Abstract
Lateral hypothalamic area (LHA) local field potentials (LFPs) were recorded in a Prader–Willi patient undergoing deep brain stimulation (DBS) for obesity. During hunger, exposure to food‐related cues induced an increase in beta/low‐gamma activity. In contrast, recordings during satiety were marked by prominent alpha rhythms. Based on these findings, we have delivered alpha‐frequency DBS prior to and during food intake. Despite reporting an early sensation of fullness, the patient continued to crave food. This suggests that the pattern of activity in LHA may indicate hunger/satiety states in humans but attest to the complexity of conducting neuromodulation studies in obesity.
Keywords: Obesity, Prader‐Willi syndrome, deep brain stimulation, hypothalamus, hunger, satiety
Introduction
Obesity is a chronic and prevalent disorder. In a substantial number of patients, pharmacological and behavioral approaches are largely ineffective. Although bariatric surgery is efficacious, the incidence of side effects and recurrence are non‐negligible.1 In recent reports, deep brain stimulation (DBS) has been used to treat obesity patients with variable results.2, 3, 4
Prader–Willi syndrome (PWS) is one of the main causes of genetic obesity. Hyperphagia associated with PWS is often refractory to pharmacological and surgical treatments.5, 6 We have recently treated a PWS patient with DBS and were able to record local field potentials (LFPs) from the lateral hypothalamic area (LHA). We found that the pattern of activity in this region was indicative of hunger and satiety states. DBS applied at rhythms recorded during satiety induced a sensation of fullness but did not influence craving, attesting to the complexity of mechanisms enticing food consumption.
Subject and Methods
The patient was a 19‐year‐old male with genetically confirmed PWS (15 g 11–13). At the time of surgery, he weighted 90 Kg and had a body mass index of 33 Kg/m2. Over the years, he has tried and failed multiple pharmacological regimens. After becoming acquainted with the potential risks and benefits of bariatric surgery, his family opted against the procedure and decided to undergo DBS. The study was approved by the Research Ethics Board of the University of São Paulo. A written informed consent was obtained from a proxy.
Surgery
Surgery was conducted under local anesthesia and sedation. Stereotactic computed tomography (CT) and 1.5T preoperative magnetic resonance images (MRI) were merged. The selected target was adjacent to the fornix, anterolateral to the mammillary bodies, and posterior to the optic tract. Once the leads were implanted, each of the four contacts was tested for side effects and efficacy (e.g., the patient was asked how hungry he was in a scale from 0 to 10). DBS delivered to the deepest contacts at 130 Hz, 90 μsec and 3.5 V induced significant increases in heart rate, with no concomitant changes in blood pressure. After testing, electrodes were connected to externalized extension cables. A new CT scan was obtained and merged with the preoperative MRI to confirm that ventral contacts were in the region of the LHA (Fig. 1). Experiments were conducted on postoperative day 3 while electrodes were still externalized. Two days later (i.e., on postoperative day 5), the patient underwent the second stage of the procedure under general anesthesia. External cables were removed and the leads connected to a pulse generator (Libra XP).
Figure 1.
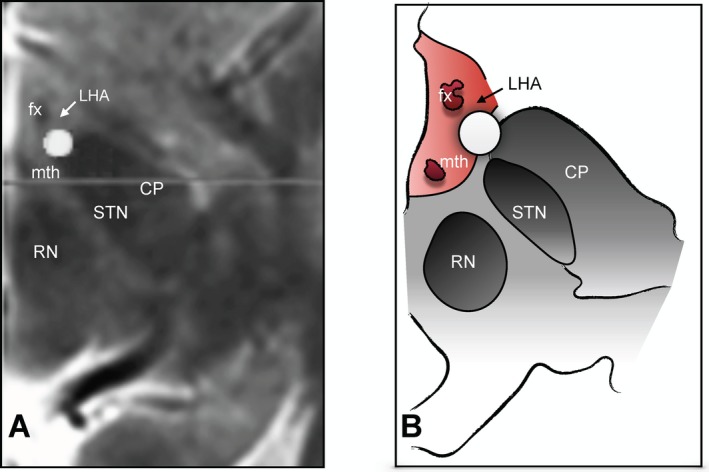
Location of ventral DBS contacts. (A) Postoperative axial computed tomography merged with preoperative magnetic resonance images and (B) a schematic representation of the same section showing the relationship between one of the contacts from which electrophysiological activity was recorded (circle) and the fornix (fx), mammilothalamic tract (mth), red nucleus (RN), cerebral peduncle (CP), subthalamic nucleus (STN), and lateral hypothalamus (LHA; arrow).
Hypothalamic electrographic recordings
Hypothalamic activity was recorded using a biosignal acquisition device (g.USBamp, g.tec, Austria). DBS contacts in the LHA region were referenced to the most dorsal ones to reduce the effect of a common reference and volume‐conducted activity.7, 8 Details of the spectral density analysis may be found in the Supplementary Materials.
Images presentation and recognition task
The patient was presented with three runs of food and animal pictures.9 Each run consisted of three blocks of 10 pictures from each category (e.g., 10 animal figures/interval/10 food figures). Figures were shown for 2.5 sec with an interstimulus interval of 0.25 sec and an interblock interval of 10 sec (details in the Supplementary Materials; Fig S1). After testing, a recognition task consisting of the presentation of 20 new and 20 previously seen images was applied to assess whether the patient was attentive during the trials.
Results
Experiments were conducted 4 h after breakfast, while the patient rated his hunger as 10/10. After stable recordings were obtained, he was presented with blocks of food and animal pictures. Compared to baseline, food images induced significant increases in beta/low‐gamma and a decrease in theta activity (Fig. 2). In contrast, the visualization of animal figures was associated with an increase in alpha activity and a decrease in theta. After testing, the patient correctly identified 80% of the presented images as novel or previously seen, discarding the possibility of attention deficits during block design experiments (Fig. S2).
Figure 2.
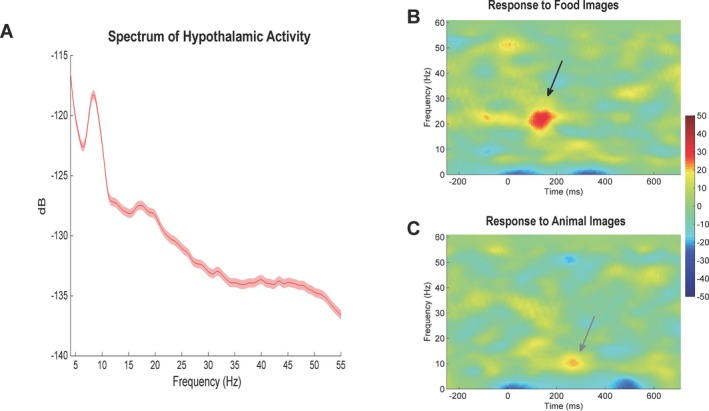
Recording from the lateral hypothalamic region in a Prader‐Willi syndrome patient during hunger. (A) Power spectrum of hypothalamic activity measured at baseline. (B) During hunger, the patient was exposed to food and animal images while hypothalamic activity was being recorded. Response to food image presentation was characterized by an increase in beta/low‐gamma activity (arrow) and a decrease in theta. (C) In contrast, exposure to animal pictures induced an increase in alpha (arrow).
We then recorded hypothalamic LFPs after the patient ate and had a hunger score of 0. As compared to the hungry state, LHA recordings during satiety were characterized by a significant increase in alpha and a decrease in beta (Fig. 3). While still sated, the patient was once again presented with the food and animal image paradigm. In contrast to preprandial recordings, food image presentation was associated with a decrease in beta and theta bands, whereas animal images continued to induce an increase in alpha (Fig. 3).
Figure 3.
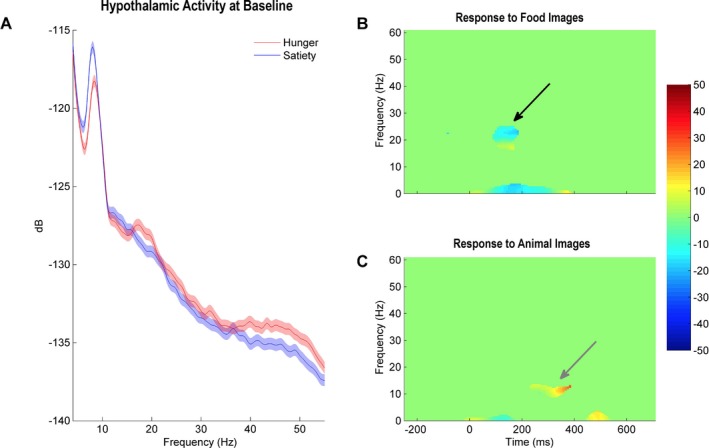
Increased hypothalamic activity in the alpha range during satiety. (A) Baseline spectral density of hypothalamic activity during hunger (red) and satiety (blue). While hunger was associated with activity peaks in the beta and gamma bands, a more prominent alpha peak marked recordings during satiety. (B and C) LHA activity recorded when the patient was presented with food or animal images during satiety. Compared to the hunger state, food image presentation was associated with a decrease in beta and theta bands, whereas animal images continued to induce an increase in alpha.
Based on these findings, we have decided to test whether satiety could be artificially driven by electrical stimulation in the alpha range following pulse generator implantation. Four hours after breakfast, electrode contacts close to the LHA were activated at 8 Hz (peak frequency recorded during satiety), 90 μsec, and 3 V (a current amplitude that was well tolerated during immediate postoperative programming sessions). Although the patient described a sensation of “fullness”, he subjectively reported a continued craving for food and was allowed to eat with the DBS electrodes activated. Overall, no differences in the pattern or amount of food consumed were noticed while he was being stimulated.
Discussion
The LHA is a heterogeneous structure comprised of distinct cell types and crossing fibers.10 In rodents and primates, LHA neurons respond to feeding and rewarding stimuli,11, 12 whereas electrical stimulation reduces food intake (for a review see 10). In line with our findings, LHA neurons in hungry non‐human primates fire at approximately 20 Hz (beta range), a frequency that drops to 5 Hz (alpha‐theta) following glucose administration.13
Mechanisms and neural circuits involved in feeding behavior are fairly complex. A variety of peptides and hormones are produced in states of energy imbalance, some able to directly modulate hypothalamic cell firing (e.g., glucose, insulin and leptin).14 In addition, peripheral information may reach the hypothalamus through the vagus and brainstem structures (e.g., gastrointestinal tract distention, hormonal release).14 From a mechanistic standpoint, fast oscillations often reflect changes in local dynamics, while slow activity is observed when distant structures communicate. Although elevated beta in the motor system may occur when distant structures interact,15 high beta and gamma activity are also thought to reflect local network dynamics. Along this line, LHA beta/low‐gamma rhythms recorded when our patient was exposed to food‐related cues could reveal local hypothalamic interactions. In contrast, the low‐frequency rhythms documented during satiety could be a consequence of the communication between distant structures, such as brainstem centers and the hypothalamus. Future studies are necessary to corroborate these inferences.
Caveats of our study include the fact that recordings were carried out in only one individual with PWS. Targeting was based on that proposed by Whiting and colleagues, who implanted some of their electrodes slightly posterior and lateral to the fornix.4 When the dimensions of the electrodes and the LHA are taken into account, it is possible that we might have recorded activity from nearby structures. For example, with the proximity of our electrodes to medial aspects of the subthalamic nucleus, we cannot completely rule out that LFP changes were not partly generated in this structure. Favoring a local response, however, electrophysiological activity recorded from dorsal contacts (i.e., near the globus pallidus) did not reveal changes associated with food pictures (Fig. S3).
Although hypothalamic abnormalities have not been extensively documented in PWS,16 hormonal dysfunction is a hallmark of the syndrome.6 In this context, it is uncertain whether our findings may be extrapolated to other pathological states. In a patient with cluster headache treated with posterior hypothalamic DBS, LFPs have been recorded following the presentation of food images but oscillatory patterns were not studied in detail.17
The regulation of food intake is comprised of both homeostatic and nonhomeostatic factors. The former involves nutritional requirements as well as the establishment of energy balance and can easily be modified by nonhomeostatic factors. While feeding initiation involves a complexity of intrinsic and extrinsic factors, the termination of feeding is predominantly driven by physiological mechanisms.18 Rewarding components involve cortical and subcortical brain regions, including the dorsal and ventral striatum.19 Taking into account that craving refers to a subjective urge or desire to gain pleasure or terminate distress,20 we hypothesize that hypothalamic stimulation in our patient might have been able to regulate physiological aspects of food intake (i.e. sensation of fullness) but not rewarding components that may lead to craving. This illustrates the complexity of mechanisms enticing food consumption and the difficulty in conducting DBS studies in obesity.
In summary, our study shows that food stimuli presented during hunger and satiety induce very different LHA responses in humans. Understanding these patterns may be important for a better appraisal of mechanisms of feeding as well as guiding neuromodulation therapies.
Conflict of Interest
CH has received honoraria from Medtronic and St Jude Medical. The other authors report no conflicts of interest related to this work.
Supporting information
Figure S1 Block design paradigm of food and animal image presentation.
Figure S2 Recognition task.
Figure S3 Recordings from electrode contacts located in the region of the globus pallidus during hunger.
References
- 1. Cheng J, Gao J, Shuai X, et al. The comprehensive summary of surgical versus non‐surgical treatment for obesity: a systematic review and meta‐analysis of randomized controlled trials. Oncotarget 2016;7:39216–39230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hamani C, McAndrews MP, Cohn M, et al. Memory enhancement induced by hypothalamic/fornix deep brain stimulation. Ann Neurol 2008;63:119–123. [DOI] [PubMed] [Google Scholar]
- 3. Harat M, Rudas M, Zielinski P, et al. Nucleus accumbens stimulation in pathological obesity. Neurol Neurochir Pol 2016;50:207–210. [DOI] [PubMed] [Google Scholar]
- 4. Whiting DM, Tomycz ND, Bailes J, et al. Lateral hypothalamic area deep brain stimulation for refractory obesity: a pilot study with preliminary data on safety, body weight, and energy metabolism. J Neurosurg 2013;119:56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cassidy SB, Schwartz S, Miller JL, Driscoll DJ. Prader‐Willi syndrome. Genet Med 2012;14:10–26. [DOI] [PubMed] [Google Scholar]
- 6. Goldstone AP. Prader‐Willi syndrome: advances in genetics, pathophysiology and treatment. Trends Endocrinol Metab 2004;15:12–20. [DOI] [PubMed] [Google Scholar]
- 7. Talakoub O, Neagu B, Udupa K, et al. Time‐course of coherence in the human basal ganglia during voluntary movements. Sci Rep 2016;6:34930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Trongnetrpunya A, Nandi B, Kang D, et al. Assessing granger causality in electrophysiological data: removing the adverse effects of common signals via bipolar derivations. Front Syst Neurosci 2015;9:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Holsen LM, Zarcone JR, Brooks WM, et al. Neural mechanisms underlying hyperphagia in Prader‐Willi syndrome. Obesity (Silver Spring) 2006;14:1028–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stuber GD, Wise RA. Lateral hypothalamic circuits for feeding and reward. Nat Neurosci 2016;19:198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brown JA, Woodworth HL, Leinninger GM. To ingest or rest? Specialized roles of lateral hypothalamic area neurons in coordinating energy balance. Front Syst Neurosci 2015;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fukuda M, Ono T, Nishino H, Nakamura K. Neuronal responses in monkey lateral hypothalamus during operant feeding behavior. Brain Res Bull 1986;17:879–883. [DOI] [PubMed] [Google Scholar]
- 13. Rolls ET, Burton MJ, Mora F. Neurophysiological analysis of brain‐stimulation reward in the monkey. Brain Res 1980;194:339–357. [DOI] [PubMed] [Google Scholar]
- 14. Williams G, Bing C, Cai XJ, et al. The hypothalamus and the control of energy homeostasis: different circuits, different purposes. Physiol Behav 2001;74(4–5):683–701. [DOI] [PubMed] [Google Scholar]
- 15. Khanna P, Carmena JM. Neural oscillations: beta band activity across motor networks. Curr Opin Neurobiol 2015;32:60–67. [DOI] [PubMed] [Google Scholar]
- 16. Swaab DF, Purba JS, Hofman MA. Alterations in the hypothalamic paraventricular nucleus and its oxytocin neurons (putative satiety cells) in Prader‐Willi syndrome: a study of five cases. J Clin Endocrinol Metab 1995;80:573–579. [DOI] [PubMed] [Google Scholar]
- 17. Nager W, Krauss JK, Heldmann M, et al. Human hypothalamus shows differential responses to basic motivational stimuli–an invasive electrophysiology study. Neuroscience 2011;189:330–336. [DOI] [PubMed] [Google Scholar]
- 18. Alonso‐Alonso M, Woods SC, Pelchat M, et al. Food reward system: current perspectives and future research needs. Nutr Rev 2015;73:296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Noori HR, Cosa Linan A, Spanagel R Largely overlapping neuronal substrates of reactivity to drug, gambling, food and sexual cues: a comprehensive meta‐analysis. Eur Neuropsychopharmacol 2016;26:1419–1430. [DOI] [PubMed] [Google Scholar]
- 20. Wise RA. The neurobiology of craving: implications for the understanding and treatment of addiction. J Abnorm Psychol 1988;97:118–132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Block design paradigm of food and animal image presentation.
Figure S2 Recognition task.
Figure S3 Recordings from electrode contacts located in the region of the globus pallidus during hunger.


