ABSTRACT
Acinetobacter baumannii is a notorious opportunistic pathogen that is prevalent mainly in hospital settings. The ability of A. baumannii to adapt and to survive in a range of environments has been a key factor for its persistence and success as an opportunistic pathogen. In this study, we investigated the effect of temperature on the clinically relevant phenotypes displayed by A. baumannii at 37°C and 28°C. Surface-associated motility was significantly reduced at 28°C, while biofilm formation on plastic surfaces was increased at 28°C. Decreased susceptibility to aztreonam and increased susceptibility to trimethoprim-sulfamethoxazole were observed at 28°C. No differences in virulence, as assayed in a Galleria mellonella model, were observed. Proteomic analysis showed differential expression of 629 proteins, of which 366 were upregulated and 263 were downregulated at 28°C. Upregulation of the Csu and iron uptake proteins at 28°C was a key finding for understanding some of the phenotypes displayed by A. baumannii at 28°C.
KEYWORDS: twitching motility, biofilm, phenyl acetate, thermoregulation
INTRODUCTION
Acinetobacter baumannii is an organism of significant interest due to its ability to resist the action of various antibiotics (1, 2). A. baumannii displays a variety of intrinsic and acquired mechanisms of resistance to antibiotics, making treatment of infections often very difficult. While Acinetobacter spp. are commonly found in the environment, natural habitats of A. baumannii remain to be properly defined. It was thought that A. baumannii tends to persist primarily within hospital environments (3); however, a number of recent studies have isolated and characterized A. baumannii from different environmental niches, although it is not clear whether human activity within those niches is responsible for the presence of A. baumannii (4–6).
A. baumannii has been reported to cause a variety of infections in hospital settings, including pneumonia, urinary tract infections, osteomyelitis, skin and soft tissue infections, bloodstream infections, and meningitis (3). It has spread rapidly in hospital settings globally, and some estimates suggest that A. baumannii causes 5 to 10% of hospital-acquired infections worldwide (7). It has been suggested that A. baumannii is responsible for disproportionately high incidences of pneumonia and bloodstream infections in hospital settings; a recent study demonstrated that, although A. baumannii was responsible for only 6% of all hospital-acquired infections, it was implicated in 60% of pneumonia cases and 25% of bloodstream infections (8). This finding speaks to the tremendous success of A. baumannii in causing hospital-acquired pneumonia and bloodstream infections.
An important characteristic of A. baumannii that partially explains its success as a hospital-acquired pathogen is its ability to persist on various environmental surfaces (9, 10). Therefore, the adaptation of A. baumannii to various environments may serve as a trigger to modulate its virulence factors. However, it is not clear how adaptation to different environmental conditions affects modulation of the antibiotic resistance and virulence of A. baumannii. Among these environmental conditions, temperature is likely to be one of the important environmental changes that A. baumannii experiences as it makes its way into the human host from the hospital environments. The purpose of this work was to study the effects of temperature on the antibiotic resistance and virulence of A. baumannii, using a combination of phenotypic and proteomic approaches.
RESULTS AND DISCUSSION
A. baumannii displays a very impressive propensity to cause multidrug-resistant infections in hospital settings; therefore, it has been studied quite extensively recently (1, 11–13). A. baumannii uses a variety of mechanisms to resist the activity of antibiotics, but antibiotic resistance is not the only factor contributing to the persistence of A. baumannii as an opportunistic nosocomial pathogen. It also contains a wide array of virulence factors (14–18) and a tremendous ability to adapt to environmental stress, leading to its persistence in hospital settings (19, 20).
For a pathogen like A. baumannii, temperature is an important environmental change that it experiences as it makes its way into its host. We observed rather fortuitously that A. baumannii ATCC 17978 displays reduced levels of surface-associated motility at 28°C, compared with those at 37°C. This suggests that the incubation temperature can affect the phenotype of A. baumannii ATCC 17978, which could modulate its virulence. Production of virulence factors by several bacterial species has been shown to be induced at 37°C. The underlying mechanisms of thermoregulation of virulence factor production and/or antibiotic resistance can be diverse. For example, in Pseudomonas aeruginosa, this phenomenon has been shown to be mediated by RNA thermometers that result in increased production of virulence factors such as rhamnolipids, pyocyanin, and elastase at 37°C (21). Further, a recent study suggested the involvement of putative diguanylate cyclase in the thermoregulation of biofilm formation in Burkholderia pseudomallei (22).
We pursued our findings further in order to study how differently A. baumannii ATCC 17978 behaves at different incubation temperatures. We carried out a comparison of the phenotypes (in particular, antibiotic resistance and virulence) of A. baumannii ATCC 17978, followed by proteomic analysis in order to understand, at the molecular level, the differences in the phenotypes.
Incubation temperature modulates the antibiotic susceptibility of A. baumannii ATCC 17978.
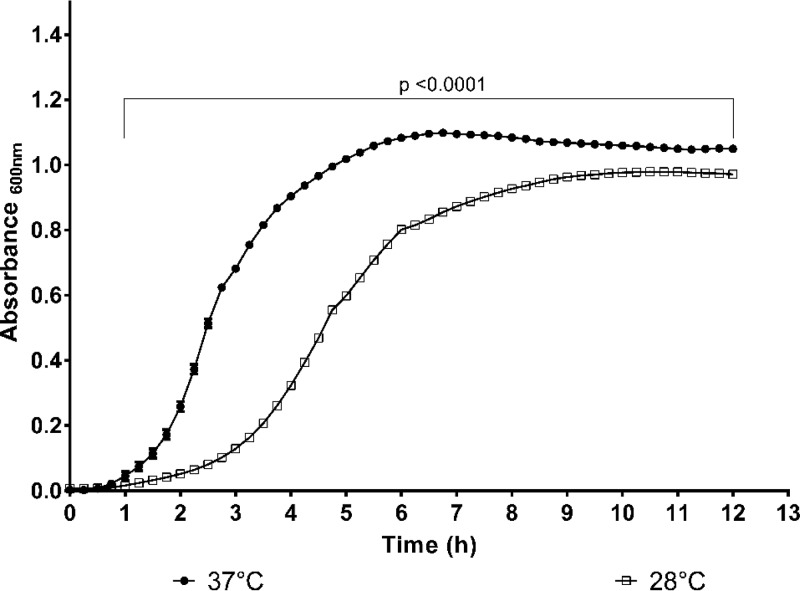
We initiated our study by comparing the growth of A. baumannii ATCC 17978 at 28°C and 37°C (Fig. 1). The initial growth of A. baumannii ATCC 17978 was slower at 28°C than at 37°C, as the absorbance at 600 nm (A600) of A. baumannii ATCC 17978 at 28°C was only about 40% of that at 37°C after 4 h of incubation (Fig. 1). This difference became less pronounced after 12 h of incubation, with the growth rate at 28°C being about 92% of that at 37°C (Fig. 1). This suggests that A. baumannii grows faster at 37°C than at 28°C, at least during the early log phase of the growth cycle.
FIG 1.
Relative growth of A. baumannii ATCC 17978 at 28°C and 37°C over 12 h. The difference in growth was most pronounced after 1 h of incubation; however, the difference was less pronounced after 6 h, and growth rates were almost the same after 8 h of incubation. Data shown are the mean of two independent assays.
Antibiotic susceptibility of A. baumannii ATCC 17978 was compared at 28 and 37°C (Table 1). A. baumannii ATCC 17978 was 4-fold less susceptible to aztreonam at 28°C, but its susceptibility to trimethoprim-sulfamethoxazole was 8-fold higher at 28°C than at 37°C. Small but reproducible changes in susceptibility to some other antibiotics were also observed (Table 1). It seems unlikely that the changes in susceptibility to aztreonam and trimethoprim-sulfamethoxazole were due to the difference in growth rates at the two temperatures tested, because there were little or no changes in susceptibility to the other antibiotics tested. However, in order to rule out the possibility that the differences in susceptibility to aztreonam and trimethoprim-sulfamethoxazole at 28°C and 37°C were due to possible differences in the diffusion of the antibiotics at these temperatures, we carried out susceptibility testing for the two antibiotics using the 2-fold broth dilution method. The results obtained using the broth dilution method showed the same trend as those obtained using the Etest method. A. baumannii ATCC 17978 was 4-fold less susceptible to aztreonam at 28°C, and its susceptibility to trimethoprim-sulfamethoxazole was 2-fold higher at 28°C than at 37°C. Currently, we are not sure why we observed opposite responses to aztreonam and trimethoprim-sulfamethoxazole at 28°C versus 37°C.
TABLE 1.
Susceptibilities of A. baumannii ATCC 17978 to various antibiotics at 37°C and 28°C
| Antibiotic(s) | MIC (μg/ml)a |
|
|---|---|---|
| 37°C | 28°C | |
| Piperacillin-tazobactam | <0.016 | <0.016 |
| Ceftriaxone | 6 | 8 |
| Cefepime | 0.75 | 1 |
| Ceftazidime | 1 | 1 |
| Imipenem | 0.125 | 0.094 |
| Doripenem | 0.094 | 0.094 |
| Ciprofloxacin | 0.064 | 0.047 |
| Moxifloxacin | 0.012 | 0.012 |
| Aztreonam | 2 | 8 |
| Trimethoprim-sulfamethoxazole | 4 | 0.5 |
| Gentamicin | 0.19 | 0.25 |
The numbers in bold indicate values that differ ≥4-fold between the two temperatures.
Temperature modulates biofilm formation and surface motility of A. baumannii ATCC 17978.
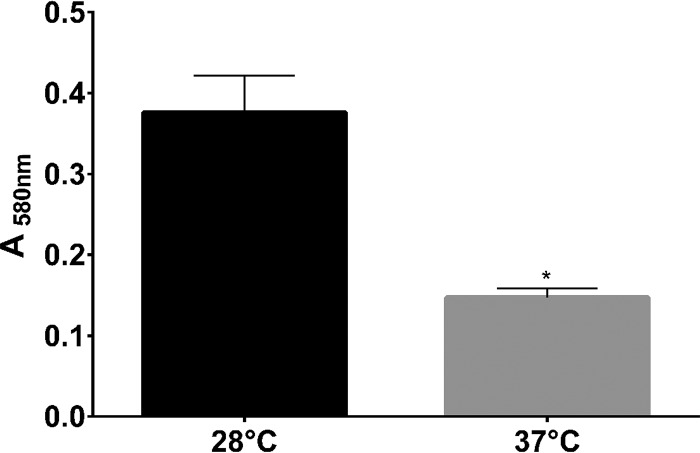
Biofilm formation by bacterial pathogens is an important virulence factor. Formation of biofilm by A. baumannii is partly responsible for its ability to cause persistent and difficult-to-treat infections (23, 24). Intriguingly, we observed that A. baumannii ATCC 17978 formed more biofilm at 28°C than at 37°C (Fig. 2), which indicates that the slower growth at 28°C does not have an impact on the ability of A. baumannii ATCC 17978 to form biofilm. Whether the thermoregulation of biofilm formation is mediated by a factor similar to that observed in B. pseudomallei (22) remains to be investigated.
FIG 2.
Comparison of biofilm formation by A. baumannii ATCC 17978 at 28°C and 37°C. Biofilm formation was significantly greater at 28°C than at 37°C. Statistical analysis was carried out using two-way analysis of variance (ANOVA). *, P = 0.001.
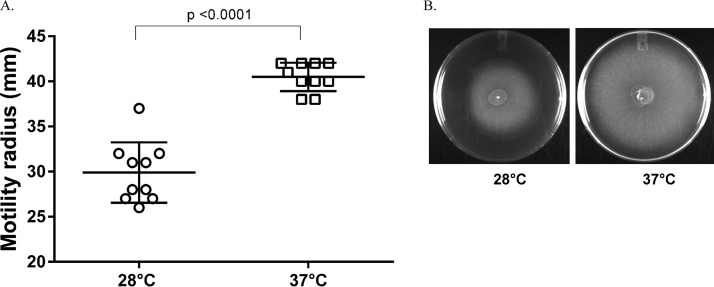
We also repeated the motility assays in order to confirm our initial observations that A. baumannii ATCC 17978 was less motile at 28°C. At 37°C, A. baumannii ATCC 17978 displayed surface-associated motility and covered the entire surface of the agarose medium 18 h after incubation (Fig. 3). Similar to our initial observations, it was less motile at 28°C, with levels of motility significantly different from those displayed at 37°C. This is an interesting observation, because at 28°C A. baumannii ATCC 17978 is less motile on agar surfaces but still forms more biofilm. Previous studies showed that, at least for some strains of A. baumannii, the ability to form biofilm is not dependent on the ability to display surface-associated twitching motility (25), which may be the case for A. baumannii ATCC 17978 as well.
FIG 3.
Comparison of twitching motility of A. baumannii ATCC 17978 at 28°C and 37°C. (A) The bacteria were able to cover the entire surface of the plate after 18 h of incubation at 37°C, but growth was significantly less at 28°C than at 37°C. Each data point represents a biological replicate. (B) The picture shows representative plates inoculated with A. baumannii ATCC 17978 and incubated at 28°C and 37°C. Statistical analysis was carried out using a paired t test.
Virulence of A. baumannii ATCC 17978 in Galleria mellonella is not affected by temperature.
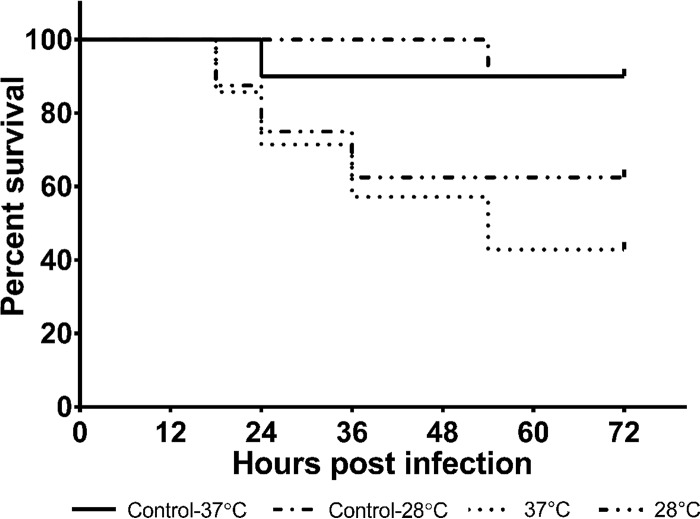
The virulence of A. baumannii ATCC 17978 in G. mellonella, a well-accepted infection model for this bacterium (26), was tested at 28°C and 37°C. The results of the virulence assays are shown in Fig. 4. Data suggest that, in spite of the phenotypic differences in A. baumannii ATCC 17978 grown at 28°C and 37°C, there was no statistically significant difference in the virulence of A. baumannii ATCC 17978 in G. mellonella at these two temperatures.
FIG 4.
Survival of Galleria mellonella inoculated with A. baumannii at 28°C and 37°C. A 10-μl inoculum (grown overnight at 28°C or 37°C) containing 1 × 106 CFU/ml in saline was injected into the second right proleg of the worm. Ten microliters of saline was injected into worms used as controls. Injected worms were incubated at 28°C and 37°C, respectively, and monitored for 72 h. The Mantel-Cox test was used for statistical analysis (P = 0.0838). Data shown are representative of two independent assays.
Proteomic analysis shows that expression of various proteins involved in antibiotic resistance and virulence is altered at different incubation temperatures.
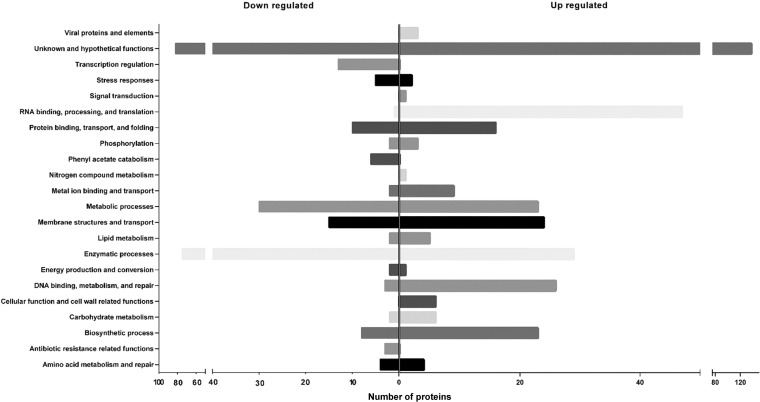
Proteomic analysis was used to help understand the molecular basis of phenotypic differences displayed by A. baumannii at different growth temperatures. Of 2,446 proteins identified (see Table S1 in the supplemental material), 629 proteins were differentially expressed, with 366 proteins being upregulated and 263 being downregulated at 28°C, compared to 37°C (Fig. 5) (the list of proteins whose expression was altered is provided in Table S2). The largest subset of proteins that could be classified whose expression was upregulated or downregulated was those involved in metabolic and enzymatic processes (Table S2). Proteins involved in RNA binding, processing, and translation were found to be largely upregulated at 28°C.
FIG 5.
Clusters of orthologous genes (COG) analysis of proteins whose expression in A. baumannii ATCC 17978 is altered at 28°C versus 37°C.
We observed downregulation of chaperonin proteins Hsp60 (A1S_2664) and Hsp10 (A1S_2665) at 28°C (Table 2). Hsp60 was shown previously to be overexpressed under ethanol stress (27), as well as with oleanolic acid (28), and is considered a stress response protein. Therefore, our data suggest that 37°C may exert stress on A. baumannii.
TABLE 2.
Select proteins whose expression was altered at 28°C versus 37°Ca
| Locus tag | Protein annotation | Log2 fold change | Function |
|---|---|---|---|
| A1S_0116 | RND-superfamily-like exporter | 3.95 | Antibiotic susceptibility |
| A1S_0908 | RND-family multidrug resistance secretion protein | 2.09 | Antibiotic susceptibility |
| A1S_1752 | AdeA membrane fusion protein | 1.37 | Antibiotic susceptibility |
| A1S_3219 | Putative RND-family drug transporter | 0.58 | Antibiotic susceptibility |
| A1S_0774 | Putative RND-family drug transporter | −0.7 | Antibiotic susceptibility |
| A1S_2737 | AdeK | 0.67 | Antibiotic susceptibility |
| A1S_1708 | β-Lactamase-like protein | −0.99 | Antibiotic susceptibility |
| A1S_1968 | Putative outer membrane protein (OmpH) | 0.79 | Virulence factor |
| A1S_1193 | OmpA/MotB | −1.27 | Virulence factor |
| A1S_1343 | PaaC | −3.33 | Virulence factor |
| A1S_1345 | PaaK | −3.09 | Virulence factor |
| A1S_1347 | PaaX | −1.04 | Virulence factor |
| A1S_2213 | CsuE | 1.6 | Biofilm formation |
| A1S_2214 | CsuD | 1.33 | Biofilm formation |
| A1S_2215 | CsuC | 2.07 | Biofilm formation |
| A1S_2216 | CsuB | 1.69 | Biofilm formation |
| A1S_2217 | CsuA | 0.73 | Biofilm formation |
| A1S_2218 | CsuA/B | 1.5 | Biofilm formation |
| A1S_1510 | Fimbrial protein | 1.16 | Biofilm formation |
| A1S_0092 | Putative ferric siderophore receptor protein | 0.64 | Iron uptake |
| A1S_1631 | Iron-binding protein | 0.82 | Iron uptake |
| A1S_1921 | Ferrichrome-iron receptor | 0.95 | Iron uptake |
| A1S_2080 | Putative siderophore receptor | 0.82 | Iron uptake |
| A1S_2081 | TonB-dependent siderophore receptor | 0.79 | Iron uptake |
| A1S_2570 | Putative siderophore biosynthesis protein; putative acetyltransferase | 0.84 | Iron uptake |
| A1S_3324 | Putative ferric siderophore receptor protein | 0.61 | Iron uptake |
| A1S_2664 | Chaperone Hsp60 | −0.97 | Stress response |
| A1S_2665 | Chaperone Hsp10 | −1.05 | Stress response |
A complete lists of proteins with altered expression at 28°C is provided in Table S1 in the supplemental material.
The expression of a number of membrane proteins potentially involved in antibiotic resistance was altered at 28°C, compared to 37°C (Table 2); these include proteins that are part of the resistance-nodulation-division (RND) efflux systems, such as A1S_0116, A1S_0908, AdeA, AdeK, A1S_3219, and A1S_0774. There was also differential expression of outer membrane porin proteins, such as A1S_1968 and OmpA. OmpA, which is considered the major porin in A. baumannii (29), has also been shown to be an important virulence factor that plays a role in the interaction of A. baumannii with eukaryotic cells (30).
We also observed downregulation of a β-lactamase protein (A1S_1708) at 28°C (Table 2). Expression of this protein appears to be at least partly regulated by the histone-like nucleoid-structuring (H-NS) protein, which regulates various virulence-associated genes in A. baumannii (14). In our study, downregulation of A1S_1708 at 28°C may explain small but reproducible increases in the susceptibility of A. baumannii to imipenem at this temperature (Table 1). However, the role of A1S_1708 in the antibiotic susceptibility of A. baumannii needs to be studied further.
Among the proteins overexpressed at 28°C, of note were those encoded by the csu operon. The csuA/BABCDE operon was shown to be involved in pilus formation and in the early steps of biofilm formation in A. baumannii ATCC 19606 (25). We observed upregulation of CsuA/B, CsuA, CsuB, CsuC, CsuD, and CsuE (Table 2). Overexpression of the fimbrial protein A1S_1510 was also observed. A1S_1510 has been shown to play a role in biofilm formation by A. baumannii (31). This is consistent with our observation that A. baumannii ATCC 17978 forms more biofilm at 28°C than at 37°C, perhaps due to upregulation of the csu operon at 28°C.
Interestingly, we also observed that proteins involved in phenyl acetate catabolism were downregulated at 28°C. The genes that encode these proteins are found in the paa operon, and PaaC, PaaJ, PaaK, PaaN, and PaaX were detected in our data set. Paa proteins were recently shown to be important for the virulence of A. baumannii in a zebra fish infection model (32). However, we did not observe any statistically significant differences in the virulence in G. mellonella of A. baumannii ATCC 17978 grown at two different temperatures. Therefore, the impact of altered expression of the paa operon under different conditions on the virulence of A. baumannii ATCC 17978 in G. mellonella needs to be investigated further.
Another intriguing observation in this study was the upregulation at 28°C of numerous iron uptake proteins, namely, A1S_0092, A1S_1631, A1S_1921, A1S_2080, A1S_2081, A1S_2570, and A1S_3324. Our data suggest that growth at 28°C may mimic the response of A. baumannii to iron-limiting conditions, which may be due to lower iron transport efficiency at this temperature. Iron is one of the most important micronutrients that plays a key role in the success of a pathogen in a host (33); it serves as an important signal for the expression of various genes, including those involved in virulence, in pathogenic bacteria such as A. baumannii (34). Therefore, it is possible that temperature serves as an additional signal for the expression of iron uptake proteins in A. baumannii.
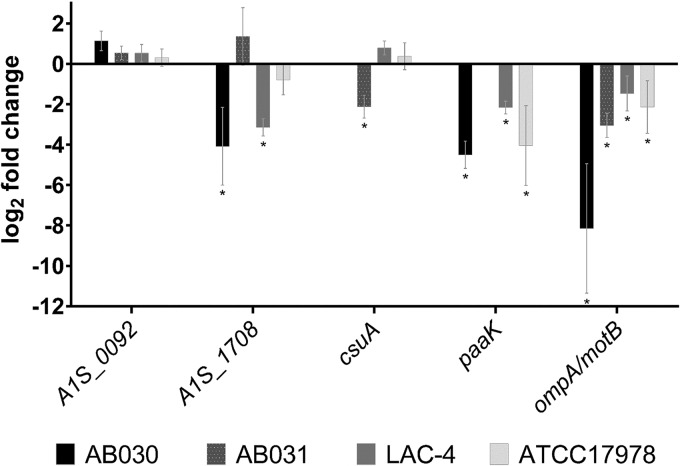
In order to determine whether the altered protein expression at 28°C versus 37°C is also displayed by clinical isolates of A. baumannii, we tested the expression of ompA, csuA, A1S_0116 (an RND transporter-encoding gene), A1S_1708 (a β-lactamase), paaK, and A1S_0092 (a putative ferrichrome receptor-encoding gene) in clinical isolates of A. baumannii using quantitative real-time reverse transcription-PCR (qRT-PCR). These genes encode a subset of proteins whose expression was altered in our proteomic analysis (Table 2). We tested gene expression in three different clinical isolates, i.e., AB030 (35), AB031 (36), and LAC-4 (37). These clinical isolates represent antibiotic-resistant and hypervirulent isolates of A. baumannii. We observed that the expression pattern of the genes at the two different temperatures in clinical isolates generally agreed with our observations in ATCC 17978 (Fig. 6). For example, the expression of paaK and ompA/motB in the clinical isolates was similar to that observed for ATCC 17978. Interestingly though, we did not detect paaK in AB031, and further analysis using endpoint PCR and genome analysis confirmed the absence of the gene in this strain. Furthermore, we did not observe any statistically significant changes in the expression of A1S_0092 (a putative ferrichrome receptor gene) at the two different temperatures in ATCC 17978 or the clinical isolates tested. Finally, we did not detect A1S_0116 transcripts in any of the clinical strains. Therefore, our observations from the expression analysis of a limited subset of genes suggest that the altered expression of proteins we observed at the two different incubation temperatures is likely to be a combination of regulation at the transcription and translation levels. The data also suggest that the altered expression of proteins in response to changes in the incubation temperature is likely to be present in the clinical isolates of A. baumannii as well.
FIG 6.
Expression of A1S_0092, A1S_1708, csuA, paaK, and ompA/motB in A. baumannii ATCC 17978 and the clinical isolates AB030, LAC-4, and AB031. Expression was determined using qRT-PCR, and 16S rRNA was used as the housekeeping control. Data shown are the relative levels of expression of each gene at 28°C versus 37°C for the respective isolate and are representative of at least two biological replicates. The csuA and paaK genes were not detected in AB030 and AB031, respectively. The paaK gene was found to be absent in AB031, which was was confirmed using the endpoint PCR as well as analysis of the genome sequence. *, P < 0.05.
Conclusions.
In conclusion, we show that traits such as antibiotic resistance, biofilm formation, and twitching motility can be thermoregulated in A. baumannii ATCC 17978. To our knowledge, this is the first study to report such findings. Using proteomic analysis, we identified >600 different proteins whose expression was altered in A. baumannii ATCC 17978 in response to the incubation temperature. We were able to establish that the expression of virulence factors such as OmpA and Paa proteins was greater at 37°C than at 28°C, which is suggestive of A. baumannii expressing virulence factors in response to the changes in growth temperature as it moves into the human host. We also observed changes in the expression of antibiotic resistance genes in response to the temperature changes. Our work shows that temperature can influence the virulence and antibiotic resistance of A. baumannii. We think that our work will lead to a better understanding of how the environment, specifically temperature, can have an impact on the antibiotic resistance and virulence of this important pathogen.
MATERIALS AND METHODS
Bacterial strain and growth conditions.
A. baumannii ATCC 17978 was used for our study. Lysogeny broth (LB) Lennox (Difco; Becton Dickinson, Sparks, MD, USA) was used to culture the organism with shaking at 250 rpm, unless otherwise indicated.
Comparison of A. baumannii ATCC 17978 growth at 28°C versus 37°C.
A. baumannii ATCC 17978 was grown overnight in 3 ml of LB Lennox at 37°C and 28°C, with shaking. Overnight cultures were then subcultured in fresh medium and grown to an early log phase (A600 of 0.6 to 0.8). Early-log-phase cultures were diluted 1:100 (vol/vol) in fresh LB in a 100-μl final volume in each well of a sterile 96-well polystyrene plate (Sarstedt, Montreal, Canada), and growth was monitored at 28°C and 37°C, with shaking, by measuring the A600 of the culture every 15 min for 12 h, using a SpectraMax M2 microplate reader (Molecular Devices, Sunnyvale, CA, USA). Results of at least two independent replicates were plotted and statistically analyzed using GraphPad Prism 6.07 (GraphPad, La Jolla, CA, USA).
Antibiotic susceptibility assays.
The susceptibility of A. baumannii ATCC 17978 to piperacillin-tazobactam, ceftriaxone, cefepime, ceftazidime, imipenem, doripenem, ciprofloxacin, moxifloxacin, aztreonam, trimethoprim-sulfamethoxazole, and gentamicin was tested using Etest strips (bioMérieux, Saint-Laurent, Canada) on Mueller-Hinton agar (Sigma-Aldrich, St. Louis, MO, USA) plates, in accordance with the manufacturer's instructions. Incubation was carried out at 28°C and 37°C for 18 h.
MIC determination using the broth dilution method was carried out using the guidelines provided by the Clinical and Laboratory Standards Institute (38). Briefly, overnight cultures grown at either 28°C or 37°C were subcultured in fresh LB and grown to the early log phase at the respective temperature. The cells were then normalized by dilution in 0.85% saline using a 0.5 McFarland standard, and 120 μl of standardized cells was mixed with 6 ml of fresh Mueller-Hinton broth (MHB). A 2-fold dilution series of aztreonam and trimethoprim-sulfamethoxazole in MHB, with 50 μl in each well, was made in a round-bottom 96-well plate (Sarstedt), and 50 μl of cells was added to each well. The plates were then incubated at either 28°C or 37°C for 18 h before the MIC values were recorded.
Biofilm assay.
Biofilm formation on plastic surfaces by A. baumannii ATCC 17978 at 28°C and 37°C was determined using a previously described method (39). Briefly, overnight cultures of A. baumannii ATCC 17978 were adjusted to an A600 of 0.05, inoculated at a 1% (vol/vol) dilution in a 100-μl final volume in a sterile 96-well polystyrene plate (Sarstedt), and incubated at either 28°C and 37°C for 48 h. The wells were then washed with distilled water and stained with 1% (wt/vol) crystal violet, and the stained biofilm was solubilized with 2% sodium dodecyl sulfate (SDS) for quantification by determination of A580 values using a SpectraMax M2 reader (Molecular Devices). Data obtained from at least two independent experiments with three or more technical replicates were plotted and statistically analyzed using GraphPad Prism 6.07.
Motility assay.
The surface motility of A. baumannii ATCC 17978 was assessed using 0.3% agarose plates, with an adapted version of a previously described method (40). Briefly, 3 μl of an overnight culture was stabbed into the middle of the motility plates and incubated for 18 h at either 28°C and 37°C. The resulting area of motility was measured and photographed. Assays were carried out with 10 biological replicates.
Virulence assay in Galleria mellonella.
The virulence of A. baumannii ATCC 17978 was assayed using Galleria mellonella larvae, following a method described previously (26), with slight modifications. A. baumannii was grown overnight (at 28°C and 37°C), and the bacterial load for infection was standardized to 1 × 106 CFU/ml in saline (0.85% NaCl) using DensiCHEK Plus (bioMérieux). Ten microliters of the standardized culture was injected into the second right proleg of the worm. Ten microliters of saline was injected in worms used as controls. Injected worms were then incubated at 28°C and 37°C for 72 h and monitored for survival every 6 h. Results of at least two independent experiments were plotted in a Kaplan-Meier survival curve and statistically analyzed using GraphPad Prism 6.0.
Proteomic analysis.
Overnight cultures of ATCC 17978 were diluted 1:100 in 500 ml of LB and incubated at 28°C and 37°C to an A600 of ∼0.8. Cells were harvested by centrifugation at 6,000 × g for 15 min at 4°C, and pellets were stored at −80°C until used. Three biological replicates were used for each growth condition. Cell pellets were homogenized in sterile Milli-Q water, mixed with 100 μl of 0.1-mm glass beads (Scientific Industries Inc., Bohemia, NY, USA), heated for 5 min at 95°C, and vortex-mixed vigorously for 3 min, followed by centrifugation and removal of the supernatant into a 15-ml conical tube. Cold sterile Milli-Q water was added to the beads, followed by vortex-mixing and centrifugation to wash the beads and to extract protein (total of six wash/extraction steps); the supernatants from each step were pooled, mixed thoroughly, and stored at −80°C.
Protein was quantified using a bicinchoninic acid (BCA) protein assay kit, with bovine serum albumin (BSA) as the standard (Pierce Protein Research Products; Thermo Fisher Scientific). A total of 100 μg of protein from each sample in triplicate for cultures of ATCC 17978 grown at 28°C and 37°C was used for each digestion/labeling reaction. Protein samples were digested with trypsin (Promega, Madison, WI, USA) overnight using a filter-assisted sample preparation (FASP) method described previously (41). iTRAQ labeling with 4-plex kits was performed according to the manufacturer's recommended protocol (Sciex, Framingham, MA, USA). Peptides from each of the three replicates of ATCC 17978 grown at 28°C and 37°C, respectively, were labeled and three sets prepared by mixing peptides from one replicate grown at 37°C and one grown at 28°C each in a 1:1 ratio. The mixture was vacuum dried and rehydrated using offline liquid chromatography (LC) buffer A (20 mM ammonium formate [pH 10]). Each set was then resolved using high pH reversed-phase liquid chromatography to yield 16 fractions per set. Each fraction was dissolved in nanoLC buffer A (2% acetonitrile [vol/vol, in mass spectrometry grade water], 0.1% formic acid) for mass spectrometry analysis.
Each peptide fraction was analyzed using a nano-flow Easy nLC II system in line with an LTQ Orbitrap Velos mass spectrometer (Thermo Fisher Scientific). The peptide sample (1 μg) was loaded onto a C18 reverse-phase analytical column (length, 20 cm; inner diameter, 75 μm; particle size, 2.4 μm) with 100% nano-LC buffer A, and peptides were eluted using a 217-min linear gradient of 2 to 30% nano-LC buffer B (98% acetonitrile, 0.1% formic acid, vol/vol in mass spectrometry-grade water). The total nano-LC-tandem mass spectrometry (MS/MS) run time was 260 min. A data-dependent acquisition method was used, dynamically choosing the top 10 abundant precursor ions from each survey scan (m/z 300 to 1,700) for fragmentation by high-energy collision dissociation (HCD). MS2 scans were acquired in the Orbitrap with a target resolution of 7,500 at 400 m/z over a dynamic m/z range.
Mascot 2.5 (Matrix Science, Boston, MA, USA) was used for peptide identification, searching against the sequence of the wild-type parent strain ATCC 17978 as the reference (accession number GCA_000015425.1) (3,803 sequences, including 1,121,966 residues); Scaffold Q+ 4 (Proteome Software, Portland, OR, USA) was used for protein filtering (protein false discovery rate [FDR] set to 1.0% and peptide FDR set to 0.1%, with a 2-peptide minimum) and for quantification of the iTRAQ labels. The Benjamini-Hochberg correction method was used to filter proteins with P values of <0.05. Final data analysis was carried out using ratios of ATCC 17978 protein data from bacteria grown at 37°C and 28°C, in Microsoft Excel software.
RNA extraction and qRT-PCR.
Bacterial cultures grown overnight in 3 ml of LB at either 28°C or 37°C, with shaking at 250 rpm, were subcultured at 1:100 (vol/vol) in fresh LB and grown to an A600 of 0.6 at the respective temperature. The cells were then pelleted and frozen at −70°C for 4 to 6 h prior to RNA extraction, to facilitate cell lysis. RNA extraction was carried out using a Qiagen RNeasy minikit (Qiagen, Mississauga, Canada), following the manufacturer's instructions, and the extracted RNA was quantified using a NanoDropLite spectrophotometer (Thermo Scientific). A total of 750 ng of RNA from each biological sample was used for the cDNA synthesis process, after digestion with DNase I (Qiagen) to remove any genomic DNA carryover. cDNA was synthesized using a SuperScript VILOcDNA synthesis kit (Invitrogen, Carlsbad, CA, USA), following the manufacturer's instructions, in a total reaction volume of 20 μl for each sample.
qRT-PCR assays were carried out using SYBR Select master mix (Applied Biosystems) in a StepOne Plus real-time PCR system (Applied Biosystems); the primers used are listed in Table 3. The 16S rRNA gene expression level was used as the internal control for normalization of the expression levels for each target gene. The fold change for each target was calculated using the cycle threshold (CT), based on the Pfaffl method (42). The calculated values were then plotted and statistically analyzed using GraphPad Prism 6.07.
TABLE 3.
Oligonucleotides used in this study
| Primer name | Sequence | Reference | Product size (bp) |
|---|---|---|---|
| 16S RT F | CTTCGGACCTTGCGCTAATA | This study | 59 |
| 16S RT R | ATCCTCTCAGACCCGCTACA | This study | |
| A1S_0092 RT F | ACGTGCCAATACCCAAGAAC | This study | 92 |
| A1S_0092 RT R | CATGAGCCACTGCTAAACGA | This study | |
| A1S_0116 RT F | CAGCCACAAGTGTTGGAGAA | This study | 114 |
| A1S_0116 RT R | CTCGATACCTTTCGCCTGAC | This study | |
| A1S_1708 RT F | CGCTGCAGATTTACTTCGTG | This study | 113 |
| A1S_1708 RT R | TGCCAACTTTAAGCATGCAA | This study | |
| csuA RT F | TTTGGTGAAGCTACCACAGC | This study | 89 |
| cusA RT R | CCAGCACACTCGATCTGAAA | This study | |
| paaK RT F | CGCTTAGGTGCAACGGTTAT | This study | 99 |
| paaK RT R | TGGCGTAACCATAATTGCAG | This study | |
| ompA RT F | TCAAGGTTTCTTGGCGACTT | This study | 103 |
| ompA RT R | GGTAAAAGCAGCCGCATAAG | This study |
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Discovery Grants program of the Natural Science and Engineering Council of Canada (grant RGPIN-2015-05550) and Research Manitoba (A.K.). P.M.D.S. was supported by a University of Manitoba Graduate Fellowship. D.M.F. was supported by a grant from the Canada Research Chair program to Peter C. Loewen, University of Manitoba. The proteomic analysis was supported, in part, by the Public Health Agency of Canada.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01514-17.
REFERENCES
- 1.Doi Y, Murray GL, Peleg AY. 2015. Acinetobacter baumannii: evolution of antimicrobial resistance—treatment options. Semin Respir Crit Care Med 36:85–98. doi: 10.1055/s-0034-1398388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peleg AY, Paterson DL. 2006. Multidrug-resistant Acinetobacter: a threat to the antibiotic era. Intern Med J 36:479–482. doi: 10.1111/j.1445-5994.2006.01130.x. [DOI] [PubMed] [Google Scholar]
- 3.Peleg AY, Seifert H, Paterson DL. 2008. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamouda A, Findlay J, Al Hassan L, Amyes SGB. 2011. Epidemiology of Acinetobacter baumannii of animal origin. Int J Antimicrob Agents 38:314–318. doi: 10.1016/j.ijantimicag.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Lupo A, Vogt D, Seiffert SN, Endimiani A, Perreten V. 2014. Antibiotic resistance and phylogenetic characterization of Acinetobacter baumannii strains isolated from commercial raw meat in Switzerland. J Food Prot 77:1976–1981. doi: 10.4315/0362-028X.JFP-14-073. [DOI] [PubMed] [Google Scholar]
- 6.Fernando DM, Khan IUH, Patidar R, Lapen DR, Talbot G, Topp E, Kumar A. 2016. Isolation and characterization of Acinetobacter baumannii recovered from Campylobacter selective medium. Front Microbiol 7:1871. doi: 10.3389/fmicb.2016.01871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Timsit J-F, Soubirou J-F, Voiriot G, Chemam S, Neuville M, Mourvillier B, Sonneville R, Mariotte E, Bouadma L, Wolff M. 2014. Treatment of bloodstream infections in ICUs. BMC Infect Dis 14:489. doi: 10.1186/1471-2334-14-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornejo-Juárez P, Vilar-Compte D, Pérez-Jiménez C, Ñamendys-Silva SA, Sandoval-Hernández S, Volkow-Fernández P. 2015. The impact of hospital-acquired infections with multidrug-resistant bacteria in an oncology intensive care unit. Int J Infect Dis 31:31–34. doi: 10.1016/j.ijid.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 9.McDonald LC, Banerjee SN, Jarvis WR, National Nosocomial Infection Surveillance System. 1999. Seasonal variation of Acinetobacter infections: 1987–1996. Clin Infect Dis 29:1133–1137. doi: 10.1086/313441. [DOI] [PubMed] [Google Scholar]
- 10.Eliopoulos GM, Maragakis LL, Perl TM. 2008. Acinetobacter baumannii: epidemiology, antimicrobial resistance, and treatment options. Clin Infect Dis 46:1254–1263. doi: 10.1086/529198. [DOI] [PubMed] [Google Scholar]
- 11.Castanheira M, Mendes RE, Jones RN. 2014. Update on Acinetobacter species: mechanisms of antimicrobial resistance and contemporary in vitro activity of minocycline and other treatment options. Clin Infect Dis 59(Suppl 6):S367–S373. doi: 10.1093/cid/ciu706. [DOI] [PubMed] [Google Scholar]
- 12.Lin MF, Lan CY. 2014. Antimicrobial resistance in Acinetobacter baumannii: from bench to bedside. World J Clin Cases 2:787–814. doi: 10.12998/wjcc.v2.i12.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kassamali Z, Jain R, Danziger LH. 2015. An update on the arsenal for multidrug-resistant Acinetobacter infections: polymyxin antibiotics. Int J Infect Dis 30:125–132. doi: 10.1016/j.ijid.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 14.Eijkelkamp BA, Stroeher UH, Hassan KA, Elbourne LDH, Paulsen IT, Brown MH. 2013. H-NS plays a role in expression of Acinetobacter baumannii virulence features. Infect Immun 81:2574–2583. doi: 10.1128/IAI.00065-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris G, Lee RK, Lam CK, Kanzaki G, Patel GB, Xu HH, Chen W. 2013. A mouse model of Acinetobacter baumannii-associated pneumonia using a clinically isolated hypervirulent strain. Antimicrob Agents Chemother 57:3601–3613. doi: 10.1128/AAC.00944-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McConnell MJ, Actis L, Pachón J. 2013. Acinetobacter baumannii: human infections, factors contributing to pathogenesis and animal models. FEMS Microbiol Rev 37:130–155. doi: 10.1111/j.1574-6976.2012.00344.x. [DOI] [PubMed] [Google Scholar]
- 17.Cerqueira GM, Kostoulias X, Khoo C, Aibinu I, Qu Y, Traven A, Peleg AY. 2014. A global virulence regulator in Acinetobacter baumannii and its control of the phenylacetic acid catabolic pathway. J Infect Dis 210:46–55. doi: 10.1093/infdis/jiu024. [DOI] [PubMed] [Google Scholar]
- 18.Tilley D, Law R, Warren S, Samis JA, Kumar A. 2014. CpaA a novel protease from Acinetobacter baumannii clinical isolates deregulates blood coagulation. FEMS Microbiol Lett 356:53–61. doi: 10.1111/1574-6968.12496. [DOI] [PubMed] [Google Scholar]
- 19.Gayoso CM, Mateos J, Mendez JA, Fernandez-Puente P, Rumbo C, Tomas M, Martinez de Ilarduya O, Bou G. 2014. Molecular mechanisms involved in the response to desiccation stress and persistence in Acinetobacter baumannii. J Proteome Res 13:460–476. doi: 10.1021/pr400603f. [DOI] [PubMed] [Google Scholar]
- 20.Wright MS, Haft DH, Harkins DM, Perez F, Hujer KM, Bajaksouzian S, Benard MF, Jacobs MR, Bonomo RA, Adams MD. 2014. New insights into dissemination and variation of the health care-associated pathogen Acinetobacter baumannii from genomic analysis. mBio 5:e00963-13. doi: 10.1128/mBio.00963-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grosso-Becerra MV, Croda-García G, Merino E, Servín-González L, Mojica-Espinosa R, Soberón-Chávez G. 2014. Regulation of Pseudomonas aeruginosa virulence factors by two novel RNA thermometers. Proc Natl Acad Sci U S A 111:15562–15567. doi: 10.1073/pnas.1402536111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plumley BA, Martin KH, Borlee GI, Marlenee NL, Burtnick MN, Brett PJ, AuCoin DP, Bowen RA, Schweizer HP, Borlee BR. 2017. Thermoregulation of biofilm formation in Burkholderia pseudomallei is disrupted by mutation of a putative diguanylate cyclase. J Bacteriol 199:e00780-16. doi: 10.1128/JB.00780-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roca I, Espinal P, Vila-Farres X, Vila J. 2012. The Acinetobacter baumannii oxymoron: commensal hospital dweller turned pan-drug-resistant menace. Front Microbiol 3:148. doi: 10.3389/fmicb.2012.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stacy DM, Welsh MA, Rather PN, Blackwell HE. 2012. Attenuation of quorum sensing in the pathogen Acinetobacter baumannii using non-native N-acyl homoserine lactones. ACS Chem Biol 7:1719–1728. doi: 10.1021/cb300351x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomaras AP, Dorsey CW, Edelmann RE, Actis LA. 2003. Attachment to and biofilm formation on abiotic surfaces by Acinetobacter baumannii: involvement of a novel chaperone-usher pili assembly system. Microbiology 149:3473–3484. doi: 10.1099/mic.0.26541-0. [DOI] [PubMed] [Google Scholar]
- 26.Peleg AY, Jara S, Monga D, Eliopoulos GM, Moellering RC, Mylonakis E. 2009. Galleria mellonella as a model system to study Acinetobacter baumannii pathogenesis and therapeutics. Antimicrob Agents Chemother 53:2605–2609. doi: 10.1128/AAC.01533-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Camarena L, Bruno V, Euskirchen G, Poggio S, Snyder M. 2010. Molecular mechanisms of ethanol-induced pathogenesis revealed by RNA-sequencing. PLoS Pathog 6:e1000834. doi: 10.1371/journal.ppat.1000834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shin B, Park W. 2015. Synergistic effect of oleanolic acid on aminoglycoside antibiotics against Acinetobacter baumannii. PLoS One 10:e0137751. doi: 10.1371/journal.pone.0137751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sugawara E, Nikaido H. 2012. OmpA is the principal nonspecific slow porin of Acinetobacter baumannii. J Bacteriol 194:4089–4096. doi: 10.1128/JB.00435-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaddy JA, Tomaras AP, Actis LA. 2009. The Acinetobacter baumannii 19606 OmpA protein plays a role in biofilm formation on abiotic surfaces and in the interaction of this pathogen with eukaryotic cells. Infect Immun 77:3150–3160. doi: 10.1128/IAI.00096-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nait Chabane Y, Marti S, Rihouey C, Alexandre S, Hardouin J, Lesouhaitier O, Vila J, Kaplan JB, Jouenne T, Dé E. 2014. Characterisation of pellicles formed by Acinetobacter baumannii at the air-liquid interface. PLoS One 9:e111660. doi: 10.1371/journal.pone.0111660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhuiyan MS, Ellett F, Murray GL, Kostoulias X, Cerqueira GM, Schulze KE, Mahamad Maifiah MH, Li J, Creek DJ, Lieschke GJ, Peleg AY. 2016. Acinetobacter baumannii phenylacetic acid metabolism influences infection outcome through a direct effect on neutrophil chemotaxis. Proc Natl Acad Sci U S A 113:9599–9604. doi: 10.1073/pnas.1523116113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carpenter BM, Whitmire JM, Merrell DS. 2009. This is not your mother's repressor: the complex role of fur in pathogenesis. Infect Immun 77:2590–2601. doi: 10.1128/IAI.00116-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nwugo CC, Gaddy JA, Zimbler DL, Actis LA. 2011. Deciphering the iron response in Acinetobacter baumannii: a proteomics approach. J Proteomics 74:44–58. doi: 10.1016/j.jprot.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loewen PC, Alsaadi Y, Fernando D, Kumar A. 2014. Genome sequence of an extremely drug-resistant clinical isolate of Acinetobacter baumannii strain AB030. Genome Announc 2:e01035-14. doi: 10.1128/genomeA.01035-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loewen PC, Alsaadi Y, Fernando D, Kumar A. 2014. Genome sequence of a tigecycline-resistant clinical isolate of Acinetobacter baumannii strain AB031 obtained from a bloodstream infection. Genome Announc 2:e01036-14. doi: 10.1128/genomeA.01036-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ou H-Y, Kuang SN, He X, Molgora BM, Ewing PJ, Deng Z, Osby M, Chen W, Xu HH. 2015. Complete genome sequence of hypervirulent and outbreak-associated Acinetobacter baumannii strain LAC-4: epidemiology, resistance genetic determinants and potential virulence factors. Sci Rep 5:8643. doi: 10.1038/srep08643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clinical and Laboratory Standards Institute. 2017. Performance standards for antimicrobial susceptibility testing, 27th ed M100-27. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 39.Iwashkiw JA, Seper A, Weber BS, Scott NE, Vinogradov E, Stratilo C, Reiz B, Cordwell SJ, Whittal R, Schild S, Feldman MF. 2012. Identification of a general O-linked protein glycosylation system in Acinetobacter baumannii and its role in virulence and biofilm formation. PLoS Pathog 8:e1002758. doi: 10.1371/journal.ppat.1002758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harding CM, Tracy EN, Carruthers MD, Rather PN, Actis LA, Munson RS. 2013. Acinetobacter baumannii strain M2 produces type IV pili which play a role in natural transformation and twitching motility but not surface-associated motility. mBio 4:e00360-13. doi: 10.1128/mBio.00360-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wisniewski JR, Zougman A, Nagaraj N, Mann M. 2009. Universal sample preparation method for proteome analysis. Nat Methods 6:359–362. doi: 10.1038/nmeth.1322. [DOI] [PubMed] [Google Scholar]
- 42.Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.