ABSTRACT
In vitro combinations of isavuconazole with echinocandins were evaluated against 30 Aspergillus strains with a two-dimensional checkerboard microdilution method and an agar-based diffusion method. With the checkerboard method, the three combinations showed indifferent interactions for all strains. With the agar-based method, indifferent interactions were found for all strains for isavuconazole-micafungin and isavuconazole-anidulafungin. For the isavuconazole-caspofungin combination, indifference was found in 24/30 strains, synergism in 4/30 strains, and antagonism in 2/30 strains.
KEYWORDS: isavuconazole, echinocandins, Aspergillus spp., EUCAST, gradient strips, MIC, combination
TEXT
Although azoles are very active against aspergillosis (1–6), acquired azole resistance in Europe and other parts of the world has been reported in recent years (7–10). It has been shown that isolates that are resistant to itraconazole and/or voriconazole may be cross resistant to isavuconazole (11, 12). Therefore, we might expect the emergence of isavuconazole-resistant isolates in the same way as occurred for the other azole drugs. The main mechanisms of azole resistance in Aspergillus fumigatus are mutations in the cyp51A gene, encoding 14α-demethylase (8, 10).
Antifungal combination is a therapeutic strategy that can be beneficial when drug interactions are synergistic, as well as in cases of infection with a resistant organism (13, 14). Indeed, it was recently proposed that a combination of voriconazole with an echinocandin may be used in clinical practice in cases of invasive aspergillosis due to azole-resistant isolates (15). In vitro and animal findings regarding voriconazole-echinocandin combinations mainly show indifferent to synergistic effects (16–24). Whether combinations of isavuconazole with echinocandins possess synergistic activity against filamentous fungi is still poorly studied. Here, we chose to examine the in vitro combinations of isavuconazole with caspofungin, anidulafungin, and micafungin against azole-susceptible and azole-resistant A. fumigatus isolates and against Aspergillus flavus, Aspergillus nidulans, Aspergillus terreus, and Aspergillus niger isolates.
(This study was presented in part at the 27th European Congress of Clinical Microbiology and Infectious Diseases, Vienna, Austria, 22 to 25 April 2017.)
Thirty isolates were tested, including 5 azole-susceptible A. fumigatus isolates, 5 azole-resistant A. fumigatus isolates, 5 A. flavus isolates, 5 A. nidulans isolates, 5 A. terreus isolates, and 5 A. niger isolates. An isolate was considered azole resistant when a MIC for itraconazole, posaconazole, or voriconazole was above the current clinical breakpoints determined by EUCAST (http://www.eucast.org/clinical_breakpoints). The 5 azole-resistant A. fumigatus isolates were resistant to itraconazole and to posaconazole. Cyp51A mutations were a 34-bp tandem repeat and L98H for 3 isolates (2 of which were resistant to voriconazole), G54W for 1 isolate, and P216L for 1 isolate (the latter 2 isolates were susceptible to voriconazole). The testing of combinations of isavuconazole and each of the echinocandins (caspofungin, anidulafungin, and micafungin) was performed using two techniques.
The first technique was a two-dimensional checkerboard microdilution method based on the EUCAST reference method (25). The final concentrations were 0.125 to 8 μg/ml for isavuconazole (Basilea, Basel, Switzerland), 0.008 to 4 μg/ml for caspofungin (MSD, Kenilworth, NJ), 0.0005 to 0.25 μg/ml for micafungin (Astellas, Tokyo, Japan), and 0.0005 to 0.25 μg/ml for anidulafungin (Pfizer, New York, NY). MICs were determined visually after 48 h of incubation at 35°C. MICs were first determined with complete inhibition endpoints (100% inhibition) for the drugs alone and in combination, and partial inhibition endpoints (50% inhibition for isavuconazole and minimal effective concentrations [MECs] for the echinocandins alone and for the combinations) were also used. Plates were also read spectrophotometrically (26). This technique was repeated twice, in two independent experiments.
For the gradient concentration strip method, RPMI agar plates were inoculated with the same spore suspension as used for the checkerboard microdilution method, already adjusted to 106 CFU/ml. Three plates were used for each isolate, i.e., one with an isavuconazole MIC test strip (Liofilchem, Roseto degli Abruzzi, Italy), one with an echinocandin MIC test strip (bioMérieux, Craponne, France), and one with the combination. Experiments were performed as described previously (23, 27). MICs were determined visually after 48 h of incubation at 35°C, using complete inhibition (100%) for isavuconazole and partial inhibition (50%) for the echinocandins and the combinations. MICs were also determined with complete inhibition endpoints for the combinations. For both techniques, Candida parapsilosis ATCC 22019, Candida krusei ATCC 6258, and Aspergillus fumigatus ATCC 204305 were used for quality control. Fractional inhibitory concentration index (FICI) values were calculated to determine whether the drug interactions were synergistic (FICI values of ≤0.5), indifferent (FICI values of >0.5 to <4), or antagonistic (FICI values of ≥4) (28).
The activities of isavuconazole and echinocandins, either alone or in combination, were first determined by checkerboard microdilution (Table 1). Isavuconazole MICs ranged from 0.5 to 8 μg/ml against azole-resistant A. fumigatus, from 0.25 to 4 μg/ml against azole-susceptible A. fumigatus, from 0.25 to 4 μg/ml against A. flavus, A. terreus, and A. nidulans, and from 4 to 8 μg/ml against A. niger. Caspofungin MICs were >4 μg/ml against all isolates from all species, and MECs ranged from 0.125 to 1 μg/ml. Micafungin MICs were >0.25 μg/ml (the highest concentration tested) against all isolates, and MECs ranged from 0.004 to 0.06 μg/ml. Anidulafungin MICs were >0.25 μg/ml against all isolates, and MECs ranged from 0.004 to 0.12 μg/ml.
TABLE 1.
Interactions of isavuconazole with echinocandins against Aspergillus spp., determined with the checkerboard broth microdilution techniquea
| Species | FICI (mean [range])b |
|||||
|---|---|---|---|---|---|---|
| Isavuconazole with caspofunginc |
Isavuconazole with micafunginc |
Isavuconazole with anidulafunginc |
||||
| MIC | MEC | MIC | MEC | MIC | MEC | |
| Azole-resistant A. fumigatus (5 isolates) | 1.025 (1–1.125) | 0.928 (0.5–2) | 1.201 (1.001–2.001) | 0.71 (0.312–1.5) | 1.201 (1.001–2.001) | 0.732 (0.531–1.25) |
| Azole-susceptible A. fumigatus (5 isolates) | 1.201 (1.001–2.001) | 0.577 (0.31–1) | 1.001 | 0.37 (0.317–0.517) | 1.401 (1.001–2.001) | 0.643 (0.5–1.033) |
| A. niger (5 isolates) | 1.126 (0.625–2.001) | 0.409 (0.059–1.062) | 1.001 | 0.621 (0.164–2.033) | 1.104 (0.516–1.001) | 0.663 (0.158–2.033) |
| A. terreus (5 isolates) | 1.001 | 0.875 (0.37–1.007) | 1.001 | 0.907 (0.375–1.062) | 1.001 | 0.873 (0.5–1.033) |
| A. flavus (5 isolates) | 0.926 (0.625–1.001) | 0.832 (0.528–1.014) | 1.201 (1.001–2.001) | 0.633 (0.317–1.017) | 1.101 (0.501–2.001) | 0.662 (0.508–1.017) |
| A. nidulans (5 isolates) | 1.001 | 1.034 (1.028–1.056) | 1.001 | 0.937 (0.562–1.033) | 1.001 | 1.031 (1.008–1.062) |
The effects of isavuconazole in combination with echinocandins against 30 Aspergillus isolates were determined with the checkerboard broth microdilution technique, using two different endpoints.
Corresponding to the lowest FICI values.
MIC corresponds to complete inhibition of growth for both drugs, and MEC corresponds to the lowest drug concentrations resulting in aberrant hyphal growth for both drugs.
Using complete inhibition endpoints, we found indifferent effects for all strains with the EUCAST-based checkerboard method. The median FICI values ranged from 0.625 to 2.001 for the isavuconazole-caspofungin combination, from 1.001 to 2.001 for the isavuconazole-micafungin combination, and from 0.501 to 2.001 for the isavuconazole-anidulafungin combination. With partial inhibition endpoints, mainly indifferent effects were found but some synergistic interactions were observed (Table 2).
TABLE 2.
Interactions of isavuconazole with caspofungin, micafungin, and anidulafungin against Aspergillus spp., determined with the checkerboard broth microdilution technique using two inhibition endpointsa
| Inhibition endpoint and species | No. of isolates |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Isavuconazole with caspofungin |
Isavuconazole with micafungin |
Isavuconazole with anidulafungin |
|||||||
| S | I | A | S | I | A | S | I | A | |
| Complete inhibition endpoint | |||||||||
| Azole-resistant A. fumigatus (5 isolates) | 0 | 5 | 0 | 0 | 5 | 0 | 0 | 5 | 0 |
| Azole-susceptible A. fumigatus (5 isolates) | 0 | 5 | 0 | 0 | 5 | 0 | 0 | 5 | 0 |
| A. niger (5 isolates) | 0 | 5 | 0 | 0 | 5 | 0 | 0 | 5 | 0 |
| A. terreus (5 isolates) | 0 | 5 | 0 | 0 | 5 | 0 | 0 | 5 | 0 |
| A. flavus (5 isolates) | 0 | 5 | 0 | 0 | 5 | 0 | 0 | 5 | 0 |
| A. nidulans (5 isolates) | 0 | 5 | 0 | 0 | 5 | 0 | 0 | 5 | 0 |
| Total (30 isolates) | 0 | 30 | 0 | 0 | 30 | 0 | 0 | 30 | 0 |
| Partial inhibition endpoint | |||||||||
| Azole-resistant A. fumigatus (5 isolates) | 1 | 4 | 0 | 1 | 4 | 0 | 0 | 5 | 0 |
| Azole-susceptible A. fumigatus (5 isolates) | 2 | 3 | 0 | 4 | 1 | 0 | 1 | 4 | 0 |
| A. niger (5 isolates) | 3 | 2 | 0 | 4 | 1 | 0 | 3 | 2 | 0 |
| A. terreus (5 isolates) | 1 | 4 | 0 | 1 | 4 | 0 | 1 | 4 | 0 |
| A. flavus (5 isolates) | 0 | 5 | 0 | 1 | 4 | 0 | 0 | 5 | 0 |
| A. nidulans (5 isolates) | 0 | 5 | 0 | 0 | 5 | 0 | 0 | 5 | 0 |
| Total (30 isolates) | 7 | 23 | 0 | 11 | 19 | 0 | 5 | 25 | 0 |
For isavuconazole, MICs were defined as the lowest concentrations of the antifungal agents that completely inhibited fungal growth. For the echinocandins, two different visual determinations of the endpoint were performed, i.e., complete inhibition of growth (MIC) and partial inhibition of growth defined as the lowest drug concentration resulting in aberrant hyphal growth (MEC), by examination with an inverted microscope. S, synergism; I, indifference; A, antagonism.
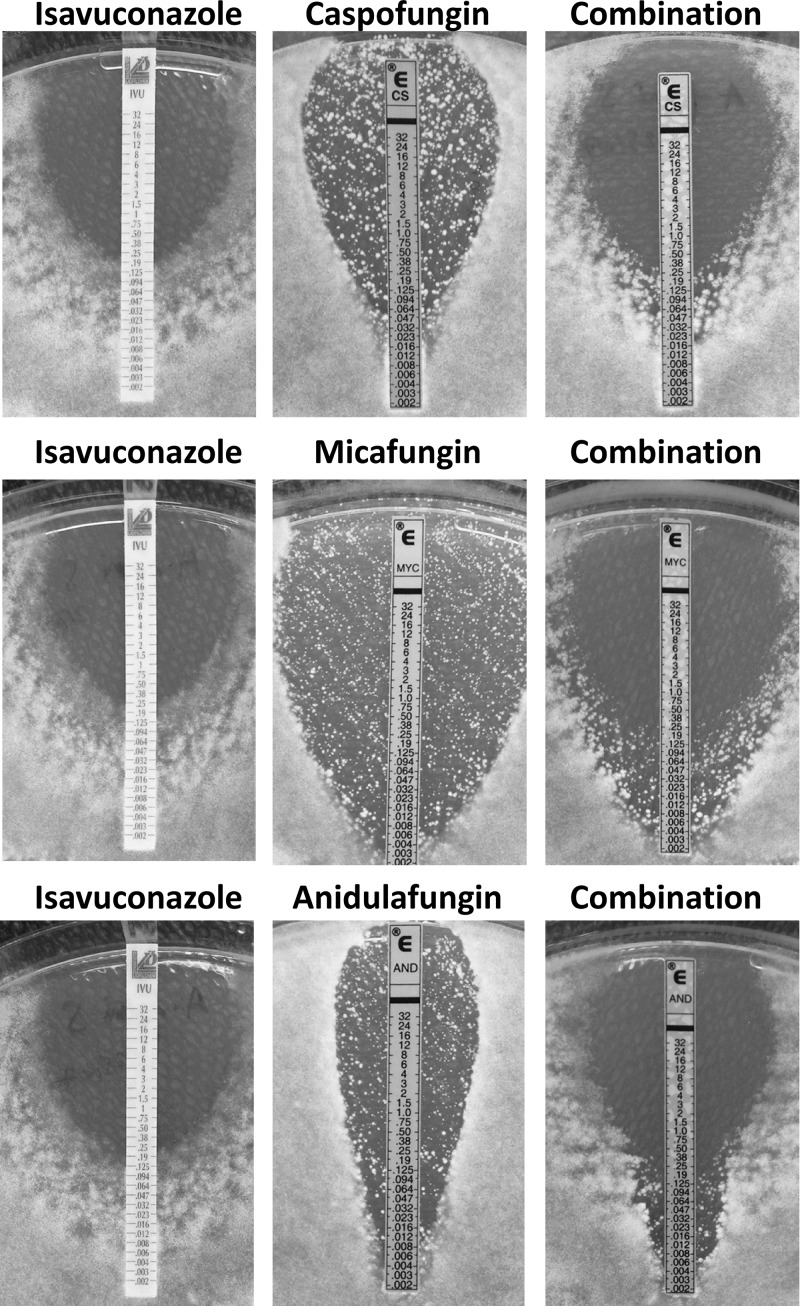
We checked our findings with spectrophotometric readings, which confirmed the indifferent effects of most of the combinations, with FICI values ranging from 0.501 to 1.001 for the isavuconazole-caspofungin combination in 26/30 strains (86.7%), for the isavuconazole-micafungin combination in 28/30 strains (93.3%), and for the isavuconazole-anidulafungin combination in 29/30 strains (96.7%). The results of agar diffusion tests are summarized in Table 3. Isavuconazole, caspofungin, micafungin, and anidulafungin MICs against all isolates ranged from 0.032 to 2 μg/ml, from 0.003 to 0.38 μg/ml, from 0.002 to 0.012 μg/ml, and from 0.002 to 0.006 μg/ml, respectively. Using complete inhibition endpoints, we found indifferent effects for all strains for the isavuconazole-micafungin combination and the isavuconazole-anidulafungin combination with the gradient concentration strip method. For the isavuconazole-caspofungin combination, the FICI values ranged from 0.630 to 3.940 (indifferent) in 24/30 strains (80%), from 0.084 to 0.424 (synergistic) in 4/30 strains (13.3%) (3 A. terreus strains and 1 azole-susceptible A. fumigatus strain), and from 4.149 to 5.601 (antagonistic) in 2/30 strains (6.7%) (2 A. niger strains). These findings were similar when interpreted with partial endpoints. Typical patterns observed by using gradient concentration strips are presented in Fig. 1.
TABLE 3.
Interactions of isavuconazole with echinocandins against Aspergillus spp., determined with gradient concentration stripsa
| Species (no. of isolates) | Isavuconazole with caspofungin |
Isavuconazole with micafungin |
Isavuconazole with anidulafungin |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FICI range | No. of isolates |
FICI range | No. of isolates |
FICI range | No. of isolates |
|||||||
| S | I | A | S | I | A | S | I | A | ||||
| Azole-resistant A. fumigatus (5) | 1.011–3.294 | 0 | 5 | 0 | 1.001–1.024 | 0 | 5 | 0 | 0.688–1.016 | 0 | 5 | 0 |
| Azole-susceptible A. fumigatus (5) | 0.425–2.291 | 1 | 4 | 0 | 1.008–1.024 | 0 | 5 | 0 | 0.774–1.024 | 0 | 5 | 0 |
| A. niger (5) | 1.016–5.601 | 0 | 3 | 2 | 1.002–1.341 | 0 | 5 | 0 | 1.001–1.502 | 0 | 5 | 0 |
| A. terreus (5) | 0.084–1.046 | 3 | 2 | 0 | 1.005–1.008 | 0 | 5 | 0 | 1.005–1.010 | 0 | 5 | 0 |
| A. flavus (5) | 1.042–2.084 | 0 | 5 | 0 | 1.005–1.349 | 0 | 5 | 0 | 0.508–1.024 | 0 | 5 | 0 |
| A. nidulans (5) | 0.630–3.061 | 0 | 5 | 0 | 0.917–1.170 | 0 | 5 | 0 | 1.004–1.042 | 0 | 5 | 0 |
| Total (30) | 0.084–5.601 | 4 | 24 | 2 | 0.917–1.349 | 0 | 30 | 0 | 0.508–1.502 | 0 | 30 | 0 |
Interactions between the antifungal agents were analyzed using FICI values. MICs were determined with partial inhibition endpoints. S, synergism; I, indifference; A, antagonism.
FIG 1.
Agar diffusion test results for the combinations of isavuconazole with caspofungin, micafungin, and anidulafungin against azole-susceptible A. fumigatus strain 292.
Because azole resistance in Aspergillus is an emerging problem in Europe (7–10, 29) and in other parts of the world (9, 11, 30), therapeutic strategy alternatives to azole monotherapy are urgently needed (15). In this study, combinations of isavuconazole with echinocandins showed mainly indifferent effects against Aspergillus spp., and antagonism was almost never observed. On the whole, these results are in accordance with those of previous studies on azole-echinocandin interactions.
Although a number of studies have reported data on the efficacy of combinations of azoles (itraconazole, voriconazole, and posaconazole) and echinocandins (caspofungin, micafungin, and anidulafungin) (18, 20, 22, 24, 31–33), whether isavuconazole possesses synergistic activity when combined with echinocandins has been poorly studied so far. Katragkou et al. showed that the interactions of isavuconazole with micafungin against A. fumigatus, A. flavus, and A. terreus were synergistic in vitro (34); in that study, however, synergism was considered when the FICI value was <1. Previous studies chose this definition to obtain a more symmetrical indifference range (FICI values of 1 to 1.25) than the conventional one (FICI values of 0.5 to 4) (24, 35). According to this definition, we would have found that the combinations were synergistic against most of the strains. Gebremariam et al. did not demonstrate any synergism of the isavuconazole-micafungin combination in treating pulmonary murine mucormycosis (36). To our knowledge, no clinical trials have been performed to study the combination of isavuconazole with echinocandins.
Because antifungal combinations are difficult to test against filamentous fungi, we used two methods, based on different principles (a checkerboard microdilution broth technique and an agar diffusion technique), and we chose to interpret the results with complete inhibition endpoints. We also used two reading methods and showed, as reported previously (26, 37–40), that spectrophotometric reading is a good alternative to visual reading for MIC determinations with the EUCAST-based technique.
Because we included azole-susceptible and azole-resistant Aspergillus isolates, we could demonstrate that the interactions between isavuconazole and echinocandins were not linked to the azole susceptibility of the isolates. In the literature, studies indicate controversy regarding this point (20, 24, 39).
In conclusion, combinations of isavuconazole and echinocandins mainly showed indifferent interactions against Aspergillus spp., and antagonism was almost never observed. Further in vivo evaluation of these combinations is warranted especially for difficult-to-treat invasive aspergillosis.
ACKNOWLEDGMENT
We thank Basilea for providing the isavuconazole gradient concentration strips.
REFERENCES
- 1.Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, Oestmann J-W, Kern WV, Marr KA, Ribaud P, Lortholary O, Sylvester R, Rubin RH, Wingard JR, Stark P, Durand C, Caillot D, Thiel E, Chandrasekar PH, Hodges MR, Schlamm HT, Troke PF, de Pauw B. 2002. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med 347:408–415. doi: 10.1056/NEJMoa020191. [DOI] [PubMed] [Google Scholar]
- 2.Maertens JA, Raad II, Marr KA, Patterson TF, Kontoyiannis DP, Cornely OA, Bow EJ, Rahav G, Neofytos D, Aoun M, Baddley JW, Giladi M, Heinz WJ, Herbrecht R, Hope W, Karthaus M, Lee D-G, Lortholary O, Morrison VA, Oren I, Selleslag D, Shoham S, Thompson GR, Lee M, Maher RM, Schmitt-Hoffmann A-H, Zeiher B, Ullmann AJ. 2016. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet 387:760–769. doi: 10.1016/S0140-6736(15)01159-9. [DOI] [PubMed] [Google Scholar]
- 3.Miceli MH, Kauffman CA. 2015. Isavuconazole: a new broad-spectrum triazole antifungal agent. Clin Infect Dis 61:1558–1565. doi: 10.1093/cid/civ571. [DOI] [PubMed] [Google Scholar]
- 4.Patterson TF, Thompson GR, Denning DW, Fishman JA, Hadley S, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Nguyen MH, Segal BH, Steinbach WJ, Stevens DA, Walsh TJ, Wingard JR, Young J-AH, Bennett JE. 2016. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 63:e1–e60. doi: 10.1093/cid/ciw326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shirley M, Scott LJ. 2016. Isavuconazole: a review in invasive aspergillosis and mucormycosis. Drugs 76:1647–1657. doi: 10.1007/s40265-016-0652-6. [DOI] [PubMed] [Google Scholar]
- 6.Tissot F, Agrawal S, Pagano L, Petrikkos G, Groll AH, Skiada A, Lass-Flörl C, Calandra T, Viscoli C, Herbrecht R. 2017. ECIL-6 guidelines for the treatment of invasive candidiasis, aspergillosis and mucormycosis in leukemia and hematopoietic stem cell transplant patients. Haematologica 102:433–444. doi: 10.3324/haematol.2016.152900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choukri F, Botterel F, Sitterlé E, Bassinet L, Foulet F, Guillot J, Costa JM, Fauchet N, Dannaoui E. 2015. Prospective evaluation of azole resistance in Aspergillus fumigatus clinical isolates in France. Med Mycol 53:593–596. doi: 10.1093/mmy/myv029. [DOI] [PubMed] [Google Scholar]
- 8.Howard SJ, Arendrup MC. 2011. Acquired antifungal drug resistance in Aspergillus fumigatus: epidemiology and detection. Med Mycol 49(Suppl 1):S90–S95. doi: 10.3109/13693786.2010.508469. [DOI] [PubMed] [Google Scholar]
- 9.van der Linden JWM, Arendrup MC, Warris A, Lagrou K, Pelloux H, Hauser PM, Chryssanthou E, Mellado E, Kidd SE, Tortorano AM, Dannaoui E, Gaustad P, Baddley JW, Uekötter A, Lass-Flörl C, Klimko N, Moore CB, Denning DW, Pasqualotto AC, Kibbler C, Arikan-Akdagli S, Andes D, Meletiadis J, Naumiuk L, Nucci M, Melchers WJG, Verweij PE. 2015. Prospective multicenter international surveillance of azole resistance in Aspergillus fumigatus. Emerg Infect Dis 21:1041–1044. doi: 10.3201/eid2106.140717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vermeulen E, Lagrou K, Verweij PE. 2013. Azole resistance in Aspergillus fumigatus: a growing public health concern. Curr Opin Infect Dis 26:493–500. doi: 10.1097/QCO.0000000000000005. [DOI] [PubMed] [Google Scholar]
- 11.Chowdhary A, Sharma C, van den Boom M, Yntema JB, Hagen F, Verweij PE, Meis JF. 2014. Multi-azole-resistant Aspergillus fumigatus in the environment in Tanzania. J Antimicrob Chemother 69:2979–2983. doi: 10.1093/jac/dku259. [DOI] [PubMed] [Google Scholar]
- 12.Gregson L, Goodwin J, Johnson A, McEntee L, Moore CB, Richardson M, Hope WW, Howard SJ. 2013. In vitro susceptibility of Aspergillus fumigatus to isavuconazole: correlation with itraconazole, voriconazole, and posaconazole. Antimicrob Agents Chemother 57:5778–5780. doi: 10.1128/AAC.01141-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baddley JW, Pappas PG. 2005. Antifungal combination therapy: clinical potential. Drugs 65:1461–1480. doi: 10.2165/00003495-200565110-00002. [DOI] [PubMed] [Google Scholar]
- 14.Spitzer M, Robbins N, Wright GD. 2017. Combinatorial strategies for combating invasive fungal infections. Virulence 8:169–185. doi: 10.1080/21505594.2016.1196300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verweij PE, Ananda-Rajah M, Andes D, Arendrup MC, Brüggemann RJ, Chowdhary A, Cornely OA, Denning DW, Groll AH, Izumikawa K, Kullberg BJ, Lagrou K, Maertens J, Meis JF, Newton P, Page I, Seyedmousavi S, Sheppard DC, Viscoli C, Warris A, Donnelly JP. 2015. International expert opinion on the management of infection caused by azole-resistant Aspergillus fumigatus. Drug Resist Updat 21-22:30–40. doi: 10.1016/j.drup.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Chandrasekar PH, Cutright JL, Manavathu EK. 2004. Efficacy of voriconazole plus amphotericin B or micafungin in a guinea-pig model of invasive pulmonary aspergillosis. Clin Microbiol Infect 10:925–928. doi: 10.1111/j.1469-0691.2004.00958.x. [DOI] [PubMed] [Google Scholar]
- 17.Clemons KV, Espiritu M, Parmar R, Stevens DA. 2005. Comparative efficacies of conventional amphotericin B, liposomal amphotericin B (AmBisome), caspofungin, micafungin, and voriconazole alone and in combination against experimental murine central nervous system aspergillosis. Antimicrob Agents Chemother 49:4867–4875. doi: 10.1128/AAC.49.12.4867-4875.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cuenca-Estrella M, Gomez-Lopez A, Garcia-Effron G, Alcazar-Fuoli L, Mellado E, Buitrago MJ, Rodriguez-Tudela JL. 2005. Combined activity in vitro of caspofungin, amphotericin B, and azole agents against itraconazole-resistant clinical isolates of Aspergillus fumigatus. Antimicrob Agents Chemother 49:1232–1235. doi: 10.1128/AAC.49.3.1232-1235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirkpatrick WR, Perea S, Coco BJ, Patterson TF. 2002. Efficacy of caspofungin alone and in combination with voriconazole in a guinea pig model of invasive aspergillosis. Antimicrob Agents Chemother 46:2564–2568. doi: 10.1128/AAC.46.8.2564-2568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krishnan-Natesan S, Wu W, Chandrasekar PH. 2012. In vitro efficacy of the combination of voriconazole and anidulafungin against voriconazole-resistant cyp51A mutants of Aspergillus fumigatus. Diagn Microbiol Infect Dis 73:135–137. doi: 10.1016/j.diagmicrobio.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Martin-Vicente A, Capilla J, Guarro J. 2017. Synergistic effect of anidulafungin combined with posaconazole in experimental aspergillosis. Med Mycol 55:457–460. [DOI] [PubMed] [Google Scholar]
- 22.Philip A, Odabasi Z, Rodriguez J, Paetznick VL, Chen E, Rex JH, Ostrosky-Zeichner L. 2005. In vitro synergy testing of anidulafungin with itraconazole, voriconazole, and amphotericin B against Aspergillus spp. and Fusarium spp. Antimicrob Agents Chemother 49:3572–3574. doi: 10.1128/AAC.49.8.3572-3574.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Planche V, Ducroz S, Alanio A, Bougnoux ME, Lortholary O, Dannaoui E. 2012. In vitro combination of anidulafungin and voriconazole against intrinsically azole-susceptible and -resistant Aspergillus spp. Antimicrob Agents Chemother 56:4500–4503. doi: 10.1128/AAC.00045-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seyedmousavi S, Meletiadis J, Melchers WJG, Rijs AJMM, Mouton JW, Verweij PE. 2013. In vitro interaction of voriconazole and anidulafungin against triazole-resistant Aspergillus fumigatus. Antimicrob Agents Chemother 57:796–803. doi: 10.1128/AAC.00980-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez-Tudela JL, Donnelly JP, Arendrup MC, Arikan S, Barchiesi F, Bille J, Chryssanthou E, Cuenca-Estrella M, Dannaoui E, Denning D, Fegeler W, Gaustad P, Lass-Florl C, Moore C, Richardson M, Schmalreck A, Velegraki A, Verweij PE. 2008. EUCAST technical note on the method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia-forming moulds. Clin Microbiol Infect 14:982–984. doi: 10.1111/j.1469-0691.2008.02086.x. [DOI] [PubMed] [Google Scholar]
- 26.Dannaoui E, Persat F, Monier MF, Borel E, Piens MA, Picot S. 1999. Use of spectrophotometric reading for in vitro antifungal susceptibility testing of Aspergillus spp. Can J Microbiol 45:871–874. doi: 10.1139/w99-075. [DOI] [PubMed] [Google Scholar]
- 27.Vitale RG, Afeltra J, Dannaoui E. 2005. Antifungal combinations. Methods Mol Med 118:143–152. [DOI] [PubMed] [Google Scholar]
- 28.Odds FC. 2003. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother 52:1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- 29.Snelders E, Melchers WJG, Verweij PE. 2011. Azole resistance in Aspergillus fumigatus: a new challenge in the management of invasive aspergillosis? Future Microbiol 6:335–347. doi: 10.2217/fmb.11.4. [DOI] [PubMed] [Google Scholar]
- 30.Lockhart SR, Frade JP, Etienne KA, Pfaller MA, Diekema DJ, Balajee SA. 2011. Azole resistance in Aspergillus fumigatus isolates from the ARTEMIS global surveillance study is primarily due to the TR/L98H mutation in the cyp51A gene. Antimicrob Agents Chemother 55:4465–4468. doi: 10.1128/AAC.00185-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seyedmousavi S, Brüggemann RJM, Melchers WJG, Rijs AJMM, Verweij PE, Mouton JW. 2013. Efficacy and pharmacodynamics of voriconazole combined with anidulafungin in azole-resistant invasive aspergillosis. J Antimicrob Chemother 68:385–393. doi: 10.1093/jac/dks402. [DOI] [PubMed] [Google Scholar]
- 32.Siopi M, Siafakas N, Vourli S, Mouton JW, Zerva L, Meletiadis J. 2016. Dose optimization of voriconazole/anidulafungin combination against Aspergillus fumigatus using an in vitro pharmacokinetic/pharmacodynamic model and response surface analysis: clinical implications for azole-resistant aspergillosis. J Antimicrob Chemother 71:3135–3147. doi: 10.1093/jac/dkw276. [DOI] [PubMed] [Google Scholar]
- 33.Zhang M, Su X, Sun W-K, Chen F, Xu X-Y, Shi Y. 2014. Efficacy of the combination of voriconazole and caspofungin in experimental pulmonary aspergillosis by different Aspergillus species. Mycopathologia 177:11–18. doi: 10.1007/s11046-013-9719-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katragkou A, McCarthy M, Meletiadis J, Petraitis V, Moradi PW, Strauss GE, Fouant MM, Kovanda LL, Petraitiene R, Roilides E, Walsh TJ. 2014. In vitro combination of isavuconazole with micafungin or amphotericin B deoxycholate against medically important molds. Antimicrob Agents Chemother 58:6934–6937. doi: 10.1128/AAC.03261-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meletiadis J, Pournaras S, Roilides E, Walsh TJ. 2010. Defining fractional inhibitory concentration index cutoffs for additive interactions based on self-drug additive combinations, Monte Carlo simulation analysis, and in vitro-in vivo correlation data for antifungal drug combinations against Aspergillus fumigatus. Antimicrob Agents Chemother 54:602–609. doi: 10.1128/AAC.00999-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gebremariam T, Wiederhold NP, Alqarihi A, Uppuluri P, Azie N, Edwards JE Jr, Ibrahim AS. 2017. Monotherapy or combination therapy of isavuconazole and micafungin for treating murine mucormycosis. J Antimicrob Chemother 72:462–466. doi: 10.1093/jac/dkw433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dannaoui E, Lortholary O, Dromer F. 2004. In vitro evaluation of double and triple combinations of antifungal drugs against Aspergillus fumigatus and Aspergillus terreus. Antimicrob Agents Chemother 48:970–978. doi: 10.1128/AAC.48.3.970-978.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dannaoui E, Meletiadis J, Mouton JW, Meis JFGM, Verweij PE, Eurofung N. 2003. In vitro susceptibilities of zygomycetes to conventional and new antifungals. J Antimicrob Chemother 51:45–52. doi: 10.1093/jac/dkg020. [DOI] [PubMed] [Google Scholar]
- 39.Mavridou E, Meletiadis J, Rijs A, Mouton JW, Verweij PE. 2015. The strength of synergistic interaction between posaconazole and caspofungin depends on the underlying azole resistance mechanism of Aspergillus fumigatus. Antimicrob Agents Chemother 59:1738–1744. doi: 10.1128/AAC.04469-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meletiadis J, Leth Mortensen K, Verweij PE, Mouton JW, Arendrup MC. 2017. Spectrophotometric reading of EUCAST antifungal susceptibility testing of Aspergillus fumigatus. Clin Microbiol Infect 23:98–103. doi: 10.1016/j.cmi.2016.10.017. [DOI] [PubMed] [Google Scholar]