ABSTRACT
Carbapenem-resistant Enterobacteriaceae (CRE) are major health care-associated pathogens and responsible for hospital outbreaks worldwide. To prevent a further increase in CRE infections and to improve infection prevention strategies, it is important to summarize the current knowledge about CRE infection prevention in hospital settings. This systematic review aimed to identify risk factors for CRE acquisition among hospitalized patients. In addition, we summarized the environmental sources/reservoirs and the most successful infection prevention strategies related to CRE. A total of 3,983 potentially relevant articles were identified and screened. Finally, we included 162 studies in the systematic review, of which 69 studies regarding risk factors for CRE acquisition were included in the random-effects meta-analysis studies. The meta-analyses regarding risk factors for CRE acquisition showed that the use of medical devices generated the highest pooled estimate (odds ratio [OR] = 5.09; 95% confidence interval [CI] = 3.38 to 7.67), followed by carbapenem use (OR = 4.71; 95% CI = 3.54 to 6.26). To control hospital outbreaks, bundled interventions, including the use of barrier/contact precautions for patients colonized or infected with CRE, are needed. In addition, it is necessary to optimize the therapeutic approach, which is an important message to infectious disease specialists, who need to be actively involved in a timely manner in the treatment of patients with known CRE infections or suspected carriers of CRE.
KEYWORDS: Enterobacteriaceae, resistance, carbapenem, risk factors, systematic review, meta-analysis
INTRODUCTION
Over the last 2 decades, a global dissemination of carbapenem-resistant Enterobacteriaceae (CRE) has been observed (1, 2). Currently, CRE are responsible for hospital outbreaks worldwide. Infections with these resistant bacteria are associated with high rates of morbidity and mortality, especially in patients with serious underlying disorders or patients admitted to the intensive care unit (ICU) (3).
Carbapenem resistance in Enterobacteriaceae is mainly mediated by the horizontal transfer of genes encoding carbapenem-hydrolyzing carbapenemase enzymes, although porin mutations or the overexpression of efflux pumps can also lead to carbapenem resistance, especially in combination with the hyperproduction of β-lactamase enzymes (4, 5). The production of carbapenemase enzymes is plasmid mediated and can be found in multiple different species of Enterobacteriaceae, such as Klebsiella pneumoniae and Escherichia coli (1, 5–7). These conjugative plasmids often carry additional genes conferring resistance to other antibiotics, such as fluoroquinolones and aminoglycosides, limiting the treatment options even more (3, 8).
To prevent a further increase in CRE infections in patients by improving infection prevention strategies, it is important to summarize the current knowledge about CRE in hospital settings. This systematic review and meta-analyses aimed to evaluate the clinical epidemiology of CRE by answering the following questions. First, what are the risk factors associated with CRE acquisition among hospitalized patients? Second, which environmental sources/reservoirs were identified in CRE outbreaks? Third, what were the essential components of effective infection control in preventing or ending hospital outbreaks?
RESULTS
During our literature search we identified 3,983 potentially relevant articles (Fig. 1). All titles and abstracts of the retrieved articles were screened against our inclusion and exclusion criteria, resulting in the exclusion of 3,720 publications. The remaining 263 articles underwent a second screening based on the full text. Seven full-text articles were received by e-mail after we contacted the corresponding authors. Finally, 162 articles were included in the systematic review (Fig. 1). For these studies, the data were extracted and the corresponding author was contacted with a request to check our completed data extraction form. Finally, the corresponding authors of 100 out of 162 articles (61.7%) responded to our request and provided feedback and additional information if necessary.
FIG 1.
Flow diagram of study selection for the systematic review of studies on carbapenem-resistant Enterobacteriaceae.
All included studies were published between 2005 and 2017. Two articles were written in Spanish, one article was written in Chinese, one article was written in Greek, and one article was written in Slovak. All other articles were written in English (n = 157, 96.9%). Most studies were conducted in Europe (n = 62; 38.3%), mainly in Greece (n = 14) and Italy (n = 11). A total of 52 studies (32.1%) were conducted in Asia, mainly in Israel (n = 18) and China (n = 16). The remaining 48 studies were conducted in North America (n = 31), South America (n = 12), Australia (n = 3), and Africa (n = 2). Thirty-seven (22.8%) out of the 162 studies used a study design involving only the ICU. The majority of studies focused on a single species of the Enterobacteriaceae family: a Klebsiella sp. (n = 103; 63.6%), an Enterobacter sp. (n = 5), E. coli (n = 4), Citrobacter freundii (n = 3), and Providencia stuartii (n = 2). The remaining 45 studies (27.8%) involved multiple Enterobacteriaceae species.
Carbapenemase production was described by 124 studies (76.5%), and these mainly involved KPC (n = 91), NDM (n = 24), and OXA (n = 22) carbapenemases. Nine studies (5.6%) mentioned the production of β-lactamase enzymes in combination with porin mutations. In addition, one study detected only porin mutations and two studies detected only β-lactamase production in their carbapenem-resistant Enterobacteriaceae isolates. Thirty-two studies (19.8%) did not mention or investigate the carbapenem resistance mechanism involved.
Factors associated with CRE acquisition.
We identified 74 studies describing factors associated with CRE acquisition with a statistically significant odds ratio (OR) or hazard ratio (HR) obtained from a multivariable analysis. All reported protective factors for CRE acquisition are summarized in Table 1. All reported risk factors were divided into two groups: factors related to antibiotic exposure and other. In addition, five studies reported risk factors associated with mortality among CRE carriers, including nine risk factors and four protective factors (9–13). The highest odds ratio was reported for the risk factor ICU stay (OR = 11.10, 95% confidence interval [CI] = 1.85 to 66.95) (12).
TABLE 1.
Summary of studies reporting protective factors for acquisition of CRE, based on multivariable analysisa
| Authors, yr (reference) | Country | Risk factor | Risk estimate |
P value | Qualityb | |
|---|---|---|---|---|---|---|
| OR | 95% CI | |||||
| Akgul et al., 2016 (31) | Turkey | Nonuse of glycopeptide | 0.143 | 0.031–0.674 | <0.05 | 14 |
| Akgul et al., 2016 (31) | Turkey | Nonuse of steroids | 0.244 | 0.072–0.822 | <0.05 | 14 |
| Akgul et al., 2016 (31) | Turkey | Absence of tracheostomy | 0.06 | 0.006–0.614 | <0.05 | 14 |
| Garbati et al., 2016 (32) | Saudi Arabia | Not being in the ICU | 0.027 | 0.001–0.496 | 0.015 | 18 |
| Gasink et al., 2009 (33) | USA | Blood isolate (compared to an isolate from other body sites) | 0.33 | 0.12–0.86 | 0.02 | 17 |
| Giuffrè et al., 2013 (34) | Italy | Administration of ampicillin-sulbactam plus gentamicin | 0.20 | 0.03–0.97 | 0.004 | 16 |
| Kwak et al., 2005 (19) | South Korea | Use of a fluoroquinolonec | 0.26 | 0.07–0.97 | 0.045 | 18 |
| Madueño et al., 2017 (35) | Spain | Corticosteroid use | 0.33 | 0.15–0.74 | 0.007 | 16 |
| Madueño et al., 2017 (35) | Spain | Antibiotic use | 0.20 | 0.65–0.62 | 0.01 | 16 |
| Mittal et al., 2016 (20) | India | Use of aminoglycosides | 0.257 | 0.068–0.975 | 0.046 | 13 |
| Mittal et al., 2016 (20) | India | Use of a ventilatorc | 0.291 | 0.097–0.871 | 0.027 | 13 |
| Schwartz-Neiderman et al., 2016 (21) | Israel | Use of cephalosporinsc | 0.2 | 0.1–0.6 | 0.005 | 18 |
| Torres-Gonzalez et al., 2015 (22) | Mexico | Admission to the ICUc | 0.42 | 0.20–0.88 | <0.05 | 19 |
Abbreviations: CRE, carbapenem-resistant Enterobacteriaceae; OR, odds radio; CI, confidence interval; ICU, intensive care unit.
According to the STROBE quality assessment scale (30).
Risk factor included in a random-effects meta-analysis study.
Risk factors related to antibiotic exposure.
All factors related to antibiotic exposure were further divided into nine smaller categories (Table 2). Carbapenem exposure (n = 26) and cephalosporin exposure (n = 15) were the most frequently mentioned risk factors associated with CRE acquisition.
TABLE 2.
Antibiotic exposure as a risk factor for acquisition of CRE, based on multivariable analysisc
| Associated risk factor | Frequency | RE | RE range | No. of cases (range) | Study reference(s) |
|---|---|---|---|---|---|
| Carbapenem use | 25 | OR | 1.83–29.17 | 9–100 | 12, 13, 16, 19, 22, 32, 36–48,d 49–53 |
| Carbapenem use | 1 | HR | 2.68 | 19 | 22 |
| Cephalosporin use | 15 | OR | 2.24–49.56 | 15–100 | 11, 12, 13, 19, 33, 38, 46, 48,d 52, 54–57, 58 |
| Quinolone use | 9 | OR | 1.18–28.9 | 18–88 | 33, 36, 43, 52, 59–63 |
| Antibiotic exposure (in general)a,b | 9 | OR | 1.66–13.37 | 26–464 | 41, 43, 61, 64–69 |
| Other β-lactam use | 9 | OR | 1.08–11.71 | 34–464 | 49, 52, 57, 60, 65, 70–73 |
| Othera | 7 | OR | 1.02–33 | 25–103 | 44, 47, 52, 58, 72, 74, 75 |
| Glycopeptide use | 5 | OR | 2.94–43.84 | 20–203 | 11, 16, 47, 73, 76 |
| No. of antibiotics administereda,b | 3 | OR | 1.6–12.60 | 59–164 | 39, 50, 77 |
| Duration of exposurea,b | 3 | OR | 1.04–9.8 | 25–104 | 74, 78, 79 |
This category was not included in a random-effects meta-analysis.
Exposure to any antibiotic.
Abbreviations: CRE, carbapenem-resistant Enterobacteriaceae; RE, risk estimate; OR, odds ratio; HR, hazard ratio.
This risk factor was identified two times in the study of Orsi et al. (48).
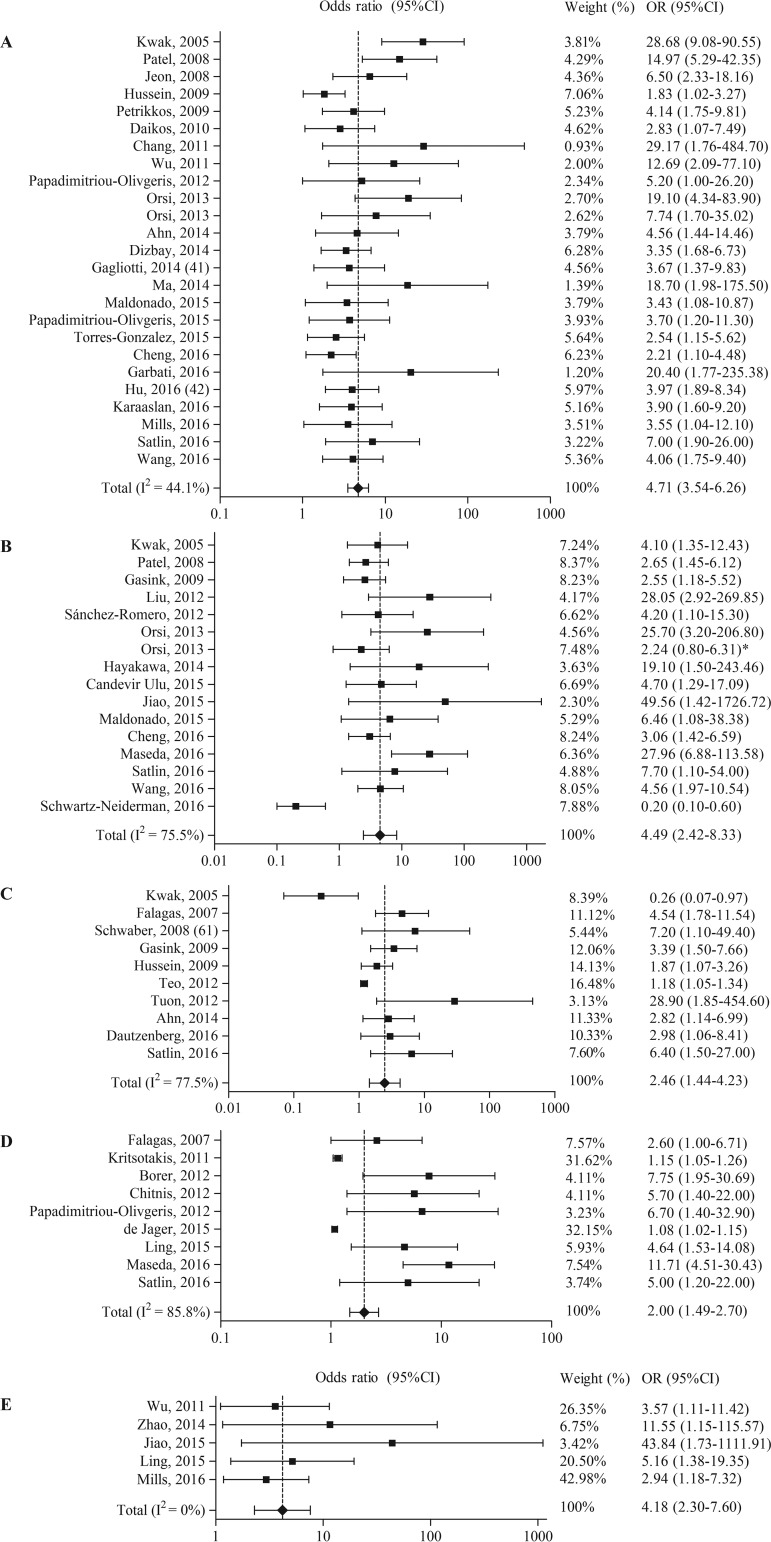
For five out of the nine categories, a random-effects meta-analysis was performed (Table 3 and Fig. 2). For the risk factor carbapenem exposure, one study was excluded because it reported a hazard ratio instead of an odds ratio. The five meta-analyses included 43 studies reporting 63 risk factors (OR > 1) and 2 protective factors (OR < 1). Carbapenem use (OR = 4.71, 95% CI = 3.54 to 6.26) and cephalosporin use (OR = 4.49, 95% CI = 2.42 to 8.33) generated the highest pooled ORs. Both publication bias indicators showed a significant result for the risk factors carbapenem use, cephalosporin use, and glycopeptide use.
TABLE 3.
Random-effects meta-analyses of antibiotic exposure and other risk factors and/or protective factors for acquisition of CREa
| Associated risk factor | No. of times identified | Pooled OR (95% CI) |
P value for risk of publication bias by use of the indicator of: |
|
|---|---|---|---|---|
| Egger et al. (28) | Begg and Mazumdar (29) | |||
| Antibiotic exposure | ||||
| Carbapenem use | 25 | 4.71 (3.54–6.26) | <0.05 | <0.05 |
| Cephalosporin use | 16 | 4.49 (2.42–8.33) | <0.05 | <0.05 |
| Quinolone use | 10 | 2.46 (1.44–4.23) | <0.05 | 0.29 |
| Other β-lactam use | 9 | 2.00 (1.49–2.70) | <0.05 | 0.26 |
| Glycopeptide use | 5 | 4.18 (2.30–7.60) | <0.05 | <0.05 |
| Other risk factors | ||||
| Underlying disease or condition | 31 | 2.54 (2.08–3.09) | <0.05 | 0.12 |
| Invasive procedures | 20 | 4.67 (3.59–6.07) | <0.05 | <0.05 |
| Medical devices | 17 | 5.09 (3.38–7.67) | <0.05 | <0.05 |
| ICU admission | 15 | 4.62 (2.46–8.69) | <0.05 | <0.05 |
| Patient demographic characteristics | 13 | 1.08 (1.03–1.14) | <0.05 | <0.05 |
| Exposure to hospital care | 12 | 1.05 (1.02–1.08) | <0.05 | <0.05 |
| Mechanical ventilation | 11 | 1.96 (1.42–2.69) | <0.05 | <0.05 |
| CRE exposure | 5 | 4.10 (1.46–11.52) | <0.05 | 0.23 |
Abbreviations: CRE, carbapenem-resistant Enterobacteriaceae; OR, odds radio; CI, confidence interval; ICU, intensive care unit.
FIG 2.
Forest plots of random-effects meta-analyses of antibiotic exposure as a risk factor and/or protective factor for the acquisition of carbapenem-resistant Enterobacteriaceae. (A) Carbapenem use; (B) cephalosporin use; (C) quinolone use; (D) β-lactam use; (E) glycopeptide use. *, nonsignificant confidence interval (Orsi et al. were contacted multiple times to receive the correct numbers; unfortunately, the authors did not respond).
In total, 26 additional meta-analyses were performed to assess the effect of the Enterobacteriaceae species studied, ICU study setting, the carbapenem resistance mechanism involved, and the study quality on the overall risk estimates (see File S4 in the supplemental material). In the additional meta-analyses, all risk factors remained significantly associated with CRE acquisition (pooled OR > 1).
Other risk factors for CRE acquisition.
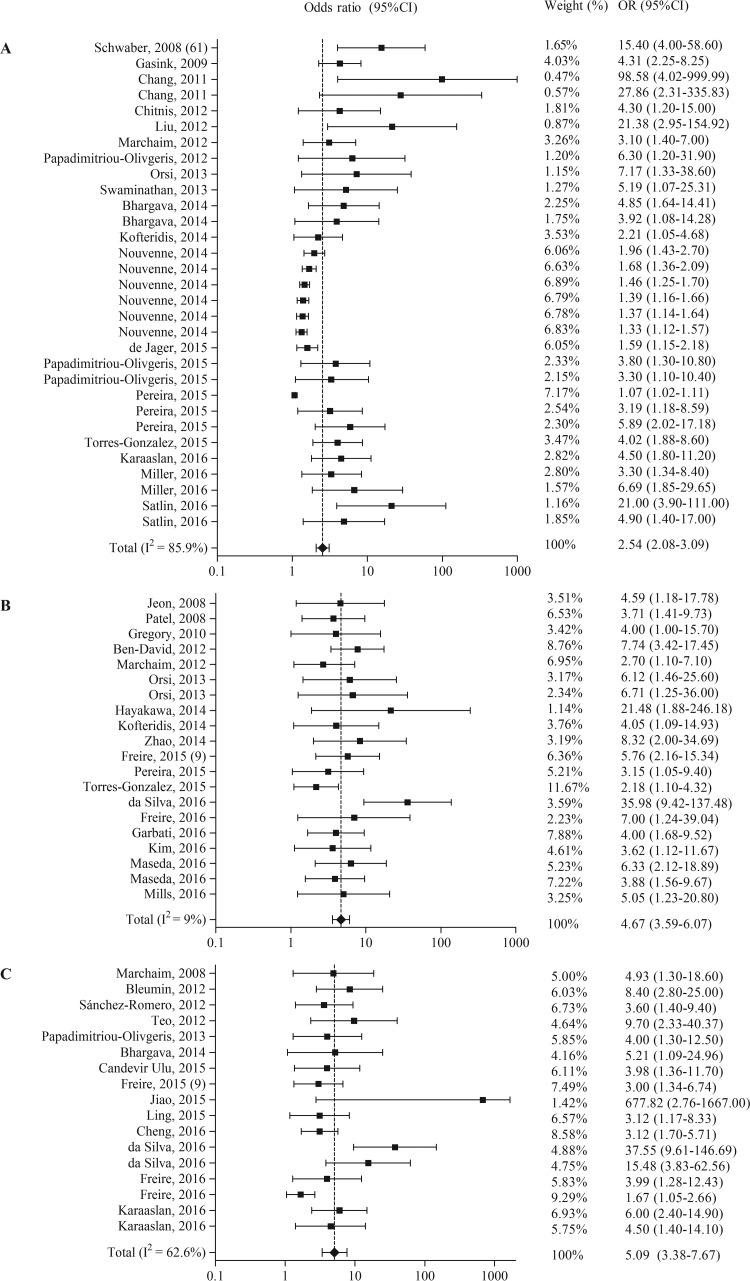
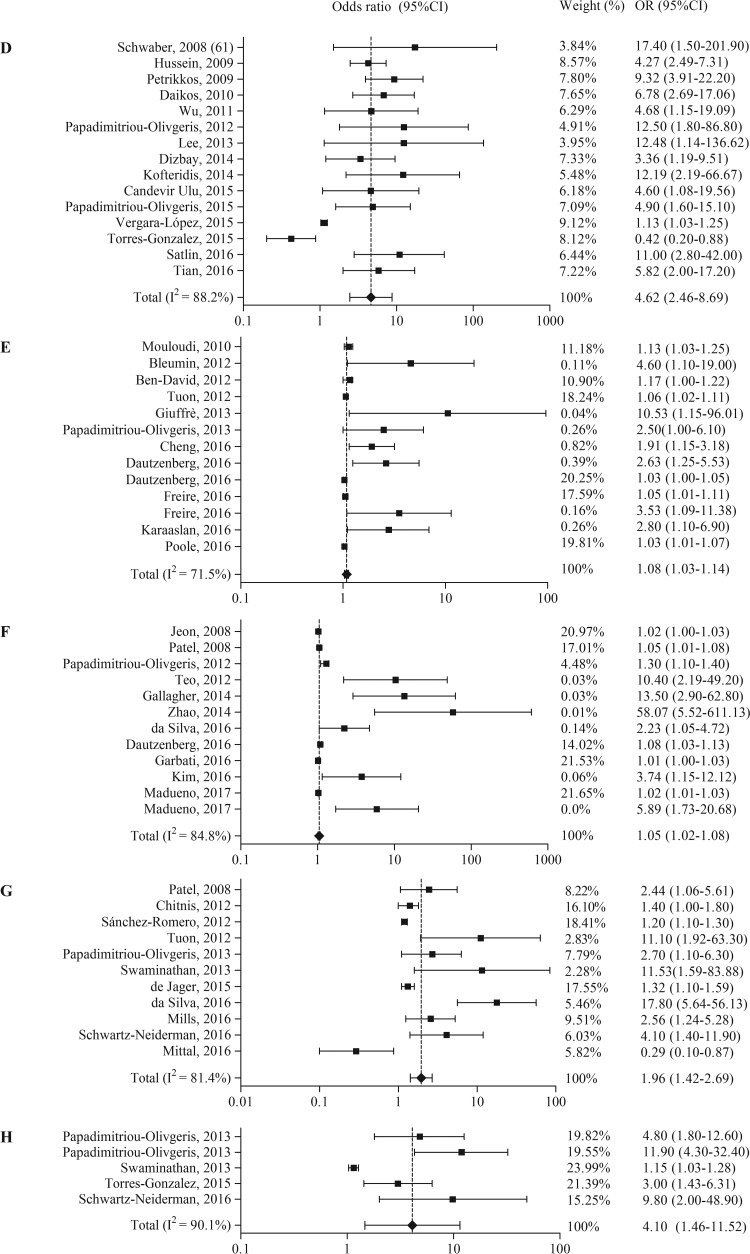
Other risk factors associated with the acquisition of carbapenem-resistant Enterobacteriaceae were divided into nine categories and are summarized in Table 4. The risk factor underlying disease or condition (n = 32 times identified) was the most frequently found. For eight out of nine categories, a meta-analysis including 59 studies was performed (Table 3 and Fig. 3). In the categories underlying disease or condition and CRE exposure, one study was excluded because it reported a hazard ratio instead of an odds ratio. In the categories exposure to hospital care and mechanical ventilation, one study was excluded because it reported relative risk instead of an odds ratio.
TABLE 4.
Other risk factors associated with acquisition of CRE, based on multivariable analysisc
| Associated risk factor | Frequency | RE type | RE range | No. of cases (range) | Study reference(s) (no. of different risk factors per reference) |
|---|---|---|---|---|---|
| Underlying disease or condition | 31 | OR | 1.07–98.58 | 17–133 | 22, 33, 37 (2), 45, 48, 49, 50 (2), 52 (2), 56, 61 67, 68 (2), 70, 71, 79, 80 (2), 81, 82 (6), 83 (3) |
| 1 | HR | 5.74 | 19 | 22 | |
| Othera | 19 | OR | 1.35–45.904 | 20–464 | 22 (2), 38, 40, 47, 50, 56, 64, 65 (2), 67 (2), 76, 82, 84, 85 (2), 86, 87 |
| 1 | RR | 5.94 | 149 | 88 | |
| 1 | HR | 19.0 | 26 | 85 | |
| Invasive procedures | 20 | OR | 2.18–35.98 | 15–99 | 9, 12, 22, 32, 44, 47, 48 (2), 55, 57 (2), 67, 76, 81, 83, 85, 89–92 |
| Medical devicesb | 17 | OR | 1.67–677.82 | 15–203 | 9, 11, 38, 45 (2), 54, 58, 62, 73, 77, 80, 84, 89 (2), 91, 93, 94 |
| ICU admission | 14 | OR | 1.13–17.4 | 25–88 | 16, 39, 40, 43, 49–52, 54, 61, 78, 81, 87, 95 |
| Patient demographic characteristics | 13 | OR | 1.03–10.53 | 10–164 | 34, 38, 45, 59 (2), 63, 69, 77, 84, 90, 91 (2), 96 |
| Exposure to hospital care | 12 | OR | 1.014–58.067 | 15–99 | 12, 32, 35 (2), 44, 49, 59, 62, 66, 76, 89, 92 |
| 1 | RR | 1.36 | 149 | 88 | |
| Mechanical ventilation | 10 | OR | 1.2–17.80 | 18–164 | 12, 21, 47, 58, 63, 70, 71, 77, 79, 89 |
| 1 | RR | 1.99 | 149 | 88 | |
| CRE exposure | 5 | OR | 1.15–11.9 | 53–165 | 21, 22, 77 (2), 79 |
| 1 | HR | 5.03 | 19 | 22 |
This category was not included in a random-effects meta-analysis.
Mechanical ventilation is excluded from this category.
Abbreviations: CRE, carbapenem-resistant Enterobacteriaceae; RE, risk estimate; OR, odds ratio; HR, hazard ratio; RR, relative risk; ICU, intensive care unit.
FIG 3.
Forest plots of random-effects meta-analyses of other risk factors and/or protective factors for the acquisition of carbapenem-resistant Enterobacteriaceae. (A) Underlying disease or condition; (B) invasive procedures; (C) medical devices; (D) ICU admission; (E) demographic patient characteristics; (F) exposure to hospital care; (G) mechanical ventilation; (H) CRE exposure.
From the eight different random-effects meta-analyses, the highest pooled OR was found for medical devices (OR = 5.09, 95% CI = 3.38 to 7.67), followed by invasive procedures (OR = 4.67, 95% CI = 3.59 to 6.07) and ICU admission (OR = 4.62, 95% CI = 2.46 to 8.69). Both publication bias indicators showed a significant result for all risk factors, except underlying disease or condition and CRE exposure.
The effects of the different variables (e.g., the CRE species studied, ICU study setting, and the mechanisms of carbapenem resistance) were reviewed by performing 47 additional meta-analyses. Surprisingly, all risk factors showed a decreased (or equal) pooled OR when only studies in which carbapenemase production was shown were included, with the OR difference ranging from 0 to −1.29 (File S5, Fig. SC). The meta-analyses of the remaining studies that described another resistance mechanism (e.g., porin mutations) or that did not investigate the resistance mechanism involved showed a large increase in the reported pooled ORs for all tested risk factors, with the mean change being +2.89.
Effective infection prevention strategies.
We identified 95 studies describing effective infection prevention strategies used to control the spread of carbapenem-resistant Enterobacteriaceae in a hospital setting. These were converted to the top 10 most successful intervention strategies (Table 5). The use of barrier and/or contact precautions was found to be the most successful intervention strategy (n = 71), followed by patient cohorting (n = 68) and active surveillance (n = 56). Control of antibiotic use was mentioned in only 17 studies and could be found in ninth place. Besides these 10 strategies, some other interventions were described in the literature, such as restricted/no admission to the affected wards (n = 9) and the use of chlorhexidine for patient disinfection (n = 9).
TABLE 5.
Top 10 strategies to control hospital outbreaks with CREa
| Intervention | No. of studies | Study references |
|---|---|---|
| 1. Barrier/contact precautions | 71 | 22, 34, 38, 53, 55, 70, 79, 82, 85, 90, 91, 94, 97–155 |
| 2. Patient transfer to single room or cohorting | 68 | 22, 34, 38, 53, 70, 82, 85, 90, 91, 94, 97, 98, 102, 103, 105–108, 110–115, 117–122, 124, 126–128, 130–132, 134, 136–141, 143, 146, 148–154, 156–170 |
| 3. Active surveillance/screening for CRE | 56 | 53, 70, 75, 79, 82, 97, 100–105, 107, 110–115, 117, 119, 120, 123–126, 128–131, 133, 135–137, 139, 140, 142, 144–146, 148–150, 154, 156–162, 165, 167–169, 171 |
| 4. Enhanced hand hygiene | 52 | 22, 34, 38, 53, 70, 85, 91, 94, 97–100, 102, 103, 105, 109, 110, 112, 113, 115–120, 124–129, 131–133, 135, 140, 142, 143, 146, 147, 150, 152, 154, 155, 157, 163, 165, 166, 168, 170, 172, 173 |
| 5. Enhanced environmental cleaning | 51 | 22, 34, 38, 70, 71, 97–100, 103, 106, 108, 109, 112, 113, 118–120, 124–126, 129–131, 133–135, 137, 142–147, 149–151, 153, 156, 157, 161, 164–170, 172–174 |
| 6. Staff educational programs | 34 | 34, 53, 70, 71, 91, 100, 106, 107, 109–111, 116, 117, 119, 121, 123, 126, 130, 131, 133, 134, 143–146, 148, 151, 157, 161, 164, 167–169, 171 |
| 7. Staff cohorting | 32 | 70, 71, 82, 85, 91, 94, 97, 98, 105, 107, 108, 110, 111, 113, 114, 120, 128, 133, 138, 140–143, 145, 149, 155, 157, 159, 162, 165, 167, 168 |
| 8. Equipment cohorting/single-use equipment | 21 | 34, 70, 71, 94, 97–99, 108, 111, 112, 121, 128, 130, 131, 135, 138, 142, 153, 156, 157, 168 |
| 9. Control of antibiotic use | 17 | 53, 85, 91, 103, 106, 110, 113, 116, 120, 121, 128, 129, 144–146, 172, 175 |
| 10. Flagging of CRE patients | 14 | 99, 103, 105, 107, 111, 114, 119, 125, 131, 132, 144, 147, 149, 160 |
CRE, carbapenem-resistant Enterobacteriaceae.
Environmental sources and reservoirs.
Twenty-seven studies provided information about the environmental sources and reservoirs identified within their hospitals. All hospital locations in which carbapenem-resistant Enterobacteriaceae were identified are summarized in Table 6. Contaminated sinks were the most frequently described (n = 10 studies), followed by patient beds (n = 6 studies) and mechanical ventilation equipment (n = 5 studies).
TABLE 6.
Identified environmental sources and reservoirs for CREb
| Environmental source or reservoir | Study reference(s) |
|---|---|
| Sinks | 125,a 137, 140, 142,a 157,a 166,a 174,a 176, 177,a 178 |
| Patient bed (e.g., bedrail, mattress) | 103, 133,a 157,a 167,a 168,a 178 |
| Mechanical ventilation equipment | 142,a 167a 168,a 172,a 179a |
| Positive cultures from nurses (hands) | 103, 151,a 152,a 157a |
| Endoscope | 92, 122, 180a |
| Duodenoscope | 105,a 181a |
| Urinary catheter | 145,a 173 |
| Monitor (e.g., vital signs, television) | 103, 167a |
| Shower/shower equipment | 137, 178 |
| Table | 157,a 172a |
| Ureteroscope | 182a |
| Razor | 108a |
| Incubator | 151a |
| Radiant warmer | 152a |
| Suction equipment | 178 |
| Wastewater drainage system | 145a |
| Stethoscope | 145a |
| Intravenous pole | 167a |
| Infusion pump | 157a |
| Janet syringe | 103 |
| Cabinet | 103 |
| Intravenous infusion counter apparatus | 103 |
| Enteral feeding formula | 103 |
The study proved the source or reservoir by molecular typing of carbapenem-resistant Enterobacteriaceae isolates.
CRE, carbapenem-resistant Enterobacteriaceae.
DISCUSSION
Summary of evidence.
In this systematic review, we identified 13 risk factors associated with the presence of carbapenem-resistant Enterobacteriaceae. These risk factors were, in order of those with the highest to those with the lowest pooled OR, (i) medical devices, (ii) carbapenem use, (iii) invasive procedures, (iv) ICU admission, (v) cephalosporin use, (vi) glycopeptide use, (vii) CRE exposure, (viii) underlying disease or condition, (ix) quinolone use, (x) β-lactam use, (xi) mechanical ventilation, (xii) demographic patient characteristics, and (xiii) exposure to hospital care (Table 3). Medical devices, antibiotic use, ICU admission, exposure to hospital care, and underlying diseases were also identified to be risk factors in systematic reviews regarding the acquisition of extended-spectrum β-lactamase (ESBL)-producing Klebsiella spp. (14) and carbapenem-resistant Pseudomonas aeruginosa (15).
Plasmids responsible for carbapenem resistance often carry additional genes conferring resistance to other antibiotics, such as fluoroquinolones and aminoglycosides. This can explain why the use of these antibiotic classes is found to be a risk factor for CRE acquisition. However, this explanation cannot be used for glycopeptide antibiotics. Wu et al. (16) and Jiao et al. (11) supposed that vancomycin treatment disrupts the intestinal microflora, promoting the colonization of Enterobacteriaceae. Glycopeptide use was also identified to be a risk factor for carbapenem-resistant P. aeruginosa (15, 17) and ESBL-producing bacteria (18) acquisition.
On the contrary, 4 out of 13 significant risk factors were also described to be protective against CRE acquisition by other authors: quinolone use (19), mechanical ventilation (20), cephalosporin use (21), and ICU admission (22). Kwak et al. speculated that fluoroquinolone use was found to be a protective factor because this antibiotic was often given as a substitute for carbapenem or cephalosporin antibiotics (19). Torres-Gonzalez et al. reported that ICU admission was protective against CRE acquisition. This observation could be explained by the fact that their CRE outbreak was initially detected in the ICU and a successful bundle of infection prevention measures was initiated in that area (22).
We also performed additional meta-analyses to estimate the influence of the following variables on the overall risk estimate: the Enterobacteriaceae species studied, the ICU study setting, the carbapenem resistance mechanism involved, and the study quality. The carbapenem resistance mechanism was found to have the highest influence on the risk estimates, especially in the meta-analyses of non-antibiotic-related risk factors for CRE acquisition (see File S4, Fig. SC, in the supplemental material). We observed that our risk factors showed a lower risk estimate only when studies in which carbapenemase-producing Enterobacteriaceae were described were included.
The most successful interventions to stop the spread of CRE were barrier/contact precautions, patient cohorting, and active surveillance. Our findings correspond to the guidelines presented by the Centers for Disease Control and Prevention (CDC), which mainly highlight active surveillance and contact precautions (23, 24). Surprisingly, antimicrobial stewardship was mentioned in only 17 out of 95 studies, although multiple antimicrobial classes were identified to be risk factors for CRE acquisition.
Only 27 out of 95 studies reported environmental sources or reservoirs for CRE within their hospitals (Table 6). This indicates that for many outbreaks the source or reservoir was not determined. Contaminated sinks were the most frequently described and correspond to the reservoirs identified for other nosocomial pathogens, such as carbapenem-resistant P. aeruginosa (15) and ESBL-producing Klebsiella spp. (14).
Strengths and limitations.
A strength of our study was the inclusion of both Enterobacteriaceae that showed in vitro resistance to any carbapenem antibiotic and Enterobacteriaceae that were found to produce carbapenemase enzymes. This is important because carbapenemase production does not always confer high-level carbapenem resistance and therefore leads to false-negative results when only phenotypic tests are used to identify carbapenem-resistant Enterobacteriaceae (25). This review included only two studies in which carbapenemase-producing but carbapenem-sensitive and -resistant isolates were studied. However, the mechanism of resistance does influence transmission and, thus, epidemiology, as we showed different risk estimates, especially for the non-antibiotic-related risk factors, when each mechanism was analyzed. With the knowledge that we have up to now, we cannot explain this difference. As we included all kinds of mechanisms, this can also be seen as a limitation of the study.
The study also has some limitations; the first is the large heterogeneity of all studies included. Studies with different target populations, for example, neonates, adults, or transplant patients, were selected. In addition, different microbiological methods were used to identify the CRE isolates, different Enterobacteriaceae species were included, and different prevention strategies were installed. To limit the influence of the study heterogeneity, the random-effects model of DerSimonian and Laird (26) was implemented in the meta-analyses, and different subgroup analyses were performed.
Second, we included both studies reporting CRE colonization and studies reporting CRE infections in hospitalized patients. However, not all studies describing risk factors for CRE infection checked whether the patients were previously colonized with CRE or not. Likewise, they did not check whether patients from the control group were colonized with CRE before or during the infection. For these studies, we cannot rule out the possibility that their reported risk factors are not specific for CRE acquisition but are specific for progression to infection after CRE colonization.
Third, both publication bias indicators showed a significant result for several risk factors (9/13), indicating that publication bias was present. To limit publication bias, the studies that we included were not limited by language, date of publication, country of publication, carbapenem resistance mechanism, study design, or patient characteristics.
Conclusions and implications.
This systematic review shows that not only antibiotic use but also many other risk factors are associated with CRE acquisition. The most significant risk estimate found in our meta-analyses was found for the risk factor medical devices, followed by carbapenem use. We identified risk factors related to the emergence/selection of CRE, but also risk factors related to the transmission of the CRE isolates. To prevent or to control hospital outbreaks, bundled interventions are needed. These interventions need to focus on both antibiotic stewardship and reduction of the use of indwelling devices to reduce the spread of CRE within the hospital. Indwelling medical devices do present a very high risk for the acquisition of CRE but are also a risk for the acquisition of infections in general. Therefore, a very useful prevention measure is the active decrease in the rate of use (deimplementation) of medical devices.
MATERIALS AND METHODS
This systematic review and meta-analyses followed the guidelines presented in the PRISMA statement (see File S1 in the supplemental material) (27). Protocol details were submitted to the PROSPERO International Prospective Register of Systematic Reviews (registration number CRD42017055455).
Study selection.
Articles related to our research questions were identified through a search of the literature in multiple databases (until 11 January 2017): Embase, Medline Ovid, Cochrane, Web of Science, and Google Scholar (File S2). The search was not limited by language, date of publication, country of publication, carbapenem resistance mechanism, study design, or patient characteristics.
We used the following inclusion criteria during the study selection: (i) studies reporting risk factors for the acquisition of CRE, (ii) studies mentioning environmental sources/reservoirs for CRE, and (iii) studies describing effective infection prevention strategies to halt nosocomial outbreaks. Risk factors for acquisition could include risk factors for infection as well as risk factors for colonization with CRE. Enterobacteriaceae were considered resistant to carbapenem antibiotics when this was shown using phenotypic tests and/or when carbapenemase genes could be identified.
We excluded studies related to nonhuman infections, nonhospital studies, conference abstracts, letters to the editor, commentaries, weekly reports, and editorials. Studies were also excluded if patients with CRE infections were compared to patients who were colonized with CRE. First, the titles and abstracts of all retrieved citations were screened independently by K.V.L. and A.F.V. After this screening, K.V.L. and A.F.V. performed a second screening based on the full text.
Data extraction.
We designed a data abstraction form, pilot tested it on three randomly selected articles, and redefined it according to the outcomes. The following data were extracted: first author, journal, year published, country, study design, study setting, patient characteristics, the carbapenem-resistant microorganism(s) studied, risk factors for acquisition/mortality, site of colonization/infection, protective factors for acquisition/mortality, potential reservoirs for CRE, and effective infection prevention strategies for CRE. The extracted data were sent to the corresponding author of the original article to verify the extracted data and to gain additional information if relevant. When we did not receive any response after the given deadline (i.e., 2 weeks), a reminder was sent. If no response was received and crucial information was missing, the study was excluded.
Data analysis. (i) Risk factors for CRE acquisition.
All risk factors associated with the acquisition of CRE for which an odds ratio (OR) with the 95% confidence interval (CI) was reported were divided into two groups: those related to antibiotic exposure and other. Risk factors that were reported as a hazard ratio or relative risk were not included in a random-effects meta-analysis and were therefore only summarized.
The first category, related to antibiotic exposure, was further divided into the following nine categories: (i) carbapenem use, (ii) cephalosporin use, (iii) quinolone use, (iv) use of other β-lactam antibiotics or β-lactam use in general, (v) glycopeptide use, (vi) antibiotic exposure (in general), (vii) number of antibiotics administered, (viii) duration of exposure, and (ix) other. The second category, other, was also divided into nine categories, as follows: (i) underlying disease or condition, (ii) invasive procedures, (iii) medical devices, (iv) ICU admission, (v) exposure to hospital care, (vi) demographic patient characteristics, (vii) mechanical ventilation, (viii) CRE exposure, and (ix) other.
Studies reporting protective factors for the acquisition of CRE were summarized and included in a meta-analysis if they could be categorized into one of the previously described categories.
(ii) Meta-analysis.
The meta-analyses were performed using StatsDirect statistical software (Altrincham, United Kingdom), including the random-effects model of DerSimonian and Laird (26). A P value of <0.05 was considered statistically significant. A meta-analysis was performed only if ≥3 studies reported the same risk factor and if the risk factors within the category were not too diverse. Publication bias was examined visually with the use of funnel plots and assessed with the indicators of Egger et al. (28) and Begg and Mazumdar (29). When both indications showed a significant result, it was assumed that publication bias was present.
Eight additional meta-analyses were performed for each risk factor category: 1a, studies including only K. pneumoniae isolates; 1b, other studies; 2a, studies with an ICU setting; 2b, studies with a different study setting; 3a, studies describing only carbapenemase production as the carbapenem resistance mechanism; 3b, studies describing another resistance mechanism or not investigating the resistance mechanism involved; 4a, studies with a moderate/high study quality; and 4b, studies with a low study quality.
(iii) Infection prevention strategies and environmental sources/reservoirs.
All effective infection prevention strategies mentioned in the included articles were categorized, and a top 10 was created on the basis of the number of studies that reported these infection prevention strategies. In addition, studies describing sources and/or reservoirs for CRE in a hospital setting were reviewed and summarized.
Study quality.
A quality assessment was performed for all studies included in a meta-analysis using the strengthening the reporting of observational studies in epidemiology (STROBE) guideline (File S3) (30). Studies with a score of ≤15 points were considered to be of relatively low methodological quality, studies receiving a quality score of 16, 17, or 18 points were rated to be of moderate quality, and studies with a score of ≥19 points were considered to have a relatively high study quality. Study quality was not considered an exclusion criterion.
Supplementary Material
ACKNOWLEDGMENTS
We thank the management of Master Infection and Immunity (Erasmus MC) for its support during the research process. We also thank Wichor M. Bramer for devising and executing our systematic literature search. In addition, we thank the corresponding authors of the studies evaluated for providing feedback on our extracted data.
This research received no specific grant from any funding agency.
We report no conflict of interest relevant to this study.
M.C.V. and A.F.V. designed the study. A.F.V. and K.V.L. conducted the literature search and selected the studies. K.V.L. performed the data extraction. M.C.V., K.V.L., and A.F.V. performed the data interpretation and analysis. K.V.L., M.C.V., and A.F.V. wrote the manuscript.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01730-17.
REFERENCES
- 1.Nordmann P, Naas T, Poirel L. 2011. Global spread of carbapenemase producing Enterobacteriaceae. Emerg Infect Dis 17:1791–1798. doi: 10.3201/eid1710.110655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwaber MJ, Carmeli Y. 2008. Carbapenem-resistant Enterobacteriaceae: a potential threat. JAMA 300:2911–2913. doi: 10.1001/jama.2008.896. [DOI] [PubMed] [Google Scholar]
- 3.Baran I, Aksu N. 2016. Phenotypic and genotypic characteristics of carbapenem-resistant Enterobacteriaceae in a tertiary-level reference hospital in Turkey. Ann Clin Microbiol Antimicrob 15:20. doi: 10.1186/s12941-016-0136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbarini D, Russello G, Brovarone F, Capatti C, Colla R, Perilli M, Moro ML, Carretto E. 2015. Evaluation of carbapenem-resistant Enterobacteriaceae in an Italian setting: report from the trench. Infect Genet Evol 30:8–14. doi: 10.1016/j.meegid.2014.11.025. [DOI] [PubMed] [Google Scholar]
- 5.Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA. 2011. Carbapenems: past, present, and future. Antimicrob Agents Chemother 55:4943–4960. doi: 10.1128/AAC.00296-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Temkin E, Adler A, Lerner A. 2014. Carbapenem-resistant Enterobacteriaceae: biology, epidemiology, and management. Ann N Y Acad Sci 1323:22–42. doi: 10.1111/nyas.12537. [DOI] [PubMed] [Google Scholar]
- 7.Wang X, Chen G, Wu X, Wang L, Cai J, Chan EW, Chen S, Zhang R. 2015. Increased prevalence of carbapenem resistant Enterobacteriaceae in hospital setting due to cross-species transmission of the blaNDM-1 element and clonal spread of progenitor resistant strains. Front Microbiol 6:595. doi: 10.3389/fmicb.2015.00595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang JT, Wu UI, Lauderdale TLY, Chen MC, Li SY, Hsu LY, Chang SC. 2015. Carbapenem-nonsusceptible Enterobacteriaceae in Taiwan. PLoS One 10:e0121668. doi: 10.1371/journal.pone.0121668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freire MP, Abdala E, Moura ML, de Paula FJ, Spadão F, Caiaffa-Filho HH, David-Neto E, Nahas WC, Pierrotti LC. 2015. Risk factors and outcome of infections with Klebsiella pneumoniae carbapenemase-producing K. pneumoniae in kidney transplant recipients. Infection 43:315–323. doi: 10.1007/s15010-015-0743-4. [DOI] [PubMed] [Google Scholar]
- 10.Freire MP, Pierrotti LC, Filho HH, Ibrahim KY, Magri AS, Bonazzi PR, Hajar L, Diz MP, Pereira J, Hoff PM, Abdala E. 2015. Infection with Klebsiella pneumoniae carbapenemase (KPC)-producing Klebsiella pneumoniae in cancer patients. Eur J Clin Microbiol Infect Dis 34:277–286. doi: 10.1007/s10096-014-2233-5. [DOI] [PubMed] [Google Scholar]
- 11.Jiao Y, Qin Y, Liu J, Li Q, Dong Y, Shang Y, Huang Y, Liu R. 2015. Risk factors for carbapenem-resistant Klebsiella pneumoniae infection/colonization and predictors of mortality: a retrospective study. Pathog Global Health 109:68–74. doi: 10.1179/2047773215Y.0000000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. 2008. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol 29:1099–1106. doi: 10.1086/592412. [DOI] [PubMed] [Google Scholar]
- 13.Wang Q, Zhang Y, Yao X, Xian H, Liu Y, Li H, Chen H, Wang X, Wang R, Zhao C, Cao B, Wang H. 2016. Risk factors and clinical outcomes for carbapenem-resistant Enterobacteriaceae nosocomial infections. Eur J Clin Microbiol Infect Dis 35:1679–1689. doi: 10.1007/s10096-016-2710-0. [DOI] [PubMed] [Google Scholar]
- 14.Hendrik TC, Voor in ‘t holt AF, Vos MC. 2015. Clinical and molecular epidemiology of extended-spectrum beta-lactamase-producing Klebsiella spp.: a systematic review and meta-analyses. PLoS One 10:e0140754. doi: 10.1371/journal.pone.0140754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voor in ‘t holt AF, Severin JA, Lesaffre EMEH, Vos MC. 2014. A systematic review and meta-analyses show that carbapenem use and medical devices are the leading risk factors for carbapenem-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother 58:2626–2637. doi: 10.1128/AAC.01758-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu D, Cai J, Liu J. 2011. Risk factors for the acquisition of nosocomial infection with carbapenem-resistant Klebsiella pneumoniae. South Med J 104:106–110. doi: 10.1097/SMJ.0b013e318206063d. [DOI] [PubMed] [Google Scholar]
- 17.Harris AD, Smith D, Johnson JA, Bradham DD, Roghmann MC. 2002. Risk factors for imipenem-resistant Pseudomonas aeruginosa among hospitalized patients. Clin Infect Dis 34:340–345. doi: 10.1086/338237. [DOI] [PubMed] [Google Scholar]
- 18.Harris AD, McGregor JC, Johnson JA, Strauss SM, Moore AC, Standiford HC, Hebden JN, Morris JG Jr. 2007. Risk factors for colonization with extended-spectrum β-lactamase-producing bacteria and intensive care unit admission. Emerg Infect Dis 13:1144–1149. doi: 10.3201/eid1308.070071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwak YG, Choi SH, Choo EJ, Chung JW, Jeong JY, Kim NJ, Woo JH, Ryu J, Kim YS. 2005. Risk factors for the acquisition of carbapenem-resistant Klebsiella pneumoniae among hospitalized patients. Microb Drug Resist 11:165–169. doi: 10.1089/mdr.2005.11.165. [DOI] [PubMed] [Google Scholar]
- 20.Mittal G, Gaind R, Kumar D, Kaushik G, Gupta KB, Verma PK, Deb M. 2016. Risk factors for fecal carriage of carbapenemase producing Enterobacteriaceae among intensive care unit patients from a tertiary care center in India. BMC Microbiol 16:138. doi: 10.1186/s12866-016-0763-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwartz-Neiderman A, Braun T, Fallach N, Schwartz D, Carmeli Y, Schechner V. 2016. Risk factors for carbapenemase-producing carbapenem-resistant Enterobacteriaceae (CP-CRE) acquisition among contacts of newly diagnosed CP-CRE patients. Infect Control Hosp Epidemiol 37:1219–1225. doi: 10.1017/ice.2016.153. [DOI] [PubMed] [Google Scholar]
- 22.Torres-Gonzalez P, Cervera-Hernandez ME, Niembro-Ortega MD, Leal-Vega F, Cruz-Hervert LP, García-García L, Galindo-Fraga A, Martinez-Gamboa A, Bobadilla-Del Valle M, Sifuentes-Osornio J, Ponce-De-Leon A. 2015. Factors associated to prevalence and incidence of carbapenem-resistant Enterobacteriaceae fecal carriage: a cohort study in a Mexican tertiary care hospital. PLoS One 10:e0139883. doi: 10.1371/journal.pone.0139883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. 2009. Guidance for control of infections with carbapenem-resistant or carbapenemase-producing Enterobacteriaceae in acute care facilities. MMWR Morb Mortal Wkly Rep 58:256–260. [PubMed] [Google Scholar]
- 24.Gupta N, Limbago BM, Patel JB, Kallen AJ. 2011. Carbapenem-resistant Enterobacteriaceae: epidemiology and prevention. Clin Infect Dis 53:60–67. doi: 10.1093/cid/cir202. [DOI] [PubMed] [Google Scholar]
- 25.Anderson KF, Lonsway DR, Rasheed JK, Biddle J, Jensen B, McDougal LK, Carey RB, Thompson A, Stocker S, Limbago B, Patel JB. 2007. Evaluation of methods to identify the Klebsiella pneumoniae carbapenemase in Enterobacteriaceae. J Clin Microbiol 45:2723–2725. doi: 10.1128/JCM.00015-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DerSimonian R, Laird N. 2015. Meta-analysis in clinical trials revisited. Contemp Clin Trials 45:139–145. doi: 10.1016/j.cct.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. 2009. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Egger M, Davey Smith G, Schneider M, Minder C. 1997. Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Begg CB, Mazumdar M. 1994. Operating characteristics of a rank correlation test for publication bias. Biometrics 50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 30.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, STROBE Initiative . 2007. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 335:806–808. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akgul F, Bozkurt I, Sunbul M, Esen S, Leblebicioglu H. 2016. Risk factors and mortality in the carbapenem-resistant Klebsiella pneumoniae infection: case control study. Pathog Global Health 110:321–325. doi: 10.1080/20477724.2016.1254976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garbati MA, Sakkijha H, Abushaheen A. 2016. Infections due to carbapenem resistant Enterobacteriaceae among Saudi Arabian hospitalized patients: a matched case-control study. Biomed Res Int 2016:3961684. doi: 10.1155/2016/3961684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gasink LB, Edelstein PH, Lautenbach E, Synnestvedt M, Fishman NO. 2009. Risk factors and clinical impact of Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Infect Control Hosp Epidemiol 30:1180–1185. doi: 10.1086/648451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giuffrè M, Bonura C, Geraci DM, Saporito L, Catalano R, Di Noto S, Nociforo F, Corsello G, Mammina C. 2013. Successful control of an outbreak of colonization by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae sequence type 258 in a neonatal intensive care unit, Italy. J Hosp Infect 85:233–236. doi: 10.1016/j.jhin.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 35.Madueño A, Gonzalez Garcia J, Ramos MJ, Pedroso Y, Diaz Z, Oteo J, Lecuona M. 2017. Risk factors associated with carbapenemase-producing Klebsiella pneumoniae fecal carriage: a case-control study in a Spanish tertiary care hospital. Am J Infect Control 45:77–79. doi: 10.1016/j.ajic.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 36.Ahn JY, Song JE, Kim MH, Choi H, Kim JK, Ann HW, Kim JH, Jeon Y, Jeong SJ, Kim SB, Ku NS, Han SH, Song YG, Yong D, Lee K, Kim JM, Choi JY. 2014. Risk factors for the acquisition of carbapenem-resistant Escherichia coli at a tertiary care center in South Korea: a matched case-control study. Am J Infect Control 42:621–625. doi: 10.1016/j.ajic.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 37.Chang HJ, Hsu PC, Yang CC, Kuo AJ, Chia JH, Wu TL, Lee MH. 2011. Risk factors and outcomes of carbapenem-nonsusceptible Escherichia coli bacteremia: a matched case-control study. J Microbiol Immunol Infect 44:125–130. doi: 10.1016/j.jmii.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 38.Cheng VCC, Chen JHK, So SYC, Wong SCY, Chau PH, Wong LMW, Ching RHC, Ng MML, Lee WM, Hung IFN, Ho PL, Yuen KY. 2016. A novel risk factor associated with colonization by carbapenemase-producing Enterobacteriaceae: use of proton pump inhibitors in addition to antimicrobial treatment. Infect Control Hosp Epidemiol 37:1418–1425. doi: 10.1017/ice.2016.202. [DOI] [PubMed] [Google Scholar]
- 39.Daikos GL, Vryonis E, Psichogiou M, Tzouvelekis LS, Liatis S, Petrikkos P, Kosmidis C, Tassios PT, Bamias G, Skoutelis A. 2010. Risk factors for bloodstream infection with Klebsiella pneumoniae producing VIM-1 metallo-β-lactamase. J Antimicrob Chemother 65:784–788. doi: 10.1093/jac/dkq005. [DOI] [PubMed] [Google Scholar]
- 40.Dizbay M, Tunccan OG, Karasahin O, Aktas F. 2014. Emergence of carbapenem-resistant Klebsiella spp. infections in a Turkish university hospital: epidemiology and risk factors. J Infect Dev Ctries 8:44–49. doi: 10.3855/jidc.3091. [DOI] [PubMed] [Google Scholar]
- 41.Gagliotti C, Giordani S, Ciccarese V, Barozzi A, Giovinazzi A, Pietrantonio AM, Moro ML, Pinelli G, Sarti M. 2014. Risk factors for colonization with carbapenemase-producing Klebsiella pneumoniae in hospital: a matched case-control study. Am J Infect Control 42:1006–1008. doi: 10.1016/j.ajic.2014.05.028. [DOI] [PubMed] [Google Scholar]
- 42.Hu Y, Ping Y, Li L, Xu H, Yan X, Dai H. 2016. A retrospective study of risk factors for carbapenem-resistant Klebsiella pneumoniae acquisition among ICU patients. J Infect Dev Ctries 10:208–213. doi: 10.3855/jidc.6697. [DOI] [PubMed] [Google Scholar]
- 43.Hussein K, Sprecher H, Mashiach T, Oren I, Kassis I, Finkelstein R. 2009. Carbapenem resistance among Klebsiella pneumoniae isolates: risk factors, molecular characteristics, and susceptibility patterns. Infect Control Hosp Epidemiol 30:666–671. doi: 10.1086/598244. [DOI] [PubMed] [Google Scholar]
- 44.Jeon MH, Choi SH, Kwak YG, Chung JW, Lee SO, Jeong JY, Woo JH, Kim YS. 2008. Risk factors for the acquisition of carbapenem-resistant Escherichia coli among hospitalized patients. Diagn Microbiol Infect Dis 62:402–406. doi: 10.1016/j.diagmicrobio.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 45.Karaaslan A, Soysal A, Altinkanat Gelmez G, Kepenekli Kadayifci E, Söyletir G, Bakir M. 2016. Molecular characterization and risk factors for carbapenem-resistant Gram-negative bacilli colonization in children: emergence of NDM-producing Acinetobacter baumannii in a newborn intensive care unit in Turkey. J Hosp Infect 92:67–72. doi: 10.1016/j.jhin.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 46.Maldonado N, Castro B, Berrio I, Manjarres M, Robledo C, Robledo J. 2015. Ertapenem resistance in 2 tertiary-care hospitals: microbiology, epidemiology, and risk factors. Enferm Infecc Microbiol Clin 35:511–515. doi: 10.1016/j.eimc.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 47.Mills JP, Talati NJ, Alby K, Han JH. 2016. The epidemiology of carbapenem-resistant Klebsiella pneumoniae colonization and infection among long-term acute care hospital residents. Infect Control Hosp Epidemiol 37:55–60. doi: 10.1017/ice.2015.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Orsi GB, Bencardino A, Vena A, Carattoli A, Venditti C, Falcone M, Giordano A, Venditti M. 2013. Patient risk factors for outer membrane permeability and KPC-producing carbapenem-resistant Klebsiella pneumoniae isolation: results of a double case-control study. Infection 41:61–67. doi: 10.1007/s15010-012-0354-2. [DOI] [PubMed] [Google Scholar]
- 49.Papadimitriou-Olivgeris M, Marangos M, Fligou F, Christofidou M, Bartzavali C, Anastassiou ED, Filos KS. 2012. Risk factors for KPC-producing Klebsiella pneumoniae enteric colonization upon ICU admission. J Antimicrob Chemother 67:2976–2981. doi: 10.1093/jac/dks316. [DOI] [PubMed] [Google Scholar]
- 50.Papadimitriou-Olivgeris M, Spiliopoulou I, Christofidou M, Logothetis D, Manolopoulou P, Dodou V, Fligou F, Marangos M, Anastassiou ED. 2015. Co-colonization by multidrug-resistant bacteria in two Greek intensive care units. Eur J Clin Microbiol Infect Dis 34:1947–1955. doi: 10.1007/s10096-015-2436-4. [DOI] [PubMed] [Google Scholar]
- 51.Petrikkos P, Kosmidis C, Psichogiou M, Tassios P, Tzouvelekis L, Avlamis A, Stefanou I, Platsouka E, Paniara O, Xanthaki A, Toutouza M, Vrionis E, Skoutelis A, Georgousi K, Bamia C, Petrikkos G, Daikos GL. 2009. Prospective study of Klebsiella pneumoniae bacteremia: risk factors and clinical significance of type VIM-1 metallo-beta-lactamases. Arch Hell Med 26:374–383. [Google Scholar]
- 52.Satlin MJ, Cohen N, Ma KC, Gedrimaite Z, Soave R, Askin G, Chen L, Kreiswirth BN, Walsh TJ, Seo SK. 2016. Bacteremia due to carbapenem-resistant Enterobacteriaceae in neutropenic patients with hematologic malignancies. J Infect 73:336–345. doi: 10.1016/j.jinf.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma MS, Wang DH, Sun XJ, Li ZH, Wang C. 2014. Risk factors for Klebsiella pneumoniae carbapenemase-producing Klebsiella pneumoniae colonization in neonates. Chin J Contemp Pediatr 16:970–974. (In Chinese.) [PubMed] [Google Scholar]
- 54.Candevir Ulu A, Kurtaran B, Inal AS, Kömür S, Kibar F, Çiçekdemir HY, Bozkurt S, Gürel D, Kılıç F, Yaman A, Aksu HSZ, Ta̧ova Y. 2015. Risk factors of carbapenem-resistant Klebsiella pneumoniae infection: a serious threat in ICUs. Med Sci Monit 21:219–224. doi: 10.12659/MSM.892516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hayakawa K, Miyoshi-Akiyama T, Kirikae T, Nagamatsu M, Shimada K, Mezaki K, Sugiki Y, Kuroda E, Kubota S, Takeshita N, Kutsuna S, Tojo M, Ohmagari N. 2014. Molecular and epidemiological characterization of IMP-type metallo-β-lactamase-producing Enterobacter cloacae in a large tertiary care hospital in Japan. Antimicrob Agents Chemother 58:3441–3450. doi: 10.1128/AAC.02652-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu SW, Chang HJ, Chia JH, Kuo AJ, Wu TL, Lee MH. 2012. Outcomes and characteristics of ertapenem-nonsusceptible Klebsiella pneumoniae bacteremia at a university hospital in northern Taiwan: a matched case-control study. J Microbiol Immunol Infect 45:113–119. doi: 10.1016/j.jmii.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 57.Maseda E, Salgado P, Anillo V, Ruiz-Carrascoso G, Gómez-Gil R, Martín-Funke C, Gimenez MJ, Granizo JJ, Aguilar L, Gilsanz F. 2016. Risk factors for colonization by carbapenemase-producing enterobacteria at admission to a surgical ICU: a retrospective study. Enferm Infecc Microbiol Clin 35:333–337. [DOI] [PubMed] [Google Scholar]
- 58.Sánchez-Romero I, Asensio Á, Oteo J, Muñoz-Algarra M, Isidoro B, Vindel A, Álvarez-Avello J, Balandín-Moreno B, Cuevas O, Fernández-Romero S, Azañedo L, Sáez D, Campos J. 2012. Nosocomial outbreak of VIM-1-producing Klebsiella pneumoniae isolates of multilocus sequence type 15: molecular basis, clinical risk factors, and outcome. Antimicrob Agents Chemother 56:420–427. doi: 10.1128/AAC.05036-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dautzenberg MJD, Ossewaarde JM, de Greeff SC, Troelstra A, Bonten MJM. 2016. Risk factors for the acquisition of OXA-48-producing Enterobacteriaceae in a hospital outbreak setting: a matched case-control study. J Antimicrob Chemother 71:2273–2279. doi: 10.1093/jac/dkw119. [DOI] [PubMed] [Google Scholar]
- 60.Falagas ME, Rafailidis PI, Kofteridis D, Virtzili S, Chelvatzoglou FC, Papaioannou V, Maraki S, Samonis G, Michalopoulos A. 2007. Risk factors of carbapenem-resistant Klebsiella pneumoniae infections: a matched case-control study. J Antimicrob Chemother 60:1124–1130. doi: 10.1093/jac/dkm356. [DOI] [PubMed] [Google Scholar]
- 61.Schwaber MJ, Klarfeld-Lidji S, Navon-Venezia S, Schwartz D, Leavitt A, Carmeli Y. 2008. Predictors of carbapenem-resistant Klebsiella pneumoniae acquisition among hospitalized adults and effect of acquisition on mortality. Antimicrob Agents Chemother 52:1028–1033. doi: 10.1128/AAC.01020-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Teo J, Cai Y, Tang S, Lee W, Tan TY, Tan TT, Kwa ALH. 2012. Risk factors, molecular epidemiology and outcomes of ertapenem-resistant, carbapenem-susceptible Enterobacteriaceae: a case-case-control study. PLoS One 7:e34254. doi: 10.1371/journal.pone.0034254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tuon FF, Rocha JL, Toledo P, Arend LN, Dias CH, Leite TM, Penteado-Filho SR, Pilonetto M, Zavascki AP. 2012. Risk factors for KPC-producing Klebsiella pneumoniae bacteremia. Braz J Infect Dis 16:416–419. doi: 10.1016/j.bjid.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 64.Ben-David D, Masarwa S, Navon-Venezia S, Mishali H, Fridental I, Rubinovitch B, Smollan G, Carmeli Y, Schwaber MJ. 2011. Carbapenem-resistant Klebsiella pneumoniae in post-acute-care facilities in Israel. Infect Control Hosp Epidemiol 32:845–853. doi: 10.1086/661279. [DOI] [PubMed] [Google Scholar]
- 65.Borer A, Saidel-Odes L, Eskira S, Nativ R, Riesenberg K, Livshiz-Riven I, Schlaeffer F, Sherf M, Peled N. 2012. Risk factors for developing clinical infection with carbapenem-resistant Klebsiella pneumoniae in hospital patients initially only colonized with carbapenem-resistant K. pneumoniae. Am J Infect Control 40:421–425. doi: 10.1016/j.ajic.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 66.Gallagher JC, Kuriakose S, Haynes K, Axelrod P. 2014. Case-case-control study of patients with carbapenem-resistant and third-generation-cephalosporin-resistant Klebsiella pneumoniae bloodstream infections. Antimicrob Agents Chemother 58:5732–5735. doi: 10.1128/AAC.03564-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marchaim D, Chopra T, Bhargava A, Bogan C, Dhar S, Hayakawa K, Pogue JM, Bheemreddy S, Blunden C, Shango M, Swan J, Lephart PR, Perez F, Bonomo RA, Kaye KS. 2012. Recent exposure to antimicrobials and carbapenem-resistant Enterobacteriaceae: the role of antimicrobial stewardship. Infect Control Hosp Epidemiol 33:817–830. doi: 10.1086/666642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miller BM, Johnson SW. 2016. Demographic and infection characteristics of patients with carbapenem-resistant Enterobacteriaceae in a community hospital: development of a bedside clinical score for risk assessment. Am J Infect Control 44:134–137. doi: 10.1016/j.ajic.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 69.Poole K, George R, Decraene V, Shankar K, Cawthorne J, Savage N, Welfare W, Dodgson A. 2016. Active case finding for carbapenemase-producing Enterobacteriaceae in a teaching hospital: prevalence and risk factors for colonization. J Hosp Infect 94:125–129. doi: 10.1016/j.jhin.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 70.Chitnis AS, Caruthers PS, Rao AK, Lamb J, Lurvey R, De Rochars VB, Kitchel B, Cancio M, Török TJ, Guh AY, Gould CV, Wise ME. 2012. Outbreak of carbapenem-resistant Enterobacteriaceae at a long-term acute care hospital: sustained reductions in transmission through active surveillance and targeted interventions. Infect Control Hosp Epidemiol 33:984–992. doi: 10.1086/667738. [DOI] [PubMed] [Google Scholar]
- 71.de Jager P, Chirwa T, Naidoo S, Perovic O, Thomas J. 2015. Nosocomial outbreak of New Delhi metallo-beta-lactamase-1-producing gram-negative bacteria in South Africa: a case-control study. PLoS One 10:e0123337. doi: 10.1371/journal.pone.0123337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kritsotakis EI, Tsioutis C, Roumbelaki M, Christidou A, Gikas A. 2011. Antibiotic use and the risk of carbapenem-resistant extended-spectrum-β-lactamase-producing Klebsiella pneumoniae infection in hospitalized patients: results of a double case-control study. J Antimicrob Chemother 66:1383–1391. doi: 10.1093/jac/dkr116. [DOI] [PubMed] [Google Scholar]
- 73.Ling ML, Tee YM, Tan SG, Amin IM, How KB, Tan KY, Lee LC. 2015. Risk factors for acquisition of carbapenem resistant Enterobacteriaceae in an acute tertiary care hospital in Singapore. Antimicrob Resist Infect Control 4:26. doi: 10.1186/s13756-015-0066-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hussein K, Raz-Pasteur A, Finkelstein R, Neuberger A, Shachor-Meyouhas Y, Oren I, Kassis I. 2013. Impact of carbapenem resistance on the outcome of patients' hospital-acquired bacteraemia caused by Klebsiella pneumoniae. J Hosp Infect 83:307–313. doi: 10.1016/j.jhin.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 75.Mantzarlis K, Makris D, Manoulakas E, Karvouniaris M, Zakynthinos E. 2013. Risk factors for the first episode of Klebsiella pneumoniae resistant to carbapenems infection in critically ill patients: a prospective study. Biomed Res Int 2013:850547. doi: 10.1155/2013/850547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao ZC, Xu XH, Liu MB, Wu J, Lin J, Li B. 2014. Fecal carriage of carbapenem-resistant Enterobacteriaceae in a Chinese university hospital. Am J Infect Control 42:e61–e64. doi: 10.1016/j.ajic.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 77.Papadimitriou-Olivgeris M, Marangos M, Fligou F, Christofidou M, Sklavou C, Vamvakopoulou S, Anastassiou ED, Filos KS. 2013. KPC-producing Klebsiella pneumoniae enteric colonization acquired during intensive care unit stay: the significance of risk factors for its development and its impact on mortality. Diagn Microbiol Infect Dis 77:169–173. doi: 10.1016/j.diagmicrobio.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 78.Lee GC, Lawson KA, Burgess DS. 2013. Clinical epidemiology of carbapenem-resistant Enterobacteriaceae in community hospitals: a case-case-control study. Ann Pharmacother 47:1115–1121. doi: 10.1177/1060028013503120. [DOI] [PubMed] [Google Scholar]
- 79.Swaminathan M, Sharma S, Blash SP, Patel G, Banach DB, Phillips M, LaBombardi V, Anderson KF, Kitchel B, Srinivasan A, Calfee DP. 2013. Prevalence and risk factors for acquisition of carbapenem-resistant Enterobacteriaceae in the setting of endemicity. Infect Control Hosp Epidemiol 34:809–817. doi: 10.1086/671270. [DOI] [PubMed] [Google Scholar]
- 80.Bhargava A, Hayakawa K, Silverman E, Haider S, Alluri KC, Datla S, Diviti S, Kuchipudi V, Muppavarapu KS, Lephart PR, Marchaim D, Kaye KS. 2014. Risk factors for colonization due to carbapenem-resistant Enterobacteriaceae among patients exposed to long-term acute care and acute care facilities. Infect Control Hosp Epidemiol 35:398–405. doi: 10.1086/675614. [DOI] [PubMed] [Google Scholar]
- 81.Kofteridis DP, Valachis A, Dimopoulou D, Maraki S, Christidou A, Mantadakis E, Samonis G. 2014. Risk factors for carbapenem-resistant Klebsiella pneumoniae infection/colonization: a case-case-control study. J Infect Chemother 20:293–297. doi: 10.1016/j.jiac.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 82.Nouvenne A, Ticinesi A, Lauretani F, Maggio M, Lippi G, Guida L, Morelli I, Ridolo E, Borghi L, Meschi T. 2014. Comorbidities and disease severity as risk factors for carbapenem-resistant Klebsiella pneumoniae colonization: report of an experience in an internal medicine unit. PLoS One 9:e110001. doi: 10.1371/journal.pone.0110001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pereira MR, Scully BF, Pouch SM, Uhlemann AC, Goudie S, Emond JE, Verna EC. 2015. Risk factors and outcomes of carbapenem-resistant Klebsiella pneumoniae infections in liver transplant recipients. Liver Transplant 21:1511–1519. doi: 10.1002/lt.24207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bleumin D, Cohen MJ, Moranne O, Esnault VLM, Benenson S, Paltiel O, Tzukert K, Mor-Yosef Levi I, Ben-Dov IZ, Levi R, Bloch A, Haviv YS. 2012. Carbapenem-resistant Klebsiella pneumoniae is associated with poor outcome in hemodialysis patients. J Infect 65:318–325. doi: 10.1016/j.jinf.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 85.Gregory CJ, Llata E, Stine N, Gould C, Santiago LM, Vazquez GJ, Robledo IE, Srinivasan A, Goering RV, Tomashek KM. 2010. Outbreak of carbapenem-resistant Klebsiella pneumoniae in Puerto Rico associated with a novel carbapenemase variant. Infect Control Hosp Epidemiol 31:476–484. doi: 10.1086/651670. [DOI] [PubMed] [Google Scholar]
- 86.Lee HJ, Choi JK, Cho SY, Kim SH, Park SH, Choi SM, Lee DG, Choi JH, Yoo JH. 2016. Carbapenem-resistant Enterobacteriaceae: prevalence and risk factors in a single community-based hospital in Korea. Infect Chemother 48:166–173. doi: 10.3947/ic.2016.48.3.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tian L, Tan R, Chen Y, Sun J, Liu J, Qu H, Wang X. 2016. Epidemiology of Klebsiella pneumoniae bloodstream infections in a teaching hospital: factors related to the carbapenem resistance and patient mortality. Antimicrob Resist Infect Control 5:48. doi: 10.1186/s13756-016-0145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lin MY, Lyles-Banks RD, Lolans K, Hines DW, Spear JB, Petrak R, Trick WE, Weinstein RA, Hayden MK, Kallen AJ, Acuna N, Albright R, Alexander P, Anthony T, Bardowski L, Berends C, Bonebrake A, Bova J, Braggs A, Burtun S, Byrnes MA, Cagle C, Chavis R, Cienkus S, Collins-Johnson S, Coomer C, Chou T, Cullen D, Deguzman D, Donceras O, Feller M, Fung S, Gasienica JAM, Garcia-Houchins S, Genovese G, Gentile M, Gonzaga G, Goodwin E, Hernandez E, Kerridge J, Kirk J, Lavin MA, Lee S, Lepinski J, Myrick S, Oats T, O'Donnell A, Pasinos V, Patton JA, Perez M, et al. 2013. The importance of long-term acute care hospitals in the regional epidemiology of Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae. Clin Infect Dis 57:1246–1252. doi: 10.1093/cid/cit500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.da Silva KE, Maciel WG, Correia Sacchi FP, Carvalhaes CG, Rodrigues-Costa F, da Silva ACR, Croda MG, Negrão FJ, Croda J, Gales AC, Simionatto S. 2016. Risk factors for KPC-producing Klebsiella pneumoniae: watch out for surgery. J Med Microbiol 65:547–553. doi: 10.1099/jmm.0.000254. [DOI] [PubMed] [Google Scholar]
- 90.Ben-David D, Ganor O, Yasmin M, David L, Nathan K, Ilana T, Dalit S, Smollan G, Galia R. 2012. Epidemiology of carbapenem resistant Klebsiella pneumoniae colonization in an intensive care unit. Eur J Clin Microbiol Infect Dis 31:1811–1817. doi: 10.1007/s10096-011-1506-5. [DOI] [PubMed] [Google Scholar]
- 91.Freire MP, de Oliveira Garcia D, Cury AP, Spadao F, Di Gioia TS, Francisco GR, Bueno MF, Tomaz M, de Paula FJ, de Faro LB, Piovesan AC, Rossi F, Levin AS, David Neto E, Nahas WC, Pierrotti LC. 2016. Outbreak of IMP-producing carbapenem-resistant Enterobacter gergoviae among kidney transplant recipients. J Antimicrob Chemother 71:2577–2585. doi: 10.1093/jac/dkw165. [DOI] [PubMed] [Google Scholar]
- 92.Kim S, Russell D, Mohamadnejad M, Makker J, Sedarat A, Watson RR, Yang S, Hemarajata P, Humphries R, Rubin Z, Muthusamy VR. 2016. Risk factors associated with the transmission of carbapenem-resistant Enterobacteriaceae via contaminated duodenoscopes. Gastrointest Endosc 83:1121–1129. doi: 10.1016/j.gie.2016.03.790. [DOI] [PubMed] [Google Scholar]
- 93.Freire MP, Oshiro ICVS, Pierrotti LC, Bonazzi PR, de Oliveira LM, Song ATW, Camargo CH, van der Heijden IM, Rossi F, Costa SF, D'Albuquerque LAC, Abdala E. 2017. Carbapenem-resistant Enterobacteriaceae acquired before liver transplantation: impact on recipient outcomes. Transplantation 101:811–820. doi: 10.1097/TP.0000000000001620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Marchaim D, Navon-Venezia S, Schwaber MJ, Carmeli Y. 2008. Isolation of imipenem-resistant Enterobacter species: emergence of KPC-2 carbapenemase, molecular characterization, epidemiology, and outcomes. Antimicrob Agents Chemother 52:1413–1418. doi: 10.1128/AAC.01103-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vergara-López S, Domínguez MC, Conejo MC, Pascual T, Rodríguez-Baño J. 2015. Lessons from an outbreak of metallo-β-lactamase-producing Klebsiella oxytoca in an intensive care unit: the importance of time at risk and combination therapy. J Hosp Infect 89:123–131. doi: 10.1016/j.jhin.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 96.Mouloudi E, Protonotariou E, Zagorianou A, Iosifidis E, Karapanagiotou A, Giasnetsova T, Tsioka A, Roilides E, Sofianou D, Gritsi-Gerogianni N. 2010. Bloodstream infections caused by metallo-β-lactamase/Klebsiella pneumoniae carbapenemase-producing K. pneumoniae among intensive care unit patients in Greece: risk factors for infection and impact of type of resistance on outcomes. Infect Control Hosp Epidemiol 31:1250–1256. doi: 10.1086/657135. [DOI] [PubMed] [Google Scholar]
- 97.Adler A, Solter E, Masarwa S, Miller-Roll T, Abu-Libdeh B, Khammash H, Najem K, Dekadek S, Stein-Zamir C, Nubani N, Kunbar A, Assous MV, Carmeli Y, Schwaber MJ. 2013. Epidemiological and microbiological characteristics of an outbreak caused by OXA-48-producing Enterobacteriaceae in a neonatal intensive care unit in Jerusalem, Israel. J Clin Microbiol 51:2926–2930. doi: 10.1128/JCM.01049-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Agodi A, Voulgari E, Barchitta M, Politi L, Koumaki V, Spanakis N, Giaquinta L, Valenti G, Romeo MA, Tsakris A. 2011. Containment of an outbreak of KPC-3-producing Klebsiella pneumoniae in Italy. J Clin Microbiol 49:3986–3989. doi: 10.1128/JCM.01242-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Alrabaa SF, Nguyen P, Sanderson R, Baluch A, Sandin RL, Kelker D, Karlapalem C, Thompson P, Sams K, Martin S, Montero J, Greene JN. 2013. Early identification and control of carbapenemase-producing Klebsiella pneumoniae, originating from contaminated endoscopic equipment. Am J Infect Control 41:562–564. doi: 10.1016/j.ajic.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 100.Balkhy HH, El-Saed A, Al Johani SM, Francis C, Al-Qahtani AA, Al-Ahdal MN, Altayeb HT, Arabi Y, Alothman A, Sallah M. 2012. The epidemiology of the first described carbapenem-resistant Klebsiella pneumoniae outbreak in a tertiary care hospital in Saudi Arabia: how far do we go? Eur J Clin Microbiol Infect Dis 31:1901–1909. doi: 10.1007/s10096-011-1519-0. [DOI] [PubMed] [Google Scholar]
- 101.Ben-David D, Maor Y, Keller N, Regev-Yochay G, Tal L, Shachar D, Zlotkin A, Smollan G, Rahav G. 2010. Potential role of active surveillance in the control of a hospital-wide outbreak of carbapenem-resistant Klebsiella pneumoniae infection. Infect Control Hosp Epidemiol 31:620–626. doi: 10.1086/652528. [DOI] [PubMed] [Google Scholar]
- 102.Ben-David D, Masarwa S, Adler A, Mishali H, Carmeli Y, Schwaber MJ. 2014. A national intervention to prevent the spread of carbapenem-resistant Enterobacteriaceae in Israeli post-acute care hospitals. Infect Control Hosp Epidemiol 35:802–809. doi: 10.1086/676876. [DOI] [PubMed] [Google Scholar]
- 103.Borer A, Eskira S, Nativ R, Saidel-Odes L, Riesenberg K, Livshiz-Riven I, Schlaeffer F, Sherf M, Peled N. 2011. A multifaceted intervention strategy for eradication of a hospital-wide outbreak caused by carbapenem-resistant Klebsiella pneumoniae in southern Israel. Infect Control Hosp Epidemiol 32:1158–1165. doi: 10.1086/662620. [DOI] [PubMed] [Google Scholar]
- 104.Borgia S, Lastovetska O, Richardson D, Eshaghi A, Xiong J, Chung C, Baqi M, McGeer A, Ricci G, Sawicki R, Pantelidis R, Low DE, Patel SN, Melano RG. 2012. Outbreak of carbapenem-resistant Enterobacteriaceae containing bla NDM-1, Ontario, Canada. Clin Infect Dis 55:e109–e117. doi: 10.1093/cid/cis737. [DOI] [PubMed] [Google Scholar]
- 105.Carbonne A, Thiolet JM, Fournier S, Fortineau N, Kassis-Chikhani N, Boytchev I, Aggoune M, Séguier JC, Sénéchal H, Tavolacci MP, Coignard B, Astagneau P, Jarlier V. 2010. Control of a multi-hospital outbreak of KPC-producing Klebsiella pneumoniae type 2 in France, September to October 2009. Euro Surveill 15(48):pii=19734 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19734. [DOI] [PubMed] [Google Scholar]
- 106.Chang LWK, Buising KL, Jeremiah CJ, Cronin K, Poy Lorenzo YS, Howden BP, Kwong J, Cocks J, Blood A, Greenough J, Waters MJ. 2015. Managing a nosocomial outbreak of carbapenem-resistant Klebsiella pneumoniae: an early Australian hospital experience. Intern Med J 45:1037–1043. doi: 10.1111/imj.12863. [DOI] [PubMed] [Google Scholar]
- 107.Ciobotaro P, Oved M, Nadir E, Bardenstein R, Zimhony O. 2011. An effective intervention to limit the spread of an epidemic carbapenem-resistant Klebsiella pneumoniae strain in an acute care setting: from theory to practice. Am J Infect Control 39:671–677. doi: 10.1016/j.ajic.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 108.Dai Y, Zhang C, Ma X, Chang W, Hu S, Jia H, Huang J, Lu H, Li H, Zhou S, Qiu G, Liu J. 2014. Outbreak of carbapenemase-producing Klebsiella pneumoniae neurosurgical site infections associated with a contaminated shaving razor used for preoperative scalp shaving. Am J Infect Control 42:805–806. doi: 10.1016/j.ajic.2014.03.023. [DOI] [PubMed] [Google Scholar]
- 109.Douka E, Perivolioti E, Kraniotaki E, Fountoulis K, Economidou F, Tsakris A, Skoutelis A, Routsi C. 2015. Emergence of a pandrug-resistant VIM-1-producing Providencia stuartii clonal strain causing an outbreak in a Greek intensive care unit. Int J Antimicrob Agents 45:533–536. doi: 10.1016/j.ijantimicag.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 110.Enfield KB, Huq NN, Gosseling MF, Low DJ, Hazen KC, Toney DM, Slitt G, Zapata HJ, Cox HL, Lewis JD, Kundzins JR, Mathers AJ, Sifri CD. 2014. Control of simultaneous outbreaks of carbapenemase-producing Enterobacteriaceae and extensively drug-resistant Acinetobacter baumannii infection in an intensive care unit using interventions promoted in the Centers for Disease Control and Prevention 2012 carbapenemase-resistant Enterobacteriaceae toolkit. Infect Control Hosp Epidemiol 35:810–817. doi: 10.1086/676857. [DOI] [PubMed] [Google Scholar]
- 111.Epson EE, Pisney LM, Wendt JM, MacCannell DR, Janelle SJ, Kitchel B, Kamile Rasheed J, Limbago BM, Gould CV, Kallen AJ, Barron MA, Bamberg WM. 2014. Carbapenem-resistant Klebsiella pneumoniae producing New Delhi metallo-β-lactamase at an acute care hospital, Colorado, 2012. Infect Control Hosp Epidemiol 35:390–397. doi: 10.1086/675607. [DOI] [PubMed] [Google Scholar]
- 112.Forcina A, Baldan R, Marasco V, Cichero P, Bondanza A, Noviello M, Piemontese S, Soliman C, Greco R, Lorentino F, Giglio F, Messina C, Carrabba M, Bernardi M, Peccatori J, Moro M, Biancardi A, Nizzero P, Scarpellini P, Cirillo DM, Mancini N, Corti C, Clementi M, Ciceri F. 2017. Control of infectious mortality due to carbapenemase-producing Klebsiella pneumoniae in hematopoietic stem cell transplantation. Bone Marrow Transplant 52:114–119. doi: 10.1038/bmt.2016.234. [DOI] [PubMed] [Google Scholar]
- 113.Fournier S, Monteil C, Lepainteur M, Richard C, Brun-Buisson C, Jarlier V, Andremont A, Armand-Lefevre L, Birgand G, Bonnal C, Lucet JC, Arlet G, Denis M, Baixench MT, Blanchard H, Casetta A, Poupet H, Barbut F, Decré D, Petit JC, Berche P, Zahar JR, Bingen E, Doit C, Cambau E, Guérin JM, Raskine L, Carbonne A, Kac G, Podglajen I, Decousser JW, Derouin V, Doucet-Populaire F, Drieux-Rouzet L, Espinasse F, Heym B, Fortineau N, Nordmann P, Herrmann JL, Lawrence C, Jansen C, Legrand P, Lesprit P, Duviquet M, Akpabie A, Kassis-Chikhani N, Marcadé G, Fihman V, Nerome S, Nicolas-Chanoine MH, et al. 2014. Long-term control of carbapenemase-producing Enterobacteriaceae at the scale of a large French multihospital institution: a nine-year experience, France, 2004 to 2012. Euro Surveill 19(19):pii=20802 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20802. [DOI] [PubMed] [Google Scholar]
- 114.Gagliotti C, Cappelli V, Carretto E, Marchi M, Pan A, Ragni P, Sarti M, Suzzi R, Tura GA, Moro ML, Alfano G, Amadori A, Ambretti S, Antonioli P, Arlotti M, Artioli S, Barbieri M, Barbolini L, Barison S, Bedosti C, Bergamini R, Bertozzi L, Bianchi S, Brambilla A, Callea E, Caminati A, Capra P, Carillo C, Carli S, Carretto E, Castellani G, Cavazzuti L, Ceccarelli P, Cugini P, Di Ruscio E, D'Erasmo D, Dodi S, Farina M, Farruggia P, Filippini F, Finzi G, Firretti A, Fusaroli P, Garlotti A, Giordani S, Govoni G, Lavezzi S, Liverani S, Liverani AL, Lombardi M, et al. 2014. Control of carbapenemase-producing Klebsiella pneumoniae: a region-wide intervention. Euro Surveill 19(43):pii=20943 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20943. [DOI] [PubMed] [Google Scholar]
- 115.Gaibani P, Ambretti S, Farruggia P, Bua G, Berlingeri A, Tamburini MV, Cordovana M, Guerra L, Mazzetti M, Roncarati G, Tenace C, Moro ML, Gagliotti C, Landini MP, Sambri V. 2013. Outbreak of Citrobacter freundii carrying Vim-1 in an Italian hospital, identified during the carbapenemases screening actions, June 2012. Int J Infect Dis 17:e714–e717. doi: 10.1016/j.ijid.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 116.Ghafur A, Nagvekar V, Thilakavathy S, Chandra K, Gopalakrishnan R, Vidyalakshmi P, Apollo Speciality Hospital, Chennai, India. 2012. “Save antibiotics, save lives”: an Indian success story of infection control through persuasive diplomacy. Antimicrob Resist Infect Control 1:29. doi: 10.1186/2047-2994-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hayden MK, Lin MY, Lolans K, Weiner S, Blom D, Moore NM, Fogg L, Henry D, Lyles R, Thurlow C, Sikka M, Hines D, Weinstein RA. 2015. Prevention of colonization and infection by Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae in long-term acute-care hospitals. Clin Infect Dis 60:1153–1161. doi: 10.1093/cid/ciu1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ho HJ, Toh CY, Ang B, Krishnan P, Lin RTP, La MV, Chow A. 2016. Outbreak of New Delhi metallo-β-lactamase-1-producing Enterobacter cloacae in an acute care hospital general ward in Singapore. Am J Infect Control 44:177–182. doi: 10.1016/j.ajic.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 119.Kanerva M, Skogberg K, Ryynänen K, Pahkamäki A, Jalava J, Ollgren J, Tarkka E, Lyytikäinen O. 2015. Coincidental detection of the first outbreak of carbapenemase-producing Klebsiella pneumonia colonisation in a primary care hospital, Finland, 2013. Euro Surveill 20(26):pii=21172 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=21172. [DOI] [PubMed] [Google Scholar]
- 120.Kassis-Chikhani N, Saliba F, Carbonne A, Neuville S, Decre D, Sengelin C, Guerin C, Gastiaburu N, Lavigne-Kriaa A, Boutelier C, Arlet G, Samuel D, Castaing D, Dussaix E, Jarlier V. 2010. Extended measures for controlling an outbreak of VIM-1 producing imipenem-resistant Klebsiella pneumoniae in a liver transplant centre in France, 2003-2004. Euro Surveill 15(46):pii=19713 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19713. [DOI] [PubMed] [Google Scholar]
- 121.Kim NH, Han WD, Song KH, Seo HK, Shin MJ, Kim TS, Park KU, Ahn S, Yoo JS, Kim ES, Kim HB. 2014. Successful containment of carbapenem-resistant Enterobacteriaceae by strict contact precautions without active surveillance. Am J Infect Control 42:1270–1273. doi: 10.1016/j.ajic.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 122.Kola A, Piening B, Pape UF, Veltzke-Schlieker W, Kaase M, Geffers C, Wiedenmann B, Gastmeier P. 2015. An outbreak of carbapenem-resistant OXA-48-producing Klebsiella pneumonia associated to duodenoscopy. Antimicrob Resist Infect Control 4:8. doi: 10.1186/s13756-015-0049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Landman D, Babu E, Shah N, Kelly P, Olawole O, Bäcker M, Bratu S, Quale J. 2012. Transmission of carbapenem-resistant pathogens in New York City hospitals: progress and frustration. J Antimicrob Chemother 67:1427–1431. doi: 10.1093/jac/dks063. [DOI] [PubMed] [Google Scholar]
- 124.Lemmenmeier E, Kohler P, Bruderer T, Goldenberger D, Kleger GR, Schlegel M. 2014. First documented outbreak of KPC-2-producing Klebsiella pneumoniae in Switzerland: infection control measures and clinical management. Infection 42:529–534. doi: 10.1007/s15010-013-0578-9. [DOI] [PubMed] [Google Scholar]
- 125.Lowe CF, Kus JV, Salt N, Callery S, Louie L, Khan MA, Vearncombe M, Simor AE. 2013. Nosocomial transmission of New Delhi metallo-beta-lactamase-1-producing Klebsiella pneumoniae in Toronto, Canada. Infect Control Hosp Epidemiol 34:49–55. doi: 10.1086/668778. [DOI] [PubMed] [Google Scholar]
- 126.Matsumura Y, Tanaka M, Yamamoto M, Nagao M, Machida K, Ito Y, Takakura S, Ogawa K, Yoshizawa A, Fujimoto Y, Okamoto S, Uemoto S, Ichiyama S. 2015. High prevalence of carbapenem resistance among plasmid-mediated AmpC β-lactamase-producing Klebsiella pneumoniae during outbreaks in liver transplantation units. Int J Antimicrob Agents 45:33–40. doi: 10.1016/j.ijantimicag.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 127.Meletis G, Oustas E, Botziori C, Kakasi E, Koteli A. 2015. Containment of carbapenem resistance rates of Klebsiella pneumoniae and Acinetobacter baumannii in a Greek hospital with a concomitant increase in colistin, gentamicin and tigecycline resistance. New Microbiol 38:417–421. [PubMed] [Google Scholar]
- 128.Meybeck A, Laurans C, Broucqsault-Dedrie C, Vanbaelinghem C, Verheyde I, Ledez R, Nyunga M, Nottebaert S, Waes S, Vachée A. 2014. Rapid containment of coincident outbreaks with 2 carbapenem-resistant Klebsiella pneumoniae strains in an intensive care unit. Am J Infect Control 42:929–931. doi: 10.1016/j.ajic.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 129.Morris D, Boyle F, Morris C, Condon I, Delannoy-Vieillard AS, Power L, Khan A, Morris-Downes M, Finnegan C, Powell J, Monahan R, Burns K, O'Connell N, Boyle L, O'Gorman A, Humphreys H, Brisse S, Turton J, Woodford N, Cormican M. 2012. Inter-hospital outbreak of Klebsiella pneumoniae producing KPC-2 carbapenemase in Ireland. J Antimicrob Chemother 67:2367–2372. doi: 10.1093/jac/dks239. [DOI] [PubMed] [Google Scholar]
- 130.Munoz-Price LS, Hayden MK, Lolans K, Won S, Calvert K, Lin M, Stemer A, Weinstein RA. 2010. Successful control of an outbreak of Klebsiella pneumoniae carbapenemase-producing K. pneumoniae at a long-term acute care hospital. Infect Control Hosp Epidemiol 31:341–347. doi: 10.1086/651097. [DOI] [PubMed] [Google Scholar]
- 131.O'Connor C, Cormican M, Boo TW, McGrath E, Slevin B, O'Gorman A, Commane M, Mahony S, O'Donovan E, Powell J, Monahan R, Finnegan C, Kiernan MG, Coffey JC, Power L, O'Connell NH, Dunne CP. 2016. An Irish outbreak of New Delhi metallo-β-lactamase (NDM)-1 carbapenemase-producing Enterobacteriaceae: increasing but unrecognized prevalence. J Hosp Infect 94:351–357. doi: 10.1016/j.jhin.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 132.Pang F, Jia XQ, Wang B, Li YH, Zhao QG. 2014. Control of an outbreak due to orthopedic infections caused by Enterobacteriaceae producing IMP-4 or IMP-8 carbapenemases. Pathol Biol 62:152–155. doi: 10.1016/j.patbio.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 133.Poulou A, Voulgari E, Vrioni G, Koumaki V, Xidopoulos G, Chatzipantazi V, Markou F, Tsakris A. 2013. Outbreak caused by an ertapenem-resistant, CTX-M-15-producing Klebsiella pneumoniae sequence type 101 clone carrying an OmpK36 porin variant. J Clin Microbiol 51:3176–3182. doi: 10.1128/JCM.01244-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Robustillo Rodela A, Díaz-Agero Pérez C, Sanchez Sagrado T, Ruiz-Garbajosa P, Pita López MJ, Monge V. 2012. Emergence and outbreak of carbapenemase-producing KPC-3 Klebsiella pneumoniae in Spain, September 2009 to February 2010: control measures. Euro Surveill 17(7):pii=20086 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20086. [PubMed] [Google Scholar]
- 135.Rossi Gonçalves I, Ferreira ML, Araujo BF, Campos PA, Royer S, Batistão DWF, Souza LP, Brito CS, Urzedo JE, Gontijo-Filho PP, Ribas RM. 2016. Outbreaks of colistin-resistant and colistin-susceptible KPC-producing Klebsiella pneumoniae in a Brazilian intensive care unit. J Hosp Infect 94:322–329. doi: 10.1016/j.jhin.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 136.Schreterova E, Takacova V, Niks M, Pastvova L, Tomco L, Siegfried L. 2014. Methods of phenotypic determination of New Delhi metallo-beta-lactamase (NDM-1) in Klebsiella pneumoniae isolated in Slovakia. Klin Mikrobiol Infekc Lek 20:79–84. (In Slovak.) [PubMed] [Google Scholar]
- 137.Seara N, Oteo J, Carrillo R, Pérez-Blanco V, Mingorance J, Gómez-Gil R, Herruzo R, Pérez-Vázquez M, Astray J, García-Rodríguez J, Ruiz-Velasco LM, Campos J, De Burgos C, Ruiz-Carrascoso G. 2015. Interhospital spread of NDM-7-producing Klebsiella pneumoniae belonging to ST437 in Spain. Int J Antimicrob Agents 46:169–173. doi: 10.1016/j.ijantimicag.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 138.Schwaber MJ, Lev B, Israeli A, Solter E, Smollan G, Rubinovitch B, Shalit I, Carmeli Y. 2011. Containment of a country-wide outbreak of carbapenem-resistant Klebsiella pneumoniae in Israeli hospitals via a nationally implemented intervention. Clin Infect Dis 52:848–855. doi: 10.1093/cid/cir025. [DOI] [PubMed] [Google Scholar]
- 139.Seiffert SN, Marschall J, Perreten V, Carattoli A, Furrer H, Endimiani A. 2014. Emergence of Klebsiella pneumoniae co-producing NDM-1, OXA-48, CTX-M-15, CMY-16, QnrA and ArmA in Switzerland. Int J Antimicrob Agents 44:260–262. doi: 10.1016/j.ijantimicag.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 140.Semin-Pelletier B, Cazet L, Bourigault C, Juvin ME, Boutoille D, Raffi F, Hourmant M, Blancho G, Agard C, Connault J, Corvec S, Caillon J, Batard E, Lepelletier D. 2015. Challenges of controlling a large outbreak of OXA-48 carbapenemase-producing Klebsiella pneumoniae in a French university hospital. J Hosp Infect 89:248–253. doi: 10.1016/j.jhin.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 141.Shilo S, Assous MV, Lachish T, Kopuit P, Bdolah-Abram T, Yinnon AM, Wiener-Well Y. 2013. Risk factors for bacteriuria with carbapenem-resistant Klebsiella pneumoniae and its impact on mortality: a case-control study. Infection 41:503–509. doi: 10.1007/s15010-012-0380-0. [DOI] [PubMed] [Google Scholar]
- 142.Snitkin ES, Zelazny AM, Thomas PJ, Stock F, Henderson DK, Palmore TN, Segre JA. 2012. Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with whole-genome sequencing. Sci Transl Med 4:148ra116. doi: 10.1126/scitranslmed.3004129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Teare L, Myers J, Kirkham A, Tredoux T, Martin R, Boasman S, Wisbey A, Charlton C, Dziewulski P. 2016. Prevention and control of carbapenemase-producing organisms at a regional burns centre. J Hosp Infect 93:141–144. doi: 10.1016/j.jhin.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 144.Thomas CP, Moore LSP, Elamin N, Doumith M, Zhang J, Maharjan S, Warner M, Perry C, Turton JF, Johnstone C, Jepson A, Duncan NDC, Holmes AH, Livermore DM, Woodford N. 2013. Early (2008-2010) hospital outbreak of Klebsiella pneumoniae producing OXA-48 carbapenemase in the UK. Int J Antimicrob Agents 42:531–536. doi: 10.1016/j.ijantimicag.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 145.Vergara-Lopez S, Dominguez MC, Conejo MC, Pascual A, Rodriguez-Bano J. 2013. Wastewater drainage system as an occult reservoir in a protracted clonal outbreak due to metallo-beta-lactamase-producing Klebsiella oxytoca. Clin Microbiol Infect 19:E490–E498. doi: 10.1111/1469-0691.12288. [DOI] [PubMed] [Google Scholar]
- 146.Viale P, Tumietto F, Giannella M, Bartoletti M, Tedeschi S, Ambretti S, Cristini F, Gibertoni C, Venturi S, Cavalli M, De Palma A, Puggioli MC, Mosci D, Callea E, Masina R, Moro ML, Lewis RE. 2015. Impact of a hospital-wide multifaceted programme for reducing carbapenem-resistant Enterobacteriaceae infections in a large teaching hospital in northern Italy. Clin Microbiol Infect 21:242–247. doi: 10.1016/j.cmi.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 147.Virgincar N, Iyer S, Stacey A, Maharjan S, Pike R, Perry C, Wyeth J, Woodford N. 2011. Klebsiella pneumoniae producing KPC carbapenemase in a district general hospital in the UK. J Hosp Infect 78:293–296. doi: 10.1016/j.jhin.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 148.Wendt C, Schütt S, Dalpke AH, Konrad M, Mieth M, Trierweiler-Hauke B, Weigand MA, Zimmermann S, Biehler K, Jonas D. 2010. First outbreak of Klebsiella pneumoniae carbapenemase (KPC)-producing K. pneumoniae in Germany. Eur J Clin Microbiol Infect Dis 29:563–570. doi: 10.1007/s10096-010-0896-0. [DOI] [PubMed] [Google Scholar]
- 149.Weterings V, Zhou K, Rossen JW, van Stenis D, Thewessen E, Kluytmans J, Veenemans J. 2015. An outbreak of colistin-resistant Klebsiella pneumoniae carbapenemase-producing Klebsiella pneumoniae in the Netherlands (July to December 2013), with inter-institutional spread. Eur J Clin Microbiol Infect Dis 34:1647–1655. doi: 10.1007/s10096-015-2401-2. [DOI] [PubMed] [Google Scholar]
- 150.Wrenn C, O'Brien D, Keating D, Roche C, Rose L, Ronayne A, Fenelon L, Fitzgerald S, Crowley B, Schaffer K. 2014. Investigation of the first outbreak of OXA-48-producing Klebsiella pneumoniae in Ireland. J Hosp Infect 87:41–46. doi: 10.1016/j.jhin.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 151.Yu F, Ying Q, Chen C, Li T, Ding B, Liu Y, Lu Y, Qin Z, Parson C, Salgado C, Qu D, Pan J, Wang L. 2012. Outbreak of pulmonary infection caused by Klebsiella pneumoniae isolates harbouring blaIMP-4 and blaDHA-1 in a neonatal intensive care unit in China. J Med Microbiol 61:984–989. doi: 10.1099/jmm.0.043000-0. [DOI] [PubMed] [Google Scholar]
- 152.Yu J, Tan K, Rong Z, Wang Y, Chen Z, Zhu X, Wu L, Tan L, Xiong W, Sun Z, Chen L. 2016. Nosocomial outbreak of KPC-2- and NDM-1-producing Klebsiella pneumoniae in a neonatal ward: a retrospective study. BMC Infect Dis 16:563. doi: 10.1186/s12879-016-1870-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zavascki AP, Carvalhaes CG, da Silva GL, Tavares Soares SP, de Alcantara LR, Elias LS, Sandri AM, Gales AC. 2012. Outbreak of carbapenem-resistant Providencia stuartii in an intensive care unit. Infect Control Hosp Epidemiol 33:627–630. doi: 10.1086/665730. [DOI] [PubMed] [Google Scholar]
- 154.Cheng VCC, Chan JFW, Wong SCY, Chen JHK, Tai JWM, Yan MK, Kwan GSW, Tse H, To KKW, Ho PL, Yuen KY. 2013. Proactive infection control measures to prevent nosocomial transmission of carbapenem-resistant Enterobacteriaceae in a non-endemic area. Chin Med J 126:4504–4509. [PubMed] [Google Scholar]
- 155.Fukigai S, Alba J, Kimura S, Iida T, Nishikura N, Ishii Y, Yamaguchi K. 2007. Nosocomial outbreak of genetically related IMP-1 β-lactamase-producing Klebsiella pneumoniae in a general hospital in Japan. Int J Antimicrob Agents 29:306–310. doi: 10.1016/j.ijantimicag.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 156.Bellodi A, Del Corso L, Favorini S, Molinari E, Coppo E, Orengo G, Del Bono V, Ghio R, Arboscello E. 2016. Management of Klebsiella pneumoniae KPC outbreak in internal medicine. Acta Med Mediterr 32:823–827. http://www.actamedicamediterranea.com/archive/2016/medica-3/management-of-klebsiella-pneumoniae-kpc-outbreak-in-internal-medicine/pdf. [Google Scholar]
- 157.Bustos-Moya G, Josa-Montero D, Perea-Ronco J, Gualtero-Trujillo S, Ortiz-Aroca J, Novoa-Bernal Á, Arias-León G, Silva-Monsalve E, Buitrago-Bernal R, Poveda-Henao M. 2016. Factors related to successful control of an outbreak by Klebsiella pneumonia-producing KPC-2 in an intensive care unit in Bogotá, Colombia. Infectio 20:25–32. doi: 10.1016/j.infect.2015.07.001. [DOI] [Google Scholar]
- 158.Chabah M, Chemsi M, Zerouali K, Alloula O, Lehlimi M, Habzi A, Benomar S. 2016. Healthcare-associated infections due to carbapenemase-producing Enterobacteriaceae: bacteriological profile and risk factors. Med Mal Infect 46:157–162. doi: 10.1016/j.medmal.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 159.Cohen MJ, Block C, Levin PD, Schwartz C, Gross I, Weiss Y, Moses AE, Benenson S. 2011. Institutional control measures to curtail the epidemic spread of carbapenem-resistant Klebsiella pneumoniae: a 4-year perspective. Infect Control Hosp Epidemiol 32:673–678. doi: 10.1086/660358. [DOI] [PubMed] [Google Scholar]
- 160.Dautzenberg MJ, Ossewaarde JM, de Kraker ME, van der Zee A, van Burgh S, de Greeff SC, Bijlmer HA, Grundmann H, Cohen Stuart JW, Fluit AC, Troelstra A, Bonten MJ. 2014. Successful control of a hospital-wide outbreak of OXA-48 producing Enterobacteriaceae in the Netherlands, 2009 to 2011. Euro Surveill 19(9):pii=20723 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20723. [DOI] [PubMed] [Google Scholar]
- 161.Delory T, Seringe E, Antoniotti G, Novakova I, Goulenok C, Paysant I, Boyer S, Carbonne A, Naas T, Astagneau P. 2015. Prolonged delay for controlling KPC-2-producing Klebsiella pneumoniae outbreak: the role of clinical management. Am J Infect Control 43:1070–1075. doi: 10.1016/j.ajic.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 162.Schwaber MJ, Carmeli Y.. 2014. An ongoing national intervention to contain the spread of carbapenem-resistant Enterobacteriaceae. Clin Infect Dis 58:697–703. doi: 10.1093/cid/cit795. [DOI] [PubMed] [Google Scholar]
- 163.Gaibani P, Colombo R, Arghittu M, Cariani L, Ambretti S, Bua G, Lombardo D, Landini MP, Torresani E, Sambri V. 2014. Successful containment and infection control of a carbapenem-resistant Klebsiella pneumoniae outbreak in an Italian hospital. New Microbiol 37:87–90. [PubMed] [Google Scholar]
- 164.Jimenez A, Castro JG, Munoz-Price LS, de Pascale D, Shimose L, Mustapha MM, Spychala CN, Mettus RT, Cooper VS, Doi Y. 2017. Outbreak of Klebsiella pneumoniae carbapenemase-producing Citrobacter freundii at a tertiary acute care facility in Miami, Florida. Infect Control Hosp Epidemiol 38:320–326. doi: 10.1017/ice.2016.273. [DOI] [PubMed] [Google Scholar]
- 165.Kochar S, Sheard T, Sharma R, Hui A, Tolentino E, Allen G, Landman D, Bratu S, Augenbraun M, Quale J. 2009. Success of an infection control program to reduce the spread of carbapenem-resistant Klebsiella pneumoniae. Infect Control Hosp Epidemiol 30:447–452. doi: 10.1086/596734. [DOI] [PubMed] [Google Scholar]
- 166.Leitner E, Zarfel G, Luxner J, Herzog K, Pekard-Amenitsch S, Hoenigl M, Valentin T, Feierl G, Grisold AJ, Högenauer C, Sill H, Krause R, Zollner-Schwetzd I. 2015. Contaminated handwashing sinks as the source of a clonal outbreak of KPC-2-producing Klebsiella oxytoca on a hematology ward. Antimicrob Agents Chemother 59:714–716. doi: 10.1128/AAC.04306-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Munoz-Price LS, De La Cuesta C, Adams S, Wyckoff M, Cleary T, McCurdy SP, Huband MD, Lemmon MM, Lescoe M, Dibhajj FB, Hayden MK, Lolans K, Quinn JP. 2010. Successful eradication of a monoclonal strain of Klebsiella pneumoniae during a K. pneumoniae carbapenemase-producing K. pneumoniae outbreak in a surgical intensive care unit in Miami, Florida. Infect Control Hosp Epidemiol 31:1074–1077. doi: 10.1086/656243. [DOI] [PubMed] [Google Scholar]
- 168.Poulou A, Voulgari E, Vrioni G, Xidopoulos G, Pliagkos A, Chatzipantazi V, Markou F, Tsakris A. 2012. Imported Klebsiella pneumoniae carbapenemase-producing K. pneumoniae clones in a Greek hospital: impact of infection control measures for restraining their dissemination. J Clin Microbiol 50:2618–2623. doi: 10.1128/JCM.00459-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Steinmann J, Kaase M, Gatermann S, Popp W, Steinmann E, Damman M, Paul A, Saner F, Buer J, Rath PM. 2011. Outbreak due to a Klebsiella pneumoniae strain harbouring KPC-2 and VIM-1 in a German university hospital, July 2010 to January 2011. Euro Surveill 16(33):pii=19944 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19944. [PubMed] [Google Scholar]
- 170.Zhou J, Li G, Ma X, Yang Q, Yi J. 2015. Outbreak of colonization by carbapenemase-producing Klebsiella pneumoniae in a neonatal intensive care unit: investigation, control measures and assessment. Am J Infect Control 43:1122–1124. doi: 10.1016/j.ajic.2015.05.038. [DOI] [PubMed] [Google Scholar]
- 171.Centers for Disease Control and Prevention. 2011. Carbapenem-resistant Klebsiella pneumoniae associated with a long-term-care facility—West Virginia, 2009-2011. MMWR Morb Mortal Wkly Rep 60:1418–1420. [PubMed] [Google Scholar]
- 172.Abboud CS, de Souza EE, Zandonadi EC, Borges LS, Miglioli L, Monaco FC, Barbosa VL, Cortez D, Bianco AC, Braz A, Monteiro J. 2016. Carbapenem-resistant Enterobacteriaceae on a cardiac surgery intensive care unit: successful measures for infection control. J Hosp Infect 94:60–64. doi: 10.1016/j.jhin.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 173.Chen S, Hu F, Liu Y, Zhu D, Wang H, Zhang Y. 2011. Detection and spread of carbapenem-resistant Citrobacter freundii in a teaching hospital in China. Am J Infect Control 39:e55–e60. doi: 10.1016/j.ajic.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 174.Clarivet B, Grau D, Jumas-Bilak E, Jean-Pierre H, Pantel A, Parer S, Lotthé A. 2016. Persisting transmission of carbapenemase-producing Klebsiella pneumoniae due to an environmental reservoir in a university hospital, France, 2012 to 2014. Euro Surveill 21(17):pii=30213. doi: 10.2807/1560-7917.ES.2016.21.17.30213. [DOI] [PubMed] [Google Scholar]
- 175.Marra AR, de Almeida SM, Correa L, Silva M Jr, Martino MDV, Silva CV, Rodrigues Cal RG, Edmond MB, dos Santos OFP. 2009. The effect of limiting antimicrobial therapy duration on antimicrobial resistance in the critical care setting. Am J Infect Control 37:204–209. doi: 10.1016/j.ajic.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 176.Domínguez Jiménez MC, Vergara-López S. 2016. Environmental sampling protocol devised and implemented to resolve a nosocomial outbreak due to carbapenem-resistant Klebsiella oxytoca. Am J Infect Control 44:1401–1403. doi: 10.1016/j.ajic.2016.04.219. [DOI] [PubMed] [Google Scholar]
- 177.Kotsanas D, Wijesooriya WRPLI, Korman TM, Gillespie EE, Wright L, Snook K, Williams N, Bell JM, Li HY, Stuart RL. 2013. “Down the drain”: carbapenem-resistant bacteria in intensive care unit patients and handwashing sinks. Med J Aust 198:267–269. doi: 10.5694/mja12.11757. [DOI] [PubMed] [Google Scholar]
- 178.Leung GHY, Gray TJ, Cheong EYL, Haertsch P, Gottlieb T. 2013. Persistence of related bla-IMP-4 metallo-beta-lactamase producing Enterobacteriaceae from clinical and environmental specimens within a burns unit in Australia—a six-year retrospective study. Antimicrob Resist Infect Control 2:35. doi: 10.1186/2047-2994-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hu L, Liu Y, Deng L, Zhong Q, Hang Y, Wang Z, Zhan L, Wang L, Yu F. 2016. Outbreak by ventilator-associated ST11 K. pneumoniae with co-production of CTX-M-24 and KPC-2 in a SICU of a tertiary teaching hospital in central China. Front Microbiol 7:1190. doi: 10.3389/fmicb.2016.01190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Marsh JW, Krauland MG, Nelson JS, Schlackman JL, Brooks AM, Pasculle AW, Shutt KA, Doi Y, Querry AM, Muto CA, Harrison LH. 2015. Genomic epidemiology of an endoscope-associated outbreak of Klebsiella pneumoniae carbapenemase (KPC)-producing K. pneumoniae. PLoS One 10:e0144310. doi: 10.1371/journal.pone.0144310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Epstein L, Hunter JC, Arwady MA, Tsai V, Stein L, Gribogiannis M, Frias M, Guh AY, Laufer AS, Black S, Pacilli M, Moulton-Meissner H, Rasheed JK, Avillan JJ, Kitchel B, Limbago BM, MacCannell D, Lonsway D, Noble-Wang J, Conway J, Conover C, Vernon M, Kallen AJ. 2014. New Delhi metallo-β-lactamase-producing carbapenem-resistant Escherichia coli associated with exposure to duodenoscopes. JAMA 312:1447–1455. doi: 10.1001/jama.2014.12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chang CL, Su LH, Lu CM, Tai FT, Huang YC, Chang KK. 2013. Outbreak of ertapenem-resistant Enterobacter cloacae urinary tract infections due to a contaminated ureteroscope. J Hosp Infect 85:118–124. doi: 10.1016/j.jhin.2013.06.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.