Abstract
Progestins used in endocrine therapies bind to multiple steroid receptors and are associated with several side-effects. It is thus important to understand the relationship between steroid receptor cross-reactivity and the side-effect profile of progestins. In cell lines that express negligible levels of steroid receptors, we report for the first time the binding affinities, potencies and efficacies of selected progestins from different generations determined in parallel. We show that the progestins bind to the androgen receptor (AR) with similar affinities to each other and progesterone, while none bind estrogen receptor (ER)-β, and only norethisterone acetate, levonorgestrel and gestodene bind ERα. Comparative dose-response analysis revealed that progestins from the first three generations display similar androgenic activity to the natural androgen dihydrotestosterone for transactivation, while norethisterone acetate, levonorgestrel and gestodene are ERα agonists. We show for the first time that the anti-androgenic properties of progesterone and drospirenone are similar to the well-known AR antagonist hydroxyflutamide, while nomegestrol acetate is more potent and nestorone less potent than both hydroxyflutamide and progesterone. Moreover, we are the first to report that the older progestins, unlike progesterone and the fourth generation progestins, are efficacious ERα agonists for transrepression, while the selected progestins from the second and third generation are efficacious AR agonists for transrepression. Considering the progestin potencies and their reported free serum concentrations relative to dihydrotestosterone and estradiol, our results suggest that the progestins are likely to exert AR-, but not ERα- or ERβ-mediated effects in vivo.
Keywords: Androgen receptor, estrogen receptor, progestins, contraception, hormone therapy
1. Introduction
A variety of progestins, most of which are structurally related to the natural progestogen, progesterone (P4), or testosterone [1,2], are used by women in contraception and menopausal hormone therapy (HT) [2,3]. Progestins are classified into four consecutive generations, with the fourth-generation reputed to be designed to have a greater affinity for the P4 receptor (PR) and elicit biological effects more similar to P4 than progestins from the earlier generations [3–5]. Despite their therapeutic benefits, clinical trials and epidemiological studies suggest that some progestins may result in side-effects including increased risk of developing breast cancer, cardiovascular disease, venous thromboembolism and increased susceptibility to genital tract infections [6–9]. Some of these adverse effects may be attributed to off-target actions via steroid receptors other than the PR [1,3,10].
Previously, the Hapgood laboratory directly compared the off-target mechanisms of the first-generation progestins medroxyprogesterone acetate (MPA) and norethisterone acetate (NET-A), and showed that they do not always mimic the effects of P4 [11–13], and can differ from one another [11,13]. For example, P4 displays anti-androgenic properties, while both MPA and NET-A are androgenic [12]. Furthermore, MPA, but not NET-A, is a partial glucocorticoid receptor agonist for transactivation [11]. However, numerous other progestins are available for clinical use, and it is surprising that studies comparing the activities of different progestins via individual steroid receptors are lacking.
Steroid receptors are ligand-activated transcription factors that activate or repress transcription of target genes [12,14]. Generally, transactivation refers to the increase in gene transcription caused by the ligand-activated steroid receptor such as the androgen- (AR) [12] or estrogen receptor (ER) [14] binding to androgen or estrogen response elements (AREs or EREs, respectively) in the promoters of target genes. Transrepression, on the other hand, can occur when the ligand-bound receptor inhibits gene transcription through protein-protein interactions with other transcription factors such as nuclear factor kappa-B (NFκB) or activator protein (AP)-1. This study is the first to directly compare the binding affinities and transcriptional activities of selected progestins from different generations relative to each other and P4, in steroid receptor-deficient cell lines exogenously expressing human AR, ERα or ERβ.
2. Materials and Methods
2.1. Inducing compounds
P4, MPA, NET-A, levonorgestrel (LNG), gestodene (GES), nestorone (NES), nomegestrol acetate (NoMAC), drospirenone (DRSP), dihydrotestosterone (DHT), hydroxyflutamide (OHF), estradiol (E2), fulvestrant (ICI 182,780) and phorbol 12-myristate13-acetate (PMA) were obtained from Sigma-Aldrich, RSA. Human tumor necrosis factor-alpha (TNFα) was obtained from Celtic Diagnostics, RSA. Unlabeled mibolerone (MIB) and [3H]-MIB (84.3 Ci/mmol) were purchased from PerkinElmer Life and Analytical Science, RSA, while [3H]-E2 (100 Ci/mmol) was obtained from AEC-Amersham, RSA.
2.2. Cell Culture
The COS-1 monkey kidney and HEK293 human embryonic kidney cell lines were obtained from the ATCC and cultured as previously described [12,15]. Only mycoplasma-negative cell lines were used in experiments.
2.3. Plasmids
The human AR expression vector (pSV-ARo) [16] was obtained from F. Claessens (University of Leuven, Belgium), while the human ERα and ERβ (pSG5-hERα and pSG5-hERβ) expression vectors [17] were received from F. Gannon (European Molecular Biology Laboratory, Germany). The pTAT-2xPRE-E1b-luciferase [18] and pGL3-2xERE-pS2-luciferase [19] constructs were gifts from G. Jenster (Erasmus University of Rotterdam, Netherlands) and B. Belandia (Institute for Biomedical Research, Spain), respectively. The 5xNFκB-luciferase plasmid was purchased from Stratagene (Houston, USA), while the p(IL6κB)350hu.IL6P-luciferase construct [20] was received from G. Haegeman (Ghent University, Belgium).
2.4. Whole cell binding assay
Competitive whole cell binding assays were performed in COS-1 cells as previously described ([12], Perkins et al., unpublished). Total binding ([3H]-MIB or [3H]-E2 in the absence of unlabeled competitor) was set as 100% and binding of the unlabeled competitors plotted relative to this. Kd values were determined from homologous displacement curves using a global fitting model [21], whilst Ki values for the competing ligands were determined from heterologous displacement curves using the equation by Cheng and Prusoff [22].
2.5. Luciferase reporter assays
Promoter-reporter assays were performed essentially as previously described for the AR [12] and ER (Perkins et al., unpublished), with a few modifications. Briefly, COS-1 or HEK293 cells were seeded into 10 cm dishes at a density of 2 × 106 cells. After 24 hours, the cells were transiently transfected using XtremeGene HP (Roche Molecular Biochemicals) as per the manufacturer’s instructions. For transactivation assays, COS-1 cells were transfected with 2 µg AR and 20 µg pTAT-2xPRE-E1b-luciferase, while HEK293 cells were transfected with 0.15 µg ERα or ERβ and 6 µg (ERα) or 3 µg (ERβ) pGL3-2xERE-pS2-luciferase. For transrepression assays, COS-1 cells were transfected with 1.35 µg AR and 2.7 µg 5xNFκB-luciferase, while HEK293 cells were transfected with 0.15 µg ERα or ERβ and 1.5 µg p(IL6κB)350hu.IL6P-luciferase as transrepression after TNFα induction was not observed in COS-1 cells (data not shown). After 24 hours, transfected cells were replated into 96-well plates at a density of 1 × 104 cells per well and treated with varying concentrations of test compounds in the absence (agonist dose-response) or presence of 0.1 nM MIB (AR) or 1 nM E2 (ER) (antagonist dose-response) for 24 hours (transactivation). For transrepression assays, the cells were treated for 24 hours with either 10 ng/ml PMA (AR) or 20 ng/ml TNFα (ER) and increasing concentrations of the test compounds in the absence (agonist dose-response) or presence of 0.1 nM MIB (AR) or 1 nM E2 (ER) (antagonist dose-response). The efficacies (maximal response) and potencies (EC50 values) were determined. Transcriptional activity of the ligands in the absence of transfected receptors was negligible in both cell lines (data not shown).
2.6. Data manipulation and statistical analysis
Graph Pad Prism® software version 5 was used for data analysis. Non-linear regression and one site competition were used for binding assays, while non-linear regression and sigmoidal dose-response were used for luciferase reporter assays. For both binding and dose-response analysis, fixed Hill slopes of 1 (transactivation) or −1 (competitive binding; transrepression) were chosen, which fitted the data with R2 values of ≥ 0.9. One-way ANOVA analysis of variance and Newman-Keuls (compares all pairs of columns) post-test were used for statistical analysis. Statistically significant differences are indicated by different letters (a, b, c). Figures show pooled results and standard error of the mean (SEM) from at least two independent experiments performed in triplicate.
3. Results
3.1. Whilst all the selected progestogens bind to the AR, only NET-A, LNG and GES bind to ERα
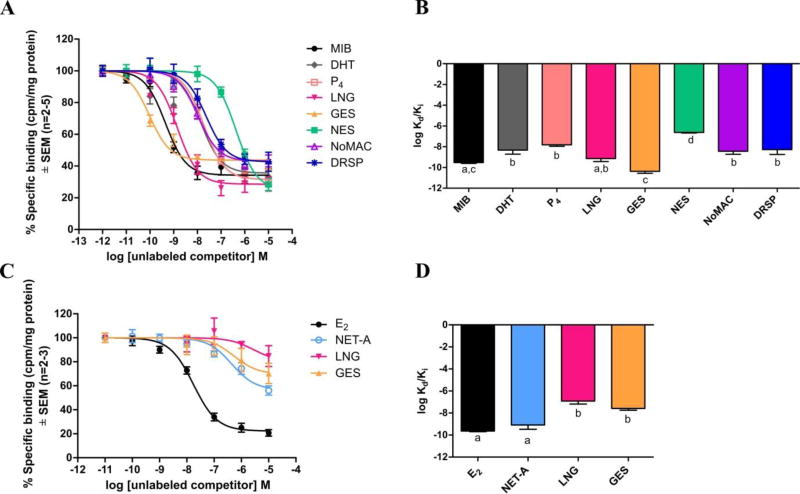
Competitive whole cell binding assays in COS-1 cells expressing exogenous human AR, ERα or ERβ showed that all progestogens investigated bind to the AR (Fig. 1A), while only NET-A, LNG and GES bind to ERα (Fig. 1C and Supplementary Fig. 1A), and none bind to ERβ (Supplementary Fig. 1B). We confirmed the Kd/Ki values previously determined for MIB, DHT, P4, MPA and NET-A [12], and showed that LNG, NoMAC and DRSP have similar binding affinities to each other and DHT for the AR, while GES exhibits a significantly higher affinity and NES a significantly lower affinity than the other progestogens and DHT (Fig. 1B; Supplementary Table 1). Surprisingly, NET-A displays a similar affinity to E2 for ERα, while LNG and GES display similar binding affinities to each other, but significantly lower than that of E2 and NET-A (Fig. 1C and 1D).
Fig. 1.
(A) The selected progestogens all compete with [3H]-MIB for binding to the human AR, (C) while only NET-A, LNG and GES bind to human ERα. COS-1 cells expressing the human (A) AR or (C) ERα expression vector, were incubated for (A) 16 hours with 0.2 nM [3H]-MIB in the absence or presence of increasing concentrations of either unlabeled MIB (●), DHT (◆), LNG (▼), GES (▲), NES (■), NoMAC (△), or DRSP (*) or (C) 4 hours with 10 nM [3H]-E2 in the absence or presence of increasing concentrations of either unlabeled E2 (●), NET-A (○), LNG (▼) and GES (▲). Counts per minute (cpm) were measured and normalized to protein concentration determined by the Bradford method [40]. Total specific binding of (A) [3H]-MIB or (C) [3H]-E2 only was set as 100% and the binding of unlabeled competitors plotted relative to this. Log Kd/Ki values of the ligands for the (B) AR and (D) ERα were plotted.
3.2. NET-A, LNG and GES are agonists for both the AR and ERα, while NES, NoMAC and DRSP are AR antagonists
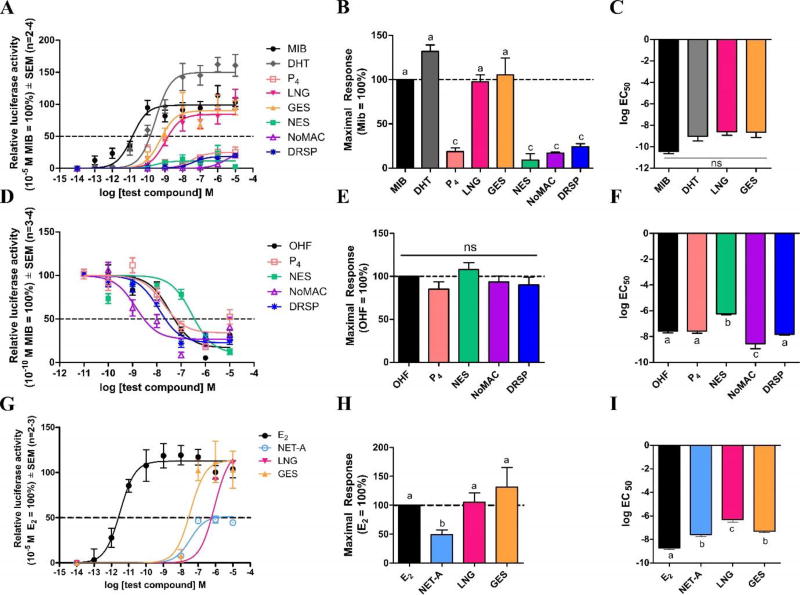
We next directly compared the relative agonist and antagonist efficacies and potencies (Supplementary Table 2 and 3) of the progestogens for transactivation on an ARE-driven reporter construct via expressed AR in COS-1 cells or an ERE-driven reporter construct via expressed ERα in HEK293 cells. Results showed that LNG and GES display similar AR agonist efficacies and potencies to each other and DHT (Fig. 2A–2C). In contrast, NES, NoMAC and DRSP, like P4, display similar AR antagonist efficacies (Fig. 2D and 2E), but differential potencies (Fig. 2D and 2F). While P4 and DRSP have similar potencies to the well-known AR antagonist OHF, NES is less potent and NoMAC more potent. We showed that although LNG and GES are full ERα agonists, while NET-A is a partial agonist (Fig. 2G and 2H), all three progestins display lower potencies than E2 (Fig. 2G and 2I).
Fig. 2.
(A) Second- and third-generation progestins display similar androgenic properties to DHT, while, like P4, the fourth-generation progestins are AR antagonists. COS-1 cells, expressing the (A and D) human AR and the pTAT-2xPRE-E1b-luciferase reporter plasmid, were treated with varying concentrations of MIB (●), DHT (◆), P4 (□), LNG (▼), GES (▲), NES (■), NoMAC (△) or DRSP (*) in the (A) absence or (D) presence of 0.1 nM MIB (set as 100%) for 24 hours. (G) LNG and GES are full agonists for transactivation via ERα, while NET-A is a partial agonist. The HEK293 cell line, expressing the (G) human ERα and the pGL3-2xERE-pS2-luciferase promoter-reporter plasmid, were treated with increasing concentrations of E2 (●), NET-A (○), LNG (▼) and GES (▲) for 24 hours. Luciferase activity was measured in relative light units and normalized to protein concentration determined by the Bradford method [40]. (B, E and H) Maximal responses and (C, F and I) log EC50 values were plotted. As P4, NES, NoMAC and DRSP displayed very weak partial AR agonist activity, these log EC50 values were not depicted in (C) and should be interpreted with caution.
3.3. Fourth-generation progestins, unlike progestins from the first three generations, are partial AR agonists for transrepression, while NET-A, LNG and GES are ERα agonists for transrepression
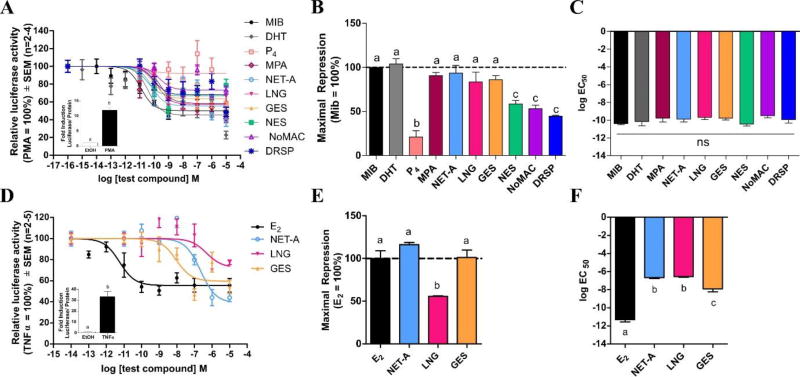
We also compared the relative agonist and antagonist efficacies and potencies of the progestogens for transrepression (Supplementary Table 4) on an NFκB-containing promoter-reporter construct, in COS-1 cells expressing the AR and HEK293 cells expressing ERα. We showed that although all progestogens display similar agonist potencies to each other and to DHT (Fig. 3A and 3C), MPA, NET-A, LNG and GES, like DHT, are full AR agonists for transrepression, while P4, NES, NoMAC and DRSP are partial agonists (Fig. 3A and 3B). Although P4, NES, NoMAC and DRSP display similar antagonist efficacies, the progestins are less potent than P4 (Supplementary Fig. 3A–3D). In terms of ERα, although NET-A and GES are full agonists for transrepression, while LNG is a partial agonist (Fig. 3D and 3E), NET-A and LNG are less potent than GES (Fig. 3D and 3F).
Fig. 3.
(A) Progestins from the first three generations are full agonist for transrepression via the AR, while the fourth-generation progestins are partial agonists. COS-1 cells expressing human AR and the 5xNFκB-luciferase reporter plasmid, were treated with vehicle (EtOH) and 10 ng/ml PMA in the absence (set as 100%) or presence of increasing concentrations of MIB (●), DHT (◆), P4 (□), MPA (◊), NET-A (○), LNG (▼), GES (▲), NES (■), NoMAC (△) or DRSP (*) for 24 hours. (D) NET-A and GES are full agonists for transrepression via ERα, while LNG is a partial agonist. HEK293 cells expressing human ERα and the p(IL6κB)350hu.IL6P-luciferase reporter plasmid, were treated with vehicle (EtOH) and 20 ng/ml TNFα in the absence (set as 100%) or presence of increasing concentrations of E2 (●), NET-A (○), LNG (▼) and GES (▲) for 24 hours. Luciferase activity was measured and normalized as in Fig. 2. Treatment with PMA or TNFα resulted in a ~12-fold and ~33-fold induction, respectively (Fig. 3A and 3D inserts). (B and E) Maximal repression and (C and F) log EC50 values were plotted.
4. Discussion
Most studies comparing the binding of progestins to the AR report EC50 values (relative binding affinities) (reviewed in [2,12,23]) rather than precise equilibrium dissociation constants (Ki values), and mostly investigate binding to the rat AR or human AR in cell lines or tissue endogenously expressing other steroid receptors to which these ligands can bind (reviewed in [2,23]). We have recently reported accurate Ki values for P4, MPA and NET-A for the human AR in COS-1 cells expressing negligible levels of endogenous steroid receptors [12]. Here we report for the first time accurate Ki values for LNG, GES, NES, NoMAC and DRSP (Supplementary Table 1). We performed detailed dose-response analysis and report both maximal responses and EC50 values for the progestins, relative to each other, natural P4 and known androgens. We show for the first time that P4 and DRSP are as potent as the well-known AR antagonist OHF, while NoMAC is more potent and NES less potent than P4 and OHF (Fig. 2D – 2F). Although others have investigated the androgenic and anti-androgenic properties of some progestins for transactivation via the human AR [2,12,24–27], these studies often did not investigate progestins from different generations in parallel, did not include the relevant AR controls, used cell lines or tissues expressing multiple steroid receptors and do not investigate transrepression. This study is the first to show that progestins from the first three generations display similar androgenic properties for transrepression to each other and DHT, while P4 and the fourth-generation progestins displayed anti-androgenic properties (Supplementary Fig. 3B) and partial agonist activity (Fig. 3A and 3B).
We showed that NET-A, LNG and GES, all structurally related to the estrogen precursor testosterone [28], bind to human ERα (Fig. 1C), but not human ERβ (Supplementary Fig. 1B), and are ERα agonists for both transactivation (Fig. 2G, Supplementary Fig. 2A) and transrepression (Fig. 3D, Supplementary Fig. 3E). Interestingly, previous studies investigated binding of progestin metabolites to ER subtypes rather than the parent compounds [29,30]. Results investigating binding of parent progestins such as MPA and NET-A are contradictory, possibly due to differences in model systems used (reviewed in [3]). Although the COS-1 and HEK293 cell lines express negligible levels of steroidogenic enzymes [31,32], we cannot exclude the possibility that the observed binding and estrogenic activity of these progestins may be due to progestin metabolites, as NET-A, LNG and GES have been shown to undergo metabolism [30], and it is known that some metabolites bind to and activate ERα [29,30]. However, as we showed no binding of NET-A, LNG and GES to ERβ, but others have reported binding of NET, LNG and GES metabolites [29,30], our results suggest that these progestins are probably not metabolized in our systems.
The physiological implications of our progestin results should be considered in the light of their affinities for the AR and ER, their serum concentrations in women using endocrine therapies and whether the progestins can bind to serum binding proteins, such as sex hormone binding globulin (SHBG). Steroids bound to SHBG are not available to enter target tissues, while unbound (free) steroids are, and can thus elicit a biological response. [33]. Both DHT and E2 bind to SHBG resulting in less than 1% of DHT and approximately 50% of E2 being available to bind to the AR and ER, respectively, in target tissues [33,34]. While MPA, NES, NoMAC and DRSP do not bind to SHBG and are 100% available, NET-A, LNG and GES can bind and the availability of these progestins is approximately 65%, 50% and 25%, respectively [2,34,35]. Considering the above, and the affinities of DHT and the progestins for the AR, plus the fact that the progestin EC50 values for the AR in our study are within the range of serum concentrations reported for MPA (0.2 – 65 nM) [13], NET-A (17.6 – 36 nM) (Jinteli package insert, Teva Pharmaceuticals USA Inc.), LNG (4.4 – 16 nM) [36], GES (6.4 – 31 nM) [36], NES (0.1 – 27.3 nM) [37], NoMAC (3 – 33 nM) [38] and DRSP (26.7 – 253 nM) [39], it is likely that the progestins will compete with DHT for binding to the AR in vivo. However, considering the affinities of the progestins and E2 for ERα, and that the EC50 values determined for NET-A, LNG and GES are 10 to 100-fold lower than the serum concentrations mentioned above, it is unlikely that these progestins will compete with E2 for binding to ERα in target tissues. Taken together, our results showing that NES, NoMAC and DRSP elicit anti-androgenic and little to no androgenic activity, while lacking estrogenic effects, reassure the claims that the fourth-generation progestins are more similar to P4 than progestins from the first three generations.
Supplementary Material
Acknowledgments
Funding: This work was supported by grants to DA from the National Research Foundation (Grant No: 99114) and Medical Research Council in South Africa, and a grant to JH from the National Institute of Health (Grant No: R01 HD083026-02S1). The funders had no role in study design, data collection and analysis, interpretation of data, decision to publish, or preparation of the manuscript. Any opinion, findings and conclusions or recommendations expressed in this material are those of the authors, and therefore the NRF does not accept any liability in regard thereto.
Abbreviations
- AR
androgen receptor
- ARE
androgen response element
- DHT
dihydrotestosterone
- DRSP
drospirenone
- ER
estrogen receptor
- ERE
estrogen response element
- E2
estradiol
- GES
gestodene
- HT
hormone therapy
- LNG
levonorgestrel
- MIB
mibolerone
- MPA
medroxyprogesterone acetate
- NES
nestorone
- NET-A
norethisterone acetate
- NoMAC
nomegestrol acetate
- OHF
hydroxyflutamide
- P4
progesterone
- PMA
phorbol 12-myristate 13-acetate
- PR
progesterone receptor
- TNFα
tumor necrosis factor alpha
References
- 1.Stanczyk FZ, Hapgood JP, Winer S, Mishell DR. Progestogens Used in Postmenopausal Hormone Therapy: Differences in Their Pharmacological Properties, Intracellular Actions, and Clinical Effects. Endocr. Rev. 2013;34:171–208. doi: 10.1210/er.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schindler AE, Campagnoli C, Druckmann R, Huber J, Pasqualini JR, Schweppe KW, Thijssen JH. Classification and pharmacology of progestins. Maturitas. 2003;46:7–16. doi: 10.1016/j.maturitas.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Africander D, Verhoog N, Hapgood JP. Molecular mechanisms of steroid receptor-mediated actions by synthetic progestins used in HRT and contraception. Steroids. 2011;76:636–652. doi: 10.1016/j.steroids.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Sitruk-Ware R. Pharmacological profile of progestins. Maturitas. 2004;47:277–283. doi: 10.1016/j.maturitas.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Sitruk-Ware R, Nath A. The use of newer progestins for contraception. Contraception. 2010;82:410–417. doi: 10.1016/j.contraception.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 6.M.W.S. Collaborators. Million Women Study Collaborators, Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362:419–27. doi: 10.1016/S0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- 7.Writing Group for the Women’s Health Initiaitive Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women’s Health Initiative randomized controlled trial. J. Am. Med. Assoc. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 8.Gronich N, Lavi I, Rennert G. Higher risk of venous thrombosis associated with drospirenone-containing oral contraceptives: a population-based cohort study. Can. Med. Assoc. J. 2011;183:E1319–E1325. doi: 10.1503/cmaj.110463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heffron R, Donnell D, Rees H, Celum C, Mugo N, Were E, de Bruyn G, Nakku-Joloba E, Ngure K, Kiarie J, Coombs RW, Baeten JM. Use of hormonal contraceptives and risk of HIV-1 transmission: a prospective cohort study. Lancet Infect. Dis. 2012;12:19–26. doi: 10.1016/S1473-3099(11)70247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hapgood JP, Koubovec D, Louw A, Africander D. Not all progestins are the same: implications for usage. Trends Pharmacol. Sci. 2004;25:554–557. doi: 10.1016/j.tips.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Koubovec D, Ronacher K, Stubsrud E, Louw A, Hapgood JP. Synthetic progestins used in HRT have different glucocorticoid agonist properties. Mol. Cell. Endocrinol. 2005;242:23–32. doi: 10.1016/j.mce.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Africander DJ, Storbeck K-H, Hapgood JP. A comparative study of the androgenic properties of progesterone and the progestins, medroxyprogesterone acetate (MPA) and norethisterone acetate (NET-A) J. Steroid Biochem. Mol. Biol. 2014;143:404–415. doi: 10.1016/j.jsbmb.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Africander D, Louw R, Hapgood JP. Investigating the anti-mineralocorticoid properties of synthetic progestins used in hormone therapy. Biochem. Biophys. Res. Commun. 2013;433:305–310. doi: 10.1016/j.bbrc.2013.02.086. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez R, Nguyen D, Rocha W, White JH, Mader S. Diversity in the mechanisms of gene regulation by estrogen receptors. Bioessays. 2002;24:244–54. doi: 10.1002/bies.10066. [DOI] [PubMed] [Google Scholar]
- 15.Mortimer M, Visser K, de Beer D, Joubert E, Louw A. Divide and Conquer May Not Be the Optimal Approach to Retain the Desirable Estrogenic Attributes of the Cyclopia Nutraceutical Extract, SM6Met. PLoS One. 2015;10:e0132950. doi: 10.1371/journal.pone.0132950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brinkmann AO, Faber PW, van Rooij HCJ, Kuiper GGJM, Ris C, Klaassen P, van der Korput JAGM, Voorhorst MM, van Laar JH, Mulder E, Trapman J. The human androgen receptor: Domain structure, genomic organization and regulation of expression. J. Steroid Biochem. 1989;34:307–310. doi: 10.1016/0022-4731(89)90098-8. [DOI] [PubMed] [Google Scholar]
- 17.Flouriot G. Identification of a new isoform of the human estrogen receptor-alpha (hER-alpha) that is encoded by distinct transcripts and that is able to repress hER-alpha activation function 1. EMBO J. 2000;19:4688–4700. doi: 10.1093/emboj/19.17.4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jenster G, Spencer TE, Burcin MM, Tsai SY, Tsai M-J, O’Malley BW. Steroid receptor induction of gene transcription: A two-step model. Proc. Natl. Acad. Sci. 1997;94:7879–7884. doi: 10.1073/pnas.94.15.7879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belandia B, Parker MG. Functional Interaction between the p160 Coactivator Proteins and the Transcriptional Enhancer Factor Family of Transcription Factors. J. Biol. Chem. 2000;275:30801–30805. doi: 10.1074/jbc.C000484200. [DOI] [PubMed] [Google Scholar]
- 20.Plaisance S, Vanden Berghe W, Boone E, Fiers W, Haegeman G. Recombination signal sequence binding protein Jkappa is constitutively bound to the NF-kappaB site of the interleukin-6 promoter and acts as a negative regulatory factor. Mol. Cell. Biol. 1997;17:3733–3743. doi: 10.1128/MCB.17.7.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Motulsky A, Christopoulos HJ, Motulsky H, Christopoulos A. Fitting models to biological data using linear and nonlinear regression. A practical guide to curve fitting. 2003 www.graphpad.com.
- 22.Cheng Y-C, Prusoff WH. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 23.Hapgood JP, Africander D, Louw R, Ray RM, Rohwer JM. Potency of progestogens used in hormonal therapy: Toward understanding differential actions. J. Steroid Biochem. Mol. Biol. 2014;142:39–47. doi: 10.1016/j.jsbmb.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Attardi BJ, Koduri S, Hild SA. Relative progestational and androgenic activity of four progestins used for male hormonal contraception assessed in vitro in relation to their ability to suppress LH secretion in the castrate male rat. Mol. Cell. Endocrinol. 2010;328:16–21. doi: 10.1016/j.mce.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 25.Kemppainen JA, Langley E, Wong C, Bobseine K, Kelce WR, Wilson EM, Hill C, Carolina N, B EBK. Agonists and Antagonists : Distinct Mechanisms of Activation by Medroxyprogesterone Acetate and Dihydrotestosterone. 2016:440–454. doi: 10.1210/mend.13.3.0255. [DOI] [PubMed] [Google Scholar]
- 26.Kumar N, Fagart J, Liere P, Mitchell SJ, Knibb AR, Petit-Topin I, Rame M, El-Etr M, Schumacher M, Lambert JJ, Rafestin-Oblin M-E, Sitruk-Ware R. Nestorone® (NES) a Novel Progestin for Non-oral Contraception: Structure-activity Relationships and Brain Metabolism Studies. Endocrinology. 2016 doi: 10.1210/en.2016-1426. en.2016-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beck V, Reiter E, Jungbauer A. Androgen receptor transactivation assay using green fluorescent protein as a reporter. Anal. Biochem. 2008;373:263–271. doi: 10.1016/j.ab.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Miller WL, Auchus RJ. The Molecular Biology, Biochemistry, and Physiology of Human Steroidogenesis and Its Disorders. Endocr. Rev. 2011;32:81–151. doi: 10.1210/er.2010-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Escande A, Pillon A, Servant N, Cravedi J-P, Larrea F, Muhn P, Nicolas J-C, Cavaillès V, Balaguer P. Evaluation of ligand selectivity using reporter cell lines stably expressing estrogen receptor alpha or beta. Biochem. Pharmacol. 2006;71:1459–1469. doi: 10.1016/j.bcp.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Larrea F, García-Becerra R, Lemus AE, García GA, Pérez-Palacios G, Jackson KJ, Coleman KM, Dace R, Smith CL, Cooney AJ. A-ring reduced metabolites of 19-nor synthetic progestins as subtype selective agonists for ERα. Endocrinology. 2001;142:3791–9. doi: 10.1210/endo.142.9.8401. [DOI] [PubMed] [Google Scholar]
- 31.Louw-du Toit R, Perkins MS, Snoep JL, Storbeck K-H, Africander D. Fourth-Generation Progestins Inhibit 3β-Hydroxysteroid Dehydrogenase Type 2 and Modulate the Biosynthesis of Endogenous Steroids. PLoS One. 2016;11:e0164170. doi: 10.1371/journal.pone.0164170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khan N, Sharma KK, Andersson S, Auchus RJ. Human 17β-hydroxysteroid dehydrogenases types 1, 2, and 3 catalyze bi-directional equilibrium reactions, rather than unidirectional metabolism, in HEK-293 cells. Arch. Biochem. Biophys. 2004;429:50–59. doi: 10.1016/j.abb.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 33.Dunn JF, Nisula BC, Rodbard D. Transport of Steroid Hormones: Binding of 21 Endogenous Steroids to Both Testosterone-Binding Globulin and Corticosteroid-Binding Globulin in Human Plasma. J. Clin. Endocrinol. Metab. 1981;53:58–68. doi: 10.1210/jcem-53-1-58. [DOI] [PubMed] [Google Scholar]
- 34.Kuhl H. Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric. 2005;8:3–63. doi: 10.1080/13697130500148875. [DOI] [PubMed] [Google Scholar]
- 35.Kuhnz W, Pfeffer M, Al-Yacoub G. Protein binding of the contraceptive steroids gestodene, 3-keto-desogestrel and ethinylestradiol in human serum. J. Steroid Biochem. 1990;35:313–8. doi: 10.1016/0022-4731(90)90290-9. http://www.ncbi.nlm.nih.gov/pubmed/2308344. [DOI] [PubMed] [Google Scholar]
- 36.Refn H, Kjær A, Lebech A-M, Borggaard B, Schierup L. Clinical and hormonal effects of two contraceptives: Correlation to serum concentrations of levonorgestrel and gestodene. Contraception. 1990;41:259–269. doi: 10.1016/0010-7824(90)90067-6. [DOI] [PubMed] [Google Scholar]
- 37.Bahamondes L, Bahamondes MV. New and emerging contraceptives: a state-of-the-art review. Int. J. Womens. Health. 2014;6:221. doi: 10.2147/IJWH.S46811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gerrits MGF, Schnabel PG, Post TM, Peeters PAM. Pharmacokinetic profile of nomegestrol acetate and 17β-estradiol after multiple and single dosing in healthy women. Contraception. 2013;87:193–200. doi: 10.1016/j.contraception.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 39.Blode H, Kowal K, Roth K, Reif S. Pharmacokinetics of drospirenone and ethinylestradiol in Caucasian and Japanese women. Eur. J. Contracept. Reprod. Heal. Care. 2012;17:284–297. doi: 10.3109/13625187.2012.677076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.