ABSTRACT
Host chitinases, chitotriosidase and acidic mammalian chitinase (AMCase), improved the antifungal activity of caspofungin (CAS) against Aspergillus fumigatus in vitro. These chitinases are not constitutively expressed in the lung. Here, we investigated whether chitosan derivatives were able to induce chitinase activity in the lungs of neutropenic rats and, if so, whether these chitinases were able to prolong survival of rats with invasive pulmonary aspergillosis (IPA) or of rats with IPA and treated with CAS. An oligosaccharide-lactate chitosan (OLC) derivative was instilled in the left lung of neutropenic rats to induce chitotriosidase and AMCase activities. Rats instilled with OLC or with phosphate-buffered saline (PBS) were subsequently infected with A. fumigatus and then treated with suboptimal doses of CAS. Survival, histopathology, and galactomannan indexes were determined. Instillation of OLC resulted in chitotriosidase and AMCase activities. However, instillation of OLC did not prolong rat survival when rats were subsequently challenged with A. fumigatus. In 5 of 7 rats instilled with OLC, the fungal foci in the lungs were smaller than those in rats instilled with PBS. Instillation of OLC did not significantly enhance the survival of neutropenic rats challenged with A. fumigatus and treated with a suboptimal dosage of CAS. Chitotriosidase and AMCase activities can be induced with OLC, but the presence of active chitinases in the lung did not prevent the development of IPA or significantly enhance the therapeutic outcome of CAS treatment.
KEYWORDS: aspergillosis, caspofungin, chitin, chitinases, chitosan, echinocandin, immunotherapy, innate immune response
INTRODUCTION
Invasive pulmonary aspergillosis (IPA) is an opportunistic infection that occurs in patients whose immune defense mechanisms are seriously weakened (1). Currently, IPA is treated mainly with voriconazole (1, 2), but resistance against this drug is emerging (3). Echinocandins, such as caspofungin (CAS), target 1,3-β-d-glucan synthesis of most pathogenic fungi and have been used as salvage treatment in patients with refractory IPA (1). Patients treated with CAS either as a first-line treatment or as salvage therapy had higher mortality rates than patients treated with voriconazole (4). Unlike amphotericin B or the azoles, echinocandins do not display typical in vitro fungicidal or fungistatic activity against Aspergillus fumigatus. Exposure to CAS provides only an altered fungal growth: the fungus grows compact and branched under the influence of CAS (5, 6). Furthermore, the cell wall composition changes, with an anticipated decrease of 1,3-β-d-glucan and a compensatory chitin increase (6).
Mammals are known to produce two types of chitinases: chitotriosidase (CHIT) and acidic mammalian chitinase (AMCase). Chitotriosidase is an endochitinase produced by macrophages and polymorphonuclear neutrophils and can be found in the lungs of mammals as well as in lacrimal glands. AMCase is an exochitinase produced by macrophages and epithelial cells and is found mainly in the gastrointestinal tract of mammals although it is also found in the lungs at low concentrations (7–9). During experimental IPA infection, pulmonary CHIT and AMCase activities increased significantly (10). Surprisingly, the location of CHIT and AMCase expression inside the infected lung differed in CAS-treated rats from that in untreated infected rats (10). In CAS-treated rats both AMCase and CHIT seemed to bind to the fungal cell wall, whereas this was not observed in untreated infected rats (10). Apparently, CAS treatment caused such an alteration in the fungal cell wall that AMCase was able to bind the fungal hyphae, and CHIT was taken up by the fungus (10). Furthermore, in in vitro experiments, lysis of the fungal cell was demonstrated when hyphae were exposed to CAS and both CHIT and AMCase simultaneously, rendering CAS fungicidal in the presence of these chitinases (10).
In uninfected (neutropenic) rats, no chitinase activity is present in the lung (10). Chitinases can be produced in the presence of chitin and chitosan (11). Chitosan has an increased water solubility and can be produced commercially by deacetylation of chitin; the degree of deacetylation can differ considerably (12). Chemically modified in various formulations, chitosan has potential as biomaterial, drug carrier, wound dressing, and transfection agent (12). As such, different chitosan formulations have been successfully administered to the lungs of mice via intratracheal (i.t.) instillation or nebulization (13, 14). Chitosan particles delivered to the lungs did not increase the number of macrophages, neutrophils, or eosinophils in bronchoalveolar lavage fluids, and there was no increase in protein content or cytokines secreted (14). Furthermore, histological examination of lung tissue sections revealed no changes in tissue morphology after exposure to chitosan particles, indicating that chitosan particles can be administered safely to the lung to induce chitinase expression in the lung (14). In this study, we determined (i) whether instillation of different chitosan particles would induce chitinase activity in the lung, (ii) whether this chitinase activity would be able to prevent the development of a lethal IPA in neutropenic rats, and (iii) whether chitinases present in the lung would improve the therapeutic efficacy of CAS in treating IPA.
RESULTS
Chitosan in vitro susceptibility test.
Since chitosan has been demonstrated to have some antifungal activity, we first set out to ascertain that the chitosan derivatives chosen for this experiment had no antifungal activity against A. fumigatus isolate EMC01 (15, 16). In vitro, neither growth inhibition nor an ≥80% reduction of mitochondrial dehydrogenase activity was observed when medium-molecular-weight chitosan (MMWC), low-molecular-weight chitosan (LMWC), and oligosaccharide-lactate chitosan (OLC) derivatives were added to A. fumigatus conidia in concentrations ranging from 0.032 to 16 μg/ml in Sabouraud and RPMI 1640 growth media. This observation indicated that none of the tested derivatives inhibited fungal growth.
LMWC and OLC can induce chitinase activity in the lung.
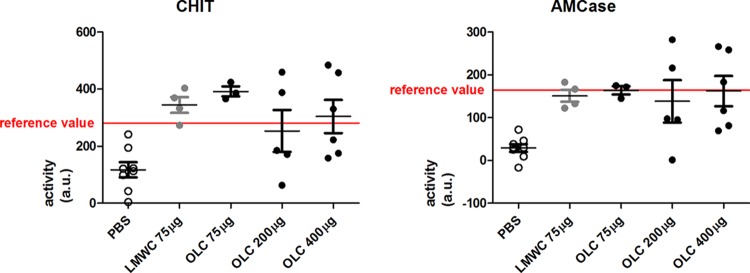
We next determined whether chitosan was able to induce CHIT and AMCase activities in the lungs of neutropenic female RP/AEur/RijHsd (RP) rats. We aimed to mimic a biological level of chitinase activity, i.e., one that is equal to that previously found in rat lungs at day 3 after fungal inoculation (10). Doses of 75 μg, 200 μg, and 400 μg of either LMWC or OLC derivatives were instilled in the left lung of uninfected, neutropenic rats at day −2. At day 0, the AMCase and CHIT activities were determined. As seen in Fig. 1, phosphate-buffered saline (PBS)-instilled rats had a median CHIT activity of 116.5 arbitrary units (AU) and AMCase activity of 29.9 AU.
FIG 1.
Chitotriosidase (CHIT) and AMCase levels after instillation of low-molecular-weight chitosan (LMWC) and oligosaccharide-lactate chitosan (OLC). (A) Activity measured at day 0 of CHIT in rat lungs instilled at day −2 with PBS, LMWC, and OLC, as indicated. As a reference value, chitotriosidase activity on day 3 in A. fumigatus-infected rats as measured by Verwer et al. (10) was used. (B) Activity measured at day 0 of AMCase in rat lungs instilled at day −2 with PBS, LMWC, and OLC, as indicated. As a reference value, chitotriosidase activity on day 3 in A. fumigatus-infected rats as measured by Verwer et al. (10) was used. au, arbitrary units.
Instillation of 75 μg/left lung LMWC or OLC significantly increased CHIT activity compared with its activity in PBS-installed rats (for LMWC, median of 350.9 AU, P = 0.004 in comparison to activity with PBS [Mann-Whitney]; for OLC, median of 386.1 AU, P = 0.012). Instillation of 200 μg/left lung and 400 μg/left lung did not further increase the CHIT activity in the lung (Fig. 1).
Instillation of 75 μg/left lung LMWC or OLC also significantly increased AMCase activity compared with that in PBS-instilled rats, with a median of 149.6 AU (Mann-Whitney, P = 0.004) and 171.9 AU (Mann-Whitney, P = 0.012), respectively. OLC at 75 μg induces slightly but nonsignificantly higher chitinase activity than 75 μg of LMWC (Fig. 1). Again, increasing the OLC concentration to 200 μg/left lung and 400 μg/left lung did not further increase the AMCase activity in the lung (P = 1.00, when activity with 75 μg/left lung was compared to that with 400 μg/left lung). These results prompted us to use OLC over LMWC for further investigation at a concentration of 400 μg/left lung as no obvious discomfort for the rats was noted. Administration of 400 μg/left lung OLC resulted in a median CHIT activity of 276 AU and a median AMCase activity of 149.5 AU, values which were comparable to the activities observed in rat lungs after a 3 days of IPA infection (median CHIT activity of 281 AU and median AMCase activity of 164 AU [10]).
Instillation of 400 μg/left lung OLC alone does not prevent IPA in neutropenic rats.
To determine whether instillation of 400 μg/left lung OLC alone will by itself enhance the survival of neutropenic rats infected with A. fumigatus, 9 rats were instilled with 400 μg/left lung OLC at day −2 (group OLC-NaCl), and 10 rats were instilled with PBS (group PBS-NaCl). On day 0, all rats were infected with A. fumigatus conidia. As seen in Fig. 2, all rats, irrespective of having OLC or PBS instilled, died between days 3 and 8 after fungal inoculation. There was no statistically significant difference in their survival rates (log rank, P = 0.39).
FIG 2.
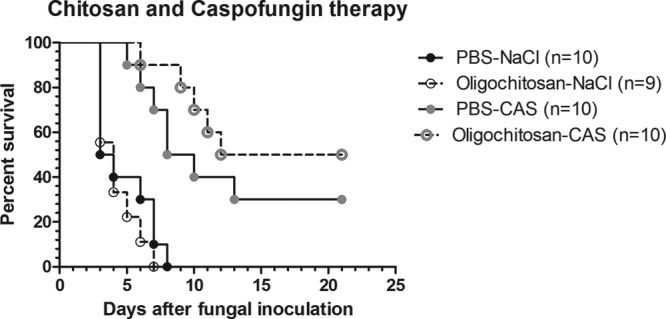
Percent survival of neutropenic rats with invasive pulmonary aspergillosis (IPA) after instillation of 400 μg/left lung oligosaccharide-lactate chitosan (OLC) with or without caspofungin (CAS) treatment. Rats were instilled with either PBS or 400 μg/left lung OLC and treated with either normal saline (PBS-NaCl or Oligochitosan-NaCl, respectively) or with a suboptimal dosage of CAS (PBS-CAS or Oligochitosan-CAS, respectively), as indicated.
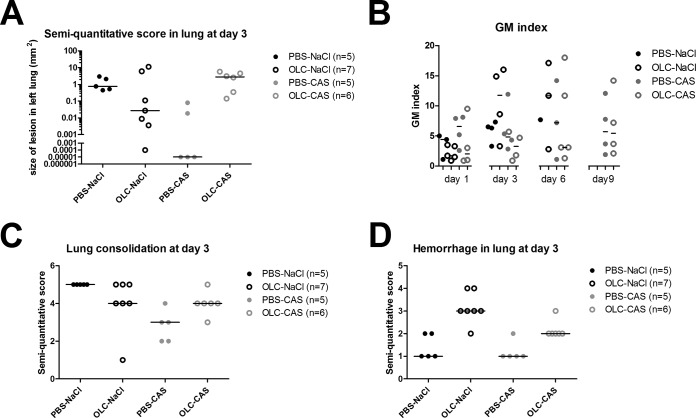
To determine whether instillation of 400 μg/left lung OLC influenced the fungal load on day 3 of infection, histopathology was performed (Fig. 3 and 4A) in a separate experiment with 5 to 7 rats per group. Compared to PBS-NaCl controls, OLC-NaCl rats showed small fungal foci in 5 out of 7 rats (Fig. 3A to H and 4A). However, the semiquantitative score was not significantly different (Mann-Whitney, P = 0.27). The heterogeneity observed in the lungs was also observed in the serum galactomannan (GM) indexes obtained for OLC-instilled rats. Again, when the total group of OLC-instilled rats was compared to the group of PBS-instilled rats on day 1 (Mann-Whitney, P = 0.39) and day 3 of infection (Mann-Whitney, P = 0.25)—when all rats were still alive—no significant difference in their GM indexes in serum was found, corresponding with the semiquantitative score. On day 6, not enough rats survived in the PBS-instilled group to perform statistical analysis.
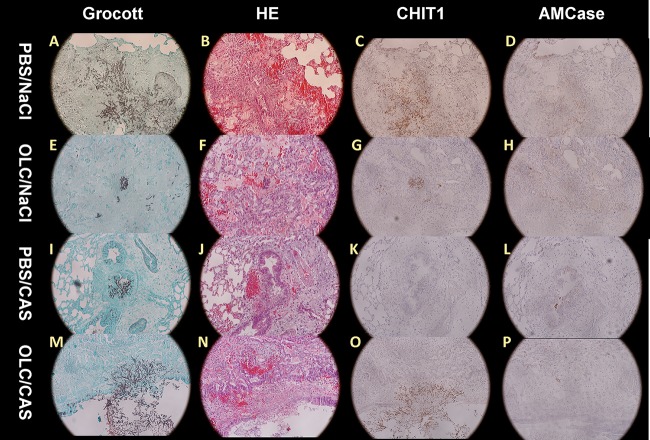
FIG 3.
Histopathology of A. fumigatus-infected lungs (magnification, ×200). Left lung sections obtained on day 3 of infection were stained with Grocott's stain, hematoxylin and eosin (H&E), chitotriosidase 1 (CHIT1), or AMCase. A. fumigatus hyphae were examined in PBS-instilled lungs treated with normal saline or with CAS and in lungs instilled with 400 μg of OLC and treated with normal saline or CAS, as indicated.
FIG 4.
Semiquantitative IPA lesion score in the lung (A), galactomannan (GM) index in serum (B), and semiquantitative histopathology in terms of lung consolidation (C) and hemorrhage in the lung (D). The GM indexes measured on days 1, 3, 6, and 9 after A. fumigatus inoculation were determined in sera of rats. The semiquantitative score in the lung was determined at day 3. Rats were divided into four groups. The number of rats differed per time point. The first group (PBS-NaCl) consisted of rats instilled with PBS on day −2, inoculated with A. fumigatus on day 0, and treated with NaCl from day 1 to day 10. There were no surviving animals at day 9; therefore no data could be displayed for the GM index for this group. The second group (OLC-NaCl) consisted of rats instilled with oligosaccharide-lactate chitosan (OLC) on day −2, inoculated with A. fumigatus on day 0, and treated with NaCl from day 1 to day 10. There were no surviving animals at day 9; therefore no data could be displayed for the GM index for this group. The third group (PBS-CAS) consisted of rats instilled with PBS on day −2, inoculated with A. fumigatus on day 0, and treated with caspofungin (CAS) from day 1 to day 10. The fourth group (OLC-CAS) consisted of rats instilled with OLC on day −2, inoculated with A. fumigatus on day 0, and treated with CAS from day 1 to day 10. Lung consolidation and hemorrhage were scored in lungs of rats at day 3 after infection. Scoring was according the following system: 5, >75% of lung affected; 4, 50 to 75% of lung affected; 3, 25 to 50% of lung affected; 2, 10 to 25% of lung affected; 1, <10% of lung affected.
Although smaller foci were obtained when rats were instilled with OLC, survival did not improve. We therefore performed hematoxylin and eosin (H&E) staining to look at the morphology in the lung. As seen in Fig. 4C it appeared that the lung consolidation of PBS-instilled rats (PBS-NaCl) was similar to that of OLC-instilled rats although the fungal foci in most lungs were smaller. In the PBS-instilled group, angioinvasion was noted in 4 out of 5 rats, while only 2 out of 7 rats had angioinvasion in the OLC-instilled group. Thrombosis was noted in all rats. However, we did observe a marked difference: OLC-instilled rats showed large zones of hemorrhage in the lungs, which were not observed in PBS-instilled rats (Fig. 4D) (Mann-Whitney, P = 0.007). To determine if the smaller fungal foci were the result of a higher chitotriosidase and/or AMCase expression level at the site of infection, immunohistochemistry was performed. As seen in Fig. 3 and 5, overall chitotriosidase expression was low. Expression was noted mostly in the epithelium of the bronchia, or CHIT was attached to the fungal hyphae (Fig. 3C and G). No marked difference was noted between rats instilled with PBS (PBS-NaCl) and those instilled with OLC (OLC-NaCl). AMCase expression was noted mostly in the goblet cells of the epithelium of the bronchia (Fig. 3D and H and 5A and B) and in macrophages in the tissue. In OLC (OLC-NaCl)-instilled rats more macrophages expressing AMCase were observed in tissue than in PBS (PBS-NaCl)-instilled rats (Fig. 5A and B).
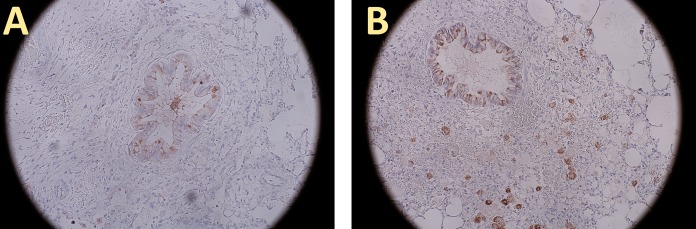
FIG 5.
AMCase expression in lungs instilled with PBS or OLC. AMCase expression, shown in brown, in PBS-instilled lungs (A) was seen mainly in goblet cells of the bronchial epithelium. Only a few macrophages stained positive. In OLC-instilled lungs (B), expression was also noted in macrophages. Magnification, ×200.
Instillation of 400 μg/left lung OLC does not enhance the therapeutic efficacy of CAS in neutropenic rats with IPA.
In a previous study it was demonstrated that AMCase and CHIT could enhance the activity of CAS in vitro (10). We therefore determined whether instillation of 400 μg/left lung OLC would enhance the survival of neutropenic rats with IPA and treated with a suboptimal dosage of CAS (OLC-CAS group). Control rats were instilled with PBS, infected with A. fumigatus, and treated with the same suboptimal dosage of CAS (PBS-CAS group). As shown in Fig. 2, 30% of infected rats in the PBS-CAS group survived this infection. This was significantly more than survived in the PBS-NaCl-treated group (log rank, P = 0.0016). Rats receiving combination treatment (OLC-CAS group) showed a further delayed death rate compared to that of the PBS-NaCl controls, and more rats (50%) survived at 21 days, a statistically significant difference (P < 0.0001). However, the difference in outcomes did not reach statistical significance (P = 0.26) when the group of rats receiving combination treatment (OLC-CAS) was compared with the group receiving CAS monotherapy (PBS-CAS). To determine if there was a difference in fungal loads between these groups on day 3 of infection, we performed histopathology and determined GM indexes. Figure 3C shows that the fungal foci were small in rats treated with CAS monotherapy (PBS-CAS), and the semiquantitative score indicated that the size of the fungal biomass was significantly reduced in rats receiving CAS monotherapy (PBS-CAS) compared to that in the untreated controls (PBS-NaCl) on day 3 of infection (Mann-Whitney, P = 0.01). However, fungal biomass was significantly larger in the OLC-CAS group than in rats in the PBS-CAS group (Mann-Whitney, P = 0.0075). This finding is not supported by the fungal marker in the serum. As can be seen in Fig. 4B, on days 1, 3, and 6 of infection, the median GM indexes of the OLC-CAS group were lower than those of PBS-CAS group. However, this difference was not statistically significant (Mann-Whitney, P = 0.49, P = 0.49, and P = 1.00, respectively, for days 1, 3, and 6).
Since the fungal biomass was larger in lungs of the OLC-CAS group than in those of the PBS-CAS group, we determined if there were also marked differences in the histopathology of the infected lungs. As seen in Fig. 4C, a larger portion of the lung was consolidated in the OLC-CAS group than in the PBS-CAS group (Mann-Whitney, P = 0.042). Furthermore, larger zones of hemorrhage were noted in the lungs of rats belonging to the OLC-CAS group than in those of rats belonging to the PBS-CAS group (Mann-Whitney, P = 0.014). No difference in the degrees of angioinvasion or thrombosis was noted between the two groups. As demonstrated in the OLC-PBS-treated group of rats, instillation of OLC did not result in marked differences in chitotriosidase expression levels, but some differences were noted in AMCase expression levels. More macrophage expression AMCase was noted in OLC-instilled rats. We therefore determined if there was a marked difference in chitotriosidase and/or AMCase expression levels between the PBS-CAS and OLC-CAS groups. Again, no difference in chitotriosidase activities was noted. In most rats chitotriosidase was hardly expressed, and if expressed, it was often attached to the fungal hyphae. AMCase expression levels again differed between OLC-instilled rats and PBS-instilled rats. In both groups AMCase expression was noted in goblet cells of the epithelium of the bronchia, but AMCase expression in macrophages occurred only in the OLC-CAS group.
DISCUSSION
We previously demonstrated that A. fumigatus hyphae exposed to CAS and both CHIT and AMCase were lysed. Exposure to caspofungin results in increased chitin content in the A. fumigatus cell wall (6, 17). In normal A. fumigatus hyphae, chitin is masked by 1,3-β-d-glucan and galactomannan (18, 19); but when hyphae are treated with CAS, 1,3-β-d-glucan is no longer formed, and galactomannan is released from the cell wall, which results in unmasking of chitin (18, 19). We therefore hypothesized that the therapeutic efficacy of CAS could be enhanced by the presence of both AMCase and CHIT at the site of infection. Indeed, the results of this study show a tendency toward a reduced death rate in infected rats instilled with OLC and treated with CAS afterwards (OLC-CAS group). The difference, however, was not statistically significant, potentially due to the small number of animals used in this study or the hemorrhage induced in the lung by OLC instillation. Also, galactomannan indexes appeared to be lower, but again the difference was not significant. Paradoxically, histopathologically, the sites of infection in the lungs appeared to be larger in rats receiving combination treatment with OLC and CAS than in rats receiving CAS monotherapy. The lower serum galactomannan indexes would indicate that dissemination of the fungus from the site of infection was prevented. On the other hand, it has been reported that there is an lack of association between decreased local fungal burden in the lung and fungal burden as determined by serum galactomannan levels, quantitative PCR (qPCR), or CFU counts (20). In the study of Hsu et al., a semiquantitative scale to assess the local burden of A. fumigatus in the lungs and qPCR and CFU counts of lung and kidney tissues were used (20). These investigators did observe statistically significant differences in the burden of infection in the lung by histopathology but not in terms of qPCR results or CFU counts in the lung or kidneys (20). This discrepancy occurs especially in the presence of CAS. In vitro it has been demonstrated that when 1,3-β-d-glucan synthesis is inhibited by, for instance, CAS, detachment of the galactomannan chains from the β-glucan polymers occurs (10), which results in enhanced release of galactomannan into culture supernatants (18). When this also happens in vivo, the detached galactomannan polymers will be released in blood, thereby resulting in a high GM index even when the fungal focus is still small.
In order to demonstrate whether the presence of CHIT and AMCase activities in the lung could enhance the therapeutic efficacy of CAS, CHIT and AMCase activities needed to be induced in the lung first. In the past, several approaches have been taken to induce chitinase activity in animals. In one study, Chinese hamster ovary (CHO) cells were transfected with the human CHIT gene in a mammalian expression system; they were encapsulated in alginate microspheres and implanted subcutaneously in mice 3 days before infection with Cryptococcus neoformans (21). CHIT activity was detected in blood samples taken from animals engrafted with these CHO cells. The median survival time was 1 day longer in the CHIT transfected mice than in the control mice, which was statistically significant (21). No significant reduction of fungal burden in lungs or brain was found (21). A more prominent level of CHIT protection was obtained in studies in which the in vivo efficacy of recombinant human CHIT was assessed in neutropenic-mouse models of systemic candidiasis and systemic aspergillosis (8). In these studies mice were treated with recombinant human CHIT by intraperitoneal (i.p.) injections for 10 consecutive days (8). In the Candida albicans-infected mice, at day 14 all mice died in the control group, while 80% of the mice receiving 100 mg/kg recombinant human CHIT were still alive at that time point (8). In the aspergillosis group, all mice in the control group had died at day 8, while 30% survival was still seen in the 80-mg/kg group (8). Based on these studies, it appears that high CHIT activity throughout the infection could help to protect against a lethal infection. However, the manner in which CHIT is provided is important, especially since CHIT seems to disappear rapidly from the circulation. In the case of recombinant CHIT, 90% of the protein was lost at 1 h postdose (8). We therefore hypothesized that CHIT and AMCase activities needed to be induced locally at the site of infection instead of systemically and that induction with a chitin- or chitosan-containing molecule might result in more stable chitinase expression.
Chitosan preparations including oligomers have been used as carriers for plasmid DNA in gene therapy (13, 22, 23). To deliver plasmid DNA to the lungs, either liquid chitosan polyplexes (13, 23) or dry-powder chitosan preparations (22) have been prepared for effective delivery directly to the lungs of mice (22). Therefore, chitosan preparations seemed promising. However, highly deacetylated chitosan oligosaccharides (24) and LMWC of 70 kDa (15) were demonstrated to inhibit the growth of several plant-pathogenic species, including Fusarium spp. (15, 24). We therefore chose two chitosan preparations, LMWC and OLC, which were demonstrated not to inhibit the growth of our A. fumigatus isolate. The two chitosans chosen were highly deacetylated. Fully deacetylated chitosan was demonstrated to result in inhibition of recombinant human CHIT activity (25). In our experimental setting we were able to induce CHIT and AMCase activities in the lung with >75% deacetylated LMWC and >90% deacetylated OLC, with CHIT and AMCase activities comparable to those measured after 3 days of infection with A. fumigatus (10). When we looked histologically, we noted that of the two chitinases studied, AMCase was more prominently expressed at 3 days after infection than CHIT. Expression of AMCase was noted in goblet cells and macrophages, while some expression of CHIT was found in the bronchial epithelium and on fungal hyphae in A. fumigatus-infected rats. A difference was noted between OLC-instilled rats and PBS-instilled rats only with respect to AMCase. More AMCase-expressing macrophages were seen in OLC-instilled rats infected with A. fumigatus than in PBS-instilled rats infected with A. fumigatus. These results indicate that in our experimental setting, we were also able to induce the secretion of chitinase proteins and induce the activity thereof and that, at least at the time of infection, histologically, mainly AMCase expression was noted. Gudmundsdottir et al. (12) also demonstrated that it was possible to induce the secretion of CHIT protein after addition of either >90% deacetylated OLC or chitosan to primary human macrophages (12).
As demonstrated in this study, the CHIT and AMCase activities induced in the lungs of neutropenic rats were able to reduce the size of the A. fumigatus lesions in the left lung in 5 out of 7 rats, but they were not able to prolong the survival of A. fumigatus-infected rats when no antifungal agent was added. The reduction in size of the fungal foci could be the result of the direct antifungal activities of CHIT and AMCase or a combination thereof, but based on our immunohistochemistry results, the most likely candidates are the AMCase-expressing macrophages as more AMCase-expressing macrophages were noted in OLC-instilled lungs than in PBS-instilled lungs. These chitinases could have a direct fungicidal effect on fungi as Boot et al. had demonstrated that recombinant human CHIT can degrade cell wall chitin of Candida albicans (26). van Eijk et al. demonstrated that C. albicans, C. neoformans, and Mucor rouxii were growth inhibited when recombinant CHIT was added to the culture medium (8). A. fumigatus hyphae collapsed in the presence of recombinant AMCase and CHIT but only after exposure to caspofungin (10), which likely resulted in the unmasking of chitin in the fungal cell wall. The reduction of the fungal foci after induction of AMCase expression was not enough to prolong rat survival. The reason for this could be that although the fungal focus was reduced in size, lung consolidation was not. It was expected that with smaller fungal foci, less consolidation of the lung would be observed. In our experimental setting, less consolidation was indeed observed in the PBS-CAS group compared to the PBS-NaCl group. However, in the OLC-NaCl group, consolidation was similar to that of the PBS-NaCl group. Furthermore, when the PBS-NaCl and OLC-NaCl groups were examined histopathologically, it was noted that more hemorrhage in the lung was observed in the OLC-NaCl group. Hemorrhage was not noted in the PBS-CAS group. In addition to inducing chitinases, chitosan has hemostatic activity. However, the hemostatic mechanisms differ with the type of chitosan used. Highly deacetylated chitosan is less effective in forming erythrocyte clots and absorbs fewer platelets than chitosan with a low degree of deacetylation (27). The hemorrhagic regions found in the lung after OLC instillation and the large regions of lung consolidation could be the reason why no enhanced survival was noted after OLC instillation. This enhanced hemorrhage was also noted in rats instilled with OLC and treated with CAS (OLC-CAS), but the extent of hemorrhage was less than that in OLC-NaCl-treated rats (Mann-Whitney, P = 0.02). Since hemostatic activity differs with the chitosan molecule used, more promising results could be obtained when a different chitosan molecule is used, especially since we did observe more AMCase-producing macrophages in the lungs of OLC-induced rats than in those of PBS-induced rats.
Surprisingly, the fungal foci of the OLC-CAS group were larger than the fungal foci of the OLC-NaCl group and the PBS-CAS group and were comparable to those of the PBS-NaCl group, but a much higher overall survival was obtained in the OLC-CAS group than in the PBS-NaCl group even though fungal foci were similar. Lung consolidation appeared to be slightly less in the OLC-CAS group than in the PBS-NaCl group, and the degree of hemorrhage was slightly greater. This indicates that chitinase induction with caspofungin treatment indeed does have a beneficial effect on overall survival as rats are able to survive with a much higher fungal burden in the lung, but that survival could be enhanced even more when a different chitosan molecule is chosen. The optimal chitosan molecule would be one that does not induce hemorrhage and lung consolidation in the host, that induces AMCase and CHIT expression, and that is able to inhibit fungal growth on its own. In future experiments, these chitosan molecules should be explored to determine if they would enhance the therapeutic efficacy of CAS. In conclusion, in this study we demonstrated that >90% deacetylated chitosan could induce CHIT and AMCase activities in lungs of neutropenic rats. However, the induction of CHIT and AMCase activities in the lungs of neutropenic rats did not prevent the development of IPA but resulted in smaller lesions in the lungs and less hemorrhage in the lungs. Combining a suboptimal dosage of CAS with chitosan instillation did not prolong survival statistically significantly. Since other investigators have reported that administration of recombinant CHIT did prolong survival in laboratory animals, induction of CHIT and/or AMCase might still be a feasible approach to prevent the formation of IPA, but other methods need to be developed for more prominent and persistent chitinase production in the lung. An option might be to use an inhalation device in which chitosan can be administered once daily to help prevent or treat infection with A. fumigatus. Nebulization or inhalation of chitosan polyplexes might offer a more constant exposure to chitosan and induce more stable CHIT and AMCase activities over time.
MATERIALS AND METHODS
A. fumigatus strain and chitosan derivatives.
A. fumigatus strain EMC01 originally isolated from a hematological patient with IPA was used in all experiments. To maintain the strain's virulence, it was regularly passed through neutropenic rats and maintained on Sabouraud maltose agar slants. Medium-molecular-weight chitosan (MMWC) (catalog number 448877; Sigma-Aldrich Chemie GmbH, Steinheim, Germany), low-molecular-weight chitosan (LMWC) (448869; Sigma-Aldrich Chemie GmbH, Steinheim, Germany), and oligosaccharide-lactate chitosan (OLC) (523682; Sigma-Aldrich Chemie GmbH, Steinheim, Germany) derivatives were tested to evaluate the effect of chitosan on the growth of A. fumigatus in vitro. MMW and LMW chitosans were 75 to 85% deacetylated and had molecular masses of 190 to 310 kDa and 50 to 190 kDa, respectively. OLC was >90% deacetylated and had a molecular mass of 5 kDa.
Chitosan in vitro susceptibility test.
For MMW and LMW chitosans, stock dilutions of 20 mg/ml were prepared as described in Palma Guerrero et al. and Benhamou et al. (15, 16). In brief, chitosans were dissolved in 0.25 mol/liter HCl under continuous stirring, and pH was adjusted to 5 to 6 with 1 mol/liter NaOH. Subsequently the solution was dialyzed and autoclaved prior to its use in fungal growth assays. A stock solution of 20 mg/ml for OLC was prepared in sterile PBS. In vitro experiments were performed in Sabouraud and RPMI 1640 media as described in the M38-A2 microdilution protocol of the Clinical and Laboratory Standards Institute (CLSI) (28). The final concentrations of the chitosans ranged from 0.032 to 16 μg/ml, and MIC endpoints were determined visually. After the samples were incubated at 37°C for 48 h, the inhibitory concentration endpoints were defined as the first concentration at which spectrophotometrically an 80% or more reduction of mitochondrial dehydrogenase activity occurred.
In vivo induction of chitinase activity with chitosan.
Female strain RP/AEur/RijHsd albino rats were immunosuppressed by the administration of cyclophosphamide (Endoxan; Baxter, Utrecht, The Netherlands) in doses of 75 mg/kg bodyweight and of 60 mg/kg body weight at day 5 and day 1, respectively, before A. fumigatus infection to induce neutropenia as described earlier (10).
To induce chitinase activity, both LMWC and OLC were investigated. The MMWC was difficult to dissolve under the experimental conditions and was, therefore, not investigated in rats. A stock solution of 10 mg/ml for LMWC was prepared in 0.1 M PBS (pH 5.9) under continuous stirring. Subsequently, the stock was further diluted to obtain the indicated experimental concentration in PBS (final pH of 6.3) (Fig. 1). Purified OLC was dissolved under continuous stirring in (PBS) (final pH, 6.2). For the vehicle solution PBS (final pH, 6.4) was used (20 μl 0.1 M HCl was added to the PBS to obtain pH 6.25, comparable to the chitosan solutions). To induce chitinase activity in vivo, the neutropenic rats were intratracheally (i.t.) inoculated by passing a cannula through a tube into the left lung and administering 40 μl of chitosan solution on day −2 to obtain chitosan dosages of 75, 200, or 400 μg/left lung. In total, 4 rats received 75 μg/left lung LMWC, 3 rats received 75 μg/left lung OLC, 5 rats received 200 μg/left lung OLC, and 6 rats received 400 μg/left lung OLC. As a negative control, a subgroup of rats were inoculated with 40 μl of PBS/left lung (n = 8 rats). The aim was to obtain CHIT and AMCase activities of 281 arbitrary units (AU) and 164 AU per lung, respectively (values comparable to those measured 3 days after inoculation of A. fumigatus in our neutropenic-rat model of IPA [10]). Subsequently, the rats were sacrificed on day 0 by CO2 exposure. The left lung was removed and stored at −80°C until further analysis.
Measurement of chitinase activity.
CHIT and AMCase activities were measured in homogenized lungs with a commercial fluorimetric chitinase kit (Sigma-Aldrich Chemie GmbH, Steinheim, Germany). The enzyme activities of 4-methylumbelliferyl N,N′-diacetyl-β-d-chitobioside (corresponding with AMCase activity) and 4-methylumbelliferyl-β-d-N,N′,N″-triacetylchitotriose (corresponding with CHIT activity) were determined. The chitinase activity was expressed as the number of arbitrary units per lung, as described previously (10).
In vivo survival of A. fumigatus-infected neutropenic rats after induction of chitinase activity.
To determine whether 400 μg/left lung OLC would prevent the development of lethal IPA in A. fumigatus-infected rats, 9 or 10 rats were immunosuppressed by the administration of cyclophosphamide (Endoxan; Baxter, Utrecht, The Netherlands) in doses of 75, 60, 50, and 40 mg/kg bodyweight at day 5 and day 1 before fungal inoculation and at day 3 and day 7 after fungal inoculation, respectively (10). In the first group of rats (group OLC-NaCl; n = 9), chitinase activity was induced on day −2 by intratracheal instillation of 40 μl of OLC to obtain a final concentration of 400 μg/left lung OLC. A second group of rats (group PBS-NaCl; n = 10) was inoculated with PBS and served as a negative control. On day 0 a left-sided pulmonary infection was induced with the A. fumigatus strain EMC01 by the instillation 20 μl of PBS containing 6 × 104 conidia of A. fumigatus, as described earlier (10, 29, 30).
To determine whether 400 μg/left lung OLC would enhance the efficacy of CAS treatment in 10 rats that were immunosuppressed by the above-mentioned regimen, a suboptimal CAS dose of 0.75 mg/kg was administered intraperitoneally at 16 h after fungal inoculation and then once daily for 10 days. Treatment with this CAS dosage resulted in a suboptimal effect in terms of only ∼31% of animal survival (31). Hence, this CAS dosage was chosen to investigate the potential increase in therapeutic efficacy after chitinase priming of the lung. Two different control groups were used. In the first group, rats were instilled with the chitosan solvent, infected with A. fumigatus, and treated with the suboptimal dosage of CAS (group PBS-CAS; n = 10). In the second group, rats were instilled with 400 μg/left lung OLC, infected with A. fumigatus, and treated with CAS solvent (group OLC-CAS; n = 10). The survival of rats was monitored for 21 days after fungal inoculation. The infection, if untreated, resulted in 100% mortality of rats within 3 to 10 days.
Histological semiquantitative assessment of fungal burden and lung pathology in rats infected with A. fumigatus.
In a separate group of rats, lungs were dissected at day 3 postinfection. In this experiment, group PBS-NaCl consisted of 5 rats, group OLC-NaCl consisted of 7 rats, group PBS-CAS consisted of 5 rats, and group OLC-CAS consisted of 6 rats. Lungs were fixed in buffered formalin and embedded in paraffin and processed for histology using standard techniques. A cross section of the lung was taken, and histopathology slides were stained with hematoxylin and eosin (H&E) and Grocott's stain. To determine the extent of the lesion, fungal foci were measured under a microscope using a reticule at a magnification of ×100. To determine the extent of lung consolidation and hemorrhage in the lung, lungs were observed under a microscope, and the region of consolidation or hemorrhage was determined semiquantitatively according to the following scoring system: consolidation or hemorrhage of <10% of the lung, 1; 10 to 25% of the lung, 2; 25 to 50% of the lung, 3; 50 to 75%,4; >75%, 5. Furthermore, presence or absence of angioinvasion or thrombosis was recorded.
Histological assessment of chitotriosidase and AMCase expression.
In the same group used for the histological semiquantitative assessment of the fungal burden, chitotriosidase and AMCase expression was demonstrated by immunohistochemistry using antibodies against chitotriosidase (H-66 at 1:75; Santa Cruz Biotechnology, Santa Cruz, CA) and AMCase (Y-14 at 1:50; Santa Cruz Biotechnology, Santa Cruz, CA) using a previously described protocol (10).
Galactomannan index.
Venous blood was obtained from the tail vein to assess the fungal load in blood on days 1, 3, 6, and 9 by measuring serum galactomannan (GM index), using a commercially available system according to the manufacturer's instructions (Platelia Aspergillus EIA; Bio-Rad, Marnes-la-Coquette, France). In this experiment, groups consisted of 9 to 10 animals at the start of the experiment. On days 1 and 6, blood was taken from 5 animals per group, and on days 3 and 9 blood was taken from the other 5 animals in that group. Since during infection animals died, the actual number of animals differed per day measured, and at day 9, no GM index could be determined for groups PBS-NaCl and OLC-NaCl due to the lack of surviving animals.
Ethical statement.
The experimental protocols used in this study adhered to the rules laid down in the Dutch Animal Experimentation Act and the EU Animal Directive 2010/63/EU. The Institutional Animal Care and Use Committee of the Erasmus University Medical Centre Rotterdam approved the present protocols (permit number EMC 2884).
Statistical analysis.
Kaplan-Meier survival curves were generated, and the differences in rat survival rates were assessed by a log rank test (GraphPad Prism, version 5.0). The (semi)quantitative parameters of fungal infection were assessed by using a nonparametric Mann-Whitney U-test (GraphPad Prism, version 5.0). A P value of <0.05 was considered statistically significant.
REFERENCES
- 1.Zmeili OS, Soubani AO. 2007. Pulmonary aspergillosis: a clinical update. QJM 100:317–334. doi: 10.1093/qjmed/hcm035. [DOI] [PubMed] [Google Scholar]
- 2.Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, Oestmann JW, Kern WV, Marr KA, Ribaud P, Lortholary O, Sylvester R, Rubin RH, Wingard JR, Stark P, Durand C, Caillot D, Thiel E, Chandrasekar PH, Hodges MR, Schlamm HT, Troke PF, de Pauw B. 2002. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med 347:408–415. doi: 10.1056/NEJMoa020191. [DOI] [PubMed] [Google Scholar]
- 3.Verweij PE, Chowdhary A, Melchers WJ, Meis JF. 2016. Azole resistance in Aspergillus fumigatus: can we retain the clinical use of mold-active antifungal Azoles? Clin Infect Dis 62:362–368. doi: 10.1093/cid/civ885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raad II, Zakhem AE, Helou GE, Jiang Y, Kontoyiannis DP, Hachem R. 2015. Clinical experience of the use of voriconazole, caspofungin or the combination in primary and salvage therapy of invasive aspergillosis in haematological malignancies. Int J Antimicrob Agents 45:283–288. doi: 10.1016/j.ijantimicag.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Lamaris GA, Lewis RE, Chamilos G, May GS, Safdar A, Walsh TJ, Raad II, Kontoyiannis DP. 2008. Caspofungin-mediated beta-glucan unmasking and enhancement of human polymorphonuclear neutrophil activity against Aspergillus and non-Aspergillus hyphae. J Infect Dis 198:186–192. doi: 10.1086/589305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verwer PE, van Duijn ML, Tavakol M, Bakker-Woudenberg IA, van de Sande WW. 2012. Reshuffling of Aspergillus fumigatus cell wall components chitin and β-glucan under the influence of caspofungin or nikkomycin Z alone or in combination. Antimicrob Agents Chemother 56:1595–1598. doi: 10.1128/AAC.05323-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boot RG, Blommaart EF, Swart E, Ghauharali-van der Vlugt K, Bijl N, Moe C, Place A, Aerts JM. 2001. Identification of a novel acidic mammalian chitinase distinct from chitotriosidase. J Biol Chem 276:6770–6778. doi: 10.1074/jbc.M009886200. [DOI] [PubMed] [Google Scholar]
- 8.van Eijk M, van Roomen CP, Renkema GH, Bussink AP, Andrews L, Blommaart EF, Sugar A, Verhoeven AJ, Boot RG, Aerts JM. 2005. Characterization of human phagocyte-derived chitotriosidase, a component of innate immunity. Int Immunol 17:1505–1512. doi: 10.1093/intimm/dxh328. [DOI] [PubMed] [Google Scholar]
- 9.Reese TA, Liang HE, Tager AM, Luster AD, Van Rooijen N, Voehringer D, Locksley RM. 2007. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature 447:92–96. doi: 10.1038/nature05746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verwer PE, ten Kate MT, Falcone FH, Morroll S, Verbrugh HA, Bakker-Woudenberg IA, van de Sande WW. 2013. Evidence supporting a role for mammalian chitinases in efficacy of caspofungin against experimental aspergillosis in immunocompromised rats. PLoS One 8:e75848. doi: 10.1371/journal.pone.0075848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorzelanny C, Poppelmann B, Pappelbaum K, Moerschbacher BM, Schneider SW. 2010. Human macrophage activation triggered by chitotriosidase-mediated chitin and chitosan degradation. Biomaterials 31:8556–8563. doi: 10.1016/j.biomaterials.2010.07.100. [DOI] [PubMed] [Google Scholar]
- 12.Gudmundsdottir S, Lieder R, Sigurjonsson OE, Petersen PH. 2015. Chitosan leads to downregulation of YKL-40 and inflammasome activation in human macrophages. J Biomed Mater Res A 103:2778–2785. doi: 10.1002/jbm.a.35417. [DOI] [PubMed] [Google Scholar]
- 13.Koping-Hoggard M, Issa MM, Kohler T, Tronde A, Varum KM, Artursson P. 2005. A miniaturized nebulization catheter for improved gene delivery to the mouse lung. J Gene Med 7:1215–1222. doi: 10.1002/jgm.762. [DOI] [PubMed] [Google Scholar]
- 14.Aragao-Santiago L, Hillaireau H, Grabowski N, Mura S, Nascimento TL, Dufort S, Coll JL, Tsapis N, Fattal E. 2016. Compared in vivo toxicity in mice of lung delivered biodegradable and non-biodegradable nanoparticles. Nanotoxicology 10:292–302. doi: 10.3109/17435390.2015.1054908. [DOI] [PubMed] [Google Scholar]
- 15.Palma-Guerrero J, Jansson HB, Salinas J, Lopez-Llorca LV. 2008. Effect of chitosan on hyphal growth and spore germination of plant-pathogenic and biocontrol fungi. J Appl Microbiol 104:541–553. [DOI] [PubMed] [Google Scholar]
- 16.Benhamou N, Lafontaine PJ, Nicole M. 1994. Induction of systemic resistance to Fusarium crown and root rot in tomato plants by seed treatment with chitosan. Phytopathology 84:1432–1444. doi: 10.1094/Phyto-84-1432. [DOI] [Google Scholar]
- 17.Walker LA, Lee KK, Munro CA, Gow NA. 2015. Caspofungin treatment of Aspergillus fumigatus results in ChsG-dependent upregulation of chitin synthesis and the formation of chitin-rich microcolonies. Antimicrob Agents Chemother 59:5932–5941. doi: 10.1128/AAC.00862-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dichtl K, Samantaray S, Aimanianda V, Zhu Z, Prevost MC, Latge JP, Ebel F, Wagener J. 2015. Aspergillus fumigatus devoid of cell wall beta-1,3-glucan is viable, massively sheds galactomannan and is killed by septum formation inhibitors. Mol Microbiol 95:458–471. doi: 10.1111/mmi.12877. [DOI] [PubMed] [Google Scholar]
- 19.Erwig LP, Gow NA. 2016. Interactions of fungal pathogens with phagocytes. Nat Rev Microbiol 14:163–176. doi: 10.1038/nrmicro.2015.21. [DOI] [PubMed] [Google Scholar]
- 20.Hsu JL, Khan MA, Sobel RA, Jiang X, Clemons KV, Nguyen TT, Stevens DA, Martinez M, Nicolls MR. 2013. Aspergillus fumigatus invasion increases with progressive airway ischemia. PLoS One 8:e77136. doi: 10.1371/journal.pone.0077136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gordon-Thomson C, Kumari A, Tomkins L, Holford P, Djordjevic JT, Wright LC, Sorrell TC, Moore GP. 2009. Chitotriosidase and gene therapy for fungal infections. Cell Mol Life Sci 66:1116–1125. doi: 10.1007/s00018-009-8765-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohri K, Okuda T, Mori A, Danjo K, Okamoto H. 2010. Optimized pulmonary gene transfection in mice by spray-freeze dried powder inhalation. J Control Release 144:221–226. doi: 10.1016/j.jconrel.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 23.Mohammadi Z, Dorkoosh FA, Hosseinkhani S, Gilani K, Amini T, Najafabadi AR, Tehrani MR. 2011. In vivo transfection study of chitosan-DNA-FAP-B nanoparticles as a new non viral vector for gene delivery to the lung. Int J Pharm 421:183–188. doi: 10.1016/j.ijpharm.2011.09.029. [DOI] [PubMed] [Google Scholar]
- 24.Kim SW, Park JK, Lee CH, Hahn BS, Koo JC. 2016. Comparison of the antimicrobial properties of chitosan oligosaccharides (COS) and EDTA against Fusarium fujikuroi causing rice bakanae disease. Curr Microbiol 72:496–502. doi: 10.1007/s00284-015-0973-9. [DOI] [PubMed] [Google Scholar]
- 25.Chen L, Zhou Y, Qu M, Zhao Y, Yang Q. 2014. Fully deacetylated chitooligosaccharides act as efficient glycoside hydrolase family 18 chitinase inhibitors. J Biol Chem 289:17932–17940. doi: 10.1074/jbc.M114.564534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boot RG, Renkema GH, Strijland A, van Zonneveld AJ, Aerts JM. 1995. Cloning of a cDNA encoding chitotriosidase, a human chitinase produced by macrophages. J Biol Chem 270:26252–26256. doi: 10.1074/jbc.270.44.26252. [DOI] [PubMed] [Google Scholar]
- 27.Yang J, Tian F, Wang Z, Wang Q, Zeng YJ, Chen SQ. 2008. Effect of chitosan molecular weight and deacetylation degree on hemostasis. J Biomed Mater Res B Appl Biomater 84:131–137. doi: 10.1002/jbm.b.30853. [DOI] [PubMed] [Google Scholar]
- 28.CLSI. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard—2nd ed CLSI document M38-A2. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 29.van de Sande WW, van Vianen W, ten Kate MT, Vissers J, Laurijsens J, Tavakol M, Rijnders BJ, Mathot RA, Bakker-Woudenberg IA. 2008. Caspofungin prolongs survival of transiently neutropenic rats with advanced-stage invasive pulmonary aspergillosis. Antimicrob Agents Chemother 52:1345–1350. doi: 10.1128/AAC.00536-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Vianen W, de Marie S, ten Kate MT, Mathot RA, Bakker-Woudenberg IA. 2006. Caspofungin: antifungal activity in vitro, pharmacokinetics, and effects on fungal load and animal survival in neutropenic rats with invasive pulmonary aspergillosis. J Antimicrob Chemother 57:732–740. doi: 10.1093/jac/dkl015. [DOI] [PubMed] [Google Scholar]
- 31.Refos JM, Vonk AG, ten Kate MT, Eadie K, Verbrugh H, Bakker-Woudenberg IAJM, van de Sande WWJ. 2017. Addition of 17-(allylamino)-17-demethoxygeldanamycin to a suboptimal caspofungin treatment regimen in neutropenic rats with invasive pulmonary aspergillosis delays the time to death but does not enhance the overall therapeutic efficacy. PLoS One 12:e0180961. doi: 10.1371/journal.pone.0180961. [DOI] [PMC free article] [PubMed] [Google Scholar]