Abstract
Virulent parasites can depress the densities of their hosts. Taxa that reduce disease via dilution effects might alleviate this burden. However, ‘diluter’ taxa can also depress host densities through competition for shared resources. The combination of disease and interspecific competition could even drive hosts extinct. Then again, genetically variable host populations can evolve in response to both competitors and parasites. Can rapid evolution rescue host density from the harm caused by these ecological enemies? How might such evolution influence dilution effects or the size of epidemics? In a mesocosm experiment with planktonic hosts, we illustrate the joint harm of competition and disease: hosts with constrained evolutionary ability (limited phenotypic variation) suffered greatly from both. However, populations starting with broader phenotypic variation evolved stronger competitive ability during epidemics. In turn, enhanced competitive ability—driven especially by parasites—rescued host densities from the negative impacts of competition, disease, and especially their combination. Interspecific competitors reduced disease (supporting dilution effects) even when hosts rapidly evolved. However, this evolutionary response also elicited a potential problem. Populations that evolved enhanced competitive ability and maintained robust total densities also supported higher densities of infections. Thus, rapid evolution rescued host densities but also unleashed larger epidemics.
Keywords: disease ecology, rapid evolution, eco-evolutionary dynamics, dilution effect, friendly competition, Daphnia
1. Introduction
Virulent parasites can regulate host populations and depress their densities [1]. In theory, a dilution effect—i.e. reduced disease risk in diverse communities—could alleviate this cost [2]. Dilution effects arise broadly when resistant ‘diluter taxa’ interfere with transmission among focal hosts [3]. However, diluter taxa also frequently compete with focal hosts for resources or space (e.g. [4,5–8]). In these situations, parasites and diluters (more specifically: competitors/diluters) could each depress the densities of focal hosts. In fact, the combination of competition and disease—despite the promise of a dilution effect—could even drive focal hosts extinct (see [9,10]).
Then again, this gloomy outlook assumes that focal hosts suffering from competition and disease cannot evolve. Yet interspecific competitors and parasites can themselves drive rapid evolution of enhanced competitive ability (e.g. [11,12]) and disease resistance (e.g. [13,14]), respectively. In theory, given sufficient standing variation, rapid evolution of these traits in focal host populations can feed back to mitigate the ecological density costs imposed by competition [15] and disease [16]. Thus, eco-evolutionary dynamics might rescue the density of focal hosts during epidemics, even while competitors/diluters reduce disease.
However, the eco-evolutionary dynamics of focal hosts interacting with both parasites and interspecific competitors (i.e. potential diluters) remain difficult to predict for several reasons. First, traits of focal hosts frequently evolve in surprising ways. For example, the removal of parasites can rapidly increase host resistance [17], small epidemics can decrease host resistance [18], and predators [19] and parasites [20] can increase the competitive ability of hosts. With these precedents, how should competitor- and parasite-mediated selection influence focal host traits, and hence densities? Second, key traits of focal hosts—competitive ability and resistance—frequently covary [21]. Given such a trade-off, how should focal hosts evolve in response to both competition and disease? Finally, focal host traits also influence impacts of diluters on disease [6]. At the extremes, if focal hosts evolve lower susceptibility (i.e. resist infection more strongly), diluters could become irrelevant for disease transmission. On the other hand, if focal hosts evolve stronger competitive ability, they could outcompete competitors/diluters, perhaps driving them extinct. Such eco-evolutionary possibilities remain largely untested and unknown.
Here, we grapple with eco-evolutionary dynamics of focal hosts facing disease, competition and dilution. Our mesocosm experiment features planktonic focal hosts that rapidly evolve in nature [18], a virulent parasite, and a competitor/diluter that reduces disease in lakes [22]. We manipulated the presence of parasites and/or competitors/diluters in a two-by-two factorial design. We further crossed these ecological treatments with two levels of standing trait (co)variation of the focal host population (eight treatments total). Constrained populations featured moderate mean competitive ability and susceptibility. We expected competition and disease to strongly depress densities in these eco-evolutionary ‘controls’, because focal hosts had little phenotypic trait space to evolve (e.g. [23]). By contrast, variable populations featured a broader range of both traits, but also imposed a trade-off between competitive ability and susceptibility (Fig. 1; see [24]). We expected competitors and parasites to drive evolution in opposite directions [18], potentially rescuing the densities of focal hosts from either competition or disease. However, we could not a priori predict eco-evolutionary outcomes—especially for a dilution effect—in treatments with both parasites and competitors.
In the experiment, parasite-mediated evolution of competitive ability rescued focal hosts from near extinction—but also elicited a warning for disease control. As predicted, competition and disease both strongly depressed the density of focal hosts when their evolutionary potential was constrained. By contrast, densities in variable populations dropped much less. Surprisingly, this rescue arose because parasites drove the rapid evolution of enhanced competitive ability (not lower susceptibility). Competitors/diluters drove relatively weak evolution of the same trait, but only before epidemics. Evolution of increased competitive ability then buffered the densities of focal hosts in variable populations from competition, disease, and especially both together. This evolutionary rescue from ecological harm seems optimistic from a perspective centred on maintaining host density. However, it also poses a challenge from a perspective centred on disease control. True, diluters reduced the density of infected hosts in both variable and constrained populations: we detected dilution effects. However, the density of infected hosts became higher in variable versus constrained populations, because of parasite-mediated evolution of competitive ability. These higher densities of infected hosts could be dangerous, depending on management goals (e.g. reducing risk of spillover). Thus, eco-evolutionary dynamics can bolster densities of focal hosts, but simultaneously unleash larger epidemics.
2. Materials and methods
(a). Natural history of the study system
The focal host, the cladoceran Daphnia dentifera, dominates grazer communities in many North American lakes [25]. It frequently suffers autumnal epidemics caused by the virulent fungal parasite Metschnikowia bicuspidata [18,22]. Focal hosts incidentally consume infectious spores while filter-feeding for algal resources [26]. Infected hosts suffer decreased birth rate, die within one week of infection, and release spores after death [27]. Another cladoceran, Ceriodaphnia sp., lowers focal host density through competition. It also consumes fungal spores while foraging, but rarely becomes infected [6]. These key competitors/diluters reduce disease in models [28], experiments [6] and lakes [22]. Focal hosts can rapidly evolve via clonal selection (generation time 7–10 days). In lakes, they can evolve lower susceptibility during large epidemics, but higher susceptibility during smaller ones [18]. These divergent outcomes probably stem from a foraging-based trade-off: fast feeders suffer high infection risk, but acquire resources rapidly, probably improving their competitive ability [24,29]. Therefore, high resistance may only be optimal during especially large epidemics. Surprisingly, genetic variation among parasite strains does not appear to impact transmission [16]. Thus, this system is ideal for focusing on evolution of host traits, rather than host–parasite coevolution.
(b). Eco-evolutionary mesocosm experiment
We measured competitive ability and susceptibility for eight previously studied isoclonal lines of Daphnia focal hosts (see ‘Trait Measurements’ in electronic supplementary material for details). In short, we estimated an index of competitive ability by calculating growth rate of juveniles (i.e. mass accrual) feeding on low resources. We estimated an index of susceptibility (i.e. the transmission coefficient, β) by fitting a mathematical model to infection assays (e.g. [26]). In a previous mesocosm experiment (A.T.S., unpublished data), we grew each of these clonal lines with and without competitors/diluters and initiated epidemics. Variation in ‘competitive ability’ predicted the densities of focal hosts versus competitors/diluters. Variation in ‘susceptibility’ predicted the size of epidemics. Thus, these traits accurately predicted ecological processes in the previous experiment, and therefor might also influence host evolution.
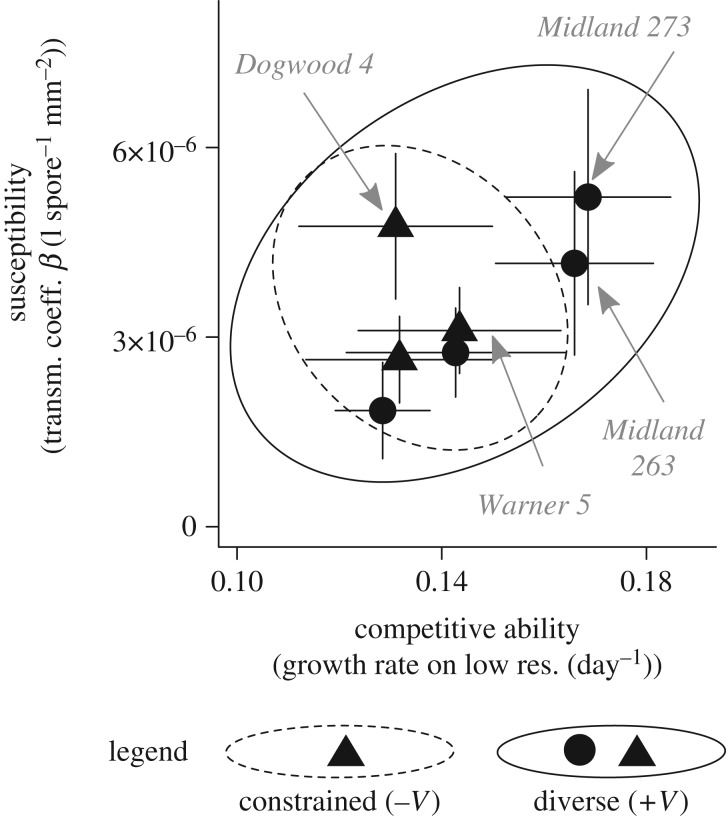
Here, we designed focal host populations with two levels of standing trait variation. Constrained populations included three specific isoclonal lines (hereafter: ‘genotypes’) with moderate competitive ability and susceptibility. Variable populations combined all eight of the previously studied genotypes and spanned a broader range of both traits, which covaried positively (figure 1). Thus, both populations began with similar mean traits and only varied in their standing phenotypic variation. Although we did not manipulate genotypic diversity per se (i.e. all constrained populations began with the same genotypes), this choice allowed us to more robustly evaluate the impacts of standing trait variation on evolution. One ‘genotype’ combined a pair of isoclonal lines that were genetically indistinguishable. Three additional genotypes were initially present (three in variable; one in constrained populations). However, they failed to sustain populations even in monoculture and remained extremely rare in evolving populations (3% of individuals sampled at the end of the experiment; see ‘Genotype Frequencies’ in electronic supplementary material). We do not have measurements of both traits for these genotypes (therefore, they are not pictured in figure 1). However, owing to their low genotype frequencies, they would have had negligible impacts on our calculation of ‘mean focal host traits’ (see below).
Figure 1.
Focal host genotypes vary in two key traits. Mixtures of these genotypes create populations with constrained or variable standing traits. Two key traits: growth rate on low resources indexes competitive ability; the transmission coefficient β indexes susceptibility to disease. Error bars are bootstrapped standard errors around each genotype. Constrained populations (dashed ellipse outline; −V) contain three genotypes (triangles) with moderate competitive ability and susceptibility. Variable populations (solid ellipse outline; +V) contain the same three (triangles), plus four additional genotypes (circles), spreading the range of both traits. The traits covary positively. Thus, standing trait variation is higher for variable (+V) than constrained populations (−V). The four most dominant genotypes are identified by name (see electronic supplementary material, figure S2).
Our mesocosm experiment crossed standing trait variation of the Daphnia focal host population (constrained [−V] or variable [+V]) with the presence/absence of Ceriodaphnia competitors/diluters (+/−C) and with the presence/absence of Metschnikowia parasites (+/−P). Thus, focal host populations experienced selection imposed by interspecific competitors alone, parasites alone, neither or both. To minimize complicating coevolutionary potential and focus on host evolution, we used a single genotype of competitors/diluters and standard laboratory-reared parasites (see [16]). All treatments were replicated five times, and each replicate was maintained in 60 l of artificial lake water. We stimulated algal growth (Ankistrodesmus falcatus) with nitrogen, phosphorus and light (see ‘Mesocosm Experiment’ in electronic supplementary material for details). We added focal hosts (mean concentration 2.1 l−1 per genotype) and competitors/diluters (2.1 l−1) on day 0. Constrained treatments began at a lower overall density of focal hosts (8 versus 21 l−1), but reached comparable densities as variable treatments before epidemics began. We sampled weekly for three weeks (mixing and sieving 1 l per tank), added parasite spores (concentration 5000 l−1) on day 21 and then continued sampling twice weekly until day 70. The experiment lasted approximately 7–10 focal host generations in total.
During the experiment, we tracked ecological and evolutionary dynamics. We counted samples with microscopes and visually diagnosed infections (50×). We recorded densities of focal hosts, competitors/diluters and infected hosts. All counted samples were then preserved in 70% ethanol with 5% 0.5 mM EDTA and stored at 2°C. Initial genotype frequencies were estimated from the starting densities of each genotype. Then, using the preserved samples, we genotyped approximately 10 individuals per tank twice: immediately before epidemics began—day 25—and at the end of the experiment—day 70 (718 individuals in total). We identified genotypes by comparing alleles at microsatellite loci (see ‘Genotyping’ in electronic supplementary material for details). Finally, we calculated mean competitive ability and susceptibility of focal host populations as trait averages weighted by genotype frequencies. Trait assays for each genotype are detailed in the electronic supplementary material. We may not have detected all rare genotypes in the experiment by only sampling 10 individuals per tank per time. However, mean traits were probably insensitive to these rare genotypes, especially relative to the large differences we detected among treatments (see Results).
(c). Statistics
Density of Focal Hosts (figure 2): All statistical analyses were conducted in R [30]. We summarized the log-transformed densities of focal hosts in each tank by integrating (trapezoid rule) over the epidemic period (days 25–70). An ANOVA attributed variation in integrated density to initial trait variation (+/−V), presence of competitors/diluters (+/−C), presence of parasites (+/−P) and all of their interactions.
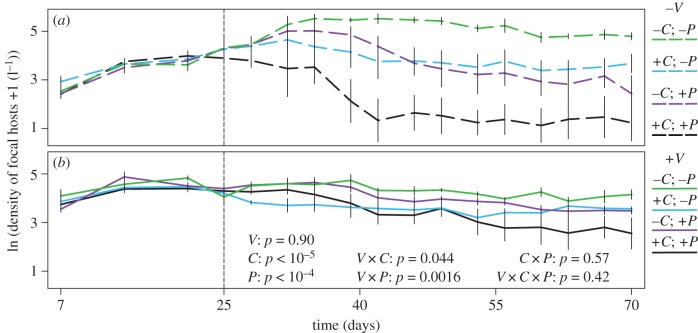
Figure 2.
Density of Focal Hosts: Competition and disease strongly depress the density of focal hosts, especially in populations with constrained traits. Epidemics begin on day 25 (vertical dashed line). (a) Constrained populations (dashed lines; −V) suffer reduced density due to competition only (blue lines; +C; −P), parasites only (purple lines; −C; +P), and especially competition and parasites together (black lines; +C; +P). (b) Variable populations (solid lines; +V) maintain more robust densities despite competition and/or disease. Abbreviations: V = standing trait variation; C = competitors/diluters; P = parasites. Error bars are standard errors.
Evolution (figure 3): We tracked changes in mean competitive ability and susceptibility during epidemics using repeated measures mixed models (NLME package in R: [31]). All models included tank as a random effect and time (t) and standing trait variation (V) as crossed fixed effects. Likelihood ratio tests determined whether we added presence of competitors/diluters (C) or presence of parasites (P) as additional crossed fixed effects (see ‘Repeated Measures Mixed Models’ and table S1 in electronic supplementary material).
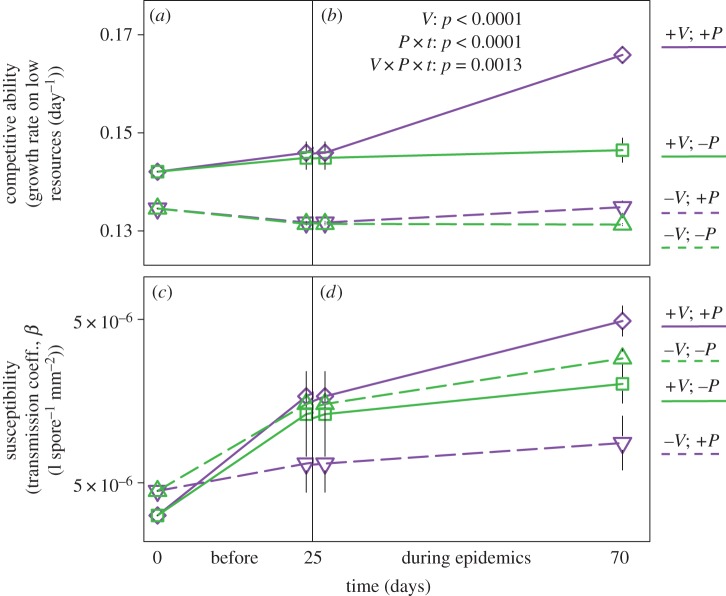
Figure 3.
Evolution: Parasites drive rapid evolution of competitive ability, especially in populations with higher initial trait variability. Mean traits are averages weighted by genotype frequencies. p-values (from repeated measures mixed models) indicate all significant changes during epidemics. (a) Competitive Ability begins higher in variable populations (solid lines; −V), and (b) is still higher as epidemics begin (V effect). Then, during epidemics, competitive ability increases in treatments with parasites (P × t interaction; purple lines), especially in variable populations (V × P × t interaction; solid purple line). (c) Susceptibility, the second trait, begins slightly higher in constrained populations and increases before epidemics. (d) It continues to increase during epidemics, although neither trait variation nor parasites impact it significantly. Abbreviations: V = standing trait variation; P = parasites; t = time. Error bars are standard errors; data include treatments with and without competitors/diluters.
Eco-Evo-Buffered Densities (figure 4): We evaluated whether final competitive ability (an index of evolution) predicted the final density of focal hosts (an index of ecology). Final densities were integrated over the last three weeks of the experiment, when ecological dynamics appeared to stabilize (+/−one sampling period to ensure robust results). We standardized final densities relative to treatments without competitors/diluters or parasites (−C, −P), because these baselines differed between trait variation treatments. Thus, we asked how strongly competition and/or disease reduced the density of focal hosts, within each level of trait variability. We also analysed the unscaled densities. The index of evolution, final mean competitive ability, was calculated from genotypes sampled on day 70 if possible, or day 25 if focal hosts had previously gone extinct. Finally, linear GLS models with flexible variance functions (fitted in NLME) linked final evolved competitive ability to the final scaled densities of focal hosts in treatments with competition, disease and both together.
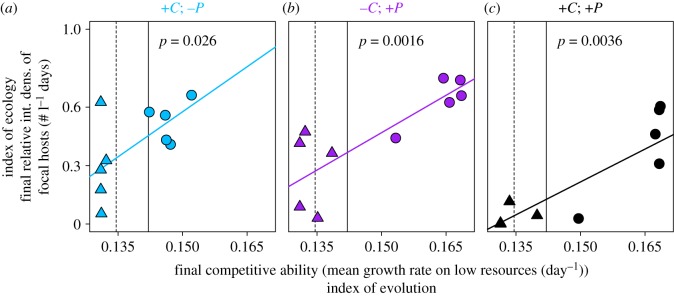
Figure 4.
Eco-Evo-Buffered Densities: Rapid evolution of competitive ability buffers final densities of focal hosts from impacts of competition and disease. Vertical lines indicate initial mean competitive ability (dashed = constrained; solid = variable). Final competitive ability (an index of evolution) is a weighted average of the final sampled genotypes. The final density of focal hosts (an index of ecology) is integrated during the last three weeks of the experiment (days 49–70; see figure 2) and standardized relative to treatments without competition or disease, for constrained (triangles) and variable (circles) treatments. Thus, y-axis values equal to 1 indicate no reduction in density. Higher final competitive ability buffers final density of focal hosts in treatments with (a) competitors only (blue; +C −P), (b) parasites only (purple; −C +P) and (c) competitors/diluters and parasites together (black; +C +P). Linear models (p-values) include flexible variance functions to account for heteroscedasticity.
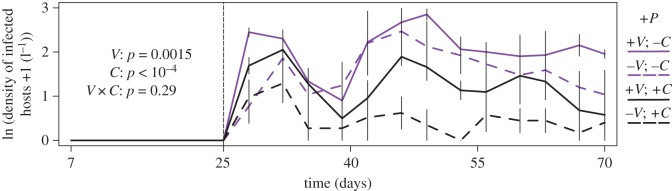
Infections and Dilution Effect (figure 5): Infections began after day 25. To quantify the size of epidemics, we integrated the log density of infected hosts (trapezoid rule) from days 25 to 70. An ANOVA attributed variation in this integrated metric of epidemic size to initial trait variation (+/−V), presence of competitors/diluters (+/−C; i.e. a dilution effect) and their interaction.
Figure 5.
Infections and Dilution Effect: The density of infected hosts is lowered by competitors/diluters but elevated by higher standing trait variation. Diluters reduce the integrated density of infected hosts at both levels of trait variability (C effect; black versus purple lines). However, higher standing trait variation allows the rapid evolution of enhanced competitive ability (driven especially by parasites; figure 3). In turn, higher competitive ability buffers total densities of focal hosts from competition and disease (figure 4). Consequently, these higher total densities lead to a higher density of infected hosts (V effect; solid versus dashed lines). Presence of competitors/diluters and trait variability do not interact (no C × V interaction). Thus, rapid evolution does not undermine the dilution effect. Abbreviations: V = standing trait variation; C = competitors/diluters. Error bars are standard errors.
3. Results
(a). Density of focal hosts
Interspecific competition and disease strongly reduced the density of Daphnia focal hosts in constrained populations (−V; dashed lines; figure 2a), but variable populations were much more robust (+V; solid lines; figure 2b). As predicted, the presence of Ceriodaphnia competitors (+C; blue lines) and Metschnikowia parasites (+P; purple lines) each lowered the integrated density of focal hosts (green lines; C and P main effects: both p < 10−4). Moreover, these reductions of density were magnified in populations with constrained trait variation (V × C interaction: p = 0.044; V × P interaction: p = 0.0016). Competitors and parasites together reduced the (log) density of focal host additively (black lines; non-significant C × P and V × C × P interactions; both p > 0.4). Thus, interspecific competition and disease each reduced the density of focal hosts, especially when evolutionary potential was constrained.
The density of competitors/diluters is presented in the electronic supplementary material. It was not significantly impacted by trait variation or the presence of parasites (electronic supplementary material, figure S1a).
(b). Evolution
Surprisingly, parasites elicited the rapid evolution of enhanced competitive ability—not lower susceptibility to infection. Mean competitive ability started higher in variable populations (figure 3a), and remained higher as epidemics began (figure 3b; V effect: p < 0.0001). Before epidemics began, competitive ability did not differ between treatments with versus without parasites (+/−P). Then, as epidemics proceeded, the presence of parasites accelerated the evolution of higher competitive ability (combining both treatments with and without competitors/diluters). Specifically, parasites drove rapid evolution of higher competitive ability at both levels of trait variation (figure 3b; P × t effect: p < 0.0001), and especially in variable populations (V × P × t effect: p = 0.0013).
This parasite-driven evolution of enhanced competitive ability occurred despite a potential cost of increased susceptibility (since the two traits covaried). Mean susceptibility—the second trait—started slightly higher in constrained populations (figure 3c) and increased at both levels of variation before epidemics. Then, if anything, it continued to increase during epidemics (figure 3d), although not significantly. A post hoc analysis confirmed that this increase became significant after removing trait variation (V) and parasites (P) from the model (p = 0.0014). Thus, parasites drove rapid evolution of competitive ability, despite incurring a potential cost of higher susceptibility.
Competitors/diluters were less important drivers of evolution, but also increased competitive ability (but only before epidemics: see electronic supplementary material, table S1 and figure S4). Changes in individual genotype frequencies are also presented in electronic supplementary material (electronic supplementary material, figures S2 and S3).
(c). Eco-evo-buffered densities
The rapid evolution of higher competitive ability buffered densities of focal hosts from impacts of competition and disease. These eco-evolutionary impacts emerged as positive relationships between tank-level values for final (i.e. evolved) competitive ability versus the density of hosts, scaled to disease- and competition-free baselines. Values closer to 1 reveal less harm to density over the final six sampling periods. Such positive relationships arose in treatments with competitors/diluters alone (figure 4a; +C, −P treatment: p = 0.026), parasites alone (figure 4b; −C, +P: p = 0.0016) and both competitors and parasites together (figure 4c; +C, +P: p = 0.0036). Hence, rapid trait evolution ‘buffered’ final densities of focal hosts. (See electronic supplementary material for unscaled densities and alternative temporal cut-offs for ‘final’ host density: electronic supplementary material, figure S5 and table S2.)
(d). Infections and dilution effect
The evolution of increased competitive ability elicits a mixed message from a disease control perspective. On the one hand, competitors/diluters lowered the integrated density of infected hosts (figure 5; C effect: p < 10−4). Thus, we detected dilution effects. Moreover, presence of competitors/diluters and trait variation did not interact (V × C effect: p = 0.29). In other words, trait variation (and hence, the evolution of competitive ability) did not undermine diluters' reduction of disease. On the other hand, the density of infected hosts was elevated by higher standing trait variation (V effect: p = 0.0015). This effect stemmed from the evolution of competitive ability. First, higher trait variation allowed parasites to drive the evolution of enhanced competitive ability (figure 3). In turn, evolution of this trait buffered total densities of focal hosts (figure 4). Finally, robust total densities supported higher densities of infections (figure 5). Thus, via host evolution, density of infected hosts remained high in variable populations despite a dilution effect.
The presence of competitors/diluters also lowered integrated infection prevalence (electronic supplementary material, figure S1b). However, it was not affected by trait variation (see electronic supplementary material for details). Thus, infection prevalence illustrated a simpler dilution effect, without any eco-evolutionary complications.
4. Discussion
Competitors and parasites can both depress the density of focal hosts, even when competitors (i.e. competitors/diluters) reduce disease and drive dilution effects (e.g. [4,5,6]). We illustrated this ecological danger of disease dilution via competitors in a mesocosm experiment with planktonic hosts. Ceriodaphnia competitors and Metschnikowia parasites strongly depressed the density of Daphnia focal hosts when we constrained their ability to evolve. This combination proved disastrous for focal host density. However, competitors and parasites can also drive rapid evolution of competitive ability and/or susceptibility (e.g. [11,12,14,32]). Here, in populations with higher standing trait variation, parasites (somewhat surprisingly) catalysed rapid evolution of enhanced competitive ability—not lower susceptibility. This evolutionary response enabled a rescue: enhanced competitive ability buffered densities of focal hosts from ecological impacts. Did this eco-evolutionary response undermine the dilution effect? Here, it did not: diluters reduced disease within both treatments of trait variation. However, a key caveat emerged between variation treatments: variable populations maintained higher densities of infections (with and without diluters), owing to their evolution of increased competitive ability. Thus, this experiment revealed potential advantages and disadvantages of eco-evolutionary dynamics when hosts interact with parasites and competitors/diluters. Rapid evolution rescued host densities, but simultaneously unleashed larger densities of infected hosts.
Rapid evolution rescued focal host populations by buffering their densities from competition and disease. Low standing trait variation prevented focal hosts from evolving either increased competitive ability or decreased susceptibility. In these treatments, interspecific competitors and parasites both strongly reduced the density of focal hosts. Moreover, these dual dangers of competition and disease operated additively (on a log scale). This outcome seems disappointing from a disease dilution perspective: diluters might have ameliorated the parasite-driven reduction of host density. Instead, parasites and competitors/diluters together nearly drove focal hosts extinct (see [9,10]). Yet, in populations with higher initial trait variation, diluters and parasites harmed host density less. In these variable treatments, rapid evolution of competitive ability dampened ecological costs (see [23]). This result follows from our focus on phenotypic constraint. Constrained populations could not sufficiently evolve, because they never included the most competitive genotypes. An alternative approach might focus instead on genotypic constraint and randomize the subset of genotypes in constrained populations. Then, we would expect similar mean densities between constrained and variable populations, but much higher variation within the constrained treatment. However, we would still expect support for our key eco-evolutionary result: populations with higher final (i.e. evolved) competitive ability maintained higher relative densities with competition, disease and especially both.
This evolutionary rescue arose via enhanced competitive ability; however, counter to our expectations, it was catalysed by parasites. Before epidemics began, interspecific competition increased mean competitive ability of focal hosts in variable populations. This competitor-driven evolution was predictable [15], but proved fairly weak. Less intuitively, parasites strongly accelerated the evolution of higher competitive ability once epidemics began. Specifically, with parasites, the focal host genotypes that competed best replaced weaker conspecific competitors in both variable and constrained populations, regardless of the presence or absence of competitors/diluters (see electronic supplementary material). In turn, this parasite-mediated clonal selection elevated mean competitive ability, especially when higher initial trait variation allowed a stronger response. Most likely, disease-enhanced death rate of hosts accelerated clonal turnover towards these superior competitors. This phenomenon is potentially general, as other natural enemies accelerate evolution of competitive ability in Daphnia (e.g. [20]) and aphids (e.g. [19]). However, to anticipate this outcome, better mechanistic theory must merge consumer–resource, host–parasite and eco-evolutionary dynamics.
Also contrary to expectations, focal hosts did not evolve enhanced resistance during epidemics. Daphnia hosts can rapidly evolve resistance against various parasites (e.g. [13,32]), including this fungus [16,18]. Moreover, higher susceptibility (as indexed here) led to larger epidemics for these focal host genotypes grown independently (A.T.S. unpublished data). However, in this study system, a trade-off links lower susceptibility with inferior resource acquisition [24,29] and probably weaker competitive ability. Moreover, this parasite does not castrate [27] or readily evolve virulence [16]. These features could allow a strongly competitive focal host population to ‘outgrow’ fitness costs of infection, especially during smaller epidemics (e.g. [18]). Here, susceptibility increased before epidemics, probably because it correlated with higher competitive ability (at least in variable populations). Then, if anything, it continued to increase during epidemics, dragged along by parasite-driven increases in competitive ability. This result probably hinges upon moderate, non-evolving virulence of the parasite, covariance between competitive ability and susceptibility, and underlying host–resource ecology (see [21,33,34]).
While parasite-mediated evolution rescued host densities, it did not undermine the dilution effect: we detected dilution effects at both levels of trait variability. Specifically, the presence of diluters reduced both the density and prevalence of infected hosts equally in constrained and variable populations. Evolution of lower susceptibility might have obviated a dilution effect if focal hosts became too resistant to fuel epidemics [6]. However, susceptibility of focal hosts did not decline (hosts did not evolve higher resistance). Alternatively, evolution of higher competitive ability could have suppressed a dilution effect, if populations of diluters were constrained or driven extinct by more competitive focal hosts [6]. However, despite the rapid evolution of competitive ability, competitors/diluters remained sufficiently numerous to reduce disease. Thus, focal hosts in variable populations benefited simultaneously from diluter-reduced prevalence of infection and evolutionary rescue. This combination suggests that, together, trait diversity and diluter taxa could help to conserve charismatic taxa (e.g. [35]), native species (e.g. [7]), crops (e.g. [36]) or livestock (e.g. [37]).
However, the parasite-mediated evolutionary rescue of focal hosts also elevated the density of infected hosts, with or without diluters. This outcome creates a potential problem for disease-control scenarios, especially when higher densities of infected wildlife hosts could increase transmission to humans. Our crossed experimental design uncovered an eco-evolutionary tension from this disease-control perspective. While an ecological force (presence of competitors/diluters) depressed the density of infected hosts, an evolutionary force (increased competitive ability) elevated it. Here, these ecological and evolutionary forces exerted roughly equal effects on the density of infected hosts. In fact, the density of infected hosts was similar in variable treatments with diluters versus constrained treatments without. In other cases, this balance might differ: depression by diluters or elevation by host evolution could exert larger effects. Perhaps evolution-mediated increases in the density of infected hosts could counterbalance or even overwhelm diluter-mediated control of diseases like hantavirus [8] or schistosomiasis [38]. In these examples, the relative sensitivity of disease transmission to community ecology versus host evolution remains, to our knowledge, unknown.
This experiment highlights the need for more mechanistic theory at the intersection of consumer–resource, host–parasite and eco-evolutionary dynamics. First, the parasite-mediated evolutionary rescue of host density via enhanced competitive ability needs mathematical explanation. Our verbal model invokes mortality-enhanced turnover of clones fuelled by resource release. However, given the feedbacks involved, evaluation of this hypothesis requires models parameterized with natural covariation in focal host traits (e.g. [29]). Such a model could delineate when evolution of competitive ability versus resistance should arise (e.g. [17,18]). Second, more expansive eco-coevolutionary theory for disease dilution—including coevolution of parasites and diluters—is also needed (see [13,14]). Here, to provide a starting point, we only allowed focal hosts to evolve. If diluters could evolve higher competitive ability, they might strengthen dilution effects, or alternatively simply drive focal hosts extinct. If parasites could evolve higher virulence, they might depress all focal host densities, even in variable populations. Higher virulence could even feed back to favour coevolution of enhanced resistance (instead of competitive ability) of focal hosts. Such possibilities represent expansive, promising areas for future research.
In this mesocosm experiment, rapid evolution transformed the ecology of focal hosts interacting with parasites and competitors/diluters. Our core results inspire several broader questions. First, when trait evolution was constrained, the combination of competition and disease strongly depressed densities of focal hosts. How often do diluters compete with focal hosts and depress their densities? Second, parasites drove the rapid evolution of competitive ability. When should we expect parasites to catalyse the evolution of competitive ability versus disease resistance? How commonly does evolution of competitive ability rescue hosts from the harm inflicted by their parasites? Finally, is parasite-mediated evolution of competitive ability a desirable outcome for disease control? Here, density of infected hosts was lowered by a dilution effect, but elevated by rapid host evolution. Can host evolution increase the density of infected wildlife that transmits zoonoses to humans? Could rapid evolution of competitive ability lead to higher disease risk despite dilution effects? The planktonic case study here illustrates these questions awaiting discovery.
Supplementary Material
Acknowledgements
A.T.S. was supported by the NSF GRFP. O. Schmidt assisted with trait measurement assays, and S. Duple assisted with the mesocosm experiment. C. Holmes and P. Lee assisted with genotyping conducted at the W.M. Keck Center for Comparative and Functional Genomics (University of Illinois at Urbana-Champaign).
Data accessibility
All data are available on Dryad Digital Repository http://dx.doi.org/10.5061/dryad.tm041 [39].
Authors' contributions
A.T.S., S.R.H., C.E.C. and M.A.D. designed the study. A.T.S. led trait measurements, assisted by J.L.H. and S.R.H. A.T.S, J.L.H. and M.S.S. sampled the mesocosm experiment. A.T.S. led DNA extractions (assisted by J.L.H.) and genotyping (assisted by M.S.S. and C.E.C.). A.T.S. conducted statistical analyses and wrote the first draft of the manuscript. All authors contributed to revisions.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by NSF DEB 1120316, 1353749, 1354407, 1353806, and 1406846, as well as NSF DUE 1129198.
References
- 1.Anderson RM, May RM. 1979. Population biology of infectious diseases - 1. Nature 280, 361–367. ( 10.1038/280361a0) [DOI] [PubMed] [Google Scholar]
- 2.Keesing F, Holt RD, Ostfeld RS. 2006. Effects of species diversity on disease risk. Ecol. Lett. 9, 485–498. ( 10.1111/j.1461-0248.2006.00885.x) [DOI] [PubMed] [Google Scholar]
- 3.Civitello DJ, et al. 2015. Biodiversity inhibits parasites: broad evidence for the dilution effect. Proc. Natl Acad. Sci. USA 112, 8667–8671. ( 10.1073/pnas.1506279112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson PTJ, Preston DL, Hoverman JT, Henderson JS, Paull SH, Richgels KLD, Redmond MD. 2012. Species diversity reduces parasite infection through cross-generational effects on host abundance. Ecology 93, 56–64. ( 10.1890/11-0636.1) [DOI] [PubMed] [Google Scholar]
- 5.Mitchell CE, Tilman D, Groth JV. 2002. Effects of grassland plant species diversity, abundance, and composition on foliar fungal disease. Ecology 83, 1713–1726. ( 10.1890/0012-9658(2002)083%5B1713:EOGPSD%5D2.0.CO;2) [DOI] [Google Scholar]
- 6.Strauss AT, Civitello DJ, Cáceres CE, Hall SR. 2015. Success, failure and ambiguity of the dilution effect among competitors. Ecol. Lett. 18, 916–926. ( 10.1111/ele.12468) [DOI] [PubMed] [Google Scholar]
- 7.Thieltges DW, Reise K, Prinz K, Jensen KT. 2009. Invaders interfere with native parasite-host interactions. Biol. Invasions. 11, 1421–1429. ( 10.1007/s10530-008-9350-y) [DOI] [Google Scholar]
- 8.Clay CA, Lehmer EM, Jeor SS, Dearing MD. 2009. Sin nombre virus and rodent species diversity: a test of the dilution and amplification hypotheses. PLoS ONE 4, e6467 ( 10.1371/journal.pone.0006467) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rudolf VHW, Antonovics J. 2005. Species coexistence and pathogens with frequency-dependent transmission. Am. Nat. 166, 112–118. ( 10.1086/430674) [DOI] [PubMed] [Google Scholar]
- 10.Dallas T, Hall RJ, Drake JM. 2016. Competition-mediated feedbacks in experimental multispecies epizootics. Ecology 97, 661–670. [PubMed] [Google Scholar]
- 11.Steiner CF, Cáceres CE, Smith SDP. 2007. Resurrecting the ghost of competition past with dormant zooplankton eggs. Am. Nat. 169, 416–422. ( 10.1086/510728) [DOI] [PubMed] [Google Scholar]
- 12.Rowe CLJ, Leger EA. 2011. Competitive seedlings and inherited traits: a test of rapid evolution of Elymus multisetus (big squirreltail) in response to cheatgrass invasion. Evol. Appl. 4, 485–498. ( 10.1111/j.1752-4571.2010.00162.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Decaestecker E, De Gersem H, Michalakis Y, Raeymaekers JAM. 2013. Damped long-term host-parasite Red Queen coevolutionary dynamics: a reflection of dilution effects? Ecol. Lett. 16, 1455–1462. [DOI] [PubMed] [Google Scholar]
- 14.Frickel J, Sieber M, Becks L. 2016. Eco-evolutionary dynamics in a coevolving host-virus system. Ecol. Lett. 19, 450–459. ( 10.1111/ele.12580) [DOI] [PubMed] [Google Scholar]
- 15.Vellend M. 2006. The consequences of genetic diversity in competitive communities. Ecology 87, 304–311. ( 10.1890/05-0173) [DOI] [PubMed] [Google Scholar]
- 16.Duffy MA, Sivars-Becker L. 2007. Rapid evolution and ecological host-parasite dynamics. Ecol. Lett. 10, 44–53. ( 10.1111/j.1461-0248.2006.00995.x) [DOI] [PubMed] [Google Scholar]
- 17.Dargent F, Scott ME, Hendry AP, Fussmann GF. 2013. Experimental elimination of parasites in nature leads to the evolution of increased resistance in hosts. Proc. R. Soc. B 280, 20132371 ( 10.1098/rspb.2013.2371) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duffy MA, Ochs JH, Penczykowski RM, Civitello DJ, Klausmeier CA, Hall SR. 2012. Ecological context influences epidemic size and parasite-driven evolution. Science 335, 1636–1638. ( 10.1126/science.1215429) [DOI] [PubMed] [Google Scholar]
- 19.Turcotte MM, Reznick DN, Hare JD. 2011. The impact of rapid evolution on population dynamics in the wild: experimental test of eco-evolutionary dynamics. Ecol. Lett. 14, 1084–1092. ( 10.1111/j.1461-0248.2011.01676.x) [DOI] [PubMed] [Google Scholar]
- 20.Zbinden M, Haag CR, Ebert D. 2008. Experimental evolution of field populations of Daphnia magna in response to parasite treatment. J. Evol. Biol. 21, 1068–1078. ( 10.1111/j.1420-9101.2008.01541.x) [DOI] [PubMed] [Google Scholar]
- 21.Duncan AB, Fellous S, Kaltz O. 2011. Reverse evolution - selection against costly resistance in disease-free microcosm poulations of Paramecium caudatum. Evolution 65, 3462–3474. ( 10.1111/j.1558-5646.2011.01388.x) [DOI] [PubMed] [Google Scholar]
- 22.Strauss AT, Shocket MS, Civitello DJ, Hite JL, Penczykowski RM, Duffy MA, Cáceres CE, Hall SR. 2016. Habitat, predators, and hosts regulate disease in Daphnia through direct and indirect pathways. Ecol. Monogr. 86, 393–411. ( 10.1002/ecm.1222) [DOI] [Google Scholar]
- 23.Yoshida T, Ellner SP, Jones LE, Bohannan BJM, Lenski RE, Hairston NG. 2007. Cryptic population dynamics: rapid evolution masks trophic interactions. PLoS Biol. 5, 1868–1879. ( 10.1371/journal.pbio.0050235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hall SR, Becker CR, Duffy MA, Cáceres CE. 2010. Variation in resource acquisition and use among host clones creates key epidemiological trade-offs. Am. Nat. 176, 557–565. ( 10.1086/656523) [DOI] [PubMed] [Google Scholar]
- 25.Tessier AJ, Woodruff P. 2002. Cryptic trophic cascade along a gradient of lake size. Ecology 83, 1263–1270. ( 10.1890/0012-9658(2002)083%5B1263:CTCAAG%5D2.0.CO;2) [DOI] [Google Scholar]
- 26.Hall SR, Sivars-Becker L, Becker C, Duffy MA, Tessier AJ, Cáceres CE. 2007. Eating yourself sick: transmission of disease as a function of foraging ecology. Ecol. Lett. 10, 207–218. ( 10.1111/j.1461-0248.2007.01011.x) [DOI] [PubMed] [Google Scholar]
- 27.Hall SR, Simonis JL, Nisbet RM, Tessier AJ, Cáceres CE. 2009. Resource ecology of virulence in a planktonic host-parasite system: an explanation using dynamic energy budgets. Am. Nat. 174, 149–162. ( 10.1086/600086) [DOI] [PubMed] [Google Scholar]
- 28.Cáceres CE, Davis G, Duple S, Hall SR, Koss A, Lee P, Rapti Z. 2014. Complex Daphnia interactions with parasites and competitors. Math. Biosci. 258, 148–161. ( 10.1016/j.mbs.2014.10.002) [DOI] [PubMed] [Google Scholar]
- 29.Auld S, Penczykowski RM, Ochs JH, Grippi DC, Hall SR, Duffy MA. 2013. Variation in costs of parasite resistance among natural host populations. J. Evol. Biol. 26, 2479–2486. ( 10.1111/jeb.12243) [DOI] [PubMed] [Google Scholar]
- 30.R Core Team. 2017. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 31.Pinheiro J, Bates D. 2000. Mixed-effects models in S and S-PLUS. New York, NY: Springer. [Google Scholar]
- 32.Capaul M, Ebert D. 2003. Parasite-mediated selection in experimental Daphnia magna populations. Evolution 57, 249–260. ( 10.1111/j.0014-3820.2003.tb00260.x) [DOI] [PubMed] [Google Scholar]
- 33.Day T, Gandon S. 2007. Applying population-genetic models in theoretical evolutionary epidemiology. Ecol. Lett. 10, 876–888. ( 10.1111/j.1461-0248.2007.01091.x) [DOI] [PubMed] [Google Scholar]
- 34.Nuismer SL, Doebeli M. 2004. Genetic correlations and the coevolutionary dynamics of three-species systems. Evolution 58, 1165–1177. ( 10.1111/j.0014-3820.2004.tb01697.x) [DOI] [PubMed] [Google Scholar]
- 35.Johnson PTJ, Preston DL, Hoverman JT, Richgels KLD. 2013. Biodiversity decreases disease through predictable changes in host community competence. Nature 494, 230–233. ( 10.1038/nature11883) [DOI] [PubMed] [Google Scholar]
- 36.Boudreau MA. 2013. Diseases in intercropping systems. Ann. Rev. Phytopathol. 51, 499–519. ( 10.1146/annurev-phyto-082712-102246) [DOI] [PubMed] [Google Scholar]
- 37.Huang ZYX, de Boer WF, van Langevelde F, Xu C, Ben Jebara K, Berlingieri F, Prins HHT. 2013. Dilution effect in bovine tuberculosis: risk factors for regional disease occurrence in Africa. Proc. R. Soc. B 280, 20130624 ( 10.1098/rspb.2013.0624) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson PTJ, Lund PJ, Hartson RB, Yoshino TP. 2009. Community diversity reduces Schistosoma mansoni transmission, host pathology and human infection risk. Proc. R. Soc. B 276, 1657–1663. ( 10.1098/rspb.2008.1718) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strauss AT, Hite JL, Shocket MS, Cáceres CE, Duffy MA, Hall SR.2017. Data from: Rapid evolution rescues hosts from competition and disease but—despite a dilution effect—increases the density of infected hosts. Dryad Digital Repository. ( ) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Strauss AT, Hite JL, Shocket MS, Cáceres CE, Duffy MA, Hall SR.2017. Data from: Rapid evolution rescues hosts from competition and disease but—despite a dilution effect—increases the density of infected hosts. Dryad Digital Repository. ( ) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
All data are available on Dryad Digital Repository http://dx.doi.org/10.5061/dryad.tm041 [39].