Abstract
Genes of the major histocompatibility complex (MHC) have been shown to influence social signalling and mate preferences in many species, including humans. First observations suggest that MHC signalling may also affect female fertility. To test this hypothesis, we exposed 191 female horses (Equus caballus) to either an MHC-similar or an MHC-dissimilar stimulus male around the time of ovulation and conception. A within-subject experimental design controlled for non-MHC-linked male characteristics, and instrumental insemination with semen of other males (n = 106) controlled for potential confounding effects of semen or embryo characteristics. We found that females were more likely to become pregnant if exposed to an MHC-dissimilar than to an MHC-similar male, while overall genetic distance to the stimulus males (based on microsatellite markers on 20 chromosomes) had no effect. Our results demonstrate that early pregnancy failures can be due to maternal life-history decisions (cryptic female choice) influenced by MHC-linked social signalling.
Keywords: sexual selection, cryptic female choice, major histocompatibility complex, mammals, fertility, female reproductive strategy
1. Introduction
The major histocompatibility complex (MHC) is a group of polymorphic genes that play a crucial role in the adaptive immune response of vertebrates [1]. The MHC also plays an important role in social signalling, from parent–progeny and other kinds of kin recognition to mate choice and further contexts of inter-sexual communication [1,2]. Ruff et al. [2] list over 20 species (including humans, see also [3,4]) where some form of MHC social signalling could be identified, and further examples have been added since their review, including the horse (Equus caballus) [5,6]. In the case of MHC-dependent mate choice, the typical observation is that mating with MHC similar individuals is avoided and some degree of MHC-dissimilarity is preferred [1,2] (see [7,8] for a recent discussion of alternative models). MHC-linked social signalling is either based on volatile chemical signals (e.g. [9]) or on non-volatile MHC peptide ligands [10,11]. The signals are recognized in the vomeronasal organ [10] and/or in the main olfactory system [3,12] (i.e. there seem to exist several independent mechanisms of MHC-linked social signalling, but the full pathways have not been solved yet).
In the context of sexual selection, the MHC may either be used as a marker for kinship to avoid inbreeding, or MHC-based mate preferences may serve to enhance the frequency of heterozygotes or of rare alleles among offspring [13] (even if MHC heterozygotes may not do better against a given infection than the respective homozygotes [14], they often show superiority during coinfections [15,16]).
Mate choice is only one of several possible levels at which sexual selection may influence offspring genotype [2]. Cryptic female choice includes selection against certain types of sperm within the female reproductive tract [17,18], non-random gamete fusion [19,20] and non-random second meiotic division in the egg after gamete fusion [19,21]. The possibility that MHC-linked signals affect female decisions at the earliest stages of a pregnancy (i.e. before implantation in the endometrium) has received little attention despite its potential relevance in mammals [22]. The frequency of early pregnancy failure can be high (e.g. around 22% in humans when diagnosed on daily urine samples, compared with 9% that happened after clinical detection of pregnancy [23]). If some of these early pregnancy failures were indeed due to maternal life-history decisions influenced by MHC-linked social signalling, human couples sharing MHC alleles would be expected to have longer periods of unprotected intercourse until a pregnancy would be diagnosed. This prediction was confirmed in studies on Hutterites [24], a group of people whose doctrine generally discourages contraception. Ober et al.'s [24] observations suggest that cryptic female rejection of early embryos could be a form of sexual selection that contributes to the excess of MHC heterozygotes that is often observed in, for example, human populations [25]. Here, we test this possibility, using horses (E. caballus) as experimental model, and concentrating on European warmbloods (WB) to avoid potential breed effects.
2. Methods
(a). Experimental procedures
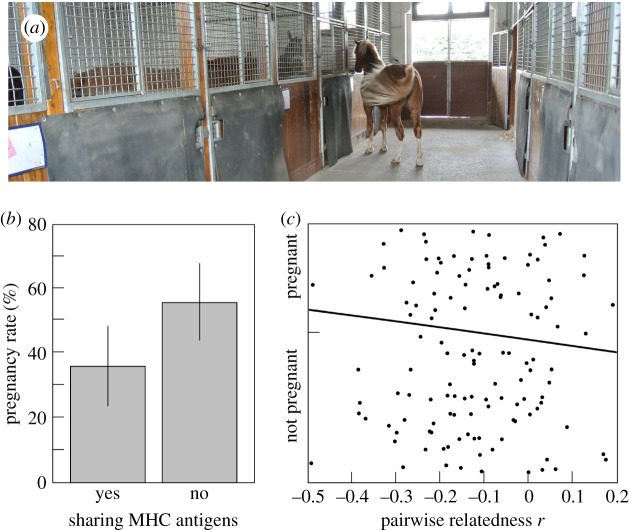
The experimental infrastructure consisted of a corridor and eight boxes (figure 1a). Over a period of 5 years, in total 191 oestrous mares without foal (only WB, including 18 Franches-Montagnes (FM) horses, i.e. a WB-related local breed) were individually stabled in one of the boxes during 62 h (after feeding and water installations had been cleaned with water, faeces and urinated litter in the boxes had been removed and new straw had been added). The mares were exposed to one of the ten stimulus stallions (all FM) who could freely move in his box and the corridor for 17 h d−1. These stimulus stallions were all non-related and unfamiliar to the mares, and the mares were haphazardly assigned to them. There were usually several mares in the same stable (up to seven of the eight boxes were occupied with mares).
Figure 1.
Experimental set-up and female fertility in response to the sharing of MHC or microsatellite alleles. (a) Exposure of oestrous mares (in boxes) to one of the ten stimulus stallions. (b) Pregnancy rate per stimulus stallion (means ± 95% CI) in response to MHC sharing between mares (n = 191) and stimulus stallions. (c) Logistic plot illustrating that pairwise relatedness r is a poor predictor of pregnancy. To improve visibility, points are jittered randomly along the y axis within the range that corresponds to pregnant or not pregnant (see table 1 for statistics). (Online version in colour.)
Before a mare would be exposed to a stimulus stallion, her cycle activity was monitored daily by rectal ultrasonographic examinations. When at least one follicle reached a diameter of greater than or equal to 35 mm, a pronounced uterus oedema was present, and the absence of any corpus luteum was confirmed, 1500 IU human chorionic gonadotropin (hCG; Chorulon, Intervet, Boxmeer, the Netherlands) was applied intravenously the following evening (in order to induce an ovulation approx. 36–42 h later [26]). The mare was then introduced into the experiment (i.e. exposed to a stimulus stallion). Instrumental inseminations were performed 24 and 38 h after hCG application with fresh or frozen-thawed semen (of one of 106 other breeding stallions). Instrumental insemination allowed controlling for potential effects of semen characteristics [5] and of embryo genetics. Sixty-two hours after hCG injection (i.e. before blastocysts are expected to leave the oviduct [27]) mares were stabled elsewhere without contact to any stallions. They were examined for pregnancy 14–17 days after ovulation by transrectal ultrasound [28].
(b). Major histocompatibility complex and microsatellite typing
Equine leucocyte antigens (ELA) were determined serologically as in Burger et al. [5,6] in microcytotoxicity tests with alloantisera detecting 18 internationally recognized MHC class I ELA-A alleles (A1–A10, A14, A15, A19, W11, W16, W17, W18 and W20), five locally defined specificities belonging to this same locus (Be22, Be25, Be27, Be28, Be108), the non-ELA-A class I antigens BeIII and W21, the MHC class II alleles DW13, DW22, DW23, DBe200 and DBeVIII, and the antigen W12 that could not yet be assigned to class I or II. Briefly, peripheral blood lymphocytes were obtained by Ficoll density gradient centrifugation, washed twice in PBS, resuspended in RPMI and diluted to 2 × 106 cells ml−1. Two microlitres of cell suspension were added to wells on Terasaki typing plates. After 20 min incubation at room temperature, 2 μl rabbit complement was added to each well. Visualization of the reaction was performed 1 h later by adding 5 μl eosin followed by 5 μl formaldehyde for fixation. A positive reaction led to killing of more than 50% of target cells. ELA was determined in all 191 mares, all 10 stimulus stallions and 31 of the 106 semen donors. MHC sharing to the semen donor could be determined for 80 mares. All MHC types were determined after the experiments (i.e. they were not known during handling of the animals). Most mares shared 0 or 1 antigen with the stimulus stallion, but sharing of up to 3 antigens could be found. Of the 112 mares that shared at least one antigen with the stimulus stallion, 4 shared only one class II antigen, 14 shared class II and class I antigens, and the rest only class I antigens (i.e. MHC sharing refers mostly to sharing of class I antigens here). FM mares were not more likely than other WB to share MHC antigens with the FM stimulus stallions (there was even a non-significant tendency of lower sharing within FM than between WB and FM; FM mares: 39% sharing, WB mares: 61% sharing, χ2 = 3.1, p > 0.05).
Genetic similarity between individuals was estimated from 20 polymorphic microsatellite loci located on 20 different chromosomes (electronic supplementary material, table S1). Genomic DNA was extracted from blood (EDTA) with the Qiagen BioSprint robotic workstation. Markers were amplified with GoTaq DNA polymerase except AHT36, UMNe567 and UD457 which were pooled and amplified with the Qiagen Multiplex PCR Kit. PCR reactions with GoTaq DNA polymerase were performed in 10 µl reaction volume using 1X GoTaq green reaction buffer, 0.5 µl of DNA, 2.5 mM MgCl2 in total, a mix of 0.2 mM dNTPs, 0.5 µM of each primer and 0.25 µ of GoTaq DNA polymerase. PCR programme with varying annealing temperatures and a general procedure was used as follows: 4 min of initial denaturation at 94°C, 38 cycles of 30 s at 94°C, 60 s at optimal annealing temperature, 40 s at 72°C, then 5 min of final extension at 72°C. PCR with the Qiagen Multiplex PCR Kit was performed in 10 µl reaction volume using 1× Qiagen Multiplex PCR Master Mix (3 mM Mg2+), 1 µl of DNA, 0.2 µM of each primer. PCR programme was used as follows: 15 min of initial denaturation and activation step at 94°C, 38 cycles of 30 s at 94°C, 90 s at 60°C, 30 s at 72°C, then 10 min of final extension at 72°C. All amplicons were subsequently analysed on an ABI-3100 sequencer and allele sizes scored using the size standards ROX-350 (Genemapper v. 4.0, Applied Biosystems).
Typing of microsatellite loci was based on new blood samples taken in some cases long after the experiments. Some samples could no more be taken because the mare had meanwhile died or because of other constraints. Therefore, the microsatellite-related analyses were based on a haphazardly reduced sample of mares. Pairwise relatedness was calculated after Wang [29] using the R package Demerelate [30] based on all 20 microsatellite loci (we had the full genotypes of all stimulus stallions and of 126 mares).
We constructed generalized linear models (GLM) and generalized linear mixed models (GLMM) on pregnancy (yes/no) as dependent variable, with MHC sharing and genetic relatedness based on microsatellite diversity as fixed factors, and the identity of the stimulus stallion as random factor. In order to test the significance of an effect, a model lacking or including an effect was compared to a reference model in likelihood ratio tests. The analyses were performed in R v. 3.3.3 [31] with the lme4 package [32], and in Jmp 11.2 (www.jmp.com).
3. Results
Sharing of at least one MHC antigen between mares and stimulus stallion was frequent (58.6%) and significantly reduced the rate of pregnancies after instrumental insemination (table 1a and figure 1b). The identity of the stimulus stallion did not seem to play role here (table 1a), and excluding the 18 mares of the FM breed (a WB-related local breed that was included in this study) did not change the conclusion that MHC sharing affected pregnancy (electronic supplementary material, table s2). As expected, MHC sharing between mares and semen donors did not correlate with MHC sharing between mares and stimulus stallions (n = 80 mares; χ2 = 0.7, p = 0.40). Whether fresh or frozen-thawed semen was used did not significantly affect pregnancy rate (likelihood ratio test: χ2 = 1.3, p = 0.26), nor did mare age (logistic fit: χ2 = 0.9, p = 0.35).
Table 1.
Effects of genetic similarity on female fertility (pregnant yes/no). Likelihood ratio tests comparing GLMMs and GLMs with genetic markers (fixed factors) and/or stallion identity (‘ID', random factor) and reference models (indicated in italics) to test the effects of (a) MHC antigen sharing (‘MHC', yes/no) and (b) pairwise genetic relatedness ‘r’ based on 20 polymorphic microsatellite loci. (c) Direct comparison of effects of MHC and r in the subsample of 126 mares that allow for such a test. Significant p-values are emphasized in bold.
| model | effect tested | d.f. | logL | χ2 | p |
|---|---|---|---|---|---|
| (a) MHC effects (191 mares) | |||||
| MHC + ID | 3 | −126.4 | |||
| ID | MHC sharing | 2 | −129.2 | 5.47 | 0.019 |
| MHC | stallion ID | 2 | −126.5 | 0.14 | 0.71 |
| MHC + ID + ID × MHC | stallion ID × MHC | 4 | −126.4 | 0.12 | 0.73 |
| (b) diversity on microsatellites (126 mares) | |||||
| r + ID | 3 | −85.5 | |||
| ID | r | 2 | −85.7 | 0.54 | 0.46 |
| r | stallion ID | 2 | −85.5 | 0 | 1.0 |
| r + ID + ID × r | stallion ID × r | 4 | −85.5 | 0 | 1.0 |
| (c) MHC versus microsatellites (126 mares) | |||||
| MHC + r + ID | 4 | −79.9 | |||
| r + ID | MHC sharing | 3 | −85.5 | 11.2 | <0.001 |
| MHC + ID | r | 3 | −80.0 | 0.1 | 0.70 |
| MHC + r | stallion ID | 3 | −79.9 | 0 | 1.0 |
There was no linkage disequilibrium among the 20 microsatellite markers (electronic supplementary material, figure S1; as expected from microsatellites that are located on different chromosomes). Electronic supplementary material, table S3 provides the observed and the expected heterozygosity per locus. In total 8 of the 20 loci showed an excess of homozygotes (average Fst = 0.078; electronic supplementary material, table S3) that was partly due to a genetic differentiation between WB and FM horses (mean Fst = 0.078; electronic supplementary material, table S3). Average pairwise relatedness r between stimulus stallion and mare was −0.111 (±0.137 s.d.). This indicator of relatedness was no significant predictor of MHC sharing (mean r of MHC-dissimilar pairs = −0.09 ± 0.019 (s.e.), of MHC-similar pairs = −0.12 ± 0.016; t = −1.2, p = 0.22) and did not predict pregnancy after the instrumental fertilization (table 1b and figure 1c). When directly comparing the effects of the MHC and genetic distance on microsatellites, we found again MHC sharing to be a significant predictor of female fertility, while r showed no effects (table 1c).
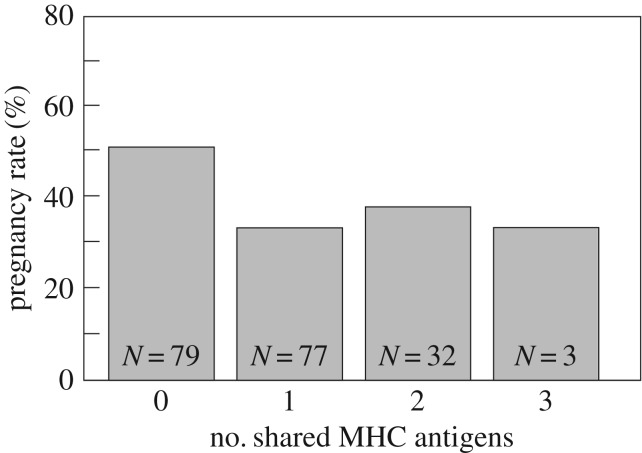
Figure 2 suggests that the number of shared MHC antigens did not play a role on the outcome of the instrumental fertilization. Nevertheless, models that compare the effects of MHC and r based on numbers of shared MHC antigens instead of the dichotomous sharing/no sharing still confirm that MHC signalling affects pregnancy (electronic supplementary material, tables S4 and S5).
Figure 2.
Pregnancy rates relative to number of MHC antigens shared between stimulus stallion and mare. The numbers in the boxes give the number of mares.
4. Discussion
Feral mares live in, or disperse between, social groups (bands) that usually include one or two stallions (i.e. male–male dominance is important in this species but female choice by dispersal is possible and likely [33,34]). Moreover, horses have long gestation periods (approx. 11 months), and foal condition crucially depends on maternal investment for some time after gestation [35]. Differential maternal reproductive strategies are therefore expected [36] and have indeed been found at early stages of pregnancy [37,38]. Our findings demonstrate that differential maternal reproductive strategies can also depend on MHC-linked signals emitted by stallions. There may be other male traits that signal, for example, health and vigour or male–male dominance, and that could potentially influence female reproductive decisions [39,40]. Our experimental design separated these other potential effects from effects of MHC sharing. It turned out that no stimulus stallion was significantly superior in affecting pregnancy rates. What mattered was whether stimulus stallions and mares shared MHC antigens.
In mice, post-mating exposure to MHC-linked odours from a male who is not the progenitor can induce pregnancy termination [41]. This so-called ‘Bruce effect’ [42–44] serves females to avoid costs of embryogenesis if offspring are likely to be killed by a new territory holder [45]. Evidence for the Bruce effect has been reported in horses [38,46] and other equids [47], and it is possible that such pregnancy terminations are induced via MHC signalling in social communication, too. However, the selection mechanism we discovered here is different. A pregnancy termination in anticipation of male infanticide would require a stimulus that implies a take-over of a territory or band by a new male, i.e. a female would have to compare new male stimuli to the ones she received at the time of conception. If MHC-linked signals were involved here, the stimulus male's MHC would have to be different to the MHC of the genetic father of the embryo. Whether the stimulus male is similar or dissimilar to the female's MHC would not be expected to matter in such situations, as experimentally confirmed in mice [48]. However, the very early pregnancy failures that we observed here happened before embryonic implantation and pregnancy can be diagnosed by ultrasonography [49]. They were directly dependent on whether the mare shared MHC antigens with the stimulus stallion (i.e. they mirrored mate choice decisions that have been found in various vertebrates [2]).
In humans, the prevalence of recurrent spontaneous abortions (of clinically diagnosed pregnancies) could frequently be linked to higher than usual MHC sharing between couples, but the evidence remains mixed [50,51]. The kind of selection we found here would mostly go unnoted or only delay menses in humans [23]. Maternal life-history decisions could play a role here, because human couples sharing MHC alleles have been found to have longer periods of unprotected intercourse between diagnosed pregnancies in Hutterites [24]. These first observations in humans [24] and our experimental findings in horses suggest that cryptic female acceptance or rejection of early embryos is an important further stage at which the MHC influences sexual selection.
In conclusion, cryptic female choice allows females to control male reproductive success after mating. In mammals, cryptic female choice may even include maternal decisions about survival of early embryos. If so, mate preferences are expected to influence female fertility. We tested this hypothesis with horses, controlling for potentially confounding effects of ejaculate characteristics, embryo genetics and genetic distance between mare and stimulus stallion. We found that mares are more likely to become pregnant if exposed to MHC-dissimilar than MHC-similar stallions around the time of instrumental insemination. It remains to be shown whether these negative effects of MHC-similar stallions on female reproductive decisions have evolved as a means to avoid inbreeding or to promote heterozygosity in the MHC region [13].
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank the horse owners for giving their informed consent, C. Berney, J. Janda, E. Jeannerat, R. Käser, S. Lazary, J. Pannell, M. Walker, B. Wespi, the ‘vet team’ of the Swiss Institute of Equine Medicine for discussion and/or assistance, and I. Cuthill, T. Pizzari and the reviewers for comments.
Ethics
Ethical clearance was granted by the Vaud canton, Switzerland (Service Vétérinaire, permission 2539 and 2227). Daily experimental handling time of mares and stallions was minimized. No manipulation resulted in injuries.
Data accessibility
The datasets supporting this article can be accessed at Dryad Digital Repository: (http://dx.doi.org/10.5061/dryad.04k5q) [52].
Authors' contributions
D.B., H.S. and C.W. designed the study. D.B. supervised the experiments. H.A. and M.D. tested the protocols and performed the experiments in the first 2 years. G.F. did the microsatellite genotyping. S.T. and E.M. performed the ELA typing, and D.B., M.R.R. and C.W. analysed the data. D.B. and C.W. wrote the manuscript, which was then critically revised by all authors.
Competing interests
We declare we have no competing interests.
Funding
This research was supported by the ISMEquine Research and the Swiss National Science Foundation (310030-138295, 310030-129837/1 and 31003A-159579).
References
- 1.Davies DM. 2013. The compatibility gene. London, UK: Allen Lane. [Google Scholar]
- 2.Ruff JS, Nelson AC, Kubinak JL, Potts WK. 2012. MHC signaling during social communication. Adv. Exp. Med. Biol. 738, 290–313. ( 10.1007/978-1-4614-1680-7_17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Milinski M, Croy I, Hummel T, Boehm T. 2013. Major histocompatibility complex peptide ligands as olfactory cues in human body odour assessment. Proc. R. Soc. B 280, 20122289 ( 10.1098/rspb.2012.2889) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kromer J, Hummel T, Pietrowski D, Giani AS, Sauter J, Ehninger G, Schmidt AH, Croy I. 2016. Influence of HLA on human partnership and sexual satisfaction. Sci. Rep. 6, 32550 ( 10.1038/srep32550) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burger D, Dolivo G, Marti E, Sieme H, Wedekind C. 2015. Female major histocompatibility complex type affects male testosterone levels and sperm number in the horse (Equus caballus). Proc. R. Soc. B 282, 20150407 ( 10.1098/rspb.2015.0407) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burger D, Meuwly C, Marti E, Sieme H, Oberthur M, Janda J, Meinecke-Tillmann S, Wedekind C. 2017. MHC-correlated preferences in diestrous female horses (Equus caballus). Theriogenology 89, 318– 323.e311 ( 10.1016/j.theriogenology.2016.09.015) [DOI] [PubMed] [Google Scholar]
- 7.Kamiya T, O'Dwyer K, Westerdahl H, Senior A, Nakagawa S. 2014. A quantitative review of MHC-based mating preference: the role of diversity and dissimilarity. Mol. Ecol. 23, 5151–5163. ( 10.1111/mec.12934) [DOI] [PubMed] [Google Scholar]
- 8.Winternitz J, Abbate JL, Huchard E, Havlicek J, Garamszegi LZ. 2017. Patterns of MHC-dependent mate selection in humans and nonhuman primates: a meta-analysis. Mol. Ecol. 26, 668–688. ( 10.1111/mec.13920) [DOI] [PubMed] [Google Scholar]
- 9.Leclaire S, Strandh M, Mardon J, Westerdahl H, Bonadonna F. 2017. Odour-based discrimination of similarity at the major histocompatibility complex in birds. Proc. R. Soc. B 284, 2016.2466 ( 10.1098/rspb.2016.2466) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leinders-Zufall T, et al. 2004. MHC class I peptides as chemosensory signals in the vomeronasal organ. Science 306, 1033–1037. ( 10.1126/science.1102818) [DOI] [PubMed] [Google Scholar]
- 11.Milinski M, Griffiths S, Wegner KM, Reusch TBH, Haas-Assenbaum A, Boehm T. 2005. Mate choice decisions of stickleback females predictably modified by MHC peptide ligands. Proc. Natl Acad. Sci. USA 102, 4414–4418. ( 10.1073/pnas.0408264102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spehr M, Kelliher KR, Li XH, Boehm T, Leinders-Zufall T, Zufall F. 2006. Essential role of the main olfactory system in social recognition of major histocompatibility complex peptide ligands. J. Neurosci. 26, 1961–1970. ( 10.1523/jneurosci.4939-05.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Penn DJ, Potts WK. 1999. The evolution of mating preferences and major histocompatibility complex genes. Am. Nat. 153, 145–164. ( 10.1086/303166) [DOI] [PubMed] [Google Scholar]
- 14.Wedekind C, Walker M, Little TJ. 2005. The course of malaria in mice: major histocompatibility complex (MHC) effects, but no general MHC heterozygote advantage in single-strain infections. Genetics 170, 1427–1430. ( 10.1534/genetics.105.040683) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Penn DJ, Damjanovich K, Potts WK. 2002. MHC heterozygosity confers a selective advantage against multiple-strain infections. Proc. Natl Acad. Sci. USA 99, 11 260–11 264. ( 10.1073/pnas.162006499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McClelland EE, Penn D, Potts WK. 2003. Major histocompatibility complex heterozygote superiority during coinfection. Infect. Immun. 71, 2079–2086. ( 10.1128/IAI.71.4.2079-2086.2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lovlie H, Gillingham MAF, Worley K, Pizzari T, Richardson DS. 2013. Cryptic female choice favours sperm from major histocompatibility complex-dissimilar males. Proc. R. Soc. B 280, 20131296 ( 10.1098/rspb.2013.1296) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Firman RC, Gasparini C, Manier MK, Pizzari T. 2017. Postmating female control: 20 years of cryptic female choice. Trends Ecol. Evol. 32, 368–382. ( 10.1016/j.tree.2017.02.010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wedekind C, Chapuisat M, Macas E, Rülicke T. 1996. Non-random fertilization in mice correlates with the MHC and something else. Heredity 77, 400–409. ( 10.1038/hdy.1996.160). [DOI] [PubMed] [Google Scholar]
- 20.Firman RC, Simmons LW. 2015. Gametic interactions promote inbreeding avoidance in house mice. Ecol. Lett. 18, 937–943. ( 10.1111/ele.12471) [DOI] [PubMed] [Google Scholar]
- 21.Rülicke T, Chapuisat M, Homberger FR, Macas E, Wedekind C. 1998. MHC-genotype of progeny influenced by parental infection. Proc. R. Soc. Lond. B 265, 711–716. ( 10.1098/rspb.1998.0351) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stearns SC. 2012. Evolutionary medicine: its scope, interest and potential. Proc. R. Soc. B 279, 4305–4321. ( 10.1098/rspb.2012.1326) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilcox AJ, Weinberg CR, Oconnor JF, Baird DD, Schlatterer JP, Canfield RE, Armstrong EG, Nisula BC. 1988. Incidence of early loss of pregnancy. N. Engl. J. Med. 319, 189–194. ( 10.1056/nejm198807283190401) [DOI] [PubMed] [Google Scholar]
- 24.Ober C, Elias S, Kostyu DD, Hauck WW. 1992. Decreased fecundability in Hutterite couples sharing HLA-DR. Am. J. Hum. Genet. 50, 6–14. [PMC free article] [PubMed] [Google Scholar]
- 25.Hedrick PW, Thomson G. 1983. Evidence for balancing selection at HLA. Genetics 104, 449–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newcombe J. 2011. Human chorionic gonadotropin. In Equine reproduction (eds McKinnon AO, Squires EL, Vaala WE, Varner DD), pp. 1804–1810, 2nd edn Chichester, UK: Wiley-Blackwell. [Google Scholar]
- 27.Betteridge KJ. 2007. Equine embryology: an inventory of unanswered questions. Theriogenology 68, S9–S21. ( 10.1016/j.theriogenology.2007.04.037) [DOI] [PubMed] [Google Scholar]
- 28.McCue PM, McKinnon AO. 2011. Pregnancy examination. In Equine reproduction (eds McKinnon AO, Squires EL, Vaala WE, Varner DD), pp. 2245–2261, 2nd edn Chichester, UK: Wiley-Blackwell. [Google Scholar]
- 29.Wang J. 2002. An estimator for pairwise relatedness using molecular markers. Genetics 160, 1203–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kraemer P, Gerlach G. 2017. Demerelate: calculating interindividual relatedness for kinship analysis based on codominant diploid genetic markers using R. Mol. Ecol. Resour. 17, 1371–1377. ( 10.1111/1755-0998.12666) [DOI] [PubMed] [Google Scholar]
- 31.R Development Core Team. 2011. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. See http://www.r-project.org/. [Google Scholar]
- 32.Bates D, Maechler M, Bolker B. 2011. lme4: linear mixed-effects models using S4 classes. R package version 0.999375-39 See http://lme4.r-forge.r-project.org.
- 33.Bowling AT, Touchberry RW. 1990. Parentage of Great-Basin feral horses. J. Wildl. Manag. 54, 424–429. ( 10.2307/3809652) [DOI] [Google Scholar]
- 34.Linklater WL, Cameron EZ. 2009. Social dispersal but with philopatry reveals incest avoidance in a polygynous ungulate. Anim. Behav. 77, 1085–1093. ( 10.1006/anbe.1999.1155) [DOI] [Google Scholar]
- 35.Boyd L, Keiper R. 2005. Behavioral ecology of feral horses. In The domestic horse: the origins, development, and management of its behaviour (eds Mills DS, McDonnell SM), pp. 55–82. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 36.Pischedda A, Rice WR. 2012. Partitioning sexual selection into its mating success and fertilization success components. Proc. Natl Acad. Sci. USA 109, 2049–2053. ( 10.1073/pnas.1110841109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cameron EZ, Linklater WL. 2007. Extreme sex ratio variation in relation to change in condition around conception. Biol. Lett. 3, 395–397. ( 10.1098/rsbl.2007.0089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bartos L, Bartosova J, Pluhacek J, Sindelarova J. 2011. Promiscuous behaviour disrupts pregnancy block in domestic horse mares. Behav. Ecol. Sociobiol. 65, 1567–1572. ( 10.1007/s00265-011-1166-6) [DOI] [Google Scholar]
- 39.Roberts SC, Gosling LM. 2003. Genetic similarity and quality interact in mate choice decisions by female mice. Nat. Genet. 35, 103–106. ( 10.1038/ng1231) [DOI] [PubMed] [Google Scholar]
- 40.Sherborne AL, Thom MD, Paterson S, Jury F, Ollier WER, Stockley P, Beynon RJ, Hurst JL. 2007. The genetic basis of inbreeding avoidance in house mice. Curr. Biol. 17, 2061–2066. ( 10.1016/j.cub.2007.10.041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamazaki K, Beauchamp GK, Wysocki CJ, Bard J, Thomas L, Boyse EA. 1983. Recognition of H-2 types in relation to the blocking of pregnancy in mice. Science 221, 186–188. ( 10.1126/science.6857281) [DOI] [PubMed] [Google Scholar]
- 42.Bruce HM. 1959. Exteroceptive block to pregnancy in the mouse. Nature 184, 105 ( 10.1038/184105a0) [DOI] [PubMed] [Google Scholar]
- 43.Marashi V, Rülicke T. 2012. The Bruce effect in Norway rats. Biol. Reprod. 86, 17 ( 10.1095/biolreprod.111.093104) [DOI] [PubMed] [Google Scholar]
- 44.Roberts EK, Lu A, Bergman TJ, Beehner JC. 2012. A Bruce effect in wild geladas. Science 335, 1222–1225. ( 10.1126/science.1213600) [DOI] [PubMed] [Google Scholar]
- 45.Hausfater G, Hrdy SB. 2008. Infanticide: comparative and evolutionary perspectives. New York, NY: Aldine. [Google Scholar]
- 46.Berger J. 1983. Induced abortion and social factors in wild horses. Nature 303, 59–61. ( 10.1038/303059a0) [DOI] [PubMed] [Google Scholar]
- 47.Pluhacek J, Bartos L. 2000. Male infanticide in captive plains zebra, Equus burchelli. Anim. Behav. 59, 689–694. ( 10.1006/anbe.1999.1371) [DOI] [PubMed] [Google Scholar]
- 48.Rülicke T, Guncz N, Wedekind C. 2006. Early maternal investment in mice: no evidence for compatible-genes sexual selection despite hybrid vigor. J. Evol. Biol. 19, 922–928. ( 10.1111/j.1420-9101.2005.01039.x) [DOI] [PubMed] [Google Scholar]
- 49.Vanderwall DK. 2008. Early embryonic loss in the mare. J. Equine Vet. Sci. 28, 691–702. ( 10.1016/j.jevs.2008.10.001) [DOI] [Google Scholar]
- 50.Beydoun H, Saftlas AF. 2005. Association of human leucocyte antigen sharing with recurrent spontaneous abortions. Tissue Antigens 65, 123–135. ( 10.1111/j.1399-0039.2005.00367.x) [DOI] [PubMed] [Google Scholar]
- 51.Meuleman T, Lashley L, Dekkers OM, van Lith JMM, Claas FHJ, Bloemenkamp KWM. 2015. HLA associations and HLA sharing in recurrent miscarriage: a systematic review and meta-analysis. Hum. Immunol. 76, 362–373. ( 10.1016/j.humimm.2015.02.004). [DOI] [PubMed] [Google Scholar]
- 52.Burger D, Thomas S, Aepli H, Dreyer M, Fabre G, Marti E, Sieme H, Robinson MR, Wedekind C. 2017. Data from: Major histocompatibility complex-linked social signalling affects female fertility Dryad Digital Repository. ( 10.5061/dryad.04k5q) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Burger D, Thomas S, Aepli H, Dreyer M, Fabre G, Marti E, Sieme H, Robinson MR, Wedekind C. 2017. Data from: Major histocompatibility complex-linked social signalling affects female fertility Dryad Digital Repository. ( 10.5061/dryad.04k5q) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The datasets supporting this article can be accessed at Dryad Digital Repository: (http://dx.doi.org/10.5061/dryad.04k5q) [52].