Abstract
Diagnosis, treatment, and secondary management of cryptogenic stroke patients pose a formidable challenge. The scenario is further complicated in patients with native and prosthetic valvular heart disease. We present a case study of a 36‐year‐old man who received intravenous thrombolysis (IV‐tPA) and endovascular thrombectomy (EVT) for presumed “cryptogenic” complete middle cerebral artery infarction who made a surprisingly excellent clinical recovery despite poor baseline and postintervention neuroimaging. Retrospective gram stain of his clot confirmed a diagnosis of infective endocarditis. This raises an important issue regarding need for more routine histopathological analysis of clot retrieved after EVT in “cryptogenic” stroke patients particularly those with valvular heart disease.
Introduction
Acute ischemic stroke (AIS) secondary to septic emboli is a life‐threatening complication of infective endocarditis (IE), with an incidence rate of 20–40%.1 Although the typical manifestation in such cases is the presentation of ischemic stroke, some cases may also present with hemorrhagic complications especially in patients with septic arteritis, anticoagulant‐induced secondary hemorrhagic transformations (HT), or mycotic aneurysms.2 A hospital‐based case series study suggested mortality as high as 56% in patients with stroke due to septic embolus.1 Initial severity of brain injury may determine the long‐term functional outcome in this subgroup of patients.3
There is a lack of consensus, due to insufficient evidence, on acute treatment options in patients with infective endocarditis (IE) with large vessel occlusion (LVO).4 Intravenous tissue‐plasminogen activator (IV‐tPA) may potentiate the risk of bleeding.1, 4 Intra‐arterial endovascular thrombectomy (EVT) potentially offers a safer first‐line treatment strategy to patients with IE.5, 6 Recent consensus guidelines of thrombus analysis in AIS do not address the need for routine gram staining of clots retrieved post‐EVT.7 Pathologic confirmation of retrieved clots can potentially provide diagnostic information on suspected IE in patients with prosthetic valvular heart disease.8 We present a case study to highlight the clinical importance of histopathological analyses of the retrieved clot and its implications for secondary stroke management.
Case Study
A 36‐year‐old man with a history of bioprosthetic aortic valve replacement 7 months previously developed an acute onset left hemiparesis while exercising on a cross‐trainer at a gymnasium. He had a large tattoo placed, 3‐months prior. On initial presentation, NIHSS score was 16 consistent with a moderate‐to‐severe stroke. CT angiography confirmed right M1 occlusion (Fig. 1D).
Figure 1.
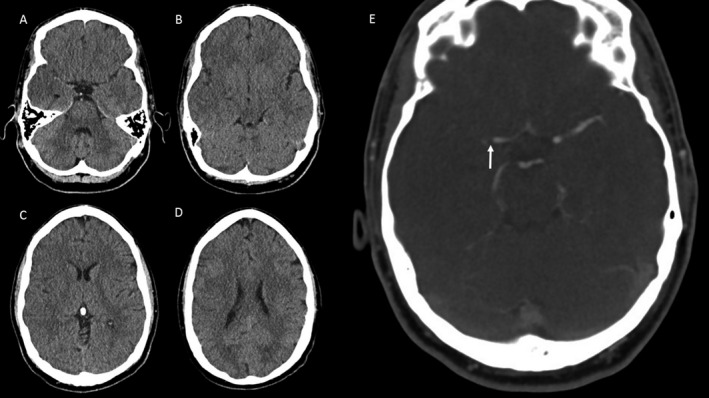
Pre‐endovascular thrombectomy (EVT), (A–D) noncontrast computed tomography (NCCT), and (E) raw computed tomography angiography (CTA) imaging showing right M1 occlusion (white arrow) in a 36‐year‐old stroke patient. Baseline ASPECTS score of 4 is consistent with a large evolving infarct prior to EVT.
The final diagnosis underpinning the stroke was not immediately apparent and the patient was treated with an intravenous tissue‐plasminogen activator (IV‐tPA) without complication, followed by EVT with aspiration through a 0.070‐inch intermediate catheter achieving complete reperfusion by 3 h 30 min (Fig. 2A, B). The patient was afebrile with an admission white cell count (WCC) 10.8 × 109/L, erythrocyte sedimentation rate (ESR) 17 mm/hr, and C‐reactive protein 11. Post‐EVT imaging indicated a completed right middle cerebral artery infarction (Fig. 2C, D); however, the patient's clinical recovery was excellent with a 24‐h NIHSS of 2. Transthoracic (TTE) and transesophageal echocardiography (TOE) showed a normal appearing bioprosthetic aortic valve and patent foramen ovale. The patient was discharged home at day 5 post‐EVT with a modified Rankin Scale (mRS) score of 1. Three weeks postdischarge, the patient developed polyarthralgias without evident fever or weight loss. Repeat TTE and thence TOE 5 weeks after his original ischemic stroke event identified a large mobile echo density, 18 mm long, on his bioprosthetic aortic valve. Blood cultures grew Staphylococcus epidermis, and retrospective gram stain of the initial clot showed gram‐positive cocci consistent with septic embolism (Fig. 2G, H). Aseptic techniques were applied in the handling and transfer of the clot sample to formalin.
Figure 2.
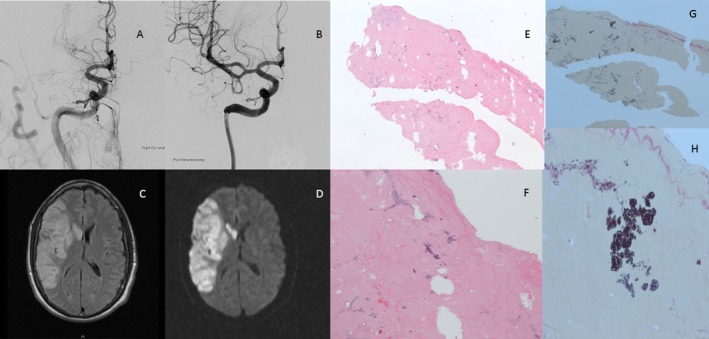
(A) Cerebral digital subtraction angiography (DSA) showing right M1 occlusion. (B) DSA after endovascular thrombectomy with aspiration through an intermediate catheter. Complete right middle cerebral artery (MCA), infarction post‐EVT on (C) T2‐weighted‐fluid‐attenuated inversion recovery (T2‐FLAIR), and (D) diffusion‐weighted imaging (DWI). Hematoxylin and eosin (H&E) stains at (E) low (20X) and (F) high magnification (40X), respectively. Gram stain of the clot showing gram‐positive cocci consistent with septic embolus at (G) low (20X) and (H) high magnification (40X), respectively.
The next day, the patient had urgent redo aortic valve surgery and then was commenced on a 6‐week course of intravenous antibiotics; he made an uncomplicated recovery from this.
Discussion
This case report presents both an uncommon phenomenon and a potential lesson. The uncommon diagnosis of embolism from IE could have been correctly made in hospital – had the retrieved clot been examined pathologically for infection.
The patient received acute intervention in accordance with time‐based principles, but his imaging suggested an evolving total MCA infarction despite the favorable time window. The surprising excellent recovery observed in this case, even though there was an unfavorable imaging profile prior to and following intervention, may possibly be attributable to the benign trajectory often observed clinically in younger patients with EVT of LVO.9 At the time of discharge, the patient's stroke was etiologically classified as cryptogenic. Cryptogenic strokes defined according to the trial of ORG 10172 in acute stroke treatment (TOAST) criteria contribute to more than 30% of ischemic stroke cases admitting to stroke units.10 The TOAST system was designed in the early 1990s to guide classification of stroke pathophysiology for a secondary prevention trial. Cryptogenic stroke patients currently receive antiplatelet medications for secondary prevention. The Post Warfarin vs. Aspirin for Recurrent Stroke Study trial did not resolve the debate for cryptogenic stroke patients.11 We acknowledge that our patient did not meet criteria for use of the term embolic stroke of unknown source (ESUS) due to the presence of a bioprosthetic valve.12 The diagnostic work‐up for an underlying source of embolism was extensive including prolonged ambulatory electrocardiogram (ECG) monitoring, CT, and digital subtraction cerebral angiography and procoagulant studies, all of which were negative. Advances in both brain and vascular imaging along with EVT providing embolic material for analysis offer new opportunities for determination of stroke etiology, particularly in cryptogenic stroke.7
Our patient's noncontrast CT (Fig. 1A) demonstrated a dense artery sign consistent with clot pathology demonstrating a predominance of RBCs (Fig. 2F). Studies have explored the correlation between clot characteristics, imaging features and treatment selection.13, 14, 15, 16 Liebeskind et al. found a correlation of the early imaging, hyperdense middle cerebral artery (HDMCA) sign, and gradient‐echo (GRE) MRI blooming artifact (BA) performed before EVT in 50 consecutive cases, with the pathology of retrieved thrombi.17 The authors concluded that the RBC content determines the appearance of HDMCA and BA signs, and the absence of these early signs may be due to the thrombus rich in fibrin, which is less likely to respond to IV‐tPA.17 Another study demonstrated a strong association of susceptibility vessel sign on GRE with an increased RBC content in the clot and a diminished presence of fibrin and platelets in EVT‐retrieved clots.13 A recent systematic review found no association between the histopathological characteristics of thrombi and etiology and angiographic outcomes.16 However, it found a significant association of HDMCA sign with RBC‐rich thrombi and improved recanalization rates.16
There are concerns about the safety and efficacy of IV‐tPA in the setting of septic emboli.18 Known endocarditis is a contraindication to IV‐tPA primarily due to concerns about bleeding risk.4 At the time of our patient's presentation, there were no clinical symptoms to raise suspicion of IE. A lack of warning symptoms such as fever has also been previously described in case studies of patients with diagnostically confirmed IE who received EVT.4 The recommended treatment for IE is to institute effective antibiotic therapy as soon as possible in an attempt to avoid further embolic complications and heart failure. However, there has been no comparative research or clear consensus on how to manage ischemic stroke patients with LVO due to suspected or diagnosed IE. Embolization occurs more frequently with larger (>10 mm), left‐sided vegetations, particularly on the mitral valve,4 and when antiphospholipid antibodies are present.5 Furthermore, vegetations comprise inflammatory cells, platelets, and microorganisms in addition to a rich fibrin network, which may explain refractoriness and complications associated with IV‐tPA.8
EVT has proven utility as a reperfusion strategy in stroke with large artery occlusion up to 8 h since symptom onset, with revascularization rates up to 90%, and a lower risk of hemorrhage compared with pharmacological thrombolysis.19 The selection strategy for intervention in suspected septic emboli remains elusive with lack of guidelines as to whether to avoid stent retrievers. However, previous case series have not confirmed advantage of aspiration over stent retrievers.5, 20
Early use of antibiotics can potentially minimize risk of further septic embolism.4 Use of daily antiplatelet and statin therapy prior to IE diagnosis may thwart septic embolization. However, post‐IE diagnosis, aspirin has been associated with negligible or poor response and heightened risk of hemorrhage21, 22; and therefore, use of aspirin is not indicated in the early management of patients with IE.21, 22 In our patient, the delay in diagnosis of IE, fortunately, did not result in such complications.
Making the diagnosis of IE can be challenging; however, delayed or missed diagnosis can carry a high risk of morbidity and mortality. In the era of reperfusion therapy with EVT, the opportunity to undertake routine clot histopathology including gram stain should be considered in cryptogenic stroke, particularly in patients with native or prosthetic valvular heart disease.
Conclusion
Given the background of suspected septic embolus or IE, and increasing adoption of EVT in acute stroke care, EVT‐retrieved clots should be sent for routine advanced pathological analyses. The key message emerging out of this report is a compelling need to consider use of clot histopathology including gram stain more routinely, especially if there are risk factors for IE and/or in patients in whom a clear cardiac source cannot be verified. Furthermore, quantification of metabolites and/or RBC/WBC, fibrin and their correlation with advanced brain imaging, such as high‐risk morphological (HRM) features, may provide insights into the underlying pathogenesis of the ischemic stroke, particularly in cases of cryptogenic stroke.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
We would like to thank Dr Richard Jin, Department of Pathology at Liverpool Hospital for providing the images of the clot.
Contributor Information
Sonu Bhaskar, Email: S.Bhaskar@westernsydney.edu.au.
Dennis Cordato, Email: Dennis.Cordato@sswahs.nsw.gov.au.
References
- 1. Walker KA, Sampson JB, Skalabrin EJ, Majersik JJ. Clinical characteristics and thrombolytic outcomes of infective endocarditis‐associated stroke. Neurohospitalist 2012;2:87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hart RG, Foster JW, Luther MF, Kanter MC. Stroke in infective endocarditis. Stroke 1990;21:695–700. [DOI] [PubMed] [Google Scholar]
- 3. Ruttmann E, Willeit J, Ulmer H, et al. Neurological outcome of septic cardioembolic stroke after infective endocarditis. Stroke 2006;37:2094–2099. [DOI] [PubMed] [Google Scholar]
- 4. Kim JM, Jeon JS, Kim YW, et al. Forced arterial suction thrombectomy of septic embolic middle cerebral artery occlusion due to infective endocarditis: an illustrative case and review of the literature. Neurointervention 2014;9:101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liang JJ, Bishu KG, Anavekar NS. Infective endocarditis complicated by acute ischemic stroke from septic embolus: successful solitaire FR thrombectomy. Cardiol Res 2012;3:277–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sveinsson O, Herrman L, Holmin S. Intra‐arterial mechanical thrombectomy: an effective treatment for ischemic stroke caused by endocarditis. Case Rep Neurol 2016;8:229–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. De Meyer SF, Andersson T, Baxter B, et al. Analyses of thrombi in acute ischemic stroke: a consensus statement on current knowledge and future directions. Int J Stroke 2017;12:606–614. [DOI] [PubMed] [Google Scholar]
- 8. Boeckh‐Behrens T, Kleine JF, Zimmer C, et al. Thrombus histology suggests cardioembolic cause in cryptogenic stroke. Stroke 2016;47:1864–1871. [DOI] [PubMed] [Google Scholar]
- 9. Chalouhi N, Tjoumakaris S, Starke RM, et al. Endovascular stroke intervention in young patients with large vessel occlusions. Neurosurg Focus 2014;36:E6. [DOI] [PubMed] [Google Scholar]
- 10. Adams HP Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 11. Hankey GJ. Warfarin‐Aspirin Recurrent Stroke Study (WARSS) Trial. is warfarin really a reasonable therapeutic alternative to aspirin for preventing recurrent noncardioembolic ischemic stroke?. Stroke 2002;33:1723–1726. [DOI] [PubMed] [Google Scholar]
- 12. Nouh A, Hussain M, Mehta T, Yaghi S. Embolic strokes of unknown source and cryptogenic stroke: implications in clinical practice. Front Neurol 2016;7:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim SK, Yoon W, Kim TS, et al. Histologic analysis of retrieved clots in acute ischemic stroke: correlation with stroke etiology and gradient‐Echo MRI. AJNR Am J Neuroradiol 2015;36:1756–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Niesten JM, van der Schaaf IC, van Dam L, et al. Histopathologic composition of cerebral thrombi of acute stroke patients is correlated with stroke subtype and thrombus attenuation. PLoS ONE 2014;9:e88882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Qazi EM, Sohn SI, Mishra S, et al. Thrombus characteristics are related to collaterals and angioarchitecture in acute stroke. Can J Neurol Sci 2015;42:381–388. [DOI] [PubMed] [Google Scholar]
- 16. Brinjikji W, Duffy S, Burrows A, et al. Correlation of imaging and histopathology of thrombi in acute ischemic stroke with etiology and outcome: a systematic review. J Neurointerv Surg 2017;9:529–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liebeskind DS, Sanossian N, Yong WH, et al. CT and MRI early vessel signs reflect clot composition in acute stroke. Stroke 2011;42:1237–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brownlee WJ, Anderson NE, Barber PA. Intravenous thrombolysis is unsafe in stroke due to infective endocarditis. Intern Med J 2014;44:195–197. [DOI] [PubMed] [Google Scholar]
- 19. Gomis M, Dávalos A. Recanalization and reperfusion therapies of acute ischemic stroke: what have We Learned, what are the major research questions, and where are we headed? Front Neurol 2014;5:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Deshaies EM, Singla A, Villwock MR, et al. Early experience with stent retrievers and comparison with previous‐generation mechanical thrombectomy devices for acute ischemic stroke. J Neurosurg 2014;121:12–17. [DOI] [PubMed] [Google Scholar]
- 21. Chan KL, Tam J, Dumesnil JG, et al. Effect of long‐term aspirin use on embolic events in infective endocarditis. Clin Infect Dis 2008;46:37–41. [DOI] [PubMed] [Google Scholar]
- 22. Chan KL, Dumesnil JG, Cujec B, et al. A randomized trial of aspirin on the risk of embolic events in patients with infective endocarditis. J Am Coll Cardiol 2003;42:775–780. [DOI] [PubMed] [Google Scholar]


