ABSTRACT
Vitamin D analogs were identified as compounds that induced lysis of planktonic cultures of Streptococcus mutans in a high-throughput screen of FDA-approved drugs. Previous studies have demonstrated that certain derivatives of vitamin D possess lytic activity against other bacteria, though the mechanism has not yet been established. Through the use of a combinatorial approach, the vitamin D derivative doxercalciferol was shown to act synergistically with bacitracin, a polypeptide-type drug that is known to interfere with cell wall synthesis, suggesting that doxercalciferol may act in a bacitracin-related pathway. Innate resistance to bacitracin is attributed to efflux by a conserved ABC-type transporter, which in S. mutans is encoded by the mbrABCD operon. S. mutans possesses two characterized mechanisms of resistance to bacitracin, the ABC transporter, S. mutans bacitracin resistance (Mbr) cassette, consisting of MbrABCD, and the rhamnose-glucose polysaccharide (Rgp) system, RgpABCDEFGHI. Loss of function of the transporter in ΔmbrA and ΔmbrD mutants exacerbated the effect of the combination of doxercalciferol and bacitracin. Despite conservation of a transporter homologous to mbrABCD, the combination of doxercalciferol and bacitracin appeared to be synergistic only in streptococcal species. We conclude that vitamin D derivatives possess lytic activity against S. mutans and act through a mechanism dependent on the bacitracin resistance mechanism of MbrABCD.
KEYWORDS: Streptococcus mutans, vitamin D, bacitracin, drug screening
INTRODUCTION
Streptococcus mutans is the primary etiological agent of dental caries, a disease that affects individuals of all ages, especially those with limited health care accessibility and poor socioeconomic circumstances (1, 2). S. mutans is an early colonizer of the tooth surface that promotes binding of other oral microorganisms to form a biofilm known as dental plaque. As one of its primary virulence mechanisms, S. mutans is acidogenic, resulting in an environmental pH below that at which tooth demineralization occurs (pH ∼5.5) (3–8). The organism is also aciduric and therefore can survive the low-pH environments that it creates, thereby outcompeting other oral organisms to dominate the ecological niche (3–8).
According to the Centers for Disease Control and Prevention, overuse and incorrect prescribing of antibiotics have led to a dangerous increase in acquired resistance in the United States (9), often leaving clinicians with relatively few, often dangerous, treatment options. Efficient high-throughput strategies for identification of novel antibiotic classes, as well as adjuvants that circumvent resistance mechanisms, in combination with thorough characterization of compounds, are essential to successful clinical outcomes. Adjuvants tend to have little to no antimicrobial capability but, in combination with specific drugs, can increase potency and block resistance.
Vitamin D is an essential nutrient and hormone that must be obtained either from the diet or from dermal synthesis. It has essential roles in the absorption of calcium, iron, magnesium, phosphate, and zinc—all of which contribute to the formation of hard tissues, such as enamel and dentin (10). The health benefits of vitamin D are wide ranging, and it has been shown to influence various metabolic systems in the body. Serum vitamin D levels vary slightly depending on ethnicity, region, gender, season, and age, with acceptable values reported to be in the 30- to 68-ng/ml range, whereas deficiency was characterized as <20 ng/ml (<50 nmol/liter) (11). Vitamin D has been suggested to play a role in the etiology of many chronic diseases, so that deficiency is correlated with a negative outcome, as in the cases of rheumatoid arthritis (12), respiratory infections (13), asthma (11), cancer (14, 15), periodontitis (16), and gingivitis (17).
The link between vitamin D levels and caries is multifactorial and includes genetic, environmental, nutritional, and socioeconomic factors. There are detectable amounts of vitamin D in saliva ranging from 105 to 1,000 pg/ml, depending on the individual, diet, and time of day (18). One study found that increased serum vitamin D levels were associated with lower occurrence of dental maladies, including caries and hypomineralization (19). In combination with calcium supplementation, vitamin D has been shown to improve overall periodontal health relative to individuals with no supplementation, in addition to reducing the severity of preexisting cases of periodontitis (20, 21). A review has suggested that increasing serum levels to greater than 40 ng/ml would greatly reduce caries (22), as low serum levels of 25-hydroxyvitamin D [25(OH)D] have been associated with elevated caries (23). Children with severe ECC, a chronic disease with tooth decay, have been found to have significantly lower levels of vitamin D than caries-free children (24). Moreover, studies have reported that prenatal vitamin D was correlated with reduced occurrence of caries in infants and supported development of healthy dentition (25, 26). Interestingly, the evidence linking improved oral health and vitamin D has led to additional work investigating vitamin-coated dental implants to promote surrounding bone mineralization and tissue growth (27, 28).
In addition to contributing to overall well-being, many reports examining the relationship between vitamin D and infection focus on the direct, significant immunomodulatory role of vitamin D (reviewed in reference 29). Vitamin D alters the innate immune response (30), and in turn, immune cells differentially regulate vitamin D-metabolizing enzymes during infection (31). One mechanism for this is stimulation by vitamin D of the production of antimicrobial peptides, such as cathelicidin and human β-defensin 2, as well as stimulation of cell-specific receptors involved in pathogen clearance (32–34).
Single nucleotide polymorphisms of the vitamin D receptor (VDR) gene have been correlated with patients with and without dental caries (35). Other genetic evidence for a connection between vitamin D and oral health includes work demonstrating that vitamin D-associated rickets (also referred to as hypophosphatemic rickets) is resistant to vitamin D supplementation. This condition is, in part, characterized by osteomalacia (a defect in the mineralization of bones), caused by mutation of the vitamin D receptor (36), resulting in severe dental caries (37, 38).
Vitamin D has been associated with bacterial-infection clearance and other disease processes. For example, the link between tuberculosis and vitamin D levels has been investigated since the 1940s and has continued to be the focus of numerous studies (39–41) and reviews (42–44). Vitamin D levels have also been shown to affect the frequency of Staphylococcus aureus infections (45, 46), as well as macrophage clearance of Pseudomonas aeruginosa (47). Interestingly, cytomegalovirus (CMV) has been shown to actively downregulate expression of the vitamin D receptor in host cells (48). A vitamin D decomposition product was shown to have direct bactericidal activity against Helicobacter pylori (49). In a monocyte model, vitamin D inhibited the growth and virulence factor expression of Porphyromonas gingivalis (50). In S. aureus, both vitamin D and E compounds have been shown to interact with antibiotic efflux (51).
Here, we report the identification of vitamin D drug derivatives that exhibit direct bactericidal activity against the cariogenic bacterium S. mutans through a mechanism involving the bacitracin-associated efflux pump MbrA.
RESULTS
A large-scale drug screen revealed that vitamin D analogs are active against S. mutans.
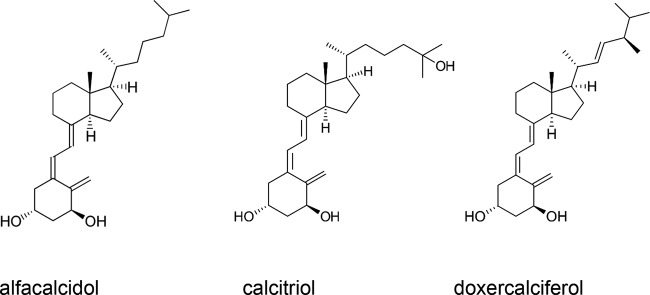
The adenylate kinase (AK) assay was used to screen a library of FDA-approved drugs from Selleck Chemical (Houston, TX) for activity against S. mutans (52). Of the 853 drugs in the Selleck library, we found 126 drugs that significantly induced lysis of planktonic cells (2-fold above background). Surprisingly, one class of compounds that showed activity against S. mutans comprised various derivatives of the fat-soluble secosteroid vitamin D. Analysis of the structures and activities of these compounds revealed that breakage of the steroid ring is required for detection of significant adenylate kinase release, as in the example of lithocholic acid, which did not exhibit bactericidal activity. The three compounds in this class that exhibited the greatest lytic activity against planktonic cells were alfacalcidol, doxercalciferol, and calcitriol (Fig. 1), all possessing two hydroxyl groups on the methylene-cyclohexane ring, implying a potential role for the structure in the activity of the compound. Vitamin D derivatives that did not possess the two hydroxyl groups did not result in significant lysis relative to background.
FIG 1.
Structures of vitamin D analogs that exhibited activity in the AK assay.
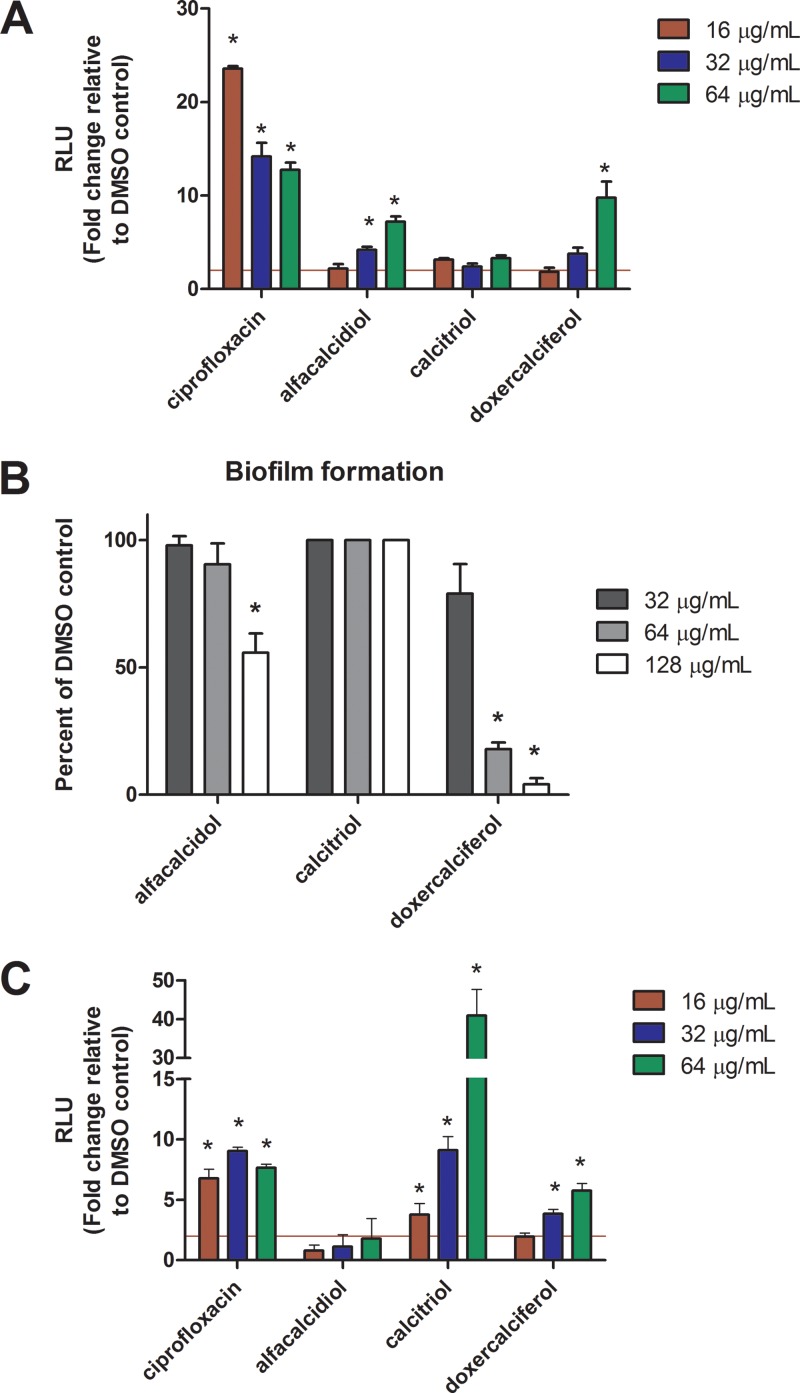
All three compounds inhibited the growth of WT cells at an MIC of 16 μg/ml. In order to further characterize the activities of the vitamin D-like compounds, we tested a range of concentrations using the AK assay. S. mutans planktonic cultures (optical density at 600 nm [OD600], ∼0.5) were exposed to the test drugs or ciprofloxacin (positive control; MIC, 2 μg/ml) ranging from 0.25× to 4× the MIC for 4 h, followed by read-out with the AK assay. These conditions were chosen to mimic the parameters established in our primary screen and to further validate those results. Treatment with alfacalcidol, calcitriol, or doxercalciferol resulted in at least 2-fold-higher signal relative to background (dimethyl sulfoxide [DMSO]) in the AK assay between 1× and 2× MIC (16 to 32 μg/ml), which is in the range of the concentrations used during the initial drug screen (52). Similar to our initial screen, alfacalcidol and doxercalciferol exhibited similar activities against planktonic cultures, whereas calcitriol signal was slightly above the 2-fold-above-background cutoff (52). These results demonstrate that, in agreement with our initial screen, the vitamin D analogs tested exhibited lytic activity against S. mutans.
Vitamin D analogs have activity against biofilms of S. mutans.
S. mutans is found in the oral cavity of humans as part of a multispecies biofilm known as dental plaque. Therefore, it was essential to test whether alfacalcidol, calcitriol, or doxercalciferol had potential to prevent biofilm formation. Despite having similar structures, as well as the ability to lyse planktonic cells, the three vitamin D analogs exhibited the ability to prevent S. mutans biofilm formation. The minimum biofilm inhibitory concentrations (MBIC) of doxercalciferol and alfacalcidol were 64 μg/ml (MBIC90) and 128 μg/ml (MBIC50), respectively, whereas, calcitriol did not inhibit biofilm formation at any concentration tested (Fig. 2B).
FIG 2.
(A) Vitamin D analogs induce adenylate kinase release from S. mutans planktonic cells. S. mutans UA159 cultures were grown in TY medium plus 1% (wt/vol) glucose to exponential phase and used to inoculate fresh medium containing ciprofloxacin (positive control), DMSO (negative control), alfacalcidol, calcitriol, or doxercalciferol at 16, 32, and 64 μg/ml for 4 h. Cell lysis was detected using the AK assay as described in Materials and Methods. The data are represented as relative luminescence units (RLU) normalized to background (DMSO) and are representative of three replicate cultures performed in triplicate. *, P < 0.05; Student's t test, two tailed. (B) Biofilm formation of S. mutans UA159 in the presence of vitamin D analogs. Cultures of S. mutans UA159 were grown in TY medium plus 1% (wt/vol) sucrose to exponential phase and used to inoculate fresh medium containing alfacalcidol, calcitriol, or doxercalciferol at concentrations from 0 to 128 μg/ml. Biofilm formation was quantitated by crystal violet staining as described in Materials and Methods. The values were normalized to DMSO control (n = 3). *, P < 0.05; Student's t test, two tailed. (C) AK assay of biofilm cultures exposed to vitamin D analogs. S. mutans UA159 cultures were seeded in 96-well plate format in TY medium plus 1% (wt/vol) sucrose and grown for 24 h to establish biofilms. The wells were washed with PBS, followed by addition of ciprofloxacin (positive control), DMSO (negative control), alfacalcidol, calcitriol, or doxercalciferol in fresh TYS at 16, 32, and 64 μg/ml for 18 h. Cell lysis was detected using the AK assay as described in Materials and Methods. The data are represented as RLU normalized to background (DMSO) and are representative of three replicates performed in triplicate. The red horizontal line represents the 2-fold cutoff used to classify active compounds (52). *, P < 0.05; Student's t test, two tailed. The error bars indicate standard deviations.
The AK assay also serves as a rapid and sensitive method to detect compounds with activity against preformed biofilms. Unlike the results from the AK assay using planktonic cultures, only calcitriol and doxercalciferol were able to induce lysis of preformed biofilms, suggesting that these similarly structured drugs have different activities under different conditions (compare Fig. 2A and C). Addition of alfacalcidol did not result in significant signal relative to the DMSO control, indicating that it does not possess activity against preformed biofilms. Addition of calcitriol resulted in a 40-fold increase in signal relative to the DMSO control, making it the most active compound tested under these conditions (Fig. 2C). These results demonstrate that analogs of vitamin D are able to prevent S. mutans biofilm formation, as well as having activity against preformed biofilms.
Doxercalciferol exhibits synergistic activity in combination with bacitracin.
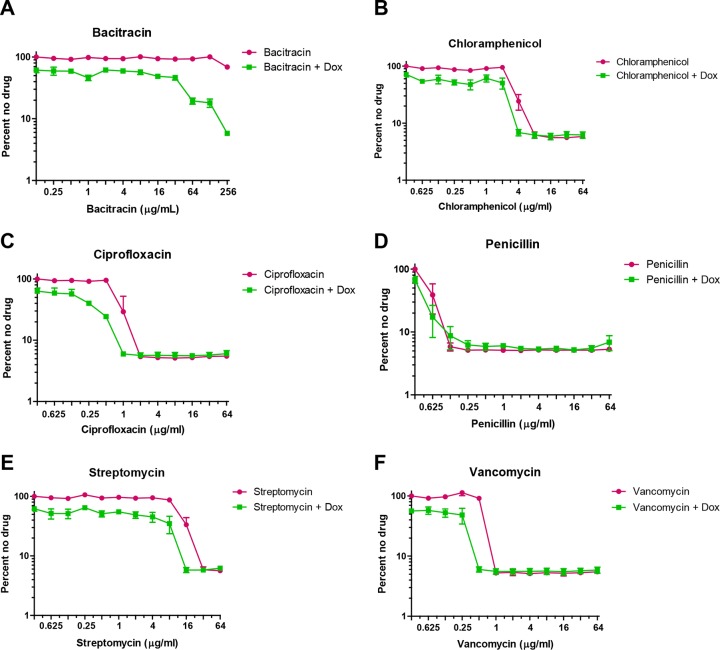
Recently, 1,25(OH)2D3 (vitamin D) was shown to inhibit the growth of the oral pathogen P. gingivalis with a MIC between 3.2 and 6.25 μg/ml (50). In addition to its effect on growth, the vitamin D derivative was shown to potentially interact with a cell wall-targeting antibiotic. In order to dissect the mechanism of growth inhibition by vitamin D on S. mutans, we used a combinatorial approach to assay a 2-fold dilution series of drugs known to target the bacterial cell membrane. As alfacalcidol, doxercalciferol, and calcitriol possessed different activities in initial characterization of the vitamin D derivatives, we proceeded with doxercalciferol, as it had the most consistent activity in our secondary assays. Twofold dilution series of the antibiotics bacitracin, chloramphenicol (control), ciprofloxacin (control), penicillin, streptomycin, and vancomycin were all tested in the presence or absence of doxercalciferol (16 μg/ml) (Fig. 3). The growth of S. mutans in the presence of bacitracin was unaltered relative to the no-drug control, which was expected, as bacitracin resistance in S. mutans has been characterized (53). However, in the presence of doxercalciferol, bacitracin inhibited the growth of S. mutans at concentrations well below that of bacitracin alone (>256 μg/ml). The MICs of chloramphenicol and ciprofloxacin were not altered in the presence of doxercalciferol, suggesting that the combinatorial effect of vitamin D and bacitracin is not a generic phenomenon. Further, the absence of synergy with other tested cell membrane-targeting drugs suggests that the target for vitamin D-mediated growth inhibition might be analogous to pathways of bacitracin resistance.
FIG 3.
Effects of doxercalciferol on the MICs of cell wall-targeting antibiotics. Cultures of S. mutans UA159 were grown in TY medium plus 1% (wt/vol) glucose to exponential phase and used to inoculate fresh medium containing 2-fold serial dilutions of the test drugs (bacitracin, chloramphenicol, penicillin, vancomycin, and streptomycin) in the presence or absence of doxercalciferol (16 μg/ml). The error bars represent the standard deviations of the results of three independent experiments.
The ABC transporter MBR has a role in the interaction between doxercalciferol and bacitracin.
There are two proposed mechanisms for the innate resistance to bacitracin in S. mutans, including rhamnose-glucose polysaccharide (RGP)-associated formation within the cell and the S. mutans-associated bacitracin resistance (MBR) efflux pump (53, 54). In order to distinguish between these mechanisms, we tested the combination of bacitracin and doxercalciferol in S. mutans strains carrying deletions in rgpF (SMU.830) and mbrA (SMU.1006), which encode key subunits associated with each mechanism. We predicted a mutant in the target would be more sensitive to the combination of bacitracin and doxercalciferol than the parent strain, MX804, which is an erythromycin-resistant (Ermr) knock-in strain similar to S. mutans UA159 (Table 1) (7). The erythromycin-selectable marker had no detectable effect on susceptibility to bacitracin or doxercalciferol (data not shown).
TABLE 1.
Strains used in this study
a Strain was obtained from the University of Rochester microbiology laboratory stock collection.
Using a checkerboard approach of serial dilution of bacitracin along the x axis and doxercalciferol along the y axis, we measured the fractional inhibitory concentration (FIC). In the presence of bacitracin alone, the MIC for the parent strain, MX804, was >128 μg/ml, confirming that S. mutans exhibits innate resistance to bacitracin (Table 2). Loss of components of the MBR transporter in the ΔmbrA or ΔmbrD strain resulted in a significant reduction in the bacitracin MIC to 2 μg/ml and 8 μg/ml, respectively, indicating a loss of bacitracin resistance. Similarly, the ΔrgpF strain exhibited a reduction in the MIC of bacitracin, confirming its previously documented role in resistance (53). Strains MX804, ΔmbrA, and ΔrgpF each exhibited an MIC of 16 μg/ml for doxercalciferol, while that for the ΔmbrD strain was 8 μg/ml. The presence of doxercalciferol significantly reduced the amount of bacitracin that inhibited growth of the MX804 (FICI, 0.125), ΔmbrA (FICI, 0.25), and ΔmbrD (FICI, 0.5) strains, indicating a synergistic interaction, according to standard definitions (55). In contrast, the FICI for the combination of bacitracin and doxercalciferol in the ΔrgpF strain was 1.125, indicating no interaction.
TABLE 2.
Fractional inhibitory concentrations of doxercalciferol plus bacitracin in planktonic cultures of S. mutansa
| Strain | MIC of drug (μg/ml) |
FIC (μg/ml) | |||
|---|---|---|---|---|---|
| Alone |
In combination |
||||
| Doxercalciferol | Bacitracin | Doxercalciferol | Bacitracin | ||
| MX804 | 16 | >128 | 4 | 4 | ∼0.125 |
| ΔmbrA | 16 | 2 | 2 | 0.25 | 0.25 |
| ΔmbrD | 8 | 4 | 2 | 1 | 0.5 |
| ΔpmrA | 4 | >128 | 4 | 32 | ∼1.125 |
| ΔrgpF | 16 | 4 | 2 | 4 | 1.125 |
| mbrA+ | 32 | 8 | 4 | 2 | 0.375 |
Cultures were grown in TY medium plus 1% (wt/vol) glucose to exponential phase and used to inoculate fresh medium in a 96-well plate. The fractional inhibitory concentrations for doxercalciferol plus bacitracin were determined as detailed in Materials and Methods. For bacitracin MICs outside the tested range (i.e., >128 μg/ml), a value of 256 μg/ml was used to calculate the FIC.
In order to establish if doxercalciferol was acting as a general efflux pump inhibitor or was specific to bacitracin-associated efflux, we tested the combination of doxercalciferol and bacitracin in a deletion strain lacking a subunit of a well-characterized efflux pump encoded by pmrA. The pmrA transporter has previously been described in Streptococcus pneumoniae and is associated with efflux of fluoroquinolone class antibiotics (56). Addition of doxercalciferol to the ΔpmrA strain in the presence of bacitracin or ciprofloxacin (as a control) resulted in a susceptibility pattern similar to that of MX804 (Table 2). The lack of synergy between doxercalciferol and bacitracin suggests that doxercalciferol is not a general efflux pump inhibitor and may act directly with the MBR efflux pump.
Kinetics of doxercalciferol activity in combination with bacitracin.
Results from the AK assay demonstrated the bactericidal activity of doxercalciferol, and other vitamin D analogs, against S. mutans (Fig. 1), and fractional inhibition studies elucidated an interaction between bacitracin and doxercalciferol (Fig. 3B). In order to further characterize the efficacy of the combination of bacitracin and doxercalciferol, we examined the killing kinetics via a time-kill assay. The MX804, ΔmbrA, and mbrA+ strains were grown to mid-log phase and seeded into fresh TYG medium (see Materials and Methods) with and without bacitracin (32 μg/ml) or doxercalciferol (16 μg/ml). The cells were enumerated at 0, 2, 4, 24, and 48 h after inoculation, as described in Materials and Methods.
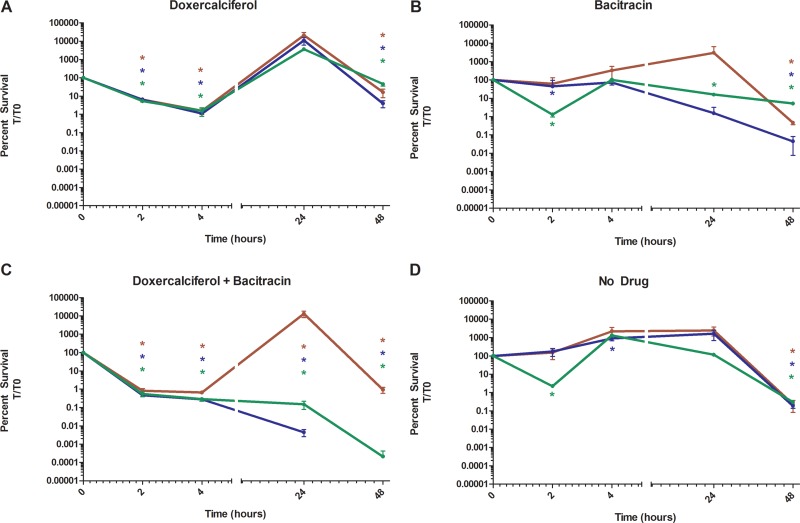
Addition of doxercalciferol to cultures of the MX804, ΔmbrA, or mbrA+ strain resulted in a 3-log-order decrease in survival after 4 h of exposure (Fig. 4A). This finding is in agreement with the results from the AK assay, as significant levels of signal were detected after the 4-h incubation period with drug for that assay (Fig. 2A). Although the initial effect of doxercalciferol appears to be bactericidal, cell counts of all strains were recovered between 4 and 24 h. Bacitracin alone did not alter the survival of MX804 cultures, in contrast to the ΔmbrA strain, whose survival decreased by 2 log orders within 48 h after inoculation (Fig. 4B), confirming the previously described role of the MBR efflux system in bacitracin resistance (53, 57). The viability of the mbrA+ complement strain was intermediate to MX804 and ΔmbrA, indicating that addition of mbrA back into the genome partially restored function with respect to bacitracin resistance.
FIG 4.
Kinetics of doxercalciferol and bacitracin interaction in MX804 (red), ΔmbrA (blue), and mbrA+ (green) strains. Cultures of S. mutans MX804, ΔmbrA, and mbrA+ were grown in TY medium plus 1% (wt/vol) glucose to exponential phase, and 105 CFU was used to inoculate fresh medium. The cultures were serially diluted and plated on BHI agar medium. Drugs were then added to the cultures as follows: doxercalciferol (16 μg/ml) (A), bacitracin (32 μg/ml) (B), a combination of doxercalciferol (16 μg/ml) and bacitracin (32 μg/ml) (C), or no-drug control (D). Aliquots were removed at 2, 4, 24, and 48 h following addition of drug and plated for enumeration. Percent survival was calculated by enumeration of CFU per milliliter at each time point. The data are averages of at least 3 independent replicates and are normalized to CFU per milliliter at time zero for each strain. *, P < 0.05; Student's t test, two tailed. The error bars indicate standard deviations.
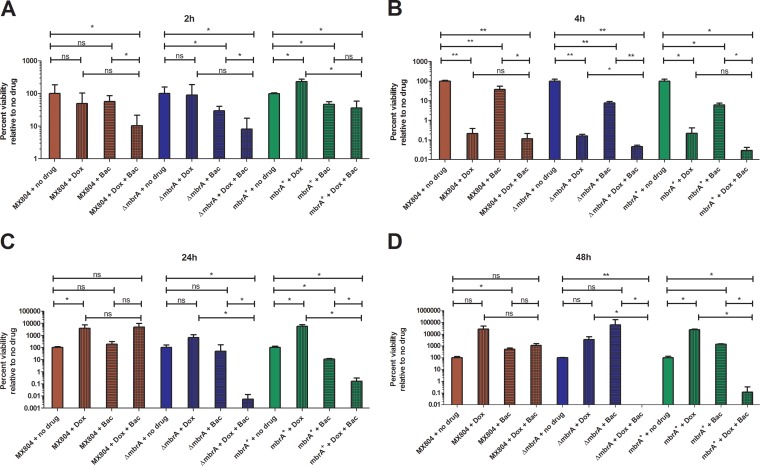
The combination of doxercalciferol and bacitracin resulted in a significant decrease in survival of strain MX804 at 2, 4, and 48 h (Fig. 4C). Interestingly, the CFU at 24 h in the MX804 cultures appeared elevated compared to the earlier time points. However, as shown in Fig. 5C, there was no statistically significant increase relative to the no-drug control. The ΔmbrA strain displayed a similar reduction in viability after 2 and 4 h of exposure to both drugs; however, unlike MX804, it did not recover at 24 h and was nonviable by 48 h. Complementation of the ΔmbrA strain resulted in partial restoration of resistance, observed at 24 and 48 h in the mbrA+ strain. These results are consistent with an interaction between bacitracin and doxercalciferol and suggest that the MBR transporter is involved in this mechanism.
FIG 5.
Doxercalciferol-bacitracin interaction occurs through an MbrA-dependent mechanism. Time-kill experiments were performed as described in the legend to Fig. 4 and Materials and Methods. Aliquots were taken at 2 h (A), 4 h (B), 24 h (C), and 48 h (D) after inoculation. The bars represent cultures with doxercalciferol (16 μg/ml; bars with vertical lines), bacitracin (32 μg/ml; bars with horizontal lines), or a combination of doxercalciferol and bacitracin (bars with crosshatching). Percent survival was calculated by enumeration of CFU per milliliter at each time point and normalized to the no-drug control. The data represent the averages and standard deviations of the results of at least three independent replicates. Note that the y axis is different for each panel. *, P < 0.05; **, P < 0.001; Student's t test, two tailed; ns, not significant.
All of the strains tested (MX804, ΔmbrA, and mbrA+) had similar growth rates and similar reductions in viability between 24 and 48 h (Fig. 4D). In order to determine that doxercalciferol and bacitracin, alone or in combination, were responsible for the decrease in the cell count, we normalized the time-kill data to a no-drug control at each time point to allow assessment of the relative effects of both drugs at individual time points.
After 2 h treatment, addition of bacitracin only did not alter the number of CFU recovered from MX804 cultures, but both the ΔmbrA and mbrA+ strains showed a slight decrease (0.53 and 0.33 log order, respectively) relative to no drug (Fig. 5A, compare the bars with horizontal lines). While there was no significant decrease in viability in cultures to which doxercalciferol was added (Fig. 5A, bars with vertical lines), the combination of bacitracin and doxercalciferol resulted in a greater decrease in recovery of the MX804 (1 log order), ΔmbrA (1 log order), and mbrA+ (0.44 log order) strains relative to no drug (Fig. 5A, compare bars with crosshatching).
All the strains showed a reduction in cell viability of approximately 2.5 log orders after exposure to doxercalciferol alone relative to the no-drug control (Fig. 4B), confirming the results observed with the AK assay (Fig. 2A). Addition of bacitracin resulted in a slight, but significant, reduction in survival of all strains at 4 h. The combination of bacitracin and doxercalciferol exhibited the greatest reduction in survival of the ΔmbrA strain (3.3 log orders) compared to either compound alone (cf. Fig. 4C to A and B).
Cultures of S. mutans MX804 were fully recovered after 24 h treatment with all the drugs, which may in part be due to resistance to bacitracin, as well as drug concentrations either at or below the MIC used during the time-kill experiments. The mbrA mutant and complement strains exhibited a phenotype similar to that of MX804, with the exception of the combination of doxercalciferol and bacitracin, which exhibited 4- and 3.5-log-order decreases in viability at 24 h, respectively, relative to the no-drug control.
MX804 cultures fully recovered after 48 h treatment with all the drugs, similar to cultures of the ΔmbrA and mbrA+ strains treated with both compounds (Fig. 5D). Cultures of the ΔmbrA strain were completely nonviable after 48 h, whereas the mbrA+ strain exhibited an approximately 3-log-order decrease relative to the no-drug control. These results support our hypothesis that doxercalciferol possesses bactericidal activity and acts through a bacitracin-associated efflux pump mechanism.
The interaction of doxercalciferol with bacitracin may be specific to streptococci.
The mbrA membrane component of the bacitracin-associated efflux pump is conserved among Gram-positive and Gram-negative bacteria. In fact, many streptococci and lactococci carry genes for multiple ABC transporter proteins with significant sequence homology to MbrA, many of which have been investigated for their drug efflux-associated functions (58, 59).
Therefore, we investigated whether the presence of MbrA contributes to the mechanism by which doxercalciferol interacts with bacitracin in organisms that carry genes homologous to mbrA. To test this hypothesis, we measured the MICs of bacitracin in the presence or absence of doxercalciferol in a variety of Gram-positive and Gram-negative bacteria, many of which have been used to study mechanisms of bacitracin resistance (60–65). We chose a fixed concentration of doxercalciferol that exhibited a significant effect on S. mutans growth in the presence of bacitracin (4 μg/ml).
The results outlined in Table 3 indicate that the presence of mbrA-like genes does not require that doxercalciferol produce a significant reduction in the bacitracin MIC. The Gram-negative organisms examined as part of this study were not sensitive to bacitracin, and the presence of doxercalciferol did not alter their sensitivity. Of the remaining 10 organisms tested, 5 (50%) exhibited a >2-fold reduction in the MIC of bacitracin in the presence of 4 μg/ml doxercalciferol (Table 3). The data suggest that this may be a streptococcus-specific phenomenon, as we observed that 5 of the 6 streptococcal species (including the near neighbor of streptococci, Enterococcus faecalis) tested here demonstrated similar reductions in the MIC of bacitracin when measured in the presence of doxercalciferol. Other Gram-positive bacteria, such as Lactococcus lactis, S. aureus, and Streptococcus sanguinis, exhibited a slight (2-fold) reduction in the MIC of bacitracin in the presence of doxercalciferol, possibly due to the doxercalciferol-specific antibacterial activity rather than to potential interaction with an efflux pump.
TABLE 3.
Effects of doxercalciferol on the MIC of bacitracin for Gram-positive and Gram-negative bacteria that possess MbrA-like efflux pumps
| Species | MICBac (μg/ml)a |
|
|---|---|---|
| Without Dox | With Dox | |
| Streptococcus mutansb | >128 | 4 |
| Bacillus subtilis | >128 | >128 |
| Enterobacter aerogenes | >128 | >128 |
| Enterococcus faecalisb | >128 | 8 |
| Escherichia coli | >128 | >128 |
| Klebsiella pneumoniae | >128 | >128 |
| Lactococcus lactis | 4 | 2 |
| Mycobacterium smegmatis | >128 | >128 |
| Pseudomonas aeruginosa | >128 | >128 |
| Staphylococcus aureus | 128 | 64 |
| Streptococcus gordoniib | 128 | 32 |
| Streptococcus oralisb | 64 | 4 |
| Streptococcus salivariusb | 4 | 1 |
| Streptococcus sanguinis | 32 | 16 |
MICs were determined for bacitracin in select bacterial strains that possess ABC transporters that are homologous to the S. mutans mbrA efflux pump subunit. The MIC of bacitracin (MICBac) was also measured in the presence of 4 μg/ml doxercalciferol (Dox). The results are the lowest concentrations that inhibited growth in at least two independent experiments, in duplicate.
Species in which addition of doxercalciferol resulted in a significant decrease in the bacitracin MIC.
DISCUSSION
Using the AK assay to screen the Selleck library of 853 FDA-approved drugs, we identified 126 compounds with activity against S. mutans planktonic cultures (52). One class of compounds with novel activity against S. mutans included several derivatives of the secosteroid vitamin D. Doxercalciferol is a synthetic vitamin D2 analog used in the treatment of hyperparathyroidism. Although other groups have reported the antibacterial properties of vitamin D derivatives, this is the first report, to our knowledge, of interaction with bacitracin as a possible mechanism.
Previous reports have demonstrated antibacterial activity of vitamin D-like compounds against both Gram-positive and Gram-negative bacteria. Treatment with doxercalciferol led to a loss of bacterial viability and cell lysis, similar to the results seen previously with other vitamin D analogs (49, 50). Interestingly, treatment with doxercalciferol initially led to a significant decrease in cell viability, followed by recovery to levels similar to those of the no-drug control (Fig. 4A). These data may suggest that the mechanism of doxercalciferol has different targets that may change throughout time or that the cellular machinery needs to be induced in order to overcome the effects of doxercalciferol. In S. aureus, a combination of vitamin D and antibiotics in efflux-associated resistant strains resulted in restoration of antibiotic activity, suggesting that vitamin D may have a role in actively inhibiting antibiotic efflux (51). The activity observed with doxercalciferol is consistent with the definition of an adjuvant, which is a small molecule with little to no antimicrobial activity alone that enhances the activity of another drug.
Bacitracin resistance has been explored in both Gram-positive (62–67) and, to a lesser extent, Gram-negative (60, 61, 68) bacteria. Although in S. mutans it has been partially attributed to the RGP-associated genes, a commonly recognized mechanism of resistance is the role of ABC transporters, such as mbrABCD. In S. mutans, the mbrA gene is located downstream of the glucosyltransferase-encoding genes gtfB and gtfC. The MBR operon consists of mbrAB (SMU.1006 and SMU.1007), the ABC transporter, and mbrCD (SMU.1008 and SMU.1009), a putative two-component system (57).
The mbrABCD ABC transporter is conserved in Gram-positive and Gram-negative bacteria. We tested a variety of bacteria, which all had ABC transporters with significant homology to mbrAB (E < 1e−45). We found that despite the presence of transport systems homologous to S. mutans mbrABCD (SmmbrABCD), the additive effect of doxercalciferol on bacitracin susceptibility is limited to streptococci (Table 3).
The mbrAB transporter is similar to the well-characterized ABC-type lipoprotein and macrolide export system of Escherichia coli known as LolCDE (EcLolCDE) (reviewed in reference 69). Addition of a LolCDE inhibitor resulted in upregulation of stress response, as well as transport, including that of multidrug efflux systems (70). Dysregulation of transport systems in doxercalciferol-treated samples or mbrABCD mutant strains may explain the altered antibiotic susceptibilities that were observed (Fig. 3) (53). In contrast to the effect of doxercalciferol on S. mutans, the inhibitor had a low MIC (0.25 μg/ml), likely because components of EcLolCDE are essential (71). While loss of mbrA or mbrB in S. mutans did not exhibit any appreciable phenotypes relative to the parent strain, MX804, under the conditions tested (7), mutants in the E. coli LolCDE system are nonviable, which results in the accumulation of lipoproteins in the inner membrane (71). Lipoprotein transport and membrane synthesis function have also been attributed to both mbrABCD and LolCDE.
Bacitracin inhibits dephosphorylation of C55-isoprenyl pyrophosphate, thus interfering with peptidoglycan synthesis. There are several possible mechanisms of action that account for the lytic activity of doxercalciferol (and other vitamin D analogs), as well as the synergism with bacitracin. Based on the FIC of bacitracin and doxercalciferol in mutants of the bacitracin-associated efflux system (Table 2), it is possible that doxercalciferol circumvents the bacitracin resistance mechanism in S. mutans by directly inhibiting bacitracin efflux. However, doxercalciferol failed to inhibit general efflux systems in standard ethidium bromide efflux assays (data not shown). As with many efflux pump inhibitors, doxercalciferol and analogs are lipid soluble and may interact with the membrane, thus exacerbating the effect of bacitracin. However, other strains carrying deletions in cell wall-related genes were tested, and they did not exhibit altered susceptibility to either doxercalciferol or the combination of doxercalciferol and bacitracin (data not shown). Although these mechanisms are not mutually exclusive, further characterization of the effect of doxercalciferol on S. mutans will be necessary to identify specific targets.
In conclusion, we found that addition of doxercalciferol, a vitamin D derivative, to S. mutans cultures resulted in time-dependent lytic activity that acts via a bacitracin resistance-dependent mechanism. Further, this activity is specific to streptococcal (and closely related) species. Other vitamin D analogs may prove to be more potent inhibitors of streptococcal species. The broader implications of a compound with robust immunomodulatory roles and growing evidence of antimicrobial activity are exciting.
MATERIALS AND METHODS
Strains and growth conditions.
S. mutans strain UA159 (72) was maintained on brain heart infusion (BHI) agar medium (BD/Difco, Franklin Lakes, NJ). Cultures were grown at 37°C in a 5% (vol/vol) CO2-95% air atmosphere in either BHI or TY medium (3% tryptone, 0.1% yeast extract, 0.5% KOH, 1 mM H3PO4) plus 1% (wt/vol) glucose (TYG). For biofilm analyses, cells were grown in TY medium plus 1% (wt/vol) sucrose (TYS). Non-mutans bacteria, obtained from laboratory stocks, were maintained on BHI and grown at 37°C (in a 5% [vol/vol] CO2-95% air atmosphere, for streptococcal strains).
Adenylate kinase assay.
Adenylate kinase assays were performed as previously described (52, 73), with minor modifications. Briefly, overnight cultures of S. mutans UA159 were diluted 1:50 in 50 ml fresh TYG medium and grown to exponential phase (OD600, ∼0.5). In a 96-well opaque plate (Corning Inc., Corning, NY), 106 cells/well were combined with the test molecule (in DMSO; final concentration of DMSO, <0.5%) in a final volume of 100 μl. The plates were incubated at 37°C in a 5% (vol/vol) CO2-95% air atmosphere for 3 h and then equilibrated to room temperature for 1 h. Reconstituted AK detection reagent (ToxiLight nondestructive cytotoxicity bioassay kit; 100 μl; Lonza, Walkersville, MD) was added to each well, and the plate was incubated in the dark for 1 h at room temperature. Luminescence was measured with an integration time of 1,000 ms per well on a SpectraMax M5 plate reader (Molecular Devices, Sunnyvale, CA). Ciprofloxacin (positive control) and DMSO (negative control) were included on all plates.
For biofilm cultures, logarithmic-phase cells were seeded in flat-bottom 96-well plates (Corning Inc., Corning, NY) and grown in TYS at 37°C in a 5% (vol/vol) CO2-95% air atmosphere for ∼18 h. The plates were washed 3 times with sterile PBS to remove planktonic cells. Drugs were serially diluted (concentrations ranged from 0 to 64 μg/ml) in fresh TYS and added to the wells, followed by incubation for an additional 18 h. After 1 h equilibration at room temperature, 50 μl culture supernatant from each well was transferred to opaque 96-well plates with an equal volume of AK reagent (reconstituted according to the manufacturer's instructions; see above). The reaction was allowed to proceed for 1 h, followed by measurement of luminescence, as described above.
MIC testing.
Compounds used in MIC testing were as follows: alfacalcidol and calcitriol (Selleck Chemical, Houston, TX); doxercalciferol (ApexBio, Houston, TX); and ciprofloxacin, bacitracin, chloramphenicol, penicillin, streptomycin, and vancomycin (Sigma-Aldrich, St. Louis, MO). To determine the MICs of test compounds against S. mutans UA159, an overnight culture grown in BHI medium was diluted 1:50 in fresh TYG medium and grown to exponential phase (OD600, ∼0.3). A 96-well plate (Corning, Inc., Corning, NY) containing fresh TYG medium was inoculated with 105 CFU. A dilution series of the test compound (concentrations ranged from 0 to 64 μg/ml) was added to the plate. The plates were incubated at 37°C in a 5% (vol/vol) CO2-95% air atmosphere for 24 h. The MIC was considered the lowest compound concentration that inhibited bacterial growth, as measured by the OD600.
The combinatorial effect of bacitracin and doxercalciferol was determined as follows. A serial dilution of bacitracin was added to a 96-well plate in the presence or absence of a subinhibitory doxercalciferol concentration (4 μg/ml). Logarithmic-phase (OD600, ∼0.5) cultures were added to a final OD600 of 0.05. The plates were incubated at 37°C (in a 5% [vol/vol] CO2-95% air atmosphere, for streptococcal strains).
FIC testing.
Synergy was assessed by identifying the fractional inhibitory concentration (FIC), given by the following equation: FIC = (MICA/MICAB) + (MICB/MICAB), where A and B are the two drugs tested, alone or in combination (AB), measured using the standard checkerboard method (55). FICs were interpreted according to standard definitions, where “synergy” is defined as a fractional inhibitory concentration index (FICI) score of ≤0.5, “antagonism” is defined as a FICI score of >4.0, and “no interaction” is a score of 0.5 ≤ FICI ≤ 4.0.
Measurement of biofilm growth with crystal violet.
Biofilm cultures were assayed with test compounds to examine their abilities to prevent biofilm formation. Bacterial cultures grown to logarithmic phase in TYS were added to 96-well plates and incubated at 37°C in a 5% (vol/vol) CO2-95% air atmosphere for ∼18 h. Planktonic cells were removed by washing the wells 3 times with distilled water. The plates were dried overnight at 70°C. Biofilms were stained with 100 μl crystal violet (0.1%) for 15 min and then washed 5 times with distilled water. Adherent crystal violet was reconstituted with acetic acid (500 mM), and the plates were read with a Bio-Rad BenchMark Plus spectrophotometer at 575 nm (Bio-Rad, Hercules, CA) (7). The MBIC was defined as the lowest concentration of compound that inhibited S. mutans biofilm formation (≥90%), as measured by crystal violet, relative to the vehicle control.
Construction of the mbrA+ complement strain.
The ΔmbrA mutant strain was complemented using a single-copy genomic insertion of the SMU.1006 (mbrA) locus, including the intergenic region between mbrA and gtfC, into the gtfA (SMU.881) locus using the streptococcal integration vector pSUGK-Bgl (74). pSUGKBgl was linearized with the restriction enzyme BglII. Primers mbrA-comple-F (5′-GAGCTCGAATAGATCTGAAGTCTGAGCTGTAAATTTCTCAGG-3′) and mbrA-comple-R (5′-ATTTAAAAATAGATCTTTACTCACCTCCTAACAGCGCTGCC-3′) were used to amplify mbrA and the intergenic region between mbrA and gtfC. The resulting amplicon was ligated into the linearized pSUGKBglII using an In-Fusion HD cloning kit (Clontech, Mountain View, CA) to produce pSUGKBgl-mbrA. The cloning reaction was transformed into E. coli Stellar (Clontech, Mountain View, CA), and positive clones were selected on LB agar medium containing kanamycin. The integrity of the construct was confirmed by sequencing. pSUGKBgl-mbrA was transformed into S. mutans ΔmbrA and selected on BHI agar medium containing kanamycin. The complemented strain was designated mbrA+, and the integrity of the complemented locus was confirmed by sequencing with gtfA-Seqkan (5′-GATGTTCAACACTGCCATCTG-3′) (75).
Time-kill assay.
The kinetics of the bacitracin-doxercalciferol interaction were analyzed by a standard bacterial time-kill assay. S. mutans MX804, ΔmbrA, and mbrA+ strains were grown to logarithmic phase (OD600, ∼0.5) and added to TYG (106 cells/ml final concentration) containing doxercalciferol (16 μg/ml), bacitracin (32 μg/ml), doxercalciferol and bacitracin, or no drug (DMSO control). Aliquots were taken at 0, 2, 4, 24, and 48 h after inoculation; serially diluted; and plated on BHI agar medium for enumeration. The data were either normalized to CFU per milliliter at 0 h and plotted over time or normalized to CFU per milliliter of the no-drug control for each time point.
ACKNOWLEDGMENTS
We thank Steve Gill for critical reading of the manuscript.
This study was supported by the Drug Discovery Program of the University of Rochester School of Medicine and Dentistry, the Training Program in Oral Sciences NIH/NIDCR T90 DE021985-05 (S.S.), DE-013683 (R.G.Q.), and DE-017425 (R.G.Q.).
Footnotes
For a companion article on this topic, see https://doi.org/10.1128/AAC.01674-17.
REFERENCES
- 1.Dye BA, Barker LK, Selwitz RH, Lewis BG, Wu T, Fryar CD, Ostchega Y, Beltran ED, Ley E. 2007. Overview and quality assurance for the National Health and Nutrition Examination Survey (NHANES) oral health component, 1999-2002. Community Dent Oral Epidemiol 35:140–151. doi: 10.1111/j.1600-0528.2007.00310.x. [DOI] [PubMed] [Google Scholar]
- 2.Dye BA, Thornton-Evans G. 2010. Trends in oral health by poverty status as measured by healthy people 2010 objectives. Public Health Rep 125:817–830. doi: 10.1177/003335491012500609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo L, McLean JS, Lux R, He X, Shi W. 2015. The well-coordinated linkage between acidogenicity and aciduricity via insoluble glucans on the surface of Streptococcus mutans. Sci Rep 5:18015. doi: 10.1038/srep18015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lemos JA, Burne RA. 2008. A model of efficiency: stress tolerance by Streptococcus mutans. Microbiology 154:3247–3255. doi: 10.1099/mic.0.2008/023770-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker JL, Faustoferri RC, Quivey RG Jr. 2017. Acid-adaptive mechanisms of Streptococcus mutans—the more we know, the more we don't. Mol Oral Microbiol 32:107–117. doi: 10.1111/omi.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loesche WJ. 1986. Role of Streptococcus mutans in human dental decay. Microbiol Rev 50:353–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quivey RG Jr, Grayhack EJ, Faustoferri RC, Hubbard CJ, Baldeck JD, Wolf AS, MacGilvray ME, Rosalen PL, Scott-Anne K, Santiago B, Gopal S, Payne J, Marquis RE. 2015. Functional profiling in Streptococcus mutans: construction and examination of a genomic collection of gene deletion mutants. Mol Oral Microbiol 30:474–495. doi: 10.1111/omi.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lemos JA, Quivey RG Jr, Koo H, Abranches J. 2013. Streptococcus mutans: a new Gram-positive paradigm? Microbiology 159:436–445. doi: 10.1099/mic.0.066134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hampton T. 2013. Report reveals scope of US antibiotic resistance threat. JAMA 310:1661–1663. doi: 10.1001/jama.2013.280695. [DOI] [PubMed] [Google Scholar]
- 10.Berdal A, Papagerakis P, Hotton D, Bailleul-Forestier I, Davideau JL. 1995. Ameloblasts and odontoblasts, target-cells for 1,25-dihydroxyvitamin D3: a review. Int J Dev Biol 39:257–262. [PubMed] [Google Scholar]
- 11.Shaikh MN, Malapati BR, Gokani R, Patel B, Chatriwala M. 2016. Serum magnesium and vitamin D levels as indicators of asthma severity. Pulm Med 2016:1643717. doi: 10.1155/2016/1643717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cutolo M, Otsa K, Laas K, Yprus M, Lehtme R, Secchi ME, Sulli A, Paolino S, Seriolo B. 2006. Circannual vitamin D serum levels and disease activity in rheumatoid arthritis: Northern versus Southern Europe. Clin Exp Rheumatol 24:702–704. [PubMed] [Google Scholar]
- 13.Miragliotta G, Miragliotta L. 2014. Vitamin D and infectious diseases. Endocr Metab Immune Disord Drug Targets 14:267–271. doi: 10.2174/1871530314666141027102627. [DOI] [PubMed] [Google Scholar]
- 14.Yao S, Kwan ML, Ergas IJ, Roh JM, Cheng TD, Hong CC, McCann SE, Tang L, Davis W, Liu S, Quesenberry CP Jr, Lee MM, Ambrosone CB, Kushi LH. 2017. Association of serum level of vitamin D at diagnosis with breast cancer survival: a case-cohort analysis in the pathways study. JAMA Oncol 3:351–357. doi: 10.1001/jamaoncol.2016.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hatse S, Lambrechts D, Verstuyf A, Smeets A, Brouwers B, Vandorpe T, Brouckaert O, Peuteman G, Laenen A, Verlinden L, Kriebitzsch C, Dieudonne AS, Paridaens R, Neven P, Christiaens MR, Bouillon R, Wildiers H. 2012. Vitamin D status at breast cancer diagnosis: correlation with tumor characteristics, disease outcome, and genetic determinants of vitamin D insufficiency. Carcinogenesis 33:1319–1326. doi: 10.1093/carcin/bgs187. [DOI] [PubMed] [Google Scholar]
- 16.Laky M, Bertl K, Haririan H, Andrukhov O, Seemann R, Volf I, Assinger A, Gruber R, Moritz A, Rausch-Fan X. 2017. Serum levels of 25-hydroxyvitamin D are associated with periodontal disease. Clin Oral Investig 21:1553–1558. doi: 10.1007/s00784-016-1965-2. [DOI] [PubMed] [Google Scholar]
- 17.Dietrich T, Nunn M, Dawson-Hughes B, Bischoff-Ferrari HA. 2005. Association between serum concentrations of 25-hydroxyvitamin D and gingival inflammation. Am J Clin Nutr 82:575–580. [DOI] [PubMed] [Google Scholar]
- 18.Fairney A, Saphier PW. 1987. Studies on the measurement of 25-hydroxy vitamin D in human saliva. Br J Nutr 57:13–25. doi: 10.1079/BJN19870005. [DOI] [PubMed] [Google Scholar]
- 19.Kuhnisch J, Thiering E, Kratzsch J, Heinrich-Weltzien R, Hickel R, Heinrich J, GINIplus Study Group, LISAplus Study Group. 2015. Elevated serum 25(OH)-vitamin D levels are negatively correlated with molar-incisor hypomineralization. J Dent Res 94:381–387. doi: 10.1177/0022034514561657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia MN, Hildebolt CF, Miley DD, Dixon DA, Couture RA, Spearie CL, Langenwalter EM, Shannon WD, Deych E, Mueller C, Civitelli R. 2011. One-year effects of vitamin D and calcium supplementation on chronic periodontitis. J Periodontol 82:25–32. doi: 10.1902/jop.2010.100207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miley DD, Garcia MN, Hildebolt CF, Shannon WD, Couture RA, Anderson Spearie CL, Dixon DA, Langenwalter EM, Mueller C, Civitelli R. 2009. Cross-sectional study of vitamin D and calcium supplementation effects on chronic periodontitis. J Periodontol 80:1433–1439. doi: 10.1902/jop.2009.090077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grant WB. 2011. A review of the role of solar ultraviolet-B irradiance and vitamin D in reducing risk of dental caries. Dermatoendocrinology 3:193–198. doi: 10.4161/derm.15841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schroth RJ, Rabbani R, Loewen G, Moffatt ME. 2016. Vitamin D and dental caries in children. J Dent Res 95:173–179. doi: 10.1177/0022034515616335. [DOI] [PubMed] [Google Scholar]
- 24.Schroth RJ, Levi JA, Sellers EA, Friel J, Kliewer E, Moffatt ME. 2013. Vitamin D status of children with severe early childhood caries: a case-control study. BMC Pediatr 13:174. doi: 10.1186/1471-2431-13-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schroth RJ, Lavelle C, Tate R, Bruce S, Billings RJ, Moffatt ME. 2014. Prenatal vitamin D and dental caries in infants. Pediatrics 133:e1277–1284. doi: 10.1542/peds.2013-2215. [DOI] [PubMed] [Google Scholar]
- 26.Karras SN, Fakhoury H, Muscogiuri G, Grant WB, van den Ouweland JM, Colao AM, Kotsa K. 2016. Maternal vitamin D levels during pregnancy and neonatal health: evidence to date and clinical implications. Ther Adv Musculoskelet Dis 8:124–135. doi: 10.1177/1759720X16656810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Satue M, Gomez-Florit M, Monjo M, Ramis JM. 2016. Improved human gingival fibroblast response to titanium implants coated with ultraviolet-irradiated vitamin D precursor and vitamin E. J Periodont Res 51:342–349. doi: 10.1111/jre.12313. [DOI] [PubMed] [Google Scholar]
- 28.Satue M, Monjo M, Ronold HJ, Lyngstadaas SP, Ramis JM. 2017. Titanium implants coated with UV-irradiated vitamin D precursor and vitamin E: in vivo performance and coating stability. Clin Oral Implants Res 28:424–431. [DOI] [PubMed] [Google Scholar]
- 29.Youssef DA, Miller CW, El-Abbassi AM, Cutchins DC, Cutchins C, Grant WB, Peiris AN. 2011. Antimicrobial implications of vitamin D. Dermatoendocrinology 3:220–229. doi: 10.4161/derm.3.4.15027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kundu R, Theodoraki A, Haas CT, Zhang Y, Chain B, Kriston-Vizi J, Noursadeghi M, Khoo B. 2017. Cell-type-specific modulation of innate immune signalling by vitamin D in human mononuclear phagocytes. Immunology 150:55–63. doi: 10.1111/imm.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stoffels K, Overbergh L, Giulietti A, Verlinden L, Bouillon R, Mathieu C. 2006. Immune regulation of 25-hydroxyvitamin-D3-1alpha-hydroxylase in human monocytes. J Bone Miner Res 21:37–47. doi: 10.1359/JBMR.050908. [DOI] [PubMed] [Google Scholar]
- 32.Kroner JDC, Sommer A, Fabri M. 2015. Vitamin D every day to keep the infection away? Nutrients 7:4170–4188. doi: 10.3390/nu7064170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gombart AF. 2009. The vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future Microbiol 4:1151–1165. doi: 10.2217/fmb.09.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo C, Gombart AF. 2014. The antibiotic effects of vitamin D. Endocr Metab Immune Disord Drug Targets 14:255–266. doi: 10.2174/1871530314666140709085159. [DOI] [PubMed] [Google Scholar]
- 35.Hu XP, Li ZQ, Zhou JY, Yu ZH, Zhang JM, Guo ML. 2015. Analysis of the association between polymorphisms in the vitamin D receptor (VDR) gene and dental caries in a Chinese population. Genet Mol Res 14:11631–11638. doi: 10.4238/2015.September.28.15. [DOI] [PubMed] [Google Scholar]
- 36.Malloy PJ, Zhou Y, Wang J, Hiort O, Feldman D. 2011. Hereditary vitamin D-resistant rickets (HVDRR) owing to a heterozygous mutation in the vitamin D receptor. J Bone Miner Res 26:2710–2718. doi: 10.1002/jbmr.484. [DOI] [PubMed] [Google Scholar]
- 37.Hochberg Z, Weisman Y. 1995. Calcitriol-resistant rickets due to vitamin D receptor defects. Trends Endocrinol Metab 6:216–220. doi: 10.1016/1043-2760(95)00126-3. [DOI] [PubMed] [Google Scholar]
- 38.Sabandal MM, Robotta P, Burklein S, Schafer E. 2015. Review of the dental implications of X-linked hypophosphataemic rickets (XLHR). Clin Oral Invest 19:759–768. doi: 10.1007/s00784-015-1425-4. [DOI] [PubMed] [Google Scholar]
- 39.Zeng J, Wu G, Yang W, Gu X, Liang W, Yao Y, Song Y. 2015. A serum vitamin D level <25nmol/l pose high tuberculosis risk: a meta-analysis. PLoS One 10:e0126014. doi: 10.1371/journal.pone.0126014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salamon H, Bruiners N, Lakehal K, Shi L, Ravi J, Yamaguchi KD, Pine R, Gennaro ML. 2014. Cutting edge: vitamin D regulates lipid metabolism in Mycobacterium tuberculosis infection. J Immunol 193:30–34. doi: 10.4049/jimmunol.1400736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tukvadze N, Sanikidze E, Kipiani M, Hebbar G, Easley KA, Shenvi N, Kempker RR, Frediani JK, Mirtskhulava V, Alvarez JA, Lomtadze N, Vashakidze L, Hao L, Del Rio C, Tangpricha V, Blumberg HM, Ziegler TR. 2015. High-dose vitamin D3 in adults with pulmonary tuberculosis: a double-blind randomized controlled trial. Am J Clin Nutr 102:1059–1069. doi: 10.3945/ajcn.115.113886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turnbull ER, Drobniewski F. 2015. Vitamin D supplementation: a comprehensive review on supplementation for tuberculosis prophylaxis. Expert Rev Respir Med 9:269–275. doi: 10.1586/17476348.2015.1042458. [DOI] [PubMed] [Google Scholar]
- 43.Facchini L, Venturini E, Galli L, de Martino M, Chiappini E. 2015. Vitamin D and tuberculosis: a review on a hot topic. J Chemother 27:128–138. doi: 10.1179/1973947815Y.0000000043. [DOI] [PubMed] [Google Scholar]
- 44.Davies PD, Martineau AR. 2015. Vitamin D and tuberculosis: more effective in prevention than treatment? Int J Tuberc Lung Dis 19:876–877. doi: 10.5588/ijtld.15.0506. [DOI] [PubMed] [Google Scholar]
- 45.Wang JW, Hogan PG, Hunstad DA, Fritz SA. 2015. Vitamin D sufficiency and Staphylococcus aureus infection in children. Pediatr Infect Dis J 34:544–545. doi: 10.1097/INF.0000000000000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomason J, Rentsch C, Stenehjem EA, Hidron AI, Rimland D. 2015. Association between vitamin D deficiency and methicillin-resistant Staphylococcus aureus infection. Infection 43:715–722. doi: 10.1007/s15010-015-0815-5. [DOI] [PubMed] [Google Scholar]
- 47.Nouari W, Ysmail-Dahlouk L, Aribi M. 2016. Vitamin D3 enhances bactericidal activity of macrophage against Pseudomonas aeruginosa. Int Immunopharmacol 30:94–101. doi: 10.1016/j.intimp.2015.11.033. [DOI] [PubMed] [Google Scholar]
- 48.Rieder FJ, Groschel C, Kastner MT, Kosulin K, Laengle J, Zadnikar R, Marculescu R, Schneider M, Lion T, Bergmann M, Kallay E, Steininger C. 2017. Human cytomegalovirus infection downregulates vitamin-D receptor in mammalian cells. J Steroid Biochem Mol Biol 165:356–362. doi: 10.1016/j.jsbmb.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hosoda K, Shimomura H, Wanibuchi K, Masui H, Amgalanbaatar A, Hayashi S, Takahashi T, Hirai Y. 2015. Identification and characterization of a vitamin D(3) decomposition product bactericidal against Helicobacter pylori. Sci Rep 5:8860. doi: 10.1038/srep08860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grenier D, Morin MP, Fournier-Larente J, Chen H. 2016. Vitamin D inhibits the growth of and virulence factor gene expression by Porphyromonas gingivalis and blocks activation of the nuclear factor kappa B transcription factor in monocytes. J Periodont Res 51:359–365. doi: 10.1111/jre.12315. [DOI] [PubMed] [Google Scholar]
- 51.Tintino SR, Morais-Tintino CD, Campina FF, Pereira RL, Costa MDS, Braga MF, Limaverde PW, Andrade JC, Siqueira-Junior JP, Coutinho HD, Balbino VQ, Leal-Balbino TC, Ribeiro-Filho J, Quintans-Junior LJ. 2016. Action of cholecalciferol and alpha-tocopherol on Staphylococcus aureus efflux pumps. EXCLI J 15:315–322. doi: 10.17179/excli2016-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saputo S, Faustoferri RC, Quivey RG Jr. 2018. A drug repositioning approach reveals that Streptococcus mutans is susceptible to a diverse range of established antimicrobials and nonantibiotics. Antimicrob Agents Chemother 62:e01674-17. doi: 10.1128/AAC.01674-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsuda H, Yamashita Y, Shibata Y, Nakano Y, Koga T. 2002. Genes involved in bacitracin resistance in Streptococcus mutans. Antimicrob Agents Chemother 46:3756–3764. doi: 10.1128/AAC.46.12.3756-3764.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kitagawa N, Shiota S, Shibata Y, Takeshita T, Yamashita Y. 2011. Characterization of MbrC involved in bacitracin resistance in Streptococcus mutans. FEMS Microbiol Lett 318:61–67. doi: 10.1111/j.1574-6968.2011.02238.x. [DOI] [PubMed] [Google Scholar]
- 55.Odds FC. 2003. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother 52:1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- 56.Piddock LJ, Johnson MM, Simjee S, Pumbwe L. 2002. Expression of efflux pump gene pmrA in fluoroquinolone-resistant and -susceptible clinical isolates of Streptococcus pneumoniae. Antimicrob Agents Chemother 46:808–812. doi: 10.1128/AAC.46.3.808-812.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mikami Y, Suzuki N, Takahashi T, Otsuka K, Tsuda H. 2011. Bacitracin upregulates mbrAB transcription via mbrCD to confer bacitracin resistance in Streptococcus mutans. J Pharmacol Sci 117:204–207. doi: 10.1254/jphs.11052SC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perreten V, Schwarz FV, Teuber M, Levy SB. 2001. Mdt(A), a new efflux protein conferring multiple antibiotic resistance in Lactococcus lactis and Escherichia coli. Antimicrob Agents Chemother 45:1109–1114. doi: 10.1128/AAC.45.4.1109-1114.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Becker P, Hakenbeck R, Henrich B. 2009. An ABC transporter of Streptococcus pneumoniae involved in susceptibility to vancoresmycin and bacitracin. Antimicrob Agents Chemother 53:2034–2041. doi: 10.1128/AAC.01485-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cain BD, Norton PJ, Eubanks W, Nick HS, Allen CM. 1993. Amplification of the bacA gene confers bacitracin resistance to Escherichia coli. J Bacteriol 175:3784–3789. doi: 10.1128/jb.175.12.3784-3789.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.El Ghachi M, Bouhss A, Blanot D, Mengin-Lecreulx D. 2004. The bacA gene of Escherichia coli encodes an undecaprenyl pyrophosphate phosphatase activity. J Biol Chem 279:30106–30113. doi: 10.1074/jbc.M401701200. [DOI] [PubMed] [Google Scholar]
- 62.Hiron A, Falord M, Valle J, Debarbouille M, Msadek T. 2011. Bacitracin and nisin resistance in Staphylococcus aureus: a novel pathway involving the BraS/BraR two-component system (SA2417/SA2418) and both the BraD/BraE and VraD/VraE ABC transporters. Mol Microbiol 81:602–622. doi: 10.1111/j.1365-2958.2011.07735.x. [DOI] [PubMed] [Google Scholar]
- 63.Manson JM, Keis S, Smith JM, Cook GM. 2004. Acquired bacitracin resistance in Enterococcus faecalis is mediated by an ABC transporter and a novel regulatory protein, BcrR. Antimicrob Agents Chemother 48:3743–3748. doi: 10.1128/AAC.48.10.3743-3748.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Matos R, Pinto VV, Ruivo M, Lopes MDF. 2009. Study on the dissemination of the bcrABDR cluster in Enterococcus spp. reveals that the BcrAB transporter is sufficient to confer high-level bacitracin resistance. Int J Antimicrob Agents 34:142–147. doi: 10.1016/j.ijantimicag.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 65.Radeck J, Gebhard S, Orchard PS, Kirchner M, Bauer S, Mascher T, Fritz G. 2016. Anatomy of the bacitracin resistance network in Bacillus subtilis. Mol Microbiol 100:607–620. doi: 10.1111/mmi.13336. [DOI] [PubMed] [Google Scholar]
- 66.Chen MY, Lira F, Liang HQ, Wu RT, Duan JH, Liao XP, Martinez JL, Liu YH, Sun J. 2016. Multilevel selection of bcrABDR-mediated bacitracin resistance in Enterococcus faecalis from chicken farms. Sci Rep 6:34895. doi: 10.1038/srep34895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang S, Li X, Wang X, Li Z, He J. 2016. The two-component signal transduction system YvcPQ regulates the bacterial resistance to bacitracin in Bacillus thuringiensis. Arch Microbiol 198:773–784. doi: 10.1007/s00203-016-1239-z. [DOI] [PubMed] [Google Scholar]
- 68.Harel YM, Bailone A, Bibi E. 1999. Resistance to bacitracin as modulated by an Escherichia coli homologue of the bacitracin ABC transporter BcrC subunit from Bacillus licheniformis. J Bacteriol 181:6176–6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tokuda H, Matsuyama S. 2004. Sorting of lipoproteins to the outer membrane in E. coli. Biochim Biophys Acta 1694:IN1–IN9. [PubMed] [Google Scholar]
- 70.Lorenz C, Dougherty TJ, Lory S. 2016. Transcriptional responses of Escherichia coli to a small-molecule inhibitor of LolCDE, an essential component of the lipoprotein transport pathway. J Bacteriol 198:3162–3175. doi: 10.1128/JB.00502-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Narita S, Tanaka K, Matsuyama S, Tokuda H. 2002. Disruption of lolCDE, encoding an ATP-binding cassette transporter, is lethal for Escherichia coli and prevents release of lipoproteins from the inner membrane. J Bacteriol 184:1417–1422. doi: 10.1128/JB.184.5.1417-1422.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ajdic D, McShan WM, McLaughlin RE, Savic G, Chang J, Carson MB, Primeaux C, Tian R, Kenton S, Jia H, Lin S, Qian Y, Li S, Zhu H, Najar F, Lai H, White J, Roe BA, Ferretti JJ. 2002. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc Natl Acad Sci U S A 99:14434–14439. doi: 10.1073/pnas.172501299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jacobs AC, Didone L, Jobson J, Sofia MK, Krysan D, Dunman PM. 2013. Adenylate kinase release as a high-throughput-screening-compatible reporter of bacterial lysis for identification of antibacterial agents. Antimicrob Agents Chemother 57:26–36. doi: 10.1128/AAC.01640-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Derr AM, Faustoferri RC, Betzenhauser MJ, Gonzalez K, Marquis RE, Quivey RG Jr. 2012. Mutation of the NADH oxidase gene (nox) reveals an overlap of the oxygen- and acid-mediated stress responses in Streptococcus mutans. Appl Environ Microbiol 78:1215–1227. doi: 10.1128/AEM.06890-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gonzalez K, Faustoferri RC, Quivey RG Jr. 2012. Role of DNA base excision repair in the mutability and virulence of Streptococcus mutans. Mol Microbiol 85:361–377. doi: 10.1111/j.1365-2958.2012.08116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]