Abstract
Background and Purpose
Synthetic cannabinoids (often sold as Spice or K2) have become a very popular alternative to cannabis due to their easy access and portrayed safety. Controlled studies on the behavioural effects of synthetic cannabinoids are currently lacking, which hampers risk assessments of these compounds.
Experimental Approach
This is a first attempt to assess the influence of a synthetic cannabinoid, JWH‐018, on neurocognition and subjective experience in humans after controlled administration. JWH‐018, 2 and 3 mg, was administered to six healthy cannabis‐experienced volunteers in a placebo‐controlled, cross‐over study following an escalating dosing schedule. Participants were monitored for 12 h after drug administration, and several neurocognitive measures and subjective questionnaires were taken.
Key Results
Serum concentrations of JWH‐018 were highest after the 2 mg dose but generally low after administration of both doses. Both doses of JWH‐018 were well tolerated, and no serious side effects were reported. Participants reported feeling more ‘high’ at 1 and 2 h after administration, particularly after the 2 mg dose. Behavioural impairments also emerged despite the low serum concentrations of JWH‐018. The low dose of JWH‐018 impaired performance on the tracking, divided attention and stop signal task.
Conclusion and Implications
JWH‐018 dosing in the present study resulted in drug concentrations that were generally low and not fully representative of common use. Yet initial impairments of neurocognitive function and subjective feelings of high did emerge despite low levels of JWH‐018 in serum. Higher doses are needed to obtain a more representative risk profile of JWH‐018.
Abbreviations
- CADSS
clinician‐administered dissociative states scale
- CTT
critical tracking test
- DAT
divided attention task
- DSMB
data safety monitoring board
- DSST
digit symbol substitution task
- MCQ
marijuana craving questionnaire
- POMS
profile of moods states
- SCRQ
Sensitivity to Cannabis Reinforcement Questionnaire
- SMT
spatial memory task
- SST
stop signal task
- THC
Δ9‐tetrahydrocannabinol
- TOL
Tower of London
- VAS
visual analogue scale
Introduction
Cannabis has been used for centuries as a medicinal and recreational drug. Its sought‐after psychoactive effects include relaxation, euphoria and dreaminess, but it can also lead to feelings of anxiety and paranoia. In addition, Δ9‐tetrahydrocannabinol (THC), the major psychotropic compound in cannabis, has repeatedly been demonstrated to impair cognition and psychomotor performance (Ramaekers et al., 2006a,b; 2009).
Recreational cannabis use is prohibited in most countries, and is one reason why potential users have turned to synthetic cannabinoids available on the market as legal highs. ‘Spice’ products were among the first herbal blends that were freely advertised over the internet. ‘Spice’ refers to a wide variety of herbal mixtures that produce experiences similar to cannabis. It is sold under many names, including Spice, K2, fake weed, Yucatan Fire, Skunk, Moon Rocks and others, and package labels often indicate that they are ‘not for human consumption’. Spice products have become very popular in several countries not only due to its easy access and portrayed safety but also due to the fact that they are not detectable in standard drug tests (Lindigkeit et al., 2009; Sedefov et al., 2009; Griffiths et al., 2010; Vardakou et al., 2010; Vandrey et al., 2012).
Labels on Spice products often claim that they contain ‘natural’ psycho‐active material taken from a variety of plants. However, chemical analyses show that although it contains dried plant material, the active ingredients are synthetic (or designer) cannabinoid compounds (http://www.drugabuse.gov/publications/drugfacts/spice‐synthetic‐marijuana). In 2008, a German company reported the synthetic cannabinoid JWH‐018 as an active ingredient of Spice (Steup, 2008). JWH‐018 is one of the synthetic cannabinoids synthesized by J.W. Huffman, as part of research into cannabinoid receptor‐ligand binding (Wiley et al., 2011; Rosenbaum et al., 2012). It is an aminoalkylindole and produces cannabis‐like effects when smoked (WHO, 2014). Effects of JWH‐018 are evident after much lower doses than the typical THC dose (Ginsburg et al., 2012; Ford et al., 2017), and the side effects reported after JWH‐018 use are similar to the side effects associated with cannabis use (Rosenbaum et al., 2012). However, as JWH‐018 acts as a full CB1 receptor agonist, it could lead to life‐threatening conditions such as, for example, seizures or convulsions (Havenon et al., 2011). In addition, the fact that the constitution of Spice products constantly changes with respect to active ingredients and dosages means that people can easily overdose and experience serious side effects (Sedefov et al., 2009; WHO, 2014). Due to these factors, from about 2008 quite a few countries have added several synthetic cannabinoids, including JWH‐018, to the list of controlled substances (Sedefov et al., 2009).
Unfortunately, clinical trials assessing the effects of Spice objectively are lacking. Much of what we know is based on case reports or hospital admission reports, stating that its effects are similar but stronger than those of cannabis (Zimmermann et al., 2009; WHO, 2014). Adverse effects reported include tachycardia, agitation, hallucination, hypertension, minor elevation of blood glucose, vomiting, chest pain, seizures, anxiety, panic attacks and acute psychosis (Auwärter et al., 2009; Zimmermann et al., 2009; Sobolevsky et al., 2010; Every‐Palmer, 2011; Simmons et al., 2011; Hermanns‐Clausen et al., 2013; WHO, 2014). In most cases, these symptoms disappeared after a couple of hours (Harris and Brown, 2012).
Two self‐experiments, describing the effects of JWH‐018, have been published. In one study, the researchers self‐administered Spice Diamond which contained JWH‐018 and the C8homologue of CP47,497 and reported cannabis‐like effects after 10 min. These effects slowly diminished over a period of 6 h (Auwärter et al., 2009). In the second study, two cannabis‐naïve volunteers smoked 50 μg·kg−1 JWH‐018 (resp. 3.6 and 4.3 mg per person) and reported typical cannabis‐like physical effects including thought disruptions (Teske et al., 2010, Personal correspondence with the author).
An important factor in the health risks associated with Spice is the disparity of the mixtures with regard to the content of the active ingredients. Consequently, we do not know which dose of ‘Spice’ or JWH‐018 is typically used to achieve a desired high. Several studies reported that JWH‐018 is four to five times more potent than THC (Aung et al., 2000). Therefore, a JWH‐018 dose, four to five times smaller than common THC doses used in controlled studies, was expected to produce comparable effects. THC has repeatedly been administered in controlled experimental studies (e.g. Ramaekers et al., 2006a, 2016) in doses up to approximately 35 mg (500 μg·kg−1 bodyweight). In the current study, single doses of 2 and 3 mg JWH‐018 were administered. It was expected that a dose of 3 mg JWH‐018 would produce pharmacological effects comparable to a dose of 15 mg of THC. The latter has been shown to produce significant behavioural effects in controlled studies while keeping adverse events to a minimum (Ramaekers et al., 2006b).
Methods
The study was approved by the standing Medical Ethics Committee of Maastricht University and was carried out in compliance with the current revision of the Declaration of Helsinki (amended in 2013, Fortaleza) and the International Conference on Harmonization guidelines for Good Clinical Practice. A permit for obtaining, storing and administering JWH‐018 was obtained from the Dutch drug enforcement administration. All subjects gave written informed consent and received financial compensation for their participation.
Participants
A total of seven occasional users of cannabis were recruited via advertisements placed around Maastricht University. Participants were screened using a health questionnaire and underwent a medical examination (including an ECG, haematology and blood chemistry, urinalysis and drug and pregnancy screening). The following inclusion criteria applied to participants: occasional use of cannabis (minimal 1 year experience, with a minimum and maximum use of 24 and 104 times a year); free‐from psychotropic medication; good physical health as determined by medical examination and laboratory analysis; absence of any major medical, endocrine and neurological conditions; body mass index (weight/length2) between 18 and 28 kg·m−2; and written informed consent. Exclusion criteria were as follows: history of drug abuse (excluding cannabis) as assessed by drug urine screens and questionnaires; excessive drinking (consumption of >20 alcoholic units a week); pregnancy or lactation or failure to use contraceptives; hypertension (diastolic > 90 mmHg; systolic > 140 mmHg); and history of psychiatric disorders.
Design and treatments
The study was conducted according to a placebo‐controlled, single‐blind, within‐subjects design and was performed in healthy, regular users of cannabis. On separate test days, each subject inhaled the vapour of a placebo, 2 and 3 mg of JWH‐018. An escalating dosing scheme was used, making sure that no subject received the high dose of JWH‐018 before having received the low dose. Subjects were quasi randomly assigned to receive one of the following treatment orders: 0–2–3 mg; 2–0–3 mg; or 2–3–0 mg.
JWH‐018 powder was mixed with a small amount of Knaster plant material and heated in a 10 cm glass pipe (‘crack pipe’). Glass pipes were only used once and replaced for every new administration. A 30 cm plastic tube was connected to the end of the pipe, while the bowl of the pipe contained the treatment. While the air holes were closed off, the bowl was heated for about 15 s. When the vapour was formed, the air holes were opened and the subject was instructed to immediately inhale the vapour in one take via the plastic tube. Drug preparation and administration was done by a different researcher from the ones performing the drug tests.
Procedures
Prior to the first test day, subjects were trained extensively in all cognitive tests in order to become familiar with all tests and minimize practice effects. Subjects were not allowed to use alcohol or caffeine on the test day or the day prior to testing. Smoking was prohibited for 30 min prior to and during test days. Subjects were instructed to continue their cannabis use as normal but were requested to abstain from cannabis from about 5 days prior to the test day, to make sure they were negative on the test day. Subjects were asked to arrive at the site well rested. On each test day, subjects were instructed to have a standard breakfast before coming to the site. They received a lunch and dinner at the site.
Test days took place at the testing facilities at Maastricht University. Participants would spend the day in a test room equipped with a bed, chair and table with laptop. The test room was adjacent to a bathroom and a room where the researchers were seated. Drug tests were performed upon arrival using a urine drug screen (assessing the presence of morphine, cocaine, cannabis, methamphetamine and amphetamine), and subsequently, an intravenous catheter was placed in the lower arm. Urine and blood samples were taken at baseline and at the end of the test day to determine laboratory safety (haematology, clinical chemistry and urinalysis). Oral fluid, blood and urine samples were taken at regular intervals during the test days to determine pharmacokinetics. Blood pressure and heart rate were measured at regular intervals during the test day using an Omron upper arm blood pressure monitor. Cognitive tests and subjective questionnaires were taken at regular intervals up until 12 h after administration (see Tables 1 and 2). In between test batteries, subjects could relax at the site, where they could watch TV, read or use the internet. Test days always started at the same time in the morning and were separated by a minimum wash‐out period of 7 days to avoid cross‐condition contamination (average of 37 days between test days, with a maximum of 92 days).
Table 1.
Cognitive tests taken during test days relative to time of administration (T0)
| Time (h) to T0 | DSST | SST | CTT | TOL | DAT | SMT |
|---|---|---|---|---|---|---|
| Baseline | x | – | x | – | – | – |
| 0:15 | – | – | x | – | – | – |
| 0:30 | – | – | – | – | – | x |
| 1:00 | – | x | – | – | x | x |
| 2:30 | – | – | x | x | x | – |
| 4:30 | x | x | – | – | – | – |
| 6:30 | – | – | x | – | – | – |
| 8:30 | x | x | – | – | – | – |
| 10:30 | – | – | x | x | x | – |
CTT, critical tracking task; DSST, digit symbol substitution test; TOL, tower of london; SST, stop signal test; DAT, divided attention task; SMT, spatial memory test.
Table 2.
Time of subjective questionnaires taken during test days, relative to time of administration (T0)
| Time (h) to T0 | VAS | SCRQ | MCQ | POMS | CADSS | B‐VAS |
|---|---|---|---|---|---|---|
| Baseline | x | – | – | x | – | – |
| 0:05 | x | x | – | – | x | – |
| 1:00 | x | – | x | x | – | x |
| 2:00 | x | – | – | – | – | – |
| 3:00 | x | – | – | – | – | – |
| 4:00 | x | – | – | – | x | – |
| 5:00 | x | – | – | x | – | x |
| 6:00 | x | – | – | – | – | – |
| 7:00 | x | – | – | – | – | – |
| 8:00 | x | – | – | – | – | – |
| 10:00 | x | x | – | – | x | – |
| 12:00 | x | – | x | x | – | x |
B‐VAS, Bowdle visual analogue scales; POMS, profile of mood states; .SCRQ, sensititvity to cannabis reinforcement questionnaire; MCQ, marijuana craving questionnaire; CADSS, clinician dissociative states scale.
Safety
A Dyna‐Vision ambulatory patient monitoring system (Techmedic International, The Netherlands) was used to continuously measure and transmit ECG and vital signs in real time to the medical supervisor. The signal was transmitted to the laptop as well as the smartphone of the medical supervisor. A patch with sensors was attached to the subject's chest and connected to a receiver which was carried by the participant around his/her neck or middle. The system transmits three lead ECGs, saturation of peripheral oxygen, plethysmogram, respiration and skin temperature and beat‐to‐beat non‐invasive blood pressure. Alert signals were given every time one of the measures was above or below the normal range. The medical doctor would determine whether the value was clinically significant or not. The medical supervisor was present at the facility during the whole test day and could check on the participant whenever he/she experienced any complaints or when any of the ECG or vital sign measures indicated a value outside the normal range.
Laboratory safety was determined at baseline and at the end of the test day. Blood and urine samples were used to perform haematology, clinical chemistry and urinalysis. Reference ranges were used to determine whether laboratory values were within the normal range. In case a measured parameter was outside the normal ranges, the medical supervisor decided whether or not it was considered a clinically significant deviation. In this decision, the other parameters, the overall condition of the participant and the baseline values were taken into account. At the end of the test day, participants were given a diary in which they were asked to take note of any possible side effect that they experienced up until 72 h after administration of the drug.
An independent data safety monitoring board (DSMB) was installed to inspect and evaluate all vital, safety and behavioural data collected throughout the study. Data were submitted to the DSMB when the third and the last participant had completed an active dose condition. The study was continued only after a positive evaluation from the DSMB. The 3 mg dose condition was initiated after all subjects had successfully and safely completed the 2 mg dose condition.
Pharmacokinetics
Fourteen blood (5 mL) and oral fluid samples and at least five urine samples were taken during each test day. Blood samples were centrifuged and serum was frozen at −20°C while oral fluid samples were stored refrigerated until pharmacokinetic assessments. Urine samples were stored at −20°C until analyses.
Performance tests
Digit symbol substitution task (DSST)
The DSST is a computerized version of the original paper and pencil test taken from the Wechsler Adult Intelligence Scale (Mcleod et al., 1982). The participant is required to match each digit with a symbol from the encoding list as rapidly as possible. The number of digits correctly encoded within 3 min is the performance measure.
Critical tracking test (CTT)
The CTT measures the subject's ability to control a displayed error signal in a first‐order compensatory tracking task (Jex et al., 1966). Error is displayed as a horizontal deviation of a cursor from the midpoint on a horizontal, linear scale. Compensatory joystick movements null the error by returning the cursor to the midpoint. The frequency at which the subject loses control is the critical frequency or λc. The test included five trials of which the lowest and the highest score were removed; the average of the remaining scores is taken as the final score.
Divided attention task (DAT)
The DAT measures the ability to divide attention between two tasks performed simultaneously (Moskowitz, 1973). Subjects have to perform the same tracking task as described above but now at a constant level of difficulty. As a secondary task, the subject monitors 24 single digits which are presented in the corners of the computer screen. The subjects are instructed to react to the target number ‘2’ by removing their foot as fast as possible from a pedal switch. Mean absolute tracking error (in mm) and number of control losses are the performance measures of the primary task. Number of correct responses and mean reaction time (ms) of the responses to the target number are the performance measures in the secondary subtask.
Stop signal task (SST)
The SST measures motor impulsivity, which is defined as the inability to inhibit an activated or pre‐cued response leading to errors of commission. The current test is adapted from an earlier version (Fillmore et al., 2002) and has been validated for showing stimulant and sedative drug effects (Ramaekers and Kuypers, 2006). The task requires subjects to make quick responses to visual go signals and to inhibit their response if a subsequent visual stop signal, that is, ‘*’, appeared in one of the four corners of the screen. Dependent variables are go reaction time, stop reaction time, response accuracy, omission (not responding on go‐trials) and commission errors (not inhibiting a no go trial). Stop reaction time represents the estimated mean time required to inhibit a response. Stop reaction time is calculated by subtracting the stop signal delay from the reaction time on go‐trials associated with n‐th percentile of the reaction time distribution (Logan, 1994).
Tower of London (TOL)
The TOL is a decision‐making task that measures executive function and planning (Shallice, 1982). The task consists of computer‐generated images of begin and end arrangements of three coloured balls on three sticks. The subject's task is to determine as quickly as possible whether the end arrangement can be accomplished by ‘moving’ the balls in two to five steps from the beginning arrangement by pushing the corresponding number coded button. The total number of correct decisions is the main performance measure.
Spatial memory task (SMT)
Ten black‐and‐white pictures are presented subsequently in 10 different locations on a computer screen. After presentation, each picture is presented alone with two possible locations where it appeared. Participants' task is to choose the correct location, a measure of immediate recall phase. This procedure is repeated six times with different stimuli and locations. After a 30 min delay, the recall phase is repeated; this test serves as a delayed recall measure (adapted from (Kessels et al., 1999).
Subjective questionnaires
Subjective high
Subjective high is self‐rated on a 10 cm visual analogue scale (VAS), with 0 indicating ‘not high at all’ and 10 indicating ‘extremely high’.
Profile of moods states (POMS)
The POMS is a self‐assessment mood questionnaire with 72 items, rated on a 5‐point Likert scale, with 0 being ‘not at all’ to 4 ‘extremely’. Subjects have to indicate to what extent these items were representative of their mood at that moment in time. Eight mood states are classified and quantified by calculating the sum score of associated items for each mood state, that is, anxiety, depression, anger, vigour, fatigue, confusion, friendliness and elation. Two composite scales are derived, arousal and positive mood (De Wit et al., 2002).
Bowdle visual analogue scales
Psychedelic effects are assessed using a 13‐item VAS (Bowdle et al., 1998). Two scales measure subjective ‘high’ and ‘drowsiness’. From the other scales, composite scores of ‘internal perception’ and ‘external perception’ are calculated.
The marijuana craving questionnaire (MCQ)
The MCQ is a 12‐item self‐report instrument that assesses marijuana craving/wanting along four dimensions: (i) compulsivity, an inability to control marijuana use; (ii) emotionality, use of marijuana in anticipation of relief from withdrawal or negative mood; (iii) expectancy, anticipation of positive outcomes from smoking marijuana; and (iv) purposefulness, intention and planning to use marijuana for positive outcomes. Items are scored on a 7‐point scale ranging from strongly disagree to strongly agree (Heishman and Singleton, 2006).
Sensitivity to Cannabis Reinforcement Questionnaire (SCRQ)
This questionnaire asks subjects to rate their liking and wanting of cannabis use during their present condition and in general. Subjects are asked four questions: how pleasant is using cannabis right now? How much do you want to use cannabis right now? How pleasant is using cannabis in general? How much do want to use cannabis in general? Subjective valence of liking and wanting is scored on a 5‐point scale: 1 = somewhat; 2 = slightly; 3 = moderately; 4 = very; and 5 = extremely.
Clinician‐administered dissociative states scale (CADSS)
The CADSS (Bremner et al., 1998) comprises 19 subjective items, ranging from 0 ‘not at all’ to 4 ‘extremely’. It is divided into three components: (i) depersonalization, (ii) derealization and (iii) amnesia. Summed together, these subscales form a total dissociative score. The CADSS is specifically designed to be a standardized measure of present‐state dissociative symptomatology.
Statistics
For cognitive performance tests, scores of different timings were summed, in order to normalize the data and get an overall score. Paired sample t‐tests were conducted to test whether performance differed between drug conditions. Subjective measures were analysed using separate paired sample t‐tests for each time point. The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015).
One sided‐testing was used, as we expected JWH‐018 to cause impairment compared to placebo. A P‐value < 0.05 was considered statistically significant. All statistical tests were conducted using IBM SPSS statistics, version 24.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2015), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017).
Results
One subject decided to withdraw from the study, after finishing the first test day. Data from the remaining six subjects (two males, four females) were analysed. On average (SD, min, max), participants were 23.5 years old (3.57, 18.8, 28.75), had a body mass index of 23.08 (2.37, 20.76, 27.25) and used cannabis for 6.17 years (4.26, 1, 13), 1.67 times a week (0.36, 1.08, 2.08). Measured body weight of the participants was 58, 59, 60, 65, 76 and 82 kg.
Although subjects were instructed to abstain from cannabis as of 5 days prior to each test day, two subjects tested positive for THC on the drug test taken at baseline. Baseline measurements showed that these participants had 2.1 and 0.51 ng·mL−1 THC in serum, which indicates that last use of cannabis was probably a couple of hours or days before the start of the test day. THC concentrations below 2 ng·mL−1 are not associated with psychomotor impairment (Ramaekers et al., 2009). This indicates that psychoactive effects of THC were negligible at the start of the test day, which was indeed confirmed by the baseline subjective high scores of these participants.
Safety
Laboratory safety analyses (haematology, clinical chemistry and urinalyses) showed no clinically relevant deviations from the normal ranges. ECG patterns and vital signs measured with the Dyna‐vision were also normal during all test days. Average (and range) blood pressure and heart rate measured manually are presented in Table 3. Paired sample t‐tests on separate time points showed incidental significant differences between treatments; however, these effects were not consistent, and all values were well within the normal ranges.
Table 3.
Average (and range) values for systolic and diastolic BP and heart rate (HR)
| Placebo | 2 mg | 3 mg | |
|---|---|---|---|
| Systolic BP (mmHg) | 112.1 (94–151) | 112.1 (92–136) | 110.5 (93–134) |
| Diastolic BP (mmHg) | 69.9 (53–129) | 70.8 (58–96) | 68.2 (47–91) |
| HR (beats.min‐1) | 69.8 (48–92) | 67.7 (43–94) | 69 (49–104) |
No side effects were reported during the test days, except for one participant feeling light headed during blood taking in the placebo condition. Four participants reported side effects after the end of the test day; two participants reported headaches in the placebo condition while one participant reported a headache after the 3 mg JWH‐018 treatment. One participant reported low energy/tiredness after the low and high dose of JWH‐018.
Pharmacokinetics
Mean pharmacokinetics for JHW‐018 after administration of 2 and 3 mg doses is given in Figure 1. A detailed report on the pharmacokinetics of this study has been published elsewhere (Toennes et al., 2017). In short, there was no clear dose–concentration relationship across the 2 and 3 mg doses. Overall, drug concentrations reached their maximum 0.08 h after inhalation and were lower after the 3 mg dose as compared to the 2 mg dose. Analysis of the residuals in the inhalation devices revealed that a substantial proportion (sometimes up to 70%) of the doses was not inhaled (Toennes et al., 2017).
Figure 1.
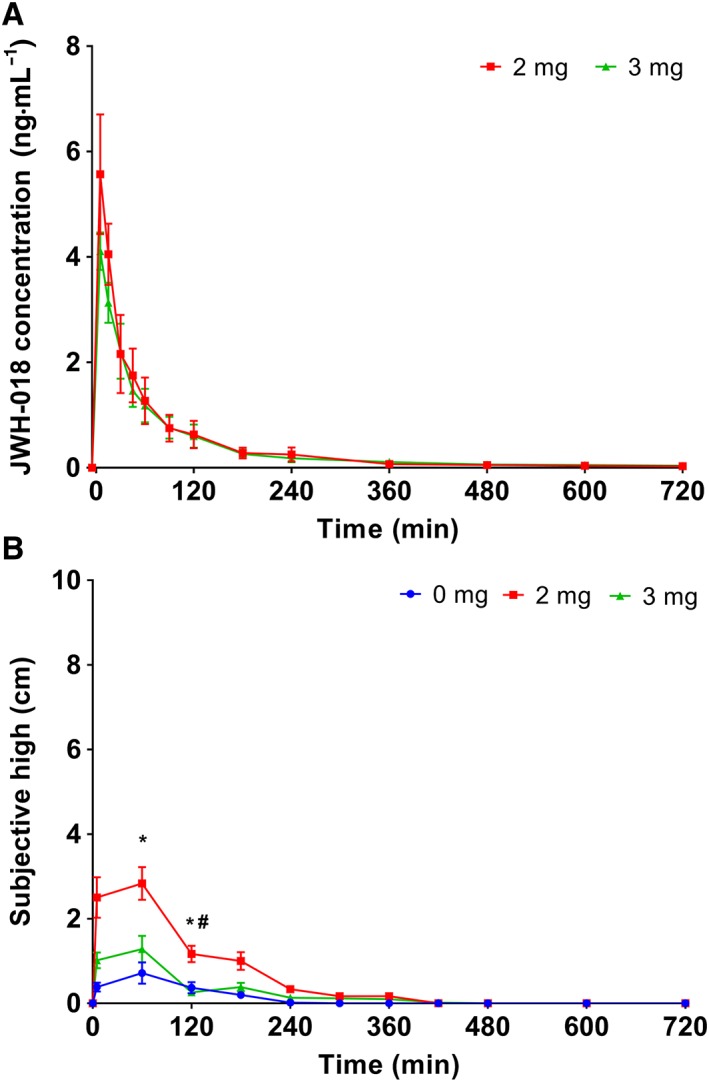
(A) JWH‐018 concentrations in serum for both JWH‐018 doses, in the 14 samples taken during test days; (B) subjective high reported during the test days after placebo, 2 and 3 mg JWH‐018. Results are presented as mean±SEM. * Significant difference from placebo (P < 0.05); # significant difference from 3 mg dose (P < 0.05).
Cognitive performance
Baseline CTT scores did not show significant differences between treatments. CTT scores for the four tests taken after administration were summed to get an overall score. Paired sample t‐tests showed a significant lower score after participants were treated with the low dose of JWH‐018 (M = 12.18) compared to placebo (M = 13.16) (t5 = 2.31; P = 0.0.35) (see Figure 2A).
Figure 2.
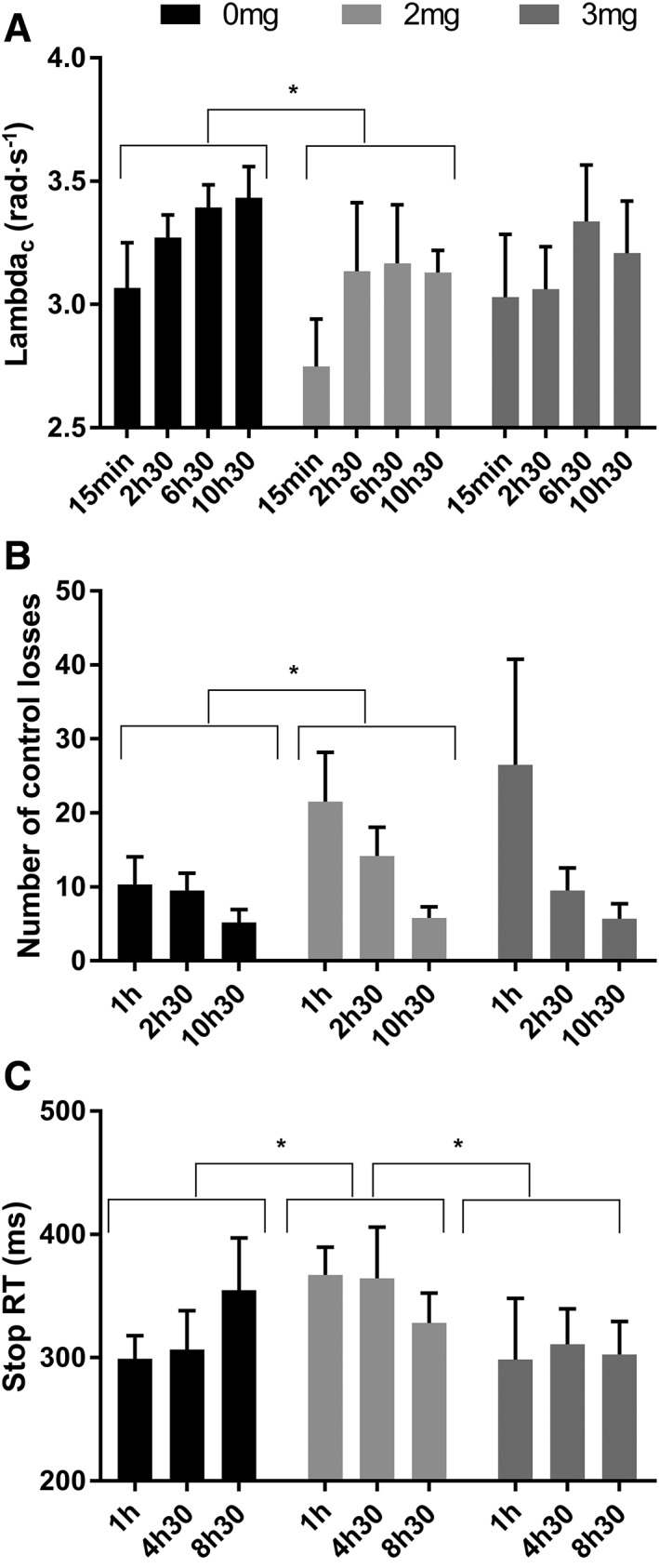
Values for (A) λ‐c in the critical tracking task (CTT), (B) number of control losses in the divided attention task (DAT) and (C) stop reaction time (RT) in the stop signal test (SST) as a function of time after treatment with placebo, 2 and 3 mg JWH‐018. Results are presented as mean±SEM. * Significant difference between treatment conditions (P < 0.05).
DAT variables were summed across repetitions. No significant drug effects were found on tracking error, reaction time or number of correct hits. Paired sample t‐tests did show significantly more control losses in the low dose of JWH‐018 (M = 41.5) than in the placebo condition (M = 25) (t5 = −2.08; P = 0.046) (see Figure 2B).
SST measures of the 3 time points were summed. Paired sample t‐tests showed significantly longer stop reaction times in the low JWH‐018 dose (M = 1059.5), compared to placebo (M = 960.2; t5 = −2.513; P = 0.027), and compared to the high dose of JWH‐018 (M = 911.67; t5 = 3.232; P = 0.012) (see Figure 2C). No significant effects were found for the other variables of the SST.
No significant differences were found between treatment conditions for the performance scores of the TOL, SMT or DSST.
Subjective questionnaires
Average subjective high scores are shown in Figure 1B. Subjects reported their subjective high 12 times during the test day. Separate paired sample t‐tests showed that subjects reported to feel more high after a low JWH‐018 dose compared to placebo 1 and 2 h after drug administration (M = −2.11; t5=‐2.43; P = 0.03 and M = −0.80; t5 = −2.619; P = 0.024). At 1 h after administration, participants also felt more high in the low dose compared to the high dose (M = 1.55; t5 = 2.675; P = 0.022). In addition, subjective high scores correlated significantly with maximum concentrations in serum immediately (r = 0.587, n = 18, P = 0.01) and 4 h after drug administration (r = 0.530; n = 18, P = 0.024).
Profile of mood states were taken at baseline and repeated four times after drug administration. Separate paired sample t‐tests showed that subjects reported more fatigue after the high dose compared to placebo at 5 and 12 h after administration (M = −1.33; t5 = −2.39; P = 0.031 and M = −1, t5 = 5; P = 0.020) (see Figure 3A). They also reported more fatigue after the high dose compared to the low dose at 12 h after drug intake (M = −1.4;t5 = 2.746 P = 0.026) (see Figure 3B). Arousal was reported to be higher 12 h after administration, after a low dose of JWH‐018, compared to placebo (M = −3.6; t5 = 2.794; P = 0.025) and the high dose (M = 4.8; t5 = 5.58 P = 0.0025).
Figure 3.
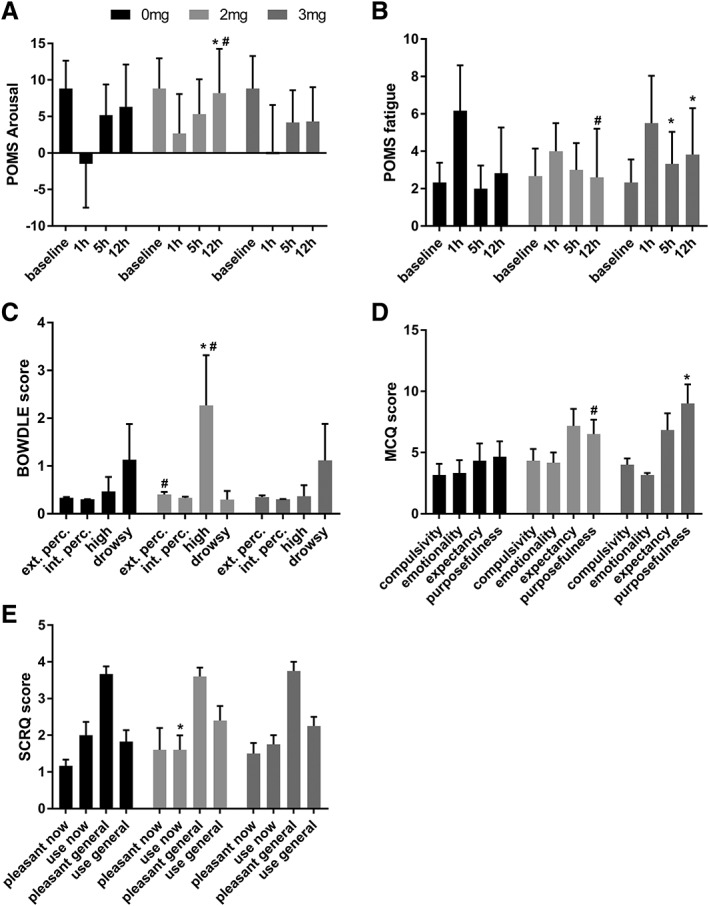
Scores for (A) the POMS arousal, and (B) POMS fatigue measured at the different times after treatment, (C) Bowdle measured at 1 h, (D) MCQ measured at 12 h and (E) SCRQ measured at 10 h after treatment. Results are presented as mean±SEM. * Significant difference from placebo (P < 0.05); # significant difference from 3 mg dose (P < 0.05).
The Bowdle VAS was administered three times during the test day. At 1 h after administrations, paired sample t‐tests showed that participants scored significantly higher on external perception in the low dose condition compared to the high dose (M = 0.053; t5 = 2.181; P = 0.041). Participants also scored higher on the ‘high’ scale in the low dose condition compared to placebo (M = −1.8; t5 = −2.171; P = 0.041) and the high dose condition (M = 1.9; t5 = 2.044; P = 0.048) (see Figure 3B).
The MCQ was administered 1 and 12 h post drug. After 12 h, paired sample t‐tests showed that participants scored higher on the purposefulness scale, in the high dose compared to placebo (M = −4.33; t5 = −2.14; P = 0.043) and the low dose condition (M = −2.50; t5 = −3.27; P = 0.011) (see Figure 3D).
The SCRQ was taken immediately after administration and 10 h later. Paired sample t‐tests showed that after 10 h, participants in the low dose condition reported a decreased feeling of wanting to use at that time (M = 0.06; t5 = 2.45 P = 0.035) compared to placebo (see Figure 3E).
Discussion
The current study is the first controlled clinical trial with the synthetic cannabinoid JWH‐018. We selected JWH‐018 as it is one of first psychoactive substances that was found in the popular Spice products. Though JWH‐018 has largely been replaced by a host of other synthetic cannabinoids since its introduction, it never fully disappeared from the market. Traces of JWH‐018 are still regularly found in products containing synthetic cannabinoids (Musshoff et al., 2014; WHO, 2014). Thus, the long‐standing history of JWH‐018 was one of the main reasons to select this compound for a phase 1 evaluation rather than any other synthetic cannabinoids. In addition, JWH‐018 is one of the few synthetic cannabinoids for which animal toxicology and receptor potency data were available prior to this trial (Aung et al., 2000; Atwood et al., 2010; Ginsburg et al., 2012; Wiebelhaus et al., 2012; WHO, 2014). Given that typical doses of JWH‐018 taken for recreational use are unknown, we estimated relevant doses of JWH‐018 that were minimally active on such toxicology and potency data.
The administration doses of of 2 and 3 mg JWH‐018 produced relatively low concentrations of this drug in serum. Concentrations of metabolites, of which some have been found to have CB1 receptor activity (Brents et al., 2011), were also low (Toennes et al., 2017). Drug concentrations were certainly lower than some of those that have been reported in actual case reports (Hermanns‐Clausen et al., 2013; WHO, 2014). However, concentrations in case reports are probably higher, as these often come from people who have overdosed with the drug. Nevertheless, the current low concentrations were partly caused by suboptimal delivery of JWH‐018 with the glass crack pipe. This could be due to differences in puff volume (Azorlosa et al., 1995) or active substance escaping the smoking device. Analyses of the delivery devices, however, revealed the presence of substantial amounts of residual JWH‐018. This demonstrates that subjects did not inhale the full amount of the JWH‐018 doses. Administration of both doses of JWH‐018 was done in the same way, and subjects were given the same instructions. Pure JWH‐018 was mixed in a glass pipe with some Knaster and heated for about 15 s. The resulting smoke was inhaled in one take. Nevertheless, the residues in the pipes indicated that a considerable amount of the active substance was not inhaled. As this was a post hoc analysis, we unfortunately have no way of knowing whether this was more the case for the high dose than the low dose (Toennes et al., 2017). However, it may very well explain why drug concentrations were lower in the 3 mg dose as compared to the 2 mg dose condition. It can therefore be concluded that our method of administration might not have been optimal for administering the JWH‐018 powder. However, when users are smoking herbal mixtures of JWH‐018, a considerable amount of the psychoactive substance probably goes up in smoke as well. In fact, the maximum concentrations measured in the current study are very comparable with the concentrations found in subjects who smoked a similar dose of JWH‐018 in an herbal mixture in an earlier study (Teske et al., 2010; Kacinko et al., 2011). Yet, as stated before, higher concentrations have also been reported in hospital cases (WHO, 2014).
In terms of vital signs, low concentrations of JWH‐018 were well tolerated by the subjects. ECG, vital signs and laboratory safety measures did not show any clinically significant deviations from the normal ranges. Side effects after JWH‐018 administration were limited to one report of headache and two of tiredness/low energy. Subjects did report subjective feelings of high for 2 h after JWH‐018 administration, particularly in the low dose condition. This confirms that drug concentrations in the low dose condition were high enough to elicit a behavioural response. Yet maximal levels of subjective high were only half of those that have typically been reported in controlled cannabis trials in our lab (Ramaekers et al., 2009, 2016). Likewise, the duration of subjective high has been two or three times longer in previous studies with cannabis (Ramaekers et al., 2009, 2016). Other subjective evaluations only revealed incidental elevations in fatigue and arousal after JWH‐018 administration. This indicates once again that JWH‐concentrations in the current study may have been too low to produce a stronger subjective or physiological response that one would typically expect after a recreational dose of cannabis.
Despite the low serum concentrations, JWH‐018 did cause behavioural impairments. JWH‐018 impaired motor performance (CTT), divided attention (DAT) and response inhibition (SST), particularly after the 2 mg dose. Executive functioning (TOL), spatial memory (SMT), speed and information processing (DSST) were not affected by JWH‐018. This demonstrates that the JWH‐018 concentrations in the current study are about the minimum levels that are needed to produce behavioural impairment. Overall, however, impairments were relatively mild and present in a subsample of neurocognitive functions which are usually impaired after regular doses of cannabis (Fried et al., 2005; Ranganathan and D'souza, 2006; Ramaekers et al., 2006a,b, 2009). The behavioural data therefore also indicate that higher doses of JWH‐018 would be needed to achieve a behavioural impairment profile that is similar to a typical cannabis dose.
Preclinical studies have shown that JWH‐018, inhaled or injected, demonstrates potent THC‐like effects in mice. These effects include hypothermia, locomotor suppression, analgesia and antinociception (Brents et al., 2011; Wiley et al., 2012; Marshell et al., 2014; Vigolo et al., 2015). However, comparing preclinical data with the current results is tricky, as dosages administered to animals are not easily translated to doses used in humans. Using an inter species dose scaling, Ossato et al. (2015), demonstrated that side effects such as convulsions, myoclonia and hyperreflexia were only evident in mice after doses which are comparable to 0.5 mg·kg−1 in humans. Low doses, which are comparable to doses between 0.0008 and 0.08 mg·kg−1 in humans, impaired motor functioning and working memory in mice (Ossato et al., 2015; Barbieri et al., 2016). These effects of low doses of JWH‐018 are somewhat comparable to the results we show in humans. The doses used in the current study are within the latter range (2 and 3 mg equals approximately 0.03 and 0.04 mg·kg−1), but as already discussed, the actual dose that was inhaled was much lower due to suboptimal delivery of the drug.
The current study was the very first attempt to assess physiological, subjective and behavioural effects of JWH‐018 in a placebo controlled study in humans. As this was a first‐in‐man study, the number of participants included was kept to a minimum. Larger sample studies are needed to better define health risk profiles of synthetic cannabinoids and novel psychoactive substances (NPS) in general. EMCDDA (2009) guidelines recommend that individual health risks of NPS should be directly assessed in pharmacological studies in humans and animals as a function of a number of key risk variables such as dose and frequency of use (EMCDDA, 2009). Human studies should furthermore include psychological and behavioural measures. Moreover, the EMCDDA guidelines also advise to assess the risk of novel NPS relative to traditional drugs of abuse. Yet, to date, systematic toxicological and pharmacological studies with NPS in humans and animals are virtually missing which hampers full scale evaluation of NPS risk, also in relation to scheduled drugs. As a consequence, current science is lagging behind in providing reliable and well‐validated information on individual health risks of NPS that are badly needed to provide a full‐scale risk assessment according to EMCDDA guidelines. The present study clearly demonstrates that controlled studies with NPS in humans are feasible and can be carried out safely in a phase 1 setting. The present study design therefore can serve as a blueprint for follow‐up research with other NPS and with higher doses/concentrations of JWH‐018. Phase 1 trials are particularly useful for NPS for which typical user doses are unknown, and dose‐finding is needed in the laboratory as in the present case. Previous controlled studies with mephedrone, another classical NPS, have also demonstrated that phase 1 trials significantly contribute to drug and risk profiling of NPS (de Sousa Fernandes Perna et al., 2016; Papaseit et al., 2016).
In summary, JWH‐018 dosing in the present study resulted in drug concentrations that were generally low and not fully representative of common use. Yet initial impairments of neurocognitive function and subjective feelings of high did emerge despite the low levels of JWH‐018 in serum. The administration of higher doses is needed to obtain a more representative risk profile of JWH‐018. The phase 1 approach for evaluating risk profiles of synthetic cannabinoids can serve as a blueprint for future research.
Author contributions
E.T, K.K. and J.R. designed the research study; N.H., N.M., E.S. and E.T. performed the study; S.T. performed the pharmacokinetic analyses; E.T. and J.R. analysed the data and wrote the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Acknowledgements
The authors thank Cees van Leeuwen and Lizzy Vuurman for medical supervision, Frans Jan Leferink for helping with data acquisition and the DSMB members (Wim Riedel, Therese van Amelsvoort and Jan Schepers) for evaluating the data and providing advice. We also thank the European Commission [HOME/2014/JDRU/AG/DRUG/7082, Predicting Risk of Emerging Drugs with In silico and Clinical Toxicology (PREDICT)] and the Bund gegen Alkohol und Drogen im Strassenverkehr (B.A.D.S.) for financial support.
Theunissen, E. L. , Hutten, N. R. P. W. , Mason, N. L. , Toennes, S. W. , Kuypers, K. P. C. , de Sousa Fernandes Perna, E. B. , and Ramaekers, J. G. (2018) Neurocognition and subjective experience following acute doses of the synthetic cannabinoid JWH‐018: a phase 1, placebo‐controlled, pilot study. British Journal of Pharmacology, 175: 18–28. doi: 10.1111/bph.14066.
References
- Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA et al (2017). The Concise Guide To PHARMACOLOGY 2017/18: G protein‐coupled receptors. Br J Pharmacol 174: S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood BK, Huffman J, Straiker A, Mackie K (2010). JWH018, a common constituent of 'Spice' herbal blends, is a potent and efficacious cannabinoid CB receptor agonist. Br J Pharmacol 160: 585–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aung MM, Griffin G, Huffman JW, Wu MJ, Keel C, Yang B et al (2000). Influence of the N‐1 alkyl chain length of cannabimimetic indoles upon CB1 and CB2 receptor binding. Drug Alcohol Depend 60: 133–140. [DOI] [PubMed] [Google Scholar]
- Auwärter V, Dresen S, Weinmann W, Müller M, Pütz M, Ferreirós N (2009). ‘Spice’ and other herbal blends: harmless incense or cannabinoid designer drugs? J Mass Spectrom 44: 832–837. [DOI] [PubMed] [Google Scholar]
- Azorlosa JL, Greenwald MK, Stitzer ML (1995). Marijuana smoking: effects of varying puff volume and breathhold duration. J Pharmacol Exp Ther 272: 560–569. [PubMed] [Google Scholar]
- Barbieri M, Ossato A, Canazza I, Trapella C, Borelli A, Beggiato S et al (2016). Synthetic cannabinoid JWH‐018 and its halogenated derivatives JWH‐018‐Cl and JWH‐018‐Br impair novel object recognition in mice: behavioral, electrophysiological and neurochemical evidence. Neuropharmacology 109: 254–269. [DOI] [PubMed] [Google Scholar]
- Bowdle AT, Radant AD, Cowley DS, Kharasch ED, Strassman RJ, Roy‐Byrne PP (1998). Psychedelic effects of ketamine in healthy volunteers: relationship to steady‐state plasma concentrations. Anesthesiology 88: 82–88. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS et al (1998). Measurement of dissociative states with the clinician‐administered dissociative states scale (CADSS). J Trauma Stress 11: 125–136. [DOI] [PubMed] [Google Scholar]
- Brents LK, Reichard EE, Zimmerman SM, Moran JH, Fantegrossi WE, Prather PL (2011). Phase I hydroxylated metabolites of the K2 synthetic cannabinoid JWH‐018 retain in vitro and in vivo cannabinoid 1 receptor affinity and activity. PLoS One 6: e21917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA et al (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sousa Fernandes Perna E, Papaseit E, Pérez‐Mañá C, Mateus J, Theunissen E, Kuypers K et al (2016). Neurocognitive performance following acute mephedrone administration, with and without alcohol. J Psychopharmacol 30: 1305–1312. [DOI] [PubMed] [Google Scholar]
- De Wit H, Enggasser JL, Richards JB (2002). Acute administration of d‐amphetamine decreases impulsivity in healthy volunteers. Neuropsychopharmacology 27: 813–825. [DOI] [PubMed] [Google Scholar]
- EMCDDA (2009). Risk Assessment of New Psychoactive Substances: Operating Guidelines. The Publication Office of the European Union: Luxemburg. [Google Scholar]
- Every‐Palmer S (2011). Synthetic cannabinoid JWH‐018 and psychosis: an explorative study. Drug Alcohol Depend 117: 152–157. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR, Hays L (2002). Acute effects of oral cocaine on inhibitory control of behavior in humans. Drug Alcohol Depend 67: 157–167. [DOI] [PubMed] [Google Scholar]
- Ford BM, Tai S, Fantegrossi WE, Prather PL (2017). Synthetic pot: not your grandfather's Marijuana. Trends Pharmacol Sci 38: 257–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried PA, Watkinson B, Gray R (2005). Neurocognitive consequences of marihuana – a comparison with pre‐drug performance. Neurotoxicol Teratol 27: 231–239. [DOI] [PubMed] [Google Scholar]
- Ginsburg BC, Schulze DR, Hruba L, Mcmahon LR (2012). JWH‐018 and JWH‐073: Δ9‐tetrahydrocannabinol‐like discriminative stimulus effects in monkeys. J Pharmacol Exp Ther 340: 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths P, Sedefov R, Gallegos A, Lopez D (2010). How globalization and market innovation challenge how we think about and respond to drug use: ‘Spice’ a case study. Addiction 105: 951–953. [DOI] [PubMed] [Google Scholar]
- Harris CR, Brown A (2012). Synthetic cannabinoid intoxication: a case series and review. J Emerg Med 44: 360–366. [DOI] [PubMed] [Google Scholar]
- Havenon AD, Chin B, Thomas KC, Afra P (2011). The secret “spice”: an undetectable toxic cause of seizure. Neurohospitalist 1: 182–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heishman SJ, Singleton EG (2006). Assessment of cannabis craving using the Marijuana Craving Questionnaire In: Onaivi ES. (eds) Marijuana and Cannabinoid Research. Methods In Molecular Medicine™, 123: Humana Press. [DOI] [PubMed] [Google Scholar]
- Hermanns‐Clausen M, Kneisel S, Hutter M, Szabo B, Auwärter V (2013). Acute intoxication by synthetic cannabinoids – four case reports. Drug Test Anal 5: 790. [DOI] [PubMed] [Google Scholar]
- Jex HR, Mcdonnell JD, Phatak AV (1966). A ‘critical’ tracking task for man‐machine research related to the operator's effective delay time. I. Theory and experiments with a first‐ order divergent controlled element. NASA CR‐616. NASA Contract Rep NASA CR, 1–105. [PubMed]
- Kacinko SL, Xu A, Homan JW, Mcmullin MM, Warrington DM, Logan BK (2011). Development and validation of a liquid chromatography‐tandem mass spectrometry method for the identification and quantification of JWH‐018, JWH‐073, JWH‐019, and JWH‐250 in human whole blood. J Anal Toxicol 35: 386–393. [DOI] [PubMed] [Google Scholar]
- Kessels RP, Postma A, De Haan EH (1999). Object relocation: a program for setting up, running, and analyzing experiments on memory for object locations. Behav Res Methods 31: 423–428. [DOI] [PubMed] [Google Scholar]
- Lindigkeit R, Boehme A, Eiserloh I, Luebbecke M, Wiggermann M, Ernst L et al (2009). Spice: a never ending story? Forensic Sci Int 191: 58–63. [DOI] [PubMed] [Google Scholar]
- Logan GD (1994). On the ability to inhibit thought and action In: Dagenbach D, Carr TH. (eds). Inhibitory Processes in Attention, Memory and Language. Academic Press: San Diego. [Google Scholar]
- Marshell R, Kearney‐Ramos T, Brents L, Hyatt W, Tai S, Prather P et al (2014). In vivo effects of synthetic cannabinoids JWH‐018 and JWH‐073 and phytocannabinoid Δ 9‐THC in mice: inhalation versus intraperitoneal injection. Pharmacol Biochem Behav 124: 40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcleod DR, Griffiths RR, Bigelow GE, Yingling J (1982). An automated version of the digit symbol substitution test (DSST). Behav Res Methods 14: 463–466. [Google Scholar]
- Moskowitz H (1973). Laboratory studies of the effects of alcohol on some variables related to driving. J Safety Res 5: 185–192. [Google Scholar]
- Musshoff F, Madea B, Kernbach‐Wighton G, Bicker W, Kneisel S, Hutter M et al (2014). Driving under the influence of synthetic cannabinoids (“Spice”): a case series. Int J Leg Med 128: 59–64. [DOI] [PubMed] [Google Scholar]
- Ossato A, Vigolo A, Trapella C, Seri C, Rimondo C, Serpelloni G et al (2015). JWH‐018 impairs sensorimotor functions in mice. Neuroscience 300: 174–188. [DOI] [PubMed] [Google Scholar]
- Papaseit E, Pérez‐Mañá C, Mateus J‐A, Pujadas M, Fonseca F, Torrens M et al (2016). Human pharmacology of mephedrone in comparison with MDMA. Neuropsychopharmacology 41: 2704–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaekers JG, Kuypers KPC (2006). Acute effects of 3,4‐methylenedioxymethamphetamine (MDMA) on behavioral measures of impulsivity: alone and in combination with alcohol. Neuropsychopharmacology 31: 1048–1055. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Kauert G, Van Ruitenbeek P, Theunissen EL, Schneider E, Moeller MR (2006a). High‐potency marijuana impairs executive function and inhibitory motor control. Neuropsychopharmacology 31: 2296–2303. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Moeller MR, Van Ruitenbeek P, Theunissen EL, Schneider E, Kauert G (2006b). Cognition and motor control as a function of delta9‐THC concentration in serum and oral fluids: limits of impairment. Drug Alcohol Depend 85: 114–122. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Kauert G, Theunissen EL, Toennes SW, Moeller MR (2009). Neurocognitive performance during acute THC intoxication in heavy and occasional cannabis users. J Psychopharmacol 23: 266–277. [DOI] [PubMed] [Google Scholar]
- Ramaekers J, Van Wel J, Spronk D, Toennes S, Kuypers K, Theunissen E et al (2016). Cannabis and tolerance: acute drug impairment as a function of cannabis use history. Sci Rep 6: 26843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan M, D'souza DC (2006). The acute effects of cannabinoids on memory in humans: a review. Psychopharmacology (Berl) 188: 425–444. [DOI] [PubMed] [Google Scholar]
- Rosenbaum CD, Carreiro SP, Babu KM (2012). Here today, gone tomorrow … and back again? A review of herbal marijuana alternatives (K2, Spice), synthetic cathinones (bath salts), kratom, Salvia divinorum, methoxetamine, and piperazines. J Med Toxicol 8: 15–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedefov R, Gallegos A, King L, Lopez D, Auwärter V, Hughes B et al (2009). Understanding the ‘Spice’ phenomenon. Thematic papers, European Monitoring Centre for Drugs and Drug Addiction.
- Shallice T (1982). Specific impairments of planning. Philos Trans R Soc Lond B Biol Sci 298: 199–209. [DOI] [PubMed] [Google Scholar]
- Simmons J, Cookman L, Kang C, Skinner C (2011). Three cases of ‘spice’ exposure. Clin Toxicol 49: 431–433. [DOI] [PubMed] [Google Scholar]
- Sobolevsky T, Prasolov I, Rodchenkov G (2010). Detection of JWH‐018 metabolites in smoking mixture post‐administration urine. Forensic Sci Int 200: 141–147. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH et al (2015). The IUPHAR/BPS guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steup C (2008). Untersuchung des Handelsproduktes 'Spice'. Frankfurt am Main: THC Pharm GmbH. [Google Scholar]
- Teske J, Weller JP, Fieguth A, Rothämel T, Schulz Y, Tröger HD (2010). Sensitive and rapid quantification of the cannabinoid receptor agonist naphthalen‐1‐yl‐(1‐pentylindol‐3‐yl) methanone (JWH‐018) in human serum by liquid chromatography‐tandem mass spectrometry. J Chromatogr B 878: 2659–2663. [DOI] [PubMed] [Google Scholar]
- Toennes SW, Geraths A, Pogoda W, Paulke A, Wunder C, Theunissen EL et al (2017). Pharmacokinetic properties of the synthetic cannabinoid JWH‐018 and of its metabolites in serum after inhalation. J Pharm Biomed Anal 140: 215–222. [DOI] [PubMed] [Google Scholar]
- Vandrey R, Dunn KE, Fry JA, Girling ER (2012). A survey study to characterize use of Spice products (synthetic cannabinoids). Drug Alcohol Depend 120: 238–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardakou I, Pistos C, Spiliopoulou C (2010). Spice drugs as a new trend: mode of action, identification and legislation. Toxicol Lett 197: 157–162. [DOI] [PubMed] [Google Scholar]
- Vigolo A, Ossato A, Trapella C, Vincenzi F, Rimondo C, Seri C et al (2015). Novel halogenated derivates of JWH‐018: behavioral and binding studies in mice. Neuropharmacology 95: 68–82. [DOI] [PubMed] [Google Scholar]
- WHO (2014). World Health Organization. JWH‐018 Critical Review Report. World Health Organization.
- Wiebelhaus JM, Poklis JL, Poklis A, Vann RE, Lichtman AH, Wise LE (2012). Inhalation exposure to smoke from synthetic ‘marijuana’ produces potent cannabimimetic effects in mice. Drug Alcohol Depend 126: 316–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Marusich JA, Huffman JW, Balster RL, Thomas BF (2011). Hijacking of basic research: the case of synthetic cannabinoids. Methods report (RTI Press). [DOI] [PMC free article] [PubMed]
- Wiley JL, Marusich JA, Martin BR, Huffman JW (2012). 1‐Pentyl‐3‐phenylacetylindoles and JWH‐018 share in vivo cannabinoid profiles in mice. Drug Alcohol Depend 123: 148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann US, Winkelmann PR, Pilhatsch M, Nees JA, Spanagel R, Schulz K (2009). Withdrawal phenomena and dependence syndrome after the consumption of ‘spice gold’. Dtsch Arztebl Int 106: 464–467. [DOI] [PMC free article] [PubMed] [Google Scholar]


