LETTER
Besides Acinetobacter baumannii, Acinetobacter pittii is an important nosocomial pathogen (1, 2). Carbapenems are the antimicrobial agents of choice for the treatment of infections with multidrug-resistant Acinetobacter spp. However, the incidence of carbapenem-resistant strains has risen over the last decade, mainly because of the acquisition of oxacillinases (OXA-23, -40, -58, -143, and -235) and less frequently of metallo-beta-lactamases (e.g., NDM, GIM, IMP) and the overexpression of the intrinsic OXA-51-like oxacillinase (2, 3). Also for human A. pittii isolates, different carbapenemase genes have been determined, including blaOXA-58 and blaNDM-1 (4–12). However, in animals, there is only one report of a carbapenem-resistant A. pittii isolate, namely, an OXA-40-positive strain from a rabbit in Lebanon (13).
In the present study, a PCR-based screening of 200 clinical A. pittii isolates from dogs (n = 110), cats (n = 48), rabbits (n = 13), horses (n = 10), and other animals (n = 19) collected between 2008 and 2017 for β-lactamase genes blaOXA-23-like, blaOXA-40-like, blaOXA-58-like, blaVIM-like, blaNDM-like, and blaIMP-like (14, 15) revealed five (2.5%) blaOXA-58-positive strains (Table 1). All of the carriers had different owners and had been admitted to two epidemiologically unlinked veterinary clinics in Germany between February 2014 and September 2016. Pulsed-field gel electrophoresis (15) divided the strains into pulsotypes A and B according to their origin from veterinary clinics A and B, respectively. This suggests the maintenance of pulsotype A strains in clinic A for >2 years and pulsotype B strains in clinic B for about 1 month.
TABLE 1.
Characteristics of OXA-58-producing A. pittii isolates from companion animals in Germany
| Strain | Date of isolation | Host | Sample (disease) | Other bacteria isolated | Veterinary clinic | Pulsotype | ST | Antimicrobial resistancea | Resistance genes |
|---|---|---|---|---|---|---|---|---|---|
| IHIT24944 | 2/2014 | Dog 1 | Nose swab (rhinitis, sinusitis) | Acinetobacter baumannii, Bacillus sp. Micrococcus sp. | A | A | 93 | FEP, CTZ, FEP, CFP, CTU, GEN, CIP, MOX, ENR, MAR, TET, TOB, NIT, SXT | aacC2, aph(3′)-Ic, strA, strB, sul2, blaOXA-500, blaADC-18-like, blaOXA-58, tet(39) |
| IHIT32473 | 7/2016 | Dog 2 | Bronchial fluid (respiratory tract infection) | Acinetobacter johnsonii, Pseudomonas sp., Candida sp. | A | A | 93 | FEP, CTZ, FEP, CFP, CTU, CPD, GEN, CIP, MOX, ENR, MAR, TET, TOB, NIT, SXT | aacC2, aph(3′)-Ic, strA, strB, sul2, blaOXA-500, blaADC-18-like, blaOXA-58, tet(39) |
| IHIT32685 | 9/2016 | Rabbit | Nose (none, screening) | Bacillus sp., Candida sp., Streptococcus sp. | A | A | 93 | FEP, CTZ, FEP, CFP, CTU, CPD, GEN, CIP, MOX, ENR, MAR, TET, TOB, NIT, SXT | aacC2, aph(3′)-Ic, strA, strB, sul2, blaOXA-500, blaADC-18-like, blaOXA-58, tet(39) |
| IHIT29469 | 5/2015 | Cat 1 | Nose swab (suppurating rhinitis) | Bordetella bronchiseptica, Staphylococcus simulans, Micrococcus sp. | B | B | 93 | CPD, CTU, GEN, CIP, MOX, ENR, TET, NIT, SXT | aacC2, aph(3′)-Ic, strA, strB, sul2, blaOXA-500, blaADC-18-like, blaOXA-58, tet(39) |
| IHIT29592 | 6/2015 | Cat 2 | Skin swab (purulent dermatitis) | Acinetobacter sp., Bacillus sp., Pasteurella multocida | B | B | 93 | CPD, CTU, CFP, GEN, CIP, MOX, ENR, TET, NIT, SXT | aacC2, aph(3′)-Ic, strA, strB, sul2, blaOXA-500, blaADC-18-like, blaOXA-58, tet(39) |
All isolates were resistant to ampicillin (AMP), amoxicillin-clavulanate (AMC), chloramphenicol (CHL), fosfomycin (FOS), cephalexin (LEX), and piperacillin (PIP) in accordance with the definitions of intrinsic resistance for members of the Acinetobacter baumannii complex set by EUCAST and CLSI. All isolates were susceptible to amikacin (AMK), imipenem (IPM), meropenem (MER), polymyxin B (PMB), tigecycline (TGC), tobramycin (TOB), and piperacillin/tazobactam (TZP). CPD, cefpodoxime; CTZ, ceftazidime; FEP, cefepime; CFP, cefpirome; CTU, ceftiofur; ENR, enrofloxacin; GEN, gentamicin; MAR, marbofloxacin; TET, tetracycline; SXT, sulfamethoxazole-trimethoprim; CIP, ciprofloxacin; MOX, moxifloxacin; FOS, fosfomycin; NIT, nitrofurantoin; CHL, chloramphenicol; TZP, piperacillin-tazobactam.
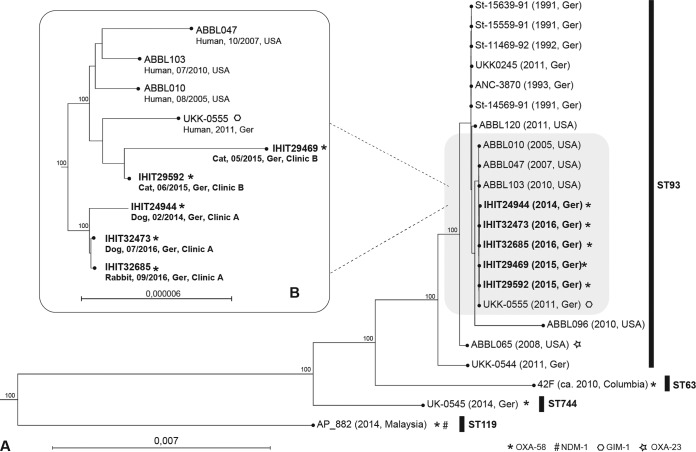
On the basis of whole-genome sequencing data and the use of MLST (multilocus sequence type) Finder 1.8 (https://cge.cbs.dtu.dk/services/), all isolates were assigned to sequence type 93 (ST93) according to the Pasteur scheme (http://pubmlst.org/abaumannii). A maximum common genome (MCG) analysis based on 2,770 orthologous genes of 17 publicly available genomes of ST93 and OXA-58-positive A. pittii isolates from humans and our isolates was performed (16, 17). Pulsotype A isolates clustered separately from pulsotype B isolates but together with strains isolated from human patients in Germany and the United States (Fig. 1). The high relatedness (88 single nucleotide polymorphisms in the MCG) of feline OXA-58 strain IHIT29469 to GIM-1-positive clinical strain UKK-0555, which was isolated in 2011 in Germany, indicates that humans and companion animals may be infected by similar A. pittii genotypes, which has already been reported for other bacteria, including methicillin-resistant Staphylococcus aureus, extended-spectrum beta-lactamase producers, and multidrug-resistant A. baumannii (15, 18, 19).
FIG 1.
(A) Maximum-likelihood tree (based on 2,770 orthologous genes) of 17 publicly available genomes of ST93 and/or OXA-58-positive A. pittii isolates from humans (lightface) and five animal isolates (bold) from this study. (B) Excerpt of the tree in panel A providing higher resolution of closely related animal and human strains. The host, isolation date, and country are shown with the strain number. Scales indicate the numbers of nucleotide substitutions per site. Bootstrap values are based on 1,000 iterations. Ger, Germany.
We detected aacC2, aph(3′)-Ic, strA, strB, sul2, and tet(39) as additional resistance genes in our A. pittii isolates (https://cge.cbs.dtu.dk/services/ResFinder/). They all showed resistance to ampicillin, amoxicillin-clavulanate, piperacillin, cephalexin, ceftiofur, gentamicin, enrofloxacin, marbofloxacin, tetracycline, nitrofurantoin, and sulfamethoxazole-trimethoprim but were susceptible to imipenem (VITEK2, bioMérieux, AST-card GN38), as interpreted according to breakpoints defined for human Acinetobacter spp. by either EUCAST or CLSI (20, 21). The hydrolytic activity of oxacillinases is normally low, and OXA-58 only confers a resistance phenotype when its expression is enhanced via insertion elements (22). Nevertheless, in A. pittii, OXA-58 does not always confer resistance, even when associated with insertion elements (9). We reassessed the MIC of imipenem after serial passages in broth containing increasing concentrations of meropenem (Sigma-Aldrich, Munich, Germany). The imipenem MICs for the OXA-58 isolates increased >8-fold (from ≥1 to 8 mg/liter), while this was not the case for an A. pittii isolate without an acquired oxacillinase gene, which served as a control.
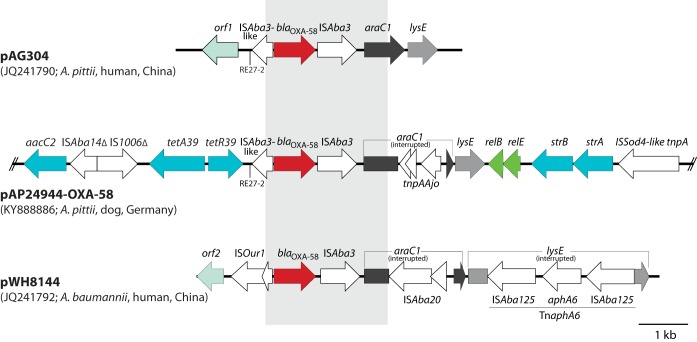
Only one kind of genetic context of blaOXA-58, containing ISAba3, araC, and lysE, was present among the A. pittii isolates (Fig. 2). The direct surroundings of blaOXA-58 in strain IHIT24944 resemble those of carbapenem-susceptible A. pittii AG304 (23) but differ from those of carbapenem-resistant A. baumannii strain WH8144 (Fig. 2). In strain IHIT24944, blaOXA-58 was localized on a 53-kb contig that could be circularized by PCR (see Table S1 in the supplemental material) and sequencing. Plasmid pAP24944 (GenBank accession no. KY888886.1) was 53,802 bp in size, was non-self-transmissible, and additionally harbored genes for streptomycin (strA and strB), aminoglycoside (aacC2), sulfonamide (sul2), and tetracycline [tetA(39)/tetR(39)] resistance. We could determine OXA-58 plasmids similar in size and structure in all of the strains, and they only partially overlapped published Acinetobacter sp. plasmid sequences, as identified by a BLAST search (https://blast.ncbi.nlm.nih.gov/Blast.cgi) (Fig. S1).
FIG 2.
Comparison of the genetic region surrounding blaOXA-58 in pAP24944-OXA-58 (GenBank accession no. KY888886.1; only a partial sequence is shown) with the corresponding regions of pAG304 (JQ241790) of A. pittii strain AG304 and pWH8144 (JQ241792) of A. baumannii strain WH8144. Arrows indicate the extent and direction of genes and open reading frames. The genes (and encoded proteins) shown are araC1 (transcription-regulating protein), lysE (threonine excretion pump protein), aacC2 (aminoglycoside-3-N-acetyltransferase), tetA(39)/tetR(39) (tetracycline efflux pump), relB/relE (putative toxin-antitoxin system), and strA/strB (aminoglycoside 3′-phosphotransferases). orf1, DNA-binding response regulator gene; orf2, putative exodeoxyribonuclease VII subunit. The region with significant DNA sequence identity is shown as a gray box. Re27-2 regions (ATTTAACATAATGGCTGTTATACGAAA) are indicated by vertical lines. The images are drawn to scale from the GenBank entries indicated in parentheses.
This study provides the first evidence of the presence of OXA-58 plasmids in carbapenem-susceptible A. pittii isolates from pets. Colocalization of various resistance genes on these plasmids might enable their spread despite the rare use of carbapenems in these animals.
Accession number(s).
The whole genome sequences of A. pittii strains have been assigned accession numbers NWWC01000000 (IHIT24944), NWWB01000000 (IHIT29469), NWWA01000000 (IHIT29592), NWVZ01000000 (IHIT32473), and NWVY01000000 (IHIT32685). The complete sequence of pAP24944-OXA-58 is available from the GenBank database under accession number KY888886.
Supplementary Material
ACKNOWLEDGMENTS
We thank the team of curators of the Institut Pasteur Acinetobacter MLST system for curating the data and making them publicly available at http://pubmlst.org/abaumannii/.
Peter Klotz received grants from the Engemann Foundation and the Schaumann Foundation. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01993-17.
REFERENCES
- 1.Peleg AY, de Breij A, Adams MD, Cerqueira GM, Mocali S, Galardini M, Nibbering PH, Earl AM, Ward DV, Paterson DL, Seifert H, Dijkshoorn L. 2012. The success of Acinetobacter species; genetic, metabolic and virulence attributes. PLoS One 7:e46984. doi: 10.1371/journal.pone.0046984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dijkshoorn L, Nemec A, Seifert H. 2007. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol 5:939–951. doi: 10.1038/nrmicro1789. [DOI] [PubMed] [Google Scholar]
- 3.Zander E, Bonnin RA, Seifert H, Higgins PG. 2014. Characterization of blaOXA-143 variants in Acinetobacter baumannii and Acinetobacter pittii. Antimicrob Agents Chemother 58:2704–2708. doi: 10.1128/AAC.02618-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang J, Chen Y, Jia X, Luo Y, Song Q, Zhao W, Wang Y, Liu H, Zheng D, Xia Y, Yu R, Han X, Jiang G, Zhou Y, Zhou W, Hu X, Liang L, Han L. 2012. Dissemination and characterization of NDM-1-producing Acinetobacter pittii in an intensive care unit in China. Clin Microbiol Infect 18:E506–E513. doi: 10.1111/1469-0691.12035. [DOI] [PubMed] [Google Scholar]
- 5.Pailhoriès H, Hadjadj L, Mahieu R, Crochette N, Rolain JM, Kempf M. 2017. Fortuitous diagnosis of NDM-1-producing Acinetobacter pittii carriage in a patient from France with no recent history of travel. J Antimicrob Chemother 72:942–944. doi: 10.1093/jac/dkw505. [DOI] [PubMed] [Google Scholar]
- 6.Al Atrouni A, Joly-Guillou ML, Hamze M, Kempf M. 2016. Emergence of NDM-1 and OXA-72 producing Acinetobacter pittii clinical isolates in Lebanon. New Microbes New Infect 12:43–44. doi: 10.1016/j.nmni.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammerum AM, Hansen F, Littauer P. 2015. Use of whole-genome sequencing for characterisation of a ST119 NDM-1-producing Acinetobacter pittii from a patient in Denmark with no history of recent travel. Int J Antimicrob Agents 46:351–352. doi: 10.1016/j.ijantimicag.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Pagano M, Poirel L, Martins AF, Rozales FP, Zavascki AP, Barth AL, Nordmann P. 2015. Emergence of NDM-1-producing Acinetobacter pittii in Brazil. Int J Antimicrob Agents 45:444–445. doi: 10.1016/j.ijantimicag.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 9.Zander E, Fernandez-Gonzalez A, Schleicher X, Dammhayn C, Kamolvit W, Seifert H, Higgins PG. 2014. Worldwide dissemination of acquired carbapenem-hydrolysing class D β-lactamases in Acinetobacter spp. other than Acinetobacter baumannii. Int J Antimicrob Agents 43:375–377. doi: 10.1016/j.ijantimicag.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Kaase M, Szabados F, Pfennigwerth N, Anders A, Geis G, Pranada AB, Rossler S, Lang U, Gatermann SG. 2014. Description of the metallo-β-lactamase GIM-1 in Acinetobacter pittii. J Antimicrob Chemother 69:81–84. doi: 10.1093/jac/dkt325. [DOI] [PubMed] [Google Scholar]
- 11.Zhou S, Chen X, Meng X, Zhang G, Wang J, Zhou D, Guo X. 2015. “Roar” of blaNDM-1 and “silence” of blaOXA-58 co-exist in Acinetobacter pittii. Sci Rep 5:8976. doi: 10.1038/srep08976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schleicher X, Higgins PG, Wisplinghoff H, Korber-Irrgang B, Kresken M, Seifert H. 2013. Molecular epidemiology of Acinetobacter baumannii and Acinetobacter nosocomialis in Germany over a 5-year period (2005-2009). Clin Microbiol Infect 19:737–742. doi: 10.1111/1469-0691.12026. [DOI] [PubMed] [Google Scholar]
- 13.Rafei R, Hamze M, Pailhoriès H, Eveillard M, Marsollier L, Joly-Guillou ML, Dabboussi F, Kempf M. 2015. Extrahuman epidemiology of Acinetobacter baumannii in Lebanon. Appl Environ Microbiol 81:2359–2367. doi: 10.1128/AEM.03824-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gröbner S, Linke D, Schutz W, Fladerer C, Madlung J, Autenrieth IB, Witte W, Pfeifer Y. 2009. Emergence of carbapenem-non-susceptible extended-spectrum beta-lactamase-producing Klebsiella pneumoniae isolates at the university hospital of Tübingen, Germany. J Med Microbiol 58:912–922. doi: 10.1099/jmm.0.005850-0. [DOI] [PubMed] [Google Scholar]
- 15.Ewers C, Klotz P, Leidner U, Stamm I, Prenger-Berninghoff E, Gottig S, Semmler T, Scheufen S. 2017. OXA-23 and ISAba1-OXA-66 class D β-lactamases in Acinetobacter baumannii isolates from companion animals. Int J Antimicrob Agents 49:37–44. doi: 10.1016/j.ijantimicag.2016.09.033. [DOI] [PubMed] [Google Scholar]
- 16.von Mentzer A, Connor TR, Wieler LH, Semmler T, Iguchi A, Thomson NR, Rasko DA, Joffre E, Corander J, Pickard D, Wiklund G, Svennerholm AM, Sjoling A, Dougan G. 2014. Identification of enterotoxigenic Escherichia coli (ETEC) clades with long-term global distribution. Nat Genet 46:1321–1326. doi: 10.1038/ng.3145. [DOI] [PubMed] [Google Scholar]
- 17.Klotz P, Gottig S, Leidner U, Semmler T, Scheufen S, Ewers C. 2017. Carbapenem-resistance and pathogenicity of bovine Acinetobacter indicus-like isolates. PLoS One 12:e0171986. doi: 10.1371/journal.pone.0171986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ewers C, Stamm I, Pfeifer Y, Wieler LH, Kopp PA, Schonning K, Prenger-Berninghoff E, Scheufen S, Stolle I, Gunther S, Bethe A. 2014. Clonal spread of highly successful ST15-CTX-M-15 Klebsiella pneumoniae in companion animals and horses. J Antimicrob Chemother 69:2676–2680. doi: 10.1093/jac/dku217. [DOI] [PubMed] [Google Scholar]
- 19.Ewers C, Bethe A, Wieler LH, Guenther S, Stamm I, Kopp PA, Grobbel M. 2011. Companion animals: a relevant source of extended-spectrum beta-lactamase-producing fluoroquinolone-resistant Citrobacter freundii. Int J Antimicrob Agents 37:86–87. doi: 10.1016/j.ijantimicag.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Clinical and Laboratory Standards Institute. 2016. Performance standards of antimicrobial susceptibility testing, 26th ed CLSI supplement M100S. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 21.European Committee on Antimicrobial Susceptibility Testing. 2016. Breakpoint tables for interpretation of MICs and zone diameters, version 6.0, 2016. European Committee on Antimicrobial Susceptibility Testing, Basel, Switzerland: http://eucast.org. [Google Scholar]
- 22.Evans BA, Amyes SG. 2014. OXA beta-lactamases. Clin Microbiol Rev 27:241–263. doi: 10.1128/CMR.00117-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu Y, Jiang J, Zhou H, Jiang Y, Fu Y, Yu Y, Zhou J. 2014. Characterization of a novel plasmid type and various genetic contexts of blaOXA-58 in Acinetobacter spp. from multiple cities in China. PLoS One 9:e84680. doi: 10.1371/journal.pone.0084680. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.