Abstract
Phenotypic variability is increasingly assessed through functional response and effect traits, which provide a mechanistic framework for investigating how an organism responds to varying ecological factors and how these responses affect ecosystem functioning. Covariation between response and effect traits has been poorly examined at the intraspecific level, thus hampering progress in understanding how phenotypic variability alters the role of organisms in ecosystems. Using a multi-trait approach and a nine-month longitudinal monitoring of individual red-swamp crayfish (Procambarus clarkii), we demonstrated that most of the measured response and effect traits were partially stable during the ontogeny of individuals. Suites of response and effect traits were associated with a response syndrome and an effect syndrome, respectively, which were correlated to form a functional syndrome. Using a bioenergetic model, we predicted that differences in the response syndrome composition of hypothetical populations had important ecological effects on a key ecosystem process (i.e. whole-lake litter decomposition) to a level similar to those induced by doubling population size. Demonstrating the existence of a functional syndrome is likely to improve our understanding of the ecological impacts of phenotypic variation among individuals in wild populations across levels of biological organization, and the linkage between ecosystem and evolutionary ecology.
Keywords: interindividual variability, functional traits, biological invasions, ecosystem functioning, eco-evolutionary dynamics, ecosystem modelling
1. Introduction
While trait variability among individuals has been historically accounted for in evolutionary sciences, it has merely been seen as noise around the average phenotype of a species by community and ecosystem ecologists [1–3]. Recent advances have, however, suggested that not only organism phenotypes are affected by their environment, but that they can reciprocally act on it [1,4], coupling ecological and evolutionary processes in a dynamic relationship [5–7]. In this context, the ecological consequences of interindividual variability are increasingly recognized, and recent studies have demonstrated broad consequences of phenotypic variability on key ecosystem processes such as primary production and leaf litter decomposition [8–11]. To date, however, most studies have focused on the ecosystem effects of a single phenotypic trait (e.g. morphology, body mass) despite the fact that individuals can simultaneously vary in multiple phenotypic traits [12]. Therefore, a multi-trait approach is needed to provide an integrative understanding of the effects of individuals on ecosystems.
From a functional perspective, phenotypic traits have been classified as functional effect traits or functional response traits [13,14]. On the one hand, functional effect traits determine how and to what extent an organism influences energy flow and matter transformation in an ecosystem [14]. For instance, nitrogen excretion rate is considered as an effect trait because it induces changes in nutrient availability resulting in altered algal growth, thus modifying primary productivity [15]. On the other hand, functional response traits determine how an organism responds to environmental conditions [13,14]. For instance, the presence of predators may reduce individual activity [16]; therefore, activity level is considered as a response trait. Studies linking intraspecific trait variability to ecosystem functioning have mostly focused on response trait variations (e.g. sex ratio [17], morphology [18] or behaviour [19]). However, response traits and ecosystem processes are not proximately related, and therefore such relationships are conditional on covariations between response and effect traits. For instance, phenotypic variations in guppies (Poecilia reticuata) have been demonstrated to impact primary productivity through a correlation between individual life history and nitrogen excretion rate [8].
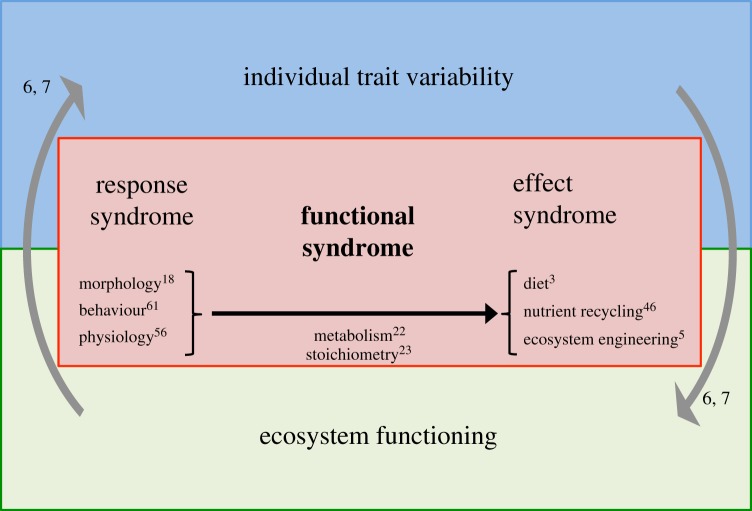
Evolutionary biologists have shed light on various patterns of covariations in life history and behavioural traits. These correlations among phenotypic traits are termed syndrome, including the life history and behavioural syndromes [12,20]. The ‘pace of life syndrome’ hypothesis further states that trait covariation extends over several phenotypic aspects including the life history, behaviour and physiology of organisms [21]. These syndromes are best understood as having emerged from evolutionary processes and, therefore, lack relevance to infer the relationship between response and effect which are underlined in eco-evolutionary dynamics [6,7]. This leads us to introduce the concept of ‘functional syndrome’ which we define as the association between correlated suites of response and effect traits (i.e. between a ‘response syndrome’ and an ‘effect syndrome’, figure 1). The dependence of effect traits upon response traits may arise from metabolic and stoichiometric constraints (metabolic theory of ecology [22] and ecological stoichiometry [23]). For instance, fast-paced individuals with high metabolic and nutrient requirements are expected to exert stronger top-down control on their resources than slow-growing individuals. Nutrient immobilization—contributing to growth rate—may result in a slower rate of nutrient excretion of fast-paced individuals than slow-paced individuals [23].
Figure 1.
Conceptual representation of the functional syndrome. Association between response and effect traits (represented by the central arrow) is at the core of the interactions between individual trait variability and ecosystem functioning that are linked through eco-evolutionary dynamics (represented by the grey arrows). Examples of categories of response traits (through which individuals adapt to their environment) and of effect traits (by which individuals act on their ecosystem) are provided under their respective syndrome. The superscripts refer to cited references. (Online version in colour.)
The aim of the present study was to test the existence of a functional syndrome linking functional response and effect traits and to use this association to predict the effects of intraspecific variability on ecosystem functioning. Using the red-swamp crayfish (Procambarus clarkii) as the model species, we monitored individuals longitudinally (nine months) and repeatedly quantified several response and effect traits. We then applied a mass-balance bioenergetic model in a Bayesian framework to predict the ecosystem consequences of hypothetical populations varying in their phenotypic traits. First, we tested the hypotheses that (i) response traits (boldness, anxiety, chelae morphology and growth rate) were consistent over time and correlated to form a response syndrome, and that (ii) effect traits (voracity, predation rate, leaf consumption rate, egestion rate and ammonium excretion rate) were also consistent over time and correlated to form an effect syndrome. Second, we tested the hypothesis that the response and effect syndromes were associated to form a functional syndrome. Because traits were quantified across several ontogenetic stages, we also tested the body mass independency of these associations. Third, we simulated hypothetical populations differing in size and response syndrome composition to predict ecosystem consequences (whole-lake litter decomposition dynamics).
2. Material and methods
(a). Model species
Native to North and Central America, the red-swamp crayfish has been introduced and established throughout Europe [24]. Described as an opportunistic and omnivorous species, it is considered as an ecosystem engineer and its ecological impacts include decreased macrophyte biomass through direct consumption [24], increased water turbidity driven by burrowing behaviour [25] and changes in the phenology of litter decomposition [26]. Importantly, it has been demonstrated to display anxiety-like behaviour [27].
(b). Animal rearing and experimental design
In June 2014, 240 juveniles (carapace length range: 20.95–35.14 mm) were collected from a single population (Lake Lamartine, southwestern France; 43°30'21.5″ N, 1°20'32.7″ E). To avoid any behavioural bias caused by the sampling method [28], individuals were captured using several active and passive methods in all habitats of the lake. In the laboratory, we maintained crayfish in 50 l tanks and marked them individually using PIT (Passive Integrated Transponder) tags (8 × 1.4 mm, FDX-B ‘skinny’ PIT tag, Oregon RFID, USA), inserted at the base of the fifth pereiopod pair through an incision made with a sterile scalpel [29]. Sixty-four individuals were chosen for the experiment to maximize interindividual variability based on boldness measurements performed in July 2014. For selection, we classified individuals into three categories (electronic supplementary material) and randomly picked 20 individuals from each category (and two extra individuals from the extreme categories). We chose to maximize interindividual variability in order to increase our statistical power to detect existing correlations among traits. The experiment lasted 289 days (see electronic supplementary material for rearing conditions) and a total of 55 individuals survived.
(c). Functional trait measurements
(i). Response traits
Boldness and anxiety-like behaviour were assessed six times (see details in electronic supplementary material, table S1 and figure S1) using corridors covered with 2 cm of sand at the bottom and filled with 37.5 l of dechlorinated tap water and 2 l of water from tanks with conspecifics. The corridors (electronic supplementary material, figure S2) contained two dark areas separated by a light area. Each corridor (n = 5) was separated by opaque walls to avoid visual contact between individuals and surrounded by curtains to obtain homogeneous light conditions. Individuals were first acclimated for 20 min in an opaque container to reduce stress level induced by handling. After being gently released in one of the dark areas for 10 min of acclimation, the sliding door was opened and individuals were filmed for 10 min. Video footage was subsequently analysed using Observer (Noldus Information Technology, Wageningen, The Netherlands). Boldness was quantified as the time before emergence from the acclimation area. We inversed the scores to associate higher values with higher boldness [30]. Anxiety-like behaviour was assessed as the proportion of time spent in dark zones after original emergence from the acclimation zone, representing a stress avoidance behaviour [27]. The order and the corridor in which individuals were assayed were randomly attributed at the first trial and were fixed for all repetitions. All behavioural assays were performed in the morning (08.00–12.00 h) to minimize the potential effects of circadian rhythms. Individuals were starved for 2 days prior to each behavioural assay.
Chelae are extremely important and costly organs for crayfish [31] and chelae morphology was selected as a response trait because they are used for individual defence against predators and competition with conspecifics [32]. Although they require a higher energy investment, large and arched chelae make individuals stronger competitors and less vulnerable prey [33]. Chelae morphology was quantified using two complementary approaches: morphometric ratio and shape determination using landmark coordinates. Individual right chela and body were pictured and measurements (chela length CLL, carapace length CL, chela width CW and palm length PL) were performed using ImageJ. Morphometric ratios that represent energy allocation to the chelae (CLL/CL) and chelae relative width (CW/PL; CW/CL) were then calculated [34]. We digitalized seven landmarks (adapted from [33] using TpsDig2 [35]) to evaluate chelae shape. A principal component analysis (PCA; package ade4 on R [36,37]) was performed on partial warp scores (TpsRelw) to obtain a chelae shape score for each individual. A second PCA was then performed on residuals of morphometric ratios with sex (because of potential sexual dimorphism) and chelae shape to obtain an integrative score of chelae morphology.
Growth rate is strongly dependent on individual food intake, metabolism and assimilation efficiency of nutrients and was quantified six times by weighing individuals (nearest 0.01 g) on seven occasions (electronic supplementary material, figure S1). Specific growth rate (SGR, % d−1) was calculated as
where Wf and Wi were the final and initial body mass, respectively, and T the time interval between two measurements, expressed in days.
(ii). Effect traits
Predation rate is an important effect trait because it can impact ecosystem functioning (e.g. trophic cascade). Predation was quantified using individual containers filled with 2.5 l of dechlorinated tap water and 20 previously frozen chironomids per container. Chironomids were selected because they are one of the most abundant littoral invertebrates in the study area and are commonly consumed by red-swamp crayfish outside of its native range [38]. Commercially available frozen chironomids were purchased at the start of the experiment to ensure that all measurements of predation were performed using prey similar in size and origin throughout the experiment. After 10 min of acclimation, individuals were allowed to access the chironomids for 10 min. Individuals were then removed and the number of remaining chironomids was counted. Predation rate was quantified twice for each individual at the beginning of the experiment (electronic supplementary material, figure S1) when individuals were the youngest because juveniles have a more carnivorous diet than subadults [39]. Hunger state was controlled by starving individuals for 2 days before experimentation.
Voracity (i.e. individual foraging activity [32] associated with individual behaviour and physiology) was quantified nine times for each individual, in the home tank at 09.00, three times per week during three consecutive weeks (electronic supplementary material, figure S1). Individuals were starved for 2 days before each measurement. The voracity test consisted of introducing four pellets of food in each tank and quantifying the number of pellets consumed after 20 min. We cumulated the scores for each week to obtain three measurements of voracity.
Leaf consumption and egestion rates were used as relevant effect traits depicting crayfish impacts on detritus dynamics and carbon cycle [40]. Consumption reduces stock of coarse particulate organic matter [26], while egestion of faeces enhances nutrient recycling by microorganisms [41]. Consumption rate (g d−1) of abscised leaves of black poplar (Populus nigra) was quantified three times (electronic supplementary material, figure S1) for each individual. Prior to the experiment, leaves were submerged for two weeks in a pond to allow microbial conditioning, a process that improves leaf palatability to detritivores [42]. Batches of air-dried leaves (4.0 g) were enclosed in 0.5 mm nylon mesh bags to prevent invertebrates in the pond from accessing the leaves. At retrieval, the leaves were rinsed with demineralized water to remove fine sediments. Crayfish were placed in a container filled with 2.5 l of dechlorinated tap water with an air stone for oxygenation. After 5 h of acclimation, conditioned leaves were introduced and left for 72 h. The remaining leaf material was then oven-dried at 70°C for 48 h and subsequently weighed to the nearest 0.01 g. Five controls without crayfish were used to quantify leaf mass loss due to microbial decomposition and leaching; this mass loss was accounted for when calculating crayfish consumption rate. Water from each container was filtered through two sieves: 1 mm mesh size to remove small leaf fragments and 50 µm mesh size to collect the faeces released by crayfish. The faecal matter was oven-dried at 60°C for 72 h and weighed to the nearest 0.01 mg. Egestion rate was expressed in grams per day.
Nitrogen excretion rate was quantified by measuring excretion of dissolved ammonium NH4+, which is a metabolic waste produced during the breakdown of proteins and amino acids [43]. Changes in NH4+ concentration can affect ecosystem functioning through an increase in nutrient availability [44] and primary production [45,46]. Excretion rate was quantified three times for each individual (electronic supplementary material, figure S1). All individuals were fed ad libitum the day before and 2 h prior to the start of the excretion experiment by adding three pellets to each tank. Individuals were then placed in plastic bags containing 500 ml of spring bottled water for 2 h [47]. Individuals were finally removed and 100 ml of water was filtered through a glass microfibre filter (Whatman, GF/C, diameter = 25 mm) and samples were frozen at −20°C. Excretion rate (NH4+, mg l−1 h−1) was determined using a high-performance ionic chromatograph (Dionex DX-120).
(d). Statistical analysis and modelling
(i). Response trait syndrome
First, we assessed the level of individual repeatability of each response trait and the correlations among them. Boldness, growth rate, morphology and voracity were measured for a total of 55 individuals. Because some individuals never left the acclimation area, anxiety-like behaviour was measured on 50 individuals. Generalized linear mixed models (package lme4 [48]) were used to test the repeatability of traits assuming Gaussian errors. For all models, we fitted time as a fixed effect and individuals as a random effect. Additional random effects were included to control for potential sources of variation owing to experimental design (corridor for boldness and anxiety-like behaviour tests and shelf for growth rate). Repeatability was quantified using the intraclass coefficient correlation [49]. The significance of the repeatability (i.e. variance explained by between individual differences) was tested using a likelihood ratio test by comparing the model with individual as random effect to an alternative model without this random effect.
Correlations among response traits were tested based on averaged trait values calculated across temporal replicates. Boldness, anxiety-like behaviour and growth rate were, however, averaged for repetitions made in 2015 to compare response traits measured at the same time as effect traits. We assessed correlations among response traits using averaged values instead of all repeats. This approach prevents comparing intra- and interindividual correlations, but, in the present study, not all traits were measured at the same time and we primarily focused on interindividual correlation. Correlations among response traits were tested using Spearman's rank correlations.
(ii). Effect trait syndrome
Because of moulting, consumption and egestion rates were measured on 52 individuals, excretion rate on 53 and predation rate on 55. The repeatability of effect traits was tested as previously described (shelves used as additional random term). Effect traits were then averaged across temporal replicates and correlations among effect traits were tested using Spearman's ranks correlations.
(iii). Relationship between response and effect traits
We used partial least-squares path modelling (PLS-PM, plspm package [50]) to summarize the trait covariance structure and to compute latent (i.e. proxy) variables representing response and effect syndromes. This technique is a robust form of structural equation modelling that relies on fewer assumptions than does covariance-based structural equation modelling [50,51]. PLS-PM is suitable for examining relationships between blocks of associated traits because latent variables are formed as linear combinations of traits without imposing any restrictions on within-block covariances. We constructed a simple path model wherein effect traits were conditioned upon response traits and individual body mass (averaged over three measurements) was specified as a mediator of this relationship. Body mass is known to be correlated with both effect and response traits; therefore, some variations in effect traits may be due to differences in crayfish body mass. Standardized path coefficients were used to evaluate the strength of relationships tested in the model. We calculated the product of the path coefficients along the mediation pathway to assess the strength of the mass-dependent relationship between response and effect traits. The construction of response and effect syndromes was examined using correlations between traits and the latent variables they form (i.e. loadings). We removed the traits with the lowest contribution to the latent variables (i.e. boldness and predation) to obtain stable and accurate parameter estimates [50]. Significance was assessed using 95% percentile confidence intervals (CIs) calculated on 200 bootstrap samples. PLS-PM was performed on a subset of 47 individuals for which no missing trait values occurred.
(iv). Modelling consequences on ecosystem functioning
A mass-balance bioenergetic model (electronic supplementary material, table S2) was used to assess the ecosystem impact of the link between the response and effect traits. Variations among individuals in their consumption rates can act on litter decomposition, a key ecosystem function of freshwater ecosystems [40]. As consumption is linked to individual growth rate, population biomass is also associated with response traits. The bioenergetic model was based on individual consumption and was modified to include the link between the response trait syndrome and consumption rate. To do so, we first evaluated the effect of the response syndrome (the latent variable extracted from the PLS-PM) on leaf consumption rate based on experimental data using a linear regression in a Bayesian framework. We then used these outputs (i.e. estimated regression parameters) and projected values of response traits syndrome and daily temperature (electronic supplementary material, table S2) to simulate growth rate, consumption rate and population biomass over a year using an individual bioenergetic model (see electronic supplementary material).
Simulations were performed on a sequence of 11 hypothetical populations composed of individuals with different syndrome values sampled along the observed distribution. We also added one control population composed of individuals with fully random syndrome values. Each population was modelled with 11 densities ranging from 1000 to 2000 individuals (simulating a biological invasion process). To estimate the effects of the simulated populations on decomposition rate and population biomass, the environmental factors were sourced from a realistic ecosystem (daily temperature and litter input). The decomposition rate was estimated over 1 year as k = −ln(X)/t [52], where X is the proportion of litter remaining after consumption by the crayfish and t the time elapsed in years. To assess whether different population induced differences in final biomass, we quantified the difference between biased response trait syndrome and control populations. All statistical analyses were performed using R software [36] unless specified otherwise.
3. Results
Overall, we observed various levels of trait variability among individuals. For instance, the mean boldness varied from 44 s (±32 s.d.) to 473 s (±87 s.d.) across the 55 individuals. The mean anxiety-like behaviour ranged from 0.43 (±0.05 s.d.) to 0.74 (±0.14 s.d.). Growth rate was also variable among individuals, ranging from 0.14 (±0.34 s.d.) to 0.63 (±0.47 s.d.) % d−1. Effect traits varied among individuals. Predation rate varied from 4.0 (±5.5 s.d.) to 17.5 (±3.5 s.d.) chironomids eaten in 10 min, leaf consumption rate ranged from 0.00 (±0.00 s.d.) to 0.31 (±0.01 s.d.) g d−1, voracity ranged from 0.1 (±0.3 s.d.) to 4.0 (±0.0 s.d.) pellets eaten in 20 min, egestion rate ranged from 0.002 (±0.001 s.d.) to 0.18 (±0.033 s.d.) g d−1 and excretion rate from 0.05 (±0.02 s.d.) to 0.18 (±0.06 s.d.) mg l−1 h−1.
(a). Response and effect syndromes
Boldness and anxiety-like behaviour were significantly repeatable over nine months (electronic supplementary material, table S3; generalized linear mixed model, boldness: ICC = 0.31, χ2 = 49.49, p < 0.001, anxiety-like behaviour: ICC = 0.14, χ2 = 9.67, p = 0.002). However, growth rate was not repeatable (ICC = 0.00, χ2 = 0.00, p = 1). The morphological axis (first PCA axis: 51% of total variance explained) was explained by the energy allocation to chelae compared with the body (loading component: 0.51), chelae width (0.42 and 0.58) and chelae shape (−0.46); that is individuals with higher morphological scores had, proportionally to their body, larger, longer and more arched chelae. Morphology was significantly and positively correlated with boldness (ρ = 0.25, p = 0.043), while boldness and anxiety-like behaviour were negatively correlated (ρ = −0.30, p = 0.034). Other correlations were non-significant (electronic supplementary material, table S4).
Predation, leaf consumption and voracity were significantly repeatable over time (electronic supplementary material, table S3; ICC = 0.34, χ2 = 6.86, p = 0.009, ICC = 0.24, χ2 = 8.11, p = 0.004 and ICC = 0.78, χ2 = 114.24, p < 0.001, respectively). Egestion rate was repeatable (ICC = 0.37, χ2 = 19.01, p < 0.001) while ammonium excretion rate was not significantly repeatable (ICC = 0.00, χ2 = 0.00, p = 1). Consumption, egestion and excretion rates were all correlated among each other (consumption–egestion: ρ = 0.94, p < 0.001, consumption–excretion: ρ = 0.38, p = 0.006 and egestion–excretion: ρ = 0.40, p = 0.003; electronic supplementary material, table S5). Voracity was correlated with the rates of leaf consumption, egestion and excretion (ρ = 0.65, p < 0.001, ρ = 0.64, p < 0.001 and ρ = 0.29, p = 0.031, respectively). Predation rate was not correlated with any other effect traits (electronic supplementary material, table S5).
(b). Functional syndrome
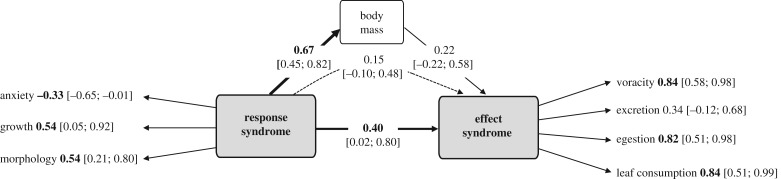
The latent variable corresponding to the response syndrome was positively associated with growth rate and chelae morphology and negatively with anxiety-like behaviour (figure 2). The second latent variable correlated with voracity, leaf consumption, egestion and, to a lesser extent, excretion rates to form an effect syndrome (figure 2). The response syndrome and body mass were positively linked (path coefficient = 0.67, 95% percentile CIs = (0.45; 0.82), R2 = 0.46). The effect syndrome was directly and positively associated with the response syndrome (path coefficient = 0.40, CI = (0.02; 0.80)) but not with body mass (path coefficient = 0.22, CI = (−0.22; 0.58), R2 = 0.37). This led to an indirect association of response syndrome on effect syndrome with a strength that was not significant and lower than the direct effect (path coefficient = 0.15, CI = (−0.10; 0.48)).
Figure 2.
Representation of the PLS-PM model assessing relationships between response syndrome, body mass and effect syndrome (goodness-of-fit of the overall model = 0.42). The width of arrows connecting boxes is proportional to the mean value of standardized path coefficient (displayed on the arrows). The dashed arrow represents the mass-dependent relationship between the response syndrome and effect syndrome. Its strength was calculated as the product of the path coefficient from the response syndrome to body mass and the path coefficient from body mass to the effect syndrome (i.e. 0.15). Loadings associated with response and effect traits indicate how they contribute to the response and effect syndromes (i.e. latent variables), respectively. Values reported in square brackets represent 95% percentile CIs calculated on 200 bootstrap samples and significant path coefficients and loadings are displayed in bold. Boldness (response trait) and predation (effect trait) were removed from the model due to their weak correlation with their respective latent variable.
(c). Consequences of trait variability on ecosystem functioning
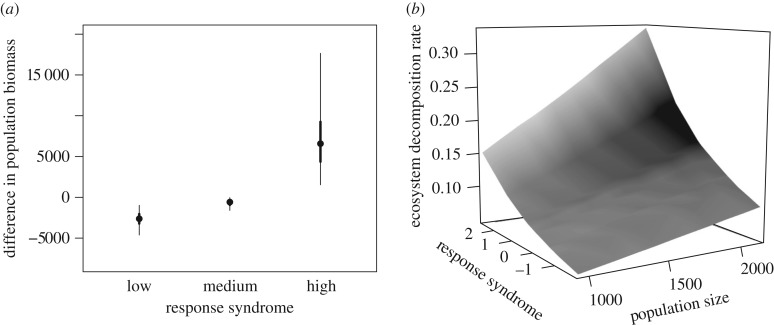
The model predicted that differences in response trait syndrome values induced a change in final population biomass when compared with a control population with individuals with random trait values (figure 3a). Specifically, population biomass was higher for a population with high response trait syndrome than for the control population, while it was lower for a population with low response trait syndrome (figure 3a). The model also predicted a higher decomposition rate for hypothetical populations with higher response trait values, independent of population density (figure 3b). The decomposition rate after 1 year was twice as high for populations with high syndrome values compared with populations with low values (figure 3b). Doubling population size (i.e. from 1000 to 2000 individuals) led to the same effect on decomposition rate than changing from lowest to highest syndrome values. For instance, the decomposition rate for a population composed of 2000 individuals with a low response trait syndrome value was similar to that of a population of 1000 individuals with a high response trait syndrome (k = 0.094 yr−1, 95% predictive interval (PI) = (0.058; 0.142) and k = 0.153 yr−1, PI = (0.087; 0.295), respectively).
Figure 3.
(a) Difference in crayfish biomass between populations composed of individuals with different response syndromes and a control population (random trait values). Circles represent the medians of the posterior predictive distribution, the thin error bars represent 95% posterior predictive distribution and the bold error bars represent 50% posterior predictive distribution. (b) Three-dimensional representation of the relationship between response syndrome, population size (number of individuals) and decomposition rate (k) based on medians of the posterior predictive distribution.
4. Discussion
Providing a mechanistic and integrative framework to understand how variations in phenotypic traits affect ecosystem functioning is crucial. Using a multi-trait approach that explicitly integrated functional response and effect traits, we first demonstrated the existence of a response syndrome based on the covariation among a suite of response traits (morphology, growth rate and anxiety-like behaviour). We then found that effect traits (voracity, leaf consumption, egestion and excretion rates) formed an effect syndrome, which was associated with the response syndrome, revealing the existence of a more general, integrative and mass-independent functional syndrome linking response to effect syndromes. We finally predicted that differences in response syndrome composition of hypothetical populations led to differences in ecosystem functioning.
Our results demonstrated that the ecological effects of intraspecific variability at higher levels of biological organization were rooted in the covariations between response and effect traits. Therefore, the functional syndrome may represent an underappreciated link between genetic and environmental factors acting on individuals [53] and the consequences of individuals on their environment [13]. This confirms the essential role of functional traits in eco-evolutionary dynamics [23], where variations in response traits are the basis for organisms to evolve when facing environmental variability and where the subsequent covariation with effect traits can influence environmental conditions (figure 1). Consequently, the functional syndrome should provide novel insights into eco-evolutionary studies and could therefore represent a new linkage between ecosystem and evolutionary ecology [5]. To test for the existence of a functional syndrome and fully embrace the importance of phenotypic variability for ecosystems, we suggest using a multi-trait and multi-step approach. First, we recommend measuring several phenotypic traits on each individual and explicitly discriminating response and effect trait when designing individual phenotypic studies. Second, associations among response traits (response syndrome) and effects traits (effect syndrome) should be tested independently. Third, linkage between the two aforementioned syndromes should be tested. While the repeatability of traits involved can inform on the stability of impacts of intraspecific trait variability on ecosystem functioning, we suggest that functional syndromes may result from correlations among traits arising from both intra- and interindividual covariations of traits [54,55] and might therefore not require the repeatability of all traits involved in the syndrome. The functional syndrome may further vary with the environmental contexts, due to selective pressures and plastic changes, even if its structure in a given context is still crucial for ecosystem functioning. Quantifying the temporal consistency and environmental dependency of the functional syndrome should provide an integrative understanding of the ecosystem response to phenotypic variability.
Our response syndrome suggested that some individuals grew more, had higher energy investment in costly organs such as chelae and were less anxious. This syndrome was correlated with trophic traits such as voracity and leaf consumption, and with non-trophic traits such as the rates of nitrogen excretion and egestion. This is not surprising because growth rate and energy investment in chelae are probably linked to ‘trophic traits’ such as leaf consumption rate and to ‘physiologic traits’ such as egestion rate. Importantly, we found that the functional syndrome was partially body mass-independent. Several intrinsic linkages (e.g. hormonal or physiological) exist between individual traits [56]. The covariation between response and effect traits could, for instance, be driven by metabolism [57,58]. Indeed, metabolism can vary among individuals with similar body mass [59] and this variation can in turn impact effect traits. Response trait syndrome was also associated with egestion and excretion rates which are closely related to metabolic activity [43]. Therefore, integrating metabolism (e.g. standard metabolic rate) in the functional syndrome might provide new mechanistic insights into the linkages among functional traits. Almost all traits involved in the functional syndrome were significantly repeatable at a level near the 0.34 average value reported in the literature for behavioural traits [60]. Conversely to previous observations [61], we found that growth rate was not repeatable. Growth patterns are strongly affected by the timing of moulting in crayfish, which was not recorded in the present study. However, as individuals got older, moulting became more asynchronous and less frequent. As growth rate was measured at intervals that were independent of moulting, it probably explains the absence of repeatability in growth rate. Individuals displayed consistent behavioural and physiological states, which may explain the temporal consistency of effect traits because of their interconnections. Importantly, we confirmed that effect traits could be repeatable over a relatively long period of crayfish lifetime (here 71 days, e.g. [61]), indicating that the effects of phenotypic variability on ecosystem functioning could be stable throughout individual life.
Our multi-trait approach suggested that response traits variability could impact several key ecosystem processes through correlation with effect traits (e.g. excretion rate affecting primary productivity and nutrient cycling [45], consumption and egestion rates acting on decomposition rate and detritus dynamics [40]). In addition, our modelling approach predicted impacts on litter stock dynamics and population biomass depending upon the composition in response traits of hypothetical populations. These impacts were similar to those induced by large changes in population size. This is particularly relevant in the context of biological invasions because invasive populations can display strongly phenotypically biased populations [62]. In addition, many natural (e.g. temperature [63]) and human-induced (e.g. biological invasions [64], pollution [65]) changes have been reported to alter the phenotypic structure of wild populations. Our knowledge on the distribution of phenotypic biases observed in the wild along gradients of environmental conditions is limited, and quantifying how functional syndromes vary across populations is clearly needed to quantify the ecosystem consequences of intraspecific variability. Nevertheless, changes in litter decomposition dynamics could ultimately have strong direct and indirect implications on invertebrates community [66], elemental cycling (release of dissolved organic and inorganic carbon [67]), food web dynamics and the phenology of ecosystem functioning [26].
In conclusion, our findings support the claim that, in community and ecosystem ecology, conspecific individuals should not be considered as functionally identical [1,2]. Because trait variability among individuals was structured and stable, we suggested the existence of a functional syndrome that we defined as the covariation between functional response traits and functional effect traits. Interesting perspectives would be to test the variability of this syndrome among populations. Indeed, as correlations among traits are context-dependent [68,69], determining how the local conditions (e.g. density, prey abundance or abiotic factors) modulate the functional syndrome is needed. It would also be of interest to assess how it is affected by species characteristics as this may modulate specific eco-evolutionary dynamics.
Supplementary Material
Acknowledgements
We are grateful to the gravière team and our numerous colleagues for their help. We also thank Robby Stoks and two anonymous reviewers for their constructive and useful comments on a previous version of the manuscript.
Ethics
Authorizations to collect and transport the crayfish and to perform the experiment were provided by the ‘Arrête Préfectoral 08-04-2014’.
Data accessibility
Data from trait measurements are available at Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.rm230) [70].
Authors' contributions
J.Cu., A.L. and J.Co. conceived the study. A.R., R.L. and J.Cu. carried the experiments with the A.L. and J.Co contributions. A.R. and R.L. collected and prepared the samples. M.B. wrote the bioenergetic model. A.R., A.L. and J.Co. designed the statistical plan. A.R. and M.B. performed statistical analyses and Bayesian modelling. A.R. and J.Cu. wrote the article and all authors made corrections. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
Financial support was provided by ONEMA (projects ISOLAC and ERADINVA) and by an ‘ERG Marie Curie’ grant (PERG08-GA-2010-276969). The department EDB is supported by the French Laboratory of Excellence Project ‘TULIP’ (ANR-10-LABX.41; ANR-11-IDEX-002-02).
References
- 1.Bolnick DI, Amarasekare P, Araújo MS, Bürger R, Levine JM, Novak M, Rudolf VH, Schreiber SJ, Urban MC. 2011. Why intraspecific trait variation matters in community ecology. Trends Ecol. Evol. 26, 183–192. ( 10.1016/j.tree.2011.01.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Violle C, Enquist BJ, McGill BJ, Jiang L, Albert CH, Hulshof C, Jung V, Messier J. 2012. The return of the variance: intraspecific variability in community ecology. Trends Ecol. Evol. 27, 244–252. ( 10.1016/j.tree.2011.11.014) [DOI] [PubMed] [Google Scholar]
- 3.Bolnick DI, Svanbäck R, Fordyce JA, Yang LH, Davis JM, Hulsey CD, Forister ML. 2003. The ecology of individuals: incidence and implications of individual specialization. Am. Nat. 161, 1–28. ( 10.1086/343878) [DOI] [PubMed] [Google Scholar]
- 4.Odling-Smee J, Laland KN, Feldman MW. 2003. Niche construction: the neglected process in evolution. Princeton, NJ: Princeton University Press. [Google Scholar]
- 5.Matthews B, De Meester L, Jones CG, Ibelings BW, Bouma TJ, Nuutinen V, van de Koppel J, Odling-Smee J. 2014. Under niche construction: an operational bridge between ecology, evolution, and ecosystem science. Ecol. Monogr. 84, 245–263. ( 10.1890/13-0953.1) [DOI] [Google Scholar]
- 6.Pelletier F, Garant D, Hendry AP. 2009. Eco-evolutionary dynamics. Phil. Trans. R. Soc. B 364, 1483–1489. ( 10.1098/rstb.2009.0027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hendry AP. 2016. Eco-evolutionary dynamics. Princeton, NJ: Princeton University Press. [Google Scholar]
- 8.Bassar RD, et al. 2010. Local adaptation in Trinidadian guppies alters ecosystem processes. Proc. Natl Acad. Sci. USA 107, 3616–3621. ( 10.1073/pnas.0908023107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rudolf VHW, Rasmussen NL. 2013. Population structure determines functional differences among species and ecosystem processes. Nat. Commun. 4, 2318 ( 10.1038/ncomms3318) [DOI] [PubMed] [Google Scholar]
- 10.Rudolf VH, Rasmussen NL. 2013. Ontogenetic functional diversity: size structure of a keystone predator drives functioning of a complex ecosystem. Ecology 94, 1046–1056. ( 10.1890/12-0378.1) [DOI] [PubMed] [Google Scholar]
- 11.El-Sabaawi RW, Bassar RD, Rakowski C, Marshall MC, Bryan BL, Thomas SN, Pringle C, Reznick DN, Flecker AS. 2015. Intraspecific phenotypic differences in fish affect ecosystem processes as much as bottom-up factors. Oikos 124, 1181–1191. ( 10.1111/oik.01769) [DOI] [Google Scholar]
- 12.Sih A, Bell A, Johnson JC. 2004. Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 19, 372–378. ( 10.1016/j.tree.2004.04.009) [DOI] [PubMed] [Google Scholar]
- 13.Violle C, Navas M-L, Vile D, Kazakou E, Fortunel C, Hummel I, Garnier E. 2007. Let the concept of trait be functional! Oikos 116, 882– 892 ( 10.1111/j.2007.0030-1299.15559.x) [DOI] [Google Scholar]
- 14.Díaz S, Purvis A, Cornelissen JHC, Mace GM, Donoghue MJ, Ewers RM, Jordano P, Pearse WD. 2013. Functional traits, the phylogeny of function, and ecosystem service vulnerability. Ecol. Evol. 3, 2958–2975. ( 10.1002/ece3.601) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Sabaawi RW, Marshall MC, Bassar RD, López-Sepulcre A, Palkovacs EP, Dalton C. 2015. Assessing the effects of guppy life history evolution on nutrient recycling: from experiments to the field. Freshw. Biol. 60, 590–601. ( 10.1111/fwb.12507) [DOI] [Google Scholar]
- 16.Wooster D, Sih A. 1995. A review of the drift and activity responses of stream prey to predator presence. Oikos 73, 3 ( 10.2307/3545718) [DOI] [Google Scholar]
- 17.Fryxell DC, Arnett HA, Apgar TM, Kinnison MT, Palkovacs EP. 2015. Sex ratio variation shapes the ecological effects of a globally introduced freshwater fish. Proc. R. Soc. B 282, 20151970 ( 10.1098/rspb.2015.1970) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harmon LJ, Matthews B, Des Roches S, Chase JM, Shurin JB, Schluter D. 2009. Evolutionary diversification in stickleback affects ecosystem functioning. Nature 458, 1167–1170. ( 10.1038/nature07974) [DOI] [PubMed] [Google Scholar]
- 19.Royauté R, Pruitt JN. 2015. Varying predator personalities generates contrasting prey communities in an agroecosystem. Ecology 96, 2902–2911. ( 10.1890/14-2424.1) [DOI] [PubMed] [Google Scholar]
- 20.Roff DA. 1992. The evolution of life histories: theory and analysis. New York, NY: Chapman & Hall. [Google Scholar]
- 21.Reale D, Garant D, Humphries MM, Bergeron P, Careau V, Montiglio P-O. 2010. Personality and the emergence of the pace-of-life syndrome concept at the population level. Phil. Trans. R. Soc. B 365, 4051–4063. ( 10.1098/rstb.2010.0208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown JH, Gillooly JF, Allen AP, Savage VM, West GB. 2004. Toward a metabolic theory of ecology. Ecology 85, 1771–1789. ( 10.1890/03-9000) [DOI] [Google Scholar]
- 23.Leal MC, Seehausen O, Matthews B. 2016. The ecology and evolution of stoichiometric phenotypes. Trends Ecol. Evol. 32, 108–117. ( 10.1016/j.tree.2016.11.006) [DOI] [PubMed] [Google Scholar]
- 24.Gherardi F. 2006. Crayfish invading Europe: the case study of Procambarus clarkii. Mar. Freshw. Behav. Physiol. 39, 175–191. ( 10.1080/10236240600869702) [DOI] [Google Scholar]
- 25.Twardochleb LA, Olden JD, Larson ER. 2013. A global meta-analysis of the ecological impacts of non-native crayfish. Freshw. Sci. 32, 1367–1382. ( 10.1899/12-203.1) [DOI] [Google Scholar]
- 26.Alp M, Cucherousset J, Buoro M, Lecerf A. 2016. Phenological response of a key ecosystem function to biological invasion. Ecol. Lett. 19, 519–527. ( 10.1111/ele.12585) [DOI] [PubMed] [Google Scholar]
- 27.Fossat P, Bacque-Cazenave J, De Deurwaerdere P, Delbecque J-P, Cattaert D. 2014. Anxiety-like behavior in crayfish is controlled by serotonin. Science 344, 1293–1297. ( 10.1126/science.1248811) [DOI] [PubMed] [Google Scholar]
- 28.Biro PA, Dingemanse NJ. 2009. Sampling bias resulting from animal personality. Trends Ecol. Evol. 24, 63–66. ( 10.1016/j.tree.2008.10.004) [DOI] [PubMed] [Google Scholar]
- 29.Bubb DH, Lucas MC, Thom TJ, Rycroft P. 2002. The potential use of PIT telemetry for identifying and tracking crayfish in their natural environment. Hydrobiologia 483, 225–230. ( 10.1023/A:1021352217332) [DOI] [Google Scholar]
- 30.Cote J, Fogarty S, Weinersmith K, Brodin T, Sih A. 2010. Personality traits and dispersal tendency in the invasive mosquitofish (Gambusia affinis). Proc. R. Soc. B 277, 1571–1579. ( 10.1098/rspb.2009.2128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stein RA. 1976. Sexual dimorphism in crayfish chelae: functional significance linked to reproductive activities. Rev. Can. Zool. 54, 220–227. ( 10.1139/z76-024) [DOI] [Google Scholar]
- 32.Pintor LM, Sih A, Bauer ML. 2008. Differences in aggression, activity and boldness between native and introduced populations of an invasive crayfish. Oikos 117, 1629–1636. ( 10.1111/j.1600-0706.2008.16578.x) [DOI] [Google Scholar]
- 33.Claverie T, Smith IP. 2007. Functional significance of an unusual chela dimorphism in a marine decapod: specialization as a weapon? Proc. R. Soc. B 274, 3033–3038. ( 10.1098/rspb.2007.1223) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larson ER, Abbott CL, Usio N, Azuma N, Wood KA, Herborg L-M, Olden JD. 2012. The signal crayfish is not a single species: cryptic diversity and invasions in the Pacific Northwest range of Pacifastacus leniusculus. Freshw. Biol. 57, 1823–1838. ( 10.1111/j.1365-2427.2012.02841.x) [DOI] [Google Scholar]
- 35.Rohlf FJ. 2005. TpsDig. Stony Brook, NY: Department of Ecology and Evolution, State University of New York.
- 36.R Core Team. 2013. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 37.Chessel D, Dufour AB, Dray S. 2007. ade4: analysis of ecological data: exploratory and Euclidean methods in multivariate data analysis and graphical display. R package version 1-4.
- 38.Alcorlo P, Geiger W, Otero M. 2004. Feeding preferences and food selection of the red swamp crayfish, Procambarus clarkii, in habitats differing in food item diversity. Crustaceana 77, 435–453. ( 10.1163/1568540041643283) [DOI] [Google Scholar]
- 39.Correia AM. 2003. Food choice by the introduced crayfish Procambarus clarkii. Ann. Zool. Fenn. 40, 517–528. [Google Scholar]
- 40.Gessner MO, Chauvet E, Dobson M. 1999. A perspective on leaf litter breakdown in streams. Oikos 85, 377–384. ( 10.2307/3546505) [DOI] [Google Scholar]
- 41.Wotton RS, Malmqvist B. 2001. Feces in aquatic ecosystems. BioScience 51, 537–544. ( 10.1641/0006-3568(2001)051%5B0537:FIAE%5D2.0.CO;2) [DOI] [Google Scholar]
- 42.Lecerf A, Dobson M, Dang CK, Chauvet E. 2005. Riparian plant species loss alters trophic dynamics in detritus-based stream ecosystems. Oecologia 146, 432–442. ( 10.1007/s00442-005-0212-3) [DOI] [PubMed] [Google Scholar]
- 43.Freire CA, Onken H, McNamara JC. 2008. A structure–function analysis of ion transport in crustacean gills and excretory organs. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 151, 272–304. ( 10.1016/j.cbpa.2007.05.008) [DOI] [PubMed] [Google Scholar]
- 44.Capps KA, Flecker AS. 2013. Invasive aquarium fish transform ecosystem nutrient dynamics. Proc. R. Soc. B 280, 20131520 ( 10.1098/rspb.2013.1520) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmitz OJ, Hawlena D, Trussell GC. 2010. Predator control of ecosystem nutrient dynamics. Ecol. Lett. 13, 1199–1209. ( 10.1111/j.1461-0248.2010.01511.x) [DOI] [PubMed] [Google Scholar]
- 46.Vanni MJ. 2002. Nutrient cycling by animals in freshwater ecosystems. Annu. Rev. Ecol. Syst. 33, 341–370. ( 10.1146/annurev.ecolsys.33.010802.150519) [DOI] [Google Scholar]
- 47.Villéger S, Grenouillet G, Suc V, Brosse S. 2012. Intra- and interspecific differences in nutrient recycling by European freshwater fish. Freshw. Biol. 57, 2330–2341. ( 10.1111/fwb.12009) [DOI] [Google Scholar]
- 48.Bates D, Maechler M, Bolker B, Walker S. 2014. lme4: linear mixed-effects models using Eigen and S4. R package version 1.1-7.
- 49.Nakagawa S, Schielzeth H. 2010. Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol. Rev. 85, 935–956. ( 10.1111/j.1469-185X.2010.00141.x) [DOI] [PubMed] [Google Scholar]
- 50.Sanchez G. 2013. PLS path modelling with R. Berkeley, CA: Trowchez Editions. [Google Scholar]
- 51.Henseler J, et al. 2014. Common beliefs and reality about PLS: comments on Rönkkö and Evermann (2013). Organ. Res. Methods 17, 182–209. ( 10.1177/1094428114526928) [DOI] [Google Scholar]
- 52.Zhang D, Hui D, Luo Y, Zhou G. 2008. Rates of litter decomposition in terrestrial ecosystems: global patterns and controlling factors. J. Plant Ecol. 1, 85–93. ( 10.1093/jpe/rtn002) [DOI] [Google Scholar]
- 53.Torres-Dowdall J, Handelsman CA, Reznick DN, Ghalambor CK. 2012. Local adaptation and the evolution of phenotypic plasticity in trinidian guppies (Poecilia reticulata). Evolution 66, 3432–3443. ( 10.1111/j.1558-5646.2012.01694.x) [DOI] [PubMed] [Google Scholar]
- 54.Brommer JE. 2013. On between-individual and residual (co)variances in the study of animal personality: are you willing to take the ‘individual gambit’? Behav. Ecol. Sociobiol. 67, 1027–1032. ( 10.1007/s00265-013-1527-4) [DOI] [Google Scholar]
- 55.Brommer JE, Class B. 2017. Phenotypic correlations capture between-individual correlations underlying behavioral syndromes. Behav. Ecol. Sociobiol. 71, 50 ( 10.1007/s00265-017-2278-4) [DOI] [Google Scholar]
- 56.Ketterson ED, Atwell JW, McGlothlin JW. 2009. Phenotypic integration and independence: hormones, performance, and response to environmental change. Integr. Comp. Biol. 49, 365–379. ( 10.1093/icb/icp057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Biro PA, Stamps JA. 2008. Are animal personality traits linked to life-history productivity? Trends Ecol. Evol. 23, 361–368. ( 10.1016/j.tree.2008.04.003) [DOI] [PubMed] [Google Scholar]
- 58.Burton T, Killen SS, Armstrong JD, Metcalfe NB. 2011. What causes intraspecific variation in resting metabolic rate and what are its ecological consequences? Proc. R. Soc. B 278, 3465–3473. ( 10.1098/rspb.2011.1778) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Careau V, Thomas D, Humphries MM, Réale D. 2008. Energy metabolism and animal personality. Oikos 117, 641–653. ( 10.1111/j.0030-1299.2008.16513.x) [DOI] [Google Scholar]
- 60.Bell AM, Hankison SJ, Laskowski KL. 2009. The repeatability of behaviour: a meta-analysis. Anim. Behav. 77, 771–783. ( 10.1016/j.anbehav.2008.12.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Biro PA, Adriaenssens B, Sampson P. 2014. Individual and sex-specific differences in intrinsic growth rate covary with consistent individual differences in behaviour. J. Anim. Ecol. 83, 1186–1195. ( 10.1111/1365-2656.12210) [DOI] [PubMed] [Google Scholar]
- 62.Juette T, Cucherousset J, Cote J. 2014. Animal personality and the ecological impacts of freshwater non-native species. Curr. Zool. 60, 417–427. ( 10.1093/czoolo/60.3.417) [DOI] [Google Scholar]
- 63.Biro PA, Beckmann C, Stamps JA. 2010. Small within-day increases in temperature affects boldness and alters personality in coral reef fish. Proc. R. Soc. B 277, 71–77. ( 10.1098/rspb.2009.1346) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zavorka L, Koeck B, Cucherousset J, Brijs J, Näslund Joacim, Aldvén D, Höjesjö J, Fleming IA, Johnsson JI. 2017. Co-existence with non-native brook trout breaks down the integration of phenotypic traits in brown trout parr. Funct. Ecol. 31, 1582–1591. ( 10.1111/1365-2435.12862) [DOI] [Google Scholar]
- 65.Brodin T, Fick J, Jonsson M, Klaminder J. 2013. Dilute concentrations of a psychiatric drug alter behavior of fish from natural populations. Science 339, 814–815. ( 10.1126/science.1226850) [DOI] [PubMed] [Google Scholar]
- 66.Pope RJ, Gordon AM, Kaushik NK. 1999. Leaf litter colonization by invertebrates in the littoral zone of a small oligotrophic lake. Hydrobiologia 392, 99–112. ( 10.1023/A:1003537232319) [DOI] [Google Scholar]
- 67.Schmitz OJ, et al. 2014. Animating the carbon cycle. Ecosystems 17, 344–359. ( 10.1007/s10021-013-9715-7) [DOI] [Google Scholar]
- 68.Bell AM, Sih A. 2007. Exposure to predation generates personality in threespined sticklebacks (Gasterosteus aculeatus). Ecol. Lett. 10, 828–834. ( 10.1111/j.1461-0248.2007.01081.x) [DOI] [PubMed] [Google Scholar]
- 69.Zavorka L, Aldven D, Naslund J, Hojesjo J, Johnsson JI. 2015. Linking lab activity with growth and movement in the wild: explaining pace-of-life in a trout stream. Behav. Ecol. 26, 877–884. ( 10.1093/beheco/arv029) [DOI] [Google Scholar]
- 70.Raffard A, Lecerf L, Cote J, Buoro M, Lassus R, Cucherousset J. 2017. Data from: The functional syndrome: linking individual trait variability to ecosystem functioning Dryad Digital Repository. ( 10.5061/dryad.rm230) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Raffard A, Lecerf L, Cote J, Buoro M, Lassus R, Cucherousset J. 2017. Data from: The functional syndrome: linking individual trait variability to ecosystem functioning Dryad Digital Repository. ( 10.5061/dryad.rm230) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data from trait measurements are available at Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.rm230) [70].