ABSTRACT
Drug efflux pumps play important roles in intrinsic and acquired drug resistance. Verapamil, an efflux inhibitor that enhances the activity of bedaquiline, clofazimine, and other drugs against Mycobacterium tuberculosis, has been proposed as a potential adjunctive agent for treatment of tuberculosis (TB). However, the extent to which verapamil enhances in vivo efficacy by inhibiting bacterial efflux pumps versus inhibiting mammalian drug transporters to improve oral bioavailability has not been delineated. We found that verapamil potentiated the in vitro activity of bedaquiline and clofazimine against M. tuberculosis clinical isolates, including those harboring rv0678 mutations. Verapamil increased the efficacy of bedaquiline in a murine TB model by the same extent to which it increased systemic bedaquiline exposure. However, verapamil showed no effect on the oral bioavailability or efficacy of clofazimine in mice. The addition of verapamil increased the sterilizing activity of a regimen composed of bedaquiline, clofazimine, and pyrazinamide. These results confirm that verapamil has adjunctive activity in vivo, but they also demonstrate that the adjunctive effect is likely due to enhanced systemic exposure to companion drugs via effects on mammalian transporters, rather than inhibition of bacterial pumps. Therefore, there may be no advantage to administering verapamil versus increasing the doses of companion drugs.
KEYWORDS: Mycobacterium tuberculosis, bedaquiline, bioavailability, efflux pump, verapamil
INTRODUCTION
Bedaquiline was conditionally approved by the U.S. Food and Drug Administration as part of combination therapy for the treatment of multidrug-resistant tuberculosis (MDR-TB) in late 2012 (1). Mutations in rv0678, which encodes the transcriptional repressor of the mycobacterial MmpS5-MmpL5 transporter, were subsequently reported to cause cross resistance to bedaquiline and clofazimine (2–4), implying a role for drug efflux in acquired resistance to both drugs. In vitro studies also suggested a role for drug efflux in intrinsic resistance to these agents, as the efflux pump inhibitor verapamil decreased the MICs of bedaquiline and clofazimine against Mycobacterium tuberculosis H37Rv by 4- to 16-fold (3, 5, 6). Verapamil was further evaluated as an adjunctive therapeutic agent in murine models of tuberculosis (TB) treatment (7, 8). Gupta et al. found that 12.5 mg/kg verapamil augmented the bactericidal activity of bedaquiline in an aerosol infection model in BALB/c mice, where it increased the effect of 12.5 mg/kg bedaquiline to a greater extent than it increased the effect of 25 mg/kg bedaquiline (8). Similarly, Andries et al. showed that 25 mg/kg verapamil increased the bactericidal effect of 6.25 mg/kg bedaquiline but not that of 50 mg/kg bedaquiline in a more acute intravenous infection model in Swiss mice (3). The seemingly discrepant results with different bedaquiline doses may be explained by previous results suggesting that 25 mg/kg bedaquiline approaches the maximally effective dose (9).
Potentiation of antibiotic activity by verapamil in vivo could be due to increasing oral bioavailability through inhibition of drug transporters in the gut and/or liver (10), decreasing efflux from infected macrophages (11, 12), and/or decreasing efflux from mycobacteria (13, 14). It is important to elucidate the extent to which in vivo potentiation by verapamil is mediated by enhanced oral bioavailability versus reduced efflux from mycobacteria and infected host cells, since the former effect may be reproduced by simply increasing the dose of the drug of interest and avoiding verapamil exposure altogether. Therefore, we set out to determine whether verapamil increases plasma bedaquiline and clofazimine concentrations and, if it does, the extent to which the increase in systemic exposure might explain its in vivo potentiating effect.
Clofazimine kills M. tuberculosis synergistically with bedaquiline through increased production of reactive oxygen species (15). The combination of bedaquiline and clofazimine leads to significantly better bactericidal and sterilizing activity in a mouse TB model, although their combined use can lead to selective amplification of cross-resistant mutants with mutations in rv0678 or pepQ (6, 16, 17). The addition of pyrazinamide to the bedaquiline-clofazimine combination results in even more rapid sterilizing activity (6, 16). Therefore, we hypothesized that the addition of verapamil would further potentiate the activity of bedaquiline plus clofazimine plus pyrazinamide (BCZ). If this hypothesis is true and the effect is greater than that expected to result from enhanced oral bioavailability of companion drugs with verapamil, then this combination could constitute the nucleus of new, highly active, TB treatment regimens.
RESULTS
Verapamil decreases the MICs of bedaquiline and clofazimine in vitro.
To evaluate the effects of verapamil on the activities of bedaquiline and clofazimine, we determined their MICs against laboratory strains and clinical isolates with different susceptibilities to each drug, in the presence or absence of verapamil. The MIC of verapamil against M. tuberculosis H37Rv was 180 μg/ml. At 50 μg/ml, verapamil decreased the MICs of bedaquiline and clofazimine against all strains tested, irrespective of clofazimine and bedaquiline susceptibility, although the magnitude of the verapamil effect varied from strain to strain (Table 1). On average, verapamil caused greater reductions in the MICs of bedaquiline than those of clofazimine (fold reductions [mean ± standard deviation] in MICs for all strains of 9.1 ± 6.0 and 3.3 ± 1.7 for bedaquiline and clofazimine, respectively; P < 0.01).
TABLE 1.
MICs for clofazimine and bedaquiline against M. tuberculosis laboratory strains and clinical isolates in the absence and presence of verapamil
| Strain | MIC (μg/ml)a |
Fold change in MIC, C/V+C | MIC (μg/ml) |
Fold change in MIC, B/V+B | Genotypeb |
Drug resistancec | |||
|---|---|---|---|---|---|---|---|---|---|
| C | V+C | B | V+B | rv0678 | atpE | ||||
| H37Rv | 0.24 | 0.06 | 4.0 | 0.06 | 0.0075 | 8.0 | WT | WT | Susceptible |
| 13529 | 1.03 | 0.421 | 2.4 | 0.151 | 0.022 | 6.9 | WT | WT | INH, RIF, STR, OFX, LVX |
| 12897 | 0.9 | 0.244 | 3.7 | 0.19 | 0.016 | 11.9 | WT | WT | INH, RIF, STR, EMB |
| 16119 | 0.6 | 0.341 | 1.8 | 0.191 | 0.008 | 23.9 | WT | WT | INH |
| 11195 | 0.53 | 0.276 | 1.9 | 0.095 | 0.012 | 7.9 | WT | WT | INH, RIF, OFX, LVX, ETO |
| 16839 | 0.25 | 0.06 | 4.2 | 0.049 | 0.017 | 2.9 | WT | WT | INH, RIF, OFX, LVX |
| H37Rv-Cr | 7.48 | 0.941 | 7.9 | 3.658 | 0.335 | 10.9 | C148T (R50W) | WT | ND |
| 12657 | 2.09 | 1.075 | 1.9 | 0.39 | 0.028 | 13.9 | C158T (S53L) | WT | INH, RIF, STR, EMB, OFX, LVX, ETO |
| 10149 | 1.2 | 0.346 | 3.5 | 0.78 | 0.209 | 3.7 | T437C (M146T) | WT | INH, RIF, EMB, CAP, AMK, OFX, LVX, ETO |
| 13476 | 4.16 | 1.6 | 2.6 | 1.54 | ND | ND | T350G (L117R) | WT | INH, RIF, STR, EMB, OFX, LVX, CAP, AMK, ETO, PAS |
| 10601 | 4 | 1.351 | 3.0 | 0.732 | 0.15 | 4.9 | G5T (S2I) | WT | INH, RIF, STR, EMB, OFX, LVX, ETO |
| 13480 | 2.77 | 1.049 | 2.6 | 0.757 | 0.153 | 4.9 | G215A (R72Q) | WT | INH, RIF, STR, EMB, OFX, LVX, ETO, PAS |
The verapamil concentration was 50 μg/ml. C, clofazimine; V, verapamil; B, bedaquiline; ND, not done.
WT, wild type.
Drug resistance was defined as resistance to any of the following drugs: isoniazid (INH), rifampin (RIF), streptomycin (STR), ethambutol (EMB), ofloxacin (OFX), levofloxacin (LVX), capreomycin (CAP), amikacin (AMK), ethionamide (ETO), or p-aminosalicylic acid (PAS), using the absolute concentration method, with 0.2, 40, 10, 2, 2, 2, 40, 30, 40, and 1 g/ml, respectively, as the critical concentrations on Löwenstein-Jensen medium.
Verapamil increases the bioavailability of bedaquiline but not clofazimine in mice.
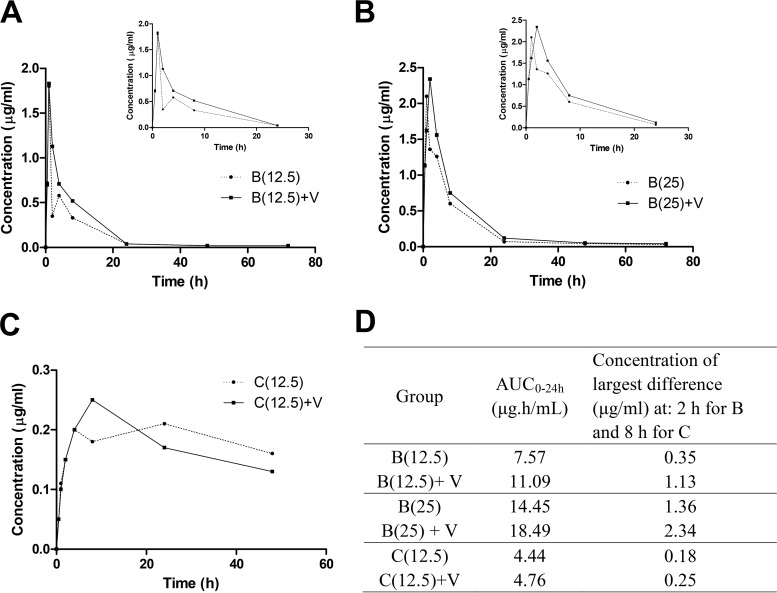
To test whether verapamil affects the pharmacokinetic (PK) profiles of clofazimine and bedaquiline in mice, we evaluated the bedaquiline and clofazimine PK profiles after a single oral dose in the presence or absence of verapamil. As shown in Fig. 1, the area under the plasma concentration-time curve over 24 h (AUC0–24) for bedaquiline at 12.5 mg/kg was increased by 46% (from 7.57 to 11.09 μg · h/ml) upon coadministration with verapamil. Similarly, the AUC0–24 for bedaquiline at 25 mg/kg was increased by 28% (from 14.45 to 18.49 μg · h/ml) upon coadministration with verapamil. The largest differences in median concentrations at any time point were 217.6% for 12.5 mg/kg bedaquiline and 72.6% for 25 mg/kg bedaquiline at 2 h postdose. For clofazimine, the largest difference in median concentration was 35.6% at 8 h. However, the AUC0–24 of clofazimine did not change significantly with verapamil coadministration.
FIG 1.
Pharmacokinetic profiles of bedaquiline and clofazimine, with or without verapamil, in mice. (A to C) Median plasma concentration-time profiles for 12.5 mg/kg bedaquiline [B(12.5)] (A), 25 mg/kg bedaquiline [B(25)] (B), and 12.5 mg/kg clofazimine [C(12.5)] (C) after administration alone or with verapamil (V). The insets in panels A and B depict the first 24 h only, to allow clearer inspection of the curves at the early time points for bedaquiline. (D) Pharmacokinetic parameters calculated from the profiles, using the median concentration at each time point.
Verapamil enhances the efficacy of bedaquiline but not clofazimine in a murine model of tuberculosis.
To determine whether verapamil increases the in vivo bactericidal activity of bedaquiline by the same degree to which it increases bedaquiline bioavailability, we compared the effect of adding verapamil to bedaquiline to the effect of increasing the bedaquiline dose to match the increase in bedaquiline AUC caused by adding verapamil, in a mouse infection model. The mean lung CFU count on the day after aerosol infection (day −13) was 3.98 ± 0.09 log10 CFU. The bacterial burden at the start of the treatment 14 days postinfection (day 0) was 7.95 ± 0.25 log10 CFU. As expected, 12.5 mg/kg verapamil alone had no activity. After 2 weeks of treatment, untreated mice and those in the verapamil-only group became moribund and required euthanasia; lung CFU counts had reached 9.62 ± 0.48 log10 CFU and 9.57 ± 0.08 log10 CFU, respectively (Table 2). After 4 weeks of treatment, 12.5 mg/kg bedaquiline alone reduced the lung CFU count by 3.9 log10 CFU, compared to day 0, whereas 12.5 mg/kg bedaquiline with verapamil reduced the CFU count by 4.7 log10 CFU (Student's t test, P < 0.05). The bactericidal effect of 12.5 mg/kg bedaquiline with verapamil was very similar to that of 18 mg/kg bedaquiline alone (P = 0.3). The lung CFU count in mice that received 12.5 mg/kg clofazimine was similar to that of mice that received 12.5 mg/kg clofazimine plus verapamil (P = 0.4). Taken together, these results suggest that the potentiation of bedaquiline, but not clofazimine, activity by verapamil in mice is mediated primarily by increased oral bioavailability of bedaquiline, but not clofazimine, upon verapamil coadministration and that any other effects of verapamil on intracellular and/or intrabacillary drug concentrations are less important.
TABLE 2.
Lung CFU counts assessed during treatment and proportions of mice relapsing after treatment completion
| Treatment | Log10 CFU (mean ± SD)a |
% of mice relapsing (no. relapsing/total no.) at week 4(+12) | |||
|---|---|---|---|---|---|
| Day −13 | Day 0 | Week 2 | Week 4 | ||
| None | 3.98 ± 0.09 | 7.95 ± 0.25 | 9.57 ± 0.08 | ||
| Verapamil | 9.62 ± 0.48 | ||||
| Bedaquiline at 12.5 mg/kg | 4.02 ± 0.18 | ||||
| Bedaquiline at 12.5 mg/kg plus verapamil | 3.29 ± 0.73 | ||||
| Bedaquiline at 18 mg/kg | 3.52 ± 0.33 | ||||
| Clofazimine at 12.5 mg/kg | 5.32 ± 0.18 | ||||
| Clofazimine at 12.5 mg/kg plus verapamil | 5.42 ± 0.16 | ||||
| BCZ | 0.84 ± 0.28 | 60 (6/10) | |||
| BCZV | 0.63 ± 0.39 | 27 (4/15) | |||
Time points are shown as days (day −13 or day 0) or weeks (week 2 or week 4) of treatment. Week 4(+12) indicates that the mice were held for 12 additional weeks after completing 4 weeks of treatment.
Relapse after treatment completion.
The addition of verapamil did not significantly increase the activity of BCZ after treatment for 4 weeks (P = 0.2). However, very few CFU were detected in mice in either group. To evaluate for durable cure of the infection, we assessed the relapse rates 12 weeks after BCZ or bedaquiline plus clofazimine plus pyrazinamide plus verapamil (BCZV) treatment. Five mice allocated to receive BCZ died early in the treatment phase, due to cage flooding. Of the remaining BCZ-treated mice, 6 (60%) of 10 experienced relapse, whereas only 4 (27%) of 15 BCZV-treated mice experienced relapse (Fisher's exact test, P < 0.01) (Table 2). These results indicate that verapamil potentiated the sterilizing activity of the BCZ combination in vivo. Considering that more than five-sixths of each lung homogenate was plated on bedaquiline-free medium, there was less than a 1 in 6 chance that a mouse relapsing with only a single CFU in the entire lung would go undetected. Since the lung CFU counts of relapsing mice after treatment with BCZ and BCZV were much higher (3.70 ± 0.58 and 3.63 ± 0.29 log10 CFU, respectively), it is unlikely that any relapsing mouse was missed with our plating scheme.
To investigate whether verapamil affects the selection of mutants with reduced bedaquiline susceptibility, the lung homogenates of BCZ- and BCZV-treated mice were also plated on selective 7H11 agar containing 0.06 μg/ml bedaquiline. No growth was observed on bedaquiline-containing plates at the end of 4 weeks of BCZ or BCZV treatment. Colonies on bedaquiline-containing plates were found for 3 of 4 mice experiencing relapse after 4 weeks of treatment with BCZV, representing approximately 0.5 to 6.1% of the total CFU count. Growth was observed on bedaquiline-containing plates for all mice (6/6 mice) experiencing relapse 12 weeks after treatment with BCZ, representing 0.5 to 5.7% or 66.7% of the total CFU count. However, amplification of DNA and sequencing of colonies revealed a pepQ mutation (insertion of G at nucleotide position 812) in the isolate from the one mouse in the BCZ group with 66.7% of the total CFU count on bedaquiline-containing plates. No pepQ mutations were found in other isolates from the BCZ and BCZV groups, and mutations in rv0678 were not found in isolates from either group.
DISCUSSION
The potentiation of the in vitro and in vivo activities of rifampin, bedaquiline, and clofazimine by verapamil, a FDA-approved antihypertensive drug, has led to interest in using verapamil as an adjunctive agent in TB therapy (5, 7, 8, 14, 19). By acting on bacterial efflux transporters to reduce drug efflux, verapamil could counteract efflux-based mechanisms of both intrinsic and acquired resistance. Although verapamil has been shown to decrease the MICs of bedaquiline and clofazimine against M. tuberculosis H37Rv and resistant mutants selected in an H37Rv background (3, 5, 6), there has been only one report of its effects on clinical isolates. Gupta et al. reported that verapamil reduced bedaquiline MICs against bedaquiline-susceptible clinical isolates from Mali by 8- to 16-fold (5). Furthermore, there are no reports of verapamil's drug-potentiating effects on clinical isolates harboring rv0678 mutations. In our study, we used 10 clinical isolates from China, 5 of which had mutations in the rv0678 gene and 4 of which had bedaquiline MICs of >0.5 μg/ml, and we tested the effects of verapamil on both bedaquiline and clofazimine MICs. We found that verapamil consistently reduced the MICs of both drugs, irrespective of baseline susceptibility, but it produced greater reductions in the MICs of bedaquiline than in those of clofazimine.
In order to exert its effect on mycobacterial drug transporters in vivo, verapamil must reach adequate concentrations at the site of infection. On its own, verapamil has very weak activity against M. tuberculosis H37Rv in vitro, as evidenced by the MIC of 180 μg/ml against our H37Rv strain. Verapamil potentiates bedaquiline and clofazimine at lower concentrations of 40 to 50 μg/ml. However, the maximum concentration (Cmax) in serum and the AUC of verapamil after a dose of 12.5 mg/kg in mice are approximately 0.5 μg/ml and 0.8 μg · h/ml, respectively, compared to a similar human AUC of 0.8 μg · h/ml observed when 240 mg of verapamil was administered with food (7). Therefore, we did not expect this dose to have anti-TB activity when used alone in mice. Indeed, verapamil monotherapy was equivalent to no treatment. This finding confirms and extends previous findings in mice treated with a dose of verapamil lower than that used in our study (19). Verapamil also failed to increase the bactericidal effect of clofazimine in mice although verapamil decreases the MIC of clofazimine in vitro by 4-fold. This discrepancy may be similarly explained by the fact that the plasma Cmax of verapamil in mice is 100 times lower than the concentration used to potentiate clofazimine activity in vitro. Verapamil also had no effect on the oral bioavailability of clofazimine in mice.
Unlike the findings with clofazimine, verapamil did potentiate the bactericidal activity of bedaquiline against M. tuberculosis in mice and appeared to do so by increasing the oral bioavailability of bedaquiline. Coadministration of 12.5 mg/kg bedaquiline with verapamil increased the plasma exposure for bedaquiline by 46% after a single oral dose. Our mouse efficacy study showed that coadministration of verapamil with 12.5 mg/kg bedaquiline produced the same bactericidal effect as did simply increasing the bedaquiline dose by 46% to 18 mg/kg. Since the bedaquiline AUC increases in a linear fashion over the dose range of 6.25 to 25 mg/kg (9), the bedaquiline AUC at 18 mg/kg should be equivalent to that produced by 12.5 mg/kg bedaquiline administered with verapamil in our PK study. Therefore, we conclude that the potentiation of bedaquiline by verapamil in mice is likely due to the increase in bedaquiline oral bioavailability alone and not the effect of verapamil on bacterial efflux transporters, and there may be no advantage to administering verapamil versus simply increasing the doses of companion drugs.
Shortening the duration of TB treatment and achieving stable cures without relapse are major goals of new drug and drug regimen development programs. Our result indicating that the addition of verapamil to BCZ reduced the proportion of mice relapsing after 4 weeks of treatment suggests that verapamil could help to shorten the treatment of TB. However, the preceding evidence suggests that the addition of verapamil was tantamount to increasing the bedaquiline dose by approximately 25 to 50%. It was recently shown that adding verapamil also potentiated the activity of rifampin and the first-line TB treatment regimen in mice (7, 19). However, verapamil is also known to increase the oral bioavailability of rifampin (20), and it is reasonable to suspect that this increase in systemic rifampin exposure, rather than the inhibition of bacterial efflux pumps, was responsible for verapamil's potentiating effects in vivo. This issue should be addressed experimentally before clinical trials evaluating the potentiating activity of verapamil are warranted.
In our study, we found an isolate with a pepQ mutation conferring reduced susceptibility to bedaquiline in one mouse from the BCZ-treated group relapsing 12 weeks after treatment. Mutations in pepQ confer low-level resistance to bedaquiline and clofazimine in M. tuberculosis, which appears to be mediated by drug efflux and is reduced in the presence of verapamil in vitro (6). Almeida et al. previously isolated a mutant with the same G812 insertion from a mouse treated with bedaquiline plus clofazimine and confirmed an 8-fold increase in the bedaquiline MIC (17), suggesting that this could be a common mutation in pepQ associated with reduced bedaquiline susceptibility. Gupta et al. also isolated strains with reduced susceptibility to bedaquiline from 2 of 10 mice treated with bedaquiline for 6 weeks but not from any of the 10 mice treated with bedaquiline plus verapamil (8). Although the numbers are small, these data support the idea that greater bedaquiline exposure may be useful for more quickly eradicating mutants with reduced bedaquiline susceptibility. This possibility is also supported by the previously described dose-dependent activity of bedaquiline against both pepQ and rv0678 mutants in vivo (3, 6). Although we think that the greater bedaquiline exposures associated with verapamil coadministration were sufficient to restrict selection of mutants with low-level reduced bedaquiline susceptibility, we cannot exclude the possibility that direct effects of verapamil on bacterial efflux pumps contributed to the observed results. Because bedaquiline and clofazimine accumulate in tissues with repeat dosing, one limitation of the present study is that we did not perform PK studies during treatment to document drug concentrations at steady state and to show that they are still equivalent in mice receiving verapamil plus bedaquiline at 12.5 mg/kg versus bedaquiline alone at 18 mg/kg and in mice receiving verapamil plus clofazimine at 12.5 mg/kg versus clofazimine alone at 12.5 mg/kg.
MATERIALS AND METHODS
Mycobacterial strains.
M. tuberculosis H37Rv was used for in vitro studies and mouse infection. Strain H37Rv-Cr was isolated after repeated subculture of M. tuberculosis H37Rv on selective 7H11 medium containing clofazimine at concentrations ranging from 0.5× MIC to 32× MIC. Ten clinical isolates were collected from individual patients attending Beijing Chest Hospital. Five of the isolates were resistant to clofazimine without known prior exposure of the patient to clofazimine or bedaquiline (4). All clofazimine-resistant mutants harbored rv0678 mutations resulting in the substitution of a single amino acid and resistance to bedaquiline as well as clofazimine. Middlebrook 7H9 broth with 0.2% glycerol, 10% oleic acid-albumin-dextrose-catalase (OADC) (Becton-Dickinson), and 0.05% Tween 80 was used for in vitro cultivation.
Antimicrobials.
Bedaquiline, clofazimine, and verapamil were obtained and formulated for oral administration as described previously (4, 6). Pyrazinamide was purchased from Acros Organics (Thermo Fisher Scientific). Verapamil was purchased from Sigma (St. Louis, MO).
MIC determination.
MICs of bedaquiline and clofazimine were determined in the presence and absence of 50 μg/ml verapamil with the microplate alamarBlue assay (MABA), as described previously (4, 5, 18).
DNA sequencing.
Genomic DNA from M. tuberculosis was isolated and subjected to PCR amplification using rv0678, pepQ, and atpE primers, and the PCR products were sequenced as described previously (4).
Pharmacokinetics of bedaquiline and clofazimine, with or without verapamil.
All animal procedures were approved by the Johns Hopkins University Animal Care and Use Committee. Single doses of clofazimine at 12.5 mg/kg or bedaquiline at 12.5 mg/kg or 25 mg/kg were administered per os, by gavage, either alone or in combination with verapamil at 12.5 mg/kg, to Swiss mice (5 weeks of age, weighing 19.4 to 21.8 g). Verapamil or vehicle (water) was administered 15 min before bedaquiline or clofazimine. Blood was sampled from 3 mice per group per time point, at 0.5, 1, 2, 4, 8, 24, 48, and 72 h after bedaquiline or clofazimine dosing. Drug concentrations in plasma were determined by validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) assays. The clofazimine standard curve was from 0.020 to 2.00 μg/ml, and the bedaquiline curve was from 0.010 to 2.00 μg/ml. For clofazimine, the within-sample precision (as coefficient of variance [%CV]) was 5.30% and the overall precision for all validation standard curves ranged from 3.83% to 10.30%. For bedaquiline, the within-sample precision was 3.87% and the overall precision for all validation standard curves ranged from 5.62% to 12.31%. A noncompartmental analysis was performed using Phoenix software version 6.2. We used AUC0–24 values for comparison, since the drugs were given every 24 h for evaluation of activity in vivo.
Aerosol infection model.
Female BALB/c mice (5 to 6 weeks of age) were infected with M. tuberculosis H37Rv using the inhalation exposure system (Glas-Col, Terre Haute, IN) and a fresh log-phase broth culture with an optical density at 600 nm of approximately 0.9. Two and three mice from each aerosol infection run were sacrificed 1 day after infection (day −13) and on the day of treatment initiation (day 0), respectively, to determine the numbers of CFU implanted in the lungs and present at the start of treatment.
Assessment of treatment efficacy and contribution of verapamil.
Since verapamil increased the AUC0–24 of bedaquiline by 46%, we compared the activity of bedaquiline alone at 18 mg/kg to that of bedaquiline at 12.5 mg/kg with or without verapamil. Although verapamil did not significantly alter the oral bioavailability of clofazimine, it did potentiate the in vitro activity of clofazimine. Therefore, we also investigated whether verapamil potentiated the activity of clofazimine in vivo, despite not increasing systemic exposures. To determine whether the addition of verapamil would further potentiate the activity of the BCZ combination, we also compared the activity of this regimen with and without verapamil.
Treatment was administered once daily, 5 days per week, by gavage. Test mice received 12.5 mg/kg bedaquiline, 12.5 mg/kg clofazimine, 25 mg/kg bedaquiline plus 12.5 mg/kg clofazimine plus 150 mg/kg pyrazinamide (BCZ), with or without 12.5 mg/kg verapamil, or 18 mg/kg bedaquiline. Control mice went untreated or received 12.5 mg/kg verapamil alone. Lung homogenates were plated in serial 10-fold dilutions on 0.4% charcoal-containing selective 7H11 plates, to reduce carryover effects (21), and were incubated for 6 weeks before final CFU counts were determined. Lung CFU counts were assessed in 5 mice per treatment group at each time point.
Cohorts of 15 mice treated with either BCZ or BCZV were held for an additional 12 weeks after completing 4 weeks of treatment [week 4(+12)] before sacrifice, to determine the proportions of mice relapsing with detectable CFU in the lungs. Lungs were homogenized in 3 ml of phosphate-buffered saline (PBS), and 2.5 ml of each lung homogenate was plated onto 5 selective 7H11 plates for relapse assessment, at least 1 of which was supplemented with 0.4% charcoal to control for drug carryover. Therefore, more than 83% of the lung homogenate was plated on bedaquiline-free medium, and the lower limit of detection approached 1 CFU per lung. Positive lung cultures were defined with ≥1 CFU of M. tuberculosis detected on any plate.
Evaluation of resistance selection.
The remaining 0.5 ml of lung homogenate from each mouse in the BCZ- and BCZV-treated groups was plated on selective 7H11 agar containing 0.06 μg/ml bedaquiline, to determine the proportions of CFU able to grow on bedaquiline at week 4 and at the week 4(+12) relapse time point. Colonies were selected for analysis by PCR and DNA sequencing of the rv0678 and pepQ genes, as described previously (6).
Statistical analysis.
Lung CFU counts (x) were log transformed (as x + 1) before analysis, and mean CFU counts were compared using Student's t test. The proportions of mice relapsing were compared using Fisher's exact test. All analyses were performed with GraphPad Prism version 5 (GraphPad, San Diego, CA).
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health (grant R01-AI111992), the Beijing Natural Science Foundation (grant 7164246), the National S&T Major Special Project on Major New Drug Innovations (grant 2015ZX09102007-015), the Beijing Municipal Administration of Hospitals' Youth Programme (grant QML20161603), and the China Scholarship Council (grant 201609110001).
REFERENCES
- 1.Cohen J. 2013. Infectious disease: approval of novel TB drug celebrated—with restraint. Science 339:130. doi: 10.1126/science.339.6116.130. [DOI] [PubMed] [Google Scholar]
- 2.Hartkoorn RC, Uplekar S, Cole ST. 2014. Cross-resistance between clofazimine and bedaquiline through upregulation of MmpL5 in Mycobacterium tuberculosis. Antimicrob Agents Chemother 58:2979–2981. doi: 10.1128/AAC.00037-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andries K, Villellas C, Coeck N, Thys K, Gevers T, Vranckx L, Lounis N, de Jong BC, Koul A. 2014. Acquired resistance of Mycobacterium tuberculosis to bedaquiline. PLoS One 9:e102135. doi: 10.1371/journal.pone.0102135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu J, Wang B, Hu M, Huo F, Guo S, Jing W, Nuermberger E, Lu Y. 2017. Primary clofazimine and bedaquiline resistance among isolates from patients with multidrug-resistant tuberculosis. Antimicrob Agents Chemother 61:e00239-. doi: 10.1128/AAC.00239-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta S, Cohen KA, Winglee K, Maiga M, Diarra B, Bishai WR. 2014. Efflux inhibition with verapamil potentiates bedaquiline in Mycobacterium tuberculosis. Antimicrob Agents Chemother 58:574–576. doi: 10.1128/AAC.01462-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Almeida D, Ioerger T, Tyagi S, Li S-Y, Mdluli K, Andries K, Grosset J, Sacchettini J, Nuermberger E. 2016. Mutations in pepQ confer low-level resistance to bedaquiline and clofazimine in Mycobacterium tuberculosis. Antimicrob Agents Chemother 60:4590–4599. doi: 10.1128/AAC.00753-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta S, Tyagi S, Almeida DV, Maiga MC, Ammerman NC, Bishai WR. 2013. Acceleration of tuberculosis treatment by adjunctive therapy with verapamil as an efflux inhibitor. Am J Respir Crit Care Med 188:600–607. doi: 10.1164/rccm.201304-0650OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta S, Tyagi S, Bishai WR. 2015. Verapamil increases the bactericidal activity of bedaquiline against Mycobacterium tuberculosis in a mouse model. Antimicrob Agents Chemother 59:673–676. doi: 10.1128/AAC.04019-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andries K, Verhasselt P, Guillemont J, Gohlmann HW, Neefs JM, Winkler H, Van Gestel J, Timmerman P, Zhu M, Lee E, Williams P, de Chaffoy D, Huitric E, Hoffner S, Cambau E, Truffot-Pernot C, Lounis N, Jarlier V. 2005. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 307:223–227. doi: 10.1126/science.1106753. [DOI] [PubMed] [Google Scholar]
- 10.Shugarts S, Benet LZ. 2009. The role of transporters in the pharmacokinetics of orally administered drugs. Pharm Res 26:2039–2054. doi: 10.1007/s11095-009-9924-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ughachukwu P, Unekwe P. 2012. Efflux pump-mediated resistance in chemotherapy. Ann Med Health Sci Res 2:191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seral C, Carryn S, Tulkens PM, Van Bambeke F. 2003. Influence of P-glycoprotein and MRP efflux pump inhibitors on the intracellular activity of azithromycin and ciprofloxacin in macrophages infected by Listeria monocytogenes or Staphylococcus aureus. J Antimicrob Chemother 51:1167–1173. doi: 10.1093/jac/dkg223. [DOI] [PubMed] [Google Scholar]
- 13.Amaral L, Martins M, Viveiros M. 2007. Enhanced killing of intracellular multidrug-resistant Mycobacterium tuberculosis by compounds that affect the activity of efflux pumps. J Antimicrob Chemother 59:1237–1246. doi: 10.1093/jac/dkl500. [DOI] [PubMed] [Google Scholar]
- 14.Adams KN, Szumowski JD, Ramakrishnan L. 2014. Verapamil, and its metabolite norverapamil, inhibit macrophage-induced, bacterial efflux pump-mediated tolerance to multiple anti-tubercular drugs. J Infect Dis 210:456–466. doi: 10.1093/infdis/jiu095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lamprecht DA, Finin PM, Rahman MA, Cumming BM, Russell SL, Jonnala SR, Adamson JH, Steyn AJ. 2016. Turning the respiratory flexibility of Mycobacterium tuberculosis against itself. Nat Commun 7:12393. doi: 10.1038/ncomms12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams K, Minkowski A, Amoabeng O, Peloquin CA, Taylor D, Andries K, Wallis RS, Mdluli KE, Nuermberger EL. 2012. Sterilizing activities of novel combinations lacking first- and second-line drugs in a murine model of tuberculosis. Antimicrob Agents Chemother 56:3114–3120. doi: 10.1128/AAC.00384-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Almeida D, Li S-Y, Nuermberger E. 2017. Characterisation of mutations causing resistance to bedaquiline and clofazimine in the murine model of tuberculosis, poster Sunday-177. Abstr ASM Microbe 2017. [Google Scholar]
- 18.Collins L, Franzblau SG. 1997. Microplate Alamar blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob Agents Chemother 41:1004–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Louw GE, Warren RM, Gey van Pittius NC, Leon R, Jimenez A, Hernandez-Pando R, McEvoy CR, Grobbelaar M, Murray M, van Helden PD, Victor TC. 2011. Rifampicin reduces susceptibility to ofloxacin in rifampicin-resistant Mycobacterium tuberculosis through efflux. Am J Respir Crit Care Med 184:269–276. doi: 10.1164/rccm.201011-1924OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hosagrahara V, Reddy J, Ganguly S, Panduga V, Ahuja V, Parab M, Giridhar J. 2013. Effect of repeated dosing on rifampin exposure in BALB/c mice. Eur J Pharm Sci 49:33–38. doi: 10.1016/j.ejps.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 21.Tasneen R, Li SY, Peloquin CA, Taylor D, Williams KN, Andries K, Mdluli KE, Nuermberger EL. 2011. Sterilizing activity of novel TMC207- and PA-824-containing regimens in a murine model of tuberculosis. Antimicrob Agents Chemother 55:5485–5492. doi: 10.1128/AAC.05293-11. [DOI] [PMC free article] [PubMed] [Google Scholar]