ABSTRACT
Studies of daily emtricitabine-tenofovir disoproxil fumarate (FTC-TDF) for HIV preexposure prophylaxis (PrEP) in men who have sex with men (MSM) modeled intracellular tenofovir-diphosphate (TFV-DP) in dried blood spots (DBS) to assess adherence and corresponding PrEP outcomes. We conducted a prospective, randomized, crossover pharmacokinetic study of TFV-DP in DBS during 33%, 67%, or 100% of daily dosing under directly observed therapy (DOT). Participants were assigned to two 12-week dosing regimens, separated by a 12-week washout. Forty-eight adults (25 women) from Denver and San Francisco were included. TFV-DP exhibited a median half-life of 17 days, reaching steady state in 8 weeks. TFV-DP was dose proportional with mean (SD) steady-state concentrations of 530 (159), 997 (267), and 1,605 (405) fmol/punch for the 33%, 67%, and 100% arms, respectively. Prior work in MSM demonstrated clinically meaningful TFV-DP thresholds of 350, 700, and 1,250 fmol/punch, which were estimated 25th percentiles for 2, 4, and 7 doses/week. In the present study, corresponding TFV-DP was within 3% of the prior estimates, and subgroups by site, race, and sex were within 14% of prior estimates, although males had 17.6% (95% confidence intervals [CIs], 6.5, 27.4%) lower TFV-DP than females. The thresholds of 350, 700, and 1,250 fmol/punch were achieved by 75% of men taking ≥1.2, 3.2, and 6 doses/week and 75% of women taking ≥0.6, 2.0, and 5.3 doses/week, indicating that lower dosing reached these thresholds for both sexes. In conclusion, TFV-DP arising from DOT was similar to previous estimates and is useful for interpreting PrEP adherence and study outcomes. (This study has been registered at ClinicalTrials.gov under identifier NCT02022657.)
KEYWORDS: HIV, preexposure prophylaxis, intracellular, nucleoside analog, adherence, pharmacokinetics
INTRODUCTION
Variable adherence is a powerful predictor of effectiveness for HIV preexposure prophylaxis (PrEP) with emtricitabine-tenofovir disoproxil fumarate (FTC-TDF) (1–5). Unfortunately, adherence to PrEP is difficult to quantify, and surrogate adherence measures for prevention, akin to HIV load assessment for treatment, are lacking. In major PrEP trials, nonpharmacological adherence measures such as self reporting, pill counts, and refill records all reflected high adherence (∼90% or more) despite wide variations in trial efficacy (from ∼0% to 75%) (1–5). In the same studies, adherence was also assessed by quantifying drug concentrations in stored specimens (e.g., plasma and peripheral blood mononuclear cells [PBMC]). These objective measurements indicated wide variability in adherence among the studies (∼30% to 80%), which correlated strongly with PrEP efficacy, thereby providing at least a partial explanation for study outcomes (2, 3, 6–9). Thus, assessing and interpreting adherence using drug concentrations has become critically important for understanding PrEP effectiveness.
Samples to measure drug concentrations (such as those described above) are often collected at convenience times without regard to time postdose. The adherence information gleaned from convenience sampling depends on the drug pharmacokinetics and assay sensitivity. Short half-life moieties such as tenofovir (TFV) and FTC in plasma, saliva, or urine (∼10- to 15-h half-lives) do not accumulate appreciably with multiple doses, such that concentrations from a single dose nearly match those at steady state (10–13). This means that convenience samples of short half-life moieties only provide information about whether a dose was recently ingested (yes or no) but not whether cumulative doses were ingested. A limitation of these moieties is “white-coat” adherence, where a subject is consistently nonadherent but ingests a dose immediately preceding their clinic visit, thereby appearing as adherent as someone who dosed every day (14, 15). Conversely, an advantage of these moieties is that one can infer how long ago the most recent dose was ingested (e.g., 2 to 7 days) based on assay sensitivity and knowledge of washout kinetics (10, 12). Similarly, emtricitabine-triphosphate (FTC-TP) in red blood cells, measured in dried blood spots (DBS), has an intermediate half-life (∼35 h). Given the close proximity of FTC-TP in DBS to the lower limit of quantitation, its quantitation is an indicator of a recent dose (within 48 h) and strongly correlated with quantitation of TFV and FTC in plasma when the lower limit of TFV quantitation is 10 ng/ml (15).
Tenofovir-diphosphate (TFV-DP) exhibits a longer half-life in PBMC (3 to 4 days), which translates to steady-state concentrations that are 5- to 8-fold higher than a single dose (10, 11). Thus, higher concentrations indicate repeated dosing in the preceding week or two. Analogously, drug concentrations in 1 to 1.5 cm of hair proximal to the scalp (representing 4 to 6 weeks of growth) provide cumulative dosing information from the preceding 4 to 6 weeks of therapy (16). The STRAND study defined TFV-DP in PBMC and TFV in hair using directly observed therapy (DOT) of three different dosing regimens that included 2, 4, and 7 doses per week (7, 16). The resulting drug concentrations provided critical benchmarks for interpreting drug concentrations and adherence in clinical trials, such as iPrEx, iPrEx OLE, VOICE, and PrEP-Demo (6, 7, 17).
The present study focused on TFV-DP and FTC-TP in red blood cells, measured in a 3-mm punch from a DBS, a specimen type that eases collection and processing. A previous study among 17 HIV-negative individuals receiving daily FTC-TDF for 30 days (not DOT) estimated that the TFV-DP half-life in DBS was 17 days, translating to a predicted 25-fold accumulation from first dose to steady state (18). This degree of accumulation provides a large dynamic range for distinguishing different levels or gradients of cumulative dosing (i.e., adherence) in the preceding 4 to 8 weeks, which presumably includes the time of exposure to HIV in PrEP studies. That study used a simple pharmacokinetic model to estimate steady-state concentrations (18), assigning adherence categories according to rounded 25th percentiles for TFV-DP in DBS. The adherence categories were ≥1,250 fmol/punch (7 doses/week), 700 to 1,249 fmol/punch (4 to 6 doses/week), 350 to 699 fmol/punch (2 to 3 doses/week), and below the limit of quantitation (BLQ) to 349 fmol/punch (<2 doses/week) (19). These categories were used in PrEP demonstration projects among men who have sex with men (MSM) to estimate gradients of adherence, where strong correlations with PrEP effectiveness were observed (19–21). The present study used DOT to address the shortcomings of the previous study (18), which included not achieving steady state and assuming a constant 30% coefficient of variation, linearity in pharmacokinetics, and that TFV-DP varied in direct proportion to dose/adherence (i.e., was dose proportional).
RESULTS
Fifty-two subjects participated in the study. Of these, 48 completed at least one dosing regimen and were included in the analysis. Of the four participants not included, three withdrew from the study prior to reaching steady state on their first regimen (range of 11 to 45 days on study). The fourth subject was diagnosed with hereditary elliptocytosis during participation in the study, a condition that could influence RBC kinetics, and an exclusion criterion. Two of the remaining 48 participants completed the first regimen but not the second; they were included in the analyses (one withdrew due to stomach cramping and the other moved out of the country). The characteristics for the 48 participants, split by sex, are shown in Table 1.
TABLE 1.
Baseline characteristics of DOT-DBS study population
| Characteristic | Value fora: |
||
|---|---|---|---|
| Male (n = 23) | Female (n = 25) | Overall (n = 48) | |
| Location | |||
| Denver site | 11 | 13 | 24 |
| San Francisco site | 12 | 12 | 24 |
| Race/ethnicity | |||
| White | 14 (61%) | 12 (48%) | 26 (54%) |
| Hispanic | 6 (26%) | 8 (32%) | 14 (29%)b |
| Black | 3 (13%) | 5 (20%) | 8 (17%) |
| Age (yr) | 29 (22–45) | 29 (21–49) | 29 (21–49) |
| Weight (kg) | 82 (60–155) | 63 (51–114) | 77 (51–155) |
| BMI (kg/m2) | 26.7 (20.4–53.9) | 23.9 (17.0–45.6) | 25.7 (17.0–53.9) |
| Estimated GFR (ml/min) | 92 (69–156) | 100 (69–129) | 98 (69–156) |
| Hematocrit (%) | 45 (40–49) | 42 (35–46) | 43 (35–49) |
Results are given as median (range) or number (percent).
Two Hispanic individuals were black, one male and one female.
TFV-DP dose proportionality.
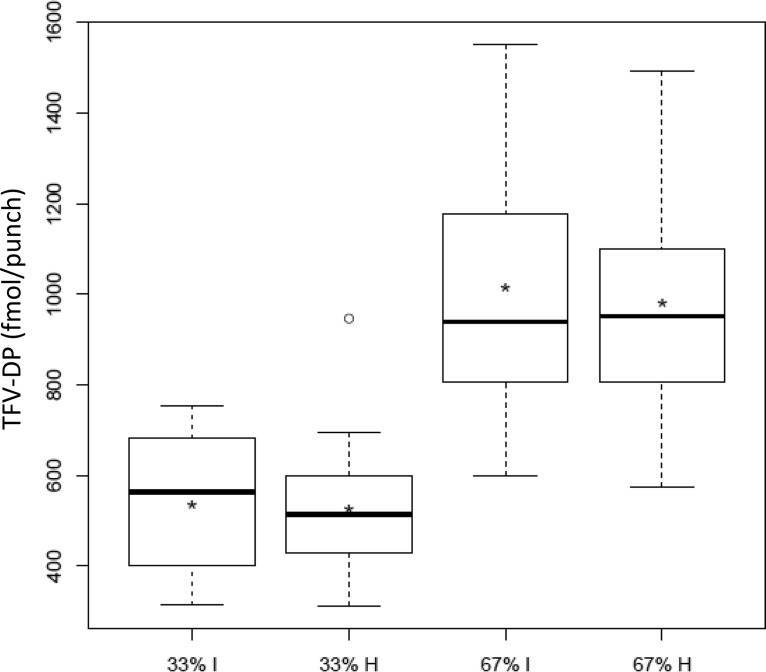
The average (range) percentage of prescribed doses taken per individual was 99.7% (91.1 to 100%), representing “as treated” data. All as treated data were confirmed by directly observed dosing. Table 2 summarizes observed TFV-DP concentrations at steady state (week 12 and week 36). For both 33% and 67% adherence, the intermittent and holiday arms exhibited nearly identical TFV-DP concentrations in DBS within 4% of each other (Fig. 1). Combining the intermittent and holiday arms yielded dose proportionality for TFV-DP; the estimated slope, β (90% confidence intervals [CI]), was 1.01 (0.92, 1.1) per randomization arm and 1.04 (0.97, 1.11) for as treated data. This indicated that TFV-DP varied in direct proportion to dosing (i.e., adherence). Dose proportionality was also demonstrated individually for the intermittent and holiday dosing patterns, 1.06 (0.97, 1.14) and 1.02 (0.90, 1.13), respectively. Finally, dose proportionality was maintained after separately adjusting for sex, site, hematocrit, mean corpuscular volume, and treatment period, all with 90% confidence intervals within 0.97 and 1.11.
TABLE 2.
Observed TFV-DP concentrations at steady state (i.e., weeks 12 and 36)
| Dose category | Observed concn (fmol/punch) by dosing regimena |
||
|---|---|---|---|
| 33% | 67% | 100% | |
| Mean overall | 530 | 997 | 1,605 |
| Intermittent/holiday | 535/525 | 1,014/982 | |
| Median overall | 518 | 946 | 1,542 |
| Intermittent/holiday | 564/514 | 940/952 | |
| Min/max overall | 312/947 | 576/1,551 | 1,092/2,677 |
| SD overall | 159 | 267 | 405 |
| Intermittent/holiday | 155/169 | 290/253 | |
The total number of participants was 48. Each participant received 2 of 3 dosing regimens for a total of 32 participants each; 2 participants completed only the first regimen. For the 33% arm, there were 30 participants; 67% arm, 32 participants; 100% arm, 32 participants. The following overall CV were found: 33% arm, 30%; 67% arm, 27%; 100% arm, 25%. The CV (%) for intermittent/holiday were as follows: 33% arm, 29/32; 67% arm, 29/26.
FIG 1.
Observed TFV-DP from week 12 or 36 (y axis) according to intermittent (I) or holiday (H) study arm (x axis). The limits of the box represent the 1st and 3rd data quartiles (Q1 and Q3), the interior horizontal line denotes the median, and the asterisk represents the mean. The whiskers extend to the most extreme point within a value of 1.5 × (Q3 − Q1) of the corresponding hinge, beyond which outliers are represented by a circle.
TFV-DP pharmacokinetics.
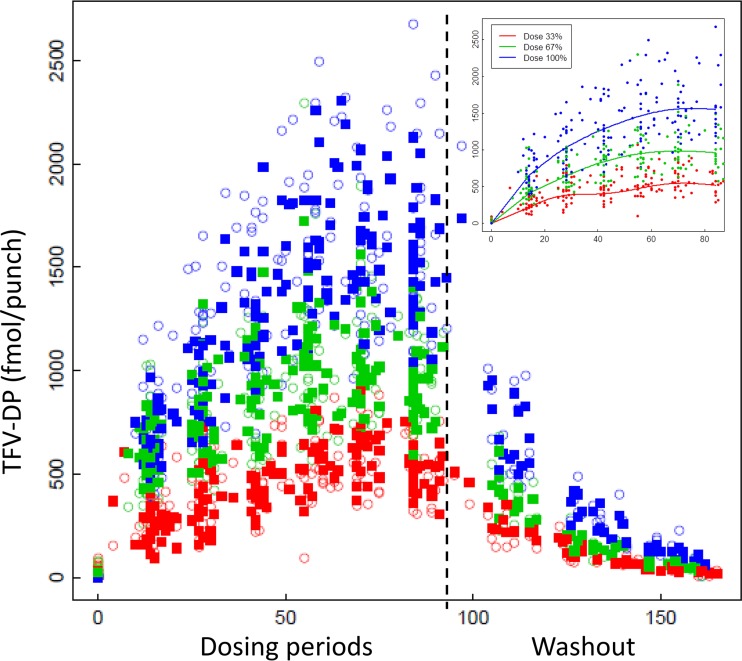
Figure 2 shows the fitted TFV-DP concentrations (base model) overlaid on the observed concentrations. The median (range) half-life in the washout phase was 17 (14 to 23) days. Consistent with this long half-life, TFV-DP concentrations accumulated during the first weeks of dosing until week 8, when values were within 10% of week 12 values (i.e., steady state was achieved). The fitted TFV-DP medians for 100% dosing for weeks 4, 8, and 12 were 1,011, 1,493, and 1,534 fmol/punch. Table S1 in the supplemental material shows the fitted median concentrations for weeks 2 through 12 for the study arms as well as 2 (28.6%) and 4 doses/week (57.1%) given the importance of these adherence thresholds in previous studies.
FIG 2.
TFV-DP (y axis) by actual day of therapy (x axis) for 100% (blue), 67% (green), and 33% (red) dosing regimens. Intermittent and holiday are combined, and both dosing periods are included from days 0 to ∼84, followed by the washout between dosing periods (right of dashed line). Open symbols depict observed concentrations, and closed symbols are fitted concentrations. The inset shows the modeled average for each regimen overlaid on the observed data during dosing periods.
Once steady state was achieved, concentrations were stable within an individual. For example, 95% of participants with consistent adherence would be expected to have a TFV-DP concentration within −5.4% and 5.7% of the previous value (or 99% between −6.7% and 7.1%).
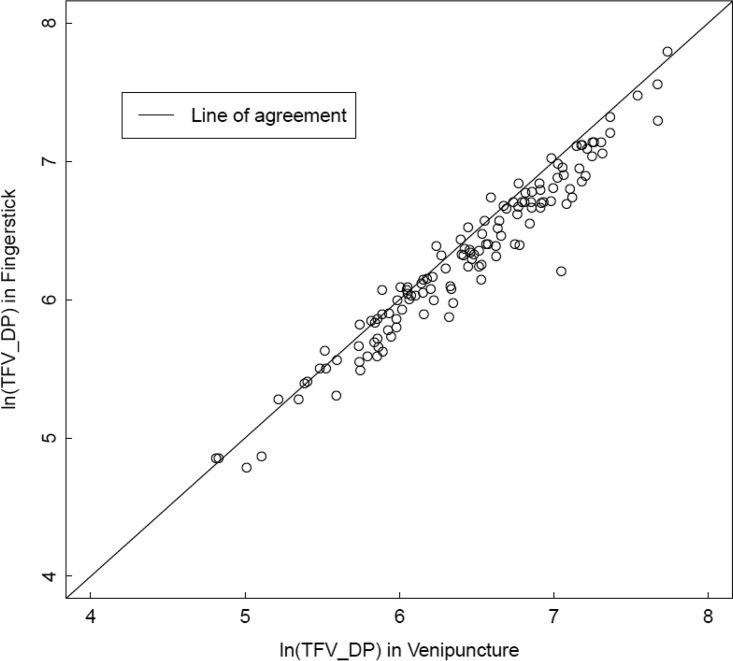
Given that food impacts TFV bioavailability, dosing with meals was recorded, and the median (range) percentage of doses taken within 2 h of a meal was 97% (49% to 100%), indicating that most participants took the majority of their doses near a meal. The only significant predictor of TFV-DP concentrations was sex. Concentrations were 17.6% (6.5%, 27.4%) lower in males than females in univariate analysis and were 19.4% (8.1%, 29.3%) lower after adjusting for dosing period (1st versus 2nd), hematocrit, body mass index (BMI), estimated glomerular filtration rate (GFR), and percentage of doses with a meal. Of the other prespecified variables, none were significant in univariate analysis or the adjusted model. In the adjusted model, TFV-DP was 14.0% (−3.1, 28.3%) lower in African Americans than in Caucasians and 8.9% (−3.8%, 23.3%) lower in subjects from San Francisco versus Denver, but these findings were not statistically significant. TFV-DP was not significantly associated with hematocrit in the adjusted model (95% CI, −3.4, 3.9) (P = 0.92). Finally, TFV-DP obtained by fingerstick was, on average, 11.7% lower (9.5%, 13.9%) than that for venipuncture, but the interclass correlation was high (0.89), as shown in Fig. 3.
FIG 3.
Natural log TFV-DP from DBS derived from fingerstick (y axis) versus TFV-DP from a paired DBS collected from venipuncture (samples were collected at the same time). The solid line is the line of agreement. The interclass correlation was 0.89.
Table 3 shows fitted 50th (25th and 75th) percentiles for subgroups, focusing on 2, 4, and 7 doses/week on average (i.e., 28.6%, 57.1%, and 100% adherence). The 25th percentiles compared well with the rounded 25th percentiles from the previous pharmacokinetic model, i.e., 350, 700, and 1,250 fmol/punch (18). The base model values (347, 714, and 1,280 fmol/punch) were within 3%, and all of the subgroups were within 14% of these values (i.e., 311 to 384, 642 to 792, and 1,153 to 1,423 fmol/punch). Values for the 10th and 90th percentiles are listed in Table S2 in the supplemental material.
TABLE 3.
Fitted TFV-DP at steady state for 2, 4, and 7 doses/week according to prespecified predictors
| Model | Fitted 50th (25th, 75th) percentiles by dosing regimen |
||
|---|---|---|---|
| 28.6% (2 doses/wk) | 57.1% (4 doses/wk) | 100% (7 doses/wk) | |
| Base | 416 (347, 498) | 856 (714, 1,026) | 1,534 (1,280, 1,837) |
| Women | 455 (384, 538) | 939 (792, 1,112) | 1,685 (1,423, 1,997) |
| Men | 375 (316, 444) | 774 (653, 917) | 1,389 (1,173, 1,646) |
| Denver | 442 (370, 527) | 912 (764, 1,088) | 1,636 (1,371, 1,953) |
| San Francisco | 390 (326, 465) | 804 (674, 960) | 1,443 (1,209, 1,722) |
| Caucasian | 433 (362, 518) | 894 (748, 1,069) | 1,604 (1,342, 1,918) |
| Hispanic | 416 (348, 498) | 859 (719, 1,027) | 1,542 (1,290, 1,843) |
| African American | 372 (311, 445) | 768 (642, 918) | 1,378 (1,153, 1,647) |
Adherence based upon TFV-DP concentration.
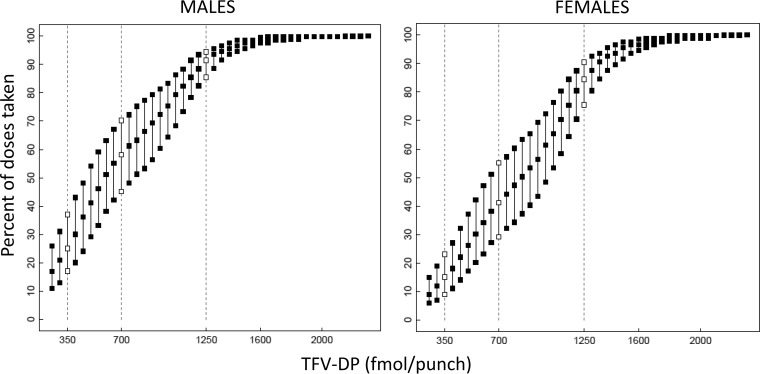
Figure 4 shows the estimated median, 25th, and 75th percentiles for percent dosing/adherence given a specific TFV-DP concentration in men and women. As expected, an observed TFV-DP concentration may be associated with more than one dosing regimen/adherence rate. For example, in men, a concentration of 700 fmol/punch, the threshold associated with high PrEP efficacy in prior MSM studies, was associated with a median adherence of 4.1 doses/week, with 75% of individuals taking 3.2 doses/week or more. For a value of 350 fmol/punch, the median adherence rate was 1.8 doses/week with 75% of individuals taking 1.2 doses/week or more. The median adherence for 1,250 fmol/punch was 6.4 doses/week, with 75% of individuals taking 6 doses/week or more.
FIG 4.
Estimated 25th, 50th, and 75th percentiles for the percentage of doses taken (y axis) for a given TFV-DP concentration in fmol/punch (x axis). Open symbols and dashed lines represent 300, 700, and 1,250 fmol/punch, values used in previous clinical studies. Estimates for males are on the left and females on the right.
Women had higher TFV-DP concentrations than men, so the estimated dosing was lower for a given TFV-DP dose. For example, 75% of women were taking 0.6, 2.0, and 5.3 doses/week or more for 350, 700, and 1,250 fmol/punch, respectively, compared with 1.2, 3.1, and 6 doses/week or more in men. For both sexes, as concentrations approached and exceeded ∼1,500 fmol/punch, the range of possible doses leveled off at 100%.
Information from FTC-TP.
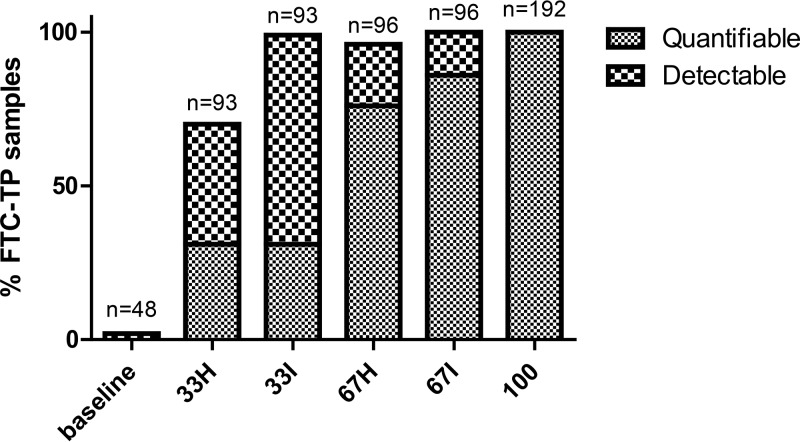
FTC-TP detection was defined as an FTC-TP sample/lower limit of quantitation (LLOQ) peak area ratio of ≥20%. Detection was indicative of an observed dose taken within 7 days with 92% (95% CI, 84%, 100%) sensitivity and 100% (100%, 100%) specificity. The area under the receiver operating characteristic (ROC) was 0.95, and there were 3/37 false negatives and 0/15 false positives. Thus, FTC-TP from the study fell into three categories: quantifiable (above the LLOQ), detectable (sample/LLOQ peak area ratio of ≥20%), and undetectable (sample/LLOQ peak area ratio of <20%). The percentage of FTC-TP in these categories was assessed by study arm. The median (interquartile range [IQR]) times postdose for 33% intermittent, 33% holiday, 67% intermittent, and 67% holiday arms were 2.03 days (0.02, 3.04), 7.29 days (3.05, 9.86), 1.97 days (0.01, 2.0), and 5.8 days (3.0, 7.93), respectively. This was consistent with convenience sampling and the intermittent and holiday dosing patterns. A total of 57 times postdose were beyond 7 days, 46 from the 33% Holiday arm, 10 from the 67% Holiday arm, and one from the 33% intermittent arm. Figure 5 illustrates the percentage of FTC-TP samples that were detectable or quantifiable by study arm. One false positive was observed at baseline. All samples from the 100% dosing arm were quantifiable, as were most of the 67% intermittent samples. The addition of FTC-TP detection captured additional recent doses (within 7 days) for a significant fraction of BLQ doses, particularly for the 33% regimens.
FIG 5.
DBS were assayed for quantitation or detection of FTC-TP (numbers of samples assayed are listed above the bars). The percentage of FTC-TP samples (y axis) either quantifiable (hatched pattern) or detectable (checkered pattern) per study arm (x axis) is shown.
DISCUSSION
This study utilized directly observed dosing in HIV-uninfected volunteers to show that TFV-DP concentration varied in direct proportion to dose/adherence (i.e., was dose proportional), exhibited linear pharmacokinetics consistent with a 17-day half-life, demonstrated a coefficient of variation (CV) at steady state of 25% to 30%, and largely recapitulated concentration estimates from a previous pharmacokinetic model (18). The previous model used rounded 25th percentiles of 350, 700, and 1,250 fmol/punch to interpret adherence categories. The present study affirms that these were accurate estimates for expected concentrations arising from 2, 4, and 7 doses/week on average across site (sea level to 5,280 feet above sea level), sex, and race. TFV-DP concentrations were approximately 19% lower in males than in females in models adjusted for BMI, hematocrit, creatinine clearance, and dosing with a meal. However, even with this difference, the estimates for men and women both were within 14% of the 350-, 700-, and 1,250-fmol/punch thresholds. The mechanism for the sex difference is unclear. Men were shown in previous studies to have lower nucleoside analog-triphosphate concentrations in PBMC, but the mechanism(s) was not elucidated (22–24). Comparatively, other studies did not reproduce these sex differences in PBMC (11, 25–27). There are a myriad of potential explanations, including differential expression of transporters or enzymes that influence tenofovir disposition, such as carboxylesterase 2, P-glycoprotein (ABCB1), and/or breast cancer resistance protein (BCRP) (28, 29). Other possibilities include different levels of red blood cell turnover or differential expression of enzymes that control the uptake, efflux, or phosphorylation of TFV in the red blood cell, including adenylate kinase I, pyruvate kinase, and nucleoside diphosphate kinase (30).
As described above, this study and the previous model assumed a constant dose (i.e., 2, 4, or 7 doses/week) and estimated the range of TFV-DP concentrations associated with that dose. Conversely, the present study also examined the relationship of assuming a constant TFV-DP concentration and estimating the range of dosing/adherence consistent with that concentration. This provided a different perspective, particularly for midrange concentrations such as 700 fmol/punch, which could arise from several dosing/adherence rates because of interindividual variability (Fig. 4). For illustration, using the former interpretation (assuming a constant dose), approximately 75% of men and women dosing with 4 doses/week on average will achieve a concentration of 700 fmol/punch or greater. Using the latter interpretation (assuming a constant concentration), approximately 75% of men with a concentration of 700 fmol/punch will be dosing with ≥3.2 doses/week (as will 25% of individuals dosing with <3.2 doses/week). For women with a concentration of 700 fmol/punch, approximately 75% will be dosing with ≥2.0 doses/week (and 25% will be dosing with <2.0 doses/week). Thus, this analysis takes into account individuals with lower adherence who also achieve 700 fmol/punch.
Taken together, these analyses provided different interpretations with complementary utility. For example, 700 fmol/punch was found to be associated with high PrEP efficacy in MSM (discussed below) (19–21, 31). Our first interpretation indicates that ≥4 doses/week would be required to achieve the ≥700-fmol/punch threshold in the majority of individuals. This would be useful for predicting efficacy for novel dosing regimens, such as nondaily event-driven dosing, where ≥4 doses/week would be highly effective. On the other hand, our second interpretation could be applied to samples from studies where the adherence-response relationships are unknown. For example, given a concentration of ≥700 fmol/punch, approximately 75% of women would be taking ≥2.0 doses/week. This information, combined with efficacy data from a trial, would be useful for defining the adherence-response relationship in women to understand dose response in this population and for application to future studies.
TFV-DP in DBS has been utilized in several PrEP studies among MSM to estimate adherence. For example, in the iPrEx OLE, ATN 110/113, and PrEP-Demo studies, which comprised over 2,000 person-years of follow up, there were 37 HIV infections (19–21, 31). TFV-DP in DBS was measured in the 37 participants who acquired HIV at the time point closest to HIV seroconversion, and 23 (62%) were BLQ, 13 (35%) were between BLQ and 349 fmol/punch, 1 (3%) was between 350 and 699 fmol/punch, and none were ≥700 fmol/punch (19–21, 31). The iPrEx OLE study, which had 28 of the 37 cases, estimated 84% (95% CI, 21%, 99%) and 100% (86%, 100%) HIV risk reduction for 350 to 699 fmol/punch and ≥700 fmol/punch, respectively (19). The previous interpretation for these categories was 2 to 3 doses/week and ≥4 doses/week on average (18). The present study adds the interpretation that, given a concentration of 350 and 700 fmol/punch, approximately 75% of men will be dosing with ≥1.2 and ≥3.2 doses/week, respectively. For MSM, these interpretations are comparable and support that PrEP was highly effective even with less than daily dosing. This interpretation is consistent with results from the IPERGAY study, which used event-driven dosing (i.e., on demand) and showed 86% (95% CI, 40%, 98%) efficacy for a median of 3.75 doses/week (15 doses per month) (32). In a subanalysis of IPERGAY, similar efficacy was observed for infrequent sexual encounters (median of 1.25/week), which resulted in a median of 2.4 doses/week (33). It must be noted that three cases of HIV infection have now been reported among MSM with TFV-DP in DBS ranging from 1,478 to 2,297 fmol/punch (34–36). These concentrations are consistent with 75% of individuals taking >94% of their doses on average. Two of these cases involved transmission of a drug-resistant virus (34, 35), and the third involved transmission of wild-type virus following numerous condomless sexual acts per day over several months (36). Thus, HIV acquisition exhibits complexities, and PrEP breakthrough infections can occur, albeit rarely, in highly adherent MSM.
Effective TFV-DP concentrations in DBS have not been estimated for women, although studies are under way to fill this gap (e.g., https://www.hptn.org/research/studies/hptn082). The concentration-adherence-efficacy estimates described above in MSM should not be extrapolated to women without supporting outcomes from trials. This is due to the different concentration-adherence findings from the present study, as well as evidence that women may require higher adherence levels for similar PrEP efficacy than MSM because of lower active drug concentrations in genital versus rectal compartments or other unknown variables (11, 37, 38). Short half-life moieties (TFV in plasma) suggested poor adherence (30 to 40% detection) in the VOICE and FEMPREP trials among young women, which failed to demonstrate efficacy (2, 3). However, a long half-life moiety (TFV in hair) in a subset of VOICE participants showed dosing consistent with 0.2 doses/week (3% adherence), suggesting worse adherence than that indicated by TFV detection in plasma, perhaps due to white-coat dosing (6). Similarly, in FEM-PrEP, an adherence score was derived from TFV in plasma and TFV-DP in upper layer packed cells (ULPC; mostly red cells), a long half-life moiety (39, 40). The study found that ∼43% were in the lowest score group versus only 11% in the highest score group. Of note, TFV-DP findings from the present study suggest that some women with relatively low adherence have been misclassified into the higher adherence categories. Taken together, future studies with long half-life moieties such as TFV-DP in DBS will be useful for estimating the adherence-response relationship for women.
Although not statistically significant, TFV-DP was 14.0% (−3.1%, 28.3%) lower in African Americans than Caucasians, and 8.9% (−3.8%, 23.3%) lower in subjects from San Francisco than Denver. Some PrEP studies have observed lower TFV-DP in DBS in African Americans than in Caucasians. For example, the PrEP-Demo study observed protective PrEP concentrations (TFV-DP of ≥700 fmol/punch) at 91.1% of visits from Caucasians versus only 56.8% of visits from African Americans (20). A discrepancy of this magnitude most likely is due to adherence differences, although pharmacokinetics and pharmacogenomics may play a small role. On an individual basis, these factors (including sex) resulted in small differences (i.e., <14%) from the estimated thresholds of 350, 700, and 1,250 fmol/punch, as described above. However, the influence of multiple factors on these estimates was not assessed in this small study, and the possibility of additive effects warrants additional research. Furthermore, the two methods of DBS collection were highly correlated, but fingerstick resulted in 11.7% (9.5%, 13.9%) lower results than those with venipuncture, presumably due to dilution of capillary blood with interstitial fluid. For this reason, studies should use a consistent method of DBS collection.
FTC-TP was measured on the same assay as TFV-DP, and the combination of TFV-DP and FTC-TP in DBS provided cumulative and recent adherence information. TFV-DP informed about average dosing over weeks, reaching steady state in approximately 8 weeks, but concentrations did not inform about recent dosing. This was evident in that TFV-DP concentrations were similar for the intermittent and holiday dosing patterns. On the other hand, FTC-TP informed about recent dosing. It was 100% quantifiable for daily dosing, consistent with being quantifiable for approximately 48 h postdose (15). Similarly, it was quantifiable in 86% of samples from the 67% intermittent arm. This study added that FTC-TP could be detected up to 7 days postdose, which increased the number of detectable samples in the 33% arms. In terms of clinical application, FTC-TP can inform white coat dosing if it is quantifiable and TFV-DP is relatively low. If FTC-TP is undetectable or unquantifiable, this fact can inform that an individual stopped dosing within 2 or up to 7 days ago, which is useful for understanding patterns of recent nonadherence.
In summary, TFV-DP concentrations varied in direct proportion to dose/adherence, exhibited linear kinetics defined by a 17-day half-life, and were similar to those from a previous model used in several clinical trials. Thus, TFV-DP concentration provides an estimate of long-term adherence, presumably encompassing times around HIV exposures in PrEP studies. TFV-DP is not significantly influenced by recent or white-coat dosing, whereas FTC-TP quantitation/detection provides evidence of recent or white-coat dosing. DBS collection and processing are convenient, and analytes are stable for 5 days at room temperature, although long-term storage should be at −20°C or −80°C to prevent analyte breakdown (41). DBS can be shipped at ambient temperature with 2-day delivery, but shipping on dry ice avoids problems in the event of delivery delays (41). The main limitations of using TFV-DP and FTC-TP for adherence assessment include the possibility of misspecification and the inability to identify different patterns of nonadherence, both of which were discussed above. Taken together, FTC-TP and TFV-DP concentrations are useful for interpreting PrEP study outcomes and may facilitate discussions of protection and residual risk for HIV acquisition in individuals receiving daily TDF/FTC for HIV prevention.
MATERIALS AND METHODS
DOT-DBS was conducted at the University of Colorado Anschutz Medical Campus (Denver) and San Francisco Department of Public Health (San Francisco). The study was approved by local Institutional Review Boards (IRBs) and registered at clinicaltrials.gov (NCT02022657), and informed consent was obtained from all participants. Inclusion eligibility consisted of 18- to 50-year-old HIV-negative adults who were deemed able to comply with the study procedures, including directly observed dosing. Because participants were given less than daily dosing with a washout period, participants at high risk of HIV acquisition were excluded from the study. Other major exclusion criteria were inability to give informed consent, HIV+ enzyme-linked immunosorbent assay (ELISA), symptoms of acute HIV infection, hepatitis B virus surface antigen positivity, pregnancy or breast-feeding, estimated GFR of <60 ml/min (MDRD) or urine protein level of ≥2+, history of nontraumatic bone fractures, medical conditions that alter red blood cell kinetics such as hemoglobinopathies or hemolysis, or laboratory abnormalities/medical/concomitant medications/psychiatric/social/alcohol/drug use conditions that, in the opinion of the investigators, might interfere with the study.
The study medication was emtricitabine-tenofovir (Truvada; 200 mg FTC and 300 mg TDF), which was donated by Gilead Sciences. The study was a prospective, randomized, crossover design with a washout period between dosing regimens. Each dosing regimen was ∼12 weeks, as was the washout period, for a total study duration of ∼36 weeks. Participants completed two of five dosing regimens: 100% daily dosing, 67% of daily dosing (intermittent or holiday), or 33% of daily dosing (intermittent or holiday). Intermittent meant that doses were skipped by days (e.g., 33% means dosing on day one followed by skipped doses on days two and three, repeated for 12 weeks, and 67% means dosing on days one and two followed by a skipped dose on day three, repeated for 12 weeks). Holiday dosing means that doses were skipped by weeks (e.g., 33% holiday means daily dosing for 1 week followed by 2 weeks of skipped dosing, repeated for 12 weeks, and 67% means daily dosing for 2 weeks followed by 1 week of skipped doses, repeated for 12 weeks).
Dosing started on the first day of the study. Doses could be anytime in the 24-h period of a dosing day. All doses were directly observed in person or by live video streaming by cell phone or computer. Following ingestion, subjects were asked to open their mouths to show the dose was swallowed. Study personnel recorded dosing time and whether dosing was within 2 h of a meal for every dose.
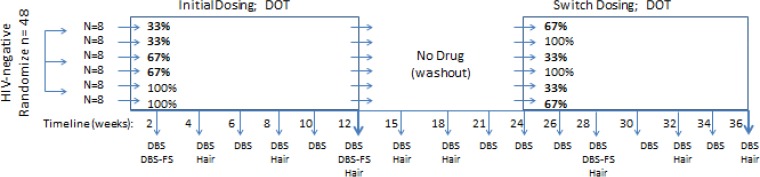
During dosing regimens, blood was collected in an EDTA tube approximately every 2 weeks for plasma, PBMC, and DBS; collections were every 3 weeks in the washout period. For DBS, 25 μl of whole blood was transferred to a Whatman 903 card using a pipette. On three occasions, a paired Whatman 903 card was also collected via fingerstick. Cards were air dried for at least 3 h or overnight at room temperature and then stored at −80°C until analysis. Hair was collected every 4 weeks during dosing and at the first three washout visits; analysis of hair, plasma, and PBMC is not included in this communication. All sampling visits were scheduled by convenience without regard to time or day since the last dose. The study schema and collection times are summarized in Fig. 6.
FIG 6.
HIV-negative volunteers were randomized to one of six 12-week sequences of two directly observed (DOT) dosing regimens (left rectangle and then right rectangle) separated by a 12-week washout (open space). Thirty-three and 67% dosing were split into intermittent and holiday patterns (not shown). The timeline and collection of DBS, DBS by fingerstick (DBS-FS), and hair are indicated along the bottom.
TFV-DP and FTC-TP were quantified from a 3-mm punch using a previously validated methodology (41). The 3-mm punch was extracted with 500 μl of 70:30 methanol-water, creating a lysed cellular matrix; 400 μl of this lysate, which comprised the sample, was assayed. The assay was linear from 25 to 6,000 fmol/sample for TFV-DP and 0.1 to 200 pmol/sample for FTC-TP.
Statistical methods.
Dose proportionality was determined based upon the observed TFV-DP concentrations following 12 weeks of dosing (week 12 or 36) using the Power model (on the natural log scale), ln(Yijk) = μ + Si + Pj + βlog(Dk) + εijk, where Yijk is TFV-DP for the kth dose (k = 33%, 67%, and 100%), jth period (j = 1 and 2), and ith subject (i = 1,…48). μ is the overall mean, Si is a random subject effect, Pj is the period effect, and εijk is random error (42). Dose proportionality dictates that β = 1 for dose-dependent parameters, and dose proportionality was assumed if the 90% CI was contained within the limits (0.8, 1.25). For the primary analysis, no effect of holiday and intermittent regimens was assumed. Dose proportionality was assessed per randomization arm (primary analysis) and as treated, using only observed doses ingested. If dose proportionality was demonstrated for 33% to 100% dosing, predictions were also made for dosing rates such as 2 and 4 doses/week on average (i.e., 28.6% and 57.1%), which were the same doses evaluated in STRAND and highly correlated with PrEP efficacy in demonstration studies (16, 19–21).
The effects of demographic and study variables were evaluated using all available concentration data. A mixed-effects model with tensor product of natural b-spline transformations of study day and dose regimen (calculated at each sampling time) was used to model log(TFV-DP) concentration as a smooth, nonlinear function of day and dose regimen. The following demographic and study variables were evaluated as predictors of TFV-DP: intermittent versus holiday, site, sex, and race. Models were adjusted for treatment period and BMI, estimated GFR, average percentage of doses taken with meal, and hematocrit (41, 43–45). A generalized linear mixed model with a logit link was used to estimate dose given the week 12 (steady-state) concentration.
A previously published analysis of DOT-DBS showed that FTC-TP was quantifiable for approximately 48 h after the last dose (15). However, upon further review, many FTC-TP samples that were below the limit of quantitation (BLQ) were still detectable, between the detection limit and the lower limit of quantitation (LLOQ). In order to determine FTC-TP detection versus assay background noise, the FTC-TP ratio of the peak area from the sample chromatograph relative to that of the LLOQ was determined. Initially, a receiver operating characteristic (ROC) analysis was carried out using all BLQ data up to 14 days postdose to probe the optimal FTC-TP peak area ratio and persistence postdose. A final analysis used a single sample from each participant within 7 days postdose to define ROC curve characteristics. Baseline samples prior to dosing were true negatives. Once the optimal peak area ratio for detection was defined, the rate of FTC-TP detection was applied to all BLQ samples from the dosing phases of the study.
Supplementary Material
ACKNOWLEDGMENTS
We thank the study participants, past and present members of the Colorado Antiviral Pharmacology Laboratory, staff at the University of Colorado CTRC and San Francisco Department of Health, Ariel Hodara, Julie Predhomme, and Joseph Rower.
Support for this work was provided by the NIH (K23AI104315 [J.C.-M.], U01 AI106499 [P.L.A.], T32 AI7447-23 [S.S.], and UL1 TR001082 [University of Colorado Clinical and Translational Sciences Institute]). Gilead Sciences provided study drug.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01710-17.
REFERENCES
- 1.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, Goicochea P, Casapia M, Guanira-Carranza JV, Ramirez-Cardich ME, Montoya-Herrera O, Fernandez T, Veloso VG, Buchbinder SP, Chariyalertsak S, Schechter M, Bekker LG, Mayer KH, Kallas EG, Amico KR, Mulligan K, Bushman LR, Hance RJ, Ganoza C, Defechereux P, Postle B, Wang F, McConnell JJ, Zheng JH, Lee J, Rooney JF, Jaffe HS, Martinez AI, Burns DN, Glidden DV. 2010. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, Palanee T, Nakabiito C, van der Straten A, Noguchi L, Hendrix CW, Dai JY, Ganesh S, Mkhize B, Taljaard M, Parikh UM, Piper J, Masse B, Grossman C, Rooney J, Schwartz JL, Watts H, Marzinke MA, Hillier SL, McGowan IM, Chirenje ZM. 2015. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med 372:509–518. doi: 10.1056/NEJMoa1402269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, Malahleha M, Owino F, Manongi R, Onyango J, Temu L, Monedi MC, Mak'Oketch P, Makanda M, Reblin I, Makatu SE, Saylor L, Kiernan H, Kirkendale S, Wong C, Grant R, Kashuba A, Nanda K, Mandala J, Fransen K, Deese J, Crucitti T, Mastro TD, Taylor D. 2012. Preexposure prophylaxis for HIV infection among African women. N Engl J Med 367:411–422. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, Tappero JW, Bukusi EA, Cohen CR, Katabira E, Ronald A, Tumwesigye E, Were E, Fife KH, Kiarie J, Farquhar C, John-Stewart G, Kakia A, Odoyo J, Mucunguzi A, Nakku-Joloba E, Twesigye R, Ngure K, Apaka C, Tamooh H, Gabona F, Mujugira A, Panteleeff D, Thomas KK, Kidoguchi L, Krows M, Revall J, Morrison S, Haugen H, Emmanuel-Ogier M, Ondrejcek L, Coombs RW, Frenkel L, Hendrix C, Bumpus NN, Bangsberg D, Haberer JE, Stevens WS, Lingappa JR, Celum C. 2012. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, Henderson FL, Pathak SR, Soud FA, Chillag KL, Mutanhaurwa R, Chirwa LI, Kasonde M, Abebe D, Buliva E, Gvetadze RJ, Johnson S, Sukalac T, Thomas VT, Hart C, Johnson JA, Malotte CK, Hendrix CW, Brooks JT. 2012. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 367:423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 6.Koss CA, Bacchetti P, Hillier SL, Livant E, Horng H, Mgodi N, Mirembe BG, Gomez Feliciano K, Horn S, Liu AY, Glidden DV, Grant RM, Benet LZ, Louie A, van der Straten A, Chirenje ZM, Marrazzo JM, Gandhi M. 2 March 2017. Differences in cumulative exposure and adherence to tenofovir in the VOICE, iPrEx OLE, and PrEP demo studies as determined via hair concentrations. AIDS Res Hum Retrovir doi: 10.1089/aid.2016.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson PL, Glidden DV, Liu A, Buchbinder S, Lama JR, Guanira JV, McMahan V, Bushman LR, Casapia M, Montoya-Herrera O, Veloso VG, Mayer KH, Chariyalertsak S, Schechter M, Bekker LG, Kallas EG, Grant RM. 2012. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med 4:151ra125. doi: 10.1126/scitranslmed.3004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donnell D, Baeten JM, Bumpus NN, Brantley J, Bangsberg DR, Haberer JE, Mujugira A, Mugo N, Ndase P, Hendrix C, Celum C. 2014. HIV protective efficacy and correlates of tenofovir blood concentrations in a clinical trial of PrEP for HIV prevention. J Acquir Immune Defic Syndr 66:340–348. doi: 10.1097/QAI.0000000000000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fonner VA, Dalglish SL, Kennedy CE, Baggaley R, O'Reilly KR, Koechlin FM, Rodolph M, Hodges-Mameletzis I, Grant RM. 2016. Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. AIDS 30:1973–1983. doi: 10.1097/QAD.0000000000001145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hendrix CW, Andrade A, Bumpus NN, Kashuba AD, Marzinke MA, Moore A, Anderson PL, Bushman LR, Fuchs EJ, Wiggins I, Radebaugh C, Prince HA, Bakshi RP, Wang R, Richardson P, Shieh E, McKinstry L, Li X, Donnell D, Elharrar V, Mayer KH, Patterson KB. 2015. Dose frequency ranging pharmacokinetic study of tenofovir-emtricitabine after directly observed dosing in healthy volunteers to establish adherence benchmarks (HPTN 066). AIDS Res Hum Retrovir 32:32–43. doi: 10.1089/AID.2015.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seifert SM, Chen X, Meditz AL, Castillo-Mancilla JR, Gardner EM, Predhomme JA, Clayton C, Austin G, Palmer BE, Zheng JH, Klein B, Kerr BJ, Guida LA, Rower C, Rower JE, Kiser JJ, Bushman LR, MaWhinney S, Anderson PL. 2016. Intracellular tenofovir and emtricitabine anabolites in genital, rectal, and blood compartments from first dose to steady state. AIDS Res Hum Retrovir 32:981–991. doi: 10.1089/aid.2016.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koenig HC, Mounzer K, Daughtridge GW, Sloan CE, Lalley-Chareczko L, Moorthy GS, Conyngham SC, Zuppa AF, Montaner LJ, Tebas P. 2017. Urine assay for tenofovir to monitor adherence in real time to tenofovir disoproxil fumarate/emtricitabine as pre-exposure prophylaxis. HIV Med 18:412–418. doi: 10.1111/hiv.12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fonsart J, Saragosti S, Taouk M, Peytavin G, Bushman L, Charreau I, Hance A, Goldwirt L, Morel S, Mammano F, Loze B, Capitant C, Clavel F, Mahjoub N, Meyer L, Anderson PL, Delaugerre C, Molina J-M. 2016. Single-dose pharmacokinetics and pharmacodynamics of oral tenofovir and emtricitabine in blood, saliva and rectal tissue: a sub-study of the ANRS IPERGAY trial. J Antimicrob Chemother 72:478–485. doi: 10.1093/jac/dkw412. [DOI] [PubMed] [Google Scholar]
- 14.Dai JY, Hendrix CW, Richardson BA, Kelly C, Marzinke M, Chirenje ZM, Marrazzo JM, Brown ER. 2016. Pharmacological measures of treatment adherence and risk of HIV infection in the VOICE study. J Infect Dis 213:335–342. doi: 10.1093/infdis/jiv333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castillo-Mancilla J, Seifert S, Campbell K, Coleman S, McAllister K, Zheng JH, Gardner EM, Liu A, Glidden DV, Grant R, Hosek S, Wilson CM, Bushman LR, MaWhinney S, Anderson PL. 2016. Emtricitabine-triphosphate in dried blood spots as a marker of recent dosing. Antimicrob Agents Chemother 60:6692–6697. doi: 10.1128/AAC.01017-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu AY, Yang Q, Huang Y, Bacchetti P, Anderson PL, Jin C, Goggin K, Stojanovski K, Grant R, Buchbinder SP, Greenblatt RM, Gandhi M. 2014. Strong relationship between oral dose and tenofovir hair levels in a randomized trial: hair as a potential adherence measure for pre-exposure prophylaxis (PrEP). PLoS One 9:e83736. doi: 10.1371/journal.pone.0083736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gandhi M, Glidden DV, Mayer K, Schechter M, Buchbinder S, Grinsztejn B, Hosek S, Casapia M, Guanira J, Bekker LG, Louie A, Horng H, Benet LZ, Liu A, Grant RM. 2016. Association of age, baseline kidney function, and medication exposure with declines in creatinine clearance on pre-exposure prophylaxis: an observational cohort study. Lancet HIV 3:e521–e528. doi: 10.1016/S2352-3018(16)30153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castillo-Mancilla JR, Zheng JH, Rower JE, Meditz A, Gardner EM, Predhomme J, Fernandez C, Langness J, Kiser JJ, Bushman LR, Anderson PL. 2013. Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res Hum Retrovir 29:384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grant RM, Anderson PL, McMahan V, Liu A, Amico KR, Mehrotra M, Hosek S, Mosquera C, Casapia M, Montoya O, Buchbinder S, Veloso VG, Mayer K, Chariyalertsak S, Bekker LG, Kallas EG, Schechter M, Guanira J, Bushman L, Burns DN, Rooney JF, Glidden DV. 2014. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis 14:820–829. doi: 10.1016/S1473-3099(14)70847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu AY, Cohen SE, Vittinghoff E, Anderson PL, Doblecki-Lewis S, Bacon O, Chege W, Postle BS, Matheson T, Amico KR, Liegler T, Rawlings MK, Trainor N, Blue RW, Estrada Y, Coleman ME, Cardenas G, Feaster DJ, Grant R, Philip SS, Elion R, Buchbinder S, Kolber MA. 2016. Preexposure prophylaxis for HIV infection integrated with municipal- and community-based sexual health services. JAMA Intern Med 176:75–84. doi: 10.1001/jamainternmed.2015.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hosek SG, Rudy B, Landovitz R, Kapogiannis B, Siberry G, Rutledge B, Liu N, Brothers J, Mulligan K, Zimet G, Lally M, Mayer KH, Anderson P, Kiser J, Rooney JF, Wilson CM. 2017. An HIV preexposure prophylaxis demonstration project and safety study for young MSM. J Acquir Immune Defic Syndr 74:21–29. doi: 10.1097/QAI.0000000000001179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pruvost A, Negredo E, Theodoro F, Puig J, Levi M, Ayen R, Grassi J, Clotet B. 2009. Pilot pharmacokinetic study of human immunodeficiency virus-infected patients receiving tenofovir disoproxil fumarate (TDF): investigation of systemic and intracellular interactions between TDF and abacavir, lamivudine, or lopinavir-ritonavir. Antimicrob Agents Chemother 53:1937–1943. doi: 10.1128/AAC.01064-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moyle G, Boffito M, Fletcher C, Higgs C, Hay PE, Song IH, Lou Y, Yuen GJ, Min SS, Guerini EM. 2009. Steady-state pharmacokinetics of abacavir in plasma and intracellular carbovir triphosphate following administration of abacavir at 600 milligrams once daily and 300 milligrams twice daily in human immunodeficiency virus-infected subjects. Antimicrob Agents Chemother 53:1532–1538. doi: 10.1128/AAC.01000-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson PL, Kakuda TN, Kawle S, Fletcher CV. 2003. Antiviral dynamics and sex differences of zidovudine and lamivudine triphosphate concentrations in HIV-infected individuals. AIDS 17:2159–2168. doi: 10.1097/00002030-200310170-00003. [DOI] [PubMed] [Google Scholar]
- 25.Else LJ, Jackson A, Puls R, Hill A, Fahey P, Lin E, Amara A, Siccardi M, Watson V, Tjia J, Emery S, Khoo S, Back DJ, Boffito M. 2012. Pharmacokinetics of lamivudine and lamivudine-triphosphate after administration of 300 milligrams and 150 milligrams once daily to healthy volunteers: results of the ENCORE 2 study. Antimicrob Agents Chemother 56:1427–1433. doi: 10.1128/AAC.05599-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rower JE, Meditz A, Gardner EM, Lichtenstein K, Predhomme J, Bushman LR, Klein B, Zheng JH, Mawhinney S, Anderson PL. 2012. Effect of HIV-1 infection and sex on the cellular pharmacology of the antiretroviral drugs zidovudine and lamivudine. Antimicrob Agents Chemother 56:3011–3019. doi: 10.1128/AAC.06337-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aweeka FT, Rosenkranz SL, Segal Y, Coombs RW, Bardeguez A, Thevanayagam L, Lizak P, Aberg J, Watts DH. 2006. The impact of sex and contraceptive therapy on the plasma and intracellular pharmacokinetics of zidovudine. AIDS 20:1833–1841. doi: 10.1097/01.aids.0000244202.18629.36. [DOI] [PubMed] [Google Scholar]
- 28.Laizure SC, Herring V, Hu Z, Witbrodt K, Parker RB. 2013. The role of human carboxylesterases in drug metabolism: have we overlooked their importance? Pharmacotherapy 33:210–222. doi: 10.1002/phar.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tong L, Phan TK, Robinson KL, Babusis D, Strab R, Bhoopathy S, Hidalgo IJ, Rhodes GR, Ray AS. 2007. Effects of human immunodeficiency virus protease inhibitors on the intestinal absorption of tenofovir disoproxil fumarate in vitro. Antimicrob Agents Chemother 51:3498–3504. doi: 10.1128/AAC.00671-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lade JM, To EE, Hendrix CW, Bumpus NN. 2015. Discovery of genetic variants of the kinases that activate tenofovir in a compartment-specific manner. EBioMedicine 2:1145–1152. doi: 10.1016/j.ebiom.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hosek S, Landovitz R, Kapogiannis B, Siberry G, Rudy B, Rutledge B, Liu N, Mulligan K, Zimet G, Mayer K, Lally M, Brothers J, Bojan K, Anderson PL, Kiser J, Rooney J, Wilson C. 2017. 5 September 2017 Antiretroviral pre-exposure prophylaxis (PrEP) for adolescent men who have sex with men (MSM) ages 15-17 years in the United States. JAMA Pediatr doi: 10.1001/jamapediatrics.2017.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Molina JM, Capitant C, Spire B, Pialoux G, Cotte L, Charreau I, Tremblay C, Le Gall JM, Cua E, Pasquet A, Raffi F, Pintado C, Chidiac C, Chas J, Charbonneau P, Delaugerre C, Suzan-Monti M, Loze B, Fonsart J, Peytavin G, Cheret A, Timsit J, Girard G, Lorente N, Preau M, Rooney JF, Wainberg MA, Thompson D, Rozenbaum W, Dore V, Marchand L, Simon MC, Etien N, Aboulker JP, Meyer L, Delfraissy JF. 2015. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med 373:2237–2246. doi: 10.1056/NEJMoa1506273. [DOI] [PubMed] [Google Scholar]
- 33.Antoni G, Tremblay C, Charreau I, Cua E, Rojas D, Hall N, Chas J, Huleux T, Spire B, Capitant C, Cotte L, Meyer L, Molina JM. 2017. Is on-demand PrEP with TDF/FTC effective among MSM with infrequent sexual intercourse? Abstr 9th Int AIDS Conf HIV Sci. NATAP, New York, NY. [Google Scholar]
- 34.Knox DC, Anderson PL, Harrigan PR, Tan DH. 2017. Multidrug-resistant HIV-1 infection despite preexposure prophylaxis. N Engl J Med 376:501–502. doi: 10.1056/NEJMc1611639. [DOI] [PubMed] [Google Scholar]
- 35.Grossman H, Anderson PL, Grant R, Gandhi M, Mohri H, Markowitz M. 2016. Newly acquired HIV-1 infection with multi-drug resistant (MDR) HIV-1 in a patient on TDF/FTC-based PrEP, abstr 0A03.06LB, p 44. Abstr HR4P Meet 2016 Conference Solutions, Portland, OR. [Google Scholar]
- 36.Hoornenborg E, De Bree G. 2017. Acute infection with a wild-type HIV-1 virus in PrEP user with high TDF levels, abstr 953. Abstr Conf Retrovir Opportun Infect. CROI Foundation, San Francisco, CA. [Google Scholar]
- 37.Patterson KB, Prince HA, Kraft E, Jenkins AJ, Shaheen NJ, Rooney JF, Cohen MS, Kashuba AD. 2011. Penetration of tenofovir and emtricitabine in mucosal tissues: implications for prevention of HIV-1 transmission. Sci Transl Med 3:112re114. doi: 10.1126/scitranslmed.3003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cottrell ML, Yang KH, Prince HM, Sykes C, White N, Malone S, Dellon ES, Madanick RD, Shaheen NJ, Hudgens MG, Wulff J, Patterson KB, Nelson JA, Kashuba AD. 2016. A translational pharmacology approach to predicting outcomes of preexposure prophylaxis against HIV in men and women using tenofovir disoproxil fumarate with or without emtricitabine. J Infect Dis 214:55–64. doi: 10.1093/infdis/jiw077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corneli AL, Deese J, Wang M, Taylor D, Ahmed K, Agot K, Lombaard J, Manongi R, Kapiga S, Kashuba A, Van Damme L. 2014. FEM-PrEP: adherence patterns and factors associated with adherence to a daily oral study product for pre-exposure prophylaxis. J Acquir Immune Defic Syndr 66:324–331. doi: 10.1097/QAI.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adams JL, Sykes C, Menezes P, Prince HM, Patterson KB, Fransen K, Crucitti T, De Baetselier I, Van Damme L, Kashuba AD. 2013. Tenofovir diphosphate and emtricitabine triphosphate concentrations in blood cells compared with isolated peripheral blood mononuclear cells: a new measure of antiretroviral adherence? J Acquir Immune Defic Syndr 62:260–266. doi: 10.1097/QAI.0b013e3182794723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng JH, Rower C, McAllister K, Castillo-Mancilla J, Klein B, Meditz A, Guida LA, Kiser JJ, Bushman LR, Anderson PL. 2016. Application of an intracellular assay for determination of tenofovir-diphosphate and emtricitabine-triphosphate from erythrocytes using dried blood spots. J Pharm Biomed Anal 122:16–20. doi: 10.1016/j.jpba.2016.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sethuraman VS, Leonov S, Squassante L, Mitchell TR, Hale MD. 2007. Sample size calculation for the Power model for dose proportionality studies. Pharm Stat 6:35–41. doi: 10.1002/pst.241. [DOI] [PubMed] [Google Scholar]
- 43.Lu Y, Goti V, Chaturvedula A, Haberer JE, Fossler MJ, Sale ME, Bangsberg D, Baeten JM, Celum CL, Hendrix CW. 2016. Population pharmacokinetics of tenofovir in HIV-1-uninfected members of serodiscordant couples and effect of dose reporting methods. Antimicrob Agents Chemother 60:5379–5386. doi: 10.1128/AAC.00559-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gervasoni C, Meraviglia P, Landonio S, Baldelli S, Fucile S, Castagnoli L, Clementi E, Riva A, Galli M, Rizzardini G, Cattaneo D. 2013. Low body weight in females is a risk factor for increased tenofovir exposure and drug-related adverse events. PLoS One 8:e80242. doi: 10.1371/journal.pone.0080242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gilead Sciences. 2017. Truvada (tenofovir disoproxil fumarate and emtricitabine). Gilead Sciences, Foster City, CA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.