
George Garas, Sheraz R. Markar, George Malietzis, Hutan Ashrafian, George B. Hanna, Emmanouil Zacharakis, Long R. Jiao, Athanassios Argiris, Ara Darzi, and Thanos Athanasiou declare no conflicts of any commercial interest.
Footnotes
This article was presented as a podium presentation at the International Surgical Congress of the Association of Surgeons of Great Britain and Ireland (ASGBI), May 3–5, 2017, Glasgow, United Kingdom.
Electronic supplementary material
The online version of this article (10.1245/s10434-017-6210-y) contains supplementary material, which is available to authorized users.
This article is dedicated to the memory of Professor Ioannis Garas, MD, PhD (1927–2013), co-founder of the European School of Oncology (ESO) and European Society of Surgical Oncology (ESSO) in 1981, and Chairman of the first Surgical Oncology Congress in Europe in 1982 (Athens, Greece).
References
- 1.Garas G, Ibrahim A, Ashrafian H, Ahmed K, Patel V, Okabayashi K, et al. Evidence-based surgery: barriers, solutions, and the role of evidence synthesis. World J Surg. 2012;36:1723–1731. doi: 10.1007/s00268-012-1597-x. [DOI] [PubMed] [Google Scholar]
- 2.Aziz O, Constantinides V, Tekkis PP, Athanasiou T, Purkayastha S, Paraskeva P, et al. Laparoscopic versus open surgery for rectal cancer: a meta-analysis. Ann Surg Oncol. 2006;13:413–424. doi: 10.1245/ASO.2006.05.045. [DOI] [PubMed] [Google Scholar]
- 3.Schwenk W, Haase O, Neudecker J, Muller JM. Short term benefits for laparoscopic colorectal resection. Cochrane Database Syst Rev 2005;(3):CD003145. [DOI] [PMC free article] [PubMed]
- 4.van der Pas MH, Haglind E, Cuesta MA, Furst A, Lacy AM, Hop WC, et al. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013;14:210–218. doi: 10.1016/S1470-2045(13)70016-0. [DOI] [PubMed] [Google Scholar]
- 5.Veldkamp R, Kuhry E, Hop WC, Jeekel J, Kazemier G, Bonjer HJ, et al. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol. 2005;6:477–484. doi: 10.1016/S1470-2045(05)70221-7. [DOI] [PubMed] [Google Scholar]
- 6.NICE Technology Appraisal Guidance . Laparoscopic surgery for colorectal cancer. London: NICE; 2006. [Google Scholar]
- 7.Weinstein GS, Levin B. Effect of crossover on the statistical power of randomized studies. Ann Thorac Surg. 1989;48:490–495. doi: 10.1016/S0003-4975(10)66846-4. [DOI] [PubMed] [Google Scholar]
- 8.Rimawi M, Hilsenbeck SG. Making sense of clinical trial data: is inverse probability of censoring weighted analysis the answer to crossover bias? J Clin Oncol. 2012;30:453–458. doi: 10.1200/JCO.2010.34.2808. [DOI] [PubMed] [Google Scholar]
- 9.Moher D, Liberati A, Tetzlaff J, Altman DG. Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Murtuza B, Pepper JR, Jones C, Nihoyannopoulos P, Darzi A, Athanasiou T. Does stentless aortic valve implantation increase perioperative risk? A critical appraisal of the literature and risk of bias analysis. Eur J Cardiothorac Surg. 2011;39:643–652. doi: 10.1016/j.ejcts.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 11.Garas G, Okabayashi K, Ashrafian H, Shetty K, Palazzo F, Tolley N, et al. Which hemostatic device in thyroid surgery? A network meta-analysis of surgical technologies. Thyroid. 2013;23:1138–1150. doi: 10.1089/thy.2012.0588. [DOI] [PubMed] [Google Scholar]
- 12.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 13.Bagshaw PF, Allardyce RA, Frampton CM, Frizelle FA, Hewett PJ, McMurrick PJ, et al. Long-term outcomes of the australasian randomized clinical trial comparing laparoscopic and conventional open surgical treatments for colon cancer: the Australasian Laparoscopic Colon Cancer Study trial. Ann Surg. 2012;256:915–919. doi: 10.1097/SLA.0b013e3182765ff8. [DOI] [PubMed] [Google Scholar]
- 14.Biere SS, van Berge Henegouwen MI, Maas KW, Bonavina L, Rosman C, Garcia JR, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet. 2012;379:1887–1892. doi: 10.1016/S0140-6736(12)60516-9. [DOI] [PubMed] [Google Scholar]
- 15.Bonjer HJ, Deijen CL, Abis GA, Cuesta MA, van der Pas MH, de Lange-de Klerk ES, et al. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med. 2015;372:1324–1332. doi: 10.1056/NEJMoa1414882. [DOI] [PubMed] [Google Scholar]
- 16.Clinical Outcomes of Surgical Therapy Study Group A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350:2050–2059. doi: 10.1056/NEJMoa032651. [DOI] [PubMed] [Google Scholar]
- 17.Colon Cancer Laparoscopic or Open Resection Study Group Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol. 2009;10:44–52. doi: 10.1016/S1470-2045(08)70310-3. [DOI] [PubMed] [Google Scholar]
- 18.Fleshman J, Sargent DJ, Green E, Anvari M, Stryker SJ, Beart RW, Jr, et al. Laparoscopic colectomy for cancer is not inferior to open surgery based on 5-year data from the COST Study Group trial. Ann Surg. 2007;246:655–662. doi: 10.1097/SLA.0b013e318155a762. [DOI] [PubMed] [Google Scholar]
- 19.Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, et al. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365:1718–1726. doi: 10.1016/S0140-6736(05)66545-2. [DOI] [PubMed] [Google Scholar]
- 20.Hewett PJ, Allardyce RA, Bagshaw PF, Frampton CM, Frizelle FA, Rieger NA, et al. Short-term outcomes of the Australasian randomized clinical study comparing laparoscopic and conventional open surgical treatments for colon cancer: the ALCCaS trial. Ann Surg. 2008;248:728–738. doi: 10.1097/SLA.0b013e31818b7595. [DOI] [PubMed] [Google Scholar]
- 21.Jayne DG, Guillou PJ, Thorpe H, Quirke P, Copeland J, Smith AM, et al. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. J Clin Oncol. 2007;25:3061–3068. doi: 10.1200/JCO.2006.09.7758. [DOI] [PubMed] [Google Scholar]
- 22.Jayne DG, Thorpe HC, Copeland J, Quirke P, Brown JM, Guillou PJ. Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br J Surg. 2010;97:1638–1645. doi: 10.1002/bjs.7160. [DOI] [PubMed] [Google Scholar]
- 23.Jeong SY, Park JW, Nam BH, Kim S, Kang SB, Lim SB, et al. Open versus laparoscopic surgery for mid-rectal or low-rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): survival outcomes of an open-label, non-inferiority, randomised controlled trial. Lancet Oncol. 2014;15:767–774. doi: 10.1016/S1470-2045(14)70205-0. [DOI] [PubMed] [Google Scholar]
- 24.Kang SB, Park JW, Jeong SY, Nam BH, Choi HS, Kim DW, et al. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol. 2010;11:637–645. doi: 10.1016/S1470-2045(10)70131-5. [DOI] [PubMed] [Google Scholar]
- 25.Kim HH, Hyung WJ, Cho GS, Kim MC, Han SU, Kim W, et al. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report: a phase III multicenter, prospective, randomized Trial (KLASS Trial) Ann Surg. 2010;251:417–420. doi: 10.1097/SLA.0b013e3181cc8f6b. [DOI] [PubMed] [Google Scholar]
- 26.Kim YW, Baik YH, Yun YH, Nam BH, Kim DH, Choi IJ, et al. Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer: results of a prospective randomized clinical trial. Ann Surg. 2008;248:721–727. doi: 10.1097/SLA.0b013e318185e62e. [DOI] [PubMed] [Google Scholar]
- 27.Kim YW, Yoon HM, Yun YH, Nam BH, Eom BW, Baik YH, et al. Long-term outcomes of laparoscopy-assisted distal gastrectomy for early gastric cancer: result of a randomized controlled trial (COACT 0301) Surg Endosc. 2013;27:4267–4276. doi: 10.1007/s00464-013-3037-x. [DOI] [PubMed] [Google Scholar]
- 28.Neudecker J, Klein F, Bittner R, Carus T, Stroux A, Schwenk W, et al. Short-term outcomes from a prospective randomized trial comparing laparoscopic and open surgery for colorectal cancer. Br J Surg. 2009;96:1458–1467. doi: 10.1002/bjs.6782. [DOI] [PubMed] [Google Scholar]
- 29.Weeks JC, Nelson H, Gelber S, Sargent D, Schroeder G. Clinical Outcomes of Surgical Therapy Study Group. Short-term quality-of-life outcomes following laparoscopic-assisted colectomy vs open colectomy for colon cancer: a randomized trial. JAMA. 2002;287:321–328. doi: 10.1001/jama.287.3.321. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto S, Inomata M, Katayama H, Mizusawa J, Etoh T, Konishi F, et al. Short-term surgical outcomes from a randomized controlled trial to evaluate laparoscopic and open D3 dissection for stage II/III colon cancer: Japan Clinical Oncology Group Study JCOG 0404. Ann Surg. 2014;260:23–30. doi: 10.1097/SLA.0000000000000499. [DOI] [PubMed] [Google Scholar]
- 31.Andersson J, Abis G, Gellerstedt M, Angenete E, Angeras U, Cuesta MA, et al. Patient-reported genitourinary dysfunction after laparoscopic and open rectal cancer surgery in a randomized trial (COLOR II) Br J Surg. 2014;101:1272–1279. doi: 10.1002/bjs.9550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arteaga Gonzalez I, Diaz Luis H, Martin Malagon A, Lopez-Tomasetti Fernandez EM, Arranz Duran J, Carrillo Pallares A, et al. A comparative clinical study of short-term results of laparoscopic surgery for rectal cancer during the learning curve. Int J Colorectal Dis. 2006;21:590–595. doi: 10.1007/s00384-005-0057-6. [DOI] [PubMed] [Google Scholar]
- 33.Braga M, Frasson M, Vignali A, Zuliani W, Carpetti G, Di Carlo V. Laparoscopic resection in rectal cancer patients: outcome and cost-benefit analysis. Dis Colon Rectum. 2007;50:464–471. doi: 10.1007/s10350-006-0798-5. [DOI] [PubMed] [Google Scholar]
- 34.Buchanan GN, Malik A, Parvaiz A, Sheffiel JP, Kennedy RH. Laparoscopic resection for colorectal cancer. Br J Surg. 2008;95:893–902. doi: 10.1002/bjs.6019. [DOI] [PubMed] [Google Scholar]
- 35.Cai J, Wei D, Gao CF, Zhang CS, Zhang H, Zhao T. A prospective randomized study comparing open versus laparoscopy-assisted D2 radical gastrectomy in advanced gastric cancer. Dig Surg. 2011;28:331–337. doi: 10.1159/000330782. [DOI] [PubMed] [Google Scholar]
- 36.Chung CC, Ng DC, Tsang WW, Tang WL, Yau KK, Cheung HY, et al. Hand-assisted laparoscopic versus open right colectomy: a randomized controlled trial. Ann Surg. 2007;246:728–733. doi: 10.1097/SLA.0b013e318123fbdf. [DOI] [PubMed] [Google Scholar]
- 37.Cui M, Li Z, Xing J, Yao Z, Liu M, Chen L, et al. A prospective randomized clinical trial comparing D2 dissection in laparoscopic and open gastrectomy for gastric cancer. Med Oncol. 2015;32:241. doi: 10.1007/s12032-015-0680-1. [DOI] [PubMed] [Google Scholar]
- 38.Curet MJ, Putrakul K, Pitcher DE, Josloff RK, Zucker KA. Laparoscopically assisted colon resection for colon carcinoma: perioperative results and long-term outcome. Surg Endosc. 2000;14:1062–1066. doi: 10.1007/s004640000092. [DOI] [PubMed] [Google Scholar]
- 39.Fleshman J, Branda M, Sargent DJ, Boller AM, George V, Abbas M, et al. Effect of Laparoscopic-Assisted Resection vs Open Resection of Stage II or III Rectal Cancer on Pathologic Outcomes: The ACOSOG Z6051 Randomized Clinical Trial. JAMA. 2015;314:1346–1355. doi: 10.1001/jama.2015.10529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fujii S, Ishibe A, Ota M, Yamagishi S, Watanabe K, Watanabe J, et al. Short-term results of a randomized study between laparoscopic and open surgery in elderly colorectal cancer patients. Surg Endosc. 2014;28:466–476. doi: 10.1007/s00464-013-3223-x. [DOI] [PubMed] [Google Scholar]
- 41.Gong J, Shi DB, Li XX, Cai SJ, Guan ZQ, Xu Y. Short-term outcomes of laparoscopic total mesorectal excision compared to open surgery. World J Gastroenterol. 2012;18:7308–7313. doi: 10.3748/wjg.v18.i48.7308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hayashi H, Ochiai T, Shimada H, Gunji Y. Prospective randomized study of open versus laparoscopy-assisted distal gastrectomy with extraperigastric lymph node dissection for early gastric cancer. Surg Endosc. 2005;19:1172–1176. doi: 10.1007/s00464-004-8207-4. [DOI] [PubMed] [Google Scholar]
- 43.Huscher CG, Mingoli A, Sgarzini G, Sansonetti A, Di Paola M, Recher A, et al. Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg. 2005;241:232–237. doi: 10.1097/01.sla.0000151892.35922.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Janson M, Bjorholt I, Carlsson P, Haglind E, Henriksson M, Lindholm E, et al. Randomized clinical trial of the costs of open and laparoscopic surgery for colonic cancer. Br J Surg. 2004;91:409–417. doi: 10.1002/bjs.4469. [DOI] [PubMed] [Google Scholar]
- 45.Janson M, Lindholm E, Anderberg B, Haglind E. Randomized trial of health-related quality of life after open and laparoscopic surgery for colon cancer. Surg Endosc. 2007;21:747–753. doi: 10.1007/s00464-007-9217-9. [DOI] [PubMed] [Google Scholar]
- 46.Kaiser AM, Kang JC, Chan LS, Vukasin P, Beart RW., Jr Laparoscopic-assisted vs. open colectomy for colon cancer: a prospective randomized trial. J Laparoendosc Adv Surg Tech A. 2004;14:329–334. doi: 10.1089/lap.2004.14.329. [DOI] [PubMed] [Google Scholar]
- 47.Kitano S, Shiraishi N, Fujii K, Yasuda K, Inomata M, Adachi Y. A randomized controlled trial comparing open vs laparoscopy-assisted distal gastrectomy for the treatment of early gastric cancer: an interim report. Surgery. 2002;131:S306–S311. doi: 10.1067/msy.2002.120115. [DOI] [PubMed] [Google Scholar]
- 48.Lacy AM, Delgado S, Garcia-Valdecasas JC, Castells A, Pique JM, Grande L, et al. Port site metastases and recurrence after laparoscopic colectomy. A randomized trial. Surg Endosc. 1998;12:1039–1042. doi: 10.1007/s004649900776. [DOI] [PubMed] [Google Scholar]
- 49.Lacy AM, Garcia-Valdecasas JC, Delgado S, Castells A, Taura P, Pique JM, et al. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet. 2002;359:2224–2229. doi: 10.1016/S0140-6736(02)09290-5. [DOI] [PubMed] [Google Scholar]
- 50.Lee JH, Han HS, Lee JH. A prospective randomized study comparing open vs laparoscopy-assisted distal gastrectomy in early gastric cancer: early results. Surg Endosc. 2005;19:168–173. doi: 10.1007/s00464-004-8808-y. [DOI] [PubMed] [Google Scholar]
- 51.Li JC, Leung KL, Ng SS, Liu SY, Lee JF, Hon SS. Laparoscopic-assisted versus open resection of right-sided colonic cancer–a prospective randomized controlled trial. Int J Colorectal Dis. 2012;27:95–102. doi: 10.1007/s00384-011-1294-5. [DOI] [PubMed] [Google Scholar]
- 52.Liang JT, Huang KC, Lai HS, Lee PH, Jeng YM. Oncologic results of laparoscopic versus conventional open surgery for stage II or III left-sided colon cancers: a randomized controlled trial. Ann Surg Oncol. 2007;14:109–117. doi: 10.1245/s10434-006-9135-4. [DOI] [PubMed] [Google Scholar]
- 53.Liang X, Hou S, Liu H, Li Y, Jiang B, Bai W, et al. Effectiveness and safety of laparoscopic resection versus open surgery in patients with rectal cancer: a randomized, controlled trial from China. J Laparoendosc Adv Surg Tech A. 2011;21:381–385. doi: 10.1089/lap.2010.0059. [DOI] [PubMed] [Google Scholar]
- 54.Lujan J, Valero G, Hernandez Q, Sanchez A, Frutos MD, Parilla P. Randomized clinical trial comparing laparoscopic and open surgery in patients with rectal cancer. Br J Surg. 2009;96:982–989. doi: 10.1002/bjs.6662. [DOI] [PubMed] [Google Scholar]
- 55.Ng SS, Leung KL, Lee JF, Yiu RY, Li JC, Hon SS. Long-term morbidity and oncologic outcomes of laparoscopic-assisted anterior resection for upper rectal cancer: ten-year results of a prospective, randomized trial. Dis Colon Rectum. 2009;52:558–566. doi: 10.1007/DCR.0b013e31819ec20c. [DOI] [PubMed] [Google Scholar]
- 56.Ng SS, Leung KL, Lee JF, Yiu RY, Li JC, Teoh AY, et al. Laparoscopic-assisted versus open abdominoperineal resection for low rectal cancer: a prospective randomized trial. Ann Surg Oncol. 2008;15:2418–2425. doi: 10.1245/s10434-008-9895-0. [DOI] [PubMed] [Google Scholar]
- 57.Sakuramoto S, Yamashita K, Kikuchi S, Futawatari N, Katada N, Watanabe M, et al. Laparoscopy versus open distal gastrectomy by expert surgeons for early gastric cancer in Japanese patients: short-term clinical outcomes of a randomized clinical trial. Surg Endosc. 2013;27:1695–1705. doi: 10.1007/s00464-012-2658-9. [DOI] [PubMed] [Google Scholar]
- 58.Schwenk W, Bohm B, Haase O, Junghans T, Muller JM. Laparoscopic versus conventional colorectal resection: a prospective randomised study of postoperative ileus and early postoperative feeding. Langenbecks Arch Surg. 1998;383:49–55. doi: 10.1007/s004230050091. [DOI] [PubMed] [Google Scholar]
- 59.Stevenson AR, Solomon MJ, Lumley JW, Hewett P, Clouston AD, Gebski VJ, et al. Effect of Laparoscopic-Assisted Resection vs Open Resection on Pathological Outcomes in Rectal Cancer: The ALaCaRT Randomized Clinical Trial. JAMA. 2015;314:1356–1363. doi: 10.1001/jama.2015.12009. [DOI] [PubMed] [Google Scholar]
- 60.Takiguchi S, Fujiwara Y, Yamasaki M, Miyata H, Nakajima K, Sekimoto M, et al. Laparoscopy-assisted distal gastrectomy versus open distal gastrectomy. A prospective randomized single-blind study. World J Surg. 2013;37:2379–2386. doi: 10.1007/s00268-013-2121-7. [DOI] [PubMed] [Google Scholar]
- 61.Winslow ER, Fleshman JW, Birnbaum EH, Brunt ML. Wound complications of laparoscopic vs open colectomy. Surg Endosc. 2002;16:1420–1425. doi: 10.1007/s00464-002-8837-3. [DOI] [PubMed] [Google Scholar]
- 62.Zhou ZG, Hu M, Li Y, Lei WZ, Yu YY, Cheng Z, et al. Laparoscopic versus open total mesorectal excision with anal sphincter preservation for low rectal cancer. Surg Endosc. 2004;18:1211–1215. doi: 10.1007/s00464-003-9170-1. [DOI] [PubMed] [Google Scholar]
- 63.Dunn G, Maracy M, Tomenson B. Estimating treatment effects from randomized clinical trials with noncompliance and loss to follow-up: the role of instrumental variable methods. Stat Methods Med Res. 2005;14:369–395. doi: 10.1191/0962280205sm403oa. [DOI] [PubMed] [Google Scholar]
- 64.Latimer N. The role of treatment crossover adjustment methods in the context of economic evaluation PhD thesis. 2012. University of Sheffield, Sheffield.
- 65.Garas G, Cingolani I, Panzarasa P, Darzi A, Athanasiou T. Network analysis of surgical innovation: measuring value and the virality of diffusion in robotic surgery. PLoS One. 2017;12:e0183332. doi: 10.1371/journal.pone.0183332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Georgalas C, Vlastos I, Picavet V, van Drunen C, Garas G, Prokopakis E. Is chronic rhinosinusitis related to allergic rhinitis in adults and children? Applying epidemiological guidelines for causation. Allergy. 2014;69:828–833. doi: 10.1111/all.12413. [DOI] [PubMed] [Google Scholar]
- 67.Arora A, Garas G, Sharma S, Muthuswamy K, Budge J, Palazzo F, et al. Comparing transaxillary robotic thyroidectomy with conventional surgery in a UK population: A case control study. Int J Surg. 2016;27:110–117. doi: 10.1016/j.ijsu.2016.01.071. [DOI] [PubMed] [Google Scholar]
- 68.Buckley CJ, Rutherford RB, Diethrich EB, Buckley SD. Inherent problems with randomized clinical trials with observational/no treatment arms. J Vasc Surg. 2010;52:237–241. doi: 10.1016/j.jvs.2010.02.255. [DOI] [PubMed] [Google Scholar]
- 69.Tolley N, Garas G, Palazzo F, Prichard A, Chaidas K, Cox J, et al. Long-term prospective evaluation comparing robotic parathyroidectomy with minimally invasive open parathyroidectomy for primary hyperparathyroidism. Head Neck. 2016;38:E300–E306. doi: 10.1002/hed.23990. [DOI] [PubMed] [Google Scholar]
- 70.Wong KA, Hodgson L, Garas G, Malietzis G, Markar S, Rao C, et al. How can cardiothoracic and vascular medical devices stay in the market? Interact Cardiovasc Thorac Surg. 2016;23:940–948. doi: 10.1093/icvts/ivw257. [DOI] [PubMed] [Google Scholar]
- 71.Mackenzie H, Markar SR, Askari A, Ni M, Faiz O, Hanna GB. National proficiency-gain curves for minimally invasive gastrointestinal cancer surgery. Br J Surg. 2016;103:88–96. doi: 10.1002/bjs.9963. [DOI] [PubMed] [Google Scholar]
- 72.Deijen CL, Velthuis S, Tsai A, Mavroveli S, de Lange-de Klerk ES, Sietses C. COLOR III: a multicentre randomised clinical trial comparing transanal TME versus laparoscopic TME for mid and low rectal cancer. Surg Endosc. 2016;30:3210–3215. doi: 10.1007/s00464-015-4615-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.