Abstract
The calcitonin/CGRP family of peptides includes calcitonin, α and β CGRP, amylin, adrenomedullin (AM) and adrenomedullin 2/intermedin (AM2/IMD). Their receptors consist of one of two GPCRs, the calcitonin receptor (CTR) or the calcitonin receptor‐like receptor (CLR). Further diversity arises from heterodimerization of these GPCRs with one of three receptor activity‐modifying proteins (RAMPs). This gives the CGRP receptor (CLR/RAMP1), the AM1 and AM2 receptors (CLR/RAMP2 or RAMP3) and the AMY1, AMY2 and AMY3 receptors (CTR/RAMPs1–3 complexes, respectively). Apart from the CGRP receptor, there are only peptide antagonists widely available for these receptors, and these have limited selectivity, thus defining the function of each receptor in vivo remains challenging. Further challenges arise from the probable co‐expression of CTR with the CTR/RAMP complexes and species‐dependent splice variants of the CTR (CT(a) and CT(b)). Furthermore, the AMY1(a) receptor is activated equally well by both amylin and CGRP, and the preferred receptor for AM2/IMD has been unclear. However, there are clear therapeutic rationales for developing agents against the various receptors for these peptides. For example, many agents targeting the CGRP system are in clinical trials, and pramlintide, an amylin analogue, is an approved therapy for insulin‐requiring diabetes. This review provides an update on the pharmacology of the calcitonin family of peptides by members of the corresponding subcommittee of the International Union of Basic and Clinical Pharmacology and colleagues.
Abbreviations
- AM
adrenomedullin
- AM2/IMD
adrenomedullin 2/intermedin
- AMY
amylin receptor
- CLR
calcitonin receptor‐like receptor
- CRSP
calcitonin receptor‐stimulating peptide
- CT
calcitonin
- CTR
calcitonin receptor
- ECD
extracellular domain
- ECL
extracellular loop
- RAMP
receptor activity‐modifying protein
- TMD
transmembrane domain
Introduction
The peptides calcitonin (CT), calcitonin gene‐related peptide (CGRP), amylin, adrenomedullin (AM) and adrenomedullin 2/intermedin (AM2/IMD) form a family of related peptides (Figure 1). CGRP exists in two forms, αCGRP and βCGRP; in some species, βCGRP is not found, but another peptide, CT receptor‐stimulating peptide (CRSP), is found instead (Katafuchi et al., 2009). There has been considerable expansion of the family in fish, such that there are two forms of CT and five forms of AM (Watkins et al., 2013).
Figure 1.
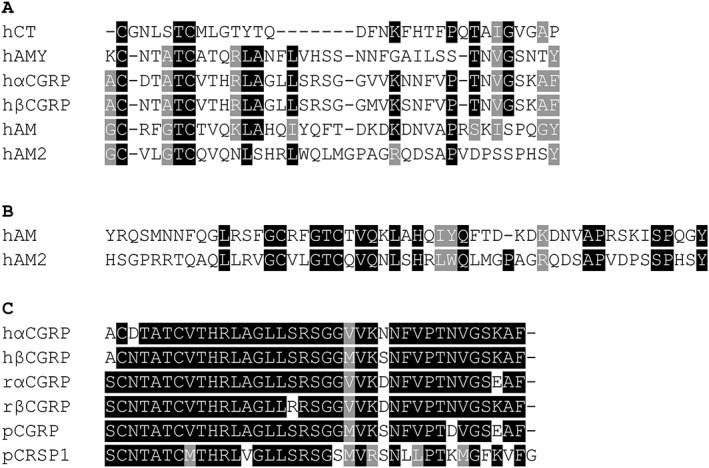
Amino acid sequence alignments of the CT peptide family. In all peptides, a disulphide bond is formed between the two N‐terminal cysteines, and they each have a C‐terminal amide. For pCRSP1, this would occur on Phe37, presuming that the glycine is removed during processing like the other peptides. (A) The human CT peptide family, omitting the N‐terminal extensions of AM and AM2. (B) Alignment of full‐length human AM and AM2. (C) Sequence alignment of human α and βCGRP, rat α and βCGRP and pig αCGRP and CRSP1. h, human; r, rat; p, pig. Alignment performed in COBALT (https://www.ncbi.nlm.nih.gov/tools/cobalt/cobalt.cgi?CMD=Web) and analysed using BoxShade (http://ch.embnet.org/software/BOX_form.html). Black indicates exact match, grey indicates 70–100% similarity and white indicates <70% similarity.
The peptides themselves, whilst showing only limited sequence homology, are related structurally by possession of a disulphide‐bonded N‐terminus, a region with strong α‐helical tendencies and a C‐terminus structured around a β‐turn and a C‐terminal amide. The peptides range in length from 32 (CT) to 52/53 amino acids (AM, AM2/IMD). In the latter two peptides, the first residues N‐terminal to the disulphide bond do not appear to be necessary for biological activity, and the AM and AM2/IMD peptides can be considered as functional ~40 amino acid peptides (Bailey and Hay, 2006; Hong et al., 2012; Watkins et al., 2013; Bower and Hay, 2016).
The peptides have a range of biological activities. CT, the first to be discovered, is a hormone produced by C cells of the thyroid, whose role is to reduce plasma calcium and promote bone formation (Findlay and Sexton, 2004), although CT‐deficient mice show a paradoxical inhibition of bone formation due to enhanced sphingosine‐1‐phosphate production (Keller et al., 2014). Amylin is produced by the pancreas and functions as a satiety hormone, regulating nutrient intake, but may also have other roles as recently reviewed (Hay et al., 2015). CGRP and AM are both potent vasodilators (Hinson et al., 2000; Russell et al., 2014). CGRP is a neuromodulator found in sensory neurons; it plays an important role in neurogenic inflammation (i.e. where sensory nerves release mediators that promote inflammation); in this case, CGRP causes vasodilatation and promotes fluid exudation from blood vessels. AM is chiefly found in endothelial cells; it is important both in vascular homeostasis and also angiogenesis and lymphangiogenesis. AM2/IMD is also found in vascular endothelial cells and probably has complementary roles to AM, although much about this peptide remains unclear. Each peptide appears to have both peripheral and central actions, although due to the complexity of this peptide‐receptor system, it is not yet clear which effects are physiological versus pharmacological or which receptors are responsible for many effects.
The peptides all act at class B GPCRs. There are seven distinct receptors for the peptides in mammals (excluding splice variants) but only two GPCRs: the CT receptor (CTR) and CT receptor‐like receptor (CLR, known as CL or CRLR in older literature). The additional functional receptors arise from the association of CTR or CLR with receptor activity‐modifying proteins (RAMPs) (McLatchie et al., 1998). There are three RAMPs. These each have an N‐terminus of around 100–120 amino acids, a single transmembrane domain (TMD) and a C‐terminus of around 10 residues (Hay and Pioszak, 2016). The receptors are shown in Figure 2.
Figure 2.
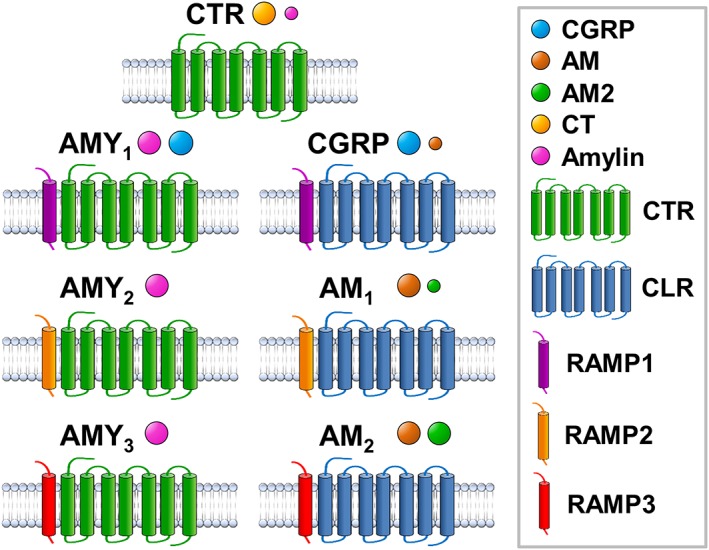
The subunit composition and current classification of human calcitonin‐family receptors. The legend is shown in the box. Ligands are indicated by spheres with relative sizes reflecting relative potency at each receptor, with the smaller sphere indicating lower potency of a given ligand.
Like other class B GPCRs, activation of CLR and CTR follows the two‐domain model, where this is achieved by binding of the C‐terminus of the peptide to the extracellular domain (ECD) of the receptor, contributing to the overall affinity of the peptide. The peptide N‐terminus binds to the TMD of the receptor. The receptors for the CT/CGRP family preferentially signal through Gs and cAMP production, although other signal transduction pathways may be activated (Walker et al., 2010). As further work characterizing the signalling of these receptors emerges, it will be important to consider how the pharmacology of these receptors compares at different signalling pathways.
The current scheme for receptor classification may be found on the IUPHAR/BPS Guide to Pharmacology website (http://www.guidetopharmacology.org/) at http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=11 and is shown in Figure 2. This is reviewed each year and is fully annotated with current references (Alexander et al., 2017; Hay and Poyner, 2017). Readers are referred to this for details of classification and also for information on receptor distribution. These pages mainly consider human receptors, and so, this information may not automatically apply to other species, where there are frequently differences in pharmacology. The structure–function relationships of RAMPs, CGRP and amylin have been recently considered elsewhere (Watkins et al., 2013; Bower and Hay, 2016; Hay and Pioszak, 2016), as has the clinical pharmacology of CGRP antagonists and antibodies (Karsan and Goadsby, 2015; Hou et al., 2017; Tso and Goadsby, 2017). In this review, the intention is to explore areas where there are significant gaps in our understanding, to guide research in this field.
Pharmacology
The current classification of the seven receptors is based on work done shortly after the discovery of the RAMP family (McLatchie et al., 1998; Christopoulos et al., 1999; Muff et al., 1999). The CLR by itself will not reach the cell surface in any significant amount and does not respond to any known ligand. With RAMP1, it becomes the CGRP receptor (i.e. CLR/RAMP1). Association with the other two RAMPs gives AM receptors: the AM1 receptor with RAMP2 (CLR/RAMP2) and the AM2 receptor with RAMP3 (CLR/RAMP3) (Figures 2 and 3). AM2/IMD shows a preference for the AM2 receptor; this is discussed further below.
Figure 3.
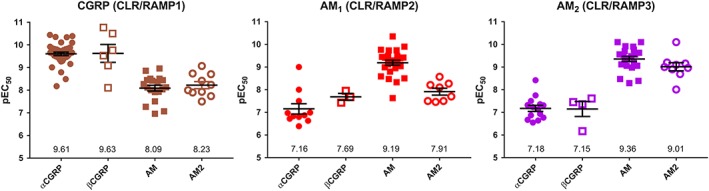
The pharmacology of selected ligands across CGRP, AM1 and AM2 receptors. All receptors are human and data are pEC50 values for cAMP production in cells transfected to express receptors. Each point is an individual value from independent publications, except where different cell lines were used within a single study and two values are therefore used from that study. The individual values and references can be found in Data S1. The mean pEC50 is shown; error bars represent SEM.
The CTR, by itself, preferentially responds to CT. The CTR can also associate with the three RAMPs to give AMY1, AMY2 and AMY3 receptors (Figure 2). As their names suggest, these respond to amylin (Poyner et al., 2002; Hay et al., 2015). There are, however, a number of important extra considerations. The CTR exists as a number of splice variants, and these are species dependent. The most significant of these for the human receptor are the absence (CT(a)) or presence (CT(b)) of a 16 amino acid insert in the first intracellular loop; this impairs coupling of the CTR to Gq whilst making little difference to Gs coupling; thus in turn gives (a) and (b) subtypes of each of the AMY receptors (Moore et al., 1995; Poyner et al., 2002). Secondly, the CTR can be expressed at the cell surface on its own, so in transient expression systems, it is highly likely that there will be mixed populations of CTR/RAMP complexes and CTR alone. This makes it very difficult to interpret the action of CT at AMY receptors in functional assays, as CT will produce a strong cAMP response via the CTR that is inevitably present. This is illustrated in Figure 4 at the AMY1(a) and AMY3(a) receptor transfected into Cos‐7 cells (Hay et al., 2005). At both of these receptors, CT fails to displace [125I]‐amylin indicating that CT and amylin do not share a common receptor (Figure 4A, B). However, at both receptors, CT stimulates a potent cAMP response (Figure 4C, D). This disconnect between binding and function could be explained by the presence of free CTR in these cells. The complex between CTR and RAMP2 is particularly difficult to observe and depends on the cell type used, and so, the pharmacology of this receptor is poorly explored.
Figure 4.
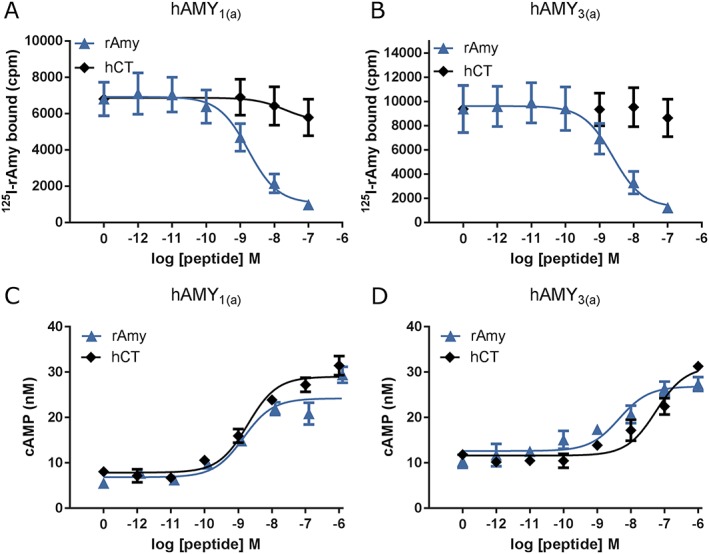
The binding of, and cAMP production by, rat amylin (rAmy) and human CT (hCT) in Cos 7 cells transfected with AMY1(a) or AMY3(a) receptors. (A) Displacement of [125I]‐amylin by amylin and CT, AMY1(a) receptor. (B) Displacement of [125I]‐amylin by amylin and CT, AMY3(a) receptor. (C) cAMP responses to amylin and CT, AMY1(a) receptor. (D) cAMP responses to amylin and CT, AMY3(a) receptor. Data replotted from Hay et al. (2005 ).
Many class B GPCRs form heterodimers. This does not seem to have been addressed in any published study for CTR or CLR. For CLR, the requirement for a RAMP may mitigate against this. For CTR, homodimerization is well described (Harikumar et al., 2010); the main ligand responsive species may be a dimer, with G protein binding causing monomer formation (Furness et al., 2016).
For most receptors, the main pharmacological tools for their characterization are the peptide agonists themselves and N‐terminally truncated peptides that usually act as antagonists. For some combinations of peptides and receptors, there is reasonable selectivity; thus at the AM receptors, there is a preference for AM over CGRP for ligand binding and cAMP production (Figure 3). However, over the entire family, it is difficult to use these agents to fully distinguish between receptors (Hay et al., 2005; Bailey and Hay, 2006). For CLR‐based receptors, non‐peptide antagonists are also available (Salvatore et al., 2006); those of the ‘gepant’ class bind to the receptor ECD at the interface between RAMP1 and CLR and have better selectivity than peptide antagonists (Moore and Salvatore, 2012), although they still need to be used with care as they may also block AMY1 receptors (Hay and Walker, 2017; Walker et al., 2017).
Heterogeneity in CGRP‐responsive receptors
The early literature on CGRP receptors was dominated by discussions on heterogeneity. Many responses could be antagonized by CGRP8–37, with a pA2 of about 8 on human and rat cells. By contrast, in a number of model systems, typified by the rat vas deferens, CGRP agonism is relatively resistant to CGRP8–37. It was suggested that CGRP was acting via another receptor, the CGRP2 receptor. Molecular cloning demonstrated that the ‘CGRP1’ receptor corresponds to the CLR/RAMP1 complex and it has been suggested that the ‘CGRP2’ receptor represented the action of CGRP at the various AM and AMY receptors (Hay et al., 2008). The high potency of CGRP in functional (cAMP) and binding assays at the AMY1(a) receptor and the AMY1(b) receptor was noted in previous studies (Leuthauser et al., 2000; Tilakaratne et al., 2000; Hay et al., 2006; Udawela et al., 2008; Hay and Walker, 2017). More recent work has confirmed that the AMY1(a) receptor can respond as well to CGRP as it does to amylin (Walker et al., 2015; 2017; Hay and Walker, 2017) (Figure 5). Even more importantly, there is evidence that in vivo, CGRP may exert effects by activating AMY1 receptors. This has potentially important implications for understanding CGRP biology and for using antagonists; it may be necessary to use agents that block both CLR/RAMP1 and CTR/RAMP1 to fully antagonize the effects of CGRP in vivo (Walker et al., 2015). Where high concentrations of non‐peptide antagonists are used, this may already be the case because these show only limited selectivity between CGRP and AMY1 receptors (Hay and Walker, 2017).
Figure 5.
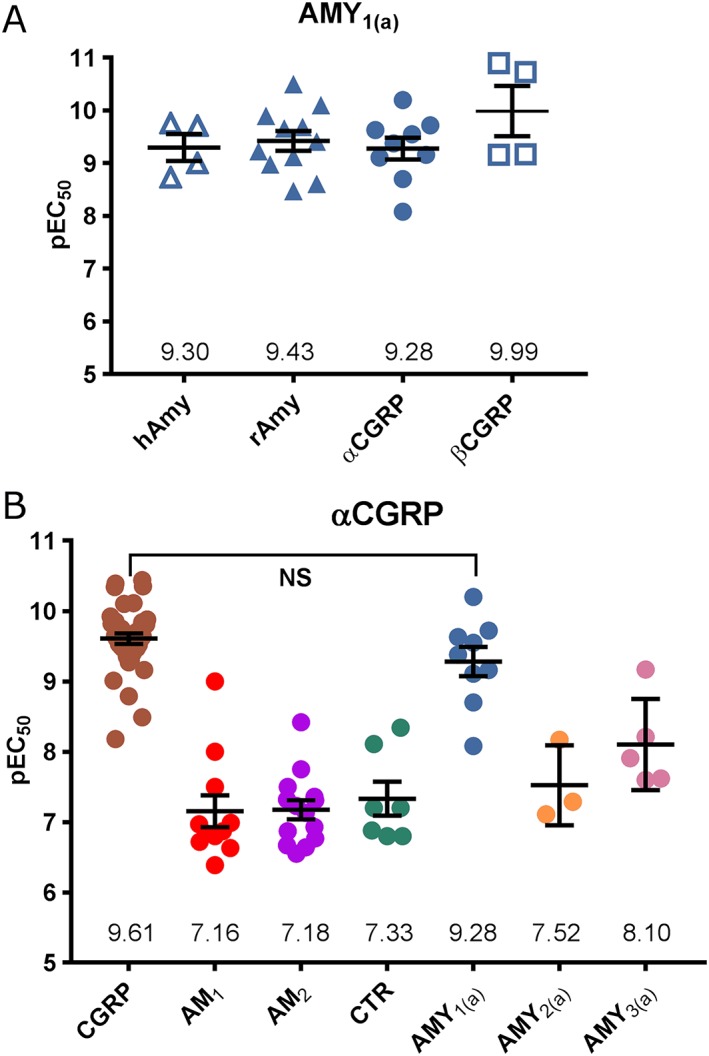
The pharmacology of (A) the AMY1(a) receptor and (B) αCGRP across various receptors. All receptors are human, and data are pEC50 values for cAMP production in cells transfected to express receptors. Each point is an individual value from independent publications, except where different cell lines were used within a single study and two values are therefore used from that study. The individual values and references can be found in Data S1. The mean pEC50 is shown; error bars represent SEM. In (B) results for the CGRP receptor (CLR/RAMP1), AM1 receptor (CLR/RAMP2), AM2 receptor (CLR/RAMP3), CTR, AMY1 receptor (CTR/RAMP1), AMY2 receptor (CTR/RAMP2) and AMY3 receptor are presented. In (A), data were analysed by one‐way ANOVA followed by Tukey's test; in (B), only the key comparison is shown.
The classification of CLR/RAMP1 as the CGRP receptor does not rule out the possibility of other endogenous CGRP receptors, such as AMY1. The ongoing interest in the CGRP system as a drug target in migraine makes it especially important to remember the early reports of functional heterogeneity, which in many cases still do not have a molecular correlate (Hay et al., 2008). Perhaps, unfortunately, the name AMY1 does not easily lend itself to an obvious role in CGRP biology. The dual activation of this receptor by both CGRP and amylin creates problems for nomenclature. There is insufficient information regarding the location and function of this receptor in vivo either as a CGRP or amylin receptor at the present time. Readers are urged to consider this receptor both in amylin and CGRP biology to assist with refining receptor nomenclature.
Endogenous agonists
AM2/IMD
AM2/IMD remains poorly understood. It has a wide range of effects on the cardiovascular system, adipose tissue and macrophages and the kidney. It increases prolactin release, and in the CNS, it reduces food intake and causes activation of the sympathetic nervous system (Hong et al., 2012; Zhang et al., 2017). It is sometimes reported to be more potent in vivo than AM, and the distribution of its mRNA is distinct from that of AM, being preferentially expressed in the thyroid and kidney, compared to the placenta and adipocytes where AM mRNA is most highly expressed (Figure 6). When reviewing the current data at human CLR‐based receptors, AM2/IMD appears to be most potent at the AM2 receptor (Figures 3 and 7), being equipotent to AM at this receptor. Equal potency for AM and AM2/IMD at the AM2 receptor has also been reported for rat and mouse receptors (Halim and Hay, 2012). This profile is different to the AM1 receptor where AM has higher potency than AM2/IMD (Figures 3 and 7); however, these data are only available for cloned human receptors. AM2/IMD can also activate CTR and AMY receptors, but there is much less data. When comparing data between species, the activity of AM2/IMD may be greater at rat AMY3(a) receptors, compared to the human receptor but this needs more investigation (Hay et al., 2005; Bailey et al., 2012). However, the pattern appears similar to the situation with AM, which may also have more activity at rat, compared to human AMY3(a) receptors (Bailey et al., 2012). It has been suggested that a distinct receptor for AM2/IMD may exist (Taylor et al., 2006; Hashimoto et al., 2007). Given its affinity at several CLR and CTR‐based receptor complexes, we consider this to be unlikely and that one or more existing complexes are likely to mediate the effects of this peptide, although we acknowledge that some results in the literature are difficult to explain (Taylor et al., 2006). The lack of useful antagonists makes this a continuing problem. Furthermore, signalling bias has been little explored at these receptors and it is unclear what distinctive features may come from AM/IMD activating each of the individual receptors. Thus, the pharmacology and physiology of AM2/IMD remain somewhat elusive.
Figure 6.
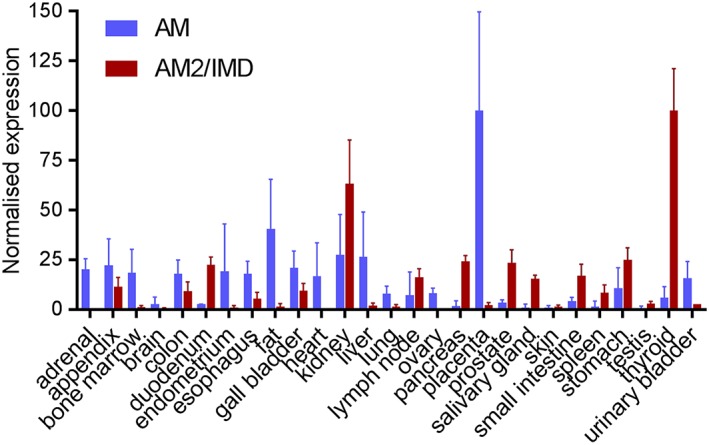
Relative distribution of the mRNA for AM and AM2/IMD. The data were taken from the HPA RNA‐seq normal tissues database, available via the NCBI Gene website (https://www.ncbi.nlm.nih.gov/gene). Values were normalized to 100% for the highest expressing tissue for both peptides. Similar expression profiles can be seen at http://www.proteinatlas.org/ENSG00000148926‐ADM/tissue and http://www.proteinatlas.org/ENSG00000128165‐ADM2/tissue.
Figure 7.
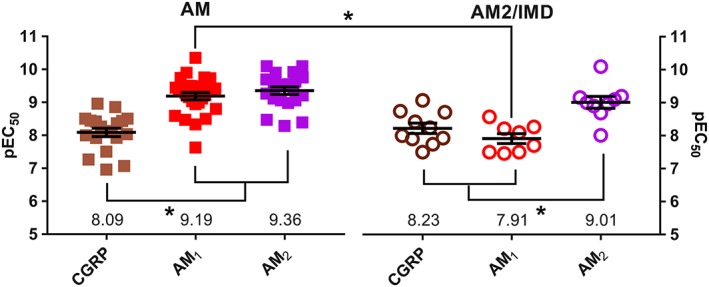
The pharmacology of AM and AM2/IMD at CGRP, AM1 and AM2 receptors. All receptors are human and data are pEC50 values for cAMP production in cells transfected to express receptors. Each point is an individual value from independent publications, except where different cell lines were used within a single study and two values are therefore used from that study. The individual values and references can be found in Data S1. The mean pEC50 is shown; error bars represent SEM. Results for the CGRP receptor (CLR/RAMP1), AM1 receptor (CLR/RAMP2) and AM2 receptor (CLR/RAMP3) are presented. Data were analysed by one‐way ANOVA followed by Tukey's test; *P < 0.05.
The actions of AM2/IMD are further complicated due to its metabolism, where it can potentially exist in a number of N‐truncated forms, all of which retain the key disulphide bond which is considered essential for full activity. It remains far from clear what the most physiologically important form of the peptide is and what are the implications of the potential metabolism for its activity (Hong et al., 2012; Zhang et al., 2017). Another AM, AM5, has also been reported in some mammals but its actions are not well understood (Takei et al., 2008).
βCGRP and CRSP
βCGRP is encoded by a different gene to αCGRP and has a different pattern of expression, being particularly prominent in the enteric nervous system. This has led to the view that βCGRP has restricted expression, but this is not necessarily the case, and is found throughout the CNS (Amara et al., 1985). It is found in only a small number of species, chiefly rodents and primates. The differences between the forms are species‐dependent (Figure 1). In rat βCGRP, there are two differences at positions 17 and 35, compared to rat αCGRP. In humans, there are three differences, at positions 3, 22 and 25. There are suggestions of subtle differences in receptor activity of human and rat α and β CGRP, although this has not been explored in any detail (Bailey and Hay, 2006, Bailey et al., 2012). In other species (but not humans), a second CGRP‐like peptide named CRSP is expressed. There is an interesting paradox with this peptide. Its sequence clearly marks it as a CGRP variant (Figure 1); however, it is reported to activate CTR‐based receptors and to have very little activity on CLR‐based receptors, including the CGRP receptor (Katafuchi et al., 2003, 2004, 2009; Katafuchi and Minamino, 2004). The reason for this is not known and this peptide would benefit from further study. However, matching sequence to pharmacology for these peptides and complex receptors is not an easy task. CGRP and amylin are the most closely related of the CT family of peptides in mammals, yet CGRP activates CTR and CLR‐based receptors with RAMP1, whereas amylin is much more selective for CTR/RAMP complexes. The nature of the C terminal amino acid seems like a good place to look to explain this, with Phe37 in CGRP and Tyr37 in amylin, yet AM shares a C‐terminal Tyr with amylin but has a strong preference for CLR/RAMP complexes.
Developments with agonists
Recent attention has focussed on the development of metabolically stable peptide agonists because the members of this peptide family can be metabolized by a range of peptidases and have several cleavage sites (Kim et al., 2013; Schonauer et al., 2016). For CGRP, a fatty acid attached to a serine at position 1 of human αCGRP gives an analogue with markedly prolonged in vivo biological activity (Nilsson et al., 2016). AM has been modified by palmitoylation, lactam cyclization and N‐methylation to produce an analogue with prolonged half‐life (Schonauer et al., 2016). For pramlinitide, a non‐aggregating analogue of human amylin, glycosylation has been used as an approach to enhance stability (Tomabechi et al., 2013; Kowalczyk et al., 2014; Yule et al., 2016). The key to these peptide mimetic development programmes is the identification of sites on the peptide that allow derivatization without compromising either receptor binding or activation. In principle, this will be facilitated by the availability of structures showing the peptides bound to their cognate, full‐length receptors, although the difficulty of predicting where an elongated substituent such as a fatty acid might bind should not be underestimated. In principle, similar problems might be anticipated in the preparation of other derivatives such as fluorescent peptides (Cottrell et al., 2005), where their use at relatively high concentrations may be needed to counter reduced affinity. The activity of analogues is usually only tested against cAMP production, leaving open the formal possibility that they have altered signalling bias.
Salmon CT has historically been used to treat Paget's disease and osteoporosis in people (Gennari and Agnusdei, 1994). However, due to side effects, relative efficacy compared to other treatments and lack of cost effectiveness, its use has declined. Particularly concerning was the suggestion that salmon CT may increase the risk of metastases. However, in a recent meta‐analysis the relationship was described as weak and there is no clear biological mechanism (Wells et al., 2016). Given its clinical usage, it is unsurprising that salmon CT has been explored as a treatment for other disorders. Numerous studies have suggested that salmon CT could treat metabolic disorders by lowering body weight, elevating energy expenditure, limiting food intake and improving glucose handling in rats (Lutz et al., 2000; Eiden et al., 2002; Wielinga et al., 2007; Feigh et al., 2012; 2014). Recently, a number of CT mimetics, known by ‘KBP’ codes, for example, KBP‐042, KBP‐088 and KBP‐089, have been described (Patent WO 2015/071229). These molecules are reported to maintain the high efficacy of salmon CT, whilst improving tolerability in rats (Gydesen et al., 2017a). A similar strategy appears to have been employed for the development of davalintide, which appears to be more effective at reducing body weight and food intake compared to amylin in rats (Mack et al., 2010). However, the development of davalintide has apparently been discontinued. Interestingly, the KBP compounds maintain the long‐acting ability of salmon CT to stimulate signalling in cell culture models (Andreassen et al., 2014a; Gydesen et al., 2016). The receptor pharmacology of the KBP peptides has not been extensively studied, and so far, the peptides have only been tested at CTR, AMY3 and CGRP receptors (Andreassen et al., 2014b; Gydesen et al., 2016; 2017a). They have been shown to activate both CT and AMY3 receptors but not CGRP receptors, similar to salmon CT, and are known as ‘DACRAs’ – dual amylin and CT receptor agonists; salmon CT is a natural DACRA. Their activity at other AMY receptors has not been tested, but the close sequence similarity to salmon CT of any peptide would make it likely that they show potent agonism at all CTR/RAMP complexes. The receptor pharmacology analysis of these peptides has relied on purchased stably transfected cell lines, which are not especially well characterized. It is not clear how much activity of the ligands occurs via free CTR in this transfected cell system (Andreassen et al., 2014b; Gydesen et al., 2016; 2017a,b). As noted above, to determine affinity at a CTR/RAMP complex, displacement of [125I]‐amylin (or 125I‐CGRP for the AMY1 receptor) is the most reliable measure of true AMY receptor affinity. Further pharmacological characterization is required to validate the DACRA nomenclature and confirm the relative activity of these peptides at different receptor complexes.
The future for novel peptides may be to follow the lead for the GLP‐1 receptor, where a ligand has been designed based on a crystal structure of the receptor (Jazayeri et al., 2017). A number of structures are available showing the ECDs of CLR/RAMP complexes or the CTR in complex with bound ligands (Table 1, Figure 8). Many of these have been reviewed (Hay and Pioszak, 2016). In addition, a cryo‐electron microscopy structure of the complete CTR bound to Gs and CT has been published (Liang et al., 2017), but the ECD and bound ligand in this are poorly resolved and are not included in the deposited co‐ordinates. Therefore, there is still some way to go before there is a complete picture to enable structure‐based peptide agonist design for these receptors.
Table 1.
Summary of structures of the of CTR and CLR/RAMP complexes with bound ligands
| RAMP | GPCR | ligand | RSCB PDB ID/Reference | Comment |
|---|---|---|---|---|
| RAMP126–117 | CLR22–133 | Telcagepant | 3N7R, (ter Haar et al., 2010) | – |
| RAMP126–117 | CLR22–133 | Olcegepant | 3N7S, (ter Haar et al., 2010) | – |
| MBP‐RAMP124–111‐(GSA)3‐CLR29–144‐(H)6 | CGRP27–37 [D31,P34,F35] | 4RWG, (Booe et al., 2015) | CGRP has β I‐turn, with terminal F facing W84 of RAMP1 (Figure 7 ) | |
| MBP‐RAMP2[L106R]55–140‐(GSA)3‐CLR29–144‐(H)6 | AM25–52 | 4RWF, (Booe et al., 2015) | AM has β I‐turn, with terminal Y facing E101 of RAMP2 (Figure 7 ) | |
| – | H6‐CTR25–144 | [BrPhe22]sCT8–32 | 5II0, (Johansson et al., 2016) | CT has β II‐turn, with terminal P facing W79/Y131 of CTR (Figure 7 ). |
| – | CTR with Gsαβγ and stabilizing nanobody 35 | sCT | 5UZ7, (Liang et al., 2017) | Cryo‐em structure. The ECD and ligand are not resolved. |
Figure 8.
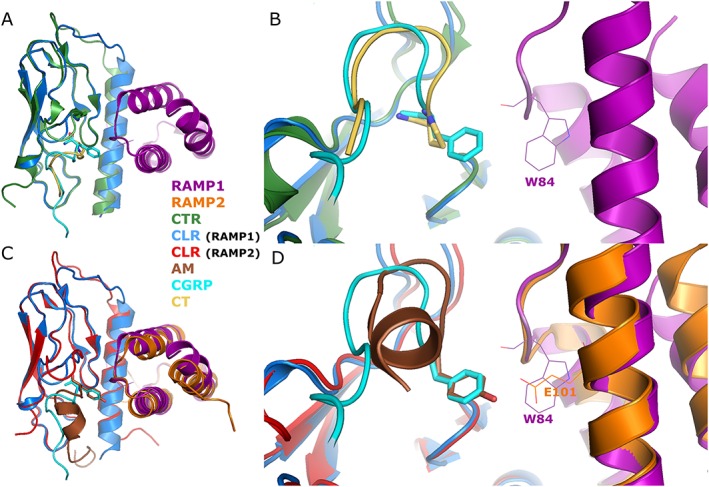
Structural alignment of CTR‐ and CLR‐based receptor ECDs with bound ligands. (A) Far and (B) near views of the CTR and CLR/RAMP1 ECDs bound to [BrPhe22]‐sCT8–32 or [D31,P34,F35]‐hαCGRP27–37 respectively. (C) Far and (D) near views of the CLR/RAMP1 and CLR/RAMP2 ECDs bound to [D31,P34,F35]‐hαCGRP27–37 or hAM22–52 respectively. All receptor ECDs are human. The C‐terminal residue of each peptide is shown in stick format, and the RAMP residue important for peptide interactions (RAMP1 W84/RAMP2 E101) is shown in line format. Images created in Pymol and aligned based on similarities between CTR and CLR or CLR and CLR. Images rotated 90° in the Z‐plane between near and far views.
A series of small molecule agonists for the CTR have been identified, and their binding site is probably at the junction of the ECD and the TMD of the receptor (Dong et al., 2009). They probably work allosterically, but their pharmacology remains largely unexplored.
Developments with antagonists
A major advance in the pharmacology of CGRP receptors came with the ‘gepant’ class of antagonists, typified by olcegepant (BIBN4096BS) and telcagepant (MK0974), which were developed as part of the global effort to develop drugs that inhibit CGRP action in migraine. These compounds have a high selectivity for CGRP as opposed to AM receptors because they bind to the interface between CLR and RAMP1. Telcagepant showed therapeutic efficacy in migraine and, although the development of this particular molecule was halted, non‐peptide CGRP receptor antagonists continue to be tested in clinical trials. The pharmacology of olcegepant and telcagepant has been extensively reviewed previously, but a number of developments should be noted. Both antagonists showed marked species‐selectivity in favour of primate receptors, restricting their use as experimental tools. Work to develop further gepant‐type compounds continues (Tora et al., 2013; Crowley et al., 2015; Civiello et al., 2016). The wider pharmacological characterization of these compounds has not been extensively pursued, but there are some significant exceptions. A study of olcegepant, telcagepant, MK‐3207 and rimagepant (BMS‐927711) on rat mesenteric arteries showed that they all behave as simple competitive antagonists with pA2 values ranging from 8.8 (MK‐3207) to 6.45 (telcagepant). They have similar affinities on mesenteric arteries and in binding assays to rat brain apart from rimagepant, which shows a 50‐fold lower affinity to brain. The reasons for this discrepancy are unclear (Sheykhzade et al., 2017). The selectivity of olcegepant and telcagepant for human CGRP and AMY1(a) receptors has been compared at receptors transfected into Cos‐7 cells. Surprisingly, for olcegepant acting on the AMY1(a) receptor, this depends on the pathway being measured; it is fivefold more potent at blocking CGRP when CREB phosphorylation is measured compared to cAMP (Walker et al., 2017). Thus, if cAMP is measured, olcegepant has over 100‐fold selectivity for CGRP over AMY1(a) receptors; for CREB, this drops to around a 25‐fold selectivity. This is not seen to the same extent with telcagepant nor is the differential antagonism observed at the CGRP receptor. The implications of this will be considered further below.
The development of the gepant antagonists has tended to draw attention away from other small molecule antagonists such as SB‐273779 (Aiyar et al., 2001) and other compounds. These compounds show little selectivity between CGRP and AM receptors; the binding site for the Merck compounds variously known as compound 4 or compound 16 appears to include part of the TMD and extracellular loop (ECL3), well away from the ECD interface between CLR and RAMP1 used by the gepants (Salvatore et al., 2006). It is possible that SB‐273779 binds in a similar place. However, mutagenesis suggests that there are RAMP effects on ECL3 and so it may be possible to develop selective antagonists, which bind to this region (Watkins et al., 2016).
There has been work to develop shortened peptide antagonists. A substituted version of the final 11 amino acids of CGRP has been reported to bind with sub‐micromolar affinity (Rist et al., 1998), and a crystal structure of this bound to the ECD of RAMP1 and CLR has been solved (Booe et al., 2015) (Table 1, Figure 8). Chimeras between CGRP8–37, AM22–52 and AM2/IMD16–47 produced analogues with novel specificities but whose activities remain difficult to understand (Robinson et al., 2009). Homology models of amylin receptors are facilitating the development of novel CTR and amylin receptor antagonists, based on the related CT family receptor ECD structures (Lee et al., 2016).
A recent development has been the use of antibodies to block the actions of CGRP, as an alternative to the use of classic antagonists for the therapy of migraine. The majority of these act against CGRP itself (Mason et al., 2017; Tso and Goadsby, 2017), but some success has been achieved with antibodies directed to the CGRP receptor, both in experimental models (Miller et al., 2016) and human studies (Shi et al., 2016; Tso and Goadsby, 2017).
The challenges of pharmacology in non‐transfected cell systems
Whilst studies with transfected cells are essential for defining the pharmacology of individual receptor subtypes, they have some limitations. In particular, if pharmacology is influenced by cell‐specific factors such as G proteins (see below) or accessory proteins, then this will only be properly revealed by experiments in the physiologically relevant cell. Even if the receptors are identical, differences in responses can be produced by their level of expression and factors such as differential expression of peptidases. There are particular considerations where CTR is expressed with RAMPs, as it is highly likely that both AMY receptors and free CTR will be present at the cell surface. This section provides some examples of the pharmacology emerging from cells that endogenously express receptors and highlights some of the challenges of using ‘model’ cell lines that endogenously express receptor components.
Primary cells
In cultured rat trigeminal ganglia neurons, CTR, CLR and RAMP1 are present, giving a particularly complex situation. The data suggest that different cells have either CLR or CTR, sometimes with RAMP1, so there are difficulties in comparing functional data of pooled responses to individual cells with one or more functional receptors. CGRP‐mediated cAMP production is blocked by the CT and amylin receptor antagonist AC187 with a pA2 appropriate to the AMY1(a) receptor. Antagonism of CGRP responses by olcegepant, however, was consistent with the presence of the CGRP receptor, CLR/RAMP1, supporting the notion that two populations of CGRP‐responsive receptors are present in these cells (Walker et al., 2015).
Somewhat similar complexities have been observed with rat embryonic dissociated spinal cord cells. In this case, CGRP, AM and AM2/IMD responses have been investigated in two separate studies. These cells express high affinity binding sites for both AM and CGRP and both peptides also produce cAMP, consistent with the presence of CGRP and AM receptors. A selection of antagonists was used to try and define the receptors that mediated cAMP responses to each agonist. The response of CGRP was effectively blocked by olcegepant. However, the data for AM and AM2/IMD are less straightforward to interpret (Takhshid et al., 2006). AM2/IMD showed biphasic high and low affinity displacement of bound [125I]‐AM but monophasic high affinity displacement of [125I]‐CGRP. Despite high affinity for the CGRP binding site, antagonism of AM2/IMD by olcegepant was weak, which is not consistent with AM2/IMD acting through a canonical CGRP receptor (Owji et al., 2008). It is likely that there are mixed populations of receptors, potentially within the same or different cells within these cultures, creating mixed pharmacology. It is possible that an amylin receptor could partially explain this. Indeed, using the same spinal cell system, amylin responses have also been studied. This highlights another mismatch between this endogenous system and transfected cells, the potency of amylin8–37. In this study, it achieved a pA2 of 7.94, which is far greater than the highest value achieved in transfected cell systems (rat) of 6.16 (Bailey et al., 2012). The reason for this is not known.
These few observations serve as examples that reflect the difficulty of working with systems that endogenously express one or more populations of receptors. A common problem is that it is difficult to test all of the different combinations of agonists and antagonists that are currently necessary to tease apart the pharmacology of these receptors. Therefore, it is common that limited concentrations and ranges of pharmacological tools are used. This is of course a consequence of using cells that are only available in small amounts and the problem with generating very pure cultures. We have used the studies discussed in the preceding paragraph because they are more helpful than many, which use only a single concentration of agonist or antagonist in an ‘all or nothing’ approach and thus cannot quantify parameters or define pharmacology in any meaningful way. For example, if CGRP were to be used in any study of rodent tissues or cells at 100 nM or a greater concentration, it could potentially act through CGRP, AM2, AMY1 or AMY3 receptors. Blockade of this response with 1 μM or more of CGRP8–37 would not rule in or out any of these receptors, because this concentration of antagonist can block all of these receptors. Hence, the concentrations and combinations of agents used are very important and further work is needed on ex vivo cells, to establish the pharmacology that they display. Similar issues are often faced in cell lines.
Cell lines
Despite the challenges associated with endogenously expressed receptors, the SK‐N‐MC cell line (derived from a human neuroblastoma) has proven invaluable for understanding CGRP receptor pharmacology. SK‐N‐MC cells have been extensively characterized and display pharmacology consistent with a functional CGRP receptor in transfected cells (Bailey and Hay, 2006). These cells have been used as a starting point for the pharmacological characterization of several CGRP receptor antagonists (Moore and Salvatore, 2012). However, this model is not perfect. They reportedly express RAMP2 in addition to CGRP receptor components (CLR and RAMP1) and lose their CGRP receptor phenotype over passages (Choksi et al., 2002). Similarly, the human breast cancer cell line, T47D, displays pharmacology consistent with a CTR and may represent an appropriate model for studying the pharmacology of this receptor (Muff et al., 1992; Zimmermann et al., 1997). Thus, SK‐N‐MC and T47D cells appear to be appropriate models to study the pharmacology of CGRP and CT receptors respectively. However, it should be noted that the compliment of downstream intracellular signalling proteins may be very different between these cell lines and a physiological tissue. Therefore, they may not be suitable for deciphering intricate biological activities.
Using a similar rationale, other human cell lines including Col‐29 (colonic epithelial) and MCF‐7 (breast cancer) have been examined for their responsiveness to CGRP and related peptides (Zimmermann et al., 1997; Hay et al., 2002). However, the pharmacology reported for these cell lines is not straight forward. Despite this, MCF‐7 cells have been used in several studies as an amylin receptor model (Sisnande et al., 2015; Shi et al., 2016). These cells are reported to express mRNA encoding two distinct splice variants of CTR, RAMP1 and RAMP3 (Chen et al., 1997; Ellegaard et al., 2010). Therefore, MCF‐7 cells have the potential to contain functional CTR, AMY1 and AMY3 receptors. In these cells, CT stimulated cAMP production potently and [125I]‐CT binding was not displaced by amylin or CGRP, suggesting that the CTR may be present. However, the potent cAMP response to amylin, coupled with the weak displacement of [125I]‐amylin binding by CGRP relative to amylin is consistent with the AMY3 receptor in transfected cell models (Zimmermann et al., 1997 ; Hay et al., 2005). Yet, in functional assays, CGRP and amylin have similar potencies for the stimulation of cAMP production (Zimmermann et al., 1997; Ellegaard et al., 2010). This suggests that these cells may contain functional AMY1 and/or CGRP receptors. Curiously in direct contradiction to this, [125I]‐CGRP was reported not to bind to MCF‐7 cells under the conditions used suggesting that neither AMY1 nor CGRP receptors were present (Zimmermann et al., 1997). It is not clear whether CLR is expressed by MCF‐7 cells. MCF‐7 cells highlight the difficulties involved in the study of this family of heterodimeric receptors, where cells may express multiple interchangeable receptor components. Overall, MCF‐7 cells probably contain a mixture of receptors and, therefore, are not recommended as a model system for this peptide family.
Receptor signalling
Biased signalling
Whilst it has been recognized for many years that CLR and CTR‐based receptors signal through a variety of pathways, most work has focussed on cAMP. Recently, work has started both to document the extent of signal bias and also to understand the underlying mechanisms.
In transfected HEK293 cells, a significant Gi‐component was observed to the response to AM at CGRP receptors and to CGRP at AM1 and AM2 receptors; this Gi‐component was not seen with CGRP or AM acting at their cognate receptors. The Gi component was not seen in HEK293S cells, perhaps reflecting the low expression of this G protein. The results are broadly consistent with data obtained in Saccharomyces cerevisiae engineered to express versions of Gs and Gi, where AM is more potent than CGRP acting through the CGRP receptor and CGRP is more potent than AM at the AM1 receptor when coupling to the Gi construct is measured (Weston et al., 2016). Taken at face value, these results suggest that ligand bias can significantly change receptor selectivity. Caution is needed; the results have only been shown in a single, transfected cell line; it remains to be established whether the effects are seen in native cells. None‐the‐less, the data indicate the potential importance of biased signalling. This conclusion is reinforced by the pathway‐selective antagonism previously discussed for olcegepant (Walker et al., 2017). In this study, strong cell‐dependent differences were seen in signalling with respect to ERK and p38. In rat trigeminal ganglion neuron cultures (which probably express both AMY1 and CGRP receptors), rat αCGRP stimulated cAMP, CREB and p38 phosphorylation but not ERK. In Cos‐7 cells transfected with human CGRP and AMY1(a) receptors, human αCGRP stimulated cAMP, CREB and ERK phosphorylation, but not p38.
There are processes, such as stimulation of angiogenesis, where cAMP‐mediated mechanisms may be expected to be of minor importance compared to stimulation of pathways such as Akt (Nikitenko et al., 2006; Walker et al., 2010) and so biased agonists might be particularly useful, either to avoid or promote this effect. However, even here, a contribution from cAMP is sometimes observed (Miyashita et al., 2003; Jin et al., 2008). There is a clear need to study signalling in physiologically relevant tissues and cells, to take into account all aspects of the inherent variability of receptor signalling.
An important contribution to understanding the mechanism behind biased signalling has come from comparing the effects of human and salmon CT on G protein activation; of the two ligands, human CT has a higher efficacy. The two agonists stabilize forms of CTR which differ in their ability to interact with Gs (Furness et al., 2016). The molecular explanation for this observation remains to be established.
Receptor internalization and recycling
In both transfected HEK cells and rat mesenteric smooth muscle cells, following challenge with CGRP, the ligand/CLR/RAMP1 complex is targeted to the early endosome. Cleavage of CGRP by endothelin‐converting enzyme‐1 within this organelle leads to the release of β arrestins and recycling of the CLR/RAMP1 complex to the cell surface (McNeish et al., 2012). There may be significant cell and tissue variability in this response. Thus, it has been reported that in human microvascular endothelial cells, AM but not CGRP could cause internalization of both AM and CGRP receptors (Nikitenko et al., 2006). Curiously, there is a report that overexpression of β arrestin 1 or 2 both inhibits AM1 receptor internalization in HEK cells (Kuwasako et al., 2017), although activation of GPCR kinases 5 and 6 cause the expected internalization (Kuwasako et al., 2016).
Internalization of the CTR is well characterized. The internalization rate differs between the hCT(a) and hCT(b), perhaps linked to the different signalling profiles of these splice variants (Moore et al., 1995). Interestingly, it has been noted that the internalized CTR may continue to stimulate adenylate cyclase when stimulated by salmon but not human CT (Andreassen et al., 2014a). The C‐terminus of the CTR plays an important role in determining the fate of the internalized receptor; the rabbit CTR can bind to the actin‐binding protein filamin and this promotes recycling (Seck et al., 2003); it is not known if this applies to other species as there are differences in the sequences of the C‐terminus. In contrast, the fate and mechanisms of CTR trafficking in the presence of RAMPs are not known.
It seems likely that internalization of CLR‐ and CTR‐based receptors depends on a combination of the cell line, the agonist, the splice variant (for CTR) and the RAMP, with both the C‐terminus (Bomberger et al., 2005a,b) and the TMD (Kuwasako et al., 2012) of the RAMP containing important determinants. The significance, if any, of signalling directed by internalized CTR or CLR complexes is not extensively explored, although a recent study has provided interesting insights relating to endosomal CGRP signalling and pain (Yarwood et al., 2017).
Unresolved questions, challenges and recommendations
Since the identification that RAMPs are required for the formation of AM, CGRP and amylin receptors, great strides have been made in understanding their biology (McLatchie et al., 1998; Christopoulos et al., 1999). However, the heterodimeric nature of these receptors results in unique challenges in understanding the pharmacological and physiological roles, and several complications or questions have arisen in the field.
Which amylin and AM receptors are biologically relevant? Although the combinations of CTR and RAMPs are described as amylin receptors, there is little protein data on the co‐expression of these subunits in tissues and it is not clear whether one or all of these ‘amylin receptors’ form functional complexes in vivo. To address this question, highly specific probes (antibodies, labelled ligand and/or antagonists) for CTR alone and individual CTR/RAMP complexes are required. A very similar situation exists for AM, when it is extremely difficult to distinguish pharmacologically between AM1 and AM2 receptors.
Amylin receptor studies may be complicated by co‐expression with free CTR. CTR can reach the cell surface in the absence of a RAMP to form a receptor for CT or in the presence of a RAMP to form an amylin receptor (Christopoulos et al., 1999). The potential contribution of free CTR to the pharmacological profiles of amylin receptors in transfected cell models was discussed earlier in this review. Whether free CTR reaches the cell surface in the presence of RAMPs in vivo is not clear. It is possible that amylin receptors are commonly co‐expressed with variable amounts of free CTR, complicating interpretation.
Is the AMY 1 receptor responsible for the physiological actions of CGRP? Although the actions of CGRP are often assumed to be via the CGRP receptor, in many cases a mixture of receptors may be involved or the receptor has simply not been identified. Given the high potency CGRP displays at the AMY1 receptor and the widespread distribution of components for the AMY1 receptor in the nervous system and peripheral tissues, it would be surprising if CGRP did not act endogenously at the AMY1 receptor (McLatchie et al., 1998; Oliver et al., 2001; Tolcos et al., 2003). This requires clarification.
AM2/IMD has two different names and has activity at several receptors. AM2 or IMD was initially described by two different research groups (Roh et al., 2004; Takei et al., 2004). No consensus has been reached regarding a single name for this peptide, and it is now generally referred to by both names as AM2/IMD (Hong et al., 2012). It is important to note that intermedin is an alternative name for melanocyte‐stimulating hormone and was also used to describe plant compounds (Li et al., 2008). The dual name for AM2/IMD may cause confusion, especially for those unfamiliar with the field. Given that IMD does not exclusively describe the AM relative, we recommend the use of AM2 or AM2/IMD but never just IMD. It is also important that when referring to the CLR/RAMP3 receptor complex, a subscript 2 character is used, that is, AM2 receptor to clearly identify descriptions of the AM2 peptide and AM2 receptor. It is likely that existing complexes of CLR and/or CTR with RAMPs can explain AM2/IMD actions without needing to invoke alternative receptors. Better antagonists are needed and an awareness of differences in pharmacology between species should be acknowledged.
βCGRP has widespread expression. βCGRP is often described as being predominantly expressed in the enteric nervous system. However, it is more correct to state that βCGRP is the predominant form of CGRP expressed in the enteric nervous system (Schutz et al., 2004). αCGRP and βCGRP are reportedly expressed throughout the nervous system with variable and overlapping distributions (Amara et al., 1985; Schutz et al., 2004). For example, αCGRP and βCGRP are both expressed in the dorsal root ganglia and dorsal horn of the spinal cord, whereas αCGRP appears to be the predominant form expressed in the ventral horn of the spinal cord and at the neuromuscular junction (Schutz et al., 2004). Hence, βCGRP should not be ignored as a widespread ligand for CGRP receptors.
Conclusions
The pharmacological classification of receptors for the CT/CGRP family as first proposed by NC‐IUPHAR in 2002 remains a useful framework. However, there are a number of conceptual challenges, many of which are highlighted in the previous section. Perhaps the most significant of these is that receptors of the AMY1 type may be activated physiologically by CGRP. There is also a lack of agents that can discriminate between AM1 and AM2 receptors, or any of the AMY receptors. This represents a major barrier to our understanding of the in vivo role of these subtypes. Whilst it is likely that coupling to Gs and cAMP is the main transduction pathway for receptors of this family, a much better exploration of ligand bias is needed. The development of new pharmacological agents will be facilitated by our increased molecular understanding of the receptors within this family, drawing on insights from both structural and computational biology. As these become available, our understanding of the physiology of these peptides and their potential therapeutic uses will increase.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017).
Conflict of interest
The authors declare no conflicts of interest.
Supporting information
Data S1 Tables of ligand potency values to support figures.
Acknowledgements
D.R.P. is supported by the Biotechnology and Biological Sciences Research Council (BB/M000176/1). D.L.H. is supported by a James Cook Fellowship of the Royal Society of New Zealand. C.S.W. is supported by a Sir Charles Hercus Health Research Fellowship from the Health Research Council of New Zealand. M.L.G. is supported by a Health Doctoral Scholarship from the University of Auckland.
Hay, D. L. , Garelja, M. L. , Poyner, D. R. , and Walker, C. S. (2018) Update on the pharmacology of calcitonin/CGRP family of peptides: IUPHAR Review 25. British Journal of Pharmacology, 175: 3–17. doi: 10.1111/bph.14075.
This article, contributed by members of the International Union of Basic and Clinical Pharmacology Committee on Receptor Nomenclature and Drug Classification (NC‐IUPHAR) subcommittee for the Calcitonin receptors, confirms the existing nomenclature for these receptors, and reviews our current understanding of their structure, pharmacology and functions, and likely physiological roles in health and disease. More information on these receptors can be found in the Concise Guide to PHARMACOLOGY (http://onlinelibrary.wiley.com/doi/10.1111/bph.13878/full), and in the corresponding open access IUPHAR/BPS Guide to PHARMACOLOGY database (http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=11).
References
- Aiyar N, Daines RA, Disa J, Chambers PA, Sauermelch CF, Quiniou M et al (2001). Pharmacology of SB‐273779, a nonpeptide calcitonin gene‐related peptide 1 receptor antagonist. J Pharmacol Exp Ther 296: 768–775. [PubMed] [Google Scholar]
- Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA et al (2017). The Concise Guide To PHARMACOLOGY 2017/18: G protein‐coupled receptors. Br J Pharmacol 174: S17‐S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amara SG, Arriza JL, Leff SE, Swanson LW, Evans RM, Rosenfeld MG (1985). Expression in brain of a messenger RNA encoding a novel neuropeptide homologous to calcitonin gene‐related peptide, Science , 229:1094–7. [DOI] [PubMed] [Google Scholar]
- Andreassen KV, Hjuler ST, Furness SG, Sexton PM, Christopoulos A, Nosjean O et al (2014a). Prolonged calcitonin receptor signaling by salmon, but not human calcitonin, reveals ligand bias. PLoS One 9: e92042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreassen KV, Feigh M, Hjuler ST, Gydesen S, Henriksen JE, Beck‐Nielsen H et al (2014b). A novel oral dual amylin and calcitonin receptor agonist (KBP‐042) exerts antiobesity and antidiabetic effects in rats. Am J Physiol Endocrinol Metab 307: E24–E33. [DOI] [PubMed] [Google Scholar]
- Bailey RJ, Hay DL (2006). Pharmacology of the human CGRP1 receptor in Cos 7 cells. Peptides 27: 1367–1375. [DOI] [PubMed] [Google Scholar]
- Bailey RJ, Walker CS, Ferner AH, Loomes KM, Prijic G, Halim A et al (2012). Pharmacological characterisation of rat amylin receptors: implications for the identification of amylin receptor subtypes. Br J Pharmacol 166: 151–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomberger JM, Parameswaran N, Hall CS, Aiyar N, Spielman WS (2005a). Novel function for receptor activity‐modifying proteins (RAMPs) in post‐endocytic receptor trafficking. J Biol Chem 280: 9297–9307. [DOI] [PubMed] [Google Scholar]
- Bomberger JM, Spielman WS, Hall CS, Weinman EJ, Parameswaran N (2005b). Receptor activity‐modifying protein (RAMP) isoform‐specific regulation of adrenomedullin receptor trafficking by NHERF‐1. J Biol Chem 280: 23926–23935. [DOI] [PubMed] [Google Scholar]
- Booe JM, Walker CS, Barwell J, Kuteyi G, Simms J, Jamaluddin MA et al (2015). Structural basis for receptor activity‐modifying protein‐dependent selective peptide recognition by a G protein‐coupled receptor. Mol Cell 58: 1040–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower RL, Hay DL (2016). Amylin structure‐function relationships and receptor pharmacology: implications for amylin mimetic drug development. Br J Pharmacol 173: 1883–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WJ, Armour S, Way J, Chen G, Watson C, Irving P et al (1997). Expression cloning and receptor pharmacology of human calcitonin receptors from MCF‐7 cells and their relationship to amylin receptors. Mol Pharmacol 52: 1164–1175. [DOI] [PubMed] [Google Scholar]
- Choksi T, Hay DL, Legon S, Poyner DR, Hagner S, Bloom SR et al (2002). Comparison of the expression of calcitonin receptor‐like receptor (CRLR) and receptor activity modifying proteins (RAMPs) with CGRP and adrenomedullin binding in cell lines. Br J Pharmacol 136: 784–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopoulos G, Perry KJ, Morfis M, Tilakaratne N, Gao Y, Fraser NJ et al (1999). Multiple amylin receptors arise from receptor activity‐modifying protein interaction with the calcitonin receptor gene product. Mol Pharmacol 56: 235–242. [DOI] [PubMed] [Google Scholar]
- Civiello RL, Han X, Beno BR, Chaturvedula PV, Herbst JJ, Xu C et al (2016). Synthesis and SAR of calcitonin gene‐related peptide (CGRP) antagonists containing substituted aryl‐piperazines and piperidines. Bioorg Med Chem Lett 26: 1229–1232. [DOI] [PubMed] [Google Scholar]
- Cottrell GS, Roosterman D, Marvizon JC, Song B, Wick E, Pikios S et al (2005). Localization of calcitonin receptor‐like receptor and receptor activity modifying protein 1 in enteric neurons, dorsal root ganglia, and the spinal cord of the rat. J Comp Neurol 490: 239–255. [DOI] [PubMed] [Google Scholar]
- Crowley BM, Stump CA, Nguyen DN, Potteiger CM, McWherter MA, Paone DV et al (2015). Novel oxazolidinone calcitonin gene‐related peptide (CGRP) receptor antagonists for the acute treatment of migraine. Bioorg Med Chem Lett 25: 4777–4781. [DOI] [PubMed] [Google Scholar]
- Dong M, Cox RF, Miller LJ (2009). Juxtamembranous region of the amino terminus of the family B G protein‐coupled calcitonin receptor plays a critical role in small‐molecule agonist action. J Biol Chem 284: 21839–21847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden S, Daniel C, Steinbrueck A, Schmidt I, Simon E (2002). Salmon calcitonin – a potent inhibitor of food intake in states of impaired leptin signalling in laboratory rodents. J Physiol 541: 1041–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellegaard M, Thorkildsen C, Petersen S, Petersen JS, Jorgensen NR, Just R et al (2010). Amylin(1‐8) is devoid of anabolic activity in bone. Calcif Tissue Int 86: 249–260. [DOI] [PubMed] [Google Scholar]
- Feigh M, Nielsen RH, Hansen C, Henriksen K, Christiansen C, Karsdal MA (2012). Oral salmon calcitonin improves fasting and postprandial glycemic control in lean healthy rats. Horm Metab Res 44: 130–134. [DOI] [PubMed] [Google Scholar]
- Feigh M, Hjuler ST, Andreassen KV, Gydesen S, Ottosen I, Henriksen JE et al (2014). Oral salmon calcitonin enhances insulin action and glucose metabolism in diet‐induced obese streptozotocin‐diabetic rats. Eur J Pharmacol 737: 91–96. [DOI] [PubMed] [Google Scholar]
- Findlay DM, Sexton PM (2004). Mini Review Calcitonin. Growth Factors 22: 217–224. [DOI] [PubMed] [Google Scholar]
- Furness SG, Liang YL, Nowell CJ, Halls ML, Wookey PJ, Dal Maso E et al (2016). Ligand‐dependent modulation of G protein conformation alters drug efficacy. Cell 167: 739–749 e11. [DOI] [PubMed] [Google Scholar]
- Gennari C, Agnusdei D (1994). Calcitonins and osteoporosis. Br J Clin Pract 48: 196–200. [PubMed] [Google Scholar]
- Gydesen S, Andreassen KV, Hjuler ST, Christensen JM, Karsdal MA, Henriksen K (2016). KBP‐088, a novel DACRA with prolonged receptor activation, is superior to davalintide in terms of efficacy on body weight. Am J Physiol Endocrinol Metab 310: E821–E827. [DOI] [PubMed] [Google Scholar]
- Gydesen S, Andreassen KV, Hjuler ST, Hellgren LI, Karsdal MA, Henriksen K (2017a). Optimization of tolerability and efficacy of dual amylin and calcitonin receptor agonist, KBP‐089, through dose escalation and combination with a GLP‐1 analogue. Am J Physiol Endocrinol Metab in press https://doi.org/10.1152/ajpendo.00419.2016. [DOI] [PubMed] [Google Scholar]
- Gydesen S, Hjuler ST, Freving Z, Andreassen KV, Sonne N, Hellgren LI et al (2017b). A novel dual amylin and calcitonin receptor agonist, KBP‐089, induces weight loss through a reduction in fat, but not lean mass, while improving food preference. Br J Pharmacol 174: 591–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Haar E, Koth CM, Abdul‐Manan N, Swenson L, Coll JT, Lippke JA et al (2010). Crystal structure of the ectodomain complex of the CGRP receptor, a class‐B GPCR, reveals the site of drug antagonism. Structure 18: 1083–1093. [DOI] [PubMed] [Google Scholar]
- Halim A, Hay DL (2012). The role of glutamic acid 73 in adrenomedullin interactions with rodent AM2 receptors. Peptides 36: 137–141. [DOI] [PubMed] [Google Scholar]
- Harikumar KG, Ball AM, Sexton PM, Miller LJ (2010). Importance of lipid‐exposed residues in transmembrane segment four for family B calcitonin receptor homo‐dimerization. Regul Pept 164: 113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto H, Hyodo S, Kawasaki M, Shibata M, Saito T, Suzuki H et al (2007). Adrenomedullin 2 (AM2)/intermedin is a more potent activator of hypothalamic oxytocin‐secreting neurons than AM possibly through an unidentified receptor in rats. Peptides 28: 1104–1112. [DOI] [PubMed] [Google Scholar]
- Hay DL, Pioszak AA (2016). Receptor activity modifying proteins: new insights and roles. Annu Rev Pharmacol Toxicol 56: 469–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay DL, Poyner DR (2017). Calcitonin receptors. IUPHAR/BPS Guide to PHARMACOLOGY Available at: http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=11 (accessed 21 September, 2017)
- Hay DL, Walker CS (2017). CGRP and its receptors. Headache 57: 625–636. [DOI] [PubMed] [Google Scholar]
- Hay DL, Howitt SG, Conner AC, Doods H, Schindler M, Poyner DR (2002). A comparison of the actions of BIBN4096BS and CGRP(8‐37) on CGRP and adrenomedullin receptors expressed on SK‐N‐MC, L6, Col 29 and Rat 2 cells. Br J Pharmacol 137: 80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay DL, Christopoulos G, Christopoulos A, Poyner DR, Sexton PM (2005). Pharmacological discrimination of calcitonin receptor: receptor activity‐modifying protein complexes. Mol Pharmacol 67: 1655–1665. [DOI] [PubMed] [Google Scholar]
- Hay DL, Christopoulos G, Christopoulos A, Sexton PM (2006). Determinants of 1‐piperidinecarboxamide, N‐[2‐[[5‐amino‐l‐[[4‐(4‐pyridinyl)‐l‐piperazinyl]carbonyl]pentyl]amino]‐1‐[(3,5‐d ibromo‐4‐hydroxyphenyl)methyl]‐2‐oxoethyl]‐4‐(1,4‐dihydro‐2‐oxo‐3(2H)‐quinazoliny l) (BIBN4096BS) affinity for calcitonin gene‐related peptide and amylin receptors‐the role of receptor activity modifying protein 1. Mol Pharmacol 70: 1984–1991. [DOI] [PubMed] [Google Scholar]
- Hay DL, Poyner DR, Quirion R (2008). International Union of Pharmacology. LXIX. Status of the calcitonin gene‐related peptide subtype 2 receptor. Pharmacol Rev 60: 143–145. [DOI] [PubMed] [Google Scholar]
- Hay DL, Chen S, Lutz TA, Parkes DG, Roth JD (2015). Amylin: pharmacology, physiology, and clinical potential. Pharmacol Rev 67: 564–600. [DOI] [PubMed] [Google Scholar]
- Hinson JP, Kapas S, Smith DM (2000). Adrenomedullin, a multifunctional regulatory peptide. Endocr Rev 21: 138–167. [DOI] [PubMed] [Google Scholar]
- Hong Y, Hay DL, Quirion R, Poyner DR (2012). The pharmacology of adrenomedullin 2/intermedin. Br J Pharmacol 166: 110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou M, Xing H, Cai Y, Li B, Wang X, Li P et al (2017). The effect and safety of monoclonal antibodies to calcitonin gene‐related peptide and its receptor on migraine: a systematic review and meta‐analysis. J Headache Pain 18: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazayeri A, Rappas M, Brown AJH, Kean J, Errey JC, Robertson NJ et al (2017). Crystal structure of the GLP‐1 receptor bound to a peptide agonist. Nature 546: 254–258. [DOI] [PubMed] [Google Scholar]
- Jin D, Harada K, Ohnishi S, Yamahara K, Kangawa K, Nagaya N (2008). Adrenomedullin induces lymphangiogenesis and amerliorates secondary lymphoedema. Cardiovasc Res 80: 339–345. [DOI] [PubMed] [Google Scholar]
- Johansson E, Hansen JL, Hansen AM, Shaw AC, Becker P, Schaffer L et al (2016). Type II turn of receptor‐bound salmon calcitonin revealed by X‐ray crystallography. J Biol Chem 291: 13689–13698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsan N, Goadsby PJ (2015). CGRP mechanism antagonists and migraine management. Curr Neurol Neurosci Rep 15: 25. [DOI] [PubMed] [Google Scholar]
- Katafuchi T, Minamino N (2004). Structure and biological properties of three calcitonin receptor‐stimulating peptides, novel members of the calcitonin gene‐related peptide family. Peptides 25: 2039–2045. [DOI] [PubMed] [Google Scholar]
- Katafuchi T, Kikumoto K, Hamano K, Kangawa K, Matsuo H, Minamino N (2003). Calcitonin receptor‐stimulating peptide, a new member of the calcitonin gene‐related peptide family. Its isolation from porcine brain, structure, tissue distribution, and biological activity. J Biol Chem 278: 12046–12054. [DOI] [PubMed] [Google Scholar]
- Katafuchi T, Hamano K, Minamino N (2004). Identification, structural determination, and biological activity of bovine and canine calcitonin receptor‐stimulating peptides. Biochem Biophys Res Commun 313: 74–79. [DOI] [PubMed] [Google Scholar]
- Katafuchi T, Yasue H, Osaki T, Minamino N (2009). Calcitonin receptor‐stimulating peptide: its evolutionary and functional relationship with calcitonin/calcitonin gene‐related peptide based on gene structure. Peptides 30: 1753–1762. [DOI] [PubMed] [Google Scholar]
- Keller J, Catala‐Lenhen P, Huebner AK, Jeschke A, Heckt T, Lueth A et al (2014). Calcitonin controls bone formation by inhibiting the release of sphingosine 1‐phosphate from osteoclasts. Nat Commun 5: 5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YG, Lone AM, Saghatelian A (2013). Analysis of the proteolysis of bioactive peptides using a peptidomics approach. Nat Protoc 8: 1730–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk R, Brimble MA, Tomabechi Y, Fairbanks AJ, Fletcher M, Hay DL (2014). Convergent chemoenzymatic synthesis of a library of glycosylated analogues of pramlintide: structure‐activity relationships for amylin receptor agonism. Org Biomol Chem 12: 8142–8151. [DOI] [PubMed] [Google Scholar]
- Kuwasako K, Kitamura K, Nagata S, Nozaki N, Kato J (2012). Characterization of the single transmembrane domain of human receptor activity‐modifying protein 3 in adrenomedullin receptor internalization. Biochem Biophys Res Commun 420: 582–587. [DOI] [PubMed] [Google Scholar]
- Kuwasako K, Sekiguchi T, Nagata S, Jiang D, Hayashi H, Murakami M et al (2016). Inhibitory effects of two G protein‐coupled receptor kinases on the cell surface expression and signaling of the human adrenomedullin receptor. Biochem Biophys Res Commun 470: 894–899. [DOI] [PubMed] [Google Scholar]
- Kuwasako K, Kitamura K, Nagata S, Sekiguchi T, Danfeng J, Murakami M et al (2017). Beta‐arrestins negatively control human adrenomedullin type 1‐receptor internalization. Biochem Biophys Res Commun 487: 438–443. [DOI] [PubMed] [Google Scholar]
- Lee SM, Hay DL, Pioszak AA (2016). Calcitonin and amylin receptor peptide interaction mechanisms: insights into peptide‐binding modes and allosteric modulation of the calcitonin receptor by receptor activity‐modifying proteins. J Biol Chem 291: 8686–8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuthauser K, Gujer R, Aldecoa A, McKinney RA, Muff R, Fischer JA et al (2000). Receptor‐activity‐modifying protein 1 forms heterodimers with two G‐protein‐coupled receptors to define ligand recognition. Biochem J 351: 347–351. [PMC free article] [PubMed] [Google Scholar]
- Li HM, Lei C, Luo YM, Li XN, Li XL, Pu JX et al (2008). Intermedins A and B; new metabolites from Schisandra propinqua var. intermedia. Arch Pharm Res 31: 684–687. [DOI] [PubMed] [Google Scholar]
- Liang YL, Khoshouei M, Radjainia M, Zhang Y, Glukhova A, Tarrasch J et al (2017). Phase‐plate cryo‐EM structure of a class B GPCR‐G‐protein complex. Nature 546: 118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz TA, Tschudy S, Rushing PA, Scharrer E (2000). Amylin receptors mediate the anorectic action of salmon calcitonin (sCT). Peptides 21: 233–238. [DOI] [PubMed] [Google Scholar]
- Mack CM, Soares CJ, Wilson JK, Athanacio JR, Turek VF, Trevaskis JL et al (2010). Davalintide (AC2307), a novel amylin‐mimetic peptide: enhanced pharmacological properties over native amylin to reduce food intake and body weight. Int J Obes (Lond) 34: 385–395. [DOI] [PubMed] [Google Scholar]
- Mason BN, Kaiser EA, Kuburas A, Loomis MM, Latham JA, Garcia‐Martinez L et al (2017). Induction of migraine‐like photophobic behavior in mice by both peripheral and central CGRP mechanisms. J Neurosci 37: 204–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N et al (1998). RAMPs regulate the transport and ligand specificity of the calcitonin‐receptor‐like receptor. Nature 393: 333–339. [DOI] [PubMed] [Google Scholar]
- McNeish AJ, Roux BT, Aylett SB, Van Den Brink AM, Cottrell GS (2012). Endosomal proteolysis regulates calcitonin gene‐related peptide responses in mesenteric arteries. Br J Pharmacol 167: 1679–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S, Liu H, Warfvinge K, Shi L, Dovlatyan M, Xu C et al (2016). Immunohistochemical localization of the calcitonin gene‐related peptide binding site in the primate trigeminovascular system using functional antagonist antibodies. Neuroscience 328: 165–183. [DOI] [PubMed] [Google Scholar]
- Miyashita K, Itoh H, Sawada N, Fukunaga Y, Sone M, Yamahara K et al (2003). Adrenomedullin promotes proliferation and migration of cultured endothelial cells. Hypertens Res 26 (SUPPL): S93–S98. [DOI] [PubMed] [Google Scholar]
- Moore EE, Kuestner RE, Stroop SD, Grant FJ, Matthewes SL, Brady CL et al (1995). Functionally different isoforms of the human calcitonin receptor result from alternative splicing of the gene transcript. Mol Endocrinol 9: 959–968. [DOI] [PubMed] [Google Scholar]
- Moore EL, Salvatore CA (2012). Targeting a family B GPCR/RAMP receptor complex: CGRP receptor antagonists and migraine. Br J Pharmacol 166: 66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muff R, Stangl D, Born W, Fischer JA (1992). Comparison of a calcitonin gene‐related peptide receptor in a human neuroblastoma cell line (SK‐N‐MC) and a calcitonin receptor in a human breast carcinoma cell line (T47D). Ann N Y Acad Sci 657: 106–116. [DOI] [PubMed] [Google Scholar]
- Muff R, Buhlmann N, Fischer JA, Born W (1999). An amylin receptor is revealed following co‐transfection of a calcitonin receptor with receptor activity modifying proteins‐1 or −3. Endocrinology 140: 2924–2927. [DOI] [PubMed] [Google Scholar]
- Nikitenko LL, Blucher N, Fox SB, Bicknell R, Smith DM, Rees MC (2006). Adrenomedullin and CGRP interact with endogenous calcitonin‐receptor‐like receptor in endothelial cells and induce its desensitisation by different mechanisms. J Cell Sci 119: 910–922. [DOI] [PubMed] [Google Scholar]
- Nilsson C, Hansen TK, Rosenquist C, Hartmann B, Kodra JT, Lau JF et al (2016). Long acting analogue of the calcitonin gene‐related peptide induces positive metabolic effects and secretion of the glucagon‐like peptide‐1. Eur J Pharmacol 773: 24–31. [DOI] [PubMed] [Google Scholar]
- Oliver KR, Kane SA, Salvatore CA, Mallee JJ, Kinsey AM, Koblan KS et al (2001). Cloning, characterization and central nervous system distribution of receptor activity modifying proteins in the rat. Eur J Neurosci 14: 618–628. [DOI] [PubMed] [Google Scholar]
- Owji AA, Chabot JG, Dumont Y, Quirion R (2008). Adrenomedullin‐2/intermedin induces cAMP accumulation in dissociated rat spinal cord cells: evidence for the existence of a distinct class of sites of action. J Mol Neurosci 35: 355–361. [DOI] [PubMed] [Google Scholar]
- Poyner DR, Andrew DP, Brown D, Bose C, Hanley MR (1992). Pharmacological characterization of a receptor for calcitonin gene‐related peptide on rat, L6 myocytes. Br J Pharmacol 105: 441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyner DR, Sexton PM, Marshall I, Smith DM, Quirion R, Born W et al (2002). International Union of Pharmacology. XXXII. The mammalian calcitonin gene‐related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol Rev 54: 233–246. [DOI] [PubMed] [Google Scholar]
- Rist B, Entzeroth M, Beck‐Sickinger AG (1998). From micromolar to nanomolar affinity: a systematic approach to identify the binding site of CGRP at the calcitonin gene‐related peptide 1 receptor. J Med Chem 41: 117–123. [DOI] [PubMed] [Google Scholar]
- Robinson SD, Aitken JF, Bailey RJ, Poyner DR, Hay DL (2009). Novel peptide antagonists of adrenomedullin and calcitonin gene‐related peptide receptors: identification, pharmacological characterization, and interactions with position 74 in receptor activity‐modifying protein 1/3. J Pharmacol Exp Ther 331: 513–521. [DOI] [PubMed] [Google Scholar]
- Roh J, Chang CL, Bhalla A, Klein C, Hsu SY (2004). Intermedin is a calcitonin/calcitonin gene‐related peptide family peptide acting through the calcitonin receptor‐like receptor/receptor activity‐modifying protein receptor complexes. J Biol Chem 279: 7264–7274. [DOI] [PubMed] [Google Scholar]
- Russell FA, King R, Smillie SJ, Kodji X, Brain SD (2014). Calcitonin gene‐related peptide: physiology and pathophysiology. Physiol Rev 94: 1099–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore CA, Mallee JJ, Bell IM, Zartman CB, Williams TM, Koblan KS et al (2006). Identification and pharmacological characterization of domains involved in binding of CGRP receptor antagonists to the calcitonin‐like receptor. Biochemistry 45: 1881–1887. [DOI] [PubMed] [Google Scholar]
- Schonauer R, Els‐Heindl S, Fischer JP, Kobberling J, Riedl B, Beck‐Sickinger AG (2016). Adrenomedullin 2.0: adjusting key levers for metabolic stability. J Med Chem 59: 5695–5705. [DOI] [PubMed] [Google Scholar]
- Schutz B, Mauer D, Salmon AM, Changeux JP, Zimmer A (2004). Analysis of the cellular expression pattern of beta‐CGRP in alpha‐CGRP‐deficient mice. J Comp Neurol 476: 32–43. [DOI] [PubMed] [Google Scholar]
- Seck T, Baron R, Horne WC (2003). Binding of filamin to the C‐terminal tail of the calcitonin receptor control recycling. J Biol Chem 278: 10408–10416. [DOI] [PubMed] [Google Scholar]
- Sheykhzade M, Amandi N, Pla MV, Abdolalizadeh B, Sams A, Warfvinge K et al (2017). Binding and functional pharmacological characteristics of gepant‐type antagonists in rat brain and mesenteric arteries. Vascul Pharmacol 90: 36–43. [DOI] [PubMed] [Google Scholar]
- Shi L, Lehto SG, Zhu DX, Sun H, Zhang J, Smith BP et al (2016). Pharmacologic characterization of AMG 334, a potent and selective human monoclonal antibody against the calcitonin gene‐related peptide receptor. J Pharmacol Exp Ther 356: 223–231. [DOI] [PubMed] [Google Scholar]
- Sisnande T, Guerreiro LH, Braga RR, Jotha‐Mattos L, Erthal LC, Tinoco P et al (2015). Monoconjugation of human amylin with methylpolyethyleneglycol. PLoS One 10: e0138803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH et al (2016). The IUPHAR/BPS guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei Y, Inoue K, Ogoshi M, Kawahara T, Bannai H, Miyano S (2004). Identification of novel adrenomedullin in mammals: a potent cardiovascular and renal regulator. FEBS Lett 556: 53–58. [DOI] [PubMed] [Google Scholar]
- Takei Y, Hashimoto H, Inoue K, Osaki T, Yoshizawa‐Kumagaye K, Tsunemi M et al (2008). Central and peripheral cardiovascular actions of adrenomedullin 5, a novel member of the calcitonin gene‐related peptide family, in mammals. J Endocrinol 197: 391–400. [DOI] [PubMed] [Google Scholar]
- Takhshid MA, Poyner DR, Chabot JG, Fournier A, Ma W, Zheng WH et al (2006). Characterization and effects on cAMP accumulation of adrenomedullin and calcitonin gene‐related peptide (CGRP) receptors in dissociated rat spinal cord cell culture. Br J Pharmacol 148: 459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MM, Bagley SL, Samson WK (2006). Intermedin/Adrenomedullin‐2 inhibits growth hormone release from cultured, primary anterior pituitary cells. Endocrinology 147: 859–864. [DOI] [PubMed] [Google Scholar]
- Tilakaratne N, Christopoulos G, Zumpe ET, Foord SM, Sexton PM (2000). Amylin receptor phenotypes derived from human calcitonin receptor/RAMP coexpression exhibit pharmacological differences dependent on receptor isoform and host cell environment. J Pharmacol Exp Ther 294: 61–72. [PubMed] [Google Scholar]
- Tolcos M, Tikellis C, Rees S, Cooper M, Wookey P (2003). Ontogeny of calcitonin receptor mRNA and protein in the developing central nervous system of the rat. J Comp Neurol 456: 29–38. [DOI] [PubMed] [Google Scholar]
- Tomabechi Y, Krippner G, Rendle PM, Squire MA, Fairbanks AJ (2013). Glycosylation of pramlintide: synthetic glycopeptides that display in vitro and in vivo activities as amylin receptor agonists. Chemistry 19: 15084–15088. [DOI] [PubMed] [Google Scholar]
- Tora G, Degnan AP, Conway CM, Kostich WA, Davis CD, Pin SS et al (2013). Preparation of imidazoles as potent calcitonin gene‐related peptide (CGRP) antagonists. Bioorg Med Chem Lett 23: 5684–5688. [DOI] [PubMed] [Google Scholar]
- Tso AR, Goadsby PJ (2017). Anti‐CGRP monoclonal antibodies: the next era of migraine prevention? Curr Treat Options Neurol 19: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udawela M, Christopoulos G, Morfis M, Tilakaratne N, Christopoulos A, Sexton PM (2008). The effects of C‐terminal truncation of receptor activity modifying proteins on the induction of amylin receptor phenotype from human CTb receptors. Regul Pept 145: 65–71. [DOI] [PubMed] [Google Scholar]
- Walker CS, Conner AC, Poyner DR, Hay DL (2010). Regulation of signal transduction by calcitonin gene‐related peptide receptors. Trends Pharmacol Sci 31: 476–483. [DOI] [PubMed] [Google Scholar]
- Walker CS, Eftekhari S, Bower RL, Wilderman A, Insel PA, Edvinsson L et al (2015). A second trigeminal CGRP receptor: function and expression of the AMY1 receptor. Ann Clin Transl Neurol 2: 595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CS, Raddant AC, Woolley MJ, Russo AF, Hay DL (2017). CGRP receptor antagonist activity of olcegepant depends on the signalling pathway measured. Cephalalgia in press. 333102417691762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins HA, Rathbone DL, Barwell J, Hay DL, Poyner DR (2013). Structure‐activity relationships for alpha‐calcitonin gene‐related peptide. Br J Pharmacol 170: 1308–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins HA, Chakravarthy M, Abhayawardana RS, Gingell JJ, Garelja M, Pardamwar M et al (2016). Receptor activity‐modifying proteins 2 and 3 generate adrenomedullin receptor subtypes with distinct molecular properties. J Biol Chem 291: 11657–11675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells G, Chernoff J, Gilligan JP, Krause DS (2016). Does salmon calcitonin cause cancer? A review and meta‐analysis. Osteoporos Int 27: 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston C, Winfield I, Harris M, Hodgson R, Shah A, Dowell SJ et al (2016). Receptor activity‐modifying protein‐directed G protein signaling specificity for the calcitonin gene‐related peptide family of receptors. J Biol Chem 291: 21925–21944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wielinga PY, Alder B, Lutz TA (2007). The acute effect of amylin and salmon calcitonin on energy expenditure. Physiol Behav 91: 212–217. [DOI] [PubMed] [Google Scholar]
- Yarwood RE, Imlach WL, Lieu T, Veldhuis NA, Jensen DD, Klein Herenbrink C et al (2017). Endosomal signaling of the receptor for calcitonin gene‐related peptide mediates pain transmission. Proc Natl Acad Sci U S A. pii: 201706656. https://doi.org/10.1073/pnas.1706656114. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yule LR, Bower RL, Kaur H, Kowalczyk R, Hay DL, Brimble MA (2016). Synthesis and amylin receptor activity of glycomimetics of pramlintide using click chemistry. Org Biomol Chem 14: 5238–5245. [DOI] [PubMed] [Google Scholar]
- Zhang SY, Xu MJ, Wang X (2017). Adrenomedullin 2/intermedin: a putative drug candidate for treatment of cardiometabolic diseases. Br J Pharmacol In press https://doi.org/10.1111/bph.13814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann U, Fluehmann B, Born W, Fischer JA, Muff R (1997). Coexistence of novel amylin‐binding sites with calcitonin receptors in human breast carcinoma MCF‐7 cells. J Endocrinol 155: 423–431. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 Tables of ligand potency values to support figures.


