Abstract
Mast seeding, or masting, is the highly variable and spatially synchronous production of seeds by a population of plants. The production of variable seed crops is typically correlated with weather, so it is of considerable interest whether global climate change has altered the variability of masting or the size of masting events. We compiled 1086 datasets of plant seed production spanning 1900–2014 and from around the world, and then analysed whether the coefficient of variation (CV) in seed set, a measure of masting, increased over time. Over this 115-year period, seed set became more variable for plants as a whole and for the particularly well-studied taxa of conifers and oaks. The increase in CV corresponded with a decrease in the long-term mean of seed set of plant species. Seed set CV increased to a greater degree in plant taxa with a tendency towards masting. Seed set is becoming more variable among years, especially for plant taxa whose masting events are known to affect animal populations. Such subtle change in reproduction can have wide-ranging effects on ecosystems because seed crops provide critical resources for a wide range of taxa and have cascading effects throughout food webs.
Keywords: masting, mast seeding, predator satiation hypothesis, climate change, weather cue, resource budget hypothesis
1. Background
Mast seeding, also called masting or masting behaviour, is the variable and synchronous production of seed crops, and is found in many species of long-lived plants [1–3]. Inter-annual variation in seed crops from perennial plant populations is important because it drives the population dynamics of numerous seed-eaters, with cascading consequences throughout ecosystems including effects on predators, invasive herbivores and human pathogens [4,5]. Several adaptive hypotheses have been proposed to explain the evolution of masting, including the satiation of seed predators [6] and increased pollination efficiency [7]. At the same time, numerous mechanistic hypotheses have been supported in various plant species to explain how plant populations, separated by hundreds of kilometres, can produce large seed crops synchronously [3,8–10]. These mechanistic hypotheses typically rely on weather and internal plant signals or resources to cause variability in seed set and to synchronize seed sets among individuals [3,9,11]. Because patterns of seed set often show close association with weather patterns and resource use, there is considerable interest as to whether global change, such as climate change, nitrogen deposition and forest decline, will affect masting.
To date, studies have relied on extrapolation from conceptual models of masting and on inference from inter-annual variation in weather and resources to anticipate the effects of global climate change on masting [9,10,12–15]. Because of differences in how researchers expect weather and resources to drive patterns of masting [16,17], these studies have proposed dramatically different hypotheses about the effects of climate change on masting ranging from more frequent mast years [12,13], more intense masting events [13,14], less intense masting events [10], less frequent mast years [18] and no difference in masting patterns [9]. To some extent, the differences in these expectations may reflect actual differences among plant species in how they will respond to climate change. Some of the uncertainty, however, reflects a lack of mechanistic understanding about how climate and resources drive seed set [3], where resource dynamics, particularly of nutrient resources, have been implicated as drivers of masting in addition to weather [14,19,20]. Global distributions of resources are rapidly changing because of CO2 increases, nutrient deposition and land use change; models predict that any of these aspects of global change could alter patterns of masting. While different plant species clearly show individualistic responses to climate and other forms of global change [21], retrospective analyses have demonstrated clear trends in northward and upward range shifts [22], earlier-flowering phenologies [23] and altered species interactions [24] over the past century.
While studies have frequently considered masting a unique reproductive strategy, plants display continuous variation in the population-level coefficient of variation (CV) of their seed crops [25] (electronic supplementary material S1), and this by itself may be a key predictor of how plants may respond to a changing climate. Those plants with the most variable seed set often respond strongly to inter-annual differences in weather or resources [26], whereas plants with invariable seed sets presumably have mechanisms to reduce seed set variability. As such, plants with highly variable seed set may respond strongly to global change, whereas those with less variable seed set may be able to compensate for such effects.
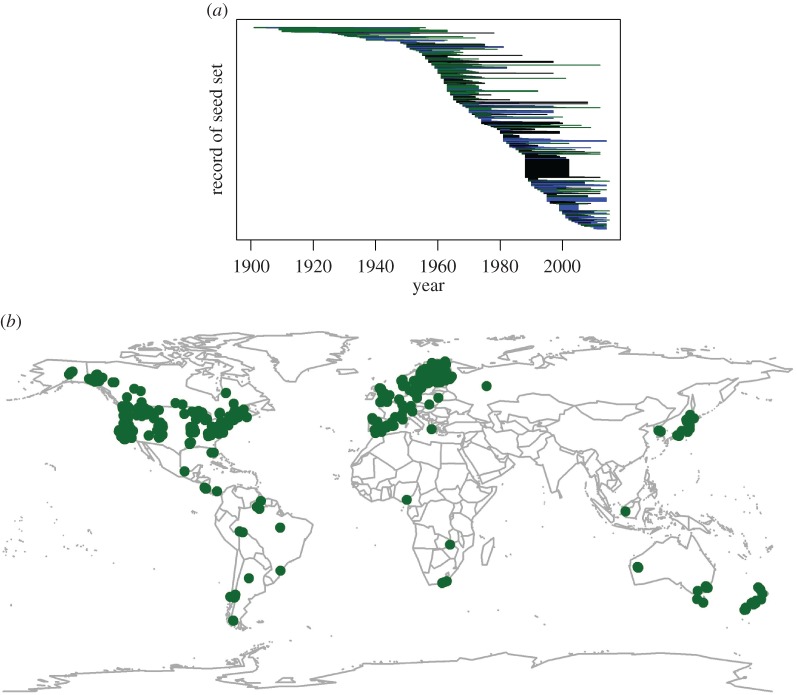
Masting is defined by high inter-annual variation in the seed set of individual plants (CVi) and high synchrony (S) between plants within a population. A high population-level coefficient of variation in seed set (CVp) can only be achieved with high CVi and S. Here, we analyse how masting patterns have changed during the past century in order to directly test whether global change has influenced masting behaviour. We compiled a comprehensive list of plant seed set records covering the time span of 1900–2014 whose durations ranged 4–56 years (figure 1a). This resulted in a dataset of 1086 quantitative records of seed set of 363 species of long-lived iteroparous plants. Plants in the dataset were located at sites from across the world, with particularly high representation from North America, Europe, East Asia and New Zealand (figure 1b).
Figure 1.
(a) Records of annual seed set (n = 1086) spanned the years 1900–2014. The longest continuous set of records from a given site was 56 years. Records in green are conifers (Pinaceae), blue are Fagaceae (primarily oaks and beeches) and black are all other records. (b) Sites with long-term records of seed set spanned the world with the highest densities of records in North America, Europe, East Asia and New Zealand. (Online version in colour.)
We ask whether CVp of seed set, or other measures of the distribution of seed set, have changed over time. We further ask whether changes in CVp are disproportionately caused by changes to CVp from plant species with greater average CVp (i.e. those with a tendency towards masting), or plants from parts of the globe that have experienced greater directional change in climate. We finally test whether changes in CVp of seed crops are accompanied by changes to the mean seed crops in plants. We use these trends to articulate and test two hypotheses, the ‘environmental stress hypothesis' and the ‘climate variability hypothesis', as possible causes of changes in CVp over time.
2. Material and methods
(a). Compilation of data
We employed four techniques to assemble records of inter-annual seed set with the goal of compiling a comprehensive list of seed/fruit set datasets. First, we searched the Web of Science using combinations of search terms such as ‘inter-annual vari*’, ‘seed set', ‘fruit set', ‘mast*' and ‘seed crop*'. Second, we scanned the references of each dataset to find additional records of seed set, and then searched references of every subsequent dataset found. Third, we contacted managers of forestry districts, wildlife surveys and regional seed surveys to ask directly for access to their information on inter-annual variation in seed set. Finally, we solicited seed set datasets from scientists contacted through the Ecolog listserv (https://listserv.umd.edu/archives/ecolog-l.html, posted 5 March 2014). Inclusion of a record of seed set in our database was contingent on several criteria that we outline in electronic supplementary material S1, resulting in a dataset of 1086 quantitative seed set records covering 363 plant species from 205 studies. Because units of seed set varied among studies, we standardized seed set for each time-series record to values between 0 and 100 based on the range of seed set values within that record [27].
Based on location data, we estimated nitrogen deposition [28] and change in surface temperature [29] for each record. We used interpolated global total nitrogen deposition estimates from [28]. These records were estimated for the early 1990s, representing twentieth-century anthropogenic nitrogen deposition. Records were coarse-grained (2.5°), so sites within a region were often given identical N deposition estimates. Preliminary analyses using specific forms of N deposition (NHx and NOx forms separately) yielded qualitatively identical results. We used global surface temperature change estimates, comparing a baseline period of 1900–1950 with a later period 2000–present from the Hansen [29] model. Global temperature change values were estimated at a grain size of 250 km. We used estimates of nitrogen deposition and global temperature change to test whether changes in CVp over time might be due to a single, global-scale driver of global change because these factors are known to have caused numerous changes to global ecosystems [30,31] and are closely tied to known drivers of variation in seed set [14,32].
(b). Statistical analysis
We divided each record of seed set into 20-year time periods corresponding to 1900–1919, 1920–1939, 1940–1959, 1960–1979, 1980–1999 and 2000–2014. A seed set record was included in a particular time period if it included at least four consecutive years of seed set data within that time period. For each record within each time period, we calculated the coefficient of variation (CVp) of seed set as the standard deviation/mean. In addition, we recorded the mean seed set for each time period, the skewness of the distribution of seed crops for each time period, the method of seed crop estimation and the number of individual plants or seed traps on which the record was based.
We constructed a linear mixed model with a Gaussian error distribution to test whether the CVp of seed set had changed between time periods. We used subject centring to account for differences in CVp among species and studies [33]. For this approach, we calculated CV(p)centred as CV(p)subject*time period—the average CV(p)subject. Plant species was considered the subject in all analyses except for one case in which time-series record was considered as the subject.
We included two linear fixed-effect covariates and three random-effect covariates in each model in addition to the effect of time period to account for potential biases among datasets. Mean seed set in a given time period was included as a numeric covariate. The length (number of years) of a seed set dataset within a given time period was included to account for differences in the calculation of CVp for records of varying length. We included seed set collection method (direct counts, seed traps, harvest records, photographs, fruit scars and timed counts) as a random effect on the intercept to account for potential differences in CVp among datasets. Plant species and dataset were also included as random effects on the intercept to account for non-independence of observations within these categories. We weighted observations proportionally to the square root of the number of individual plants or traps observed for a given record, such that records including larger samples were weighted higher.
In all mixed-model analyses, we tested the effect of time period on log-transformed CVp of seed set because a Shapiro–Wilk test indicated greater normality of log-transformed values (W = 0.99) than untransformed CVp (W = 0.95). We constructed a null model that was identical to the full model, but lacked time period as a predictor variable. The full and null models were compared using a likelihood ratio test. Because time period is an ordered rather than a continuous predictor variable, we conducted initial tests using time period as an ordered categorical variable. These tests demonstrated that CVp of seed set varied significantly between time periods (χ2 = 53.2, d.f. = 5, p < 0.001). We then specified time period as an ordered numerical predictor to test whether CVp of seed set had changed linearly over time. We focused our analysis on two subsets of the full dataset to see if trends in CVp were consistent within well-sampled groups of species. For these analyses, linear mixed models were constructed as above; however, the dataset was limited to plants in the family Fagaceae or the family Pinaceae. Lastly, we tested whether the skewness (distribution asymmetry) of seed set distribution, as opposed to CVp, had increased over time. Models of skew were conducted as described above.
To test whether our method of binning seed set records into time periods affected the observed trends in CVp of seed set, we changed the methods for binning data by using time periods of 15 years, by offsetting time periods by 10 years (i.e. binning by 20 years, but starting in 1910 as opposed to 1900) and by including a seed set record in a time period only if it included eight or more years of data. Using these permutations of our binning approach, we constructed identical mixed models as described above, and each resulted in similar trends towards an increasing CVp of seed set as with the above-described binning method (electronic supplementary material S2).
In addition, we used a simulation approach to estimate the likelihood that observed change in CVp over time was greater than expected by chance. In this approach, we randomized the order of time bins 1000 times and ran identical mixed-effects models as described above to create a distribution of slopes of the relationship between (randomized) time and CVp. We calculated the slope of CVp over time for a set of 1000 models with randomly ordered time bins using the same approach. We then compared the observed slope to the distribution of slopes from the randomized models.
We calculated the change in CVp over time for the 78 plant species with the longest records of seed set (greater than 23 years). This discarded records from all plant species whose records did not span two or more time periods. We created a linear model for each plant species in which CVp of seed set was predicted by the time period as a numeric predictor and the mean seed set within that time period. We used the model's coefficient for time period as an estimate of the change in CVp of seed set over time, and then tested for patterns among plant species that explained the change in CVp of seed set over time using a phylogenetically informed generalized least-squares (pGLS) analysis. We assembled a phylogeny of plant taxa with seed set records using phylomatic [34], and trimmed a recent large, time-calibrated plant phylogeny [35] to the taxa represented in our dataset. We specified a Brownian motion model of trait evolution as the expected covariance in the pGLS analysis and used multiple pGLS regression to test whether change in CVp over time was related to (i) the mean CVp of seed set over the entire period of observation, (ii) the mean latitude of seed set records for that plant species, (iii) estimates of nitrogen deposition over the past century at the location of a record, and (iv) estimates of change in surface temperature over the past 60 years.
To assess changes in the frequency of mast years, we used the standard deviate method [36] to categorize mast years (years of extremely high seed set) within each of the 1086 data series. We then split the data into 20-year time periods as described above. We constructed a generalized linear mixed model with a binomial error distribution in which the frequency of mast years within a given time period was predicted by time period and included random effects of plant species and observation (seed set record by time period). Models that were weighted by the number of individuals failed to converge, but the trend in the non-converged model was qualitatively identical to models that were not weighted by the number of individuals. Therefore, we present unweighted analysis for the frequency of mast years.
All statistical analyses were conducted in R using lmer and glmer functions in the lme4 package, gls function in the nlme package and the ape package for handling phylogenetic information [37–40].
3. Results
(a). Is variation in seed set (CVp) changing over time?
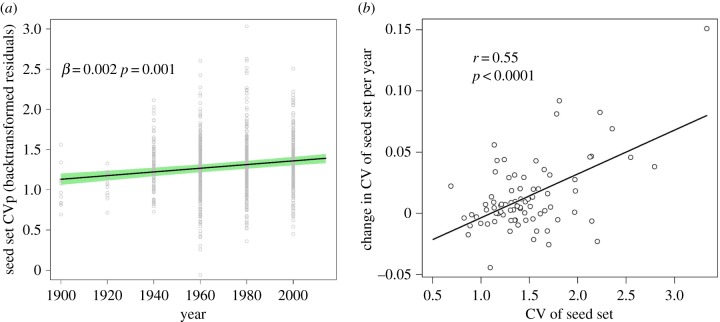
CVp of seed set increased over time (χ2 = 24.9, d.f. = 1, p < 0.001; figure 2a). We found a similar increase in CVp when we limited the dataset to the two most sampled plant families (Fagaceae and Pinaceae), as sampling within these groups has been relatively consistent between time periods (χ2 = 14.2, d.f. = 1, p = 0.001). Likewise, within each of these families, we observed an increase in CVp over time (Fagaceae: χ2 = 9.1, d.f. = 1, p = 0.002; Pinaceae: χ2 = 4.5, d.f. = 1, p = 0.035). The frequency of exceptional mast years did not increase over the past century when considering all species (χ2 = 0.1, d.f. = 1, p = 0.81; electronic supplementary material S4) or only those with high seed set CVp (upper quartile of seed set CVp; χ2 = 0.4, d.f. = 1, p = 0.53), suggesting that the increase in CVp was due to a paucity of average seed set years and not due to a change in the frequency of extreme seed set events. As direct evidence for this, skewness increased with time (χ2 = 9.6, d.f. = 1, p = 0.002), indicating that the distribution of seed set records tended to have longer right-hand tails in later years.
Figure 2.
(a) The coefficient of variation (CVp) of seed set between years increased over time. Trend line is from a subject-centred linear mixed-effects model. Error around the trend line was assessed by bootstrap estimation of model error and only reflects error in estimates of fixed effects. Points are back-transformed residual values of CVp for each species–time period from a model not informed by time period. Points were plotted at the beginning of each time period. The model accounted for species-level differences in seed set and the potentially confounding factor of mean seed set within a time period. (b) The increase in CVp of seed set over time was greater for plant species (dots) that displayed a high CVp throughout the entire sampling period. (Online version in colour.)
Because no single study covered the entire 115-year time span, differences among studies could inflate or mask changes in CVp over time. We tested this by centring data around individual studies, which made all differences in CVp over time attributable to directional changes in CVp over the course of individual studies [35]. The results still exhibited a clear pattern of increase in CVp over time (χ2 = 6.63, d.f. = 1, p = 0.010) even when differences among studies were removed. Finally, analyses indicated that the results were insensitive to the length of the period over which CVp was calculated, its start date or the minimum number of years for a record to be included (electronic supplementary material S3). In addition, we found no indication that methodological changes in assessing seed set affect our estimate of CVp change over time (electronic supplementary material S1). Likewise, the exclusion of the earliest (1900–1919) and latest (2000–2014) time periods from the data did not change the trend towards increasing CVP over time (electronic supplementary material S1). A simulation approach in which we randomized the order of time bins to create a null distribution of slopes and model fits, suggested that CVp had increased to a greater degree over time than expected by chance (electronic supplementary material S4).
(b). Which plant species show the greatest change in seed set variation (CVp) over time?
There was considerable variation among plant species in how CVp of seed set changed over time. Using the 79 plant species with the longest seed set records, we calculated the decadal increase in CVp of seed set for each species. We found the plant species that had the most variable seed set became more variable over time than plant species that had relatively consistent seed sets between years (pGLS, F1,74 = 35.5, p < 0.0001; figure 2b and table 1). We found the same relationship when change in CVp was represented as percentage increase in CV (pGLS, F1,74 = 18.8, p < 0.0001). We found no support for the hypothesis that these differences were driven geographically; that is, species in more polar habitats were not becoming more variable over time compared to species at lower latitudes (table 1). Likewise, an increase in CVp was not associated with plant species in regions that have experienced greater temperature increases over the past 60 years (table 1), nor was an increase in CVp associated with regions that had experienced greater nitrogen deposition (table 1).
Table 1.
Predictors of increase in CVp among the 78 plant species with at least 23 years of records of CVp. Comparative statistical tests took into account plant phylogenetic relationships using a pGLS framework.
| predictor | standardized β | F | p |
|---|---|---|---|
| log(mean CV) | 0.013 | 35.5 | <0.0001 |
| latitude | 0.000 | 0.0 | 0.98 |
| temperature increase | 0.001 | 0.2 | 0.65 |
| nitrogen deposition | −0.001 | 0.2 | 0.70 |
4. Discussion
There are many reasons why inter-annual variation in seed set of perennial plants may be increasing, particularly for plant species with a tendency towards masting behaviour. We carefully explored the evidence and found a directional increase in CVp over time. Our data suggest that the mechanisms driving this trend are neither a product of any obvious single factor of global change, nor are they predicted by a plant's geographical distribution.
The lack of a simple explanation for increasing CVp is perhaps not surprising. Past studies that have looked for patterns in aspects of the abiotic environment that influence mast years have yielded clear patterns in some cases, such as with the plants of New Zealand [9], but not in other cases, such as the genus Quercus [41]. The lack of a simple, single-factor explanation for an increase in CVp suggests that multiple factors may be driving the observed increase in CVp. As an analogy, multiple factors (including a changing climate, altered management practices, and introduced herbivores and diseases) have profoundly shaped forests worldwide, and this multiplicity of factors has had clear directional impacts leading to declines in forest health at a global scale [42–44].
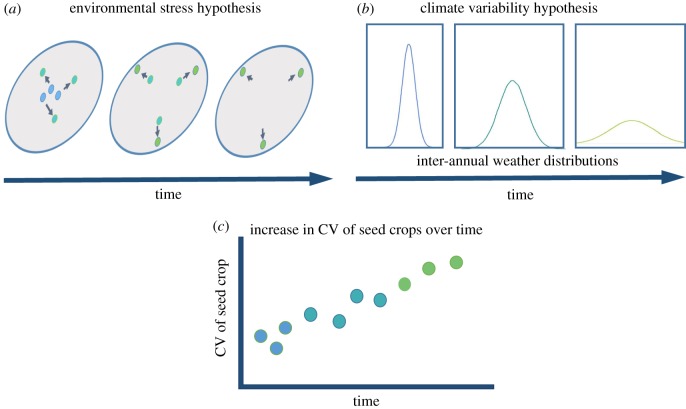
We propose two hypotheses that could explain an increase in CVp of seed set over time (figure 3). First, several studies that have tracked the variation of seed set over resource gradients have found that plants that grow in more stressful, resource-limited environments tend to have greater CV of seed set [14,15,19,45] (figure 3a). A hallmark of the environmental stress hypothesis is that more resource-limited plants also tend to produce a smaller long-term mean seed set. For example, more water-limited oaks produced smaller long-term seed crops that were more variable from year to year [45,46], and temperate tree individuals that produced lower average seed crops also tended to have greater year-to-year variability [47]. Our analysis here lends support to this hypothesis because as CVp has increased, mean seed crops have decreased. Additional support for this hypothesis could come from analysing individual-level CV of seed crops (CVi) and synchrony (S). The resource limitation hypothesis would suggest that CVi should increase over time, while S should not change. Researchers have documented multivariate changes to forests globally including changes to abiotic conditions, forest practices and biotic stressors that each contribute to greater environmental stress [42–44]. An increasing CVp of seed set may be a symptom of these changes.
Figure 3.
Proposed reasons for increased variability in seed set (CVp), illustrated in (c). (a) Changes in the multivariate environment that defines resource niche of the plant shift from a condition that is not stressful to the plant (blue dots) to a condition that is more stressful to the plant (green dots) over time. Resources that define that niche will vary by plant species and location; however, large perturbations to the environment are likely to shift a plant away from favourable conditions. Plants in stressful resource environments (defined variously as nutrient and water availability) have been shown to produce more variable seed crops (i.e. greater CVi) that translate into greater CVp. Plants in a stressful environment are expected to have a lower long-term mean seed crop. (b) Greater climatic variability directly translates into seed crop variability when seed crops directly track weather. There is evidence for increasing variability in various aspects of climate. Resource matching, a proposed direct connection between environment and seed set, has been shown to be insufficient to explain seed set variability in plants where this has been tested. More frequent climatic ‘vetoes' of seed crops may or may not affect CVi or long-term mean seed crop of an individual, but probably increase synchrony (S) of seed sets across individuals resulting in higher CVp. (Online version in colour.)
Alternatively, increasing CVp may reflect greater variability in weather conditions to the extent that seed set tracks weather (figure 3b). Consistent with the climate variability hypothesis, recent studies have found increasing variability in inter-annual weather, including a trend towards greater large-magnitude climatic events, and greater spatial synchrony in weather patterns [48–50]. Support for the climate variability hypothesis would come from a correlation between increasing CV of weather and increasing CVp of seed set. Additionally, studies that determine the mechanistic link between weather and seed set variation will be important in testing this hypothesis [3].
A clear trend in our analysis was that increases in CVp are more pronounced in species that have a tendency towards masting. This observation is consistent with both the environmental stress hypothesis and the environmental variability hypothesis because the CVp of seed set of masting species is thought to be more responsive to resource status and weather than plant species that exhibit little variation in seed set. More strongly masting species are thought to actively use weather events and internal resource dynamics to achieve the benefits of high CVp, whereas other species may benefit from low CVp if they are animal-pollinated or dispersed [2,51]. Likewise, we showed that changes in CVp are not caused by changing frequency of mast events, as some mechanistic models of masting have predicted [12,13], but rather by a further skewing of the distribution of seed sets of plants away from a normal distribution.
Increased variability in seed set is likely to have a significant effect on animal populations, which often have difficulty tracking highly variable resources [6]. Current predictions about the consequences of more variation in resources include lower herbivore populations in ecosystems such as boreal forests that are dominated by masting trees [52], increased omnivory of animals (such as increased garbage pilfering by bears in areas where acorns or piñon are primary bear resources [53]) and restriction of animal species to regions with food resources other than mast crops [54]. At the same time, because high inter-annual variation in seed set is typically thought to be adaptive due to economies of scale in seed production [2], plants could possibly benefit from higher inter-annual variation in seed set.
High inter-annual variation in seed set has shaped current food webs, and increasing variation in seed set will probably have profound consequences for plants, their herbivores and even more distantly connected taxa. We found evidence that seed set variation is increasing, especially in plant taxa that already have a tendency towards high variation, and we posit two hypotheses to explain the observed directional change in seed set variability. Our study is key to the ongoing debate about how global change will affect masting, because we demonstrate a clear historical directional change in CVp. The direction of the change towards higher CVp lends support to models of masting that can predict such a change [14,15,45], and suggests caution in extrapolating from models that predict no change or a decrease in CVp [9,10,12].
Supplementary Material
Acknowledgements
We thank Michael Lordon for help in compiling datasets as well as the many researchers who helped us compile their work on seed set into a single database.
Data accessibility
Seed set data and accompanying metadata are available at ScienceBase (doi:10.5066/F7HD7TV6). A bibliography of seed set records is provided in electronic supplementary material S5.
Authors' contributions
I.S.P. conceived of the study, compiled datasets, ran initial statistical analyses and wrote the manuscript. J.M.L. compiled datasets, refined hypothesis, ran mast year frequency statistics and edited the manuscript. W.D.K. compiled datasets, helped with statistics and edited the manuscript.
Competing interests
We have no competing interests. Any use of trade, firm or product names is for descriptive purposes only and does not imply endorsement by the US Government.
Funding
W.D.K. was supported by NSF grant DEB-1256394.
References
- 1.Kelly D. 1994. The evolutionary ecology of mast seeding. Trends Ecol. Evol. 9, 465–470. ( 10.1016/0169-5347(94)90310-7) [DOI] [PubMed] [Google Scholar]
- 2.Kelly D, Sork VL. 2002. Mast seeding in perennial plants: why, how, where? Annu. Rev. Ecol. Syst. 33, 427–447. ( 10.1146/annurev.ecolsys.33.020602.095433) [DOI] [Google Scholar]
- 3.Pearse IS, Koenig WD, Kelly D. 2016. Mechanisms of mast seeding: resources, weather, cues, and selection. New Phytol. 212, 546–562. ( 10.1111/nph.14114) [DOI] [PubMed] [Google Scholar]
- 4.Jones CG, Ostfeld RS, Richard MP, Schauber EM, Wolff JO. 1998. Chain reactions linking acorns to gypsy moth outbreaks and Lyme disease risk. Science 279, 1023–1026. ( 10.1126/science.279.5353.1023) [DOI] [PubMed] [Google Scholar]
- 5.Bogdziewicz M, Zwolak R, Crone EE. 2015. How do vertebrates respond to mast seeding? Oikos 125, 300–307. ( 10.1111/oik.03012) [DOI] [Google Scholar]
- 6.Janzen DH. 1971. Seed predation by animals. Annu. Rev. Ecol. Syst. 2, 465–492. ( 10.1146/annurev.es.02.110171.002341) [DOI] [Google Scholar]
- 7.Norton DA, Kelly D. 1988. Mast seeding over 33 years by Dacrydium cupressinum Lamb. (rimu) (Podocarpaceae) in New Zealand: the importance of economies of scale. Funct. Ecol. 2, 399–408. ( 10.2307/2389413) [DOI] [Google Scholar]
- 8.Satake A, Iwasa Y. 2000. Pollen coupling of forest trees: forming synchronized and periodic reproduction out of chaos. J. Theor. Biol. 203, 63–84. ( 10.1006/jtbi.1999.1066) [DOI] [PubMed] [Google Scholar]
- 9.Kelly D, et al. 2013. Of mast and mean: differential-temperature cue makes mast seeding insensitive to climate change. Ecol. Lett. 16, 90–98. ( 10.1111/ele.12020) [DOI] [PubMed] [Google Scholar]
- 10.Koenig WD, Knops JMH, Carmen WJ, Pearse IS. 2015. What drives masting? The phenological synchrony hypothesis. Ecology 96, 184–192. ( 10.1890/14-0819.1.sm) [DOI] [PubMed] [Google Scholar]
- 11.Isagi Y, Sugimura K, Sumida A, Ito H. 1997. How does masting happen and synchronize? J. Theor. Biol. 187, 231–239. ( 10.1006/jtbi.1997.0442) [DOI] [Google Scholar]
- 12.McKone MJ, Kelly D, Lee WG. 1998. Effect of climate change on mast-seeding species: frequency of mass flowering and escape from specialist insect seed predators. Glob. Change Biol. 4, 591–596. ( 10.1046/j.1365-2486.1998.00172.x) [DOI] [Google Scholar]
- 13.Schauber EM, et al. 2002. Masting by eighteen New Zealand plant species: the role of temperature as a synchronizing cue. Ecology 83, 1214–1225. ( 10.2307/3071937) [DOI] [Google Scholar]
- 14.Smaill SJ, Clinton PW, Allen RB, Davis MR. 2011. Climate cues and resources interact to determine seed production by a masting species. J. Ecol. 99, 870–877. ( 10.1111/j.1365-2745.2011.01803.x) [DOI] [Google Scholar]
- 15.Monks A, Monks JM, Tanentzap AJ. 2016. Resource limitation underlying multiple masting models makes mast seeding sensitive to future climate change. New Phytol. 210, 419–430. ( 10.1111/nph.13817) [DOI] [PubMed] [Google Scholar]
- 16.Pearse IS, Koenig WD, Knops JMH. 2014. Cues versus proximate drivers: testing the mechanism behind masting behavior. Oikos 123, 179–184. ( 10.1111/j.1600-0706.2013.00608.x) [DOI] [Google Scholar]
- 17.Holland EP, James A. 2015. Assessing the efficacy of population-level models of mast seeding. Theor. Ecol. 8, 121–132. ( 10.1007/s12080-014-0238-4) [DOI] [Google Scholar]
- 18.Iler AM, Inouye DW. 2013. Effects of climate change on mast-flowering cues in a clonal montane herb, Veratrum tenuipetalum (Melanthiaceae). Am. J. Bot. 100, 519–525. ( 10.3732/ajb.1200491) [DOI] [PubMed] [Google Scholar]
- 19.Tanentzap AJ, Lee WG, Coomes DA. 2012. Soil nutrient supply modulates temperature-induction cues in mast-seeding grasses. Ecology 93, 462–469. ( 10.1890/11-1750.1) [DOI] [PubMed] [Google Scholar]
- 20.Miyazaki Y, et al. 2014. Nitrogen as a key regulator of flowering in Fagus crenata: understanding the physiological mechanism of masting by gene expression analysis. Ecol. Lett. 17, 1299–1309. ( 10.1111/ele.12338) [DOI] [PubMed] [Google Scholar]
- 21.Williams JW, Shuman BN, Webb T, Bartlein PJ, Leduc PL. 2004. Late-quaternary vegetation dynamics in North America: scaling from taxa to biomes. Ecol. Monogr. 74, 309–334. ( 10.1890/02-4045) [DOI] [Google Scholar]
- 22.Parmesan C, Yohe G. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42. ( 10.1038/nature01286) [DOI] [PubMed] [Google Scholar]
- 23.Wolkovich EM, et al. 2012. Warming experiments underpredict plant phenological responses to climate change. Nature 485, 494–497. ( 10.1038/nature11014) [DOI] [PubMed] [Google Scholar]
- 24.Burkle LA, Marlin JC, Knight TM. 2013. Plant-pollinator interactions over 120 years: loss of species, co-occurrence, and function. Science 339, 1611–1615. ( 10.1126/science.1232728) [DOI] [PubMed] [Google Scholar]
- 25.Herrera CM, Jordano P, Guitián J, Traveset A. 1998. Annual variability in seed production by woody plants and the masting concept: reassessment of principles and relationship to pollination and seed dispersal. Am. Nat. 152, 576–594. ( 10.1086/286191) [DOI] [PubMed] [Google Scholar]
- 26.Kelly D, Turnbull MH, Pharis RP, Sarfati MS. 2008. Mast seeding, predator satiation, and temperature cues in Chionochloa (Poaceae). Popul. Ecol. 50, 343–355. ( 10.1007/s10144-008-0109-1) [DOI] [Google Scholar]
- 27.Koenig WD, Knops JMH. 2000. Patterns of annual seed production by northern hemisphere trees: a global perspective. Am. Nat. 155, 59–69. ( 10.1086/303302) [DOI] [PubMed] [Google Scholar]
- 28.Dentener F.2006. Global maps of atmospheric nitrogen deposition, 1860, 1993, and 2050. See http://daac.ornl.gov/CLIMATE/guides/global_N_deposition_maps.html .
- 29.Hansen J, Ruedy R, Sato M, Lo K. 2010. Global surface temperature change. Rev. Geophys. 48, 1–29. ( 10.1029/2010RG000345) [DOI] [Google Scholar]
- 30.Luo Y, et al. 2008. Modeled interactive effects of precipitation, temperature, and [CO2] on ecosystem carbon and water dynamics in different climatic zones. Glob. Change Biol. 14, 1986–1999. ( 10.1111/j.1365-2486.2008.01629.x) [DOI] [Google Scholar]
- 31.Stevens CJ, Dise NB, Mountford JO, Gowing DJ. 2004. Impact of nitrogen deposition on the species richness of grasslands. Science 303, 1876–1879. ( 10.1126/science.1094678) [DOI] [PubMed] [Google Scholar]
- 32.Koenig WD, Knops JMH, Carmen WJ, Stanback MT, Mumme RL. 1996. Acorn production by oaks in central coastal California: influence of weather at three levels. Can. J. For. Res. 26, 1677–1683. ( 10.1139/x26-189) [DOI] [Google Scholar]
- 33.Van de Pol M, Wright J. 2009. A simple method for distinguishing within versus between-subject effects using mixed models. Anim. Behav. 77, 753–758. ( 10.1016/j.anbehav.2008.11.006) [DOI] [Google Scholar]
- 34.Webb CO, Donoghue MJ. 2005. Phylomatic: tree assembly for applied phylogenetics. Mol. Ecol. Resour. 5, 181–183. ( 10.1111/j.1471-8286.2004.00829.x) [DOI] [Google Scholar]
- 35.Zanne AE, et al. 2014. Three keys to the radiation of angiosperms into freezing environments. Nature 506, 89–92. ( 10.1038/nature12872) [DOI] [PubMed] [Google Scholar]
- 36.LaMontagne JM, Boutin S. 2009. Quantitative methods for defining mast-seeding years across species and studies. J. Veg. Sci. 20, 745–753. ( 10.1111/j.1654-1103.2009.01068.x) [DOI] [Google Scholar]
- 37.R Core Team. 2013. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.R-project.org/. [Google Scholar]
- 38.Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 39.Pinheiro J, Bates D, DebRoy S, Sarkar D, The R Core Development Team 2009. nlme: linear and nonlinear mixed effects models. R package version 3.1-93. See https://cran.r-project.org/web/packages/nlme. [Google Scholar]
- 40.Paradis E, Claude J, Strimmer K. 2004. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290. ( 10.1093/bioinformatics/btg412) [DOI] [PubMed] [Google Scholar]
- 41.Koenig WD, et al. 2016. Is the relationship between mast-seeding and weather in oaks related to their life-history or phylogeny? Ecology 97, 2603–2615. ( 10.1002/ecy.1490) [DOI] [PubMed] [Google Scholar]
- 42.Choat B, et al. 2012. Global convergence in the vulnerability of forests to drought. Nature 491, 752–755. ( 10.1038/nature11688) [DOI] [PubMed] [Google Scholar]
- 43.Cohen WB, Yang Z, Stehman SV, Schroeder TA, Bell DM, Masek JG, Huang C, Meigs GW. 2016. Forest disturbance across the conterminous United States from 1985–2012: the emerging dominance of forest decline. For. Ecol. Manag. 360, 242–252. ( 10.1016/j.foreco.2015.10.042) [DOI] [Google Scholar]
- 44.Schulze E-D, Lange OL, Oren R, Asche R. 2012. Forest decline and air pollution: a study of spruce (Picea Abies) on acid soils. Berlin, Germany: Springer Science & Business Media. [Google Scholar]
- 45.Pesendorfer MB, Koenig WD, Pearse IS, Knops JMH, Funk KA. 2016. Individual resource-limitation combined with population-wide pollen availability drives masting in the valley oak (Quercus lobata). J. Ecol. 104, 637–645. ( 10.1111/1365-2745.12554) [DOI] [Google Scholar]
- 46.Barringer BC, Koenig WD, Knops JMH. 2013. Interrelationships among life-history traits in three California oaks. Oecologia 171, 129–139. ( 10.1007/s00442-012-2386-9) [DOI] [PubMed] [Google Scholar]
- 47.Minor DM, Kobe RK. 2017. Masting synchrony in northern hardwood forests: super-producers govern population fruit production. J. Ecol. 105, 987–998. ( 10.1111/1365-2745.12729) [DOI] [Google Scholar]
- 48.Coumou D, Rahmstorf S. 2012. A decade of weather extremes. Nat. Clim. Change 2, 491–496. [Google Scholar]
- 49.Koenig WD, Liebhold AM. 2016. Temporally increasing spatial synchrony of North American temperature and bird populations. Nat. Clim. Change 6, 614–617. ( 10.1038/nclimate2933) [DOI] [Google Scholar]
- 50.Schär C, Vidale PL, Lüthi D, Frei C, Häberli C, Liniger MA, Appenzeller C. 2004. The role of increasing temperature variability in European summer heatwaves. Nature 427, 332–336. ( 10.1038/nature02300) [DOI] [PubMed] [Google Scholar]
- 51.Wang Y, Zhang J, LaMontagne JM, Lin F, Li B, Ye J, Yuan Z, Wang X, Hao Z. 2017. Variation and synchrony of tree species mast seeding in an old-growth temperate forest. J. Veg. Sci. 28, 413–423. ( 10.1111/jvs.12494) [DOI] [Google Scholar]
- 52.Bergeron P, Réale D, Humphries MM, Garant D. 2011. Anticipation and tracking of pulsed resources drive population dynamics in eastern chipmunks. Ecology 92, 2027–2034. ( 10.1890/11-0766.1) [DOI] [PubMed] [Google Scholar]
- 53.Lewis DL, Breck SW, Wilson KR, Webb CT. 2014. Modeling black bear population dynamics in a human-dominated stochastic environment. Ecol. Model. 294, 51–58. ( 10.1016/j.ecolmodel.2014.08.021) [DOI] [Google Scholar]
- 54.Koenig WD, Haydock J. 1999. Oaks, acorns, and the geographical ecology of acorn woodpeckers. J. Biogeogr. 26, 159–165. ( 10.1046/j.1365-2699.1999.00256.x) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Seed set data and accompanying metadata are available at ScienceBase (doi:10.5066/F7HD7TV6). A bibliography of seed set records is provided in electronic supplementary material S5.