Abstract
Humans commonly harvest animals based on their expression of secondary sexual traits such as horns or antlers. This selective harvest is thought to have little effect on harvested populations because offtake rates are low and usually only the males are targeted. These arguments do not, however, take the relationship between secondary sexual trait expression and animal condition into account: there is increasing evidence that in many cases the degree of expression of such traits is correlated with an animal's overall well-being, which is partly determined by their genetic match to the environment. Using an individual-based model, we find that when there is directional environmental change, selective harvest of males with the largest secondary sexual traits can lead to extinction in otherwise resilient populations. When harvest is not selective, the males best suited to a new environment gain the majority of matings and beneficial alleles spread rapidly. When these best-adapted males are removed, however, their beneficial alleles are lost, leading to extinction. Given the current changes happening globally, these results suggest that trophy hunting and other cases of selective harvest (such as certain types of insect collection) should be managed with extreme care whenever populations are faced with changing conditions.
Keywords: sexual selection, trophy hunting, climate change, directional selection, insect collecting
1. Background
Humans harvest animal populations for many reasons. While many animal populations are harvested chiefly for food, others are targeted for recreational purposes, and in these latter cases the main targets for harvest are often males with large sexual ornaments. Some insect collectors, for example, will target particularly large and well-ornamented specimens of insects such as rhinoceros and stag beetles, and good specimens of some of these can sell for large sums of money (figure 1). Similarly, trophy hunters often specifically target male animals with exceptionally large antlers, horns, manes or other secondary sexual traits. A considerable amount of effort is sometimes expended by trophy hunters to take the largest and best-ornamented males in a population, and Safari Club International, the main representative body for such hunters, awards prizes every year on the basis of measurements of these traits. Well-ornamented males are prized in some non-Western societies as well. As an example, the Huli ‘Wigmen’ of Papua New Guinea use the plumes, and sometimes entire specimens, of male birds of paradise in the construction of their elaborate headwear [1]. Finally, illegal poaching of animals such as elephants for the ivory trade also targets animals with the greatest expression of secondary sexual traits [2].
Figure 1.
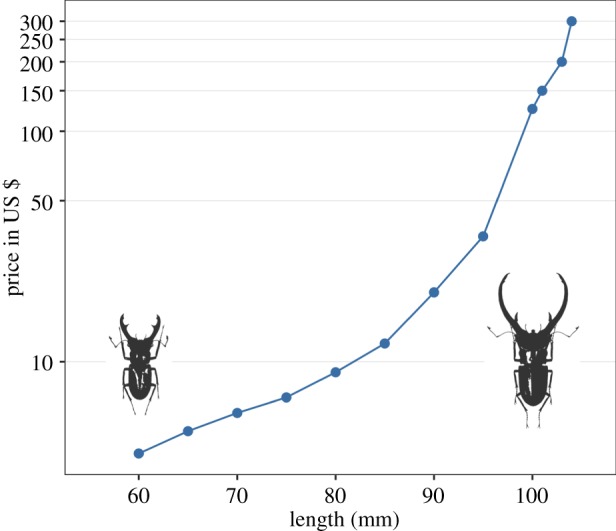
Insect collectors place a strong premium on males with extreme expression of secondary sexual traits. The figure shows prices quoted for specimens of the male stag beetles Cyclommatus elaphus plotted against the total length of the specimen. The pictured males are specimens 100 mm and 60 mm long and show that the very expensive large males are mostly differentiated from the cheap medium-sized males by the length of their mandibles. Note the log scale on the y-axis, and also that neither females nor males less than 60 mm long were even offered for sale—some male C. elaphus can be as small as 30 mm in length. Data taken from the website of an insect dealer specializing in Indonesian insects (http://www.giradis-insect.com/) on 3 April 2017. (Online version in colour.)
The impacts of selective harvest have largely been studied with regard to trophy hunting. Hunting generally is a considerable cause of mortality for many populations [3], and uncontrolled hunting has caused some well-known extinctions such as the quagga (Equus quagga) [4]. Trophy hunting of well-managed populations, however, is often thought to be unlikely to have serious consequences for the long-term population stability of the harvested animals, for two reasons. First, well-managed trophy hunting often involves low offtakes from large populations [4]. Second, in many cases the animals targeted are males, and in the polygynous systems typical of the hunted animals, females will not have difficulty acquiring mates unless a large proportion of the males are removed [5]. Consequently, recruitment into the population should be unaffected by hunting, meaning that in the absence of other threats populations of animals that are primarily harvested for trophies or specimens should not be at risk of extinction [6,7]. Instead, more subtle effects from trophy hunting are found, such as changes in the sociobiological makeup of populations arising from the removal of males, as seen in hunted lion populations [8], and evolutionary changes arising from selection against desirable traits for hunters such as large horns or large body size [9–12], although the magnitude of this latter effect is the subject of some debate [13–15].
This assumption that selective harvest is not especially threatening, however, does not take into account recent work which has found that sexual selection itself seems to have the capacity to have important effects on evolutionary processes such as adaptation to changing environments [16–19]. Traits used in sexual signalling and contests between males, including those which are often specifically targeted by humans such as the horns and antlers of bovids and cervids, are known often to show ‘condition dependence’, whereby the degree of expression of the trait is strongly affected by the overall health and well-being (the ‘condition’) of the bearer [20–23]. This condition dependence means that trait expression and therefore mating success is associated with the genetic quality of the male in question—males carrying a heavy load of deleterious alleles will not be in good condition and will not be able to express their signal traits well, whereas those with a genetic makeup that makes them particularly able to acquire resources and to develop will have particularly large, loud or colourful secondary sexual traits, and will acquire a disproportionate number of matings [24,25]. A series of studies over the last decade and a half have argued that as a consequence of this strong reproductive skew towards the fittest males, populations of strongly sexually selected animals should clear deleterious mutations faster and adapt to changing environments more quickly than populations where mating is less selective [16,19]. This benefit from sexual selection is consistently found in laboratory experiments which have shown that strong sexual selection leads to faster adaptation to novel foods [26] and to pesticides [27], to a reduced extinction risk from thermal stress [17] and to a reduction in inbreeding depression leading to improved persistence of small populations [18,28]. Field data, by contrast, mostly find that strong sexual selection is either neutral or associated with higher extinction rates, but a recent modelling study which forms the basis of the present paper appears to resolve this—we found that when populations are very small, as is the case for many of the field populations that have been studied [29], the cost of growing and bearing secondary sexual ornaments appears to increase the risk of extinction arising from demographic stochasticity, but when populations are larger the increased adaptation rate more than compensates for this risk [19].
Given this benefit to mean population fitness arising from sexual selection, especially when the populations in question are under environmental stress, it is possible that selective harvest of well-ornamented males could have a much larger effect than might be expected from simple demographic considerations. If the environment is changing, then removing those males with the largest ornaments will have the effect of removing those individuals who are best adapted to the new environment, potentially weakening, neutralizing or even reversing the increase in adaptation rates and the reduction in extinction probability that sexual selection appears to bestow upon a population. We investigated the effect of selective harvesting on adaptation and extinction using a modification of an existing individual-based simulation model, previously used to investigate the relationships between demographic effects, sexual selection and environmental change (see electronic supplementary material for full details). Individual-based models allow feedback between demographic and evolutionary processes, and are well suited for investigating so-called ‘eco-evo’ questions such as this [30,31].
2. Methods
The model used is a modification of the individual-based model used in [19] and is described in detail in the electronic supplementary material, which also includes the full model code. The model is written in R [32], and allows the population dynamics and evolution of a spatially homogeneous, age-structured population of animals to be simulated, with the strength of sexual selection being specified for each population. The model proceeds as a series of time steps of arbitrary length. These can be thought of as representing years, but the model is not necessarily constrained to this time scale and we would caution against interpreting the output from this model as making specific predictions about the time scale of evolutionary or extinction events.
Individuals in the simulation are born as juveniles and mature aged 2. In the absence of other influences, the probability of death is modelled as a quadratic function such that adults aged 5 or 6 years old experience the lowest probability of death, with young and old individuals both having an increased probability of dying. The probability of death is also related to population density, with an increasing likelihood of dying when the population approaches or exceeds the environmental carrying capacity.
The environment is modelled via a single variable, environment, which is assumed to represent a continuously variable environmental factor such as temperature or salinity. The environment can change randomly every year with the standard deviation of the changes being specified for each simulation, and larger random changes can also occur at intervals and with a magnitude which can be varied. For directional environmental change, the simulations begin with a period of 150 time steps of stability with only a small amount of random noise altering environment to allow the population to reach ‘normal’ levels of adaptation. After this, environment alters by a randomly drawn value with a mean greater than zero each time step: for the majority of simulations this value was 0.005 but for the simulations shown in figure 3 and electronic supplementary material, figure S3 this value was varied. To allow for variability in individual responses to a particular environment, each individual experiences a different value for environment calculated as the current value plus a random number drawn from a normal distribution with mean zero and standard deviation equal to 0.2.
Figure 3.
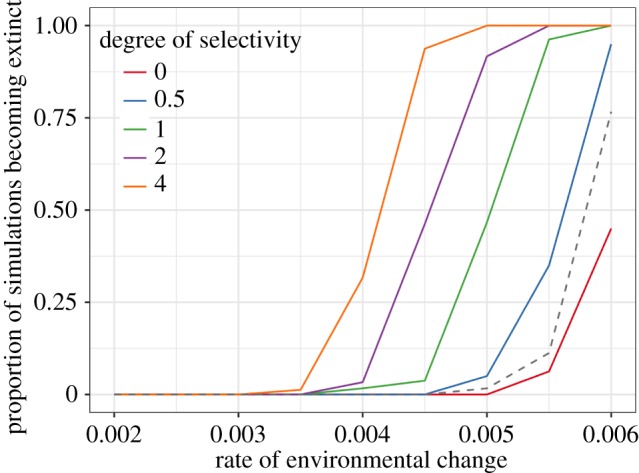
Increasingly selective harvest leads to a decreased critical rate of environmental change. The y-axis shows the proportion of simulations in which the population became extinct before 600 time steps had been completed, and the x-axis gives the amount by which the environment changes every time step. Harvest is random for a degree of selectivity of zero and is very selective when the degree of selectivity is 4 (see electronic supplementary material, figure S1). Because of the stochasticity in the model there is not a single critical rate for each set of parameter values, but this plot shows that a much lower rate of environmental change causes extinction when selectivity is high. All data compiled from 80 model runs at each combination of parameter values for 600 time steps, with the harvest rate set to 0.2, a base fecundity of 3 and a carrying capacity of 1000. The dashed line shows the proportion of simulations becoming extinct when the harvest rate is set to zero—as can also be seen in figure 2, random harvest of males only (the situation when selectivity = 0) appears to protect the population to some degree against environmental change, probably because of the ‘Hydra effect’ recently described by Osmond et al. [34].
The fit to the environment for each individual is assumed to be a complex polygenic trait controlled by a large number of loci, and so is modelled as a continuous value called genotype. genotype is calculated from the mean value for the two parents, plus a random number drawn from a normal distribution with mean 0 and s.d. 0.05 which allows for stochastic effects and mutation. For both males and females, the square of the difference between the value for genotype and the value for environment (mismatch) determines the overall health and condition of the individual, with larger values for mismatch indicating worse condition. This then leads to reduced survival and reduced fecundity in females. Phenotype (mismatch) is thus controlled by genotype but is not completely equal to it because of the variability in individual environments, and throughout the simulation there is an optimal phenotype (mismatch = 0), which a population should track.
The expression of sexual display traits by males is calculated when they reach maturity, and is determined by several factors: a genetic variable controlling the degree by which such traits tend to be expressed (t), which was itself allowed to evolve during the simulation, the condition of the individual male (mismatch) and a constant (α) which specifies the extent by which expression of the sexual display trait scales with condition, such that trait expression for an individual male is calculated as:
α was set to 4 for the majority of simulations, but the effects of varying it are shown in electronic supplementary material, figure S3.
Mate choice occurs by each female sampling a set number of males and choosing to mate with one, with the probability of choosing a particular male changing according to the difference between his display trait size and the median value for the group sampled, adjusted by the strength of female preference. The overall strength of sexual selection experienced by a population is determined by a constant specifying the number of males that a female will assess before choosing a mate: if this number is 1 then mating is random; if it is greater than 1 then the population will experience sexual selection, with the degree of reproductive skew being greater as this value increases. Following this each female who has mated (it is possible for there to be no mature males in a population) produces a number of offspring determined by the parameter O which specifies maximum fecundity per female, adjusted by that female's condition (mismatch).
Harvesting was added to the model such that either removal of mature individuals of both sexes or of mature males only could be specified. To add selectivity to this process, individual males were ranked according to their expression of secondary sexual traits and the probability of being removed weighted by
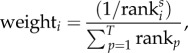 |
where S is a selectivity coefficient (‘harvest_selectivity’ in the model code) and T is the total number of adult males. This gives random sampling if S = 0 and increasingly selective sampling as S becomes larger (electronic supplementary material, figure S5).
3. Results
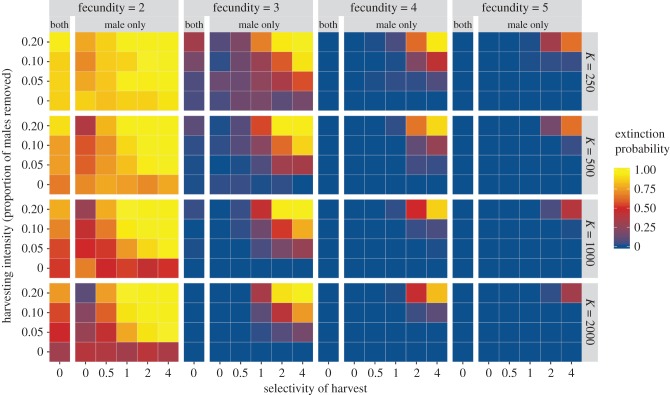
When environmental change is random, selective harvesting does not increase extinction risk, even when the amount of change is such that there is a reasonable risk of extinction (electronic supplementary material, figure S1). With directional environmental change, however, increasing selectivity of harvesting is strongly associated with an increasing risk of extinction (figure 2; electronic supplementary material, figure S2). When there is a low or medium probability that a changing environment will lead to extinction even in the absence of harvesting, selective harvest makes extinction a near-certainty. When the population is resilient to environmental change in the absence of harvesting, selective harvest can still lead to a high probability of extinction, especially when coupled with a relatively high harvest rate.
Figure 2.
When there is directional environmental change the probability of extinction increases with increasing selectivity and harvesting intensity. K indicates the carrying capacity of the environment, which will determine the population size when environmental change begins, and fecundity is the average fecundity of a female who is well adapted to the environment. The columns headed ‘both’ show probabilities of extinction when both male and female adults are harvested at random. The columns headed ‘male only’ show extinction probabilities when only adult males are harvested. The y-axes show harvesting intensity, expressed as the proportion of the male population removed per time step (for the ‘both’ columns the harvesting intensity was reduced by 50% for each sex so that the same overall number of animals were removed). The x-axes show the degree of selectivity expressed as the coefficient S from the model, with 0 indicating that harvesting is random and the degree of selectivity increasing as the value increases such that a value of 4 indicates a strong preference for the most ornamented males—see electronic supplementary material, figure S2. All probabilities calculated from 80 runs of the model over 600 time steps and with the increase in the environmental variable set to 0.005 per time step. See the electronic supplementary material for full details of the model.
Selective harvesting reduces the ability of the population to adapt as the environment changes. Chevin et al. [33] considered the question of how much environmental change a population can tolerate, and defined the critical rate of environmental change as ‘the maximum rate of sustained environmental change that allows long-term persistence of a population’. The stochastic nature of these simulations means that there is not a critical rate associated with each combination of parameter values, but whether we consider the rate of change at which no population, 10% of populations or 50% of populations become extinct to be a stochastic equivalent, we can see that the reduced capacity of selectively harvested populations to adapt means that this critical rate is reduced (figure 3). These effects are seen even when sexual selection is weak and the link between fitness and expression of the secondary sexual trait is not especially strong, and even a relatively mild degree of selectivity can increase the probability of extinction under some circumstances (electronic supplementary material, figure S3).
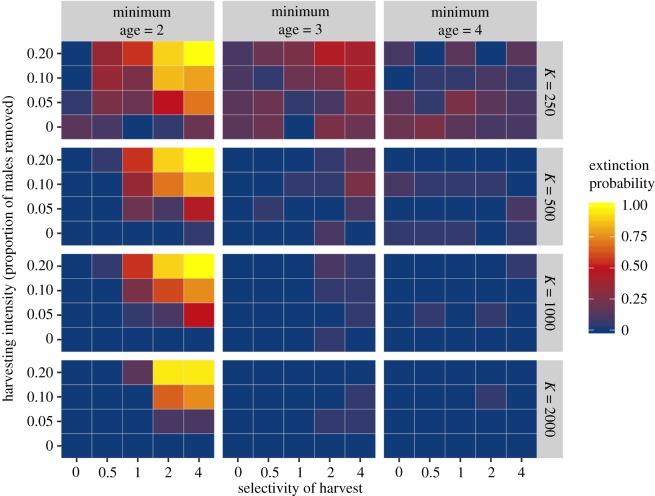
We modelled two management scenarios that could ameliorate the possible negative effect of trophy hunting. Imposing a threshold population size below which harvest is not allowed does reduce the risk of extinction but only when the threshold is a large fraction of the population carrying capacity (electronic supplementary material, figure S4). Age restrictions on harvest, however, where males are only targeted once they are over a certain age, are effective in reducing the risk of extinction (figure 4) because the ‘high-quality’ males have opportunities to breed before being removed.
Figure 4.
Increasing the minimum age for harvest reduces extinction probability. In this model, males and females become sexually mature at age 2 and harvest is restricted to these mature individuals. If the minimum age for harvest is increased (as in the centre and right hand columns), the effect of selective harvest on extinction risk seems to be largely nullified. This is because the ‘high-quality’ males that would otherwise be removed from the population have an opportunity to breed and pass on their genes. Management of trophy hunts whereby older males only are targeted is already recommended for lion populations, albeit to reduce the effects of infanticide and lack of paternal care associated with removal of breeding age males [35], and similar schemes should perhaps be considered for other hunted animals. Age-based management requires either an easy way of telling an animal's age or close management of a population whereby individuals are followed through time. Neither of these are likely to be possible for many harvested populations, however: these include many mammal populations which are not intensely managed as well as, for example, insect populations where males with large secondary sexual traits are the focus of collection. For these populations it is difficult to recommend a simple management intervention that will avoid the effects detailed here, but close monitoring and reactive management is likely to help and to give warning of declining numbers and potential problems. All extinction probabilities calculated from 80 replicate runs of the simulation, with base fecundity = 3, the rate of environmental change set to 0.005 per time step, strong sexual selection (strength of sexual selection = 5) and strong condition dependence (α = 4).
4. Discussion
It has been argued that human predation is qualitatively different from other forms of predation, with a strong bias towards the removal of large adults leading to different and more severe impacts on prey species than those caused by non-human predators [3]. Indeed, natural predation can actually enhance population persistence when prey populations are exposed to directional environmental change. This can occur via both ‘selective push’, whereby poorly adapted individuals are removed by predators leading to more rapid adaptation, and also the ‘Hydra effect’, whereby removal of individuals from a population leads to increased recruitment and enhances adaptation because generation time is effectively reduced [34]—a phenomenon also seen in our model results when harvest is non-selective (figure 3; electronic supplementary material, figure S3). Our results here contrast notably with these effects of ‘normal’ predation and reinforce the point that the sorts of selectivity associated with human predation can lead to uniquely severe impacts on harvested populations.
This demonstration that selective harvesting can potentially push otherwise resilient populations to extinction when the environment changes is concerning. As mentioned earlier, it is widely believed that selective harvest is unlikely to endanger well-managed populations, and this might well be reasonable when the environment is relatively stable, or changing at random—but directional environmental change is now a dangerous reality for considerable numbers of species [36], with global environmental change causing raised temperatures worldwide, and increasing ocean acidification and changes in seasonal timing becoming increasingly important. Populations with restricted geographical ranges, such as those on islands or which are confined to isolated habitat patches such as forest fragments or isolated conserved areas, are unable to migrate to new environments. Given that phenotypic plasticity is unlikely to be sufficient to allow population persistence in many cases [37–39], these populations will have to adapt or they will become extinct.
Our results clearly show that age restrictions on harvest which allow males to breed before they are taken are effective at reducing the impact of selective harvest on adapting populations. Such management is already recommended for lions [35], and populations where these recommendations are followed are likely to be relatively unaffected by the removal of these males. Other well-managed trophy-hunting schemes, such as those which follow the IUCN SSC Guiding Principles on Trophy Hunting [40], with low offtake and reactive management, will also be somewhat resilient to the effects described here, although managers would need to consider the effects of removal of well-ornamented males if the population were faced with an altered environment. Poorly managed populations of hunted animals, however, with higher levels of offtake, a lack of monitoring and harvesting of males of all ages are likely to be vulnerable to problems caused by the removal of the fittest males even in the absence of other threats.
The present model does not consider two phenomena which might alter the effect of selective harvesting on adaptation. These are inbreeding and intralocus sexual conflict. In the case of the former, if selective harvest reduces the degree of reproductive skew in a population by removing the most attractive or dominant males then this will change the effective population size and potentially reduce the amount of inbreeding. In species which experience severe effects from inbreeding depression this could mitigate the negative effects of removing the best adapted males to some extent. How important this effect might be is not clear, and the situation is further complicated by the potential for a history of strong sexual selection to buffer a population against inbreeding depression [18]. There is a clear requirement for further research, both theoretical and empirical, to help us understand how inbreeding will interact with selective harvesting and how this might alter population mean fitness.
In the case of intralocus sexual conflict, different phenotypic optima for males and females could mean that in some cases females who mate with the most attractive males might actually have female offspring with reduced fitness [41,42], potentially reducing population mean fitness. It is possible, therefore, that removing those most attractive males from the system would reduce this effect and mitigate the other, negative effects of selective harvest to some extent. On the basis of current knowledge, however, we would suggest that this is unlikely, especially under directional selection: both empirical [43,44] and theoretical [45] research indicates that the negative effects of sexual conflict in changing environments are reduced because the phenotypic optima for both sexes are shifted in the same direction, leading to the selection gradients for both sexes becoming more similar [45]. Nonetheless, as with inbreeding, this is a complex question which remains an area for future research.
The outputs of this model are predicated on the assumption that sexually selected traits are condition-dependent and will respond to the environment in a way that allows sexual selection to affect adaptation and persistence. As discussed in the introduction, there is now a considerable amount of laboratory data supporting this assumption, but we must be cautious because these studies were all carried out on invertebrates, whereas the targets for selective harvesting are often vertebrates, which may well have rather more sophisticated breeding systems with important contributions from social selection as well as sexual selection [46]. Age is also important in sexual selection in many vertebrate systems, with the growth of sexual ornaments increasing throughout a male's life and only ‘prime’ aged males being able to compete fully for access to females [21,35,47]. How this age effect might interact with adaptation and selective harvest is not clear, although removal of prime-aged males could potentially reverse the beneficial effects of only harvesting older males which we have found. As with all theoretical models, therefore, we do not claim that the effects found here will be universal, or even necessarily typical. Nonetheless, the effect of selective harvesting on extinction risk under environmental change appears to be strong and should at least be considered when strongly sexually selected species are harvested.
When properly regulated, trophy hunting is arguably a powerful force for conservation [4,48], with a greater area being conserved for hunting in Sub-Saharan Africa than is conserved in national parks [4]. Other forms of selective harvest such as insect collecting are much less well managed or studied. Unless a species of insect is specifically protected by national or international legislation, collection is usually unregulated and populations are not managed: a search on Web of Knowledge for a variety of combinations of ‘insect’, ‘collect*’, ‘population’ and ‘management’ returns no relevant hits. This is unlikely to be a problem when populations are large and rates of offtake from collectors are low, but when populations are small and demand for particularly showy specimens is strong, as might well be the case for the larger lucanid and dynastid species in fragmented forests, there is a risk that collection targeted at these specimens might inadvertently cause local or even global extinction even when the proportion of animals collected seems insignificant.
Supplementary Material
Acknowledgements
We are grateful to Lars Chittka and Richard Nichols for helpful comments on a draft of this manuscript, and to Luke Holman and a second, anonymous referee for constructive and helpful criticisms which have greatly improved this paper.
Data accessibility
The full model code is available in the electronic supplementary material.
Authors' contributions
R.J.K. conceived the study. C.M.-R. and R.J.K. wrote the model code and ran the simulations. R.J.K. wrote the manuscript and both authors gave final agreement for publication.
Competing interests
We declare we have no competing interests.
Funding
This research used Queen Mary's MidPlus computational facilities, supported by QMUL Research-IT and funded by EPSRC grant no. EP/K000128/1.
References
- 1.Sillitoe P. 1988. From head-dresses to head-messages: the art of self-decoration in the highlands of Papua New Guinea. Man 23, 298–318. ( 10.2307/2802807) [DOI] [Google Scholar]
- 2.Chiyo PI, Obanda V, Korir DK. 2015. Illegal tusk harvest and the decline of tusk size in the African elephant. Ecol. Evol. 5, 5216–5229. ( 10.1002/ece3.1769) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Darimont CT, Fox CH, Bryan HM, Reimchen TE. 2015. The unique ecology of human predators. Science 349, 858–860. ( 10.1126/science.aac4249) [DOI] [PubMed] [Google Scholar]
- 4.Lindsey PA, Roulet PA, Romañach SS. 2007. Economic and conservation significance of the trophy hunting industry in sub-Saharan Africa. Biol. Conserv. 134, 455–469. ( 10.1016/j.biocon.2006.09.005) [DOI] [Google Scholar]
- 5.Milner-Gulland EJ, Bukreeva OM, Coulson T, Lushchekina AA, Kholodova MV, Bekenov AB, Grachev IA. 2003. Conservation: reproductive collapse in saiga antelope harems. Nature 422, 135 ( 10.1038/422135a) [DOI] [PubMed] [Google Scholar]
- 6.Mysterud A. 2012. Trophy hunting with uncertain role for population dynamics and extinction of ungulates. Anim. Conserv. 15, 14–15. ( 10.1111/j.1469-1795.2011.00519.x) [DOI] [Google Scholar]
- 7.Mysterud A, Coulson T, Stenseth NC. 2002. The role of males in the dynamics of ungulate populations. J. Anim. Ecol. 71, 907–915. ( 10.1046/j.1365-2656.2002.00655.x) [DOI] [Google Scholar]
- 8.Davidson Z, Valeix M, Loveridge AJ, Madzikanda H, Macdonald DW. 2011. Socio-spatial behaviour of an African lion population following perturbation by sport hunting. Biol. Conserv. 144, 114–121. ( 10.1016/j.biocon.2010.08.005) [DOI] [Google Scholar]
- 9.Coltman DW, O’Donoghue P, Jorgenson JT, Hogg JT, Strobeck C, Festa-Bianchet M. 2003. Undesirable evolutionary consequences of trophy hunting. Nature 426, 655–658. ( 10.1038/nature02177) [DOI] [PubMed] [Google Scholar]
- 10.Allendorf FW, Hard JJ. 2009. Human-induced evolution caused by unnatural selection through harvest of wild animals. Proc. Natl Acad. Sci. USA 106, 9987–9994. ( 10.1073/pnas.0901069106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mysterud A. 2011. Selective harvesting of large mammals: how often does it result in directional selection? J. Appl. Ecol. 48, 827–834. ( 10.1111/j.1365-2664.2011.02006.x) [DOI] [Google Scholar]
- 12.Pigeon G, Festa-Bianchet M, Coltman DW, Pelletier F. 2016. Intense selective hunting leads to artificial evolution in horn size. Evol. Appl. 9, 521–530. ( 10.1111/eva.12358) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coulson T, Schindler S, Traill L, Kendall BE. In press Predicting the evolutionary consequences of trophy hunting on a quantitative trait. J. Wildl. Manage. ( 10.1002/jwmg.21261) [DOI] [Google Scholar]
- 14.Janeiro MJ, Coltman DW, Festa-Bianchet M, Pelletier F, Morrissey MB. 2017. Towards robust evolutionary inference with integral projection models. J. Evol. Biol. 30, 270–288. ( 10.1111/jeb.13000) [DOI] [PubMed] [Google Scholar]
- 15.Chevin L-M. 2015. Evolution of adult size depends on genetic variance in growth trajectories: a comment on analyses of evolutionary dynamics using integral projection models. Methods Ecol. Evol. 6, 981–986. ( 10.1111/2041-210X.12389) [DOI] [Google Scholar]
- 16.Lorch PD, Proulx S, Rowe L, Day T. 2003. Condition-dependent sexual selection can accelerate adaptation. Evol. Ecol. Res. 5, 867–881. [Google Scholar]
- 17.Plesnar-Bielak A, Skrzynecka AM, Prokop ZM, Radwan J. 2012. Mating system affects population performance and extinction risk under environmental challenge. Proc. R. Soc. B 279, 4661–4667. ( 10.1098/rspb.2012.1867) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lumley AJ, et al. 2015. Sexual selection protects against extinction. Nature 522, 470–473. ( 10.1038/nature14419) [DOI] [PubMed] [Google Scholar]
- 19.Martínez-Ruiz C, Knell RJ. 2017. Sexual selection can both increase and decrease extinction probability: reconciling demographic and evolutionary factors. J. Anim. Ecol. 86, 117–127. ( 10.1111/1365-2656.12601) [DOI] [PubMed] [Google Scholar]
- 20.Douhard M, Pigeon G, Festa-Bianchet M, Coltman DW, Guillemette S, Pelletier F. 2017. Environmental and evolutionary effects on horn growth of male bighorn sheep. Oikos 126, 1031–1041. ( 10.1111/oik.03799) [DOI] [Google Scholar]
- 21.Kruuk LEB, Slate J, Pemberton JM, Brotherstone S, Guinness F, Clutton-Brock T, Houle D. 2002. Antler size in red deer: heritability and selection but no evolution. Evolution 56, 1683–1695. ( 10.1111/j.0014-3820.2002.tb01480.x) [DOI] [PubMed] [Google Scholar]
- 22.Vanpé C, et al. 2007. Antler size provides an honest signal of male phenotypic quality in roe deer. Am. Nat. 169, 481–493. ( 10.1086/512046) [DOI] [PubMed] [Google Scholar]
- 23.Emlen DJ. 2008. The evolution of animal weapons. Annu. Rev. Ecol. Syst. 39, 387–413. ( 10.1146/annurev.ecolsys.39.110707.173502) [DOI] [Google Scholar]
- 24.Rowe L, Houle D. 1996. The lek paradox and the capture of genetic variance by condition dependent traits. Proc. R. Soc. B 263, 1415–1421. ( 10.1098/rspb.1996.0207) [DOI] [Google Scholar]
- 25.Tomkins JL, Radwan J, Kotiaho JS, Tregenza T. 2004. Genic capture and resolving the lek paradox. Trends Ecol. Evol. 19, 323–328. ( 10.1016/j.tree.2004.03.029) [DOI] [PubMed] [Google Scholar]
- 26.Arnqvist G. 2007. Rapid adaptation to a novel host in a seed beetle (Callosobruchus maculatus): the role of sexual selection. Evolution 61, 440–452. ( 10.1111/j.1558-5646.2007.00038.x) [DOI] [PubMed] [Google Scholar]
- 27.Jacomb F, Marsh J, Holman L. 2016. Sexual selection expedites the evolution of pesticide resistance. Evolution 70, 2746–2751. ( 10.1111/evo.13074) [DOI] [PubMed] [Google Scholar]
- 28.Jarzebowska M, Radwan J. 2010. Sexual selection counteracts extinction of small populations of the bulb mites. Evolution 64, 1283–1289. [DOI] [PubMed] [Google Scholar]
- 29.Sorci G, Møller AP, Clobert J. 1998. Plumage dichromatism of birds predicts introduction success in New Zealand. J. Anim. Ecol. 67, 263–269. ( 10.1046/j.1365-2656.1998.00199.x) [DOI] [Google Scholar]
- 30.DeAngelis DL, Mooij WM. 2005. Individual-based modeling of ecological and evolutionary processes. Annu. Rev. Ecol. Evol. Syst. 36, 147–168. ( 10.1146/annurev.ecolsys.36.102003.152644) [DOI] [Google Scholar]
- 31.DeAngelis DL, Grimm V. 2014. Individual-based models in ecology after four decades. F1000Prime Rep. 6, 39 ( 10.12703/P6-39) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.R Development Core Team. 2013. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 33.Chevin L-M, Lande R, Mace GM. 2010. Adaptation, plasticity, and extinction in a changing environment: towards a predictive theory. PLoS Biol. 8, e1000357 ( 10.1371/journal.pbio.1000357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osmond MM, Otto SP, Klausmeier CA, Goodnight CJ, Michalakis Y. 2017. When predators help prey adapt and persist in a changing environment. Am. Nat. 190, 83–98. ( 10.1086/691778) [DOI] [PubMed] [Google Scholar]
- 35.Whitman K, Starfield AM, Quadling HS, Packer C. 2004. Sustainable trophy hunting of African lions. Nature 428, 175–178. ( 10.1038/nature02395) [DOI] [PubMed] [Google Scholar]
- 36.Wiens JJ. 2016. Climate-related local extinctions are already widespread among plant and animal species. PLoS Biol. 14, e2001104 ( 10.1371/journal.pbio.2001104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phillimore AB, Hadfield JD, Jones OR, Smithers RJ. 2010. Differences in spawning date between populations of common frog reveal local adaptation. Proc. Natl Acad. Sci. USA 107, 8292–8297. ( 10.1073/pnas.0913792107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duputié A, Rutschmann A, Ronce O, Chuine I. 2015. Phenological plasticity will not help all species adapt to climate change. Glob. Chang. Biol. 21, 3062–3073. ( 10.1111/gcb.12914) [DOI] [PubMed] [Google Scholar]
- 39.van Heerwaarden B, Kellermann V, Sgrò CM. 2016. Limited scope for plasticity to increase upper thermal limits. Funct. Ecol. 30, 1947–1956. ( 10.1111/1365-2435.12687) [DOI] [Google Scholar]
- 40.IUCN SSC. 2012. IUCN SSC guiding principles on trophy hunting as a tool for creating conservation incentives Gland, Switzerland: IUCN. [Google Scholar]
- 41.Arnqvist G, Rowe L. 2005. Sexual conflict. Princeton, NJ: Princeton University Press. [Google Scholar]
- 42.Bonduriansky R, Chenoweth SF. 2009. Intralocus sexual conflict. Trends Ecol. Evol. 24, 280–288. ( 10.1016/j.tree.2008.12.005) [DOI] [PubMed] [Google Scholar]
- 43.Long TAF, Agrawal AF, Rowe L. 2012. The effect of sexual selection on offspring fitness depends on the nature of genetic variation. Curr. Biol. 22, 204–208. ( 10.1016/j.cub.2011.12.020) [DOI] [PubMed] [Google Scholar]
- 44.Berger D, Grieshop K, Lind MI, Goenaga J, Maklakov AA, Arnqvist G. 2014. Intralocus sexual conflict and environmental stress. Evolution 68, 2184–2196. ( 10.1111/evo.12528) [DOI] [PubMed] [Google Scholar]
- 45.Connallon T, Hall MD. 2016. Genetic correlations and sex-specific adaptation in changing environments. Evolution 70, 2186–2198. ( 10.1111/evo.13025) [DOI] [PubMed] [Google Scholar]
- 46.Lyon BE, Montgomerie R. 2012. Sexual selection is a form of social selection. Phil. Trans. R. Soc. B 367, 2266–2273. ( 10.1098/rstb.2012.0012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoccoz NG, Mysterud A, Langvatn R, Stenseth NC. 2002. Age- and density-dependent reproductive effort in male red deer. Proc. R. Soc. Lond. B 269, 1523–1528. ( 10.1098/rspb.2002.2047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Di Minin E, Leader-Williams N, Bradshaw CJA. 2016. Trophy hunting does and will support biodiversity: a reply to Ripple et al. Trends Ecol. Evol. 31, 496–498. ( 10.1016/j.tree.2016.03.010) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The full model code is available in the electronic supplementary material.