Abstract
To review the optimality and safety of different anti‐Amyloid‐β(Aβ) immunotherapies for Alzheimer's disease (AD). Published randomized controlled trials were comprehensively reviewed from electronic databases (Cochrane library, Embase, Pubmed, and Google scholar). Pooled outcomes as mean difference or odds ratio values with 95% confidence interval were reported. The network estimates with confidence and predictive intervals for all pairwise relative effects was evaluated. Optimal intervention was ranked by benefit‐risk ratio based on the surface under the cumulative ranking curve. Eleven eligible RCTs from 9 literatures, including 5141 patients and 5 interventions were included. The quality of evidence was rated low in comparisons. For efficacy, in terms of Mini‐Mental State Examination, aducanumab and solanezumab are significantly effective than placebo. For safety, in terms of Amyloid‐Related Imaging Abnormalities (ARIA), bapineuzumab and aducanumab are significantly worse than placebo. There were no significant differences in outcomes of Alzheimer's disease Assessment Scale‐Cognitive subscale, Disability Assessment for Dementia, Adverse Events, and mortality. Given the clinical therapeutic effects of anti‐Aβ immunotherapies for AD, aducanumab and solanezumab improve the cognitive function, while aducanumab and bapineuzumab may increase the risks of ARIA.
Introduction
Alzheimer's disease
Alzheimer's disease (AD) is a globally prevalent neurodegenerative condition, clinically characterized by progressive impairment of memory and cognitive functions.1 At present, there were approximately 36 million AD patients in the world, and it is expected to double every 20 years in future. By 2050, the number of AD patients may reach 115 million. Once in 2010, 83,494 deaths from AD were officially recorded, most of which were attributed to the complications of AD. In 2013, more than 15 million family members and other unpaid caregivers had provided an estimated 17.7 billion hours of care to people with AD and other dementias.2 The 2014 World Alzheimer's Disease report states that one in every three elderly people is killed by AD or other types of dementia, and about 500,000 people die each year from AD. At present, the clinical treatment of AD mainly includes acetylcholinesterase inhibitor for improving the cognitive ability, N‐methyl‐D‐aspartate (NMDA) antagonist such as memantine, etc., all of these drugs are only reducing the symptoms of patients, and cannot fundamentally treat AD.3 It is urgent to search strategies to alleviate the intensification of these challenges.
At present, amyloid‐β (Aβ) hypothesis is widely known as the most important pathogenesis of AD. It is believed that abnormal accumulation of Aβ into extracellular toxic plaques is responsible for the neurodegeneration and resulting dementia in AD.4 The clearance of Aβ depends on the immune mechanism of the organism. Peripheral Aβ antibodies (IgG) can be infiltrated into the brain, combined with Aβ to form immune complexes, activated microglia to remove Aβ. In addition, Aβ antibody/Aβ immune complex can also be transported across the blood‐brain barrier to peripheral blood through the neonatal Fc receptor (FcRn). Endogenous autoantibodies against Aβ appear to be effective in purifying Aβ deposition against Aβ toxicity and have no side effects, but the expression level in vivo (especially in AD patients) is low. AD treatment strategy mainly included the active immunotherapy vaccine and the passive immunotherapy antibody targeting Aβ. Studies have shown that active immunization of Aβ can effectively remove Aβ plaques and relieve AD symptoms. However, immunotherapies in some clinical trials cause aseptic meningitis or brain hemorrhage phenomenon by autoreactive T‐cell infiltration.5 These suggested the complexity of the immune clearance mechanism of Aβ in vivo, and highlight the safety of immunotherapy. Immunotherapy represents one of the first tests of the amyloid hypothesis in the clinic and holds great potential for treating or even preventing AD.
Anti‐Aβ immunotherapy
As a new treatment, immunotherapy can reduce the pathological damage of AD, delay or reverse the decline in cognitive ability. Active immunization or vaccination of AD is through the introduction of Aβ peptide fragments and adjuvant binding to stimulate the host immune response enabling the host to produce antibodies against Aβ, thereby resulting in effective removal or prevention of Aβ plaques.
Active immunotherapy
Active immunization is by injecting a complex of Aβ peptide with adjuvant to stimulate the immune system. A randomized placebo‐controlled study (Phase I) was conducted in mild to moderate AD patients in 2000 to assess the safety of human immunogenic aggregation of Aβ peptide immunization and the results showed a dose‐dependent antibody response, but there was poor correlation in plaques clearance and short‐term clinical efficacy. The results of phase I trial showed that there was no significant difference in the disability assessment score between the treatment group and the control group after 84 weeks. Two years later, the results of the phase II trial showed that the clearance of amyloid plaque removal was obvious in the treatment group, but about 6% of patients suffered aseptic meningoencephalitis leaving the trial stopped.6 This response is thought to be caused by adjuvant mediating Th1 cells infiltrated in the central nervous system, resulting an autoimmune neuritis. A 3‐year clinical trial (Phase I) was performed in 80 AD patients. The evidence for successful immunization was a reduction in plaque burden, but the immunization had less effect on cognitive function.7 Another study has found that removal of plaque alone is not enough to change the progression of AD.8
The goal of the recent vaccine design is to reduce the Th1 cellular immune response and induce the Th2 humoral immune response. CAD106 is a novel virus‐like‐particle‐based vaccine that can be used to enhance the immune response to the self‐proteins without adjuvants its viral component contains exogenous polypeptides that can escape from autoimmune T‐cell responses. The result of clinical trial (phase I) showed that the adverse reactions of CAD106 is nasopharyngeal and injection site response, but no meningitis.9 The result of phase II trial showed that mild to moderate AD patients had better safety and antibody formation, 82% of patients in the treatment group had a corresponding antibody response. Aβ peptides have been used to develop vaccines and carry out clinical trials. These synthetic peptides are shorter (6 amino) to mimic the N‐terminus of unmodified Aβ peptides and activate T cells with less cross‐reactivity.8
Passive immunotherapy
Passive immunotherapy refers to the direct use of anti‐Aβ monoclonal antibody. It is dose controlled and free of cells response but need for repeated injection with a high cost. At present, with the success of preclinical trials, clinical trials have been performing for passive immunotherapy, namely the development of monoclonal antibodies for different sites of Aβ peptide. Bapineuzumab is the first N‐terminal humanized monoclonal antibody against Aβ peptides. Clinical trials (phase II)10 found that adverse reactions were vascular edema and microhaemorrhages, but the incidence was not sufficient to discontinue further studies. Vascular edema is closely related to ApoE4 carriers, and microhaemorrhages are associated with amyloid angiopathy. Solanezumab is a monoclonal antibody against the intermediate domain of Aβ peptide. Phase II Clinical trials11 showed that it is safe and has a good effect in reducing the plaque burden, patients were benefited in cognitive effect. Gantenerumab is the first fully human anti‐Aβ monoclonal antibody, Phase III clinical trials are ongoing12 Ponezumab, a humanized monoclonal antibody, is designed to reduce T‐cell responses, it had been identified safer in animal studies and has now completed Phase I clinical trials.13 In order to determine the safest and most appropriate antigenic determinants, a small‐scale clinical trial results14 showed that Aβ decreased in cerebrospinal fluid, and cognitive function improved significantly 6 weeks after treatment. Large‐scale trials will focus on cognitive, functional, and behavioral outcomes.
Adverse effects
Immunotherapy is one of the most evolving approaches to modify the neurodegeneration and the cognitive decline present in AD,4, 15 it works together with human immune system to neutralize the aggregation process of Aβ species.15 However, the therapeutic risk should not be ignored. Amyloid‐related imaging abnormalities (ARIA) represent the major severe side effect of Aβ directed immunotherapy.16 The proportion of ARIA and mortality were not systematically reviewed in published studies. Except for ARIA, headache, urinary system, and upper respiratory tract infection are also common in immunotherapy for AD patients.
Network meta‐analysis
Network meta‐analysis (NMA) is a methodology that can synthesize direct and indirect evidence in a network of trials and rank multiple interventions according to the study outcomes.17 The Frequentist method considers all indirect and direct evidence to determine the relative treatment effects between all interventions that can be linked through shared comparators.18
Previous pairwise meta‐analyses were done to evaluate the efficacy safety of all types of immunotherapeutic agents targeting Aβ.19, 20 However, these studies were inconclusive because many therapies have not been directly compared so that the question of which immunotherapies would be preferred for the treatment of AD remains controversial. Therefore, the purpose of this NMA is to comprehensively determine the optimal therapeutic treatment and evaluate risk for AD.
Materials and Methods
This NMA was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA NMA)21 and Cochrane guidelines.22
Eligibility criteria
According to the PICOS checklist,22 inclusion criteria were listed as follows: Population were the patients diagnosed with AD based on the criteria of Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM‐IV); Intervention were any anti‐Aβ antibodies against Aβ plaques pathologically presented in patients with AD; Control were placebo or any drugs in trials comparing different monoclonal antibodies; Outcomes were measurable clinical scores referred to as “efficacy” and adverse events referred to as “risk”; Study design were randomized controlled trials.
Study search and data extraction
Three independent authors (Jia‐Jie Mo, Jin‐Yu Li, Zheng Yang) constructed the corresponding search strategies in Cochrane library, Embase and Pubmed databases until June 2017. Additionally, to identify further studies, references manually scanned from relevant reference report, clinical trials websites were also checked.
Titles and abstracts were independently reviewed for potentially relevant studies according to the predefined criteria. If multiple publications of the same trial were retrieved, only the most recent and informative publication was included.
Three authors (Jia‐Jie Mo, Jin‐Yu Li, Zheng Yang), respectively, reviewed full manuscripts of eligible studies and extracted correlated information, including study characteristics, measured outcomes, and adverse events. When essential data was not reported, we contacted corresponding authors and estimated them from summary statistics. Discrepancies were resolved by discussion and consensus.
Risk of bias assessment and evidence grading
Risk of bias was assessed using the items reported in the Cochrane Risk of Bias Tool (Revman, version 5.3 for Windows), including random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other sources of bias. Based on the above domains, the included RCTs were classified into one of three categories: low risk, high risk, or unclear risk.
The Grades of Recommendation, Assessment, Development and Evaluation (GRADE) system23 adopted to NMA24 was used to grade the quality of evidence into four levels: high, moderate, low, and very low quality. The quality can be downgraded by one (serious concern) or two levels (very serious concern) for the following reasons: risk of bias (study‐level quality), inconsistency (unexplained heterogeneity, inconsistency of results), indirectness (indirect population, intervention, control, outcomes), and imprecision (wide predictive intervals, single trial). The quality can also be upgraded by one level because of large summary effect and dose‐response gradient.
Summary measures
The overall improvement of AD symptoms from baseline to endpoint and the proportion of adverse events (AEs), ARIA and mortality related to the immunotherapies were considered for the primary issue. To measure improvement of AD symptoms, the Alzheimer's Disease Assessment Scale‐Cognitive subscale (ADAS‐Cog), Clinical Dementia Rating‐Sum of boxes (CDR‐SB), Disability Assessment for Dementia (DAD); Mini‐Mental State Examination (MMSE) were used to evaluate the “Efficacy” (Higher scores indicate greater cognitive impairment in ADAS‐Cog and CDR‐SB while conversely in DAD and MMSE). Proportion of AEs, ARIA, mortality was extracted to evaluate the “Safety” (Higher rates indicate greater risk).
Firstly, a conventional pair‐wise meta‐analysis was conducted by synthesizing all direct evidence. We used arm‐specific mean differences (MD) from baseline and odds ratios (OR) for continuous and dichotomous data, respectively. The 95% confidence interval (CI) was calculated as a measure of estimate uncertainty. The heterogeneity across studies was estimated using the ifplot command with STATA software (version 13.0) to evaluate the statistical inconsistency. To account for heterogeneity between studies, pooled estimate and 95% CI were calculated assuming random‐effects models with Inverse Variance (IV) method.
Furthermore, a network meta‐analysis (NMA) was performed for each endpoint within a Frequentist framework, implemented in STATA software (version 13.0).25
A series of graphical tools are used to provide visual graphs for the results of this NMA. Network plot of interventions is a visual representation of the evidence base and offers a concise description of its characteristics;
Contribution plots present the influence of each direct piece of evidence;
Inconsistency plots refer to detect the difference between direct and various indirect effect estimates for the same comparison;
Funnel plots is a simple scatter plot of the intervention effect estimates from individual studies against some measure of each study's size or precision.22 Funnel plots were used to detect publication bias and explain their resources; Ranking plots will be performed to provide information about ranking of all evaluated interventions for the study outcomes.26
The surface under the cumulative ranking curve (SUCRA) was created to rank these interventions. A simple numerical summary to supplement the graphical display of cumulative ranking is to estimate the SUCRA line for each treatment, which equals one when a treatment is certain to be the best and zero when a treatment is certain to be the worst.27
Results
Study characteristics
Totally 118 references from literature were identified based on the searching strategy up to May 2017. After reviewing the titles and abstracts, we excluded 105 references as they were either not relevant or duplicate publications (Fig. 1). Of the remaining 13 references, we excluded 4 studies28, 29, 30, 31 because the primary outcomes presented by these trials were not meeting the inclusion criteria.
Figure 1.
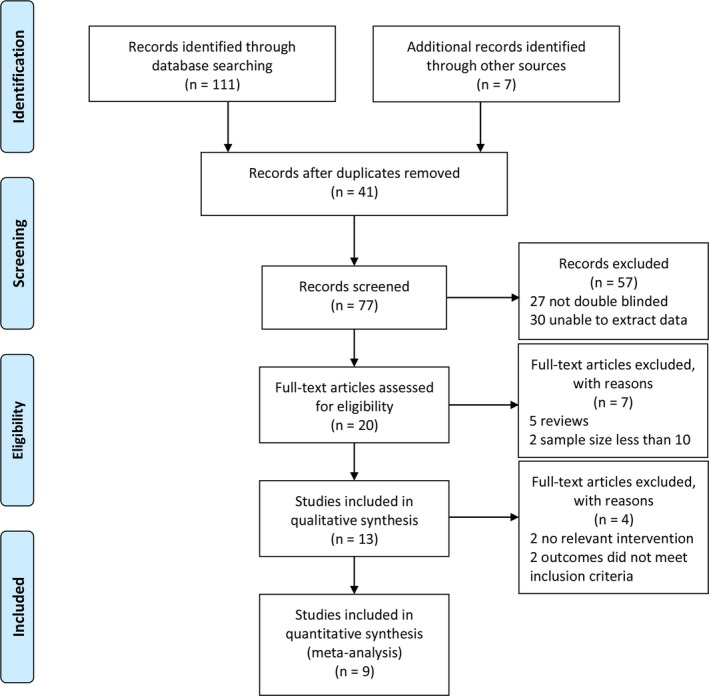
PRISMA flow diagram.
Eventually, 11 RCTs from 9 studies,9, 32, 33, 34, 35, 36, 37, 38, 39 including 5141 patients diagnosed with mild to moderate AD and 5 interventions: Aducanumab,37 AN1792 + QS21,32 Bapineuzumab,33, 36, 39 CAD106,9, 38 Solanezumab34, 35 were retrieved. The details of all included studies were demonstrated in Table S1. The mean study sample size was 571 participants, ranging from 52 to 2204 patients in the included studies. All the patients were randomly assigned to experimented group and placebo group. Almost all the studies reported complete demographic (mostly from North America and Europe) and clinical characteristics, especially one study35 did not illustrate the trial centers and the other trial9 failed to evaluate the efficacy of CAD106 for AD in appropriate rating scale. The age of sample population was ranging from 67.9 to 75.0 years; follow‐up period was ranging from 12 to 80 weeks. Changes from baseline to endpoint in different rating scores, such as ADAS‐Cog, CDR‐SB, DAD and MMSE, were calculated to evaluate the efficacy and the OR value in the proportion of AEs, the data of ARIA and mortality was used to investigate the safety.
Risk of bias within studies
In terms of quality, 5 (45.5%) trials were rated as moderate risk of bias, 6 (54.5%) trials as low (Fig. 2). 5 studies 9, 32, 33, 37, 38 adequately described the method used to generate the allocation sequence, concealment and the blinding, so that we considered them as low risks as well as 3 studies33, 35, 36 mentioned the modified intent‐to‐treat population. However, we deemed high drop‐out rate (more than 20%) as high risk in 2 studies.33, 35 Besides, 5 studies9, 32, 37, 38, 39 was funded by the pharmaceutical company in which reporting bias could not be excluded. Other respects among studies were assessed at unclear risk of bias when too few details were presented to make a judgement of “high risk” or “low risk” (Table S2).
Figure 2.
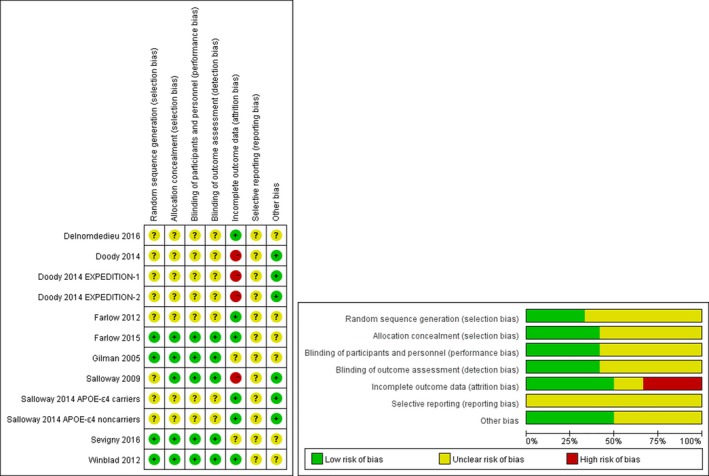
Assessment of risk bias.
Results of individual studies
For the efficacy of immunotherapy, Gilman et al.32 revealed that AN1792 (QS21) did not benefit patients with AD during 12‐month follow‐up. Sevigny et al.37 found a slowing of clinical decline in MMSE score, when patients used aducanumab. Salloway et al.33, 36 performed 2 RCTs to investigate the efficacy of bapineuzumab in the year of 2009 and 2014 but concluded that no significant differences were found in the primary efficacy analysis. Farlow et al.34 assessed efficacy of solanezumab only according to the clinical laboratory values. Doody et al.35 showed no significant improvement in the primary outcomes between solanezumab and placebo group.
For the safety of immunotherapy, the occurrence of AEs was higher in experimental group than placebo group during the follow‐up period. But the AEs are common and over 90% were mild to moderate in severity. Seven incidences of death were reported by Gilman et al.32 Only one death was reported by Salloway et al.33 in the bapineuzumab‐treated group. Farlow et al.34 unfolded 10 serious adverse events (SAEs) in 8 patients but mortality was missing. Winblad et al.9 observed that 9 patients had SAEs but no death or discontinuations due to SAEs. Doody et al.35 reported cases of deaths in both two group but revealed no clear treatment‐related pattern. Salloway et al.36 described the information of ARIA, mortality, AEs in detail. Sevigny et al.37 found that there were no drug‐related deaths. Overall, we could not underestimate the potential risks brought by these approaches.
Synthesis of results
The results of change in symptoms from baseline to endpoint and the proportion of AEs, ARIA, and mortality among included studies were shown in the Table S3. Additionally, we summarized the results of network meta‐analyses with effect sizes (MD or OR) and their 95% CI in Figure 3. Comparisons should be read from left to right. Different estimate was located at the intersection of the column‐defining interventions and the row‐defining interventions. For efficacy, the mean overall change in ADAS‐Cog and CDR‐SB below 0 favors the row‐defining interventions, however, the mean overall change in DAD and MMSE below 0 favors the column‐defining interventions. For safety (proportion of AEs, ARIA, Mortality), an OR below 1 favors the row‐defining interventions. Significant results are in bold. As shown in Figure 3, in terms of CDR‐SB score, only bapineuzumab was lower than placebo (MD: −0.70, 95% CI: −1.37 to −0.03); in terms of MMSE, aducanumab, and solanezumab are significantly effective than placebo (1.11 0.53–1.69 and 0.65 0.28–1.02). Aducanumab are solanezumab are significantly better than bapineuzumab (1.06 0.38–1.74 and 0.60 0.09–1.12). In terms of ARIA, bapineuzumab and aducanumab are significantly worse than placebo (OR: 60.88 95% CI: 15.07–245.94 and 6.54 1.49–28.65). Aducanumab is significantly worse than CAD106 and solanezumab (30.07 1.21–748.79 and 6.79 1.48–31.12). Also, bapineuzumab is significantly worse than solanezumab (63.20 14.91–267.86). There is no significant differences in ADAS‐Cog, DAD, AEs, and mortality.
Figure 3.
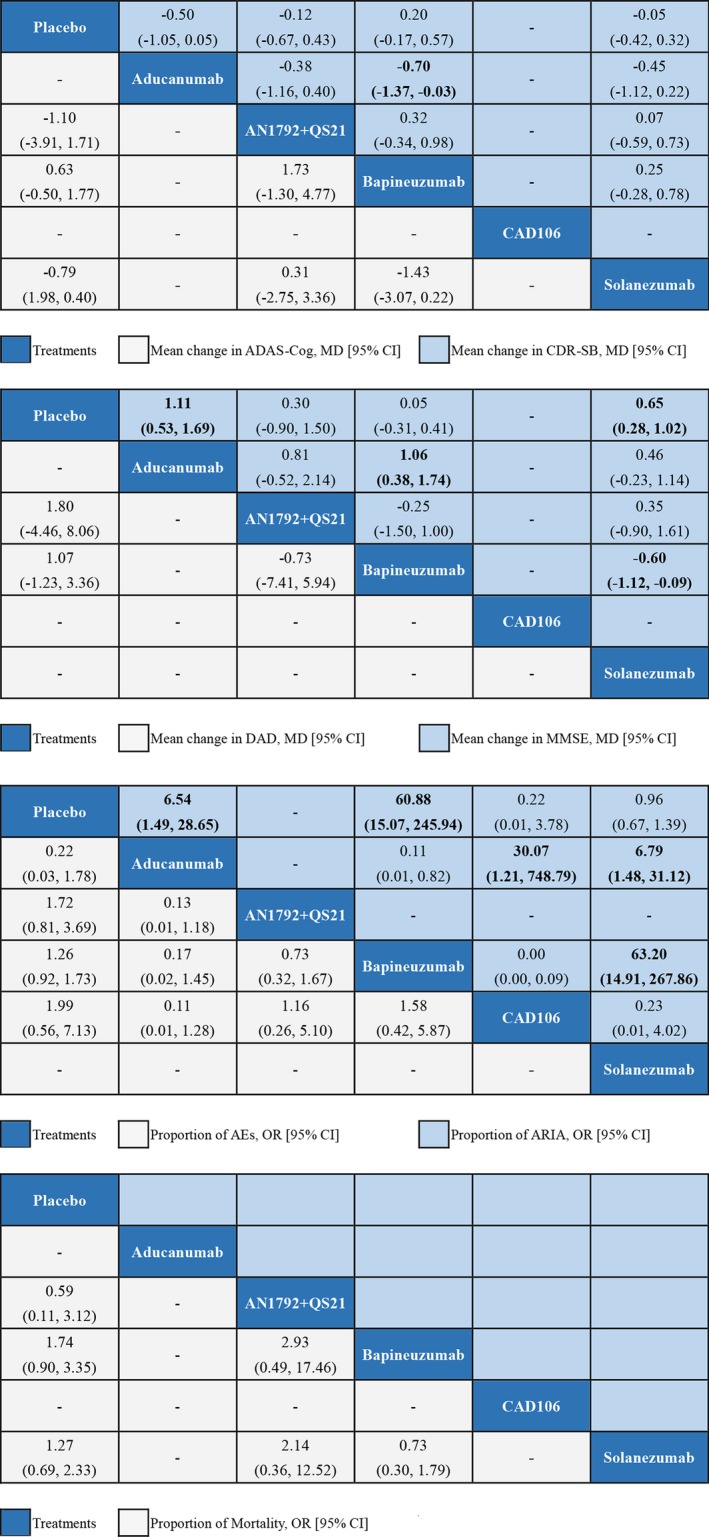
Network meta‐analysis of efficacy and safety. Treatments are reported in order of efficacy and safety ranking according to SUCRAs. Comparisons should be read from left to right. Different estimate was located at the intersection of the column‐defining interventions and the row‐defining interventions. For efficacy, the mean overall change in ADAS‐Cog and CDR‐SB below 0 favors the row‐defining interventions, however, the mean overall change in DAD and MMSE below 0 favors the column‐defining interventions. For safety (proportion of AEs, ARIA, Mortality), an OR below 1 favors the row‐defining interventions. Significant results are in bold. AEs, Adverse Events; MMSE, Mini‐mental state examination; SUCRA, The surface under the cumulative ranking curves; ‐, not available; MD, mean difference; OR, odds ratio; 95% CI, 95% credible interval. ARIA, amyloid‐related imaging abnormalities.
In the results of network meta‐analysis, SUCRA values were performed to report each evaluation in Table S4. The best intervention to improve symptom based on ADAS‐Cog, CDR‐SB, DAD and MMSE is solanezuma, aducanumab, AN1792 + QS21, and aducanumab. The safest intervention based on the proportion of AEs, ARIA, and mortality is aducanumab, CAD106, AN1792 + QS21.
According to the results of GRADE, the quality of evidence for primary studied outcomes was rated low for all comparisons (Table S5) which were attributed to the high drop‐out rates and different interventions. Preplanned sensitive analyses did not affect the primary outcomes.
Risk of bias across studies
Our study could be considered as high quality because we reduce bias as possible as we can. Reasonable search strategies and three authors’ independent contribution can reduce sampling bias. Explicit and rigid inclusion criteria, independent searching and sensitivity analysis contribute to the reduction in selection bias. Separated data extraction, quality assessment of individual study and evidence grading alleviate the study bias. Also, no funding resource influenced on the reporting bias.
Additional analyses (Interpretation of finished network graphs)
Network plots were shown in Figure 4. The size of every node represented randomly assigned participants (sample size) and the thickness of the lines represented the number of trials comparing every pair of treatments weight.
Figure 4.
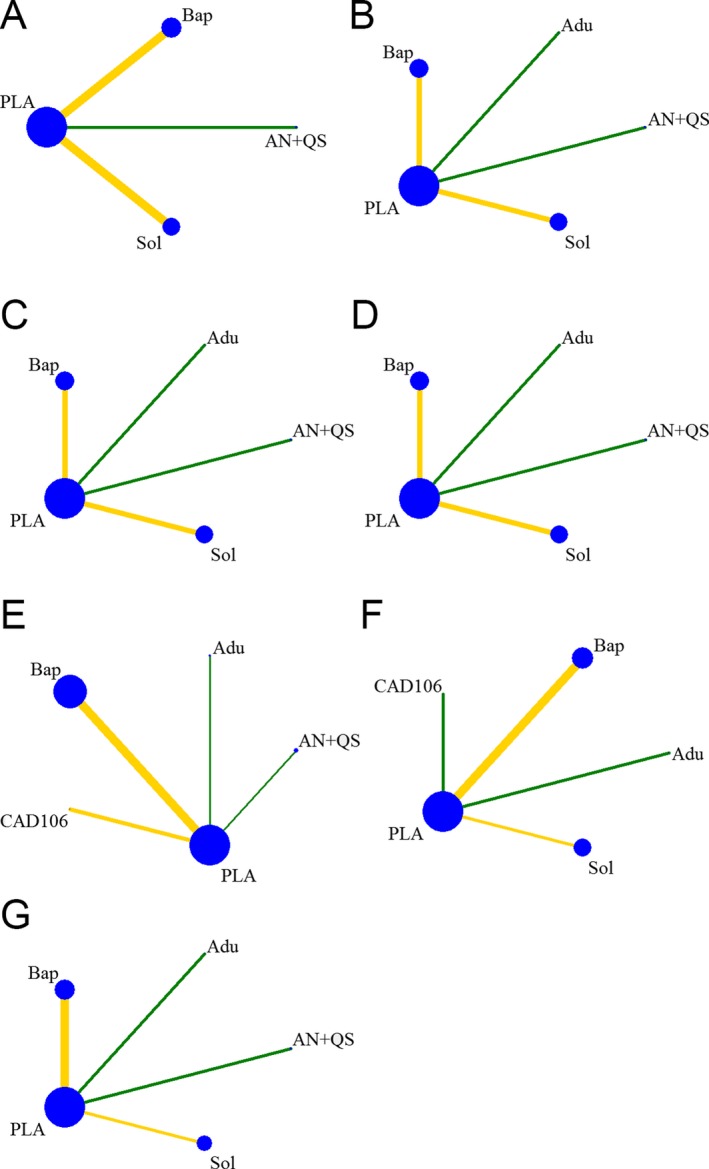
Plots for network of interventions. (A) ADAS‐Cog; (B) CDR‐SB; (C) DAD; (D) MMSE; (E) AEs; (F) ARIA; (G) Mortality. PLA, Placebo; Adu, Aducanumab; AN + QS, AN1792 + QS21; Bap, Bapineuzumab; CAD, CAD106; Sol, Solanezumab.
Contribution plots were shown in Figure S1. All direct pairwise effect sizes with variances in the entire network were calculated and the percentage contribution of each comparison was estimated. The weighted circles were created to represent the respective contributions.
Interval plots were shown in Figure S2. The horizontal lines in the forest plots represent the confidence intervals which were extended to show simultaneously the predictive intervals.
SUCRA values were used to estimate the ranking probabilities for each intervention being at order. Then, based on the SUCRA percentages and the mean ranks, the rankograms (line plots of the probabilities vs. ranks) and cumulative ranking plots (line plots of the cumulative probabilities vs. ranks) for all treatments were shown in Figures S3 and S4.
Funnel plots were shown in Figure S5. The horizontal axis shows the difference of each study's estimate from the summary effect for the respective comparison, while the vertical axis presents a measure of dispersion of estimate. Asymmetry of the funnel plots can be attributed to the presence of bias, which indicates the smaller studies with lower methodological quality may produce exaggerated estimated effects of interventions.
Inconsistency plots could not be performed because ifplot command identified all triangular and quadratic loops in a network of interventions. But all the treatments comparing with placebo in all the trials were different, lacking direct comparison between each other. In other words, no trial were conducted to compare two or more different drugs with each other. Therefore, there was no closed loop exist in the networks.
Discussion
Summary of evidence
In the past several decades, numbers of individuals were diagnosed with AD. Nowadays, we do not have an effective treatment for AD. This might be changed as much clinical trials were performed to identify the optimal treatment in slowing down the progression of AD, especially the monoclonal antibody against amyloid plaques in the brain. Many pairwise meta‐analyses were established to investigate the efficacy of anti‐Aβ immunotherapy for AD.1, 40 However, these studies were inconclusive because many drugs had not been compared directly. This NMA represents the comprehensive synthesis of data for currently available immunotherapies for patients with AD. By comparing with placebo, aducanumab and solanezumab were significantly presented more effective than placebo, based on MMSE score. Besides, aducanumab and bapineuzumab increased incidence of ARIA. Our analysis did not find evidence to suggest a significantly increased AEs and risk for mortality. Unfortunately, due to the absence of reliable data on mortality for many anti‐Aβ immunotherapies, it was not enough to comprehensively investigate the risk of all drugs. Although the AEs were mild to moderate and could be resolved by corresponding therapeutic methods, clinicians should not ignore these problems. Patients should be closely monitored and implemented individualized immunotherapies, particularly at the beginning of treatment. However, the clinical interpretation of these findings is affected by the potential bias due to selective reporting. We tried our best to identify all available unpublished information and contacted researchers for supplementary data, but we cannot rule out the possibility that some unpublished studies are still missing or that published reports might overestimate the efficacy of treatments. In the network of this NMA, we failed to analyze inconsistency for efficacy and safety, because comparisons among studies could not form triangular or quadratic loops. To reduce heterogeneity and inconsistency across trials in this NMA, we established restrict inclusion criteria, but had to agree that it decreased the external validity of the results and led to an overestimation of efficacy in this meta‐analysis.
After years of study on monoclonal antibodies for AD, some experiments had shown that aducanumab and solanezumab enabled to cross the blood‐brain barrier and get into the brain. 41, 42 The drugs are thought to target aggregated forms of Aβ including soluble oligomers and insoluble fibrils deposited into the amyloid plaque in the brain, but does not bind Aβ monomers.37 Also, the treatment can decrease amyloid plaques, as measured by positron emission tomography, and slows decline of cognition, reported from the phase 1b PRIME trial.43
In Table 1, we have shown the comprehensive research advances of immunotherapy targeting Aβ for AD. We summarized the mechanism and ongoing or completed studies about active and passive immunotherapy.
Table 1.
Clinical trials of active and passive immunotherapy for AD
| Pharmaceutical company | Compound | Epitope | Phase | Outcomes |
|---|---|---|---|---|
| Active immunotherapy | ||||
| Elan/Wyeth | AN1792 | Aggregated Aβ 1‐42/QS21 adjuvant | IIa | Discontinued, inefficacy, 6% patients suffered meningoencephalitis.6 |
| Pfizer/Jansen | ACC‐001 | Aβ 1‐7/nontoxic diphtheria/QS21 adjuvant | II | Unpublished, no detailed outcomes and AEs.49 |
| Merck | V950 | Multivalent Aβ peptide/ISCOMATRIXTM adjuvant | I | Unpublished, AEs rate is high. (ClinicalTrials.gov Identifier: NCT00464334) |
| Affinis AG/GSK | Affitope AD02 | Aβ 1–6 mimetic/keyhole limpet hemocyanin/aluminum adjuvant | II | Completed, no detailed outcomes and AEs.50 |
| Affitope AD03 | Modified Aβ 1–6 mimetic/keyhole limpet hemocyanin/aluminum adjuvant | I | Unpublished, no detailed outcomes and AEs. (ClinicalTrials.gov Identifier: NCT01309763) | |
| Novarits | CAD106 | Aβ 1–6/bacteriophage Qβ coat protein | I/II | Completed, tolerated.9, 38 |
| AC Immune | ACI‐24 | Tetra‐palmitoylated Aβ 1– 15/reconstituted in liposome | I/IIa | Discontinued, worsen symptom, tolerated.51 |
| United Biochemical | UB311 | Tetra‐palmitoylated Aβ 1– 15/recoTwo UBITh® synthetic peptides coupled to Aβ 1–14 peptide/CpG oligonucleotidenstituted in liposome | II | Uncompleted, no detailed outcomes and AEs. (ClinicalTrials.gov Identifier: NCT02551809) |
| Lundbeck/Otsuka | Lu AF20513 | Aβ 1– 12 peptides replaced with two foreign T‐helper epitopes from tetanus toxoid | I | Uncompleted, no detailed outcomes and AEs. (ClinicalTrials.gov Identifier: NCT02388152) |
| Passive immunotherapy | ||||
| Janssen/Pfizer | Bapineuzemab (AAB‐001) | Humanized versions of the anti‐Aβ murine antibody 3D6 | III | Unpublished, no detailed outcomes and AEs. (ClinicalTrials.gov Identifier: NCT00606476) |
| Bapineuzemab (AAB‐003) | Modifying bapineuzumab to reduce Fc‐receptor‐mediated effector function | I | Completed, tolerated.39 | |
| Eli Lilly | Solanezumab | Humanized monoclonal antibody/binds soluble forms of amyloid | III | Completed, inefficacy.35 |
| Roche | Gantenerumab | Fully human anti‐amyloid beta monoclonal antibody/interacts with aggregated Aβ/eliciting effector cell‐mediated clearance. | II/III | Unpublished, no detailed outcomes and AEs. (ClinicalTrials.gov Identifier: NCT02711423, NCT02051608) |
| Genentech | Crenezumab | IgG4/interacts with aggregated Aβ 16‐24 | II/III |
Phase II Completed, inefficacy. Phase III trials are ongoing.52
(ClinicalTrials.gov Identifier: NCT03114657) |
| Biogen | Aducanumab | Human monoclonal anti‐body/selectively aggregated forms of beta‐amyloid peptide | Ib/III |
Phase I b Completed, efficacy.37
Two phase III trials are ongoing. (ClinicalTrials.gov Identifier: NCT02484547, NCT02477800) |
Aducanumab is the promising new antibody for passive immunotherapy with AD. Albanian monoclonal antibody is an all‐human IgG1 antibody from a healthy, well‐cognically normal elderly person who recognizes aggregated fibrillates and oligomers Aβ. In the Phase I clinical trial,44 166 patients with mild prophylaxis or mild symptoms were given 1,3,6, and 10 mg/kg doses of Aducanumab every 4 weeks, and after 54 weeks of treatment, compared with placebo, the 3 and 10 mg/kg dose of the drug significantly reduced the Aβ content in the brain and improved the cognitive ability, and the higher dose was more pronounced. Although the results of 6 mg/kg dose group were not between the 3 mg/kg dose group and the 10 mg/kg dose group, the dose of 6 mg/kg was able to significantly reduce Aβ levels in the brain and the patient's mental decline slowed. According to the data from this clinical trial and the results of our analysis, aducanumab may be the first drug to significantly slow the cognitive decline in AD patients. At present, Biogen has carried out Phase III clinical trials in 2700 patients with early AD to determine the true value of the monoclonal antibody and is expected to be completed in 2018. The lack of specific targeting of the most toxic Aβ oligomers is a serious drawback in the current clinical trials of passive immunotherapy antibody: solanezumab is only targeted to soluble Aβ; bapineuzumab and crenezumab recognize all three forms of Aβ; Aducanumab and gantenerumab bind to fibrillation or aggregates Aβ. The antibody that targets soluble Aβ may play a role in the very early stage of the AD process by the “peripheral blood sedimentation” mechanism, which is likely to be the preclinical stage of AD. AD is a chronic disease, the long‐term treatment of antibody targeted to normal soluble Aβ, there may be interference with its normal physiological function and regulation of human long‐term potentiation and innate immune response risk. AD is characterized by the accumulation of vascular amyloid, which is also a common feature associated with ARIA. Also, the risk of ARIA increased with the dose of the antibodies suggesting a relationship between the effectiveness of amyloid clearance and the imaging abnormality. Previous reports indicated that passive and active anti‐Aβ immunotherapy increase vascular amyloid on capillary structures.45 ARIA may be related to vascular amyloid burden in the context of the disease and vascular amyloid clearance in the context of treatment.46, 47, 48 Antibodies targeting Aβ (including oligomers) in the form of aggregates have the potential for clinical efficacy, such as aducanumab showing a preliminary therapeutic effect in clinical trials. Thus, it is preferred that the antibody specifically targets the most toxic Aβ oligomers, while also avoiding the generation of related side effects. Another important issue is that these antibodies should be treated in the early stages of AD to enhance their therapeutic effect, preferably before synaptic or cognitive impairment, before they can produce significant effects. Therefore, for patients with AD symptoms of these anti‐Aβ drug treatment may have no significant effect, cannot reverse the brain injury and cognitive deficits, cannot achieve the goal of treatment.
In general, Anti‐Aβ immunotherapies have not been well studied, further research on moderators of treatment effect and possible new interventions are needed and clinicians should balance the risk–benefit profile during the treatment of AD.
Limitations
There are several limitations in this study. First, 4 of 11 trials,9, 32, 33, 37 involving aducanumab, AN1972 + QS21, bapineuzumab, CAD106, funded by pharmaceutical company and four studies 9, 34, 38, 39 with small sample size may result in an exaggerated treatment effect. Second, in the GRADE framework, all the comparisons were assessed as low quality, which largely restricts the interpretation of these results. Third, we failed to perform inconsistency plots to evaluate statistical inconsistency in networks of interventions because all the comparisons among studies were placebo and another drug which implied that a closed loop formed by three or more treatments was impossible to analyses.
Conclusions
The NMA provided available evidence to evaluate the efficacy and safety of anti‐Aβ immunotherapies for AD. Aducanumab and solanezumab improve the cognitive function, while aducanumab and bapineuzumab may increase the risks of ARIA.
Conflicts of Interest
None declared.
Supporting information
Figure S1. Contribution plots.
Figure S2. Confidence & predictive plots.
Figure S3. Rankograms.
Figure S4. Cumulative ranking curve plots.
Figure S5. Funnel plots for a network of interventions.
Table S1. Summary of characteristic of patients.
Table S2. Quality of individuals study.
Table S3. Efficacy and safety assessment.
Table S4. Outcomes of network meta‐analysis with consistency model.
Table S5. GRADE evidence profile.
Acknowledgments
This work was supported by the Medical Research Fund of Guangdong Province (A2015481), Traditional Chinese Medicine Research Projects of Guangdong Province (20171152), and Doctoral Research Fund of Guangdong Medical University (B2014004).
Funding Statement
This work was funded by Medical Research Fund of Guangdong Province grant A2015481; Traditional Chinese Medicine Research Projects of Guangdong Province grant 20171152; Doctoral Research Fund of Guangdong Medical University grant B2014004.
References
- 1. Abushouk AI, Elmaraezy A, Aglan A, et al. Bapineuzumab for mild to moderate Alzheimer's disease: a meta‐analysis of randomized controlled trials. BMC Neurol 2017;17:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alzheimer's disease facts and figures . Alzheimers Dement 2015;11:332–384. [DOI] [PubMed] [Google Scholar]
- 3. Ghezzi L, Scarpini E, Galimberti D. Disease‐modifying drugs in Alzheimer's disease. Drug Des Devel Ther. 2013;7(default):1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schenk D. Amyloid‐beta immunotherapy for Alzheimer's disease: the end of the beginning. Nat Rev Neurosci 2002;3:824–828. [DOI] [PubMed] [Google Scholar]
- 5. Ferrer I, Boada Rovira M, Sanchez Guerra ML, et al. Neuropathology and pathogenesis of encephalitis following amyloid‐beta immunization in Alzheimer's disease. Brain Pathol 2004;14:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Orgogozo J, Gilman S, Dartigues J, et al. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology 2003;61:46–54. [DOI] [PubMed] [Google Scholar]
- 7. Holmes C, Boche D, Wilkinson D, et al. Long‐term effects of Abeta42 immunisation in Alzheimer's disease: follow‐up of a randomised, placebo‐controlled phase I trial. Lancet 2008;372:216–223. [DOI] [PubMed] [Google Scholar]
- 8. Ryan JM, Grundman M. Anti‐amyloid‐beta immunotherapy in Alzheimer's disease: ACC‐001 clinical trials are ongoing. J Alzheimers Dis 2009;17:243. [DOI] [PubMed] [Google Scholar]
- 9. Winblad B, Andreasen N, Minthon L, et al. Safety, tolerability, and antibody response of active Abeta immunotherapy with CAD106 in patients with Alzheimer's disease: randomised, double‐blind, placebo‐controlled, first‐in‐human study. Lancet Neurol 2012;11:597–604. [DOI] [PubMed] [Google Scholar]
- 10. Panza F, Frisardi V, Imbimbo BP, et al. Bapineuzumab: anti‐beta‐amyloid monoclonal antibodies for the treatment of Alzheimer's disease. Immunotherapy 2010;2:767–782. [DOI] [PubMed] [Google Scholar]
- 11. Grundman M, Dibernardo A, Raghavan N, et al. 2012: a watershed year for Alzheimer's disease research. J Nutr Health Aging 2013;17:51–53. [DOI] [PubMed] [Google Scholar]
- 12. Delrieu J, Ousset PJ, Vellas B. Gantenerumab for the treatment of Alzheimer's disease. Expert Opin Biol Ther 2012;12:1077–1086. [DOI] [PubMed] [Google Scholar]
- 13. Landen JW, Zhao Q, Cohen S, et al. Safety and pharmacology of a single intravenous dose of ponezumab in subjects with mild‐to‐moderate Alzheimer disease: a phase I, randomized, placebo‐controlled, double‐blind, dose‐escalation study. Clin Neuropharmacol 2013;36:14–23. [DOI] [PubMed] [Google Scholar]
- 14. Relkin NR, Szabo P, Adamiak B, et al. 18‐Month study of intravenous immunoglobulin for treatment of mild Alzheimer disease. Neurobiol Aging 2009;30:1728–1736. [DOI] [PubMed] [Google Scholar]
- 15. Barrera‐Ocampo A, Lopera F. Amyloid‐beta immunotherapy: the hope for Alzheimer disease? Colombia Médica (Cali, Colombia). 2016;47:203–212. [PMC free article] [PubMed] [Google Scholar]
- 16. DiFrancesco JC, Longoni M, Piazza F. Anti‐abeta autoantibodies in amyloid related imaging abnormalities (ARIA): candidate biomarker for immunotherapy in alzheimer's disease and cerebral amyloid angiopathy. Front Neurol 2015;6:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chaimani A, Higgins JP, Mavridis D, et al. Graphical tools for network meta‐analysis in STATA. PLoS ONE 2013;8:e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jansen JP, Fleurence R, Devine B, et al. Interpreting indirect treatment comparisons and network meta‐analysis for health‐care decision making: report of the ISPOR Task Force on Indirect treatment comparisons good research practices: part 1. Value Health 2011;14:417–428. [DOI] [PubMed] [Google Scholar]
- 19. Matsumoto SE, Jin H, Takeda K, et al. Development of antibodies for immunotherapy of Alzheimer's disease . Rinsho shinkeigaku = Clin Neurol 2012;52:1168–1170. [DOI] [PubMed] [Google Scholar]
- 20. Yang C, Xiao S. New developments of clinical trial in immunotherapy for Alzheimer's disease. Curr Pharm Biotechnol 2015;16:484–491. [DOI] [PubMed] [Google Scholar]
- 21. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006–1012. [DOI] [PubMed] [Google Scholar]
- 22. Higgins J, Green SE. Cochrane handbook for systematic reviews of interventions Version 5.1.0. The Cochrane Collaboration (Eds):pp. 202–206. Naunyn‐Schmiedebergs Archiv für experimentelle Pathologie und Pharmakologie, 2011. [Google Scholar]
- 23. Guyatt GH, Oxman AD, Schunemann HJ, et al. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol 2011;64:380–382. [DOI] [PubMed] [Google Scholar]
- 24. Salanti G, Del Giovane C, Chaimani A, et al. Evaluating the quality of evidence from a network meta‐analysis. PLoS ONE 2014;9:e99682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. White IR, Barrett JK, Jackson D, Higgins JP. Consistency and inconsistency in network meta‐analysis: model estimation using multivariate meta‐regression. Res Synth Methods 2012;3:111–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple‐treatment meta‐analysis: an overview and tutorial. J Clin Epidemiol 2011;64:163–171. [DOI] [PubMed] [Google Scholar]
- 27. Cope S, Jansen JP. Quantitative summaries of treatment effect estimates obtained with network meta‐analysis of survival curves to inform decision‐making. BMC Med Res Methodol 2013;13:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bayer A, Bullock R, Jones R, et al. Evaluation of the safety and immunogenicity of synthetic Abeta42 (AN1792) in patients with AD. Neurology 2005;64:94–101. [DOI] [PubMed] [Google Scholar]
- 29. Rinne JO, Brooks DJ, Rossor MN, et al. 11C‐PiB PET assessment of change in fibrillar amyloid‐beta load in patients with Alzheimer's disease treated with bapineuzumab: a phase 2, double‐blind, placebo‐controlled, ascending‐dose study. Lancet Neurol 2010;9:363–372. [DOI] [PubMed] [Google Scholar]
- 30. Ostrowitzki S, Deptula D, Thurfjell L, et al. Mechanism of amyloid removal in patients with Alzheimer disease treated with gantenerumab. Arch Neurol 2012;69:198–207. [DOI] [PubMed] [Google Scholar]
- 31. Uenaka K, Nakano M, Willis B, et al. Comparison of pharmacokinetics, pharmacodynamics, safety, and tolerability of the amyloid beta monoclonal antibody solanezumab in japanese and white patients with mild to moderate Alzheimer disease. Clin Neuropharmacol [Internet] 2012;35:25–29. [DOI] [PubMed] [Google Scholar]
- 32. Gilman S, Koller M, Black RS, et al. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology 2005;64:1553–1562. [DOI] [PubMed] [Google Scholar]
- 33. Salloway S, Sperling R, Gilman S, et al. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology 2009;73:2061–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Farlow M, Arnold SE, van Dyck CH, et al. Safety and biomarker effects of solanezumab in patients with Alzheimer's disease. Alzheimers Dement 2012;8:261–271. [DOI] [PubMed] [Google Scholar]
- 35. Doody RS, Thomas RG, Farlow M, et al. Phase 3 trials of solanezumab for mild‐to‐moderate Alzheimer's disease. N Engl J Med 2014;370:311–321. [DOI] [PubMed] [Google Scholar]
- 36. Salloway S, Sperling R, Fox NC, et al. Two phase 3 trials of bapineuzumab in mild‐to‐moderate Alzheimer's disease. N Engl J Med 2014;370:322–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sevigny J, Chiao P, Bussière T, et al. The antibody aducanumab reduces Aβ plaques in Alzheimer's disease. Nature 2016;537:50–56. [DOI] [PubMed] [Google Scholar]
- 38. Farlow MR, Andreasen N, Riviere ME, et al. Long‐term treatment with active Abeta immunotherapy with CAD106 in mild Alzheimer's disease. Alzheimer's Res Ther 2015;7:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Delnomdedieu M, Duvvuri S, Li DJ, et al. First‐In‐Human safety and long‐term exposure data for AAB‐003 (PF‐05236812) and biomarkers after intravenous infusions of escalating doses in patients with mild to moderate Alzheimer's disease. Alzheimer's Res Ther 2016;8:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Penninkilampi R, Brothers HM, Eslick GD. Safety and efficacy of anti‐amyloid‐beta immunotherapy in Alzheimer's disease: a systematic review and meta‐analysis. J Neuroimmune Pharmacol 2017;12:194–203. [DOI] [PubMed] [Google Scholar]
- 41. Kastanenka KV, Bussiere T, Shakerdge N, et al. Immunotherapy with aducanumab restores calcium homeostasis in Tg2576 mice. Journal of Neuroscience 2016;36:12549–12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rhodes K, Bacskai BJ, Vaillancourt DE. Aducanumab reduces abeta plaques in Alzheimer's disease. J Neurosci 2016;31:1631. [DOI] [PubMed] [Google Scholar]
- 43. Sevigny J, Suhy J, Chiao P, et al. Amyloid PET screening for enrichment of early‐stage Alzheimer Disease Clinical trials: experience in a phase 1b clinical trial. Alzheimer Dis Assoc Disord 2016;30:1–7. [DOI] [PubMed] [Google Scholar]
- 44. Sevigny J, Chiao P, Williams L, et al. Aducanumab (BIIB037), an anti‐amyloid beta monoclonal antibody, in patients with prodromal or mild Alzheimer's disease: interim results of a randomized, double‐blind, placebo controlled, phase 1b study. Alzheimers Dement 2015;11(7 suppl. 1):P277. [Google Scholar]
- 45. Boche D, Zotova E, Weller RO, et al. Consequence of Abeta immunization on the vasculature of human Alzheimer's disease brain. Brain 2008;131(Pt 12):3299–3310. [DOI] [PubMed] [Google Scholar]
- 46. Sperling RA, Jack CR Jr, Black SE, et al. Amyloid‐related imaging abnormalities in amyloid‐modifying therapeutic trials: recommendations from the Alzheimer's Association Research Roundtable Workgroup. Alzheimers Dement 2011;7:367–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sperling R, Salloway S, Brooks DJ, et al. Amyloid‐related imaging abnormalities in patients with Alzheimer's disease treated with bapineuzumab: a retrospective analysis. Lancet Neurol 2012;11:241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zago W, Schroeter S, Guido T, et al. Vascular alterations in PDAPP mice after anti‐Abeta immunotherapy: Implications for amyloid‐related imaging abnormalities. Alzheimers Dement 2013;9(5 Suppl):S105–S115. [DOI] [PubMed] [Google Scholar]
- 49. Pasquier F, Sadowsky C, Holstein A, et al. Two phase 2 multiple ascending‐dose studies of vanutide cridificar (ACC‐001) and QS‐21 adjuvant in mild‐to‐moderate Alzheimer's disease. J Alzheimers Dis 2016;51:1131–1143. [DOI] [PubMed] [Google Scholar]
- 50. Hendrix S, Ellison N, Stanworth S, et al. Methodological aspects of the phase II study AFF006 evaluating amyloid‐beta ‐targeting vaccine AFFITOPE® AD02 in early Alzheimer's disease ‐ prospective use of novel composite scales. J Prev Alzheimers Dis 2015;2:91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sheridan C. AC immune drops ACI−91 on phase II Alzheimer's failure. Bioworld [EB/OL] http://www.bioworld.com/content/ac-immune-drops-aci-91-phase-ii-alzheimers-failure-1, 2013. [Google Scholar]
- 52. Cummings J, Cho W, Ward M, et al. A randomized, double‐blind, placebocontrolled phase 2 study to evaluate the efficacyand safety of crenezumab in patients with mild to moderate Alzheimer's disease. Alzheimers Dement 2014;10:P275. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Contribution plots.
Figure S2. Confidence & predictive plots.
Figure S3. Rankograms.
Figure S4. Cumulative ranking curve plots.
Figure S5. Funnel plots for a network of interventions.
Table S1. Summary of characteristic of patients.
Table S2. Quality of individuals study.
Table S3. Efficacy and safety assessment.
Table S4. Outcomes of network meta‐analysis with consistency model.
Table S5. GRADE evidence profile.


