LETTER
Heteroresistance to piperacillin-tazobactam has only been reported in Pseudomonas aeruginosa (1). In Escherichia coli, heteroresistance to cefepime and carbapenems (2, 3), but not to piperacillin-tazobactam, has been described. Here, we characterize the first case of heteroresistance to piperacillin-tazobactam in E. coli sequence type 131 (ST131) clinical isolates.
The piperacillin-tazobactam MICs for isolates C2-23 and C1-140 (recovered from bile and blood cultures, respectively, of the same patient admitted to the University Hospital Virgen del Rocío, Seville, Spain) determined by the standard broth microdilution method were 64 and 32 mg/liter (intermediate [I], CLSI; resistant [R], EUCAST), respectively, after 24 h of incubation. The MICs for isolates C2-23 and C1-140 determined with the automated MicroScan system were 64 mg/liter (I, CLSI; R, EUCAST) and ≤8 mg/liter (susceptible [S], CLSI and EUCAST), respectively.
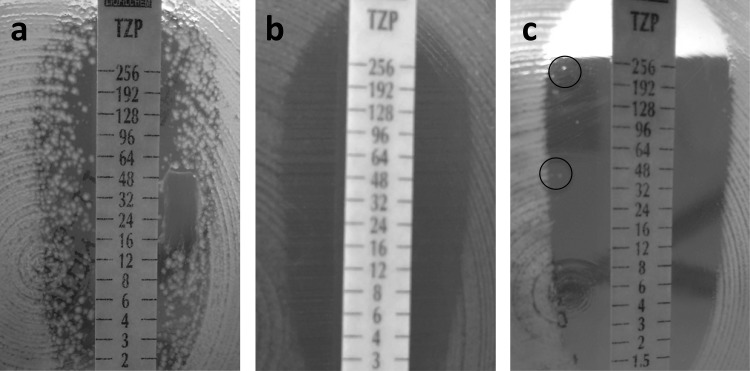
Different subpopulations of isolate C2-23 were detected when the Etest method was used, i.e., one larger susceptible subpopulation with a MIC of 1.5 mg/liter and another subpopulation forming numerous colonies in the inner zone of inhibition all along the piperacillin-tazobactam Etest strip (Fig. 1a). Isolate C1-140, with a piperacillin-tazobactam MIC of 1.5 mg/liter when an Etest was performed with a bacterial inoculum with a McFarland standard of 0.5, apparently showed only one homogeneous susceptible population (Fig. 1b). However, when the bacterial inoculum was increased to a McFarland standard of 2, a few colonies grew in the inhibition zone along the piperacillin-tazobactam Etest strip (Fig. 1c). When the Etest was repeated with the colonies from the inner zone and prior subculture on sheep blood agar, it showed resistance to piperacillin-tazobactam, with a MIC of >256 mg/liter.
FIG 1.
Etests with piperacillin-tazobactam (TZP) of isolate C2-23 with a 0.5 McFarland standard inoculum (a) and isolate C1-140 with 0.5 (b) and 2 (c) McFarland standard inocula. Colonies growing in the inner inhibition zone are characteristic of heteroresistance.
Both isolates belonged to the ST131 clone, as demonstrated by multilocus sequence typing analysis (4), and the blaTEM1 gene was detected with specific primers in both (5).
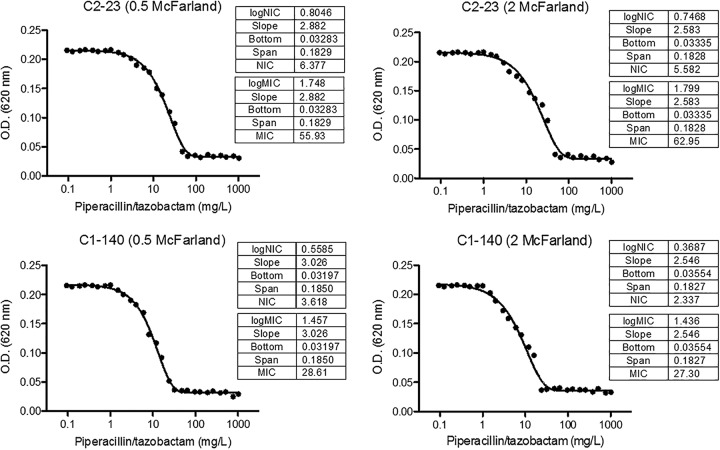
Population analysis profiling (PAP) was performed to determine the MIC and noninhibitory concentration (NIC) as previously reported (6). The PAP showed heterogeneous behavior of the two isolates when they were grown at high piperacillin-tazobactam concentrations. The MIC and NIC of strain C2-23 were 55.93 and 6.38 mg/liter, respectively, with a 0.5 McFarland standard inoculum and 62.95 and 5.58 mg/liter with a 2 McFarland standard inoculum; in both cases, the MIC/NIC ratio was >8. For strain C1-140, with a 0.5 McFarland standard inoculum, the MIC and NIC were 28.61 and 3.62 mg/liter, respectively, and the MIC/NIC ratio was 7.90; however, with the larger inoculum, these values were 27.30 and 2.34 mg/liter, respectively, and the MIC/NIC ratio was 11.67 (Fig. 2). That is probably because the subpopulation resistant to piperacillin-tazobactam is smaller than the susceptible one; however, the precise mechanism remains to be elucidated.
FIG 2.
PAP of isolates C2-23 and C1-140 when 0.5 and 2 McFarland standard inocula are used. MICs and NICs are shown. A MIC/NIC ratio of >8 defines the population as heteroresistant to the antibiotic, a MIC/NIC ratio of >2 and ≤8 defines it as intermediately heteroresistant, and a MIC/NIC ratio of ≤2 defines it as homogeneously resistant. O.D., optical density.
In summary, we report for the first time clinical isolates of E. coli heteroresistant to piperacillin-tazobactam that were clonally related although they did not have the same behavior or the same proportions of susceptible and resistant subpopulations. We can probably expect the emergence of more cases in coming years because of the extensive use of piperacillin-tazobactam.
ACKNOWLEDGMENTS
This study was supported by the Miguel Servet Tipo I Project grant, Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Economía, Industria y Competitividad (CP15/00132), and by Plan Nacional de I+D+i 2013-2016 and Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Economía, Industria y Competitividad, Spanish Network for Research in Infectious Diseases (REIPI RD12/0015/0001; RD12/0015/0012; RD16/0016/0009), cofinanced by the European Development Regional Fund “A Way to Achieve Europe,” Operative Program Intelligent Growth 2014-2020. Y.S. is supported by the Subprograma Miguel Servet Tipo I, Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Economía y Competitividad, Spain (CP15/01358).
None of the authors has a conflict of interest to declare.
REFERENCES
- 1.Pournaras S, Ikonomidis A, Neou E, Kantzanou M, Maniatis AN, Tsakris A. 2008. Piperacillin/tazobactam heteroresistant Pseudomonas aeruginosa from urinary infection, successfully treated by piperacillin/tazobactam. J Antimicrob Chemother 61:757–758. doi: 10.1093/jac/dkm528. [DOI] [PubMed] [Google Scholar]
- 2.Ma W, Sun J, Yang S, Zhang L. 2016. Epidemiological and clinical features for cefepime heteroresistant Escherichia coli infections in Southwest China. Eur J Clin Microbiol Infect Dis 35:571–578. doi: 10.1007/s10096-015-2572-x. [DOI] [PubMed] [Google Scholar]
- 3.Sun JD, Huang SF, Yang SS, Pu SL, Zhang CM, Zhang LP. 2015. Impact of carbapenem heteroresistance among clinical isolates of invasive Escherichia coli in Chongqing, southwestern China. Clin Microbiol Infect 469:e1–e10. doi: 10.1016/j.cmi.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 4.Wirth T, Falush D, Lan R, Colles F, Mensa P, Wieler LH, Karch H, Reeves PR, Maiden MC, Ochman H, Achtman M. 2006. Sex and virulence in Escherichia coli: an evolutionary perspective. Mol Microbiol 60:1136–1151. doi: 10.1111/j.1365-2958.2006.05172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Briñas L, Zarazaga M, Sáenz Y, Ruiz-Larrea F, Torres C. 2002. β-Lactamases in ampicillin-resistant Escherichia coli isolates from foods, humans, and healthy animals. Antimicrob Agents Chemother 46:3156–3163. doi: 10.1128/AAC.46.10.3156-3163.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Halfawy OM, Valvano MA. 2015. Antimicrobial heteroresistance: an emerging field in need of clarity. Clin Microbiol Rev 28:191–207. doi: 10.1128/CMR.00058-14. [DOI] [PMC free article] [PubMed] [Google Scholar]